Abstract
Objective. To investigate the possible mechanisms of oxymatrine's role in anti laryngeal squamous cell carcinoma. Methods. We examined the effects of oxymatrine on the proliferation, cell cycle phase distribution, apoptosis, and the protein and mRNA expression levels of HPV16E7 gene in laryngeal carcinoma Hep-2 cells in vitro. The HPV16E7 siRNA inhibition was also done to confirm the effect of downregulating HPV16E7 on the proliferation in Hep-2 cells. Results. Oxymatrine significantly inhibited the growth and proliferation of Hep-2 cells in a dose-dependence and time-dependence manner. Oxymatrine blocked Hep-2 cells in G0/G1 phase, resulting in an obvious accumulation of G0/G1 phase cells while decreasing S phase cells. Oxymatrine induced apoptosis of Hep-2 cells, whose apoptotic rate amounted to about 42% after treatment with 7 mg/mL oxymatrine for 72 h. Oxymatrine also downregulated the expression of HPV16E7 gene, as determined by the western blotting and reverse transcription-polymerase chain reaction analysis. Knockdown of HPV16E7 effectively inhibited the proliferation of Hep-2 cells. Conclusions. Oxymatrine inhibits the proliferation and induces apoptosis of laryngeal carcinoma Hep-2 cells, which might be mediated by a significant cell cycle arrest in G0/G1 phase and downregulation of HPV16E7 gene. Oxymatrine is considered to be a likely preventive and curative candidate for laryngeal cancer.
1. Introduction
Laryngeal squamous cell carcinoma is a common malignancy in the head and neck region [1]. In addition to the well-established risk factors for laryngeal cancer such as tobacco smoking and alcohol consumption, a number of studies suggest that human papillomavirus type 16 (HPV16) is also associated with the development of laryngeal cancer [2–4]. Carcinogenesis by HPV16 is primarily attributed to the continuous expression of viral protein E6/E7, which results in cellular resistance to apoptosis and cancer formation by, respectively, interacting with p53 and pRb in cell cycle checkpoint [5–7]. We have also demonstrated that the overexpression of HPV16E7 oncoprotein is associated with the development of laryngeal cancer [8]. Therefore, the HPV16E7 oncogene is considered to be potential therapeutic target for blocking the development of laryngeal cancer.
Treatment strategies for laryngeal cancer today are focused on larynx preservation which have the aim to preserve not only the anatomic organ, but, more importantly, also its function [9]. To achieve organ preservation, various options for treatment modalities including radiotherapy, chemotherapy, and targeted molecular therapies have been added to the conventional approaches of surgery [10]. Although a number of chemotherapeutic drugs are available for the treatment of cancer, which can be used for controlling the growth of cancer and have received certain curative efficacy, the side effects limit their application. Therefore, to discover novel natural substances that have therapeutic selectivity without significant toxicity to normal cells is an important tendency for laryngeal cancer therapy. Oxymatrine is one of the quinolizidine alkaloids extracted from the root of traditional Chinese herbal medicine Sophora japonica (Sophora flavescens Ait). It has been reported that oxymatrine plays important roles in anti-inflammation, inhibition of immune reaction, antivirus, antitumors, and so on [11–14]. Different from the usual chemotherapy medicine, oxymatrine has the selectivity kill capability to the tumor cells, with little influence to some normal cells [15].
To our knowledge, there are few studies on the application of oxymatrine in the treatment of laryngeal cancer. Similarly, there currently is no report concerning the mechanism of oxymatrine and its putative relationship with HPV16E7. In the present study, we investigated the effects of oxymatrine on laryngeal squamous cell carcinoma Hep-2 cells by examining cell proliferation, cell cycle phase distribution, apoptosis, and the protein and mRNA expression levels of HPV16E7 gene in vitro. This study aimed to explore the antitumor mechanisms of oxymatrine and provide experimental evidence for the application of oxymatrine in the prevention and treatment of laryngeal squamous cell carcinoma.
2. Materials and Methods
2.1. Oxymatrine
Oxymatrine (300 mg/mL) was purchased from Chia Tai Tianqing Pharmaceutical Group Co., Ltd., Nanjing (Jiangsu, China). In the experiment, we used the same batch of oxymatrine, whose purity is more than 99% indicated by SDS-PAGE analysis.
2.2. Cell Line and Culture
The Hep-2 human laryngeal carcinoma cell line was obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). Cells were routinely cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco Corporation, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS; Sijiqing, China), 100 U/mL penicillin G, and 100 U/mL streptomycin (Gibco, Carlsbad, CA, USA) in a humidified atmosphere of 95% air and 5% CO2 at 37°C. The medium was changed every 3 days.
2.3. Proliferation Assay
Hep-2 cells in logarithmic growth phase were seeded in 96-well microplates with 1 × 105 each well and cultured cells with DMEM growth medium for 24 h. Then the medium was replaced with DMEM growth medium containing various concentrations of oxymatrine (3, 5, and 7 mg/mL) and cultured continuously. In addition, control cells were incubated with medium only. After exposure to oxymatrine, changes of cell morphology were observed by optical microscope (Olympus, Japan) and the proliferation of Hep-2 cells was assessed by using CCK-8 assay. After 24, 72, 120, and 168 h, cells were treated with 10 μL of CCK-8 reagent (Dojindo Molecular Technologies, Kunamoto, Japan) and incubated at 37°C for 1 h. An automatic microtiter plate reader was set to zero according to the control wells. The absorbance (A) of each well was measured at a wavelength of 450 nm. The rate of inhibition was calculated as follows: cell proliferation inhibition (%) = (1 − average absorbance (A) of the experimental group/average absorbance (A) of the control group) × 100%.
2.4. Cell Cycle Analysis
After preincubation with DMEM growth medium for 24 h, Hep-2 cells were exposed to medium containing different concentrations of oxymatrine (0, 3, 5, and 7 mg/mL) for 72 h. The cells were harvested by centrifugation, fixed in 70% cooled ethanol, and then dyed with propidium iodide (PI) for 30 min. DNA content was detected by a flow cytometer (FACSCaliburTM, America BD). The relative proportions of cells in the individual cell-cycle phase fraction were determined from the flow cytometry data.
2.5. Apoptosis Assay
All the operations were based on instructions which are described as manual of cell apoptosis assay kit. After treatment with various concentrations of oxymatrine (0, 3, 5, and 7 mg/mL) for 72 h, Hep-2 cells were harvested by centrifugation, resuspended in binding buffer, and successively incubated with 5 μL of Annexin V-FITC and 5 μL of PI (Multi Sciences, China) for 15 min at room temperature. Apoptosis was determined by flow cytometric analysis using a flow cytometer (FACSCaliburTM, America BD).
2.6. Real-Time PCR
Total RNA was extracted from Hep-2 cells treated with 5 mg/mL of oxymatrine for 72 h or the control cells using Trizol reagent (Invitrogen, Shanghai, China) and converted to cDNA using MMLV reverse transcriptase kit (Promega, USA). Aliquots of cDNA were subjected to quantitative real-time PCR using Step One Plus Real-time PCR system (Applied Biosystems, USA). The mRNA levels were normalized to that of β-actin. The specific primer pairs were as follows: HPV16E7, sense: 5′-CGGGATCCATGCATGGAGATACA-3′ and antisense: 5′-GCGGGCCCTTATGGTTTCTGAGA-3′; β-actin, sense: 5′-CATGTACGTTGCTATCCAGGC-3′ and antisense: 5′-CTCCTTAATGTCACGCACGAT-3′. Data were analyzed using the 2−ΔΔCt method.
2.7. Western Blot Analysis
The protein was extracted from Hep-2 cells treated with 5 mg/mL of oxymatrine for 72 h or the control cells. Cell extracts were prepared in radioimmunoprecipitation assay buffer (150 mM NaCl, 0.1% SDS, 0.5% sodium deoxycholate, 1% Nonidet P-40, and 50 mM Tris, pH 8.0), with the addition of 2 mM phenylmethylsulfonyl fluoride. Lysis buffer was freshly prepared and added in 6-well plates (100 μL/well) on ice, which were then incubated for 10 min. Protein concentrations were determined by protein assay kit. Equal amounts of proteins (10 μg per condition) were boiled for 10 min in loading buffer before being separated on 15% SDS-PAGE gels. Separated proteins were transferred to polyvinylidene difluoride membranes at 100 V for 1 h before membrane blocking in PBS with 5% skim milk powder and Tween 20. Anti-HPV16E7 primary antibody (Biorbyt) and secondary antibodies (Abcam) were diluted by 1/500 with PBST buffer and incubated for 60 min at room temperature. Membrane was washed for 3 times by PBST before each step. Protein bands were visualized by enhanced chemiluminescence. β-actin was used as an internal control.
2.8. RNA Interference
The RNAi sequence for HPV16E7 (GCT TCG GTT GTG CGT ACA A) was identified by using the manufacturer's RNAi Designer programme, and the negative control having no homology with human genome was created by a scrambled sequence (TTC TCC GAA CGT GTC ACG T). The siRNA duplex was transfected using Lipofectamine 2000 Reagent (Invitrogen) as recommended by the manufacturer, and the cells were assayed for silencing 72 h after transfection.
2.9. Statistical Analysis
All experiments were performed in triplicate, and data were shown as the mean ± SD where they are applicable. Statistically significant differences between groups were determined by one-way ANOVA using SPSS 17.0 software (SPSS, Chicago, IL, USA), and P < 0.05 was considered statistically significant.
3. Results
3.1. Oxymatrine Inhibits the Growth and Proliferation of Hep-2 Cells
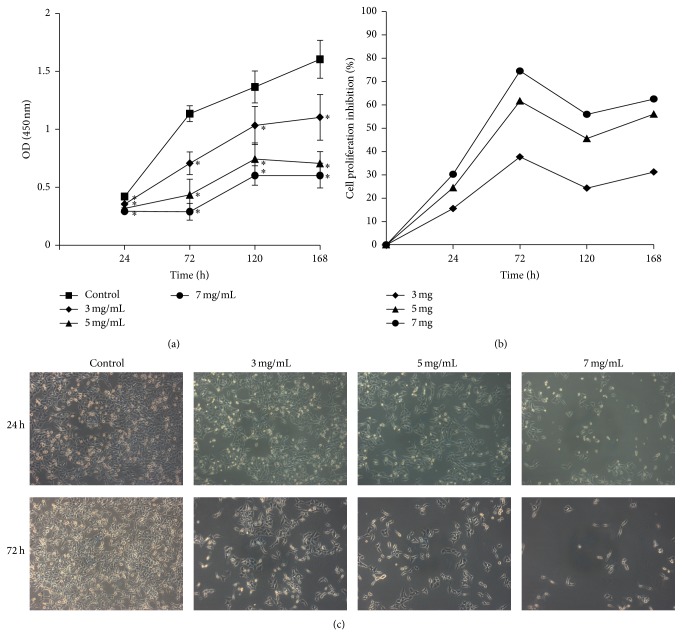
To determine whether oxymatrine inhibits the proliferation of Hep-2 cells, we examined the effect of oxymatrine on proliferation of the Hep-2 cell line by using CCK-8 assay. We found that oxymatrine significantly inhibited the growth of Hep-2 cells in a concentration-dependence and time-dependence manner, compared with that in the control cells (P < 0.05, Figure 1(a)). In the group treated with 3 mg/mL of oxymatrine at the indicated time points of 24, 72, 120, and 168 h, the percentage of Hep-2 cell proliferation inhibition was 15.6%, 37.7%, 24.3%, and 31.2%, respectively. Additionally, in the group treated with 5 mg/mL of oxymatrine, the percentage of cell proliferation inhibition at 24, 72, 120, and 168 h was 24.5%, 61.6%, 45.5%, and 56.0%, respectively. In the group treated with 7 mg/mL of oxymatrine, the percentage of cell proliferation inhibition at 24, 72, 120, and 168 h was 30.2%, 74.5%, 55.9%, and 62.5%, respectively (Figure 1(b)). At 72 h, oxymatrine showed a significantly higher inhibiting effect than that at 24 h. In contrast, there was no significant difference in cell proliferation inhibition among prolonged treatment for 120 h and 168 h. Therefore, we choose the time point of 72 h for the further investigation. Besides, we also found that the number of cells decreased with increasing concentrations of oxymatrine under a microscope (Figure 1(c)).
Figure 1.
Effects of oxymatrine on proliferation of Hep-2 cells. (a) Hep-2 cells were treated with oxymatrine at different concentrations for various times and the OD values were obtained through reading plate at 450 nm with 96-well microtest spectrophotometer by CCK-8 assay. (b) Relationship between the percentage of laryngeal cancer cell proliferation inhibition and different concentrations of oxymatrine. (c) Morphological changes induced by various concentrations of oxymatrine for 24 and 72 h in Hep-2 cells (×100).
3.2. Oxymatrine Arrests Hep-2 Cells in the G0/G1 Phase
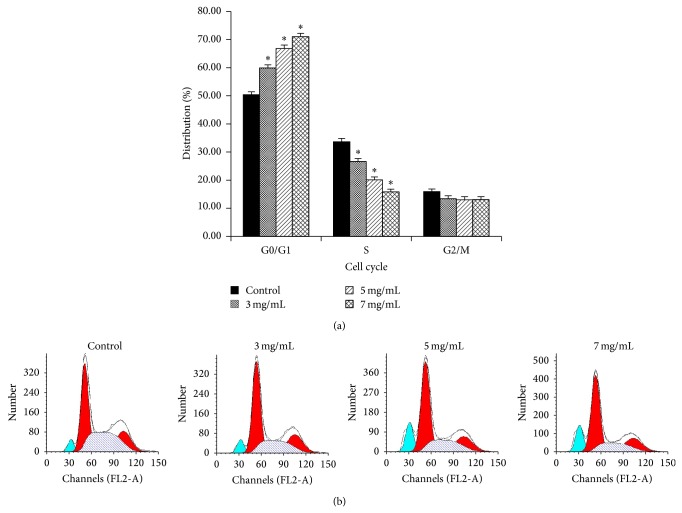
To evaluate the effect of oxymatrine on the cell cycle distribution, the FCM analysis was performed. We found that the proportion of G0/G1 phase was 59.91 ± 1.09%, 66.85 ± 1.18%, and 71.07 ± 1.12%, respectively, after treatment with 3, 5, and 7 mg/mL of oxymatrine for 72 h. For the control group, the percentage of G0/G1 phase was 50.37 ± 1.08%. The frequency of Hep-2 cell in G0/G1 phase is significantly increased while that of cells in the S-phase is decreased with the increasing concentration of oxymatrine, as compared with that in the control group (P < 0.05, n = 6, Figure 2). These data indicated that oxymatrine arrested cell cycle of Hep-2 cells in G0/G1 phase.
Figure 2.
Changes of cell cycle distribution in Hep-2 cells after treatment with oxymatrine at different concentrations for 72 h. (a) Oxymatrine increased the proportion of cells in G0/G1 phase and decreased that in the S phase, compared with the control group (* P < 0.05). (b) FCM analysis showed oxymatrine arrested Hep-2 cell cycling in the G0/G1 phase.
3.3. Oxymatrine Induces Apoptosis of Hep-2 Cells
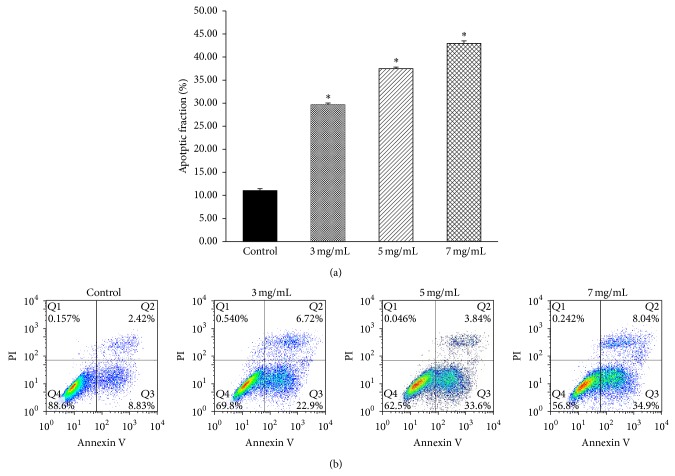
To further investigate the effects of oxymatrine on cell death of Hep-2 cells, the rate of apoptosis was evaluated by flow cytometry analysis. We found that the apoptosis rate was 29.62 ± 0.41%, 37.44 ± 0.38%, and 42.94 ± 0.56%, respectively, after treatment with 3, 5, and 7 mg/mL of oxymatrine for 72 h. For the control group, the apoptosis rate was 11.05 ± 0.40%. The percentage of apoptosis is significantly increased with the increasing concentration of oxymatrine, as compared with that in the control group (P < 0.05, n = 6, Figure 3). The results demonstrate that suppression of cell proliferation may be caused by cell death resulting from oxymatrine.
Figure 3.
Apoptosis induced by oxymatrine was detected by Annexin V-FITC/PI. (a) Oxymatrine significantly induced cell apoptosis in the treatment group, compared with that in the control group (* P < 0.05). (b) FCM data showed that the apoptotic rate of Hep-2 cells treated with oxymatrine was significantly increased.
3.4. Oxymatrine Downregulates HPV16E7 Expression in Hep-2 Cells
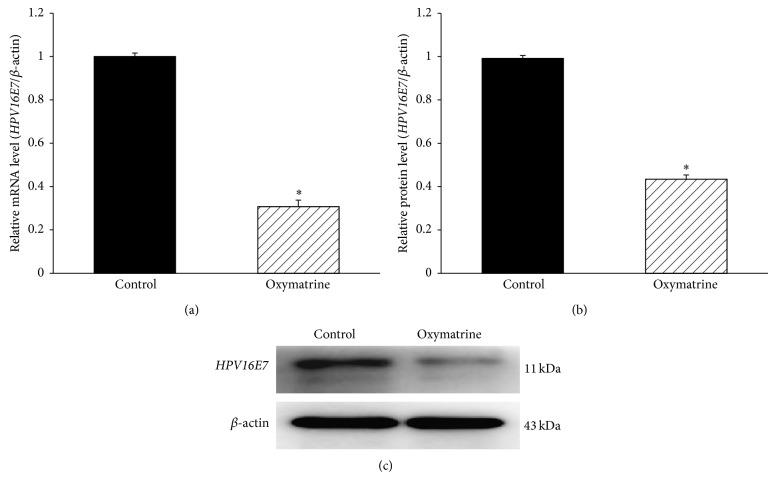
The effect of oxymatrine on the expression of HPV16E7 gene was examined by real-time PCR and Western blot analysis. We found that both mRNA and protein levels of HPV16E7 gene in the Hep-2 cells treated with 5 mg/mL of oxymatrine were sharply reduced, compared with that in the control group (P < 0.05, n = 6, Figure 4). The mRNA level is decreased by 69.3%, while the protein level is reduced by 56.3%. These data demonstrate that the expression of HPV16E7 gene is efficiently downregulated by oxymatrine in Hep-2 cells.
Figure 4.
Expressions of HPV16E7 mRNA and protein in Hep-2 cells treated with oxymatrine. (a) The expression levels of HPV16E7 mRNA were assessed by qRT-PCR (2−ΔΔCt method. 2−ΔΔCt indicated relative expression level in the treatment group, compared with that in the control group). (b) The protein level of HPV16E7 in the treatment group was significantly reduced, compared with the control group (* P < 0.05). (c) The expression levels of HPV16E7 protein were detected by Western blotting.
3.5. Knockdown of HPV16E7 Inhibits the Proliferation of Hep-2 Cells
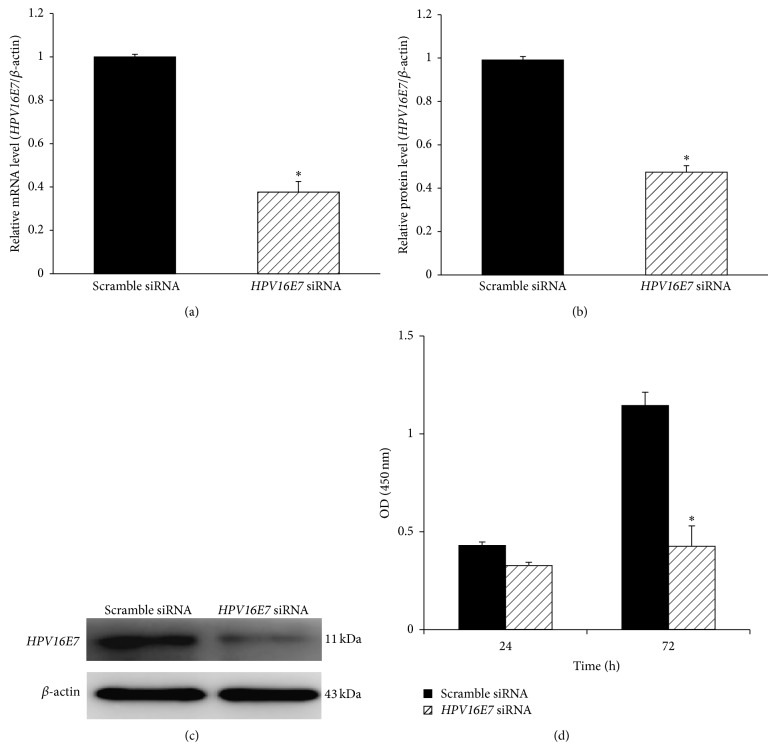
To assess the effect of downregulating HPV16E7 on the proliferation of Hep-2 cells, we knocked down HPV16E7 mRNA transcripts using HPV16E7 siRNA. HPV16E7 siRNA caused over 62.4% of reduction in HPV16E7 mRNA expression and 52.7% in protein expression compared to scrambled siRNA (Figures 5(a)–5(c)). After 72 h of culture, the proliferation inhibition of Hep-2 cells transfected by HPV16E7 siRNA was 62.8% compared with scramble control (P < 0.05, Figure 5(d)). These data indicated that downregulating HPV16E7 inhibited the growth of Hep-2 cells.
Figure 5.
Expressions of HPV16E7 mRNA and protein in Hep-2 cells treated with HPV16E7 siRNA. (a) The expression levels of HPV16E7 mRNA were assessed by qRT-PCR (2−ΔΔCt method. 2−ΔΔCt indicated relative expression level in the treatment group, compared with that in the control group). (b) The protein level of HPV16E7 in the HPV16E7 siRNA group was significantly reduced, compared with the scramble control group (* P < 0.05). (c) The expression levels of HPV16E7 protein were detected by Western blotting. (d) Hep-2 cells were treated with HPV16E7 siRNA and proliferation was measured by CCK-8 assay at 24 and 72 h after treatment.
4. Discussion
Oxymatrine is one of the main alkaloid components in the traditional Chinese herbal medicine Sophora japonica (Sophora flavescens Ait). It is commonly known that oxymatrine exhibits many biological activities and possesses a wide range of pharmacological effects, such as anti-inflammation [16], antifibrosis [17], analgesic [18], immunosuppression [19], and antivirus [20]. Oxymatrine also has been extensively studied, for their cancer chemoprevention potentially against various cancers, for instance, gastric cancer [21], breast cancer [22], hepatocellular carcinoma [23], and pancreatic cancer [24]. However, the mechanisms of the antitumor properties of oxymatrine in laryngeal cancer are not well established to date. In the present study, we treated laryngeal squamous cell carcinoma Hep-2 cells with various concentrations of oxymatrine, to investigate the effect of oxymatrine on the cell proliferation, cell-cycle progression, and apoptosis of laryngeal cancer.
The loss of regulatory control of cell proliferation and apoptosis plays an important role in tumorigenesis. It has been a well-studied area to interfere with the dysfunctions of cell proliferation and apoptosis in cancer research. In this study, we found that oxymatrine significantly inhibited the growth and proliferation in Hep-2 cells, which presented a concentration-dependence and time-dependence manner, as determined by the CCK-8 assay. Not only does the inhibitory mechanism involve alteration of the proliferation of cancer cells, but also induction of apoptosis. From apoptosis assay, we found that oxymatrine induced an obviously apoptosis in Hep-2 cells, and the apoptotic rate amounted to 42.94 ± 0.56% after being treated by 7 mg/mL oxymatrine for 72 h. In general, there is a near correlativity between apoptosis and cell cycle. If cell cycle is blocked in a phase, apoptosis would be appearing in the phase in which cell cycle is arrested. As shown from FCM analysis, oxymatrine significantly blocked the cell cycle of Hep-2 cells in G0/G1 phase, the cells in G0/G1 phase were significantly increased, while the cells in the S phase were decreased with the increasing concentration of oxymatrine, which suggests that apoptosis of Hep-2 cells induced by oxymatrine maybe occurs in G0/G1 phase. One of the basic characteristics of tumor cells is uncontrolled cell growth. The mechanism underlying uncontrolled proliferation is the destruction of the cell cycle control machinery. Regulation of cell cycle progression can potentially trigger cellular apoptosis suppressing cell proliferation to achieve antitumor effects. The results of our study demonstrate that oxymatrine may be a potential chemotherapeutics for the treatment of laryngeal cancer.
Molecular epidemiologic evidences clearly indicate that HPV16 is closely associated with the development of laryngeal cancer, which encodes the E6 and E7 oncogenes, whose expression is essential for virus replication. The viral protein E7 of HPV16 binds to hypophosphorylated members of the retinoblastoma family, resulting in their destabilization and the disruption of Rb/E2F complexes [25], which repress transcription of genes required for cell cycle progression to allow the progression of cell cycle into S phase [26] and increase antiapoptotic protein Bcl-2 to promote the survival of cells and stimulate cell proliferation [27]. In previous studies, oxymatrine has been demonstrated to exhibit specific pharmacological properties for antihepatitis virus. Although there are no reports on anti-HPV effects of oxymatrine, we found that oxymatrine downregulated the HPV16E7 expression at both mRNA and protein levels. To assess the effect of downregulating HPV16E7 on the proliferation of Hep-2 cells, we also knocked down HPV16E7 mRNA transcripts using HPV16E7 siRNA and found that downregulating HPV16E7 inhibited the growth of Hep-2 cells. Thus, we speculate that oxymatrine may inhibit proliferation of Hep-2 cells through downregulation of HPV16E7 gene. In other studies, compounds isolated from arsenic have demonstrated inhibitory effects on HPV oncogene activation and anti-HPV-associated tumors [28]. Therefore, we hypothesize that oxymatrine may also have an anti-HPV effect that may be able to treat laryngeal cancer. Moreover, oxymatrine has been found to enhance the antitumor immune response, which may also be beneficial for the treatment of laryngeal cancer.
In summary, our in vitro experiments demonstrate that oxymatrine significantly inhibits growth and proliferation and induces apoptosis of laryngeal carcinoma Hep-2 cells. This apoptosis may be in part mediated by a significant blockage in G0/G1 phase. We suggest that the observed effects of oxymatrine are likely associated with the regulation of the HPV16E7 and downstream protein expression. Due to the effective, nontoxic, and natural antitumor properties, oxymatrine is considered to be a likely preventive and curative candidate for laryngeal cancer. Additional studies are required to determine the underlying mechanisms whereby oxymatrine suppresses laryngeal cancer at the molecular level and to provide a theoretical basis for the tumoricidal and clinical utility of oxymatrine.
Acknowledgment
This work was supported by the Science and Technology Commission of Shanghai Municipality, China (13ZR1433600).
Conflict of Interests
The authors declared no conflict of interests.
References
- 1.Rudolph E., Dyckhoff G., Becher H., Dietz A., Ramroth H. Effects of tumour stage, comorbidity and therapy on survival of laryngeal cancer patients: a systematic review and a meta-analysis. European Archives of Oto-Rhino-Laryngology. 2011;268(2):165–179. doi: 10.1007/s00405-010-1395-8. [DOI] [PubMed] [Google Scholar]
- 2.Li X., Gao L., Li H., et al. Human papillomavirus infection and laryngeal cancer risk: a systematic review and meta-analysis. Journal of Infectious Diseases. 2013;207(3):479–488. doi: 10.1093/infdis/jis698. [DOI] [PubMed] [Google Scholar]
- 3.Manjarrez M. E., Ocadiz R., Valle L., et al. Detection of human papillomavirus and relevant tumor suppressors and oncoproteins in laryngeal tumors. Clinical Cancer Research. 2006;12(23):6946–6951. doi: 10.1158/1078-0432.CCR-06-1214. [DOI] [PubMed] [Google Scholar]
- 4.Franceschi S., Muñoz N., Bosch X. F., Snijders P. J. F., Walboomers J. M. M. Human papillomavirus and cancers of the upper aerodigestive tract: a review of epidemiological and experimental evidence. Cancer Epidemiology Biomarkers and Prevention. 1996;5(7):567–575. [PubMed] [Google Scholar]
- 5.Munger K., Werness B. A., Dyson N., Phelps W. C., Harlow E., Howley P. M. Complex formation of human papillomavirus E7 proteins with the retinoblastoma tumor suppressor gene product. The EMBO Journal. 1989;8(13):4099–4105. doi: 10.1002/j.1460-2075.1989.tb08594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scheffner M., Werness B. A., Huibregtse J. M., Levine A. J., Howley P. M. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63(6):1129–1136. doi: 10.1016/0092-8674(90)90409-8. [DOI] [PubMed] [Google Scholar]
- 7.Münger K., Baldwin A., Edwards K. M., et al. Mechanisms of human papillomavirus-induced oncogenesis. Journal of Virology. 2004;78(21):11451–11460. doi: 10.1128/JVI.78.21.11451-11460.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ying X.-J., Zhao S.-W., Qiu J., Ye Q., Sun A.-H. Expression of human papillomavirus type 16 E7 and retinoblastoma proteins in human laryngeal squamous. Academic Journal of Second Military Medical University. 2004;25:1364–1367. [Google Scholar]
- 9.Holsinger F. C. Swing of the pendulum: optimizing functional outcomes in larynx cancer. Current Oncology Reports. 2008;10(2):170–175. doi: 10.1007/s11912-008-0026-7. [DOI] [PubMed] [Google Scholar]
- 10.Nakayama M., Laccourreye O., Holsinger F. C., Okamoto M., Hayakawa K. Functional organ preservation for laryngeal cancer: past, present and future. Japanese Journal of Clinical Oncology. 2012;42(3):155–160. doi: 10.1093/jjco/hyr190. [DOI] [PubMed] [Google Scholar]
- 11.Guzman J. R., Koo J. S., Goldsmith J. R., Muhlbauer M., Narula A., Jobin C. Oxymatrine prevents NF-κB nuclear translocation and ameliorates acute intestinal inflammation. Scientific Reports. 2013;3, article 1629 doi: 10.1038/srep01629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu L.-G., Zeng M.-D., Mao Y.-M., et al. Oxymatrine therapy for chronic hepatitis B: a randomized double-blind and placebo-controlled multi-center trial. World Journal of Gastroenterology. 2003;9(11):2480–2483. doi: 10.3748/wjg.v9.i11.2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fan H. G., Li L. T., Zhang X. J., et al. Oxymatrine Downregulates TLR4, TLR2, MyD88, and NF-κB and protects rat brains against focal ischemia. Mediators of Inflammation. 2009;2009:10. doi: 10.1155/2009/704706.704706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song M.-Q., Zhu J.-S., Chen J.-L., et al. Synergistic effect of oxymatrine and angiogenesis inhibitor NM-3 on modulating apoptosis in human gastric cancer cells. World Journal of Gastroenterology. 2007;13(12):1788–1793. doi: 10.3748/wjg.v13.i12.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin Z., Huang C.-F., Liu X.-S., Jiang J. In vitro anti-tumour activities of quinolizidine alkaloids derived from Sophora flavescens Ait . Basic & Clinical Pharmacology and Toxicology. 2011;108(5):304–309. doi: 10.1111/j.1742-7843.2010.00653.x. [DOI] [PubMed] [Google Scholar]
- 16.Dong Y. H., Xi H. L., Yu Y. Y., Wang Q. H., Jiang K., Li L. Z. Effects of oxymatrine on the serum levels of T helper cell 1 and 2 cytokines and the expression of the S gene in hepatitis B virus S gene transgenic mice: a study on the anti-hepatitis B virus mechanism of oxymatrine. Journal of Gastroenterology and Hepatology. 2002;17(12):1299–1306. doi: 10.1046/j.1440-1746.2002.02885.x. [DOI] [PubMed] [Google Scholar]
- 17.Chai N. L., Fu Q., Shi H., et al. Oxymatrine liposome attenuates hepatic fibrosis via targeting hepatic stellate cells. World Journal of Gastroenterology. 2012;18(31):4199–4206. doi: 10.3748/wjg.v18.i31.4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu H., Sun Y., Gao Y., Chen F., Xu M., Liu Z. The analgesic effect and mechanism of the combination of sodium ferulate and oxymatrine. Neurochemical Research. 2010;35(9):1368–1375. doi: 10.1007/s11064-010-0193-4. [DOI] [PubMed] [Google Scholar]
- 19.Ma S.-C., Du J., But P. P.-H., et al. Antiviral Chinese medicinal herbs against respiratory syncytial virus. Journal of Ethnopharmacology. 2002;79(2):205–211. doi: 10.1016/s0378-8741(01)00389-0. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y.-P., Zhao W., Xue R., et al. Oxymatrine inhibits hepatitis B infection with an advantage of overcoming drug-resistance. Antiviral Research. 2011;89(3):227–231. doi: 10.1016/j.antiviral.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 21.Zhu J.-S., Xu Z.-P., Song M.-Q., Zhang Q. Effect of oxymatrine combined with low dose 5-Fu on lymphatic vessel and microvascular endothelial cell growth of gastric cancer in a severe combined immunodeficient mouse orthotopic implantation model. European Journal of Inflammation. 2011;9(1):45–51. [Google Scholar]
- 22.Zhang Y., Piao B., Hua B., et al. Oxymatrine diminishes the side population and inhibits the expression of beta-catenin in MCF-7 breast cancer cells. Medical Oncology. 2011;28(1):S99–S107. doi: 10.1007/s12032-010-9721-y. [DOI] [PubMed] [Google Scholar]
- 23.Song G., Luo Q., Qin J., Wang L., Shi Y., Sun C. Effects of oxymatrine on proliferation and apoptosis in human hepatoma cells. Colloids and Surfaces B: Biointerfaces. 2006;48(1):1–5. doi: 10.1016/j.colsurfb.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 24.Ling Q., Xu X., Wei X. Y., et al. Oxymatrine induces human pancreatic cancer PANC-1 cells apoptosis via regulating expression of Bcl-2 and IAP families, and releasing of cytochrome C. Journal of Experimental & Clinical Cancer Research. 2011;30(1, article 66) doi: 10.1186/1756-9966-30-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Münger K., Basile J. R., Duensing S., et al. Biological activities and molecular targets of the human papillomavirus E7 oncoprotein. Oncogene. 2001;20(54):7888–7898. doi: 10.1038/sj/onc/1204860. [DOI] [PubMed] [Google Scholar]
- 26.Jones D. L., Münger K. Interactions of the human papillomavirus E7 protein with cell cycle regulators. Seminars in Cancer Biology. 1996;7(6):327–337. doi: 10.1006/scbi.1996.0042. [DOI] [PubMed] [Google Scholar]
- 27.Huang H. J., Zegarra-Moro O. L., Benson D., Tindall D. J. Androgens repress Bcl-2 expression via activation of the retinoblastoma (RB) protein in prostate cancer cells. Oncogene. 2004;23(12):2161–2176. doi: 10.1038/sj.onc.1207326. [DOI] [PubMed] [Google Scholar]
- 28.Zheng J., Deng Y.-P., Lin C., Fu M., Xiao P.-G., Wu M. Arsenic trioxide induces apoptosis of HPV16 DNA-immortalized human cervical epithelial cells and selectively inhibits viral gene expression. International Journal of Cancer. 1999;82(2):286–292. doi: 10.1002/(sici)1097-0215(19990719)82:2x003C;286::aid-ijc21x0003e;3.0.co;2-k. [DOI] [PubMed] [Google Scholar]