Abstract
Background
The World Health Organization (WHO) targeted China for measles elimination by 2012. While China made significant progress, transmission continues, warranting examination of China's measles vaccination program. The WHO recommends that children receive at least two doses of a measles containing vaccine (MCV) to ensure protection. In Tianjin, China, MCV is given in three doses; 8 months (MV), 18-24 months (MMR-1), and 5 years (MMR-2). MMR-2 is important because of the young age for MV administration. This study describes MCV coverage, assesses administration timeliness, and evaluates completion of the MCV series for children living in Tianjin, China.
Methods
In July 2012, immunization records were selected from Tianjin's Immunization Information Management System. Records were abstracted for children born from 2004 to 2011, who were age 8 months or older. Descriptive statistics characterized the study population and assessed timeliness and coverage for each MCV dose.
Results
We examined records of 205,982 children living in Tianjin, China. Among children who were age-appropriate for each vaccine, 98.6% received MV, 97.6% received MMR-1, and 76.9% received MMR-2. Of the children who were old enough to receive MMR-2, 78.8% received the complete series, and 71.6% of children were fully immunized for measles by age 6.
Conclusions
Tianjin has high rates of MV and MMR-1 coverage, with lower levels for MMR-2. Most children who completed the series did so on-time. Maintaining high coverage and timely administration of MV and MMR-1 and increasing coverage of MMR-2 are necessary for China to attain the goal of national measles elimination.
Keywords: Measles, China, Vaccine Coverage
INTRODUCTION
Measles is one of the leading causes of mortality among children worldwide, resulting in approximately 158,000 deaths annually. (1) Significant progress toward disease elimination and eradication has been made globally; between 2000 and 2011, the number of new measles cases worldwide decreased from 853,500 to 355,000 and related deaths decreased from 542,000 to 158,000. (2) The World Health Organization (WHO) estimated that 20 million children did not receive the first dose of a measles-containing vaccine (MCV) in 2011. (2) Moreover, several large measles outbreaks have been reported in Africa, Asia, and Europe in the last few years, (2) indicating a need for enhanced vaccination vigilance, even in low incidence areas. While measles eradication is believed to be achievable, sustained immunization delivery programs and adequate surveillance will be needed. (3)
Effectively interrupting measles transmission requires high levels of herd immunity with at least 95% of the population non-susceptible to disease. (1, 4, 5) Infants are generally protected against measles for the first several months of life by passively acquired maternal antibodies. (6, 7) Immunity from maternal antibodies wanes at 6 to 9 months of age, when infants then require a vaccine for protection. (8, 9) Approximately 85% of 8 to 9 month old infants seroconvert after receiving an initial measles vaccine; any dose given before this age is unlikely to produce optimal immunity because of interference from maternal antibodies. (6, 8, 10) Almost all children develop protective immunity after receiving a second dose of the measles vaccine (either single antigen or in combination with other antigens such as mumps and rubella), and as such the WHO recommends all children receive at least two doses of vaccine to ensure adequate individual immunity and prevention of transmission in the population. (1, 6)
In most low-incident, western nations the first dose of an MCV is not administered until 12 months of age. It is felt that interference with the measles immune response from circulating maternal antibodies is significant enough to outweigh any potential benefit of administering the vaccine to infants in the first year of life. (19) However, China gives the first dose early based on the significant burden of disease in infants in an attempt to confer protection at a younger age at the likely cost of generating a weaker immunogenic response. As recently as 2008, Tianjin had more than 500 cases per 100,000 persons age <1 year. (Unpublished data, Tianjin Centers for Disease Control and Prevention, February 8, 2013). As a result, administration of the initial measles dose prior to 12 months with its attenuated immunogenicity makes receipt of the third dose (MMR-2) both important and necessary to insure a fully protected child. There is ample data characterizing coverage with the first dose, which is often the focus of supplemental immunization activities (SIAs) and the assessment of which receives the most attention from the WHO. However, little is known about coverage with the second and third MCV doses, despite their key role in measles control and elimination efforts. This study examines coverage of three MCV doses (MV, MMR-1, and MMR-2), and assesses predictors for completion of the three-dose MCV schedule among children in Tianjin, China.
METHODS
The WHO slated measles for elimination from China by 2012, and the average measles incidence there declined steadily from 2000 to 2012 (5, 8, 11-13) although the degree of that decrease has varied by region and by municipality. (14) Measles incidence in the municipality of Tianjin has mirrored trends countrywide, and their use of an immunization information system makes them an ideal region for examining progress in measles vaccine coverage. Tianjin is located 120 km south east of Beijing and has a population of approximately 12 million people. It is the third largest urban population in China; its port serves as the center of trade and economics for Northern China. In Tianjin between 2005 and 2007, more than 700 cases of measles were reported each year. The number of cases peaked at 1550 cases in 2010 and subsequently was as low as 20 cases in 2012, before rapidly increasing to 260 lab-confirmed cases in 2013. (Unpublished data, Tianjin Centers for Disease Control and Prevention, February 8, 2013) China employs the WHO's Expanded Programme on Immunization (EPI) as a basis for its national childhood immunization programs. Through China's EPI, the government provides vaccinations for 12 childhood diseases, including measles, free of charge. (14) The measles vaccine, in use in China since 1965, was added to the EPI in 1978. The measles immunization schedule varies regionally in China with most of the 31 provinces using either a two or three dose schedule. (10, 11, 15, 16) In Tianjin, measles vaccine (MV) is recommended for children at 8 months of age, followed by measles-mumps-rubella vaccine at 18-24 months (MMR-1) and again at 5 years (MMR-2). Current estimates of national coverage for a single dose of MCV in China range from 92-99%. (12, 17, 18)
This study utilized information from the Tianjin Centers for Disease Control and Prevention's (TJCDC) Immunization Information Management System (IMS), an electronic, patient-level immunization registry. The IMS contains individual-level records that include patient identifying information, basic demographic information, and details for all vaccines received. Since the registry is based at the TJCDC, all immunizations received in Tianjin are included for each patient, regardless of the clinical venue where vaccine administration took place. Immunization clinic staff enters immunization information into the registry each time a child receives a vaccine. At birth, children are added to the registry list at each health care facility. TJCDC estimates that all children in Tianjin are included in the IMS after age 1 month, even if they do not receive any vaccines. A child's record is updated as each immunization is received. Prior to IMS implementation in late 2010, immunization records were paper-based; these records were used to populate the system, which now contains records dating back to children born in 2004.
In July 2012, we extracted immunization data records of children who were seen in a random sample of clinics located in five of Tianjin's 15 districts, representing urban, suburban, coastal and rural districts. Forty percent of the children of Tianjin, age birth to 9 years, live within these five districts. The sampling frame included all immunization clinics in these districts, from which a random sample of 69 was selected; 8 clinics from Binhai (coastal), 28 clinics from Wuqing (rural), 9 clinics from Xiqing (suburban), 12 clinics from Hedong (urban), and 12 clinics from Nankai (urban). For each selected clinic, all patients with immunization records were selected. If a participant received any vaccine at a selected clinic, all of their immunization records were included, regardless of whether the clinic administering each vaccine was selected for the study. Study subjects included children born between January 1, 2004 and November 21, 2011; those born after this date were excluded since they were younger than 8 months and not eligible for the measles vaccine at the time of data extraction. The following patient-level data were examined for this study: sex, date of birth, clinic number, district of residence, and date of immunization for MV, MMR-1, and MMR-2.
On-time receipt of vaccine was determined based on Tianjin's recommended childhood immunization schedule and the child's date of birth. Children were considered “eligible” for MV if they were at least 8 months of age and “on-time” if they were 8 months to 9 months of age, “eligible” for MMR-1 if they were at least 18 months of age and “on-time” if they were 18 months to 25 months of age, and “eligible” for MMR-2 if they were 5 years of age and “on-time” if they were 5-6 years of age. Children who were eligible and received three doses of an MCV (MV, MMR-1, and MMR-2) at the time of data extraction (July 2012) were considered to have completed the series. Children who received three doses of MCV before age 6 were considered to have completed the series on-time.
Descriptive statistics were used to characterize the study population. Overall vaccine coverage rates among the study population were determined for each of the three MCV. Coverage rates, on-time receipt of vaccine, series completion, and on-time series completion were examined by sex, birth year, and area of residence (urban, suburban, coastal, and rural) using the Wald chi-square test. To gain a more complete picture of how these socio-demographic variables predicted vaccination status, multivariate logistic regression models were tested using series completed, series completed on-time and each dose completed on-time as the outcome variables and sex, residence and birth year as predictor variables, and interaction terms for each predictor variable combination. The final models included sex, residence, birth year, and an interaction term for residence by birth year. All analyses were conducted using SAS v. 9.3
The University of Michigan Institutional Review Board and the TJCDC Ethics Committee reviewed and approved this project.
RESULTS
Measles immunization records were examined for 205,982 children residing in Tianjin, China. The study population consisted of more males (53.6%) than females (46.4%) (Table 1); differences in the male: female ratio persisted across residency areas. Subjects ranged in age from 8 months to 8.7 years, with a mean age of 4.1 years (standard deviation (SD) = 2.2 years).
Table 1.
Sociodemographic characteristics of the study population, children born 2004 – 2011 who were over 8 months of age at data collection, Tianjin, China.
| n | Frequency (%) | |
|---|---|---|
| Total | 205,982 | 100 |
| Sex | ||
| Male | 110,321 | 53.6 |
| Female | 95,661 | 46.4 |
| Area of residence | ||
| Coastal | 30,896 | 15.0 |
| Rural | 62,949 | 30.6 |
| Suburban | 42,536 | 20.7 |
| Urban | 69,601 | 33.8 |
| Birth year | ||
| 2004 | 15,882 | 7.7 |
| 2005 | 19,222 | |
| 2006 | 21,380 | 10.4 |
| 2007 | 25,692 | 12.5 |
| 2008 | 27,356 | 13.3 |
| 2009 | 31,140 | 15.1 |
| 2010 | 32,107 | 15.6 |
| 2011 | 33,203 | 16.1 |
Vaccine Coverage in Study Population
Within the study population, 205,982 children were eligible for MV, 172,930 children were eligible for MMR-1, and 68,884 children were eligible for MMR-2. Overall, among children eligible for each vaccine, 98.6% received MV (n = 202,987), 97.6% received MMR-1 (n = 171,015), and 76.9% received MMR-2 (n = 55,124) (Table 2). Relatively few children received vaccine before the recommended age; 1,195 (0.6%) children received MV early, 2,333 (1.4%) children received MMR-1 early, and 1,226 (2.2%) children received MMR-2 early. However, children frequently received vaccine after the recommended age; 59,256 children (29.2%), 25,929 children (15.2%), and 5,078 children (9.2%) received the MV, MMR-1 and MMR-2 late, respectively.
Table 2.
Coverage of measles vaccine (MV), measles-mumps-rubella vaccine dose one (MMR-1), measles-mumps-rubella vaccine dose two (MMR-2) 2004 - 2011, Tianjin, China.
| MV | MMR1 | MMR2 | ||||
|---|---|---|---|---|---|---|
| Frequency (%) | n | Frequency (%) | n | Frequency (%) | n | |
| Total | 98.6 | 202,987 | 97.6 | 171,015 | 76.9 | 55,124 |
| Sex | ||||||
| Male | 98.5 | 108,630 | 97.5 | 91,310 | 76.8 | 29,464 |
| Female | 98.6 | 94,357 | 97.7 | 79,705 | 77.2 | 25,660 |
| Area of residence | ||||||
| Urban | 98.3 | 68,444 | 97.7 | 56,225 | 85.0 | 16,198 |
| Suburban | 98.5 | 41,891 | 97.7 | 34,659 | 86.9 | 11,934 |
| Coastal | 97.9 | 30,250 | 95.8 | 25,535 | 79.6 | 9,380 |
| Rural | 99.1 | 62,402 | 98.3 | 54,596 | 65.1 | 17,612 |
| Birth year | ||||||
| 2004 | 99.8 | 15,853 | 98.7 | 15,678 | 58.2 | 9,248 |
| 2005 | 99.9 | 19,199 | 99.5 | 19,120 | 82.2 | 15,809 |
| 2006 | 99.8 | 21,340 | 99.7 | 21,309 | 93.4 | 19,967 |
| 2007 | 99.9 | 25,662 | 99.5 | 25,553 | 66.6 | 10,100 |
| 2008 | 99.8 | 27,308 | 99.4 | 27,193 | -- | -- |
| 2009 | 99.8 | 31,066 | 99.4 | 30,956 | -- | -- |
| 2010 | 99.7 | 32,012 | 96.3 | 30,921 | -- | -- |
| 2011 | 92 | 30,547 | 11.6 | 285 | -- | -- |
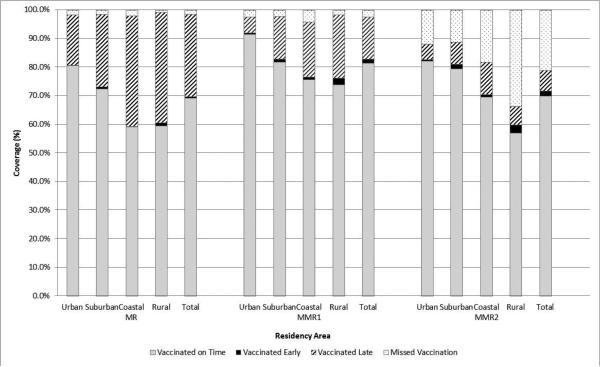
Overall coverage of MV was consistently high by birth year 2004-2010 (range: 99.7% - 99.9%) (Table 2). A similarly high level of coverage was also observed for MMR-1 in each birth year (range: 96.3% - 99.7%). In contrast, coverage of MMR-2 varied significantly by birth year, with the lowest coverage reported among those born in 2004 (58.2%) and the highest coverage reported among those born in 2006 (93.4%), the most recent year in which the entire cohort was old enough to receive MMR-2. Coverage of each dose varied by study subjects’ residency area (Table 2). In general, children in the coastal region were less likely than children in other areas to receive the vaccines, while children in rural areas were the least likely to receive MMR-2. Late receipt of vaccine was more likely in rural and coastal areas for all doses of vaccine (Figure 1).
Figure 1.
Coverage of measles vaccine (MV), measles-mumps-rubella vaccine dose one (MMR-1), measles-mumps-rubella vaccine dose two (MMR-2) 2004 – 2011, Tianjin, China.
Age at Immunization
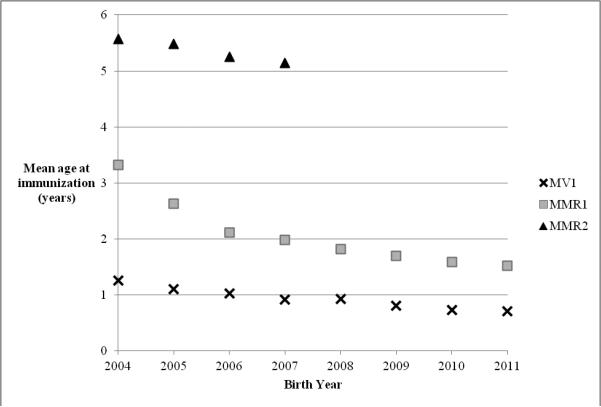
Figure 2 illustrates the mean age at receipt of each dose of MCV, by birth year. Children born in 2004 received MV at a mean age of 15 months and MMR-1 at a mean age of 39.8 months. In contrast, children born in 2010 received MV at a mean age of 8.8 months and MMR-1 at a mean age of 19.1 months. The mean age at vaccination for MMR-2 decreased for children born between 2004 and 2007, but remained within the recommended range for vaccination age (5-6 years). At the time of data collection, children born in 2008 or later were younger than the recommended age for receipt of MMR-2, however, some of these children had already received MMR-2. Ninety-one children born in 2008 received MMR-2 at a mean age of 33.5 months (SD = 8.8), 57 children born in 2009 received MMR-2 at a mean age of 24.7 months (SD = 5.7), and 20 children born in 2010 received MMR-2 at a mean age of 21.1 months (SD = 2.8 months). SIAs occurred throughout Tianjin in 2008 and 2010 and may account for some of the vaccination prior to the recommended age.
Figure 2.
Mean age at immunization with measles vaccine (MV) (n = 202,987) and measles-mumps rubella vaccine dose one (MMR-1) (n = 171,042) and dose two (MMR-2) (n = 55,345) by birth year, among children receiving each vaccine, children born 2004 – 2011, Tianjin, China.
Among those eligible, on-time immunization occurred in 69.2% of MV administrations, 81.5% of MMR-1 administrations, and 69.9% of MMR-2 administrations. For MV, 28.8% of children received the vaccine late (after 9 months of age), while 0.6% received the vaccine early (i.e. younger than 8 months old).
Series Completion
Of the 69,888 children who were eligible to receive MMR-2, 78.8% (n = 55,068) received the complete three dose MCV series. While 71.6% of children received a complete series by age 6 (i.e. on-time), just 52.5% of the children received each of the three doses on-time (Table 3). Female children were slightly more likely than male children to receive the complete series (95% CI, 0.92 – 0.99) and to receive it on time (95% CI, 0.90 – 0.97), although given the large sample size, marginal statistical significance may not indicate a meaningful difference. Series completion was highest among suburban children (88.7% of children in suburban areas received the complete series). In contrast, on-time series completion was highest among urban children (82.6% of urban children who received the complete series received it on time). Rural children were the least likely to complete the series (66.3%), receive the third dose on-time (59.7%), and receive each of the 3 doses on-time (35.4%). Children born in 2006 were most likely to receive a complete series (93.3%) compared to other years. Children born in 2004 were the least likely to receive a complete series (58.2%) and were the least likely to complete the series on time (47.5%).
Table 3.
Demographic characteristics of children by 3-dose measles containing vaccine series completion status; bivariate analyses; children born 2004 – 2007 in Tianjin, China
| Series Completed (n=69,888) | Series Completed On-time (n=69,888) | Each Dose Completed On-time (n=55,068) | |||||
|---|---|---|---|---|---|---|---|
| Proportion Complete | 95% Confidence Interval | Proportion On-time | 95% Confidence Interval | Proportion On-time | 95% Confidence Interval | ||
| Sex | Female | 79.1% | ref. | 72.3% | ref. | 53.6% | ref. |
| Male | 78.5% | 0.92 – 0.99 | 70.9% | 0.90 – 0.97 | 51.5% | 0.89 – 0.95 | |
| Residence | Urban | 88.0% | ref. | 82.6% | ref. | 74.1% | ref. |
| Suburban | 88.7% | 0.88 – 1.00 | 80.9% | 1.06 – 1.19 | 53.9% | 2.32 – 2.57 | |
| Coastal | 81.4% | 1.57 – 1.79 | 70.2% | 1.91 – 2.13 | 45.4% | 3.25 – 3.62 | |
| Rural | 66.3% | 3.55 – 3.94 | 59.7% | 3.06 – 3.35 | 35.4% | 4.97 – 5.46 | |
| Birth Year | 2004 | 58.2% | ref. | 47.5% | ref. | 54.0% | ref. |
| 2005 | 82.2% | 3.16 – 3.48 | 67.6% | 2.21 – 2.41 | 49.8% | 0.77 – 0.85 | |
| 2006 | 93.3% | 9.42 – 10.66 | 90.7% | 10.15 – 11.34 | 54.5% | 0.94 – 1.03 | |
| 2007 | 75.3% | 2.09 – 2.31 | 75.3% | 3.21 – 3.54 | 50.3% | 0.78 – 0.88 | |
| TOTAL | 78.8% | 71.6% | 52.5% | ||||
Table 4 illustrates the results of three multivariate logistic regression models. The first model examines predictors for completing the three dose measles vaccine series. Among children old enough to receive all three MCV doses, sex, residence, birth year and the interaction between residence and birth year were statistically significant predictors for completion. Similar results were found in the other two models that examined predictors for completing the overall series on time and for completing each individual dose of the series on time.
Table 4.
Multivariate analysis of demographic characteristics of children by 3-dose measles containing vaccine series completion status; children born 2004 – 2007 in Tianjin, China
| Series Completed (n=69,888) | Series Completed On-time (n=69,888) | Each Dose Completed On-time (n=55,068) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Estimate | Standard Error | P-Value | Estimate | Standard Error | P-Value | Estimate | Standard Error | P-Value | ||
| Sex | Male vs Female | −0.068 | 0.021 | 0.0016 | −0.091 | 0.019 | <.0001 | −0.095 | 0.018 | <.0001 |
| Residence | Suburban vs Urban | −1.481 | 0.086 | <.0001 | −1.008 | 0.063 | <.0001 | −0.721 | 0.063 | <.0001 |
| Coastal vs Urban | −1.063 | 0.091 | <.0001 | −0.977 | 0.065 | <.0001 | −1.118 | 0.063 | <.0001 | |
| Rural vs Urban | −3.557 | 0.078 | <.0001 | −3.027 | 0.058 | <.0001 | −2.448 | 0.069 | <.0001 | |
| Birth Year | 2005 vs 2004 | −0.061 | 0.094 | 0.5144 | −0.122 | 0.063 | 0.0522 | −0.210 | 0.057 | 0.0002 |
| 2006 vs 2004 | 0.291 | 0.094 | 0.002 | 0.779 | 0.068 | <.0001 | −0.075 | 0.055 | 0.172 | |
| 2007 vs 2004 | −1.764 | 0.081 | <.0001 | −0.757 | 0.059 | <.0001 | −0.326 | 0.059 | <.0001 | |
| Interaction: Residence & Birth Year | Suburban*2005 | 1.876 | 0.128 | <.0001 | 0.895 | 0.084 | <.0001 | −0.134 | 0.079 | 0.090 |
| Suburban*2006 | 1.641 | 0.128 | <.0001 | 1.005 | 0.096 | <.0001 | −0.238 | 0.076 | 0.002 | |
| Suburban*2007 | 2.168 | 0.105 | <.0001 | 1.695 | 0.088 | <.0001 | −0.324 | 0.086 | 0.0002 | |
| Coastal*2005 | 0.446 | 0.119 | 0.0002 | 0.084 | 0.084 | 0.3162 | −0.171 | 0.081 | 0.0340 | |
| Coastal*2006 | −0.096 | 0.118 | 0.4145 | 0.148 | 0.092 | 0.1056 | −0.078 | 0.079 | 0.3197 | |
| Coastal*2007 | 0.742 | 0.106 | <.0001 | 0.657 | 0.085 | <.0001 | −0.374 | 0.093 | <.0001 | |
| Rural*2005 | 1.689 | 0.100 | <.0001 | 1.68 | 0.073 | <.0001 | 0.695 | 0.083 | <.0001 | |
| Rural*2006 | 3.433 | 0.109 | <.0001 | 3.171 | 0.086 | <.0001 | 0.911 | 0.079 | <.0001 | |
| Rural*2007 | 4.218 | 0.095 | <.0001 | 3.688 | 0.078 | <.0001 | 1.151 | 0.087 | <.0001 | |
Among the 55,068 children who received the complete MCV series, the average age at immunization was 12.4 months (SD = 10.0 months), 24.0 months (SD = 8.7 months), and 5.3 years (SD = 0.5 years) for MV, MMR-1, and MMR-2, respectively. Among children who received each dose of the complete series on-time (n=28,891), the average age at immunization was 8.4 months (SD = 0.3 months) for MV, 21.2 months (SD = 1.2 months) for MMR-1 and 5.2 years (SD = 0.2 years) for MMR-2.
DISCUSSION
China has made great strides in improving measles immunization coverage over the last decade. However, as a country slated for measles elimination, China needs to improve on-time series completion in young children if that goal is to be realized. Given the extremely high 2-dose measles vaccine coverage in children, measles should be well controlled in childhood populations under age 8 years. The age distribution of recent measles cases in Tianjin confirms this; children age 1-9 contributed only 7.3% of cases to the total case count for 2013, while adults age 20-49 accounted for 69.2% of the total case count. However, continued high levels of vaccine coverage are important in preventing a resurgence of measles in the near future. Other countries, which have relaxed measles vaccination efforts, such as France, have experienced a major resurgence of disease in recent years. (20) As China strives to eliminate measles, additional studies need to be conducted among other age groups to identify susceptible pockets of the population, which can then guide public health programs in achieving improved coverage in age-specific targets.
Despite high levels of coverage with the first and second doses of an MCV, many children in Tianjin did not receive the third recommended dose. In particular, children residing in rural areas were least likely to receive a complete series, and most likely to receive MCV doses late, putting them at greater risk for measles for a longer period of time. This association persisted in bivariate and multivariate analyses. Variations in coverage by residency area are consistent with previous reports of urban-rural disparities in vaccine coverage in China. (21) Furthermore, urban-rural inequities in healthcare in China have been increasing, despite an overall improvement in population health. (22) Compared to urban areas, rural areas have greater under-five mortality rates, fewer births at healthcare facilities, and a higher burden of maternal deaths. (23) Those who live in poor, rural areas are reported to have the greatest difficulties accessing health care. (24, 25) In our study, children in all areas are accessing vaccination services and receiving the first two doses of MCV at a high level, indicating that vaccine is in fact available to all children. However, perhaps many of the children in Tianjin received the complete measles immunization series through SIAs. These are not directed at individual dose timeliness but rather at series completion. Access to regular and ongoing immunization clinic services, which helps insure timely administration of each dose, may be more spotty on a day to day basis in rural and suburban areas than in urban areas where they are more focused and accessible. Additional emphasis on receiving MMR-2 may be necessary to improve coverage of the complete series of MCV in rural areas. Furthermore, MMR-2 coverage needs to be improved in all residency areas – urban, suburban, rural, and coastal – to ensure ongoing high levels of protection from measles in children and to minimize transmission in this young population. There is a clear interaction between area of residence and year of birth. The measles SIAs that occurred in China in 2008 and 2010, while intended to reach all unvaccinated and under-vaccinated children in Tianjin, may have been more successful or more fully operationalized in some areas or among some age groups, than in others. Importantly, the data included vaccination information for all vaccines, but did not specify if the vaccination was provided by a regular immunization clinic or specifically as part of a measles SIA. Of course, efforts to insure that all children are caught up during SIAs and through other vaccine activities are important, regardless of area of residence.
Immunizations received during and after SIAs, which are administered regardless of current immunization status, may be important for assessing timeliness and continuity of routine immunization services, but should not be relied upon in lieu of regular immunization services. Wenzhou, China experienced an epidemic of measles in 2010. (26) Investigation into this epidemic suggested that relaxed routine immunization following the nationwide SIA accounted for a large number of cases.
While female children were more likely to receive the complete series of MCV, and to complete it on-time, male children who received the complete series were more likely to receive each dose on-time. The differences, however, were small and not likely to be meaningful in terms of overall disease control efforts.
The average age at immunization with MV and MMR-1 was shown to decrease by birth cohort. The Tianjin immunization schedule did not change between 2004 and the present. The observed trend may therefore be a result of China's increased public health efforts in recent years, such as SIAs for measles vaccination; improved infectious disease surveillance and control are hallmarks of China's success in developing its public health system. (27)
Improvements in on-time receipt of vaccine ensure that more children are adequately protected against measles at the earliest age possible. (28) While we observed decreased age at immunization for MV and MMR-1 over the birth cohorts, we did not observe a similar trend in series completion or on-time receipt by birth cohort. Further attention to series completion and on-time receipt of the series continue to be key to China's efforts to eliminate measles.
The three-dose schedule in Tianjin allows for up to 16 months between MV and MMR-1. Given that vaccine efficacy is approximately 85% in children who receive MV at age less than 12 months, children in Tianjin may be at substantial risk for measles infection for more than a year. While this age group does not have a particularly high burden of measles disease based on the current epidemiological profile for measles, it is important to continue to monitor case surveillance data for increased burden of disease in children who are between their MV and MMR-1 doses. An increase in cases may indicate a need for consideration of an earlier administration of MMR-1.
Future projects should investigate the early administrations of MV, MMR-1, and MMR-2, and the impact that they have on measles susceptibility. Although the minimum interval between doses of MCV is one month, adherence to the immunization schedule is thought to maximize the immune response to the vaccine and reduce the number of children susceptible to disease (6, 7). Understanding the reasons for early administrations could also improve health education and future immunization activities. Furthermore, the schedule should be guided by the changing epidemiology of measles in Tianjin, in this era of measles elimination.
The study has several important limitations. The study population was limited to 69 clinics in five districts in the eastern part of China, thus it may not be representative of the greater population of Tianjin or all of China. Although TJCDC estimates that all children were included in the immunization registry, some children may have been missed, particularly those who newly migrated to Tianjin. Missing data on these children may result in an overestimate of measles vaccine coverage in Tianjin. However, we believe the impact is minor, as vaccines are free, readily available, and required for school entry. Furthermore, village doctors work closely with village leaders to ensure that all children have access to immunization clinics. This study excluded children who were not old enough to receive vaccine at time of data collection, but included older children who may have received vaccines early. Consequently, we may have excluded some children who received the vaccine early, leading to an underestimate of coverage. Since our analysis revealed that relatively few children received the vaccine before the recommended age, we anticipate that the impact is minor. The large sample size of this study provided high levels of statistical power and internal validity.
Extremely high rates of immunization are observed for the first two doses of MCV, yet these rates declined for the third dose. Consequently, results from this study may be indicative of gaps in immunization services in Tianjin, China. Maintaining high coverage of MV and MMR-1, increasing coverage of MMR-2, and ensuring on-time immunizations will aid China in reaching its goal of eliminating measles in the near future.
Acknowledgments
Funding Acknowledgement: This project was funded in part by the National Institutes of Health, National Institute of Allergy and Infectious Disease, Division of Microbial and Infectious Diseases U01-AI-088671
REFERENCES
- 1.Measles Fact Sheet, World Health Organization [November 5, 2013];2013 ( http://www.who.int/mediacentre/factsheets/fs286/en/). Updated February, 2013.
- 2.WHO . Measles deaths decline, but elimination progress stalls in some regions Geneva. World Health Organization; Switzerland: 2013. [November 5, 2013]. ( http://www.who.int/mediacentre/news/notes/2013/measles_20130117/en/). Updated January 17, 2013. [Google Scholar]
- 3.World Health Organization . Global Eradication of Measles. World Health Organization; 2010. [December 17, 2013]. ( http://apps.who.int/gb/ebwha/pdf_files/wha63/a63_18-en.pdf). [Google Scholar]
- 4.Fine PE. Herd immunity: history, theory, practice. Epidemiol Rev. 1993;15(2):265–302. doi: 10.1093/oxfordjournals.epirev.a036121. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention . Progress Toward the 2012 Measles Elimination Goal --- Western Pacific Region, 1990--2008. 24. Vol. 58. Centers for Disease Control and Prevention; 2009. pp. 669–674. [PubMed] [Google Scholar]
- 6.World Health Organization (WHO) Measles vaccines: WHO position paper. Vol. 74. Weekly Epidemiologic Record; 2009. pp. 349–360. [Google Scholar]
- 7.World Health Organization (WHO) Measles vaccines: WHO Position Paper. Weekly Epidemiologic Record. 2004;79:129–144. [Google Scholar]
- 8.Caceres VM, Strebel PM, Sutter RW. Factors determining prevalence of maternal antibody to measles virus throughout infancy: a review. Clin Infect Dis. 2000;31(1):110–9. doi: 10.1086/313926. [DOI] [PubMed] [Google Scholar]
- 9.Leuridan E, Hens N, Hutse V, Leven M, Aerts M, Van Damme P. Early waning of maternal measles antibodies in era of measles elimination: longitudinal study. BMJ. May 18. 2010;340:c1626. doi: 10.1136/bmj.c1626. doi: 10.1136/bmj.c1626. [DOI] [PubMed] [Google Scholar]
- 10.Lixia W, Guang Z, Lee LA, et al. Progress in accelerated measles control in the People's Republic of China, 1991-2000. J Infect Dis. 2003;187(Suppl 1):S252–7. doi: 10.1086/368045. [DOI] [PubMed] [Google Scholar]
- 11.Measles. World Health Organization; 2013. [November 5, 2013]. ( http://www.wpro.who.int/china/mediacentre/factsheets/measles/en/index.html). [Google Scholar]
- 12.Immunization Profile -- China [Data file] World Health Organization; 2012. [November 5, 2013]. ( http://apps.who.int/immunization_monitoring/globalsummary/countries?countrycriteria%5B country%5D%5B%5D=CHN&commit=OK). [Google Scholar]
- 13.World Health Organization [September 27, 2013];Measles Reported Cases. Available at: http://apps.who.int/immunization_monitoring/globalsummary/timeseries/tsincidencemeasles.html. Updated July 14, 2013.
- 14.World Health Organization [January 29, 2014];Representative Office: China. Measles. ( http://www.wpro.who.int/china/mediacentre/factsheets/measles/en/index.html).
- 15.National Immunization Data - EPI Data by Country. World Health Organization; China: 2011. [November 5, 2013]. ( http://www.wpro.who.int/immunization/documents/epi_country_poster_2011_CHN.pdf). [Google Scholar]
- 16.Immunization Schedules [Data file] World Health Organization; 2012. [November 5, 2013]. ( http://apps.who.int/immunization_monitoring/globalsummary/schedules). [Google Scholar]
- 17.China: WHO and UNICEF estimates of immunization coverage: 2011 revision. World Health Organization; 2012. [November 5, 2013]. ( http://www.who.int/immunization_monitoring/data/chn.pdf). [Google Scholar]
- 18.Murray CJ, Shengelia B, Gupta N, et al. Validity of reported vaccination coverage in 45 countries. Lancet. 2003;362(9389):1022–7. doi: 10.1016/S0140-6736(03)14411-X. [DOI] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention (CDC) Measles. In: Atkinson W, Wolfe S, Hamborsky J, editors. Epidemiology and Prevention of Vaccine-Preventable Diseases (The Pink Book) 12th ed. Public Health Foundation; Washington, DC: 2012. [December 18, 2013]. pp. 173–191. Available at http://www.cdc.gov/vaccines/pubs/pinkbook/index.html. [Google Scholar]
- 20.World Health Organization [December 11, 2013];WHO vaccine-preventable diseases: monitoring system 2013 global summary. Available at: http://apps.who.int/immunization_monitoring/globalsummary.
- 21.Zhou YH, Wu C, Zhuang H. Vaccination against hepatitis B: the Chinese experience. Chin Med J (Engl) 2009;122(1):98–102. [PubMed] [Google Scholar]
- 22.Gao J, Qian J, Tang S, et al. Health equity in transition from planned to market economy in China. Health Policy Plan. 2002;17(Suppl):20–9. doi: 10.1093/heapol/17.suppl_1.20. [DOI] [PubMed] [Google Scholar]
- 23.Meng Q, Zhang J, Yan F, et al. One country, two worlds - the health disparity in China. Glob Public Health. 2012;7(2):124–36. doi: 10.1080/17441692.2011.616517. [DOI] [PubMed] [Google Scholar]
- 24.Cui FQ, Gofin R. Immunization coverage and its determinants in children aged 12-23 months in Gansu, China. Vaccine. 2007;25(4):664–71. doi: 10.1016/j.vaccine.2006.08.027. [DOI] [PubMed] [Google Scholar]
- 25.Hsiao WC, Liu Y. Economic reform and health--lessons from China. N Engl J Med. 1996;335(6):430–2. doi: 10.1056/NEJM199608083350611. [DOI] [PubMed] [Google Scholar]
- 26.Gao J, Chen E, Wang Z, et al. Epidemic of measles following the nationwide mass immunization campaign. BMC Infect Dis. 2013;13(139):2334–13-139. doi: 10.1186/1471-2334-13-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feng Z, Li W, Varma JK. Gaps remain in China's ability to detect emerging infectious diseases despite advances since the onset of SARS and avian flu. Health Aff (Millwood) 2011;30(1):127–35. doi: 10.1377/hlthaff.2010.0606. [DOI] [PubMed] [Google Scholar]
- 28.Bielicki JA, Achermann R, Berger C. Timing of measles immunization and effective population vaccine coverage. Pediatrics. 2012;130(3):e600–6. doi: 10.1542/peds.2012-0132. [DOI] [PubMed] [Google Scholar]