Abstract
Persistent infection with oral HPV16 is believed to drive the development of most oropharyngeal cancers. However, patterns of oral HPV16 persistence remain understudied, particularly among HIV-negative individuals. Oral HPV16 persistence was evaluated among 1626 participants of the HPV Infection in Men (HIM) Study. Twenty-three oral HPV16-positive men who provided an oral gargle sample on ≥2 study visits were included in the analysis. Archived oral samples from all follow-up visits were tested for HPV16 using Linear Array and INNO-LiPA detection methods. Persistence was evaluated using consecutive HPV16-positive visits held approximately 6 months apart and using the Kaplan-Meier method. Oral HPV16-positive men were aged 18–64 years (median, 36 years; IQR, 25–42) and were followed for a median of 44.4 months (IQR, 29.9–49.5). Of 13 incident infections, 4 (30.8%) persisted ≥12 months, 1 (10.0%) persisted ≥24 months, and none persisted ≥36 months (median infection duration, 7.3 months [95% CI, 6.4–NA]). Of 10 prevalent infections, 9 (90.0%) persisted ≥12 months, 8 (80.0%) persisted ≥24 months, 4 (57.1%) persisted ≥36 months, and 2 (40.0%) persisted ≥48 months (median infection duration, NA). Twelve-month persistence of incident infections increased significantly with age (P trend=0.028). Prevalent oral HPV16 infections in men persisted longer than newly acquired infections, and persistence appeared to increase with age. These findings may explain the high prevalence of oral HPV observed at older ages. Understanding oral HPV16 persistence will aid in the identification of men at high-risk of developing HPV-related oropharyngeal cancer.
Keywords: oral HPV, HPV16, natural history, persistence, clearance
Introduction
Mounting evidence in some economically developed countries suggests that human papillomaviruses (HPVs) cause the majority of oropharyngeal cancers (OPCs) among men (1, 2), with HPV type 16 implicated in most cases (3). In the United States (USA), OPC incidence is fourfold higher among men than women (4). Though still considered a rare cancer (<10 per 100,000 individuals) (4), HPV-positive OPC incidence has increased significantly in recent decades among men (1, 4), with the annual number of overall OPC cases now surpassing that of cervical cancers (4).
Most HPV-driven OPC cases are diagnosed at an advanced clinical stage (III–IV) (5), and treatment often causes substantial morbidity, including facial disfigurement and permanent difficulties in speaking, swallowing, and breathing. Unfortunately, there are no clinically validated screening tests available to detect OPCs at an earlier stage or to predict the risk of developing HPV-driven OPC. Though primary prevention of oral HPV through prophylactic vaccination is promising (6), there is no definitive evidence that HPV vaccines will prevent new oral HPV infection and subsequent disease. Furthermore, generations of men and women will remain unprotected, including individuals currently outside of the recommended age range for vaccination and those already infected with oral HPV (7), emphasizing the need for the development of oral HPV prevention and early OPC detection methods, particularly among high-risk individuals.
Advancing our knowledge of the natural history of genital HPV infection has led to important innovations in the prevention of cervical cancer (e.g., cervical HPV testing and prophylactic HPV vaccination). Similar data on oral HPV natural history are needed to advance the development of OPC interventions. Unfortunately, oral HPV natural history remains understudied. Like cancers at other anatomic sites, persistent oral HPV infection may be the driving factor in OPC carcinogenesis; therefore, it is crucial to examine each step along the continuum of carcinogenesis, from initial HPV infection to development of cancer. Previously, we described the prevalence (8) and acquisition (9) of oral HPV detected among otherwise healthy men enrolled in a large HPV natural history study. Here, we supplement these data with an evaluation of long-term oral HPV16 persistence among the same cohort of men.
Materials and Methods
Study population
The HPV Infection in Men (HIM) Study is an ongoing, multinational cohort study of the natural history of genital HPV infections (10, 11). More than 4,000 men aged 18–70 years were recruited from a variety of population sources within Brazil, Mexico, and the USA beginning in 2005. Men reported no previous diagnosis of anogenital cancers, had never been diagnosed with anogenital warts, and reported no symptoms of or treatment for a sexually transmitted infection, including HIV/AIDS in order to be eligible for the study. In 2007, oral sample collection was initiated, and details of the HIM Study oral HPV substudy can be found elsewhere (8, 9). Briefly, the first consecutively recruited 1626 men who provided oral gargle samples on ≥2 study visits were tested for oral HPV, none of whom reported a history of head and neck cancer (9). Twenty-eight men were positive for oral HPV16 and 23 men had sufficient data to evaluate oral HPV16 persistence (i.e., ≥6 months of follow-up after the initial infection).
The Human Subjects Committees of the University of South Florida (Tampa, FL, USA), Ludwig Institute for Cancer Research (São Paulo, Brazil), Centro de Referência e Treinamento em Doenças Sexualmente Transmissíveis e AIDS (São Paulo, Brazil), and Instituto Nacional de Salud Pública de México (Cuernavaca, Mexico) approved all study procedures, and all participants provided written informed consent.
Procedures
Participants attended a pre-enrollment (baseline) visit, an enrollment visit (two weeks post-baseline), and follow-up visits every six months for up to four years. At each visit, participants completed a computer-assisted self-interview with questions about sociodemographic characteristics and potential risk factors, and underwent a clinical examination in which genital and oral samples were collected. Each participant was asked to rinse with and gargle 15 mL of locally available mouthwash for a total of 30 seconds (8). The sample was centrifuged at 3000xg for 15 minutes at 4°C, the supernatant was decanted, and the cell pellet was resuspended in 20 mL of sterile saline. To ensure even sample distribution, centrifugation was repeated, and the cell pellet was resuspended in 1.2 mL of saline with repeated pipetting and vortexing. Samples were then stored at −80°C for PCR and genotyping analyses.
HPV DNA extraction and genotyping have been described previously (8, 9). Briefly, oral cells underwent robotic DNA extraction using the QIAamp Media MDx Kit (Qiagen, Valencia, CA, USA) in accordance with the manufacturer’s instructions. Samples were tested for the presence of HPV DNA with PGMY09/11 PCR, and HPV genotyping was conducted with Linear Array (Roche Molecular Diagnostics, Alameda, CA, USA), irrespective of HPV PCR results. The Linear Array assay detects 37 mucosal HPV types, classified as high-risk (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68) or low-risk (6, 11, 26, 40, 42, 53, 54, 55, 61, 62, 64, 66, 67, 69, 70, 71, 72, 73, 81, 82, 82 subtype IS39, 83, 84, and 89 [CP6108]) (12).
To evaluate long-term infection persistence among oral HPV16-positive study participants, we identified and retrieved all available oral gargle samples that were archived through the end of the study. Oral HPV genotyping of all archived samples from these men was completed using Linear Array. For 11 men, at least one intervening negative result occurred among a series of positive results, suggesting that oral HPV16 viral load fell below the lower limit of detection of the assay. We used a more sensitive assay, INNO-LiPA HPV Genotyping Extra (Fujirebio, Ghent, Belgium), to confirm the results of 14 specimens that, according to Linear Array, were intervening negatives (single or multiple negatives) between positive visits. Eleven specimens (78.6%) tested HPV16-positive using INNO-LiPA, although three specimens remained HPV16-negative using INNO-LiPA. If a visit tested HPV16-positive by INNO-LiPA, that visit was considered HPV16-positive, overriding the results of the Linear Array assay. If an intervening negative tested HPV16-negative by both Linear Array and INNO-LiPA, but had prior and subsequent HPV16-positive visits, the intervening negative visit was considered a false negative; this occurred for three men (two with prevalent HPV and one with incident HPV). We also used INNO-LiPA to test a random sample of five HPV16-positive and five HPV16-negative specimens previously determined using Linear Array; all (100%) of the HPV16-positive specimens remained positive and all (100%) of the HPV16-negative specimens remained negative. Using results from both assays, we evaluated oral HPV16 persistence and clearance for up to 56.4 months of follow up.
Statistical analysis
Persistence was first evaluated using a definition based on consecutive oral HPV16-positive visits. Persistence was estimated at six-month intervals, as study visits occurred approximately six months apart; however, as pre-enrollment and enrollment visits occurred two weeks apart, oral HPV16 results from these visits were combined into one visit (i.e., the combined visit was considered positive if either visit was positive). Six-month type-specific persistence was defined as the presence of oral HPV16 DNA on two or more consecutive study visits, 12-month type-specific persistence as the presence of oral HPV16 DNA on three or more consecutive study visits, and so on. If a single HPV-negative visit occurred between two HPV-positive visits, that visit was deemed a false negative and was treated as though it were an HPV-positive visit. Persistence was reported as a proportion and was calculated as the number of persistent HPV16 infections divided by the total number of HPV16 infections detected among men who had been followed for that period of time or longer. As not all men were followed for 48 months, the total number of infections (i.e., denominator) changed over time.
Persistence was also evaluated using the Kaplan-Meier method to estimate the cumulative probability of infection throughout the entire study period and median time to clearance. Infection clearance was defined as two or more HPV16-negative visits following an HPV16-positive visit. However, an infection was considered cleared if a single HPV-negative occurred at the final study visit, as there was insufficient evidence to consider this a false negative (i.e., the absence of an HPV16-positive visit following the HPV16-negative visit); this occurred for one man with a prevalent infection. Men whose infections did not clear by the final study visit were censored in the Kaplan-Meier analysis. Log-rank tests were used to identify significant differences between the cumulative probabilities of prevalent versus incidence infections.
Factors associated with oral HPV16 infection persistence were assessed if they were believed to be relevant on the basis of previous work (8, 9). At 12, 24, 36, and 48 months, estimates of persistence were stratified by each categorical risk factor, and exact Pearson chi-square tests and Cochran-Armitage tests for trend were used to identify differences and linear trends, respectively, in the proportions of persistent infections. Because of low numbers of oral HPV16 infections, factors associated with persistence were also assessed using Cox proportional-hazards regression.
All analyses were conducted separately for prevalent and incident HPV infections. All statistical tests were two-sided and attained statistical significance at α=0.05. Analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC, USA).
Results
Twenty-three men were evaluated for long-term oral HPV16 persistence in this prospective analysis. These men were followed for a median of 44.4 months (IQR 29.9–49.5) and ranged in age from 18–64 years (median 36 years; IQR 25–42). One man (4.3%) only completed four follow-up visits, four men (17.4%) completed five visits, eight men (34.8%) completed six visits, one man (4.3%) completed seven visits, six men (26.1%) completed eight visits, and three men (13.0%) completed nine visits. The sociodemographic and behavioral profile of men with oral HPV16, stratified by prevalent or incident infection, is presented in Table 1. Oral HPV16-positive men were predominantly young, white, non-Hispanic, never smokers, and well educated. No significant differences were observed between men with incident versus prevalent oral HPV16 infections.
Table 1. Characteristics of men with oral human papillomavirus (HPV) type 16 at the time of enrollment into the oral HPV substudy.
| Total (n=23) |
Prevalent (n=10) |
Incident (n=13) |
||
|---|---|---|---|---|
|
|
||||
| Characteristic | n (%) | n (%) | n (%) | P-valuea |
| Country | 0.241 | |||
| USA | 8 (34.8%) | 2 (20.0%) | 6 (46.2%) | |
| Brazil | 7 (30.4%) | 5 (50.0%) | 2 (15.4%) | |
| Mexico | 8 (34.8%) | 3 (30.0%) | 5 (38.5%) | |
| Age (years) | 0.721 | |||
| Median (IQR) | 36 (25–42) | 37 (25–43) | 32 (30–37) | |
| 18–30 | 10 (43.5%) | 4 (40.0%) | 6 (46.2%) | |
| 31–44 | 10 (43.5%) | 4 (40.0%) | 6 (46.2%) | |
| 45–64 | 3 (13.0%) | 2 (20.0%) | 1 (7.7%) | |
| Race | 1.000 | |||
| White | 12 (52.2%) | 5 (50.0%) | 7 (53.8%) | |
| Black | 2 (8.7%) | 1 (10.0%) | 1 (7.7%) | |
| Asian/Pacific Islander | 1 (4.3%) | 0 (0%) | 1 (7.7%) | |
| Mixed race | 8 (34.8%) | 4 (40.0%) | 4 (30.8%) | |
| Ethnicity | 1.000 | |||
| Non-Hispanic | 15 (65.2%) | 7 (70.0%) | 8 (61.5%) | |
| Hispanic | 8 (34.8%) | 3 (30.0%) | 5 (38.5%) | |
| Marital status | 0.594 | |||
| Single | 10 (43.5%) | 3 (30.0%) | 7 (53.8%) | |
| Married or cohabiting | 10 (43.5%) | 5 (50.0%) | 5 (38.5%) | |
| Divorced, separated, or widowed | 3 (13.0%) | 2 (20.0%) | 1 (7.7%) | |
| Education (years) | 0.754 | |||
| ≤12 | 7 (30.4%) | 4 (40.0%) | 3 (23.1%) | |
| 13–15 | 6 (26.1%) | 2 (20.0%) | 4 (30.8%) | |
| ≥16 | 10 (43.5%) | 4 (40.0%) | 6 (46.2%) | |
| Smoking status | 1.000 | |||
| Never | 16 (69.6%) | 8 (80.0%) | 8 (61.5%) | |
| Former | 1 (4.3%) | 0 (0%) | 1 (7.7%) | |
| Current | 5 (21.7%) | 2 (20.0%) | 3 (23.1%) | |
| Missing data | 1 (4.3%) | 0 (0%) | 1 (7.7%) | |
| Alcohol intake (drinks per month) | 0.861 | |||
| 0 | 4 (17.4%) | 2 (20.0%) | 2 (15.4%) | |
| 1–30 | 10 (43.5%) | 5 (50.0%) | 5 (38.5%) | |
| ≥31 | 9 (39.1%) | 3 (30.0%) | 6 (46.2%) | |
| Sexual orientation | 1.000 | |||
| MSW | 20 (87.0%) | 9 (90.0%) | 11 (84.6%) | |
| MSM | 1 (4.3%) | 0 (0%) | 1 (7.7%) | |
| MSWM | 2 (8.7%) | 1 (10.0%) | 1 (7.7%) | |
| Lifetime number of sex partners | 0.934 | |||
| Median (IQR) | 10 (4–26) | 11 (4–26) | 8 (5–20) | |
| 0–2 | 3 (13.0%) | 1 (10.0%) | 2 (15.4%) | |
| 3–7 | 6 (26.1%) | 2 (20.0%) | 4 (30.8%) | |
| 8–19 | 7 (30.4%) | 4 (40.0%) | 3 (23.1%) | |
| ≥20 | 7 (30.4%) | 3 (30.0%) | 4 (30.8%) | |
| Ever had oral sex | 0.277 | |||
| No | 4 (17.4%) | 3 (30.0%) | 1 (7.7%) | |
| Yes | 19 (82.6%) | 7 (70.0%) | 12 (92.3%) | |
| Ever had oral sex with a man | 0.622 | |||
| No | 18 (78.3%) | 7 (70.0%) | 11 (84.6%) | |
| Yes | 5 (21.7%) | 3 (30.0%) | 2 (15.4%) | |
| Had oral sex in the past 6 monthsb | 0.640 | |||
| No | 7 (31.8%) | 4 (40.0%) | 3 (23.1%) | |
| Yes | 15 (68.2%) | 6 (60.0%) | 9 (69.2%) | |
| Missing data | 1 (4.3%) | 0 (0%) | 1 (7.7%) | |
| Number of oral sex acts in the past 6 monthsb | 0.579 | |||
| 0 | 9 (39.1%) | 4 (40.0%) | 5 (38.5%) | |
| 1–6 | 1 (4.3%) | 0 (0%) | 1 (7.7%) | |
| 7–24 | 8 (34.8%) | 3 (30.0%) | 5 (38.5%) | |
| ≥25 | 4 (17.4%) | 3 (30.0%) | 1 (7.7%) | |
| Missing data | 1 (4.3%) | 0 (0%) | 1 (7.7%) | |
| Time since last oral sex (days)b | 0.519 | |||
| 0–3 | 6 (26.1%) | 3 (30.0%) | 3 (23.1%) | |
| >3–10 | 2 (8.7%) | 0 (0%) | 2 (15.4%) | |
| >10–30 | 3 (13.0%) | 2 (20.0%) | 1 (7.7%) | |
| >30 | 6 (26.1%) | 2 (20.0%) | 4 (30.8%) | |
| Never | 4 (17.4%) | 3 (30.0%) | 1 (7.7%) | |
| Missing data | 2 (8.7%) | 0 (0%) | 2 (15.4%) | |
MSW=men who have sex with women. MSM=men who have sex with men. MSWM=men who have sex with women and men.
Exact Pearson chi-square P-value using Monte Carlo estimation.
Question not asked among all men because the study questionnaire was revised in 2007 after oral sample collection began.
Ten men (43.5%) entered the study with a prevalent oral HPV16 infection, and throughout the study period, 13 men (56.5%) acquired a new, incident infection. Thirteen men (56.5%) cleared their infection by the end of the study: three (30.0%) with prevalent and 10 (76.9%) with incident infections.
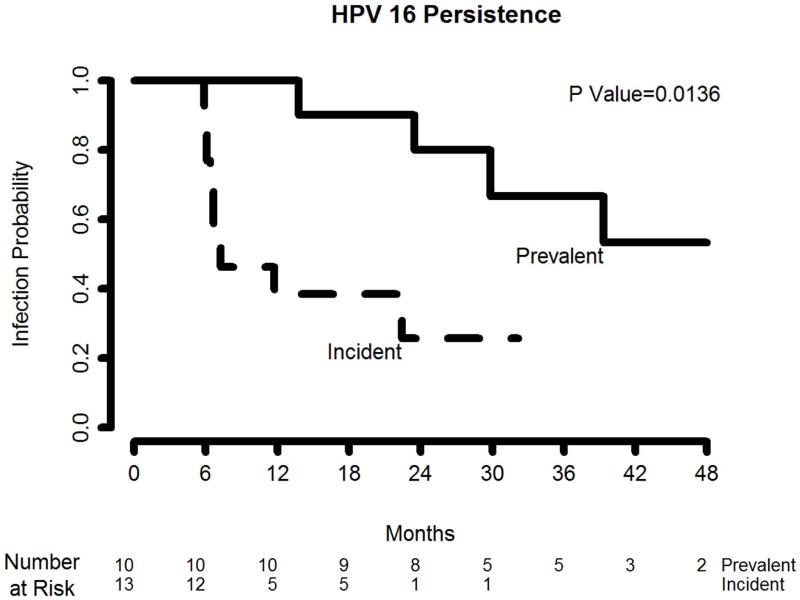
Overall, oral HPV16 persistence was substantially greater for prevalent versus incident infections (Table 2). Among men with prevalent infections, nine (90.0%) were found to persist ≥12 months, eight (80.0%) persisted ≥24 months, four (57.1%) persisted ≥36 months, and two (40.0%) persisted ≥48 months. Among men with incident oral HPV16 infections, four (30.8%) persisted ≥12 months, one (10.0%) persisted ≥24 months, and none persisted beyond 36 months. Using the Kaplan-Meier method, the cumulative probability of continued infection among men with prevalent oral HPV16 was consistently higher than that among men with an incident infection across the follow-up period (log-rank P=0.014; Figure 1).
Table 2. Long-term persistence of prevalent and incident oral human papillomavirus (HPV) type 16 infections in men (n=23).
| Prevalent (n=10) |
Incident (n=13) |
|||
|---|---|---|---|---|
| Persistence | Number of persistent infections/ number of total infectionsa |
% | Number of persistent infections/ number of total infectionsa |
% |
| ≥6 months | 10/10 | 100 | 5/13 | 38.5 |
| ≥12 months | 9/10 | 90.0 | 4/13 | 30.8 |
| ≥18 months | 9/10 | 90.0 | 4/13 | 30.8 |
| ≥24 months | 8/10 | 80.0 | 1/10 | 10.0 |
| ≥30 months | 6/8 | 75.0 | 1/10 | 10.0 |
| ≥36 months | 4/7 | 57.1 | 0/9 | 0 |
| ≥42 months | 3/6 | 50.0 | 0/9 | 0 |
| ≥48 months | 2/5 | 40.0 | 0/9 | 0 |
As not all men were followed for 48 months, the number of total infections changes over time.
Figure 1. Kaplan-Meier estimates of prevalent and incident oral human papillomavirus (HPV) type 16 persistence in men (n=23).
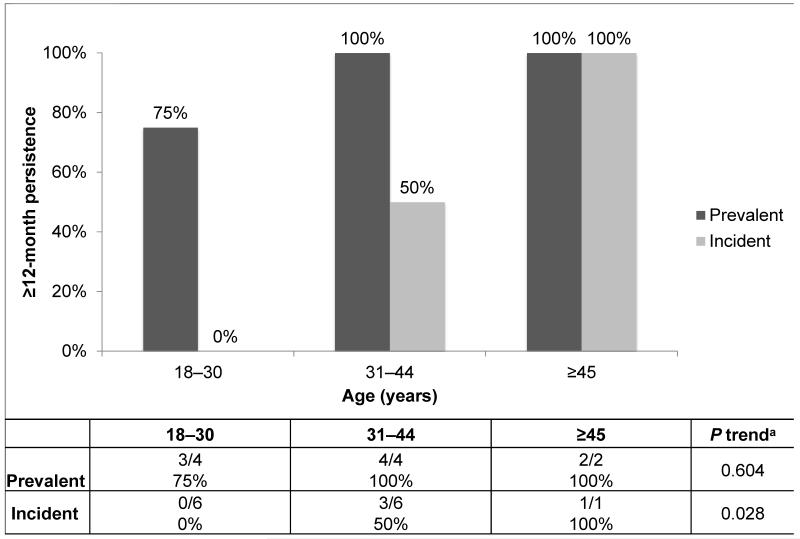
Age appeared to be associated with oral HPV16 persistence. The proportion of incident infections persisting ≥12 months increased significantly with increasing age (P trend=0.028; Figure 2); men ≥45 years of age were more likely to have a persistent oral HPV16 infection (100%) than men aged 31–44 years (50.0%) or those men aged 18–30 years (0%). Though not statistically significant, 12-month persistence of prevalent infections also increased with age (P trend=0.604). No statistically significant age trends were observed in the persistence of incident or prevalent infections at 24, 36, or 48 months (data not shown). Furthermore, no associations were observed between oral HPV16 persistence at 12, 24, 36, or 48 months and cigarette smoking, alcohol intake, country, educational attainment, marital status, sexual orientation, lifetime numbers of sexual partners, or ever/recent oral sexual behaviors (data not shown). In analyses using Cox regression, oral HPV16 persistence was not associated with any sociodemographic or behavioral characteristic, including age (data not shown).
Figure 2. ≥12-month persistence of oral human papillomavirus (HPV) type 16 in men (n=23), by age.
aCochran-Armitage test for trend using exact Monte Carlo methods.
Discussion
In this study, we describe patterns of long-term oral HPV16 persistence among otherwise healthy men. Using two sensitive HPV genotyping assays and analyzing additional specimens from a longer follow-up period allowed us to more accurately estimate long-term persistence compared to our previous studies (9). Our results indicate that prevalent HPV16 infections of the oral cavity are more likely to persist than newly acquired infections, and persistence appears to increase with age among men.
Few studies conducted to date have evaluated oral HPV16 persistence, none of which have reported data separately for prevalent and incident infections over such a long time span and across such a broad age range among otherwise healthy individuals. Furthermore, persistence estimates have varied widely, likely due to differences in definitions of persistence, study populations, study visit intervals, length of follow-up, oral sampling techniques, and HPV DNA detection methods. In a recent study by Mooij et al. conducted among 413 HIV-negative men who have sex with men (MSM), three of nine (33%) prevalent oral HPV16 infections persisted six months (13). In a small study of 59 HIV-negative women, D’Souza et al. determined that three of five (60%) prevalent oral HPV infections persisted six months (14); however, the authors did not provide separate estimates for oral HPV16. As part of the large, prospective Finnish Family HPV (FFHPV) Study, an analysis of oral HPV among 121 male and 216 female spouses observed that 11 of 20 (55%) prevalent high-risk oral HPV infections detected at baseline persisted two years among men, and 15 of 31 (50%) persisted two years among women (15); however, these estimates were likely overestimated, as persistence analyses were not genotype-specific and relied only on prevalently detected infections. In subsequent FFHPV Study analyses, HPV16 was identified as the most frequent type to persist at the oral cavity, with longer persistence observed among men (mean, 22 months) (16) than among women (mean, 19 months) (17), although these analyses combined prevalent and incident HPV infections. In our study of 1626 healthy, adult men, we report higher rates of persistence, with 100% of prevalent oral HPV16 infections persisting ≥6 months and 80% persisting ≥2 years, and 39% of incident infections persisting ≥6 months and 10% persisting ≥2 years.
Existing oral HPV16 infections (i.e., prevalent infections detected at baseline) were more likely to persist than newly acquired infections (i.e., incident infections detected at a follow-up visit), as predicted. Prevalent HPV infections are more likely to be long-term, persistent infections; the longer the duration of an HPV infection, the higher the likelihood of continued persistence, and the higher the probability of detecting HPV DNA at any one time point. In our study, 40% of prevalent oral HPV16 infections persisted beyond four years, whereas none of the incident infections persisted beyond three years. These findings are consistent with the cervical HPV literature, which supports the hypothesis that prevalent high-risk cervical HPV infections are associated with a high likelihood of persistence (18).
Recent studies have sought to understand rates and determinants of oral HPV acquisition and clearance and, in doing so, have raised important questions about the effects of gender and age. In a large, population-based study of oral HPV prevalence (19), men were three times more likely than women to have a prevalent infection, mirroring the gender ratio observed in HPV-positive OPC. In the same study, a significant bimodal age distribution was observed among men, with the peak prevalence occurring among men over 50 years, which is contrary to the established decline in cervical HPV prevalence with age (20). In a previous analysis conducted among men in the HIM Study (9), we showed that the risk of acquiring a new oral HPV infection remained constant across the lifespan, suggesting that the peak in prevalence at older ages is likely due to an increase in infection persistence. Here, we suggest that persistence of incident oral HPV16 infections increases with age among men, a finding consistent with one study conducted among women (14). Altogether, these data support the hypothesis that increased prevalence of oral HPV16 detected among men at older ages is likely due to increased duration of infections and not increased acquisition, a pattern consistent with that of cervical HPV infection persistence (21-23). Similar to factors thought to influence HPV persistence at the cervix, our observation of increased oral HPV persistence with age may be due to impairment of host immune responses (24) and elevation of inflammatory cytokines (25) that occur throughout the aging process. In a recent FFHPV study, Louvanto et al. demonstrated that oral high-risk HPV infections persist three times longer among women with long-term, persistent cervical HPV infections compared with cervical HPV-negative women (26), suggesting that similar factors may be responsible for increased HPV persistence, regardless of epithelial site of infection. Larger cohort studies are needed to elucidate the biological and behavioral mechanisms underlying oral HPV persistence.
The role of tobacco use in oral HPV natural history remains unclear. In our previous analysis, cigarette smokers were significantly more likely to acquire a new oral high-risk HPV infection (9); however, here we report no association between smoking and oral HPV16 persistence. This finding is contrary to two other studies that demonstrated that any (14) and high-risk (16) oral HPV were more likely to persist among current smokers. Smoking is known to cause local inflammation and immunosuppression (27) and is thought to increase susceptibility to a new HPV infection at the oral epithelium. However, smoking has also been found to induce expression of secretory leukocyte protease inhibitor (SLPI) (28), an innate immunity-associated protein that has been hypothesized to protect against HPV infection (29). Future studies are needed to understand the complex role of smoking and SLPI with respect to oral HPV natural history before definitive interpretations are made.
This is one of the first studies to provide detailed estimates of long-term oral HPV16 persistence using data from a large cohort of healthy, adult men. These findings are more likely to represent oral HPV16 natural history than previous reports, due to increased sensitivity of HPV DNA detection and longer follow-up of study participants. However, the following limitations should be considered. First, despite having such a large cohort, we acknowledge that oral HPV16 infection is rare among otherwise healthy men, thus limiting our ability to identify factors significantly associated with infection persistence. However, to our knowledge, the HIM Study is the only existing HPV natural history study with the ability to describe long-term oral HPV persistence in healthy men. Second, our definition of persistence was based on consecutive study visits and assumed that men returned every six months, on schedule. While most men adhered to this protocol, some may have returned for the next visit later than scheduled, increasing the time between visits. As a result, we performed an additional time-to-event analysis based on the Kaplan-Meier method. Third, we may have underestimated persistence among one individual who had a single HPV16-negative result at his final study visit. This individual’s infection was considered cleared in our analysis, although it is possible that this final specimen contained a low viral load that was undetectable by Linear Array. Lastly, intermittent negativity was observed among some HPV16-positive men, which has been described in other studies (30). These negative visits may indicate a low-copy-number infection that fell below the lower limit of assay detection (31); however, we acknowledge that this pattern may also represent reactivation of a latent infection or reinfection after a previously cleared infection. In the current study, we utilized a more sensitive assay, INNO-LiPA, to re-test samples that appeared to have fallen below the lower limit of detection, and indeed found that we would have misclassified several specimens as negative and therefore underestimated our estimates of infection persistence, had we relied on the linear array method alone.
Understanding patterns of oral HPV persistence is essential in the development of HPV-driven OPC prevention and early detection strategies. Long-term persistence of high-risk genital HPV infection is the strongest predictor of cervical precancer and cancer development in women (32). Among all high-risk types, HPV16 has been shown to persist longest and confer the highest risk of developing precancerous lesions. More than 40% of women with cervical HPV16 infections persisting at least one year develop cervical intraepithelial neoplasia grade II (CIN II) or more severe lesions within three years (33). Given that the overwhelming majority of prevalent oral HPV16 infections detected here persisted beyond one year, and 40% persisted beyond four years, there is a clear need to evaluate whether long-term persistent oral HPV16 infection can predict future OPC risk. With a constant rate of acquisition of new oral HPV infections (9) and higher likelihood of persistence with age, mid-adult and older men appear to be at the highest risk of oral HPV infection and should be the focus of prevention interventions.
Acknowledgements
The authors would like to thank the HIM Study participants and teams in the USA (Moffitt Cancer Center, Tampa, FL: JL Messina, C Gage, K Eyring, K Kennedy, K Isaacs, A Bobanic, BA Sirak, MR Papenfuss), Brazil (Centro de Referência e Treinamento em DST/AIDS, Fundação Faculdade de Medicina Instituto do Câncer do Estado de São Paulo, and Ludwig Institute for Cancer Research, São Paulo: ML Baggio, L Galan, RJ Carvalho da Silva, L Sichero, E Gomes, E Brito, F Cernicchiaro, R Cintra, R Cunha, R Matsuo, V Souza, B Fietzek, R Hessel, V Relvas, F Silva, J Antunes, G Ribeiro, R Bocalon, R Otero, R Terreri, S Araujo, M Ishibashi, CRT-DST/AIDS nursing team), and Mexico (Instituto Mexicano del Seguro Social and Instituto Nacional de Salud Pública, Cuernavaca: J Salmerón, M Quiterio Trenado, A Cruz Valdez, R Alvear Vásquez, O Rojas Juárez, R González Sosa, A Salgado Morales, A Rodríguez Galván, P Román Rodríguez, A Landa Vélez, M Zepeda Mendoza, G Díaz García, V Chávez Abarca, J Ruiz Sotelo, A Gutiérrez Luna, M Hernández Nevárez, G Sánchez Martínez, A Ortiz Rojas, C Barrera Flores). The authors also received valuable assistance from the Biostatistics Core and Tissue Core Facilities at the H. Lee Moffitt Cancer Center & Research Institute, an NCI designated Comprehensive Cancer Center, supported under NIH grant P30 CA076292.
Funding
This work was supported by the National Cancer Institute at the National Institutes of Health [R01 CA098803 to ARG], the National Cancer Institute Intramural Program [to ARK], the Investigator-Initiated Studies Program of Merck Sharp & Dohme [to ARG] and the American Cancer Society [PF-13-222-01 – MPC to CMPC].
Financial support: The infrastructure of the HIM Study cohort was supported through a grant from the National Cancer Institute (NCI), National Institutes of Health (NIH), to A Giuliano (R01 CA098803). Funding for a subset of oral sample collection and HPV testing was provided by the NCI Intramural Program to A Kreimer. Additional funding for testing of the remaining oral samples was provided through a research grant from the Investigator-Initiated Studies Program of Merck Sharp & Dohme to A Giuliano. C Pierce Campbell was supported, in part, by a Postdoctoral Fellowship (PF-13-222-01 – MPC) from the American Cancer Society. The contents of the report do not necessarily represent the official views of the NCI or the NIH, and the opinions expressed are those of the authors and do not necessarily represent those of Merck Sharp & Dohme.
ARG receives research funding from Merck Sharp & Dohme Corp. LLV and ARG are consultants of Merck Sharp & Dohme Corp. for human papillomavirus vaccines.
Footnotes
Conflicts of interest: All other authors declare that they have no conflicts of interest.
Presentation at a prior meeting: This manuscript was presented, in part, as an oral presentation during the 2013 EUROGIN Congress in Florence, Italy in November 2013 (OC 15-3).
References
- 1.Chaturvedi AK, Engels EA, Pfeiffer RM, Hernandez BY, Xiao W, Kim E, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29:4294–301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chaturvedi AK, Anderson WF, Lortet-Tieulent J, Curado MP, Ferlay J, Franceschi S, et al. Worldwide trends in incidence rates for oral cavity and oropharyngeal cancers. J Clin Oncol. 2013;31:4550–9. doi: 10.1200/JCO.2013.50.3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kreimer AR, Clifford GM, Boyle P, Franceschi S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev. 2005;14:467–75. doi: 10.1158/1055-9965.EPI-04-0551. [DOI] [PubMed] [Google Scholar]
- 4.Jemal A, Simard EP, Dorell C, Noone AM, Markowitz LE, Kohler B, et al. Annual Report to the Nation on the Status of Cancer, 1975-2009, featuring the burden and trends in human papillomavirus(HPV)-associated cancers and HPV vaccination coverage levels. J Natl Cancer Inst. 2013;105:175–201. doi: 10.1093/jnci/djs491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marur S, D’Souza G, Westra WH, Forastiere AA. HPV-associated head and neck cancer: a virus-related cancer epidemic. Lancet Oncol. 2010;11:781–9. doi: 10.1016/S1470-2045(10)70017-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herrero R, Quint W, Hildesheim A, Gonzalez P, Struijk L, Katki HA, et al. Reduced prevalence of oral human papillomavirus (HPV) 4 years after bivalent HPV vaccination in a randomized clinical trial in Costa Rica. PLoS One. 2013;8:e68329. doi: 10.1371/journal.pone.0068329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kreimer AR. Prospects for prevention of HPV-driven oropharynx cancer. Oral Oncol. 2014;50:555–9. doi: 10.1016/j.oraloncology.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kreimer AR, Villa A, Nyitray AG, Abrahamsen M, Papenfuss M, Smith D, et al. The epidemiology of oral HPV infection among a multinational sample of healthy men. Cancer Epidemiol Biomarkers Prev. 2011;20:172–82. doi: 10.1158/1055-9965.EPI-10-0682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kreimer AR, Pierce Campbell CM, Lin HY, Fulp W, Papenfuss MR, Abrahamsen M, et al. Incidence and clearance of oral human papillomavirus infection in men: the HIM cohort study. Lancet. 2013;382:877–87. doi: 10.1016/S0140-6736(13)60809-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giuliano AR, Lazcano-Ponce E, Villa LL, Flores R, Salmeron J, Lee JH, et al. The human papillomavirus infection in men study: human papillomavirus prevalence and type distribution among men residing in Brazil, Mexico, and the United States. Cancer Epidemiol Biomarkers Prev. 2008;17:2036–43. doi: 10.1158/1055-9965.EPI-08-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giuliano AR, Lee JH, Fulp W, Villa LL, Lazcano E, Papenfuss MR, et al. Incidence and clearance of genital human papillomavirus infection in men (HIM): a cohort study. Lancet. 2011;377:932–40. doi: 10.1016/S0140-6736(10)62342-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bouvard V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, et al. A review of human carcinogens--Part B: biological agents. Lancet Oncol. 2009;10:321–2. doi: 10.1016/s1470-2045(09)70096-8. [DOI] [PubMed] [Google Scholar]
- 13.Mooij SH, Boot HJ, Speksnijder AG, Meijer CJ, King AJ, Verhagen DW, et al. Six-month incidence and persistence of oral HPV infection in HIV-negative and HIV-infected men who have sex with men. PLoS One. 2014;9:e98955. doi: 10.1371/journal.pone.0098955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D’Souza G, Fakhry C, Sugar EA, Seaberg EC, Weber K, Minkoff HL, et al. Six-month natural history of oral versus cervical human papillomavirus infection. Int J Cancer. 2007;121:143–50. doi: 10.1002/ijc.22667. [DOI] [PubMed] [Google Scholar]
- 15.Rintala M, Grenman S, Puranen M, Syrjanen S. Natural history of oral papillomavirus infections in spouses: a prospective Finnish HPV Family Study. J Clin Virol. 2006;35:89–94. doi: 10.1016/j.jcv.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 16.Kero K, Rautava J, Syrjanen K, Willberg J, Grenman S, Syrjanen S. Smoking increases oral HPV persistence among men: 7-year follow-up study. Eur J Clin Microbiol Infect Dis. 2014;33:123–33. doi: 10.1007/s10096-013-1938-1. [DOI] [PubMed] [Google Scholar]
- 17.Rautava J, Willberg J, Louvanto K, Wideman L, Syrjanen K, Grenman S, et al. Prevalence, genotype distribution and persistence of human papillomavirus in oral mucosa of women: a six-year follow-up study. PLoS One. 2012;7:e42171. doi: 10.1371/journal.pone.0042171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodriguez AC, Schiffman M, Herrero R, Hildesheim A, Bratti C, Sherman ME, et al. Longitudinal study of human papillomavirus persistence and cervical intraepithelial neoplasia grade 2/3: critical role of duration of infection. J Natl Cancer Inst. 2010;102:315–24. doi: 10.1093/jnci/djq001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gillison ML, Broutian T, Pickard RK, Tong ZY, Xiao W, Kahle L, et al. Prevalence of oral HPV infection in the United States, 2009-2010. JAMA. 2012;307:693–703. doi: 10.1001/jama.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Sanjose S, Diaz M, Castellsague X, Clifford G, Bruni L, Munoz N, et al. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a meta-analysis. Lancet Infect Dis. 2007;7:453–9. doi: 10.1016/S1473-3099(07)70158-5. [DOI] [PubMed] [Google Scholar]
- 21.Castle PE, Schiffman M, Herrero R, Hildesheim A, Rodriguez AC, Bratti MC, et al. A prospective study of age trends in cervical human papillomavirus acquisition and persistence in Guanacaste, Costa Rica. J Infect Dis. 2005;191:1808–16. doi: 10.1086/428779. [DOI] [PubMed] [Google Scholar]
- 22.Hildesheim A, Schiffman MH, Gravitt PE, Glass AG, Greer CE, Zhang T, et al. Persistence of type-specific human papillomavirus infection among cytologically normal women. J Infect Dis. 1994;169:235–40. doi: 10.1093/infdis/169.2.235. [DOI] [PubMed] [Google Scholar]
- 23.Mollers M, Boot Hein J, Vriend Henrike J, King Audrey J, van den Broek Ingrid VF, van Bergen Jan EA, et al. Prevalence, incidence and persistence of genital HPV infections in a large cohort of sexually active young women in the Netherlands. Vaccine. 2013;31:394–401. doi: 10.1016/j.vaccine.2012.10.087. [DOI] [PubMed] [Google Scholar]
- 24.Garcia-Pineres AJ, Hildesheim A, Herrero R, Trivett M, Williams M, Atmetlla I, et al. Persistent human papillomavirus infection is associated with a generalized decrease in immune responsiveness in older women. Cancer Res. 2006;66:11070–6. doi: 10.1158/0008-5472.CAN-06-2034. [DOI] [PubMed] [Google Scholar]
- 25.Kemp TJ, Hildesheim A, Garcia-Pineres A, Williams MC, Shearer GM, Rodriguez AC, et al. Elevated systemic levels of inflammatory cytokines in older women with persistent cervical human papillomavirus infection. Cancer Epidemiol Biomarkers Prev. 2010;19:1954–9. doi: 10.1158/1055-9965.EPI-10-0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Louvanto K, Rautava J, Syrjanen K, Grenman S, Syrjanen S. The clearance of oral high-risk hpv infection is impaired by long-term persistence of cervical hpv infection. Clin Microbiol Infect. 2014 doi: 10.1111/1469-0691.12700. In press. [DOI] [PubMed] [Google Scholar]
- 27.Arnson Y, Shoenfeld Y, Amital H. Effects of tobacco smoke on immunity, inflammation and autoimmunity. J Autoimmun. 2010;34:J258–65. doi: 10.1016/j.jaut.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 28.Quabius ES, Moller P, Haag J, Pfannenschmidt S, Hedderich J, Gorogh T, et al. The role of the antileukoprotease SLPI in smoking-induced human papillomavirus-independent head and neck squamous cell carcinomas. Int J Cancer. 2014;134:1323–34. doi: 10.1002/ijc.28462. [DOI] [PubMed] [Google Scholar]
- 29.Woodham AW, Da Silva DM, Skeate JG, Raff AB, Ambroso MR, Brand HE, et al. The S100A10 subunit of the annexin A2 heterotetramer facilitates L2-mediated human papillomavirus infection. PLoS One. 2012;7:e43519. doi: 10.1371/journal.pone.0043519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beachler DC, D’Souza G, Sugar EA, Xiao W, Gillison ML. Natural history of anal vs oral HPV infection in HIV-infected men and women. J Infect Dis. 2013;208:330–9. doi: 10.1093/infdis/jit170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fakhry C, Sugar E, D’Souza G, Gillison M. Two-week versus six-month sampling interval in a short-term natural history study of oral HPV infection in an HIV-positive cohort. PLoS One. 2010;5:e11918. doi: 10.1371/journal.pone.0011918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rositch AF, Koshiol J, Hudgens MG, Razzaghi H, Backes DM, Pimenta JM, et al. Patterns of persistent genital human papillomavirus infection among women worldwide: a literature review and meta-analysis. Int J Cancer. 2013;133:1271–85. doi: 10.1002/ijc.27828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Castle PE, Rodriguez AC, Burk RD, Herrero R, Wacholder S, Alfaro M, et al. Short term persistence of human papillomavirus and risk of cervical precancer and cancer: population based cohort study. BMJ. 2009;339:b2569. doi: 10.1136/bmj.b2569. [DOI] [PMC free article] [PubMed] [Google Scholar]