Abstract
Background/Aims
Retaining patients in prevention of mother-to-child transmission of HIV studies can be challenging in resource limited settings, where high lost to follow-up (LTFU) rates have been reported. In this paper, we describe the effectiveness of methods used to encourage retention in the Breastfeeding, Antiretrovirals, and Nutrition (BAN) study and analyze factors associated with LTFU in the study.
Methods
The BAN clinical trial was designed to evaluate the efficacy of 3 different mother-to-child HIV transmission prevention strategies. Lower than expected participant retention prompted enhanced efforts to reduce LTFU during the conduct of the trial. Following study completion, we employed regression modeling to determine predictors of perfect attendance and variables associated with being LTFU.
Results
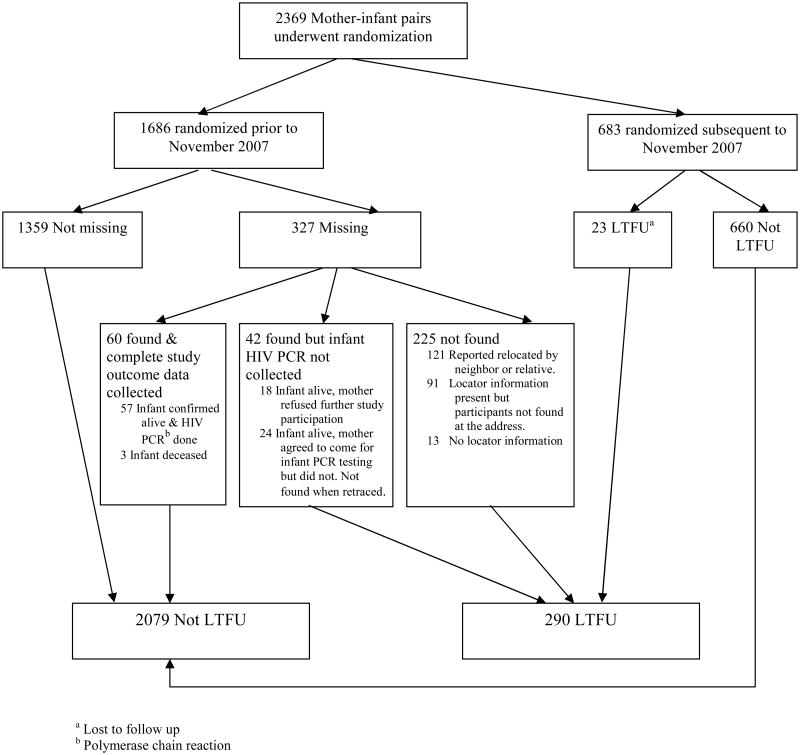
During the study, intensive tracing efforts were initiated after the first 1686 mother-infant pairs had been enrolled, and 327 pairs were missing. Sixty of these pairs were located and had complete data obtained. Among the 683 participants enrolling after initiation of intensive tracing efforts, the LTFU rate was 3.4%. At study's end, 290 (12.2%) of the 2369 mother-infant pairs were LTFU. Among successfully traced missing pairs, relocation was common and three were deceased. Log-binomial regression modeling revealed higher maternal hemoglobin and older maternal age to be significant predictors of perfect attendance. These factors and the presence of food insecurity were also significantly associated with lower rates of LTFU.
Conclusions
In this large HIV prevention trial, intensive tracing efforts centered on reaching study participants at their homes succeeded in finding a substantial proportion of LTFU participants, and were very effective in preventing further LTFU during the remainder of the trial. The association between food insecurity and lower rates of LTFU is likely related to the study's provision of nutritional support, including a family maize supplement, which may have contributed to patient retention.
Keywords: Antiretroviral therapy, human immunodeficiency virus, lost to follow up, prevention of mother to child transmission, retention, tracing
Background/Aims
Retaining patients in prevention of mother-to-child HIV transmission studies or antiretroviral treatment programs is a major challenge in resource-limited settings. Recent large prevention of mother-to-child transmission trials have experienced percentages of lost to follow-up (LTFU) ranging from 3.5% at 7 months in a study conducted in Botswana1 to 14.0% at 9 months in a trial conducted in Malawi.2 HIV treatment programs in similar settings have also reported substantial LTFU. For patients starting antiretroviral therapy between 2004 and 2007 at public sector clinics in Malawi, the reported LTFU percentage was 10.0% at 12 months.3 One large systematic review of retention in antiretroviral treatment programs in sub-Saharan Africa found a weighted average of 13.0% LTFU at 12 months for the 33 antiretroviral therapy programs evaluated.4
Factors that potentially influence LTFU in prevention of mother-to-child transmission trials and treatment programs in developing countries include stigma and lack of disclosure to family, medication side effects, transportation costs, health of participants, gender of the infant, education level, occupation, study complexity, and lack of telecommunications infrastructure.5-10 In some settings, a significant proportion of program attrition is due to unrecognized patient deaths11, which could also be a significant contributor to LTFU in prevention of mother-to-child transmission trials.
The sample size for clinical trials is determined by predictions of the comparative efficacy of study interventions and LTFU during the study period. The Breastfeeding, Antiretrovirals, and Nutrition (BAN) clinical trial was designed with a sample size of 2418 and an expected LTFU of 10% by 28 weeks.12 Higher LTFU would result in decreased statistical power to detect a significant difference between the study arms or require increased enrollment of participants with resultant increased cost and delay in determining the study outcome. In this paper, we describe the methods used to minimize LTFU in the BAN study, the effectiveness of these methods for study retention, and factors associated with LTFU in our study.
Methods
Description of the BAN study
The BAN study has been described in detail previously.12-15 Briefly, investigators recruited pregnant women who tested HIV positive through a prevention of mother-to-child transmission program from four antenatal clinics, with outreach to all pregnant women in Lilongwe, Malawi, from April 2004 to January 2009. Primary eligibility criteria included age of at least 14 years, hemoglobin > 7 g/dl, alanine aminotransferase < 2.5 times the upper limit of normal, no history of antiretroviral drug use, and a CD4 T cell count of 250/μL or more (before July 24, 2006, a CD4 T cell count of ≥200/μL was used; this change was in accordance with the Malawi Ministry of Health guidelines for HIV treatment). After delivery, women and their infants were eligible for randomization if they met the secondary eligibility criteria: presentation at the study site within 36 hours of delivery, infant birth weight ≥2000 g, no severe congenital malformations or conditions incompatible with infant survival, and no maternal condition that would preclude start of the study interventions (based on clinical assessment). Ineligible women were referred to an HIV treatment facility. Infants found to be HIV-infected at birth or in the first 2 weeks of life were dis-enrolled from the BAN study and referred for care. All women provided written informed consent. The BAN study protocol was approved by the Malawi National Health Science Research Committee and by institutional review boards at the University of North Carolina at Chapel Hill and the United States Centers for Disease Control and Prevention. The study was approved and overseen by a Vaccine and Prevention Data Safety and Monitoring Board at the National Institutes of Health.
Mother-infant pairs were randomized within one week of delivery in a 2 × 3 factorial design to a two-arm maternal nutritional intervention (nutritional supplement and no supplement) and a three-arm antiretroviral intervention for up to 28 weeks (maternal antiretrovirals, infant nevirapine, and control group). Irrespective of antiretroviral treatment assignment, all mothers in labor and their newborn infants were given single-dose nevirapine and zidovudine and lamivudine for 7 days. Additionally, all women received 2 kg/week maize flour for 48 weeks. The interventions were initiated immediately following delivery and were continued until the cessation of breast-feeding but no longer than 28 weeks postpartum. All mothers enrolled in the study were counselled to exclusively breastfeed for 24 weeks and then wean over a 4-week period.
Mother-infant pairs were followed at 1, 2, 4, 6, 8, 12, 18, 21, 24, 28, 32, 36, 42, and 48 weeks postpartum or until a study endpoint was reached. In this study three events were considered as endpoints: infant HIV infection, infant death and maternal death. Infants were tested in real-time for HIV infection at birth and at 2, 12, 28, and 48 weeks with the use of the Roche Amplicor 1.5 DNA polymerase chain reaction assay.
Retention strategies
The BAN study developed several strategies to maximize participant retention prior to beginning enrollment. In designing the study, strategies to foster community involvement and cultural acceptability included interviews, focus groups and home visits. These have been described at length elsewhere.16 During the study, routine strategies included support groups, home visits, transportation to and from study visits, frequent attempts to contact clients to reschedule missed visits, provision of treatment for intermittent infections, and involving husbands or partners by encouraging them to accompany the study participants to the clinic. Partners were offered HIV counseling and testing and referral to other health services. Study participants also received the equivalent of $4 in Malawi Kwacha at each study visit and the equivalent of $10 for the delivery visit to reimburse for transportation costs.
At enrollment, all the women were asked for locator information which was recorded on a locator form. They were asked for permission to have study staff call or visit them at their home or work place if necessary during the study. Telephone contact numbers were collected, though some participants did not have a telephone. Since there are no street addresses for most locations in Malawi, directions to participants' homes or work places were often mapped, with important landmarks, on the locator forms. Study staff asked permission to contact a spouse and another person; if granted, this contact information was recorded on the locator form. At each subsequent visit, the locator information was verified and updated if necessary by a reception nurse.
The BAN study community nurses conducted weekly support group meetings. All study participants were encouraged to attend these support group meetings as often as possible. Home visits were made to all women at least once to help provide support during breastfeeding. This gave an opportunity to verify the locator information. Extra home visits were made to women in need of additional counseling or support.
Tracing missing study participants
The BAN study included a team of six community nurses and drivers whose work focused on retention through tracing the study participants in the community by phone or vehicle. Tracing attempts were made by the community study staff within 48 hours for all women who missed a scheduled follow-up visit. Staff would determine the reason for the missed visit and try to resolve the issue so that the woman could come for her visit.
Study participants who were not found or did not report for more than two weeks after an initial tracing visit were considered missing. At a minimum, a second attempt to locate the mother-infant pair was made. If the pair was unable to be located, a termination form was completed to document the dates and outcome of tracing attempts, as well as any additional information about the pair's possible whereabouts or reasons for not reporting.
Efforts to locate missing study participants
Despite routine immediate tracing for missed visits, interim data analysis in November, 2007 after 1686 pairs had been enrolled revealed a higher-than-expected proportion of pairs without a study endpoint or a week-28 study visit. In 2008, we intensified our efforts to find pairs that had not been located by standard tracing efforts. Weekly staff meetings and additional strategy sessions were employed to increase focus on improving retention. We searched the study database for infants due to have reached their 28 week study visit but with missing HIV DNA results. For these cases, extensive tracing efforts focused on determining their vital and HIV status.
The BAN Study developed several innovative efforts to find lost participants. In 2008, the study broadcast a radio announcement in Chichewa, the local language, on two radio stations three times per day for six days over a two-week period. It identified the study without mentioning HIV and asked participants to return to the clinic if they had not completed the study. In June 2008, we hired a community educator to trace missing participants via motorcycle. A motorcycle was chosen due to input from the tracing staff that some villages were difficult to reach by larger vehicle due to poor roads, particularly during the rainy season. Logos that would identify it as belonging to an HIV study were avoided in order to minimize possible stigma. Upon arrival at a home the community educator noted if the pair was found, and if the mother and infant were alive. If either of them had died, the tracer attempted to ascertain the cause and date of death. If the pair were found alive, the community educator would request that they come to the study clinic for HIV DNA testing on the child. Additionally, we collected a specimen for maternal CD4 count to determine antiretroviral eligibility and promote referral to an antiretroviral clinic for continued care. Pairs that were found but still did not come back to the clinic were visited again and offered home collection of blood for infant HIV DNA testing and maternal CD4 count. The intensive tracing and retention policies initiated in 2008 were continued for the remainder of the study, so that all pairs who missed visits after that time were intensively traced and, if they refused further participation in the study, were offered in-home blood collection for infant HIV DNA testing. From this tracing, we learned that some missing participants had relocated to areas outside of Lilongwe. Therefore, we expanded our tracing radius to include all districts in Malawi. When a specific location for the participant's residence could be obtained, community educators organized trips throughout the country to trace missing study participants.
Statistical analysis methods
To examine predictors of missed visits, we defined the outcome as missing at least one scheduled study visit between zero and 28 weeks post-partum, choosing 28 weeks because this represented the time point for the primary outcomes of the BAN study. Pairs where an infant HIV infection, infant death, or maternal death endpoint was observed by 28 weeks were considered to have perfect attendance if no scheduled visits were missed prior to the endpoint. Pairs where the infant was not infected and neither the mother nor infant died by 28 weeks were considered to have perfect attendance if they attended all 11 scheduled study visits between 0 (birth) and 28 weeks. Log-binomial regression models were fit to assess associations between participant covariates and risk of missing at least one scheduled visit. As a sensitivity analysis, log-binomial regression models were also fit modeling the probability of missing at least two visits.
Cox regression models were employed to determine variables associated with the hazard of being LTFU. The Cox model has the advantage over the log-binomial model of being able to incorporate time-varying covariates, such as the occurrence of an adverse event. A mother-infant pair was defined as LTFU if their last study visit was prior to 28 weeks and at that visit the mother and infant were alive and the infant was HIV negative. For the Cox model, mother-infant pairs where the infant became HIV infected (prior to 28 weeks) were right censored at their first positive visit; mother-infant pairs where the mother or HIV-negative infant died (prior to 28 weeks) were right censored at the time of death. Mother-infant pairs not categorized as LTFU prior to week 28 were right censored at 29 weeks.
Covariates for possible inclusion in the log-binomial and Cox regression models were initially considered if believed a priori to be potentially associated with missed visits based on the existing literature or BAN study operational experience. Covariates marginally associated with the outcome at the 0.10 significance level were retained in the multivariate regression models. Collinearity between covariates was assessed using Pearson's correlation coefficient. As a sensitivity analysis, models were fit dropping each potentially collinear covariate, and results that differed from the final adjusted model were reported.
Results
Recruitment
The BAN study started enrolling participants in April 2004 and completed follow-up on the last participant in February 2010. By the end of the study, a total of 3572 women consented to study screening, 3109 met primary eligibility criteria, and 2791 delivered live-born infants. A total of 2382 mother-infant pairs met secondary eligibility criteria following delivery, and 2369 agreed to enroll in the study and were randomized.
Yield of intensive tracing efforts
Prior to implementation of enhanced intensive tracing efforts in November 2007, there were 327 mother-infant pairs classified as missing, based on the absence of a 28-week infant HIV DNA result in those for whom study endpoints of death or infant HIV infection had not occurred, out of 1686 enrolled (19.4%). Of these 327 mother-infant pairs, 102 (31.2%) were found. For 60 mother-infant pairs (18.3% of the 327 missing), study endpoint data were obtained, consisting of 57 infants for whom HIV DNA tests were performed and 3 infants who were confirmed to have died. The remaining 42 pairs were comprised of infants that were alive but whose mothers either withdrew from the study by refusing further participation (n=18) or failed to bring the infant for final HIV DNA testing (n=24) (Figure 1). There were 225 pairs that were not located. Of these, 13 had no locator information and 121 were reported to have relocated. There were several reports of women having moved to other parts of the country for employment, to help with the harvest, or to return to their home village after the death of a husband. Additionally, eight infants and two mothers were reported to have died after 28 weeks.
Figure 1.
Enrollment and outcomes in the BAN Study, including results of intensive tracing efforts in 2007 and 2008 on 327 missing mother-infant pairs.
At the conclusion of intensive tracing efforts, the 225 pairs that were not located and the 42 who were found but for whom study endpoint data could not be collected were classified as LTFU, a total of 267 pairs (15.8%). Over the remaining study period after the initiation of intensive tracing efforts in November 2007, an additional 23 out of 683 (3.4%) became LTFU, resulting in 290 LTFU LTFU pairs out of 2369 pairs who received treatment assignment (12.2%) (Figure 1).
Study attendance
Mother-infant pairs that were LTFU did not differ significantly from those not LTFU by the antiretroviral or nutritional arm to which they had been randomized (Table 1). Also, the mothers who were LTFU did not differ significantly from those not LTFU on some baseline demographic and laboratory characteristics, including whether living with partner, whether widowed, and baseline CD4 count. However, there were statistically significant differences in maternal age (p<0.001) and maternal body mass index at delivery (p=0.03), and whether this was the mother's first pregnancy (p<0.001). Of the 2369 mother-infant pairs randomized, 1688 (71%) had perfect attendance and an additional 218 (9.2%) missed only one visit prior to completing the study at 28 weeks postpartum or reaching a study endpoint. Among the 184 infants HIV-positive by week 28, 92% had perfect attendance prior to their HIV test. Among the 29 infants who died, 93% had perfect attendance prior to death.
Table 1.
Baseline characteristics of the mothers in the BAN trial by LTFU status at end of studya.
| LTFU (n=290) | Not LTFU (n=2079) | p-valueb | |
|---|---|---|---|
| Maternal age, median (interquartile range) | 24 (21,27) | 26 (23, 30) | <.001 |
|
| |||
| Living with partner | 266 (91.7%) | 1936 (93.1%) | 0.32 |
|
| |||
| Widowed | 0 (0.0%) | 24 (1.2%) | 0.96 |
|
| |||
| Ever married | 281 (96.9%) | 2037 (98.0%) | 0.21 |
|
| |||
| Walks to clinic | 4 (2.6%) | 44 (4.7%) | 0.31 |
|
| |||
| ARVc arm | |||
| Mother ARV | 103 (35.5%) | 749 (36.0%) | 0.58 |
| Infant NVP | 103 (35.5%) | 746 (35.9%) | |
| No ARV | 84 (29.0%) | 584 (28.1%) | |
|
| |||
| Maternal CD4 count >350 at baseline | 200 (69.0%) | 1462 (70.3%) | 0.38 |
|
| |||
| Median CD4 (interquartile range) | 448 (318, 612) | 437 (330, 573) | 0.44 |
|
| |||
| First pregnancy | 60 (21.3%) | 224 (11.2%) | <.001 |
|
| |||
| Maternal body mass index at delivery (median) | 22.8 | 23.2 | 0.03 |
|
| |||
| Infant adverse event at birth | 3 (1.0%) | 21 (1.0%) | 0.81 |
|
| |||
| Maternal adverse event at delivery | 2 (0.7%) | 10 (0.5%) | 0.54 |
Number of subjects with missing data by variable: age (n=7), walks to clinic (n=1276), first pregnancy (n=83), and maternal body mass index at delivery (n=243).
Based on Cox proportional hazards models with each characteristic as the sole covariate in the model
Antiretroviral
Predictors of missed visits
In the adjusted log-binomial regression model, maternal hemoglobin and maternal age were significantly associated with missing one or more visits (Table 2). For each one unit increase in maternal hemoglobin (g/dL), the risk of missing one or more visits was reduced by 6% (RR=0.94, 95% CI 0.89, 0.98, p=0.009). Additionally, there was a 5% decrease in the risk of missing more than one visit for each year increase in maternal age (RR=0.95, 95% CI 0.94, 0.96, p<0.001). There was a significant negative association (i.e., collinearity) between maternal age and first pregnancy (p<0.001). When either age or first pregnancy was excluded from the adjusted model, results were nearly identical except that when maternal age was excluded, the RR estimate for first pregnancy was 1.54 (95% 1.32, 1.80; p<0.001). Log-binomial regression models of predictors of two or more missed visits yielded similar results (data not shown).
Table 2.
Log-binomial regression modeling of significant predictors for one or more missed visits versus no missed visits by 28 weeks adjusted for all variables in the table. a, b
| Predictorsa | Missed one or more visits | Risk Ratio (95% CI) | P-value | |
|---|---|---|---|---|
| Maternal hemoglobin at baseline (per increase of 1 g/dl) | Observed (2274) | 660 (29.0%) | 0.94 (0.89, 0.98) | 0.009 |
|
| ||||
| Maternal age at baseline (per increase of 1 year) | Observed (2274) | 660 (29.0%) | 0.95 (0.94, 0.96) | <.001 |
|
| ||||
| First pregnancy | No (1990, 87.5 %) | 541 (27.2%) | 1.0 (referent) | 0.16 |
| Yes (284, 12.5%) | 119 (41.9%) | 1.13 (0.95, 1.35) | ||
This table only includes covariates that were significant at the 0.10 level when included as the sole covariate in the model. The following covariates were not significant at the 0.10 level in univariate (i.e., unadjusted) models: living with partner, widowed, literate, education above primary school, ever married, walks to clinic, infant birth weight, infant adverse event at birth, maternal adverse event at delivery, maternal CD4 count at baseline ≥350 cells/ul, and treatment arm.
N = 2274 mother-infant pairs were included. 95 observations were excluded due to missing values of maternal hemoglobin (n=5), maternal age (n=7), or first pregnancy (n=83).
Predictors of being LTFU
In unadjusted Cox regression models of the rate of LTFU, factors significantly associated with increased hazard of LTFU were lower infant weight-for-age, lower maternal hemoglobin, younger maternal age, lower maternal body mass index at delivery, food security, and this not being a mother's first pregnancy (Table 3). In the adjusted Cox model, infant weight-for-age, maternal body mass index at delivery, and first pregnancy were no longer significantly associated with LTFU. Maternal hemoglobin was significantly associated with LTFU in the adjusted model, with a 17% (95% CI 6%, 26%) lower rate of LTFU per one unit increase in hemoglobin (grams/deciliter) (p=0.002). Maternal age was significantly associated with a 6% (95% CI 3%, 10%) lower rate of LTFU per one year increase in age. Additionally, the presence of food insecurity, defined as any self-reported food shortage within the past 4 weeks, was significantly associated with a 42% (95% CI 10%, 63%) lower rate of LTFU (Table 3). Sensitivity analyses excluding potentially collinear covariates yielded nearly identical results, except that when maternal age was excluded the hazard ratio estimate for first pregnancy was 1.67 (95% CI 1.14, 2.44; p=0.01).
Table 3. Cox regression modeling of possible risk factors associated with rate of lost to follow-up from 0 to 28 weeksa.
| Risk factor | Unadjustedb | Adjustedc | ||
|---|---|---|---|---|
|
| ||||
| Hazard Ratio (95% CI) | P-value | Hazard Ratio (95% CI) | P-value | |
| Infant weight for age Z-score (per increase of 1 unit)d | 0.87 (0.76, 1.00) | 0.05e | 0.95 (0.80, 1.11) | 0.50 |
|
| ||||
| Maternal hemoglobin at baseline (per increase of 1 g/dl) | 0.86 (0.78, 0.94) | 0.002 | 0.83 (0.74, 0.94) | 0.002 |
|
| ||||
| Maternal age at baseline (per increase of 1 year) | 0.92 (0.89, 0.94) | <.001 | 0.94 (0.90, 0.97) | <.001 |
|
| ||||
| Maternal body mass index at delivery (per increase of 1 unit) | 0.95 (0.90, 0.99) | 0.03 | 0.96 (0.91, 1.02) | 0.17 |
|
| ||||
| First pregnancy | ||||
| Yes | 2.03 (1.51, 2.72) | <.001 | 1.15 (0.75, 1.76) | 0.52 |
| No | 1.0 (referent) | 1.0 (referent) | ||
|
| ||||
| Any food insecurity (yes vs no) d, f | ||||
| Yes | 0.67 (0.48, 0.93) | 0.02 | 0.58 (0.37, 0.90) | 0.02 |
| No | 1.0 (referent) | 1.0 (referent) | ||
This table only includes covariates that were significant at the 0.10 level in unadjusted Cox models. The following covariates were not significant at the 0.10 level: living with partner, widowed, literate, education above primary school, ever married, walks to clinic, infant birth weight, infant adverse event at birth, infant adverse event between birth and 28 weeks, maternal adverse event at delivery, maternal CD4 count at baseline ≥350 cells/ul, infant hemoglobin at delivery, and treatment arm.
Cox model with each risk factor as the sole covariate.
Cox model adjusted for all covariates in the table. N = 2033 mother infant pairs contributed to the adjusted model. 331 mother-infant pairs were excluded due to missing covariate values (n=243 missing maternal body mass index, n=83 missing first pregnancy, n=7 missing maternal age, n=5 missing maternal hemoglobin, and n=1 missing weight for age z-score, with some pairs missing more than one covariate) and 5 additional infants were HIV positive (right censored) at birth
Time-dependent covariates, with last value carried forward when missing
P-value=0.046
Any food shortage (self-report) in the last four weeks
Discussion
Intensive tracing efforts in the BAN study reduced the percent missing from 19.4% to 12.2%. Despite the decrease, this was still higher than the expected LTFU of 10% used when designing the study and determining the target sample size. Of the 327 mother-infant pairs missing at the start of intensive tracing, primary study endpoint data were able to be collected on a total of 60, representing 58.8% of those who were found and 18.3% of the total missing. The intensive tracing strategies were continued for the remainder of the study and their impact was reflected in a LTFU rate of only 3.4% in the 683 mother-infant pairs who began the study after November 2007. This striking improvement reveals the improved effectiveness of intensive tracing when conducted in real time as opposed to retroactively, by which time missing study participants could have relocated. The emphasis on retention to study staff may also have resulted in better location information and better patient counseling regarding study attendance.
In addition to reducing LTFU, tracing efforts yielded information about reasons for study attrition. Of the 327 missing pairs at the start of intensive tracing, 225 (69%) were unable to be located, highlighting the need for better tracing methods. The 121 of these reported to have relocated likely represent an underestimate of population mobility, since some of those for which no information was found may also have relocated. Previous studies in sub-Saharan Africa have implicated both mobile populations17 and transportation costs18 in loss to follow-up. In addition to the reasons suggested above, the frequent lack of street addresses in Lilongwe likely contributed. Use of global positioning system technology to more precisely identify the location of participants' homes has been reported with success in HIV prevention trials19 and has subsequently been used for a malaria vaccine study in Lilongwe, Malawi.20 Our data and analysis are insufficient to determine whether there would have been a benefit in the BAN study.
Unrecognized infant and maternal mortality may have contributed to LTFU in our study. Among successfully traced participants, we discovered three infant deaths at prior to 28 weeks and ten mother or infant deaths after 28 weeks, but there may have been additional deaths among LTFU participants who were not found. In other studies in Africa, 30-46% of successfully traced participants were found to have died.10,21,22 The high mortality seen in these cases highlights the urgency of retaining and tracing participants in studies both for accurate evaluation of study outcomes and to ensure appropriate and possibly life-saving medical care is provided.
Some of our participants did not provide a phone number. Telephone ownership has increased dramatically across Africa in recent years with the increasing affordability of mobile phones.23 Cell phone costs in Malawi fell from approximately $100 in 2004 to $20 in 2008. Providing cell phones for mothers might have been a cost-effective intervention compared to the amount spent on transportation costs and salaries for our community educators. The presence of a contact phone number was found in an antiretroviral study to double the success rate of tracing lost patients10 and found to be an acceptable and useful tool in the health sector in Africa.23,24
Our analyses of patient-specific factors associated with LTFU revealed several interesting associations. Regression modeling revealed younger mothers were more likely to become LTFU. This may reflect older women being at a more stable point in their lives in terms of day-to-day activities, ability to keep their appointments, or likelihood of remaining in one area.
Interestingly, lower baseline maternal hemoglobin was associated with missed visits and LTFU while other health and nutrition-related covariates such as maternal CD4, maternal body mass index and maternal adverse events were not. We postulate that the association with hemoglobin is due to aspects of health not captured in these other variables. Maternal body mass index was measured immediately after delivery and likely has a much less predictable relationship to health and nutrition than body mass index in non-pregnant individuals. Exclusion of mothers with low (<250) CD4 counts from the BAN study could have obscured an association of maternal CD4 with retention. Anemia could result in missed visits due to poor health that would not qualify as specific adverse events, such as via decreased energy making the trip to clinic more onerous. It could also predispose to more severe illnesses which could prompt unannounced relocation and consequent study dropout, or even lead to undiscovered deaths.
A history of food insecurity was associated with being retained in the study. Women experiencing food insecurity may have been more motivated to attend visits by the study's provision of a maternal nutritional supplement to half of the women, a family maize supplement to all participants at each visit, and a weaning supplement for all the infants. In some instances, food insecurity has been shown to be associated with non-adherence to antiretroviral therapy.25,26 However, studies have also suggested an association between the provision of food supplementation and retention in HIV programs.27,28 Our results are consistent with the idea that nutritional support can be a positive factor for adherence in settings with food shortages.
In the BAN Study, 71% of mother-infant pairs achieved perfect attendance, an impressively high percentage in light of the 11 required visits for post-partum mothers and newborn infants, as well as the limited resources and widespread poverty of the study setting. However, the 12.2% LTFU at 28 weeks in our study was higher than anticipated, but in keeping with that seen in other large prevention of mother-to-child transmission studies in sub-Saharan Africa. Our study's intensive community-based efforts and statistical analysis of predictors of LTFU may offer some insight into ways to minimize losses to follow-up in future studies. The dramatic reduction of LTFU rate to 3.4% for pairs enrolled after the implementation of intensive tracing efforts also suggests a large benefit to a proactive rather than reactive approach to retention. The BAN study experience highlights the benefits of determined, community-based tracing efforts and also the difficulties presented by lack of fixed contact information and minimal telephone contacts. This suggests a role for increased use of mobile phones to improve study retention and enhance patient care. Large clinical trials, such as the BAN study, may prove to be useful test cases for the feasibility of innovative strategies such as this.
Acknowledgments
We are grateful to the following: BAN Study Team at University of North Carolina Chapel Hill, Centers for Disease Control and Prevention, Atlanta, and UNC Project team in Lilongwe including: Linda Adair, Yusuf Ahmed, Mounir Ait-Khaled, Sandra Albrecht, Shrikant Bangdiwala, Ronald Bayer, Margaret Bentley, Brian Bramson, Emily Bobrow, Nicola Boyle, Sal Butera, Charles Chasela, Charity Chavula, Joseph Chimerang'ambe, Maggie Chigwenembe, Maria Chikasema, Norah Chikhungu, David Chilongozi, Grace Chiudzu, Lenesi Chome, Anne Cole, Amanda Corbett, Amy Corneli, Anna Dow, Ann Duerr, Henry Eliya, Sascha Ellington, Joseph Eron, Sherry Farr, Yvonne Owens Ferguson, Susan Fiscus, Valerie Flax, Ali Fokar, Shannon Galvin, Laura Guay, Chad Heilig, Irving Hoffman, Elizabeth Hooten, Mina Hosseinipour, Michael Hudgens, Stacy Hurst, Lisa Hyde, Denise Jamieson, George Joaki (deceased), David Jones, Elizabeth Jordan-Bell, Zebrone Kacheche, Esmie Kamanga, Gift Kamanga, Coxcilly Kampani, Portia Kamthunzi, Deborah Kamwendo, Cecilia Kanyama, Angela Kashuba, Damson Kathyola, Dumbani Kayira, Peter Kazembe, Caroline C. King, Rodney Knight, Athena P. Kourtis, Robert Krysiak, Jacob Kumwenda, Hana Lee, Edde Loeliger, Dustin Long, Misheck Luhanga, Victor Madhlopa, Maganizo Majawa, Alice Maida, Cheryl Marcus, Francis Martinson, Navdeep Thoofer, Chrissie Matiki (deceased), Douglas Mayers, Isabel Mayuni, Marita McDonough, Joyce Meme, Ceppie Merry, Khama Mita, Chimwemwe Mkomawanthu, Gertrude Mndala, Ibrahim Mndala, Agnes Moses, Albans Msika, Wezi Msungama, Beatrice Mtimuni, Jane Muita, Noel Mumba, Bonface Musis, Charles Mwansambo, Gerald Mwapasa, Jacqueline Nkhoma, Megan Parker, Richard Pendame, Ellen Piwoz, Byron Raines, Zane Ramdas, John Rublein, Mairin Ryan, Ian Sanne, Christopher Sellers, Diane Shugars, Dorothy Sichali, Wendy Snowden, Alice Soko, Allison Spensley, Jean-Marc Steens, Gerald Tegha, Martin Tembo, Roshan Thomas, Hsiao-Chuan Tien, Beth Tohill, Charles van der Horst, Esther Waalberg, Elizabeth Widen, Jeffrey Wiener, Cathy Wilfert, Patricia Wiyo, Innocent Zgambo, Chifundo Zimba. Finally and most especially, all the women and infants that have agreed to participate in the study.
Funding: The Breastfeeding, Antiretrovirals, and Nutrition Study was supported by the Prevention Research Centers Special Interest Project of the Centers for Disease Control and Prevention [SIP 13-01 U48-CCU409660-09, SIP 26-04 U48-DP000059-01, and SIP 22-09 U48-DP001944-01]; the National Institute of Allergy and Infectious Diseases; the University of North Carolina Center for AIDS Research [P30-AI50410]; the National Institutes of Health Fogarty AIDS International Training and Research Program [DHHS/NIH/FIC 2-D43 Tw01039-06]; the Fogarty International Clinical Research Scholars Program [R24 Tw00798]; the American Recovery and Reinvestment Act; Ad Astra Fellowship from University College Dublin, Ireland; the Bill and Melinda Gates Foundation [Grant number OPP53107]; a grant from the Gilead Foundation in support of our trainees; the National Institutes of Health / National Institute of Allergy and Infectious Diseases Training in Infectious Disease Epidemiology Grant [Grant number NIH 5T32AI070114-08]. The antiretrovirals used in the BAN study were donated by Abbott Laboratories, GlaxoSmithKline, Boehringer Ingelheim, Roche Pharmaceuticals, and Bristol-Myers Squibb. The Call to Action PMTCT program was supported by the Elizabeth Glaser Pediatric AIDS Foundation, the United Nations Children's Fund, the World Food Program, the Malawi Ministry of Health and Population, Johnson & Johnson, and the U.S. Agency for International Development.
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Conflict of Interest Statement: The authors declare that there is no conflict of interest.
References
- 1.Thior I, Lockman S, Smeaton LM, et al. Breastfeeding plus infant zidovudine prophylaxis for 6 months vs formula feeding plus infant zidovudine for 1 month to reduce mother-to-child HIV transmission in Botswana: a randomized trial: the Mashi Study. JAMA. 2006;296:794–805. doi: 10.1001/jama.296.7.794. [DOI] [PubMed] [Google Scholar]
- 2.Kumwenda NI, Hoover DR, Mofenson LM, et al. Extended antiretroviral prophylaxis to reduce breast-milk HIV-1 transmission. N Engl J Med. 2008;359:119–129. doi: 10.1056/NEJMoa0801941. [DOI] [PubMed] [Google Scholar]
- 3.Weigel R, Estill J, Egger M, et al. Mortality and loss to follow-up in the first year of ART: Malawi national ART programme. AIDS. 2012;26:365–373. doi: 10.1097/QAD.0b013e32834ed814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fox MP, Rosen S. Patient retention in antiretroviral therapy programs up to three years on treatment in sub-Saharan Africa, 2007-2009: systematic review. Trop Med Int Health. 2010;15(Suppl 1):1–15. doi: 10.1111/j.1365-3156.2010.02508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andersen JW, Fass R, van der Horst C. Factors associated with early study discontinuation in AACTG studies, DACS 200. Contemp Clin Trials. 2007;28:583–592. doi: 10.1016/j.cct.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 6.Bwirire L, Fitzgerald M, Zachariah R, et al. Reasons for loss to follow-up among mothers registered in a prevention-of-mother-to-child transmission program in rural Malawi. Trans R Soc Trop Med Hyg. 2008;102:1195–1200. doi: 10.1016/j.trstmh.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 7.Feldblum PJ, Halpern V, Lie CC, et al. What predicts non-retention in microbicide trials? Contempy Clin Trials. 2011;32:512–516. doi: 10.1016/j.cct.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 8.Gust DA, Mosimaneotsile B, Mathebula U, et al. Risk factors for non-adherence and loss to follow-up in a three-year clinical trial in Botswana. PloS One. 2011;6:e18435. doi: 10.1371/journal.pone.0018435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ioannidis JP, Taha TE, Kumwenda N, et al. Predictors and impact of losses to follow-up in an HIV-1 perinatal transmission cohort in Malawi. Int J Epidemiol. 1999;28:769–775. doi: 10.1093/ije/28.4.769. [DOI] [PubMed] [Google Scholar]
- 10.Weigel R, Hochgesang M, Brinkhof MW, et al. Outcomes and associated risk factors of patients traced after being lost to follow-up from antiretroviral treatment in Lilongwe, Malawi. BMC Infect Dis. 2011;11:31. doi: 10.1186/1471-2334-11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu JK, Chen SC, Wang KY, et al. True outcomes for patients on antiretroviral therapy who are “lost to follow-up” in Malawi. Bull World Health Organ. 2007;85:550–554. doi: 10.2471/BLT.06.037739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Horst C, Chasela C, Ahmed Y, et al. Modifications of a large HIV prevention clinical trial to fit changing realities: a case study of the Breastfeeding, Antiretroviral, and Nutrition (BAN) protocol in Lilongwe, Malawi. Contemp Clin Trials. 2009;30:24–33. doi: 10.1016/j.cct.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chasela CS, Hudgens MG, Jamieson DJ, et al. Maternal or infant antiretroviral drugs to reduce HIV-1 transmission. New Engl J Med. 2010;362:2271–2281. doi: 10.1056/NEJMoa0911486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jamieson DJ, Chasela CS, Hudgens MG, et al. Maternal and infant antiretroviral regimens to prevent postnatal HIV-1 transmission: 48-week follow-up of the BAN randomised controlled trial. Lancet. 2012;379:2449–2458. doi: 10.1016/S0140-6736(12)60321-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kayira D, Bentley ME, Wiener J, et al. A lipid-based nutrient supplement mitigates weight loss among HIV-infected women in a factorial randomized trial to prevent mother-to-child transmission during exclusive breastfeeding. Am J Clin Nutr. 2012;95:759–765. doi: 10.3945/ajcn.111.018812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corneli AL, Piwoz EG, Bentley ME, et al. Involving communities in the design of clinical trial protocols: the BAN Study in Lilongwe, Malawi. Contemp Clin Trials. 2007;28:59–67. doi: 10.1016/j.cct.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferguson L, Lewis J, Grant AD, et al. Patient attrition between diagnosis with HIV in pregnancy-related services and long-term HIV care and treatment services in Kenya: a retrospective study. J Acquir Immune Defic Syndr. 2012;60:e90–e97. doi: 10.1097/QAI.0b013e318253258a. [DOI] [PubMed] [Google Scholar]
- 18.Merten S, Kenter E, McKenzie O, et al. Patient-reported barriers and drivers of adherence to antiretrovirals in sub-Saharan Africa: a meta-ethnography. Trop Med Int Health. 2010;15:16–33. doi: 10.1111/j.1365-3156.2010.02510.x. [DOI] [PubMed] [Google Scholar]
- 19.Gappoo S, Montgomery ET, Gerdts C, et al. Novel strategies implemented to ensure high participant retention rates in a community based HIV prevention effectiveness trial in South Africa and Zimbabwe. Contemp Clin Trials. 2009;30:411–418. doi: 10.1016/j.cct.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 20.Giebultowicz S, Taylor C, Emch M, et al. Malawi-Carolina Summer Institute Poster Session. Chapel Hill, NC: 2009. Implementing a Malaria Vaccine in Malawi Using Spatial Methods. [Google Scholar]
- 21.Brinkhof MW, Pujades-Rodriguez M, Egger M. Mortality of patients lost to follow-up in antiretroviral treatment programmes in resource-limited settings: systematic review and meta-analysis. PloS One. 2009;4:e5790. doi: 10.1371/journal.pone.0005790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tweya H, Gareta D, Chagwera F, et al. Early active follow-up of patients on antiretroviral therapy (ART) who are lost to follow-up: the ‘Back-to-Care’ project in Lilongwe, Malawi. Trop Med Int Health. 2010;15(Suppl 1):82–89. doi: 10.1111/j.1365-3156.2010.02509.x. [DOI] [PubMed] [Google Scholar]
- 23.Siedner MJ, Haberer JE, Bwana MB, et al. High acceptability for cell phone text messages to improve communication of laboratory results with HIV-infected patients in rural Uganda: a crosssectional survey study. BMC Med Inform Decis Mak. 2012;12:56. doi: 10.1186/1472-6947-12-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mahmud N, Rodriguez J, Nesbit J. A text message-based intervention to bridge the healthcare communication gap in the rural developing world. Technol Health Care. 2010;18:137–144. doi: 10.3233/THC-2010-0576. [DOI] [PubMed] [Google Scholar]
- 25.Berhe N, Tegabu D, Alemayehu M. Effect of nutritional factors on adherence to antiretroviral therapy among HIV-infected adults: a case control study in Northern Ethiopia. BMC Infect Dis. 2013;13:233. doi: 10.1186/1471-2334-13-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Young S, Wheeler AC, McCoy SI, et al. A review of the role of food insecurity in adherence to care and treatment among adult and pediatric populations living with HIV and AIDS. AIDS Behav. 2014;18(Suppl 5):S505–S515. doi: 10.1007/s10461-013-0547-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Braitstein P, Katshcke A, Shen C, et al. Retention of HIV-infected and HIV-exposed children in a comprehensive HIV clinical care programme in Western Kenya. Trop Med Int Health. 2010;15:833–841. doi: 10.1111/j.1365-3156.2010.02539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rich ML, Miller AC, Niyigena P, et al. Excellent clinical outcomes and high retention in care among adults in a community-based HIV treatment program in rural Rwanda. J Acquir Immune Defic Syndr. 2012;59:e35–e42. doi: 10.1097/QAI.0b013e31824476c4. [DOI] [PubMed] [Google Scholar]