Abstract
Somatostatin (SST) deficits are common pathological features in depression and other neurological disorders with mood disturbances, but little is known about the contribution of SST deficits to mood symptoms or causes of these deficits. Here we show that mice lacking Sst (SstKO) exhibit elevated behavioral emotionality, high basal plasma corticosterone and reduced gene expression of Bdnf, Cortistatin, and Gad67, together recapitulating behavioral, neuroendocrine and molecular features of human depression. Studies in SstKO and heterozygous (SstHZ) mice show that elevated corticosterone is not sufficient to reproduce the behavioral phenotype, suggesting a putative role for Sst cell-specific molecular changes. Using laser-capture microdissection, we show that cortical SST-positive interneurons display significantly greater transcriptome deregulations after chronic stress compared to pyramidal neurons. Protein translation through eukaryotic initiation factor 2 (EIF2) signaling, a pathway previously implicated in neurodegenerative diseases, was most affected and suppressed in stress-exposed SST neurons. We then show that activating EIF2 signaling through EIF2 kinase inhibition mitigated stress-induced behavioral emotionality in mice. Together, our data suggest that (1) low SST plays a causal role in mood-related phenotypes, (2) deregulated EIF2-mediated protein translation may represent a mechanism for vulnerability of SST neurons, and (3) that global EIF2 signaling has antidepressant/anxiolytic potential.
Keywords: somatostatin, stress, interneurons, depression, translation initiation, EIF2, GABA
INTRODUCTION
Major depressive disorder (MDD) is a severe psychiatric disorder with a lifetime prevalence of 28–40%.1 It is the leading cause of years lost due to disability worldwide in women and men,2 reflecting a life-long trajectory of recurrent episodes, increasing severity and progressive treatment resistance.3 MDD is a complex polygenic disease with highly heterogeneous clinical presentation and pathology. Therapeutic approaches rely mostly on compounds targeting the monoaminergic systems. However, even under optimal conditions, only one out two patients will experience remission from symptoms after antidepressant treatment.4 Together, the heavy burden on society and individuals, and its insufficient pharmacological treatment highlight the critical need for a better understanding of the primary pathophysiology of MDD, as a first step towards developing new therapeutic approaches.
Reduced levels of the inhibitory neuropeptide somatostatin (SST; also called SOM or SRIF) are frequently observed in individuals with MDD and other neurological disorders, regardless of categorical diagnosis.5,6 Human post-mortem studies from our group have described SST deficits in MDD patients, including a down-regulation of SST gene expression in the dorsolateral prefrontal cortex (dlPFC), subgenual anterior cingulate cortex (sgACC), and amygdala.7–10 SST is co-expressed with gamma-aminobutyric acid (GABA), and SST-expressing GABA neurons preferentially target the dendrites of pyramidal neurons.11–13 Thus, it has been hypothesized that SST neurons regulate information input by providing spatiotemporal integration of postsynaptic potential in local cortical circuits.14–16 Notably, two peptides co-localized with SST, neuropeptide Y (NPY) and cortistatin, are both significantly down-regulated in the corticolimbic areas of MDD patients,7,10 further suggesting a potential role of dis-inhibition of the dendritic compartment of pyramidal neurons in mood dysregulation.
SST inhibits the release of numerous hormones from the hypothalamus, as confirmed by mice lacking SST (SstKO) displaying elevated circulating corticosterone.17 Low SST levels were initially reported in the cerebrospinal fluid of MDD patients,18–20 which negatively correlated with urinary cortisol levels.21 These observations are consistent with altered hypothalamic-pituitary-adrenal (HPA) axis function described in some depressed patients,22,23 although its direct contribution to depression is still controversial.24 Conversely, several pharmacological studies support the anti-stress role of somatostatin on the endocrine, autonomic, and visceral systems.25 Intra-cerebroventricular administration of pan-somatostatin agonist (ODT8-SST) in rats suppresses the stress-induced rise of ACTH, epinephrine and norepinephrine in the plasma.26,27 In addition, rats with intracisternal injection of ODT8-SST reduced stress-related inhibition of ghrelin, food intake and gastric emptying.28
What could be the origin of reduced SST in MDD? Exposure to chronic stress is a leading risk factor associated with mood symptoms.29 Biological stressors, such as seizure or electrical foot shock, can selectively affect SST interneurons or Sst expression.30,31 Studies in mice show that brain-derived neurotrophic factor (BDNF), a stress-sensitive growth factor, is necessary for the maintenance of normal expression of Sst, Npy, and Cortistatin.7,9,32–34 In humans, reduced BDNF signaling has been implicated in the pathophysiology of MDD through both peripheral and central measures.7,9,35
Evidence for a causal role for SST in mood regulation is inconclusive at this point. SstKO mice displayed a trend (p>0.08) toward increased anxiety-like behaviors in the light/dark-avoidance test but not in the open field test (p>0.3).17 Albrecht et al. (2013) investigated SstKO mice in two days of the dark cycle and found that unstressed SstKO mice showed increased anxiety-like behaviors during the second, but not the first day of the active phase.36 Microinjections of SST into the amygdala or ventricles exert anxiolytic effects in the elevated plus maze test.37,38 Reducing SST neuronal function acutely and chronically in the frontal cortex yields opposite effects on behavioral emotionality, suggesting that mood regulation may depend on complex network adaptations including multiple regions and on the timeframe.39 In addition, it is not known whether HPA axis dysregulation of SstKO mice contributes to these behavioral changes.
As mood dysregulation represents the distal effect of complex interactions among multiple genes, brain systems and stressors, we speculate that global dysfunction of SST and SST neurons may contribute to altered neuroendocrine function and to aberrant inhibitory neurotransmission in local cortical circuits. Therefore, SST deficits could affect the function of key corticolimbic brain areas, prime the system for deregulated stress responses, and induce physiological changes, together leading to mood dysregulation. To begin testing this hypothesis, we investigated here (1) whether global SST deficits in mice can cause behavioral, molecular and neuroendocrine phenotypes that are observed in depressed patients, and (2) how chronic stress may affect SST neurons.
MATERIAL AND METHODS
A complete description is found in the Supplementary Information.
Animals
All experiments were performed with 3- to 5 month-old male and female mice littermates, between 9 am and 3 pm. All non-stressed control mice were group housed and maintained under standard conditions (12/12-hour light/dark cycle, lights on at 07:00 h, 22±1°C, with food and water ad libitum). All mice were on the C57BL/6J background. The SstKO mouse line was obtained from the Jackson Laboratory (stock no. 008117).17 SstKO, SstHZ, SstWT littermates were generated by crossing SstHZ mice. Genotyping was done by Polymerase Chain Reaction (PCR) analysis of DNA isolated from tail cuts. To generate mice with green fluorescent protein (GFP) expression in SST cells, the mouse line carrying a somatostatin promoter-driven Cre recombinase expression (SST-ires-cre knock-in homozygous mice; Jackson Laboratory; stock no. 013044) was crossed with a RosaGFP-floxed reporter mouse line carrying a loxP-flanked STOP cassette (Ai6 Rosa26-loxP-STOP-loxP-ZsGreen; Jackson Laboratory; stock no.008242), as described previously.40
Unpredictable chronic mild stress (UCMS)
Mice were subjected to six weeks of a random schedule consisting of 1–3 environmental stressors per day, seven days per week.41 The social isolation treatment began from the fifth week until the day of euthanasia. Mice typically exhibit robust physiological changes (i.e. coat state degradation) which plateau after 4 weeks,41 and that can be exacerbated by social isolation. For the drug study (salubrinal and GSK2606414), we avoided the ceiling effects of UCMS on behavioral emotionality and used a 4 weeks of UCMS treatment. Weekly assessment of weight and fur was performed to monitor physiological progression of the UCMS syndrome.
Behavior
Anxiety/depressive-like behaviors were tested in the elevated plus maze (EPM), open field (OF), novelty suppressed feeding (NSF), and sucrose preference test (SP) separated by a minimum of 1 day. All tests were performed during the light phase of the circadian cycle, between 9 am and 3 pm, as previously described.42 Since the EPM test is considered the most sensitive test to prior behavioral testing, we started with EPM, followed by the other tests. To minimize stress of behavioral testing in the drug study and to avoid excess sucrose solution that might affect oral gavage of drug treatments, we performed EPM followed by NSF and OF. To assess the consistency of behavioral performance across related tests, emotionality and locomotion- related data were integrated using a Z-score methodology.43
Corticosterone Measurements
The stress reactivity test consisted of a 30-min restraint period, corresponding to an acute, moderate stressor. The blood samplings were drawn at three time points: immediately before, during a 30-min exposure to restraint stress, as well as 60 min after ending the stress session (recovery period). The animals were restrained in a 50-ml Falcon tube with a ventilation hole and a hole in the lid for the tail. Tail blood (30 μl) was collected into a Microtainer tube with Lithium Heparin (BD Vacutainer Systems, Franklin Lakes, NJ) at 10:00–14:00. All samples were ran in duplicate and measured by Corticosterone ELISA Kit (Enzo Life Sciences, Farmingdale, NY) according to the instructions of the manufacturer.
Chronic corticosterone treatment
As described previously,44 mice were given corticosterone (35 mg/ml; Sigma-C2505, St Louis, MO, USA) dissolved in 20% cyclodextrin (Sigma H107) as their only water supply for 6 weeks.
Visualization of individual neurons in mice
Mice were rapidly transcardially perfused with 1 ml of 1X Tris-buffered saline and 1 ml of 4% paraformaldehyde in phosphate buffer (pH 7.4) to lightly fix tissue while preserving GFP signal and RNA integrity. Cryostat sections (14 μm) were cut from coronal blocking containing cingulate cortex (between bregma +1.42 to −0.5 mm), thaw-mounted onto on polyethylene napthalate membrane coated slides (Leica Microsystems; #11505158; Bannockburn, IL, USA) that had been treated with UV at 254 nm for 30 min. Before microdissection, slides for SST neurons were dehydrated through 100% ethanol for 10 seconds. For pyramidal neurons, cryostat sections from mice with GFP-tagged SST neurons were dried briefly and stored at −80°C. Immediately before microdissection, slides were immersed in an ethanol-acetic fixation solution, stained with thionin, dehydrated with 100% ethanol, as described previously.45 Pyramidal neurons were visually identified based on their location and characteristic somal morphology including pyramidal shape, large size, and thick apical dendrite.
Laser microdissection and gene expression profiling
Laser microdissection was performed using a Leica LMD6500 system (40X objective in the fluorescence mode for SST neurons or bright-field mode for pyramidal neurons). Cutting and collection steps were subsequently examined in fluorescent or bright-field mode. 100 cells from the mouse cingulate cortex per animal were collected in 0.5 ml microtube caps (Ambion, Foster City, CA, USA) and lysed by vortexing for 30 sec in 150 μl of RLT Buffer Plus (Qiagen, Valencia, CA, USA) within a 3-hour time span, and stored at 80 °C until further processing. Total RNA was then extracted using the RNeasy Plus Micro Kit (Qiagen) according to the manufacturer’s instructions. RNA samples were amplified with NuGEN® Ovation Pico WTA System V2 (NuGen, San Carlos, CA). The fragmented labeled cDNA samples were processed and hybridized to Affymetrix® Mouse Gene 1.1 ST Array Plates (Affymetrix). Gene expression information was obtained with the Affymetrix console software. Genes showing significant differences (Student t-test p<0.005; >1.2 fold cutoff) in expression between SST neurons from stressed and unstressed mice were analyzed using the IPA software (Ingenuity Systems, Redwood, CA).
Real-time quantitative polymerase chain reaction (qPCR)
For molecular phenotypes in mice with low SST, brains were flash frozen on dry ice after sacrifice. Bilateral cingulate cortex was obtained using a cryostat and a 1-mm-bore tissue punch. Total RNA was extracted using the RNeasy Micro Kit (Qiagen) according to the manufacturer’s instructions. Reverse transcription reaction was performed using the qScript cDNA Supermix (Quanta Biosciences, Gaithersburg, MD). The comparative threshold cycle (Ct) measurement was performed for quantification with SYBR green fluorescence signal (Invitrogen, Carlsbad, CA), as described previously.45 For single-cell analyses, total RNA from mouse cells were amplified with NuGEN® Ovation Pico WTA System V2 (NuGen, San Carlos, CA). Samples were run in triplicates and the difference in ΔCt values for each transcript was determined by comparison to a non-biased control gene, neurofilament (NEFM).
RNAscope Assay for in situ RNA Detection
Probe sequences of mouse Sst conjugated to Alexa Fluor 488, and Eif2a conjugated to 546 were custom-made (Advanced Cell Diagnostics, Hayward, CA). Fresh-frozen cryostat sections (10 μm) were placed on slides and fixed in cold 4% paraformaldehyde for 1 hour, followed by dehydration in an ethanol series. Tissue sections were then dried at room temperature for 30 min, followed by protease digestion at room temperature for 10 min. Using RNAscope Fluorescent Multiplex Reagent Kit (Advanced Cell Diagnostics; # 32085), tissue sections were then rinsed in deionized water, and immediately treated with target probes, preamplifier, amplifier, and label probe in a HybEZ hybridization oven (Advanced Cell Diagnostics, Hayward, CA). Images were acquired using an Olympus IX71 fluorescent microscope (Olympus, Tokyo, Japan) and a Fast 1394 CCD Camera (QImaging, BC, Canada). Overlapping signals from different fluorophores were separated and visualized under a 20x objective lens with MetaMorph Advanced software (Molecular Devices, CA, USA). The ratio of overlapping fluorescent signal areas from EIF2A and SST mRNA transcripts was determined in the cingulate cortex, based on 5 microscopic images from 3 tissue sections of each animal.
Drug Administration
The selective EIF2A phosphatase inhibitor complexes, Salubrinal (Millipore, Billerica, MA, USA), and the EIF2AK3/PERK inhibitor, GSK2606414 (Millipore, Billerica, MA, USA) were dissolved in 100% dimethyl sulfoxide (DMSO) and later diluted in saline (0.9% NaCl). Based on previous studies,46–48 vehicle (10% DMSO/saline), Salubrinal (1.5 mg/kg), and GSK2606414 (30 mg/kg) were administered by oral gavage once daily for 7 days to mice (0.1 ml/10 mg) treated with 3 weeks of UCMS before behavioral testing. A separate group treated with GSK2606414 alone was used to assess the specificity of the drug, compared to non-stressed vehicle group. Three days of booster administrations were given on the second day after each test. Concentrations were adjusted to administer 10 ml/kg. After 7 days of drug treatments, anxiety/depressive-like behaviors and locomotion were tested in the following order: EPM, NSF, OF, separated by one day of booster administration. All tests were performed during the light phase of the circadian cycle, between 9 am and 3 pm.
Statistical Analysis
Statistical analyses were carried out using GraphPrism Version 6.0 (Graph Pad. Software Inc., San Diego, CA, USA). Student’s t test was used to compare means between two groups, and one-way or two-way analysis of variance followed by Tukey’s post hoc among means were used to determine significant differences among multiple groups. All data are expressed as means ± standard error of the mean.
RESULTS
Sst genetic ablation increases anxiety/depressive-like behaviors
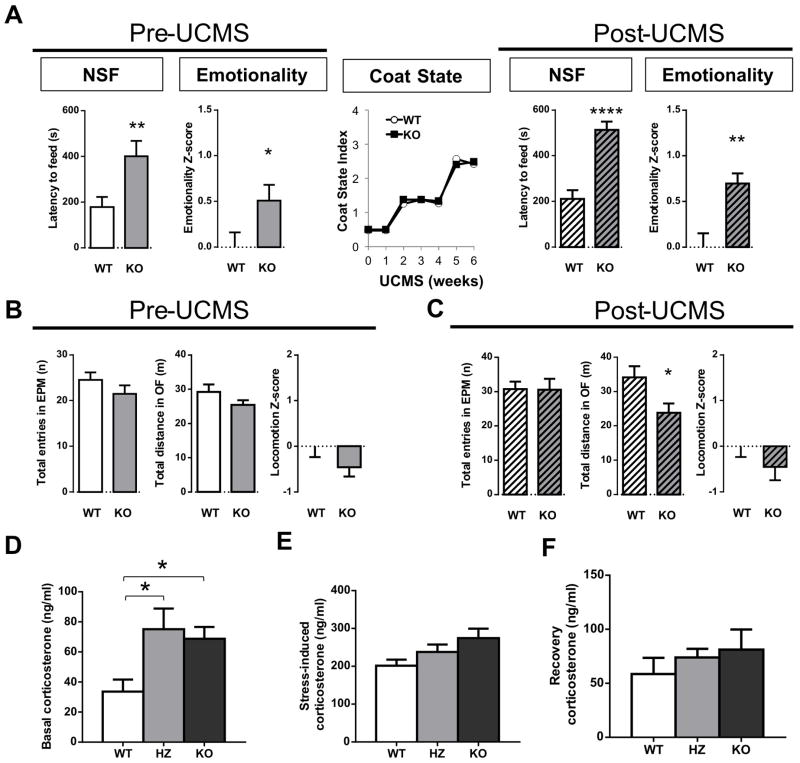
To determine whether SST plays a causal role in mood dysregulation, we utilized previously generated SstKO mice.17 SstKO and wild-type (SstWT) littermates were submitted to several behavioral tests for measuring aspects of anxiety/depressive-like behaviors. To assess the consistency of behavioral performance over time and related assays, we also applied Z-normalization to integrate emotionality-related measures across tests, as previously described.43 Here, the most robust findings were consistently observed in the novelty suppressed feeding (NSF) test, so we focus our report on this test, while also providing results from integrated emotionality Z-scores (Detailed results of all tests are provided in the supplements). Under non-stressed baseline conditions, SstKO mice showed increased latency to begin eating in the NSF compared to SstWT mice (Figure 1A). Results were variable in other tests, displaying increased anxiety/depressive like behaviors or no change (Figure S1). Integrated z-scores showed that SstKO mice overall displayed significant elevated behavioral emotionality, when compared to SstWT mice (Figure 1A, left panel).
Figure 1. Loss of somatostatin (Sst) leads to high anxious/depressive-like behavior in the novelty suppressed feeding (NSF) test and overall elevated behavioral emotionality.
(A) Before and after exposure to unpredictable mild chronic stress (UCMS), SstKO mice display increased latency in the NSF test and elevated overall emotionality Z-score (an integrated result from four related tests; Details in Fig. S1). UCMS induced a typical progressive fur coat degradation, which did not differ between groups (middle). (B) Locomotion, as measured by the total number of arm entries in the elevated plus maze (EPM) or total distance traveled in the open field (OF), and overall locomotion z-scores, did not differ between SstKO and wild-type mice at baseline. (C) After UCMS SstKO displayed reduced locomotion in the OF but not overall differences. (D) Basal corticosterone levels were elevated in SstKO and SstHZ mice. (E) Acute stress-induced corticosterone levels were normal in mice with low SST. (F) No abnormal corticosterone levels were detected in mice with low SST during the recovery period. *p<0.05, **p<0.01, ****p<0.001. Error bars represent the standard error of the mean. (n=12–15 mice per genotype, 6–9 mice per sex).
Exposure to chronic stress is a main exogenous risk factor associated with elevated mood symptoms,29 and the interaction of stress with genetic risk factors can increase susceptibility to mood dysregulation in humans.49 Unpredictable chronic mild stress (UCMS) is a paradigm that induces anxiety/depressive-like behaviors in rodents, as well as neuroendocrine and molecular changes otherwise associated with MDD.50,51 Hence, to investigate the effects of Sst ablation on behavioral emotionality in response to stress, we exposed the same cohort of SstKO and SstWT mice to UCMS. We observed a progressive degradation of the fur coat quality, indicating that both groups responded to UCMS (Figure 1A). Behavioral testing in the last two weeks of the UCMS protocol revealed increased latency to feed in the NSF test and higher emotionality z-scores in stressed SstKO mice compared to stressed SstWT mice (Figures 1A, right panel, and S1F–J). Although normal variability in behavioral testing precludes comparison of absolute values across time in a longitudinal study design (baseline followed by post-UCMS behavioral testing), the results do not suggest any genotype by stress interaction. Instead, the results show that SstKO mice maintained a similarly higher behavioral emotionality over SstWT littermates under non-stressed [1.51 ± 0.17 fold (s.e.m.) relative to control] and stressed conditions [1.69 ± 0.11 fold (s.e.m.) relative to control].
To validate these findings, we investigated the behavioral emotionality of SstKO mice in a second independent cohort of mice. Consistent with results described above, SstKO mice displayed increased latency to feed in the NSF test compared to control SstWT mice both at baseline (p<0.01) and after UCMS exposure (p<0.005; Figure S2A), demonstrating reliability across cohorts and time.
Based on a priori information of a putative sex difference (i.e. human studies), we separated the data by sex groups (male and female cohorts) and observed that the increased emotionality was more robust in female SstKO mice under chronic stress conditions (Figures S2B–C). Note that there was no statistical interaction between sex and treatment, indicating that the results in female SstKO were more robust rather than different from male SstKO. These findings parallel of the molecular findings in depressed patients, showing that down-regulated SST levels in MDD patients are more robust in women, with similarly no evidence for a gender difference.
SST is involved in feeding behaviors,52,53 so we assessed the appetitive drive in the NSF paradigm by measuring the amount of food consumed in the home cage after NSF testing. In the first cohort, we observed a small but significant reduction in food consumption in SstKO mice under baseline non-stressed conditions (Figure S3). This phenomenon was not observed after UCMS exposure (Figure S3). Using the second cohort in the replication study, we observed non-significant trends for reduced post-NSF food consumption (Figure S3). Combining the two cohorts of SstKO mice, there was no significant correlation between post-NSF food consumption and anxiety/depressive-like behaviors (i.e. latency to feed) during the NSF under baseline (r=−0.05; p=0.77) or UCMS-exposed (r=−0.23; p=0.24) conditions (Figure S4A). No significant correlation between home-cage food consumption and baseline/trait emotionality (r=0.01, p=0.95) or post-UCMS state emotionality (r=0.05, p=0.81) was found in SstKO mice (Figure S4B). In addition, SstKO mice displayed normal body weight and general locomotion across tests and conditions, as demonstrated by the absence of significant differences in locomotion z-scores (Figures 1B–C), although SstKO mice showed reduced locomotion in the open field after UCMS (Figure 1C). Together these control studies did not identify any consistent pattern of differences and thus suggest that the increased latency to feed in the NSF and the overall elevated behavioral emotionality of SstKO mice were not confounded by physiological or activity changes (See Discussion).
Neuroendocrine function does not correlate with behavioral emotionality in SstKO and SstHZ mice
We next tested the putative role of HPA function in the regulation of the SstKO phenotype. Consistent with early observations,17 we report high basal plasma levels of corticosterone in SstKO mice (Figure 1D). We extended these findings and showed that heterozygous (SstHZ) mice displayed similar elevated corticosterone levels (Figure 1D). After 30 minutes of acute restraint stress or one hour of recovery from stress, corticosterone levels were not different among SstWT, SstKO and SstHZ mice (ANOVA p>0.05; Figures 1E–F). Compared to SstWT, stress-induced corticosterone levels were only elevated in SstKO (p<0.05, Tukey’s test), but not SstHZ mice. Overall, SstKO mice had largest area under the curve of corticosterone profile (1.58 ng/ml*min*104), compared to SstHZ (1.40 ng/ml*min*104) and SstWT (1.13 ng/ml*min*104) mice. Thus, it is not surprising that only SstKO mice exhibit an increased behavioral emotionality as compared to SstWT mice. Two-way ANOVA showed main effects of genotype (p<0.005) and treatment (p<0.0001), but no interaction (p=0.47). Tukey’s post hoc comparisons revealed significant differences between SstWT and SstKO. Nonetheless, to further test whether HPA axis dysregulation (i.e. elevated baseline) may contribute to the observed behavioral differences, we characterized the behaviors of SstHZ mice. Surprisingly, SstHZ mice displayed normal anxiety/depressive-like behaviors (Figure S5) under baseline conditions. Similarly, after UCMS treatment, behaviors of stressed SstHZ mice were not different from stressed SstWT littermates (Figure S5).
Together, these observations indicate that the elevated levels of corticosterone observed in SstKO and SstHZ mice are potential biomarkers of low SST but not systematically associated with measures of behavioral emotionality. Although we cannot rule out a partial contribution of corticosterone to the high emotionality phenotype in SstKO mice, results in SstHZ mice indicated that high corticosterone levels were not sufficient to increase behavioral emotionality. Indeed, these results also suggest the presence of compensatory mechanisms in SstHZ mice or additional regulatory mechanisms in SstKO mice.
GABA-related gene expression in SstKO mice recreate a dysregulated profile reported in human depression
SST is a marker of dendritic-targeting GABA neurons which partly overlaps with neuropeptide Y (NPY), cortistatin (CORT), and that contains the 67 kDa isoform of GABA-synthesizing enzyme glutamic acid decarboxylase (GAD67), together contributing the majority of inhibitory input onto pyramidal dendrites.54,55 Expression of these GABA-related markers is down-regulated in the anterior cingulate cortex and amygdala of depressed patients, and in mice with reduced BDNF,7,9,10 suggesting a vulnerable BDNF/GABA-enriched gene module in SST neurons associated with mood regulation. To examine whether loss of SST affected this particular gene module, we performed qPCR to measure gene expression in the cingulate cortex of mice with low SST. We showed that when compared to SstWT mice, Sst ablation resulted in low expression of Cort, Bdnf, and Gad67 (Table 1), hence mimicking key gene changes observed in mice with altered BDNF function and in depressed patients (Table 1). On the other hand, SstHZ mice showed unchanged Npy and Gad67 expression, reduced Bdnf expression, but increased Cort expression (Table 1). Since Cortistatin (Cort) binds to all SST receptors and shares structural, functional and pharmacological properties with SST, including inhibitory neuromodulation,56 we speculate that upregulation in Cort may compensate for the absence of Sst and contribute to the normal behavioral emotionality in SstHZ mice. It is notable that although BDNF is a known upstream regulator of SST expression, the results show that reduced BDNF expression can also occur downstream from reduced SST expression, demonstrating bidirectional regulatory links. It is also possible that elevated levels of corticosterone in mice with low SST may lead to reduced BDNF expression. Together, the integrated results indicate that SstKO mice recapitulate behavioral (high emotionality), molecular (downregulated expression of BDNF/GABA-enriched gene module), and neuroendocrine (high corticosterone) phenotypes that are observed in depressed patients.
Table 1.
Altered expression of Bdnf and GABA-related genes in SstKO and SstHZ mice. Comparison with BDNF mutant mice and human depression.
| Gene | Mouse | Mouse (Tripp et al., 2012) | Human (Guilloux et al., 2012; Tripp et al., 2012) | |||
|---|---|---|---|---|---|---|
| SST HZ vs. WT qPCR | SST KO vs. WT qPCR | |||||
| Change (%) | p-value | Change (%) | p-value | Bdnf KIV or HZ | MDD | |
| Cort | 50.5 ± 19.8 | 0.02 | −57.7 ± 8.3 | 6.06E-03 | ↓ | ↓ |
| Npy | −16.4 ±12.9 | 0.24 | −7.8 ± 9.4 | 0.35 | ↓ | ↓ |
| Gad67 | −10.4 ± 15.7 | 0.31 | - 48.9±19.5 | 0.02 | ↔ | ↓ |
| Bdnf | - 31.5 ± 7.4 | 0.01 | - 22.3± 8.9 | 0.04 | ↓ | ↓# |
Cort, cortistatin; Npy, neuropeptide Y; Gad67, glutamic acid decarboxylase 67; Bdnf: brain-derived neurotrophic factor; HZ: heterozygous; WT: wild type; KO: knockout; KIV: knockin promotor IV. (A) (Left) Altered expression of Bdnf and GABA-related genes in SstKO and SstHZ mice. (Right) Bdnf-dependency of Cort and Npy expression is shown in BdnfHZ and Bdnf exon-IV knockout mice (From Tripp et al, 20127) and core depression-related gene profile in human subjects (From Guilloux et al., 20119; Tripp et al., 20127). (B) Number sign (#) represents decreased BDNF-TrkB signaling in postmortem corticolimbic regions of depressed subjects. Error bars represent the standard error of the mean (n=8–16/per genotype). (C). Bold entries refer to change values associated with a p-value <0.05.
Stress affects the transcriptome of SST-positive neurons but not pyramidal cells
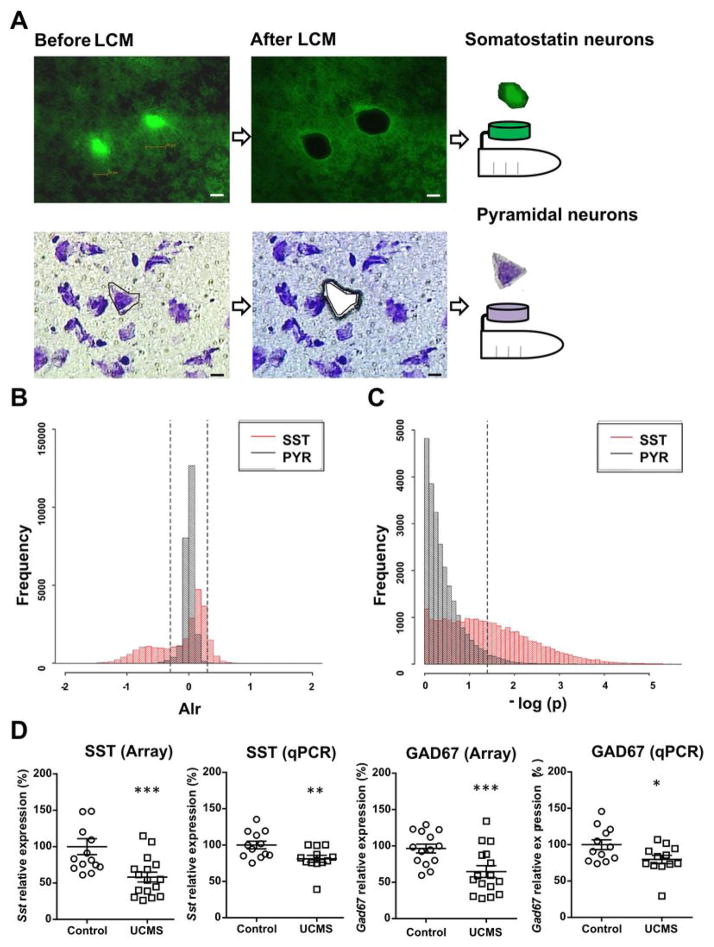
To investigate whether the above-described changes in a BDNF/GABA/SST gene module corresponded to broader molecular changes in SST neurons, we applied UCMS to mice expressing GFP in SST neurons, captured GFP-positive SST neurons and thionin-stained pyramidal neurons from cingulate cortex using laser capture microdissection (LCM), and analyzed RNA profiles using Affymetrix Gene 1.1 ST microarrays (Figure 2A). Frequency histograms demonstrate striking differences between the effects of stress on SST neurons (Figures 2B–C, red bars) and pyramidal neurons (Figures 2B–C, black bars) [Kolmogorov-Smirnov (K–S) test, p<e-15]. Specifically, UCMS induced changes in a large proportion of the transcriptome in SST neurons, as shown by frequency distributions in the histograms of effect sizes (Figure 2B) and statistical significance (Figure 2C). The shifted distributions of effect size (to left) and statistical significance (to right) were mostly characterized by down-regulated expression. In addition, we also examined the transcriptome profiles of two other types of interneurons that are partially co-localized with SST after chronic exposure to UCMS or corticosterone, including neuropeptide Y (NPY) and cortistatin-containing neurons. Interestingly, we observe very mild changes in gene expression in these two GABA neuron subpopulations (data not shown). This indicates a heterogeneous vulnerability of those related cell populations, and suggests that changes in SST neurons per se may be the critical biological event associated with heightened cellular stress and behavioral emotionality. Among those UCMS-affected genes in SST neurons were Sst and Gad67. The array results were confirmed by independent qPCR assays (Figure 2D). In contrast, we observed small and non-significant changes of gene expression in pyramidal neurons from UCMS-exposed mice (Figures 2B–C, black bars). Together, the results demonstrated a selective cellular vulnerability of SST neurons to stress.
Figure 2. Transcriptome changes induced by chronic stress reveal a selective vulnerability of Sst neurons, compared to pyramidal neurons.
(A) Scheme depicting the use of laser capture microdissection (LCM) to capture individual cell types. Bar represent 20 μm. (B–C) Distributions of stress effects for (B) effect size (Average log2 ratio; Alr; changes > 20%; vertical dashed lines) and (C) significance (p-value; p<0.05; horizontal dashed lines) of all expressed probesets in SST neurons (red lines) and pyramidal neurons (PYR; black lines) were presented as density plots. These probability distributions between SST neurons and pyramidal neurons were significantly different. p-value was calculated by Kolmogorov-Smirnov (K–S) test. (D) qPCR confirms significant down-regulation of Sst and Gad67 transcripts in UCMS-exposed mouse SST neurons.
Stress links SST neuron deficits to eukaryotic translation initiation factor 2 (EIF2) signaling
To assess the biological implications of the transcriptome results, we applied Ingenuity Pathway Canonical pathway analysis (IPA) on the sets of differentially-expressed genes at various stringency levels. IPA results indicated a common enrichment of affected protein translation initiation and mitochondrial functions. Under moderate stringency (changes ≥20%; p<0.005), the EIF2 pathway was the most significantly affected (p=7.38E-21) with multiple down-regulated genes in SST neurons of UCMS-treated mice (Tables 2, S1). Results were highly similar across different cut-off ranges. Here we showed that Sst expression was highly correlated with EIF2α subunit (Eif2a) expression (Figure S6A), suggesting that EIF2A may be associated with SST deficits. Down-regulation of Eif2a expression in SST neurons from UCMS-exposed mice was validated by qPCR in tissue extracts (Figure S6B) and RNAScope in situ hybridization on tissue sections (Figure S6C).
Table 2.
Top ranked canonical pathways affected in SST neurons of mice exposed to chronic stress.
| Canonical Pathways | P-value | Ratio |
|---|---|---|
| Eukaryotic translation initiation factor 2 Signaling | 7.38E-21 | 40.3% |
| Oxidative Phosphorylation | 8.57E-14 | 40.8% |
| Mitochondrial Dysfunction | 2.24E-12 | 28.8% |
| Remodeling of Epithelial Adherens Junctions | 2.62E-09 | 44.3% |
| GABA Receptor Signaling | 1.00E-08 | 42.9% |
| Huntington’s Disease Signaling | 3.43E-08 | 26.2% |
| Protein Kinase A Signaling | 6.84E-08 | 24.0% |
| Regulation of eIF4 and p70S6K Signaling | 1.21E-07 | 27.2% |
(A) Genes were tested for differential expression between UCMS and control groups. (B) Selected genes (fold-change>20%; p<0.005) were submitted to IPA to test for over-representation of canonical pathways. p-value was calculated by right-tailed Fisher’s exact tests. The ratio was calculated by the number of differentially expressed genes, divided by total number of genes that make up a given pathway.
EIF2 signaling plays a crucial role in protein translation initiation by binding the initiator methionyl-tRNA Met to the 40S ribosomal subunit for forming the pre-initiation complex. In response to cellular stress, increasing levels of misfolded/damaged proteins can initiate the unfolded protein response (UPR) to suppress EIF2 signaling, which blocks protein translation as a protective cellular mechanism. Several brain disorders have been associated with suppression of protein translation through phosphorylation of EIF2A, including Alzheimer’s (AD) and Parkinson’s diseases (PD), and animal models of AD, prion disease, and amyotrophic lateral sclerosis (ALS).46,48,57 Here, applying an upstream regulator prediction analysis in IPA,58 we found that the top regulators have been previously associated with neurodegenerative diseases (e.g., MAPT, APP, PSEN1, HTT; Table S2), together suggesting a similar mechanism of deregulated protein homeostasis (proteostasis) underlying heightened vulnerability of neurons across neurological diseases. Hence, these results provide additional putative links between stress-induced SST deficits and neurodegenerative processes, as least though these in silico analyses.
We next sought to independently validate these transcriptome results. Since we cannot completely rule out a contribution of elevated corticosterone to the phenotypes of SstKO mice, and chronic elevated corticosterone exposure can induce anxiety/depressive-like behaviors in mice,44,59 we speculated that SST neurons might be similarly vulnerable to elevated glucocorticoids, albeit to a lower extent than to UCMS exposure. So in a parallel study, we performed similar single-cell analyses for SST neurons in mice chronically exposed to corticosterone. We found that chronic corticosterone exposure induced transcriptome alterations in SST neurons that were highly similar to changes observed after UCMS (Pearson correlation, r=0.82; p=10−7), although with reduced effect sizes (slope=0.48; i.e. ~50% of UCMS effect size; Figure S7A). Similar to UCMS results, very few gene changes were observed in pyramidal neurons compared to SST neurons following corticosterone exposure (K–S test, p<e-15; Figures S7B–C), suggesting a similarly selective vulnerability of SST neurons to a neuroendocrine stressor. Notably, the EIF2 pathway was the most significantly affected function in SST neurons following chronic corticosterone treatment, although this result was observed at much less stringent statistical thresholds compared to results after UCMS exposure (changes ≥20%; p<0.05; IPA p=0.000463; Table S2). It suggests a central role for EIF2 signaling in response to stress.
Chronic corticosterone exerted similar but greatly reduced impact on gene changes in SST neurons, and also showed minimal effects on pyramidal neurons (Figures S7A), hence confirming the elevated vulnerability of SST neurons. These results are also consistent with a non-sufficient role for the elevated baseline corticosterone of SstKO and SstHZ mice in increasing behavioral emotionality.
EIF2 kinase inhibition blocks the development of stress-induced anxiety/depressive-like behaviors
One arm of this UPR pathway leads to the phosphorylation of protein kinase RNA-like endoplasmic reticulum (PERK) and its downstream effector, EIF2A. EIF2A phosphorylation is a well-known mechanism to suppress protein synthesis in response to cellular stress and is thought to protect cells against stress-induced apoptosis.60 Systemic modulation of EIF2A phosphorylation affects TDP-43 toxicity in mouse models of ALS, neurodegeneration in prion-infected mice, and cognitive function in mouse models of AD without overt side-effects.46–48,61 Compounds that inhibit EIF2A kinase or phosphatase have been validated for optimal use in mice, including for brain-related targets.46–48,61 Accordingly, we predicted that stress-induced anxiety/depressive-like behaviors would be affected by compounds that target the EIF2 pathway. Salubrinal is an inducer of EIF2A phosphorylation via inhibition of EIF2A phosphatase,62 i.e. activating the cellular stress response and mimicking the disease effects. GSK2606414 is an inhibitor of EIF2A phosphorylation via inhibition of PERK, an EIF2 kinase,63 i.e. inhibiting the cellular stress response and potentially exerting therapeutic/antidepressant-like effects.
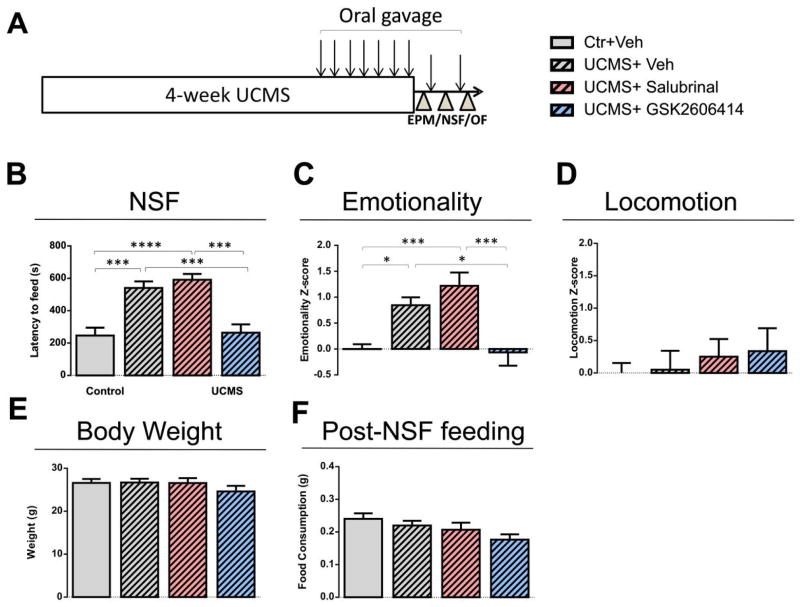
Results showed that, as predicted, anxiety/depressive-like behaviors in the NSF (Figure 3B) and overall emotionality (Figure 3C) increased after UCMS treatment in vehicle-treated mice. We found that UCMS-exposed mice treated with GSK2606414 displayed significantly less UCMS-induced anxiety/depressive-like behaviors in the NSF (Figure 3B) and emotionality z-scores (Figure 3C) compared to the vehicle-treated stressed mice. Salubrinal did not significantly exacerbate UCMS-induced anxiety/depressive-like behaviors, suggesting either no effect of salubrinal at a 1.5 mg/kg dose or a ceiling effect of stress on behavioral emotionality. Locomotion (Figure 3D), weight (Figure 3E), and post-NSF food consumption (Figure 3F) were not affected by drug treatments. Consistent with previous observations, there was no significant correlation between post-NSF food consumption and latency to feed after NSF (r=−0.10; p=0.52). A group treated with GSK2606414 alone did not display any phenotypes as compared to control vehicle mice, including behavioral emotionality, post-NSF feeding, locomotion or weight changes (Figure S8). Together, the results suggest that activating the EIF2 pathway through EIF2 kinase inhibition may relieve stress-related mood disturbances.
Figure 3. Attenuation of anxiety/depression-like behaviors in stressed mice after treatment with GSK2606414, an EIF2A-mediated inhibitor of cellular stress response.
(A) Experimental design. (B) Anxiety/depressive-like behaviors in the NSF test were increased after UCMS treatment, and GSK2606414 treatment significantly eliminated UCMS effects on behaviors. (C) The same effects of UCMS and GSK2606414 on emotionality were confirmed with emotionality Z-scores. (D–F) No treatments affected overall locomotion, body weight, or post-NSF feeding at home cage. *p<0.05, **p<0.01, ***p<0.005, ****p<0.001. Error bars represent the standard error of the mean (n =8–12/group).
DISCUSSION
We report behavioral alterations, and aberrant molecular and neuroendocrine phenotypes in SstKO mice which are consistent with changes frequently observed in MDD patients. Studies in SstKO and heterozygous (SstHZ) mice show that elevated corticosterone is not sufficient to reproduce the behavioral phenotype, suggesting a putative role for Sst cell-specific molecular changes. Gene expression of multiple components of the translational machinery, especially the EIF2 signaling pathway, was suppressed in SST neurons across two murine stress models, and that the extent of these changes paralleled behavioral changes. Activation of the EIF2 pathway reversed the elevated behavioral emotionality in mice exposed to chronic stress. Collectively, our data provide evidence in support of a causal role for SST deficits in the mood component of MDD, and suggest a mechanism of deregulated EIF2-mediated protein translation as a critical gateway for vulnerability of SST neurons and behavioral emotionality to chronic stress.
Somatostatin in mood regulation
Human postmortem brain studies have reported SST deficits in depression and other neurological disorders,5,6 raising the possibility that altered functions of SST interneurons, associated neuroendocrine, and cortical local circuit regulation, may represent common pathophysiological features associated with mood dysregulation across disorders.5 Although direct evidence for causality is not achievable with human studies alone, we now report elevated behavioral emotionality in SstKO mice under baseline and chronic stress conditions (Figures 1 and S1). Specifically, the increased latency to feed in the NSF test is typically interpreted as elevated anxiety/depressive-like behaviors, since it can be reversed by chronic but not acute antidepressant treatment.64 These results were independently replicated in a second cohort of mice. SstKO mice displayed variability in behaviors across other behavioral tests, but consistently in the same direction, together resulting in significantly elevated emotionality z-scores in both cohorts (Figures 1, S1 and S2). The reason for the more robust phenotypic result of SstKO mice in the NSF test is not known. It may reflect the fact that this test captures a different component of mood regulation or engages slightly different biological and neural network regulation.44 Zeyda et al. (2001) did not report significant changes in behavioral emotionality in SstKO mice, but nominal trends were observed in some of the performed tests and they did not use the NSF test. In addition, SstKO mice were maintained on a 129/Sv-background in that previous study, compared to the C57BL/6J background used here, and mice were only investigated under non-stressed baseline conditions. So variations in behavioral phenotype, genetic background and stress state may underlie those discrepancies.
Despite the limitations of a global knockout, SstKO mice provide an opportunity to explore the causal role of somatostatin in mood dysregulation and the underlying neural mechanisms. Such insights, however, will need to be refined with a systematic behavioral characterization with fine spatial and temporal resolution, using region-specific manipulations at different developmental stages. Studies will then need to assess the molecular and cellular systems that somatostatin manipulations converge upon, and where the exact neural circuits are affected.
Neuroendocrine and molecular correlates of depression in SstKO mice
Altered HPA axis function commonly occurs in depression, and includes increased cortisol and abnormal dexamethasone suppression.65 Previous investigations in the role of low SST in depression have largely focused on its neuroendocrine function, since SST inhibits the release of several hormones from the hypothalamus and pituitary gland.66 Our data confirm that SST deficits are an important causal factor leading to HPA axis hyperactivity, as high plasma levels of corticosterone were measured in SstHZ and SstKO mice. However, SstHZ mice exhibited normal behavioral emotionality, indicating that high corticosterone is not sufficient to induce elevated behavioral emotionality, at least under the conditions of this study. Corticosterone is implicated in the organization of daily-related events such as sleep and body growth,67 so follow-up studies investigating the circadian patterns of corticosterone in mice with low SST may uncover more subtle phenotypes in SstHZ and SstKO mice.
The anterior cingulate cortex is a key brain area involved in mood regulation, where emotional information integrates with cognitive control.68,69 Changes in cingulate cortex activity have been implicated in depression and anxiety disorders,69,70 and in regulating homologous behaviors in mice.71 On the molecular level, SST ablation induced further changes in the cingulate cortex of SstKO mice, including low Bdnf, Gad67, and Cort. These results suggest impairments in neurotrophic supply and GABAergic inhibition, which is reminiscent of observations in the cingulate cortex of depressed patients. The SstKO molecular phenotype did not extend to other related GABA markers (i.e. NPY) identified in depression, suggesting additional mechanisms at play in the human condition.7–10 In contrast to previous studies,17,72,73 we report downregulated Cort mRNA levels in the cingulate cortex of SstKO mice, but upregulated in SstHZ mice. Cortistatin (Cort) shares structural, functional and pharmacological properties with SST and binds to all SST receptors.56 So we speculate that upregulated Cort levels may compensate for low Sst function in SstHZ mice, and that additional and different mechanisms are engaged in the full knockout, including reduced GABA function since low Gad67 was observed in SstKO but not in SstHZ mice. Notably the fact that SstKO, and not SSstHZ mice, replicate the disease behavioral and molecular profiles of the illness is not uncommon in rodent studies, since the pathology of MDD is thought to include multiple molecular deregulations in addition to reduced Sst deregulations and that more robust changes (as in full KO) may be needed in rodent models. Finally, the observation of down-regulated Bdnf expression in SstHZ and SstKO mice is intriguing and worth noting since Bdnf is mostly characterized as an upstream regulator of SST expression.7 Together, our results uncover subtle and complex homeostatic changes that are likely to affect the inhibitory function of local cortical circuits, potentially reflected in an altered emotionally-salient network in the anterior cingulate cortex of MDD patients.
Biological vulnerability of SST neurons involves the Eif2a pathway
We exposed mice to two different stress models, UCMS, an environmental/social/psychological stress and chronic corticosterone exposure, a neuroendocrine stress. Although both models lead to neuroendocrine, physical and behavioral depression-like alterations, UCMS has better construct, face and overall mechanistic validity compared to corticosterone exposure since social-environmental stress induces multi-systems disruptions that are not limited to neuroendocrine changes.74,75 Accordingly, our results show that UCMS elicited similar but larger transcriptome alterations in SST neurons compared to chronic corticosterone exposure. Out of the large number of stress-induced molecular changes, suppression of EIF2 signaling in stressed Sst neurons was most prominent in both stress models, as reflected by the down-regulated expression of many genes within this pathway. EIF2 signaling decreases protein translation in response to cellular stress. Extracellular stimuli and fluctuations in intracellular homeostasis modulate endoplasmic reticulum (ER) protein-folding status. The unfolded protein response (UPR) activation is a general translational repression that is induced by ER stress. A molecular mediator of this pathway is phosphorylation of PERK which in turn phosphorylates EIF2A, leading to a general inhibition of protein synthesis.76 Notably, UPR-ER-EIF2 signaling has been implicated in the selective vulnerability of specific neurons in models of neurodegenerative diseases, including striatal neurons in Huntington’s disease, motor neurons in ALS mice, and dopaminergic neurons in PD.77–80 Our results now implicate this pathway for the first time in mechanisms of cellular vulnerability of SST neurons in the context of major depression. Depression and other neuropsychiatric disorders are typically not considered neurodegenerative but rather neuroprogressive disorders that promotes brain rewiring and vulnerability to life stress. We speculate here that related basic cellular mechanisms may link both sets of disorders, consistent with the fact that SST deficits are commonly seen in neurodegenerative and neuropsychiatric diseases.
Finally, the stress-induced dysfunctions of SST and pyramidal cell may take many forms, including activity changes (i.e. electrophysiological properties), which were not measured here. Future studies will need to investigate those changes under both baseline and stressed conditions, and to determine the conditions under which aberrant EIF2 signaling and neuronal activity are mechanistically related.
Therapeutic Implications and Conclusion
Our studies using SstKO mice demonstrate that the lack of Sst induces downregulation of Bdnf and GABA-related gene expression, elevated stress hormones and a behavioral phenotype consistent with elevated anxiety and/or a depressive-like syndrome (denoted as behavioral emotionality). Moreover chronic stress induced large-scale molecular changes in cingulate cortex SST neurons and specifically highlighted a compromised EIF2 signaling. Prolonged suppression of EIF2 signaling alters proteostasis and cell function. We speculate that suppression of EIF2 signaling in stressed SST neurons may represent an early adaptive mechanism to reduce the burden of destabilized proteins associated with increased recruitment of these neurons during stress. This putative pathological mechanism is consistent with the frequent report of reduced SST expression observed across those disorders. UPR-ER-EIF2 signaling regulates secretory cell function,81 and expression of secretory GABAergic markers, including SST and NPY through BDNF signaling.82,83 In our rodent model, we show that a one week treatment with a PERK inhibitor was sufficient to mitigate anxiety/depressive-like behaviors induced by chronic stress. This finding raises the intriguing possibility that boosting protein translation capacity of stressed SST neurons in order to maintain normal function and induce a routine protective response, such as up-regulation of stress-responsive genes (e.g., SST-GABA signaling) may avoid the negative cellular effects of stress in SST neurons, which in turn may maintain appropriate local cortical circuit function in a mood regulatory neural network. In line with this hypothesis, EIF2 kinase inhibitor treatment reversed cellular and behavioral abnormalities in mouse models of several neurodegenerative diseases including Alzheimer’s disease, amyotrophic lateral sclerosis, and prion disease.46,48,61 There are four known kinases, GCN2, PKR, PERK, HRI, which can phosphorylate EIF2A. Interestingly, the potential role of these EIF2A kinases in mood disorders is not known. Only two related studies have used PERK or PKR knockout mice, and results indicated normal anxiety levels in the elevated plus maze and open field.84,85 However, these preliminary studies will need to be followed by full behavioral assessment under baseline and stressed conditions, and by spatial and temporal genetic manipulations. Several specific biochemical, cellular and neural activation steps are involved between blocking the activation of the UPR and the behavioral responses. Although beyond the scope of this study, delineating the different EIF2 signaling branches of UPR in specific cellular types and brain regions with specificity and timing (temporal versus chronic) of the pharmacological manipulations will form the basis of follow-up studies of symptom domains in neurological disorders.
Supplementary Material
Acknowledgments
We thank Chien-Wei Lin and Ying Ding for assistance with the microarray data pre-processing, Ye Chen for assistance with behavioral testing, and Beverly French for comments on the manuscript. This work was supported by grants from National Institute of Mental Health (NIMH-MH093723, MH084060 and MH077159).
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
References
- 1.Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Arch Gen Psychiatry. 2003;60(9):929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- 2.WHO. World Health Organization - The Global Burden of Disease - 2004 update. WHO Library. 2008 [Google Scholar]
- 3.Moylan S, Maes M, Wray NR, Berk M. The neuroprogressive nature of major depressive disorder: pathways to disease evolution and resistance, and therapeutic implications. Mol Psychiatry. 2012 doi: 10.1038/mp.2012.33. [DOI] [PubMed] [Google Scholar]
- 4.Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- 5.Lin LC, Sibille E. Reduced brain somatostatin in mood disorders: a common pathophysiological substrate and drug target? Front Pharmacol. 2013;4:110. doi: 10.3389/fphar.2013.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martel G, Dutar P, Epelbaum J, Viollet C. Somatostatinergic systems: an update on brain functions in normal and pathological aging. Front Endocrinol (Lausanne) 2012;3:154. doi: 10.3389/fendo.2012.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tripp A, Oh H, Guilloux JP, Martinowich K, Lewis DA, Sibille E. Brain-derived neurotrophic factor signaling and subgenual anterior cingulate cortex dysfunction in major depressive disorder. Am J Psychiatry. 2012;169(11):1194–1202. doi: 10.1176/appi.ajp.2012.12020248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sibille E, Morris HM, Kota RS, Lewis DA. GABA-related transcripts in the dorsolateral prefrontal cortex in mood disorders. Int J Neuropsychopharmacol. 2011;14(6):721–734. doi: 10.1017/S1461145710001616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guilloux JP, Douillard-Guilloux G, Kota R, Wang X, Gardier AM, Martinowich K, et al. Molecular evidence for BDNF- and GABA-related dysfunctions in the amygdala of female subjects with major depression. Mol Psychiatry. 2012;17(11):1130–1142. doi: 10.1038/mp.2011.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tripp A, Kota RS, Lewis DA, Sibille E. Reduced somatostatin in subgenual anterior cingulate cortex in major depression. Neurobiol Dis. 2011;42(1):116–124. doi: 10.1016/j.nbd.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hendry SH, Jones EG, Emson PC. Morphology, distribution, and synaptic relations of somatostatin- and neuropeptide Y-immunoreactive neurons in rat and monkey neocortex. J Neurosci. 1984;4(10):2497–2517. doi: 10.1523/JNEUROSCI.04-10-02497.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Melchitzky DS, Lewis DA. Dendritic-targeting GABA neurons in monkey prefrontal cortex: Comparison of somatostatin- and calretinin-immunoreactive axon terminals. Synapse. 2008;62(6):456–465. doi: 10.1002/syn.20514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu X, Roby KD, Callaway EM. Immunochemical characterization of inhibitory mouse cortical neurons: three chemically distinct classes of inhibitory cells. J Comp Neurol. 2010;518(3):389–404. doi: 10.1002/cne.22229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gentet LJ, Kremer Y, Taniguchi H, Huang ZJ, Staiger JF, Petersen CC. Unique functional properties of somatostatin-expressing GABAergic neurons in mouse barrel cortex. Nat Neurosci. 2012;15(4):607–612. doi: 10.1038/nn.3051. [DOI] [PubMed] [Google Scholar]
- 15.Le Magueresse C, Monyer H. GABAergic interneurons shape the functional maturation of the cortex. Neuron. 2013;77(3):388–405. doi: 10.1016/j.neuron.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 16.DeFelipe J, Lopez-Cruz PL, Benavides-Piccione R, Bielza C, Larranaga P, Anderson S, et al. New insights into the classification and nomenclature of cortical GABAergic interneurons. Nat Rev Neurosci. 2013;14(3):202–216. doi: 10.1038/nrn3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zeyda T, Diehl N, Paylor R, Brennan MB, Hochgeschwender U. Impairment in motor learning of somatostatin null mutant mice. Brain Res. 2001;906(1–2):107–114. doi: 10.1016/s0006-8993(01)02563-x. [DOI] [PubMed] [Google Scholar]
- 18.Agren H, Lundqvist G. Low levels of somatostatin in human CSF mark depressive episodes. Psychoneuroendocrinology. 1984;9(3):233–248. doi: 10.1016/0306-4530(84)90003-9. [DOI] [PubMed] [Google Scholar]
- 19.Molchan SE, Lawlor BA, Hill JL, Martinez RA, Davis CL, Mellow AM, et al. CSF monoamine metabolites and somatostatin in Alzheimer’s disease and major depression. Biol Psychiatry. 1991;29(11):1110–1118. doi: 10.1016/0006-3223(91)90253-i. [DOI] [PubMed] [Google Scholar]
- 20.Kling MA, Rubinow DR, Doran AR, Roy A, Davis CL, Calabrese JR, et al. Cerebrospinal fluid immunoreactive somatostatin concentrations in patients with Cushing’s disease and major depression: relationship to indices of corticotropin-releasing hormone and cortisol secretion. Neuroendocrinology. 1993;57(1):79–88. doi: 10.1159/000126345. [DOI] [PubMed] [Google Scholar]
- 21.Molchan SE, Hill JL, Martinez RA, Lawlor BA, Mellow AM, Rubinow DR, et al. CSF somatostatin in Alzheimer’s disease and major depression: relationship to hypothalamic-pituitary-adrenal axis and clinical measures. Psychoneuroendocrinology. 1993;18(7):509–519. doi: 10.1016/0306-4530(93)90044-l. [DOI] [PubMed] [Google Scholar]
- 22.Swaab DF, Bao AM, Lucassen PJ. The stress system in the human brain in depression and neurodegeneration. Ageing Res Rev. 2005;4(2):141–194. doi: 10.1016/j.arr.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 23.Holsboer F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology. 2000;23(5):477–501. doi: 10.1016/S0893-133X(00)00159-7. [DOI] [PubMed] [Google Scholar]
- 24.Belmaker RH, Agam G. Major depressive disorder. NEnglJ Med. 2008;358(1):55–68. doi: 10.1056/NEJMra073096. [DOI] [PubMed] [Google Scholar]
- 25.Stengel A, Rivier J, Tache Y. Central actions of somatostatin-28 and oligosomatostatin agonists to prevent components of the endocrine, autonomic and visceral responses to stress through interaction with different somatostatin receptor subtypes. Curr Pharm Des. 2013;19(1):98–105. doi: 10.2174/13816128130114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fisher DA, Brown MR. Somatostatin analog: plasma catecholamine suppression mediated by the central nervous system. Endocrinology. 1980;107(3):714–718. doi: 10.1210/endo-107-3-714. [DOI] [PubMed] [Google Scholar]
- 27.Brown MR, Rivier C, Vale W. Central nervous system regulation of adrenocorticotropin secretion: role of somatostatins. Endocrinology. 1984;114(5):1546–1549. doi: 10.1210/endo-114-5-1546. [DOI] [PubMed] [Google Scholar]
- 28.Stengel A, Goebel-Stengel M, Wang L, Luckey A, Hu E, Rivier J, et al. Central administration of pan-somatostatin agonist ODT8-SST prevents abdominal surgery-induced inhibition of circulating ghrelin, food intake and gastric emptying in rats. Neurogastroenterol Motil. 2011;23(7):e294–308. doi: 10.1111/j.1365-2982.2011.01721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keller MC, Neale MC, Kendler KS. Association of different adverse life events with distinct patterns of depressive symptoms. Am J Psychiatry. 2007;164(10):1521–1529. doi: 10.1176/appi.ajp.2007.06091564. quiz 1622. [DOI] [PubMed] [Google Scholar]
- 30.Vezzani A, Hoyer D. Brain somatostatin: a candidate inhibitory role in seizures and epileptogenesis. Eur J Neurosci. 1999;11(11):3767–3776. doi: 10.1046/j.1460-9568.1999.00838.x. [DOI] [PubMed] [Google Scholar]
- 31.Ponomarev I, Rau V, Eger EI, Harris RA, Fanselow MS. Amygdala transcriptome and cellular mechanisms underlying stress-enhanced fear learning in a rat model of posttraumatic stress disorder. Neuropsychopharmacology. 2010;35(6):1402–1411. doi: 10.1038/npp.2010.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martinowich K, Schloesser RJ, Jimenez DV, Weinberger DR, Lu B. Activity-dependent brain-derived neurotrophic factor expression regulates cortistatin-interneurons and sleep behavior. Mol Brain. 2011;4:11. doi: 10.1186/1756-6606-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Glorioso C, Sabatini M, Unger T, Hashimoto T, Monteggia LM, Lewis DA, et al. Specificity and timing of neocortical transcriptome changes in response to BDNF gene ablation during embryogenesis or adulthood. Mol Psychiatry. 2006;11(7):633–648. doi: 10.1038/sj.mp.4001835. [DOI] [PubMed] [Google Scholar]
- 34.Grosse G, Djalali S, Deng DR, Holtje M, Hinz B, Schwartzkopff K, et al. Area-specific effects of brain-derived neurotrophic factor (BDNF) genetic ablation on various neuronal subtypes of the mouse brain. Brain Res Dev Brain Res. 2005;156(2):111–126. doi: 10.1016/j.devbrainres.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 35.Duman RS, Heninger GR, Nestler EJ. A molecular and cellular theory of depression. Arch Gen Psychiatry. 1997;54(7):597–606. doi: 10.1001/archpsyc.1997.01830190015002. [DOI] [PubMed] [Google Scholar]
- 36.Albrecht A, Thiere M, Bergado-Acosta JR, Poranzke J, Muller B, Stork O. Circadian modulation of anxiety: a role for somatostatin in the amygdala. PLoS One. 2013;8(12):e84668. doi: 10.1371/journal.pone.0084668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yeung M, Engin E, Treit D. Anxiolytic-like effects of somatostatin isoforms SST 14 and SST 28 in two animal models (Rattus norvegicus) after intra-amygdalar and intra-septal microinfusions. Psychopharmacology (Berl) 2011;216(4):557–567. doi: 10.1007/s00213-011-2248-x. [DOI] [PubMed] [Google Scholar]
- 38.Yeung M, Treit D. The anxiolytic effects of somatostatin following intra-septal and intra-amygdalar microinfusions are reversed by the selective sst2 antagonist PRL2903. Pharmacol Biochem Behav. 2012;101(1):88–92. doi: 10.1016/j.pbb.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 39.Soumier A, Sibille E. Opposing Effects of Acute versus Chronic Blockade of Frontal Cortex Somatostatin-Positive Inhibitory Neurons on Behavioral Emotionality in Mice. Neuropsychopharmacology. 2014 doi: 10.1038/npp.2014.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taniguchi H, He M, Wu P, Kim S, Paik R, Sugino K, et al. A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex. Neuron. 2011;71(6):995–1013. doi: 10.1016/j.neuron.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Surget A, Wang Y, Leman S, Ibarguen-Vargas Y, Edgar N, Griebel G, et al. Corticolimbic transcriptome changes are state-dependent and region-specific in a rodent model of depression and of antidepressant reversal. Neuropsychopharmacology. 2009;34(6):1363–1380. doi: 10.1038/npp.2008.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sibille E, Pavlides C, Benke D, Toth M. Genetic inactivation of the Serotonin(1A) receptor in mice results in downregulation of major GABA(A) receptor alpha subunits, reduction of GABA(A) receptor binding, and benzodiazepine-resistant anxiety. J Neurosci. 2000;20(8):2758–2765. doi: 10.1523/JNEUROSCI.20-08-02758.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guilloux JP, Seney M, Edgar N, Sibille E. Integrated behavioral z-scoring increases the sensitivity and reliability of behavioral phenotyping in mice: relevance to emotionality and sex. J Neurosci Methods. 2011;197(1):21–31. doi: 10.1016/j.jneumeth.2011.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.David DJ, Samuels BA, Rainer Q, Wang JW, Marsteller D, Mendez I, et al. Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron. 2009;62(4):479–493. doi: 10.1016/j.neuron.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lin LC, Lewis DA, Sibille E. A human-mouse conserved sex bias in amygdala gene expression related to circadian clock and energy metabolism. Mol Brain. 2011;4:18. doi: 10.1186/1756-6606-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ma T, Trinh MA, Wexler AJ, Bourbon C, Gatti E, Pierre P, et al. Suppression of eIF2alpha kinases alleviates Alzheimer’s disease-related plasticity and memory deficits. Nat Neurosci. 2013;16(9):1299–1305. doi: 10.1038/nn.3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moreno JA, Halliday M, Molloy C, Radford H, Verity N, Axten JM, et al. Oral treatment targeting the unfolded protein response prevents neurodegeneration and clinical disease in prion-infected mice. Sci Transl Med. 2013;5(206):206ra138. doi: 10.1126/scitranslmed.3006767. [DOI] [PubMed] [Google Scholar]
- 48.Kim HJ, Raphael AR, Ladow ES, McGurk L, Weber RA, Trojanowski JQ, et al. Therapeutic modulation of eIF2alpha phosphorylation rescues TDP-43 toxicity in amyotrophic lateral sclerosis disease models. Nat Genet. 2014;46(2):152–160. doi: 10.1038/ng.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301(5631):386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 50.Sibille E, Wang Y, Joeyen-Waldorf J, Gaiteri C, Surget A, Oh S, et al. A molecular signature of depression in the amygdala. Am J Psychiatry. 2009;166(9):1011–1024. doi: 10.1176/appi.ajp.2009.08121760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nollet M, Le Guisquet AM, Belzung C. Models of depression: unpredictable chronic mild stress in mice. Curr Protoc Pharmacol. 2013;Chapter 5(Unit 5):65. doi: 10.1002/0471141755.ph0565s61. [DOI] [PubMed] [Google Scholar]
- 52.Scalera G, Tarozzi G. Somatostatin administration modifies food intake, body weight, and gut motility in rat. Peptides. 1998;19(6):991–997. doi: 10.1016/s0196-9781(98)00053-9. [DOI] [PubMed] [Google Scholar]
- 53.Ricci GL, Fevery J. Somatostatin inhibits the effect of secretin on bile flow and on hepatic bilirubin transport in the rat. Gut. 1989;30(9):1266–1269. doi: 10.1136/gut.30.9.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.de Lecea L, del Rio JA, Criado JR, Alcantara S, Morales M, Danielson PE, et al. Cortistatin is expressed in a distinct subset of cortical interneurons. J Neurosci. 1997;17(15):5868–5880. doi: 10.1523/JNEUROSCI.17-15-05868.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Viollet C, Lepousez G, Loudes C, Videau C, Simon A, Epelbaum J. Somatostatinergic systems in brain: networks and functions. Mol Cell Endocrinol. 2008;286(1–2):75–87. doi: 10.1016/j.mce.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 56.de Lecea L. Cortistatin--functions in the central nervous system. Mol Cell Endocrinol. 2008;286(1–2):88–95. doi: 10.1016/j.mce.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 57.Nagata T, Ilieva H, Murakami T, Shiote M, Narai H, Ohta Y, et al. Increased ER stress during motor neuron degeneration in a transgenic mouse model of amyotrophic lateral sclerosis. Neurol Res. 2007;29(8):767–771. doi: 10.1179/016164107X229803. [DOI] [PubMed] [Google Scholar]
- 58.Kramer A, Green J, Pollard J, Jr, Tugendreich S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics. 2014;30(4):523–530. doi: 10.1093/bioinformatics/btt703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ardayfio P, Kim KS. Anxiogenic-like effect of chronic corticosterone in the light-dark emergence task in mice. Behav Neurosci. 2006;120(2):249–256. doi: 10.1037/0735-7044.120.2.249. [DOI] [PubMed] [Google Scholar]
- 60.Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, Schapira M, et al. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell. 2000;6(5):1099–1108. doi: 10.1016/s1097-2765(00)00108-8. [DOI] [PubMed] [Google Scholar]
- 61.Moreno JA, Radford H, Peretti D, Steinert JR, Verity N, Martin MG, et al. Sustained translational repression by eIF2alpha-P mediates prion neurodegeneration. Nature. 2012;485(7399):507–511. doi: 10.1038/nature11058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Boyce M, Bryant KF, Jousse C, Long K, Harding HP, Scheuner D, et al. A selective inhibitor of eIF2alpha dephosphorylation protects cells from ER stress. Science. 2005;307(5711):935–939. doi: 10.1126/science.1101902. [DOI] [PubMed] [Google Scholar]
- 63.Axten JM, Medina JR, Feng Y, Shu A, Romeril SP, Grant SW, et al. Discovery of 7-methyl-5-(1-{[3-(trifluoromethyl)phenyl]acetyl}-2,3-dihydro-1H-indol-5-yl)-7H-p yrrolo[2,3-d]pyrimidin-4-amine (GSK2606414), a potent and selective first-in-class inhibitor of protein kinase R (PKR)-like endoplasmic reticulum kinase (PERK) J Med Chem. 2012;55(16):7193–7207. doi: 10.1021/jm300713s. [DOI] [PubMed] [Google Scholar]
- 64.Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301(5634):805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 65.Carroll BJ, Schroeder K, Mukhopadhyay S, Greden JF, Feinberg M, Ritchie J, et al. Plasma dexamethasone concentrations and cortisol suppression response in patients with endogenous depression. J Clin Endocrinol Metab. 1980;51(3):433–437. doi: 10.1210/jcem-51-3-433. [DOI] [PubMed] [Google Scholar]
- 66.Burgus R, Ling N, Butcher M, Guillemin R. Primary structure of somatostatin, a hypothalamic peptide that inhibits the secretion of pituitary growth hormone. Proc Natl Acad Sci U S A. 1973;70(3):684–688. doi: 10.1073/pnas.70.3.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.de Kloet ER, Sarabdjitsingh RA. Everything has rhythm: focus on glucocorticoid pulsatility. Endocrinology. 2008;149(7):3241–3243. doi: 10.1210/en.2008-0471. [DOI] [PubMed] [Google Scholar]
- 68.Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: Implications for major psychiatric disorders. Biol Psychiatry. 2003;54(5):515–528. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- 69.Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, et al. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156(5):675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- 70.Brody AL, Saxena S, Mandelkern MA, Fairbanks LA, Ho ML, Baxter LR. Brain metabolic changes associated with symptom factor improvement in major depressive disorder. Biol Psychiatry. 2001;50(3):171–178. doi: 10.1016/s0006-3223(01)01117-9. [DOI] [PubMed] [Google Scholar]
- 71.Kim SS, Wang H, Li XY, Chen T, Mercaldo V, Descalzi G, et al. Neurabin in the anterior cingulate cortex regulates anxiety-like behavior in adult mice. Mol Brain. 2011;4:6. doi: 10.1186/1756-6606-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ramirez JL, Mouchantaf R, Kumar U, Otero Corchon V, Rubinstein M, Low MJ, et al. Brain somatostatin receptors are up-regulated in somatostatin-deficient mice. Mol Endocrinol. 2002;16(8):1951–1963. doi: 10.1210/me.2002-0068. [DOI] [PubMed] [Google Scholar]
- 73.Cammalleri M, Cervia D, Dal Monte M, Martini D, Langenegger D, Fehlmann D, et al. Compensatory changes in the hippocampus of somatostatin knockout mice: upregulation of somatostatin receptor 2 and its function in the control of bursting activity and synaptic transmission. Eur J Neurosci. 2006;23(9):2404–2422. doi: 10.1111/j.1460-9568.2006.04770.x. [DOI] [PubMed] [Google Scholar]
- 74.Pothion S, Bizot JC, Trovero F, Belzung C. Strain differences in sucrose preference and in the consequences of unpredictable chronic mild stress. Behav Brain Res. 2004;155(1):135–146. doi: 10.1016/j.bbr.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 75.Willner P, Towell A, Sampson D, Sophokleous S, Muscat R. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology (Berl) 1987;93(3):358–364. doi: 10.1007/BF00187257. [DOI] [PubMed] [Google Scholar]
- 76.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8(7):519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 77.Valdes P, Mercado G, Vidal RL, Molina C, Parsons G, Court FA, et al. Control of dopaminergic neuron survival by the unfolded protein response transcription factor XBP1. Proc Natl Acad Sci U S A. 2014;111(18):6804–6809. doi: 10.1073/pnas.1321845111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Leitman J, Barak B, Benyair R, Shenkman M, Ashery U, Hartl FU, et al. ER stress-induced eIF2-alpha phosphorylation underlies sensitivity of striatal neurons to pathogenic huntingtin. PLoS One. 2014;9(3):e90803. doi: 10.1371/journal.pone.0090803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Saxena S, Cabuy E, Caroni P. A role for motoneuron subtype-selective ER stress in disease manifestations of FALS mice. Nat Neurosci. 2009;12(5):627–636. doi: 10.1038/nn.2297. [DOI] [PubMed] [Google Scholar]
- 80.Hetz C, Mollereau B. Disturbance of endoplasmic reticulum proteostasis in neurodegenerative diseases. Nat Rev Neurosci. 2014;15(4):233–249. doi: 10.1038/nrn3689. [DOI] [PubMed] [Google Scholar]
- 81.Calfon M, Zeng H, Urano F, Till JH, Hubbard SR, Harding HP, et al. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature. 2002;415(6867):92–96. doi: 10.1038/415092a. [DOI] [PubMed] [Google Scholar]
- 82.Kakiuchi C, Iwamoto K, Ishiwata M, Bundo M, Kasahara T, Kusumi I, et al. Impaired feedback regulation of XBP1 as a genetic risk factor for bipolar disorder. Nat Genet. 2003;35(2):171–175. doi: 10.1038/ng1235. [DOI] [PubMed] [Google Scholar]
- 83.Hayashi A, Kasahara T, Kametani M, Kato T. Attenuated BDNF-induced upregulation of GABAergic markers in neurons lacking Xbp1. Biochem Biophys Res Commun. 2008;376(4):758–763. doi: 10.1016/j.bbrc.2008.09.059. [DOI] [PubMed] [Google Scholar]
- 84.Zhu PJ, Huang W, Kalikulov D, Yoo JW, Placzek AN, Stoica L, et al. Suppression of PKR promotes network excitability and enhanced cognition by interferon-gamma-mediated disinhibition. Cell. 2011;147(6):1384–1396. doi: 10.1016/j.cell.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Trinh MA, Kaphzan H, Wek RC, Pierre P, Cavener DR, Klann E. Brain-specific disruption of the eIF2alpha kinase PERK decreases ATF4 expression and impairs behavioral flexibility. Cell Rep. 2012;1(6):676–688. doi: 10.1016/j.celrep.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.