Abstract
Objectives
Brachytherapy plays an important role in the treatment of cervical cancer. While small trials have shown comparable survival outcomes between high (HDR) and low-dose rate (LDR) brachytherapy, little data is available in the US. We examined the utilization of HDR brachytherapy and analyzed the impact of type of brachytherapy on survival for cervical cancer.
Methods
Women with stage IB2–IVA cervical cancer treated with primary (external beam and brachytherapy) radiotherapy between 2003–2011 and recorded in the National Cancer Database (NCDB) were analyzed. Generalized linear mixed models and Cox proportional hazards regression were used to examine predictors of HDR brachytherapy use and the association between HDR use and survival.
Results
A total of 10,564 women including 2681 (25.4%) who received LDR and 7883 (74.6%) that received HDR were identified. Use of HDR increased from 50.2% in 2003 to 83.9% in 2011 (P<0.0001). In a multivariable model, year of diagnosis was the strongest predictor of use of HDR. While patients in the Northeast were more likely to receive HDR therapy, there were no other clinical or socioeconomic characteristics associated with receipt of HDR. In a multivariable Cox model, survival was similar between the HDR and LDR groups (HR=0.93; 95% 0.83–1.03). Similar findings were noted in analyses stratified by stage and histology. Kaplan-Meier analyses demonstrated no difference in survival based on type of brachytherapy for stage IIB (P=0.68), IIIB (P=0.17), or IVA (P=0.16) tumors.
Conclusions
The use of HDR therapy has increased rapidly. Overall survival is similar for LDR and HDR brachytherapy.
Introduction
Radiation therapy has long been the mainstay of treatment for advanced stage cervical cancer. Radiation is delivered in the form of external beam therapy in combination with intracavitary brachytherapy. Brachytherapy allows dose escalation to the cervix and surrounding tissues and is critical in improving local control and decreasing the risk of pelvic relapse.1,2
Intracavitary radiation typically relies on low-dose rate (LDR) brachytherapy which delivers radiation at a dose of 0.4–2 Gray (Gy)/hour.3 The radiation sources are loaded into an intrauterine tandem and vaginal ovoid delivery system that is placed while the patient is under anesthesia in the operating room. Patients are typically hospitalized after placement of the applicator for 24–72 hours to allow radiation delivery of LDR treatments. The source positions and dosing of LDR brachytherapy have been well defined for several decades.
More recently, high-dose rate (HDR) brachytherapy has been explored for the treatment of cervical cancer. HDR brachytherapy delivers a dose >12 Gy/hour, and is typically delivered in the outpatient setting through multiple applicator placements that are left in place for a short duration. Advantages of HDR brachytherapy include greater patient convenience and ease of administration, as well as the ability to optimize dosing to normal tissues.
Outcomes after LDR and HDR brachytherapy have been compared in a number of retrospective, institutional studies as well as in four prospective randomized trials.2,4–11 The randomized trials noted similar survival outcomes for the two brachytherapy techniques, however, all four studies were conducted outside of the U.S. and have been criticized for a number of methodologic limitations including the inclusion of diverse patient populations and the utilization of a variety of different radiation techniques.2,7 Given the limited data describing the safety and use of HDR brachytherapy in the U.S., we performed a population-based analysis to examine the patterns of brachytherapy use and outcomes for women with cervical cancer undergoing primary radiation therapy.
Materials and Methods
Data Source and Patient Selection
The National Cancer Data Base (NCDB) was used for analysis. NCDB is a nationwide registry developed and sponsored by the American College of Surgeons and American Cancer Society.12,13 The database records all patients with newly diagnosed invasive cancers from over 1500 Commission on Cancer (CoC) affiliated hospitals located throughout the United States. The NCDB catalogs data on patient demographic factors, tumor characteristics and treatment data, staging, and survival.12,13 Data are abstracted by trained registrars and is audited regularly to ensure accuracy. It is estimated that nearly 78% of women with invasive cervical cancer in the U.S. are recorded in NCDB.14 The Columbia University Institutional Review Board deemed the study exempt.
Women with stage IB2–IVA cervical cancer diagnosed from 2003–2011 were included. Only patients who underwent primary radiotherapy with combination external beam radiation and intracavitary brachytherapy were included in the analysis. Further, the cohort was limited to those women with specific documentation of receipt of either LDR or HDR brachytherapy. NCDB only reports survival data on patients with at least five years of follow-up. Therefore, all survival analyses were limited to patients treated from 2003–2006.
Clinical and Demographic Characteristics
Demographic data analyzed included age (<40, 40–49, 50–59, 60–69, ≥70 years), race (white, black, Hispanic, other or unknown), income (median household income in a patient’s zip code), education (percentage of adults in a patient’s zip code that did not graduate high school; <14%, 14–19.9%, 20–28.9%, ≥29%, unknown) and insurance status (commercial, Medicare, Medicaid, uninsured and unknown. Comorbidity was measured using the Deyo classification of the Charlson comorbidity score (0, 1, ≥2).15,16 Tumor stage (stages IB2–IVA) and grade (1, 2, 3, unknown) were noted for each patient. Tumor histology was classified as squamous, adenosquamous, adenocarcinoma and other.
Hospital characteristics analyzed included region (Northeast, Midwest, South, or West) and location (metropolitan, urban, rural). Based on the ACS CoC criteria, hospitals are also classified as academic/research cancer centers or community cancer centers.13 Hospital volume was estimated as annualized volume. We calculated the total number of patients treated at a given hospital divided by the number of years in which a given hospital treated at least one patient.17,18 Patients were then stratified into four approximately equal volume quartiles: lowest (<2 cases/year), second (2.00–3.25 cases/year), third (3.26–5.37 cases per year), and highest (≥5.37 cases/year).
Treatment quality was captured through measurement of use of chemotherapy (yes, no and unknown) and through duration of radiation therapy.19 Radiation therapy encompassed prior radiation treatment and was grouped as: <6 weeks, 6–10 weeks, 10 weeks-6 months, >6 months, and unknown.
Statistical Analysis
Frequency distributions between categorical variables were compared using χ2 tests and trends analyzed using Mantel-Haenszel tests. The association between the clinical and demographic characteristics and use of HDR brachytherapy was examined using multivariable mixed effects log-linear regression models. To account for hospital-level clustering, these models included a random-intercept for the hospital in which the radiation was administered. The models included all clinically relevant demographic, clinical, and oncologic variables. Results are reported as risk ratios (RR) with 95% confidence intervals (CI).
Overall survival was estimated as the number of months from diagnosis until death from any causes. Patients who were alive at last follow-up were censored. Stage-specific Kaplan-Meier curves were developed to compare survival between women who received LDR and HDR brachytherapy. The log-rank test was used to compare the curves. Marginal Cox proportional hazards models were developed to evaluate the association between type of brachytherapy and survival while adjusting for other clinical and demographic characteristics and accounting for hospital level clustering.
A number of sensitivity analyses were performed. First, separate survival analyses were performed stratified by histology (either squamous cell carcinomas or adenocarcinomas). Second, stage-specific Cox models were developed in which the analyses were limited to patients with either stage II or stage III neoplasms. All hypothesis tests were two-sided. A P-value of <0.05 was considered statistically significant. All analyses were conducted using SAS version 9.4 (SAS Institute Inc, Cary, North Carolina).
Results
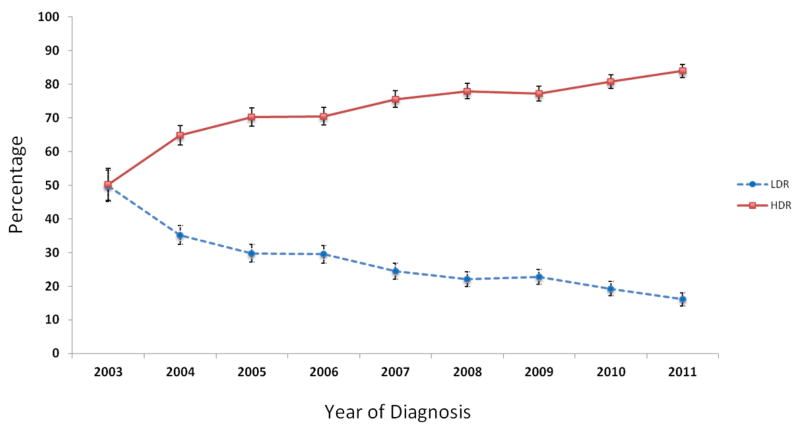
A total of 10,564 women with locally advanced cervical cancer treated at 960 hospitals were identified. The cohort included 2681 (25.4%) who received LDR brachytherapy and 7883 (74.6%) who received HDR brachytherapy. The use of HDR brachytherapy increased from 50.2% in 2003 to 83.9% in 2011 (P<0.0001) (Figure 1).
Figure 1.
Trends in use of low-dose rate (LDR) and high-dose rate (HDR) brachytherapy from 2003–2011.
Table 1 displays the clinical and demographic characteristics of the cohort. In the unadjusted analysis, HDR brachytherapy was more commonly utilized in more recent years (P<0.0001), in white women (P<0.0001), in those with higher median zip code income (P<0.0001), and in patients with commercial insurance (P=0.0004). HDR brachytherapy use was also associated with treatment in the Northeast (P<0.0001), treatment at a comprehensive community cancer program (P<0.0001) and with treatment at higher volume hospitals (P<0.0001). There was no statistically significant association between tumor histology, grade, or stage and type of brachytherapy utilized.
Table 1.
Clinical and demographic characteristics of the cohort stratified by type of brachytherapy utilized.
| LDR | HDR | P-value | |||
|---|---|---|---|---|---|
|
| |||||
| N | (%) | N | (%) | ||
| 2681 | (25.4) | 7883 | (74.6) | ||
| Year of diagnosis | <0.0001 | ||||
| 2003 | 218 | (8.1) | 220 | (2.8) | |
| 2004 | 378 | (14.1) | 696 | (8.8) | |
| 2005 | 341 | (12.7) | 805 | (10.2) | |
| 2006 | 347 | (12.9) | 829 | (10.5) | |
| 2007 | 296 | (11.0) | 917 | (11.6) | |
| 2008 | 289 | (10.8) | 1023 | (13.0) | |
| 2009 | 312 | (11.6) | 1058 | (13.4) | |
| 2010 | 267 | (10.0) | 1119 | (14.2) | |
| 2011 | 233 | (8.7) | 1216 | (15.4) | |
| Age | 0.48 | ||||
| <40 | 467 | (17.4) | 1389 | (17.6) | |
| 40–49 | 755 | (28.2) | 2086 | (26.5) | |
| 50–59 | 682 | (25.4) | 2026 | (25.7) | |
| 60–69 | 426 | (15.9) | 1328 | (16.9) | |
| ≥70 | 351 | (13.1) | 1054 | (13.4) | |
| Race | <0.0001 | ||||
| White | 1688 | (63.0) | 5111 | (64.8) | |
| Black | 575 | (21.5) | 1369 | (17.4) | |
| Hispanic | 299 | (11.2) | 918 | (11.7) | |
| Other | 97 | (3.6) | 397 | (5.0) | |
| Unknown | 22 | (0.8) | 88 | (1.1) | |
| Zip code income | <0.0001 | ||||
| <$30,000 | 592 | (22.1) | 1566 | (19.9) | |
| $30,000–$34,999 | 595 | (22.2) | 1574 | (20.0) | |
| $35,000–$45,999 | 742 | (27.7) | 2176 | (27.6) | |
| ≥$46,000 | 617 | (23.0) | 2153 | (27.3) | |
| Unknown | 135 | (5.0) | 414 | (5.3) | |
| Education | 0.006 | ||||
| <14% | 537 | (20.0) | 1685 | (21.4) | |
| 14–19.9% | 546 | (20.4) | 1767 | (22.4) | |
| 20–28.9% | 721 | (26.9) | 2093 | (26.6) | |
| ≥29% | 742 | (27.7) | 1923 | (24.4) | |
| Unknown | 135 | (5.0) | 415 | (5.3) | |
| Insurance status | 0.0004 | ||||
| Commercial | 1033 | (38.5) | 3304 | (41.9) | |
| Medicare | 548 | (20.4) | 1641 | (20.8) | |
| Medicaid | 660 | (24.6) | 1878 | (23.8) | |
| Uninsured | 364 | (13.6) | 831 | (10.5) | |
| Other | 27 | (1.0) | 87 | (1.1) | |
| Unknown | 49 | (1.8) | 142 | (1.8) | |
| Comorbidity | 0.31 | ||||
| 0 | 2301 | (85.8) | 6857 | (87.0) | |
| 1 | 307 | (11.5) | 834 | (10.6) | |
| ≥2 | 73 | (2.7) | 192 | (2.4) | |
| Region | <0.0001 | ||||
| Northeast | 457 | (17.1) | 1834 | (23.3) | |
| Midwest | 881 | (32.9) | 1973 | (25.0) | |
| South | 1183 | (44.1) | 2852 | (36.2) | |
| West | 160 | (6.0) | 1224 | (15.5) | |
| Metropolitan location | 0.10 | ||||
| Metropolitan | 2028 | (75.6) | 5993 | (76.0) | |
| Urban | 453 | (16.9) | 1308 | (16.6) | |
| Rural | 66 | (2.5) | 140 | (1.8) | |
| Unknown | 134 | (5.0) | 442 | (5.6) | |
| Hospital type | <0.0001 | ||||
| Community cancer program | 231 | (8.6) | 415 | (5.3) | |
| Comprehensive community cancer program | 1214 | (45.3) | 3891 | (49.4) | |
| Academic | 1231 | (45.9) | 3546 | (45.0) | |
| Other | 5 | (0.2) | 31 | (0.4) | |
| Hospital volume | <0.0001 | ||||
| Lowest | 876 | (32.7) | 2077 | (26.4) | |
| Second | 632 | (23.6) | 1740 | (22.1) | |
| Third | 603 | (22.5) | 2080 | (26.4) | |
| Highest | 570 | (21.3) | 1986 | (25.2) | |
| Histology | 0.18 | ||||
| Squamous | 2206 | (82.3) | 6345 | (80.5) | |
| Adenocarcinoma | 226 | (8.4) | 709 | (9.0) | |
| Adenosquamous | 60 | (2.2) | 218 | (2.8) | |
| Other | 189 | (7.1) | 611 | (7.8) | |
| Grade | 0.32 | ||||
| 1 | 121 | (4.5) | 340 | (4.3) | |
| 2 | 852 | (31.8) | 2398 | (30.4) | |
| 3 | 848 | (31.6) | 2640 | (33.5) | |
| Unknown | 860 | (32.1) | 2505 | (31.8) | |
| Stage | 0.06 | ||||
| IB2 | 243 | (9.1) | 803 | (10.2) | |
| IIA | 31 | (1.2) | 118 | (1.5) | |
| IIB | 940 | (35.1) | 2752 | (34.9) | |
| IINOS | 281 | (10.5) | 670 | (8.5) | |
| IIIA | 67 | (2.5) | 210 | (2.6) | |
| IIIB | 1006 | (37.5) | 2996 | (38.0) | |
| IIINOS | 30 | (1.1) | 99 | (1.3) | |
| IVA | 83 | (3.1) | 235 | (3.0) | |
| Chemotherapy | 0.30 | ||||
| No | 278 | (10.4) | 811 | (10.3) | |
| Yes | 2387 | (89.0) | 7000 | (88.8) | |
| Unknown | 16 | (0.6) | 72 | (0.9) | |
| Radiation duration | <0.0001 | ||||
| <6 weeks | 633 | (23.6) | 1498 | (19.0) | |
| 6–10 weeks | 1433 | (53.4) | 4327 | (54.9) | |
| 10 weeks-6 months | 511 | (19.1) | 1779 | (22.6) | |
| >6 months | 13 | (0.5) | 33 | (0.4) | |
| Unknown | 91 | (3.4) | 246 | (3.1) | |
In a multivariable model of factors associated with use of HDR brachytherapy, year of diagnosis was significantly associated with receipt of HDR treatment (Table 2). Compared to 2003, the risk ratios for receipt of HDR treatment increased each year from 1.23 (95% CI, 1.05–1.43) in 2004 to 1.61 (95% CI, 1.39–1.87) in 2011. The only other factor associated with use of HDR therapy was area of residence; compared to patients treated in the Northeast, residents in the Midwest (RR=0.87; 95% CI, 0.79–0.96) and South (RR=0.88; 95% CI, 0.80–0.97) were less likely to receive HDR therapy. There were no statistically significant associations between any of the clinical or demographic characteristics and receipt of HDR brachytherapy.
Table 2.
Multivariable model of predictors of HDR brachytherapy use.
| HDR | |
|---|---|
| Year of diagnosis | |
| 2003 | Referent |
| 2004 | 1.23 (1.05–1.43)* |
| 2005 | 1.34 (1.15–1.56)* |
| 2006 | 1.34 (1.15–1.56)* |
| 2007 | 1.42 (1.22–1.65)** |
| 2008 | 1.47 (1.26–1.70)** |
| 2009 | 1.47 (1.27–1.71)** |
| 2010 | 1.55 (1.34–1.80)** |
| 2011 | 1.61 (1.39–1.87)** |
| Age | |
| <40 | Referent |
| 40–49 | 1.02 (0.95–1.08) |
| 50–59 | 1.02 (0.94–1.10) |
| 60–69 | 1.01 (0.93–1.08) |
| ≥70 | 1.02 (0.92–1.12) |
| Race | |
| White | Referent |
| Black | 0.98 (0.87–1.11) |
| Hispanic | 0.99 (0.87–1.12) |
| Other | 1.00 (0.78–1.27) |
| Unknown | 1.00 (0.90–1.12) |
| Zip code income | |
| <$30,000 | Referent |
| $30,000–$34,999 | 1.01 (0.94–1.09) |
| $35,000–$45,999 | 1.03 (0.94–1.12) |
| ≥$46,000 | 1.03 (0.95–1.11) |
| Unknown | 0.98 (0.13–7.28) |
| Education | |
| <14% | Referent |
| 14–19.9% | 1.00 (0.93–1.07) |
| 20–28.9% | 0.98 (0.91–1.06) |
| ≥29% | 0.98 (0.90–1.07) |
| Unknown | 1.05 (0.14–7.73) |
| Insurance status | |
| Commercial | Referent |
| Medicare | 0.97 (0.89–1.06) |
| Medicaid | 1.01 (0.93–1.10) |
| Uninsured | 1.03 (0.86–1.23) |
| Other | 0.99 (0.79–1.23) |
| Unknown | 1.02 (0.96–1.09) |
| Comorbidity | |
| 0 | Referent |
| 1 | 0.98 (0.91–1.05) |
| ≥2 | 0.96 (0.83–1.11) |
| Region | |
| Northeast | Referent |
| Midwest | 0.87 (0.79–0.96)* |
| South | 0.88 (0.80–0.97)* |
| West | 1.12 (1.00–1.23) |
| Metropolitan location | |
| Metropolitan | Referent |
| Urban | 0.98 (0.82–1.17) |
| Rural | 0.98 (0.84–1.14) |
| Unknown | 1.02 (0.95–1.09) |
| Hospital type | |
| Academic | Referent |
| Comprehensive community cancer program | 1.02 (0.89–1.17) |
| Community cancer program | 1.08 (0.96–1.23) |
| Other | 1.30 (0.87–1.95) |
| Hospital volume | |
| Lowest | Referent |
| Second | 0.98 (0.89–1.07) |
| Third | 1.06 (0.96–1.17) |
| Highest | 1.05 (0.93–1.20) |
| Histology | |
| Squamous | Referent |
| Adenocarcinoma | 1.04 (0.89–1.21) |
| Adenosquamous | 1.01 (0.91–1.13) |
| Other | 1.01 (0.93–1.09) |
| Grade | |
| 1 | Referent |
| 2 | 0.99 (0.88–1.11) |
| 3 | 1.01 (0.90–1.13) |
| Unknown | 1.00 (0.89–1.12) |
| Stage | |
| IB2 | Referent |
| IIA | 0.97 (0.80–1.19) |
| IIB | 0.99 (0.91–1.07) |
| IINOS | 0.95 (0.85–1.05) |
| IIIA | 1.01 (0.87–1.19) |
| IIIB | 0.98 (0.90–1.06) |
| IIINOS | 1.03 (0.83–1.28) |
| IVA | 0.96 (0.83–1.12) |
| Chemotherapy | |
| No | Referent |
| Yes | 1.02 (0.94–1.10) |
| Unknown | 1.09 (0.86–1.38) |
| Radiation duration | |
| 6–10 weeks | Referent |
| <6 weeks | 0.97 (0.91–1.02) |
| 10 weeks-6 months | 0.93 (0.87–1.00) |
| >6 months | 0.95 (0.67–1.35) |
| Unknown | 0.95 (0.83–1.10) |
P<0.05,
P<0.0001
Within the survival cohort, the median follow-up was 46.42 months (IQR, 18.91–75.60 months) for women who received LDR brachytherapy and 47.86 months (IQR, 19.19–74.22 months) in those who received HDR treatment. In a multivariable Cox proportional hazards model there was no association between HDR brachytherapy use and survival (HR=0.93; 95% CI, 0.83–1.03) (Table 3). Age, race, insurance status, comorbidity, histology, stage, duration of radiation therapy and use of chemotherapy were all associated with survival.
Table 3.
Cox proportional hazards models of death.
| All patients (n=3834) | Stage II (n=1914) | Stage III (n=1467) | |
|---|---|---|---|
| Brachytherapy | |||
| LDR | Referent | Referent | Referent |
| HDR | 0.93 (0.83–1.03) | 0.98 (0.83–1.16) | 0.91 (0.78–1.07) |
| Year of diagnosis | |||
| 2003 | Referent | Referent | Referent |
| 2004 | 0.94 (0.80–1.11) | 0.87 (0.69–1.10) | 0.94 (0.73–1.22) |
| 2005 | 0.97 (0.83–1.15) | 1.08 (0.84–1.38) | 0.88 (0.68–1.12) |
| 2006 | 0.94 (0.80–1.11) | 0.87 (0.69–1.11) | 0.92 (0.71–1.18) |
| Age | |||
| <40 | Referent | Referent | Referent |
| 40–49 | 1.07 (0.91–1.26) | 1.34 (1.05–1.70)* | 0.85 (0.66–1.10) |
| 50–59 | 1.06 (0.89–1.25) | 0.98 (0.75–1.29) | 1.00 (0.78–1.27) |
| 60–69 | 1.14 (0.94–1.37) | 1.19 (0.89–1.58) | 1.10 (0.83–1.47) |
| ≥70 | 1.48 (1.19–1.83)* | 1.49 (1.11–2.00)* | 1.34 (0.98–1.84) |
| Race | |||
| White | Referent | Referent | Referent |
| Black | 1.03 (0.91–1.18) | 0.90 (0.73–1.12) | 1.10 (0.90–1.34) |
| Hispanic | 0.75 (0.63–0.90)* | 0.56 (0.42–0.75)* | 0.90 (0.70–1.16) |
| Other | 0.70 (0.53–0.93)* | 0.55 (0.36–0.84)* | 0.72 (0.47–1.10) |
| Unknown | 1.05 (0.70–1.58) | 1.06 (0.58–1.95) | 1.19 (0.65–2.17) |
| Zip code income | |||
| <$30,000 | Referent | Referent | Referent |
| $30,000–$34,999 | 0.87 (0.74–1.03) | 0.81 (0.63–1.04) | 0.92 (0.70–1.21) |
| $35,000–$45,999 | 0.88 (0.74–1.04) | 0.86 (0.68–1.08) | 0.98 (0.75–1.30) |
| ≥$46,000 | 0.83 (0.67–1.02) | 0.83 (0.60–1.13) | 0.86 (0.63–1.17) |
| Unknown | 0.83 (0.58–1.20) | 0.93 (0.51–1.67) | 0.91 (0.54–1.53) |
| Education | |||
| <14% | Referent | Referent | Referent |
| 14–19.9% | 0.95 (0.79–1.13) | 0.93 (0.72–1.21) | 0.98 (0.77–1.24) |
| 20–28.9% | 0.98 (0.82–1.17) | 1.05 (0.80–1.36) | 0.90 (0.70–1.15) |
| ≥29% | 0.92 (0.75–1.14) | 0.86 (0.64–1.15) | 1.01 (0.75–1.36) |
| Unknown | NA | NA | NA |
| Insurance status | |||
| Commercial | Referent | Referent | Referent |
| Medicare | 1.24 (1.06–1.46)* | 1.63 (1.30–2.06)** | 0.97 (0.77–1.22) |
| Medicaid | 1.19 (1.04–1.35)* | 1.39 (1.12–1.72)* | 0.98 (0.81–1.19) |
| Uninsured | 0.97 (0.81–1.16) | 0.97 (0.72–1.31) | 0.97 (0.75–1.25) |
| Other | 1.26 (0.77–2.04) | 1.11 (0.54–2.28) | 1.08 (0.53–2.21) |
| Unknown | 1.18 (0.86–1.62) | 1.76 (1.11–2.80)* | 0.97 (0.62–1.53) |
| Comorbidity | |||
| 0 | Referent | Referent | Referent |
| 1 | 1.25 (1.08–1.46)* | 1.01 (0.80–1.28) | 1.38 (1.13–1.69)* |
| ≥2 | 1.75 (1.31–2.33)** | 2.78 (1.74–4.45)** | 1.17 (0.82–1.68) |
| Region | |||
| Northeast | Referent | Referent | Referent |
| Midwest | 1.02 (0.89–1.16) | 1.07 (0.87–1.31) | 0.96 (0.79–1.16) |
| South | 1.04 (0.91–1.19) | 1.16 (0.94–1.43) | 1.02 (0.85–1.22) |
| West | 0.98 (0.81–1.18) | 1.32 (1.01–1.72)* | 0.90 (0.71–1.13) |
| Metropolitan location | |||
| Metropolitan | Referent | Referent | Referent |
| Urban | 1.08 (0.95–1.22) | 1.09 (0.89–1.33) | 1.09 (0.90–1.32) |
| Rural | 0.88 (0.65–1.20) | 0.69 (0.42–1.12) | 0.82 (0.50–1.34) |
| Unknown | 0.85 (0.62–1.17) | 0.58 (0.33–1.00) | 0.98 (0.65–1.47) |
| Hospital type | |||
| Academic | Referent | Referent | Referent |
| Community cancer program | 1.23 (1.00–1.51) | 1.33 (0.98–1.81) | 1.20 (0.85–1.71) |
| Comprehensive community cancer program | 1.12 (1.00–1.25) | 1.03 (0.87–1.21) | 1.20 (1.03–1.38)* |
| Other | 1.50 (0.61–3.73) | 1.73 (0.83–3.60) | 0.58 (0.05–6.11) |
| Hospital volume | |||
| Lowest | Referent | Referent | Referent |
| Second | 1.13 (0.98–1.30) | 1.13 (0.92–1.39) | 1.10 (0.89–1.35) |
| Third | 0.98 (0.85–1.14) | 0.92 (0.74–1.15) | 0.95 (0.77–1.16) |
| Highest | 1.12 (0.97–1.28) | 0.99 (0.80–1.23) | 1.18 (0.98–1.44) |
| Histology | |||
| Squamous | Referent | Referent | Referent |
| Adenocarcinoma | 1.24 (1.05–1.45)* | 1.20 (0.93–1.56) | 1.43 (1.11–1.85)* |
| Adenosquamous | 1.30 (0.96–1.76) | 1.05 (0.63–1.73) | 1.47 (0.93–2.32) |
| Other | 1.27 (1.06–1.54)* | 1.37 (1.00–1.86)* | 1.19 (0.92–1.53) |
| Grade | |||
| 1 | Referent | Referent | Referent |
| 2 | 1.12 (0.88–1.42) | 1.20 (0.80–1.80) | 0.93 (0.69–1.27) |
| 3 | 1.29 (1.01–1.64) | 1.44 (0.96–2.15) | 1.13 (0.82–1.56) |
| Unknown | 1.06 (0.83–1.34) | 1.11 (0.74–1.65) | 0.94 (0.69–1.28) |
| Stage | |||
| IB2 | Referent | - | - |
| IIA | 1.36 (0.60–3.11) | Referent | - |
| IIB | 1.38 (1.12–1.70)* | 0.94 (0.42–2.09) | - |
| IINOS | 1.30 (1.01–1.68)* | 0.86 (0.39–1.91) | - |
| IIIA | 2.22 (1.60–3.09)** | - | Referent |
| IIIB | 2.47 (2.02–3.01)** | - | 1.04 (0.78–1.39) |
| III NOS | 1.97 (1.27–3.04)* | - | 0.84 (0.51–1.36) |
| IVA | 3.52 (2.63–4.72)** | - | - |
| Chemotherapy | |||
| No | Referent | Referent | Referent |
| Yes | 0.71 (0.61–0.82)** | 0.71 (0.59–0.86)* | 0.78 (0.61–0.99)* |
| Unknown | 0.50 (0.25–0.98)** | 0.62 (0.27–1.41) | 0.51 (0.21–1.23) |
| Radiation duration | |||
| 6–10 weeks | Referent | Referent | Referent |
| <6 weeks | 1.05 (0.94–1.18) | 1.02 (0.85–1.22) | 1.16 (0.97–1.38) |
| 10 weeks-6 months | 1.16 (1.02–1.32)* | 1.33 (1.09–1.62)* | 1.04 (0.88–1.23) |
| >6 months | 1.47 (0.81–2.67) | 2.49 (1.02–6.09)* | 1.01 (0.37–2.76) |
| Unknown | 1.03 (0.81–1.32) | 0.98 (0.63–1.51) | 0.84 (0.53–1.35) |
P<0.05,
P<0.0001
When stratified by stage, use of HDR brachytherapy was not associated with survival for either stage II (HR=0.98; 95% CI, 0.83–1.16) or III (HR=0.91; 95% CI, 0.78–1.07) neoplasms (Table 3). Likewise, when the analysis was limited only to women with stage IIIB cancers, there was no association between use of HDR brachytherapy and survival (HR=0.94; 95% CI, 0.79–1.11) (Supplemental Table). Similarly, when stratified by histology, HDR brachytherapy was not associated with survival for either squamous cell carcinomas (HR=0.95; 95% CI, 0.84–1.06) or adenocarcinomas (HR=0.77; 95% CI, 0.50–1.18).
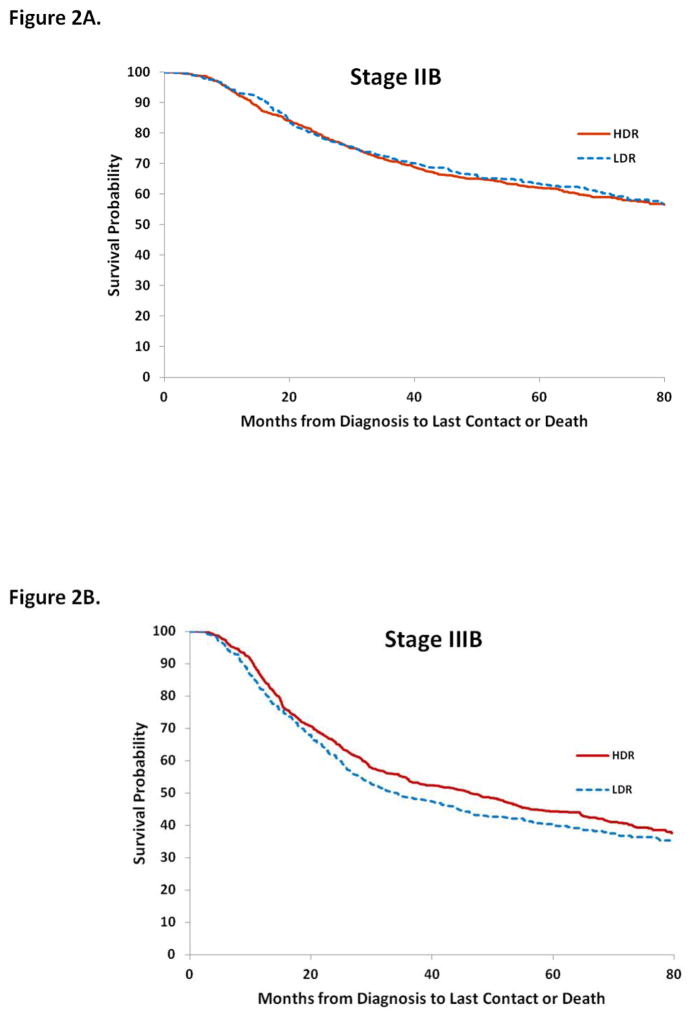
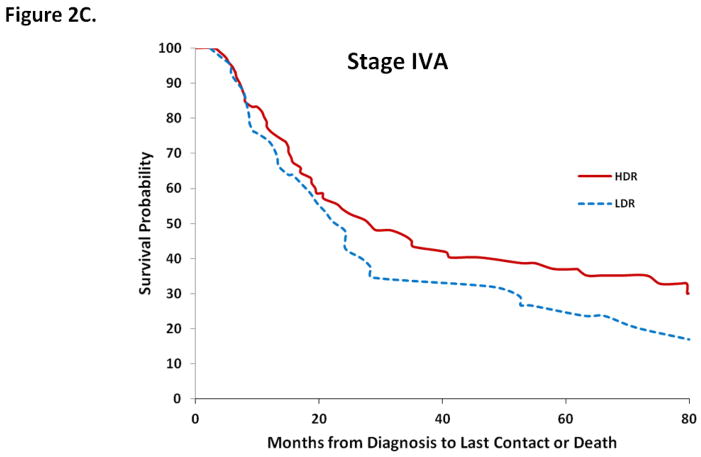
Overall survival at 1, 3, and 5-years for LDR and HDR brachytherapy were 88.0% (95% CI, 86.2–89.8%) vs. 89.9% (95% CI, 88.7–91.0%); 62.1% (95% CI, 59.4–64.8%) vs. 65.4% (95% CI, 63.5–67.3%); and 53.0% (95% CI, 50.2–55.9%) vs. 55.8% (95% CI, 53.8–57.9%), respectively (Table 4). Across all stages, 1, 3, and 5-year survival estimates overlapped for the two types of brachytherapy. Similar findings were seen in a series of Kaplan-Meier analyses that were stratified by stage. Type of brachytherapy was not associated with survival for women with stage IIB (P=0.68), IIIB (P=0.17), or stage IVA (P=0.16) tumors (Figure 2).
Table 4.
Survival by type of brachytherapy utilized.
| LDR | HDR | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Survival probability | 95%CI
|
Survival probability | 95%CI
|
|||
| Lower | Upper | Lower | Upper | |||
| All patients | ||||||
| 1-year | 88.0 | 86.2 | 89.8 | 89.9 | 88.7 | 91.0 |
| 3-year | 62.1 | 59.4 | 64.8 | 65.4 | 63.5 | 67.3 |
| 5-year | 53.0 | 50.2 | 55.9 | 55.8 | 53.8 | 57.9 |
| Stage IIB | ||||||
| 1-year | 93.2 | 91.0 | 95.5 | 92.4 | 90.7 | 94.1 |
| 3-year | 72.1 | 68.0 | 76.1 | 71.1 | 68.2 | 74.0 |
| 5-year | 63.4 | 59.0 | 67.9 | 62.2 | 59.0 | 65.3 |
| Stage IIIB | ||||||
| 1-year | 82.1 | 78.5 | 85.8 | 85.7 | 83.4 | 88.1 |
| 3-year | 48.6 | 43.7 | 53.5 | 54.3 | 50.9 | 57.6 |
| 5-year | 40.0 | 35.2 | 44.8 | 44.2 | 40.8 | 47.7 |
| Stage IVA | ||||||
| 1-year | 71.3 | 57.5 | 85.0 | 77.5 | 67.8 | 87.2 |
| 3-year | 32.0 | 17.2 | 46.7 | 43.4 | 31.6 | 55.2 |
| 5-year | 23.8 | 10.1 | 37.3 | 37.0 | 25.4 | 48.6 |
Figure 2.
Kaplan-Meier analysis of survival for cervical cancer stratified by stage and type of intracavitary therapy (LDR or HDR) administered. 2A. Stage IIB. 2B. Stage IIIB. 2C. Stage IVA.
Discussion
These data suggest that the use of HDR brachytherapy has increased rapidly in the United States. In 2011, nearly 84% of women with newly diagnosed cervical cancer who received primary radiotherapy were treated with HDR therapy. Importantly, survival is similar for women with stage IB2–IVA cervical cancer treated with LDR and HDR brachytherapy.
Four randomized trials of LDR vs. HDR brachytherapy for cervical cancer have demonstrated equivalent survival outcomes.5,6,8,10 A recent meta-analysis that pooled data from these studies and included 1265 women, found no difference in 3 (70% vs. 66%), 5 (60% vs. 55%), or 10-year (53% vs. 44%) overall survival between LDR and HDR therapy, respectively.7 Similarly, the analysis found no differences in disease specific survival, relapse-free survival, local control, or patterns of failure between the two treatment modalities.7 While the quality of evidence from these trials was judged to be low to moderate, observational data in general has also reported equivalent survival outcomes for the two types of intracavitary systems.2,4,7,9,11,20–24 Our population-based data is in accord with these findings; overall, we noted similar survival outcomes for women treated with LDR and HDR brachytherapy.
The most controversial area for the use of HDR brachytherapy has been for women with bulky stage III tumors. One report of 124 women with stage III tumors noted that 5-year survival was lower for women treated with HDR compared to LDR brachytherapy (36% vs. 46%).4 While a second study noted similar findings (survival rate 58% for LDR vs. 33% for HDR), one study found that survival for women with stage III tumors was actually better in those treated with HDR intracavitary therapy (3-year survival 37% for LDR vs. 54% for HDR).9,11 While the etiology of discrepancy in outcomes is unclear, it has been suggested that early initiation of HDR therapy may provide inadequate coverage of the outer margins of tumors, highlighting the need for 3D imaging and careful treatment planning. In our stratified analyses, we noted no difference in survival when patients with stage III tumors or specifically stage IIIB neoplasms were analyzed separately.
Prior work has suggested that patterns of use of brachytherapy are highly variable in the United States.25–27 A survey undertaken by the American Brachytherapy Society in the late 1990’s found that 40% of the providers did not utilize brachytherapy in the treatment of cervical cancer. Of those who included brachytherapy in their treatment plans, 24% utilized HDR therapy.25 A second patterns of care study found that the use of HDR brachytherapy increased from 9% in 1992–1994 to 15% in 1996–1999.26 In a follow-up study analyzing care from 2005–2007, these investigates noted a sharp increase in use of HDR to 69%.27 Our data from the NCDB suggest that HDR has become widely accepted in the U.S. HDR is now the predominant modality of brachytherapy and rose from 50% in 2003 to 84% in 2011.
While LDR brachytherapy has been in use for several decades, utilization of HDR therapy offers a number of advantages for patients and providers. Compared to LDR therapy, HDR brachytherapy allows outpatient treatment, requires shorter times, is associated with greater patient convenience, allows individualized treatment planning and source optimization, and may allow greater safety for treatment personnel.2,28,29 Importantly, our data suggest that outcomes of HDR and LDR brachytherapy among patients in the United States are similar and provide support for use of HDR therapy.
While our study benefits from the inclusion of a large sample of patients treated across over 900 hospitals, we recognize a number of important limitations. First, tumor registry data may under-report the use of brachytherapy.30,31 As the goal of our analysis was specifically to compare different types of brachytherapy, a priori we chose to restrict our cohort to only those women in whom we could specifically identify not only the use of brachytherapy, but also the particular type of treatment. However, we recognize that a small number of women who received intracavitary therapy may not have been captured. Second, we are unable to capture specific details of radiation planning and delivery including dose, fields, and fractionation schedule. While these factors influence outcomes, these features likely varied across patients in both groups. Third, it is unclear why there were some imbalances between treatment groups including in the duration of treatment. Fourth, we are unable to determine the intent of treatment and, as such, some patients may have received palliative and not curative intent treatment. Lastly, NCDB lacks data on toxicity, quality of life, and patient reported outcomes. From a patient standpoint, these outcomes have an important impact on medical decision-making and preferences and warrant future study.
These data indicate that the use of HDR brachytherapy has increased rapidly among women with cervical cancer receiving primary radiation therapy in the U.S. Encouragingly, survival appears to be similar for LDR and HDR intracavitary therapy. Given the advantages of HDR therapy for patients and providers, our findings that survival is similar for LDR and HDR brachytherapy provides support for the use of HDR therapy in women undergoing primary radiation therapy for cervical cancer. Further work is needed to compare the cost effectiveness of LDR and HDR brachytherapy and to explore the impact of each treatment modality on patient reported outcomes.
Supplementary Material
Research Highlights.
The use of HDR therapy has increased rapidly
Overall survival is similar for LDR and HDR brachytherapy.
Acknowledgments
Dr. Wright (NCI R01CA169121-01A1) and Dr. Hershman (NCI R01 CA166084) are recipients of grants and Dr. Tergas is the recipient of a fellowship (NCI R25 CA094061-11) from the National Cancer Institute.
Footnotes
The authors have no conflicts of interest or disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Eifel PJ, Thoms WW, Jr, Smith TL, Morris M, Oswald MJ. The relationship between brachytherapy dose and outcome in patients with bulky endocervical tumors treated with radiation alone. Int J Radiat Oncol Biol Phys. 1994;28:113–8. doi: 10.1016/0360-3016(94)90148-1. [DOI] [PubMed] [Google Scholar]
- 2.Stewart AJ, Viswanathan AN. Current controversies in high-dose-rate versus low-dose-rate brachytherapy for cervical cancer. Cancer. 2006;107:908–15. doi: 10.1002/cncr.22054. [DOI] [PubMed] [Google Scholar]
- 3.International Commission on Radiological Units and Measurements. Dose and Volume Specification for Reporting Intracavitary Therapy in Gynecology (International Commission on Radiation Units and Measurements//ICRUReport) 1985 Mar 1; [Google Scholar]
- 4.Ferrigno R, Nishimoto IN, Novaes PE, et al. Comparison of low and high dose rate brachytherapy in the treatment of uterine cervix cancer. Retrospective analysis of two sequential series. Int J Radiat Oncol Biol Phys. 2005;62:1108–16. doi: 10.1016/j.ijrobp.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 5.Hareyama M, Sakata K, Oouchi A, et al. High-dose-rate versus low-dose-rate intracavitary therapy for carcinoma of the uterine cervix: a randomized trial. Cancer. 2002;94:117–24. doi: 10.1002/cncr.10207. [DOI] [PubMed] [Google Scholar]
- 6.Lertsanguansinchai P, Lertbutsayanukul C, Shotelersuk K, et al. Phase III randomized trial comparing LDR and HDR brachytherapy in treatment of cervical carcinoma. Int J Radiat Oncol Biol Phys. 2004;59:1424–31. doi: 10.1016/j.ijrobp.2004.01.034. [DOI] [PubMed] [Google Scholar]
- 7.Liu R, Wang X, Tian JH, et al. High dose rate versus low dose rate intracavity brachytherapy for locally advanced uterine cervix cancer. Cochrane Database Syst Rev. 2014;10:CD007563. doi: 10.1002/14651858.CD007563.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patel FD, Sharma SC, Negi PS, Ghoshal S, Gupta BD. Low dose rate vs. high dose rate brachytherapy in the treatment of carcinoma of the uterine cervix: a clinical trial. Int J Radiat Oncol Biol Phys. 1994;28:335–41. doi: 10.1016/0360-3016(94)90055-8. [DOI] [PubMed] [Google Scholar]
- 9.Petereit DG, Sarkaria JN, Potter DM, Schink JC. High-dose-rate versus low-dose-rate brachytherapy in the treatment of cervical cancer: analysis of tumor recurrence--the University of Wisconsin experience. Int J Radiat Oncol Biol Phys. 1999;45:1267–74. doi: 10.1016/s0360-3016(99)00262-x. [DOI] [PubMed] [Google Scholar]
- 10.Teshima T, Inoue T, Ikeda H, et al. High-dose rate and low-dose rate intracavitary therapy for carcinoma of the uterine cervix. Final results of Osaka University Hospital. Cancer. 1993;72:2409–14. doi: 10.1002/1097-0142(19931015)72:8<2409::aid-cncr2820720819>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 11.Kucera H, Potter R, Knocke TH, Baldass M, Kucera E. High-dose versus low-dose rate brachytherapy in definitive radiotherapy of cervical cancer. Wiener klinische Wochenschrift. 2001;113:58–62. [PubMed] [Google Scholar]
- 12.The National Cancer Data Base. Accessed at https://http://www.facs.org/qualityprograms/cancer/ncdb.
- 13.Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15:683–90. doi: 10.1245/s10434-007-9747-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lerro CC, Robbins AS, Phillips JL, Stewart AK. Comparison of cases captured in the national cancer data base with those in population-based central cancer registries. Ann Surg Oncol. 2013;20:1759–65. doi: 10.1245/s10434-013-2901-1. [DOI] [PubMed] [Google Scholar]
- 15.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 16.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–9. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 17.Wright JD, Lewin SN, Deutsch I, Burke WM, Sun X, Herzog TJ. The influence of surgical volume on morbidity and mortality of radical hysterectomy for cervical cancer. Am J Obstet Gynecol. 2011;205:225e1–7. doi: 10.1016/j.ajog.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 18.Wright JD, Lewin SN, Deutsch I, Burke WM, Sun X, Herzog TJ. Effect of surgical volume on morbidity and mortality of abdominal hysterectomy for endometrial cancer. Obstet Gynecol. 2011;117:1051–9. doi: 10.1097/AOG.0b013e31821647a0. [DOI] [PubMed] [Google Scholar]
- 19.Fyles A, Keane TJ, Barton M, Simm J. The effect of treatment duration in the local control of cervix cancer. Radiother Oncol. 1992;25:273–9. doi: 10.1016/0167-8140(92)90247-r. [DOI] [PubMed] [Google Scholar]
- 20.Orton CG, Seyedsadr M, Somnay A. Comparison of high and low dose rate remote afterloading for cervix cancer and the importance of fractionation. Int J Radiat Oncol Biol Phys. 1991;21:1425–34. doi: 10.1016/0360-3016(91)90316-v. [DOI] [PubMed] [Google Scholar]
- 21.Akine Y, Arimoto H, Ogino T, et al. High-dose-rate intracavitary irradiation in the treatment of carcinoma of the uterine cervix: early experience with 84 patients. Int J Radiat Oncol Biol Phys. 1988;14:893–8. doi: 10.1016/0360-3016(88)90011-9. [DOI] [PubMed] [Google Scholar]
- 22.Arai T, Nakano T, Morita S, Sakashita K, Nakamura YK, Fukuhisa K. High-dose-rate remote afterloading intracavitary radiation therapy for cancer of the uterine cervix. A 20-year experience. Cancer. 1992;69:175–80. doi: 10.1002/1097-0142(19920101)69:1<175::aid-cncr2820690129>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 23.Hsu WL, Wu CJ, Jen YM, et al. Twice-per-day fractionated high versus continuous low dose rate intracavitary therapy in the radical treatment of cervical cancer: a nonrandomized comparison of treatment results. Int J Radiat Oncol Biol Phys. 1995;32:1425–31. doi: 10.1016/0360-3016(94)00484-3. [DOI] [PubMed] [Google Scholar]
- 24.Lorvidhaya V, Tonusin A, Changwiwit W, et al. High-dose-rate afterloading brachytherapy in carcinoma of the cervix: an experience of 1992 patients. Int J Radiat Oncol Biol Phys. 2000;46:1185–91. doi: 10.1016/s0360-3016(99)00383-1. [DOI] [PubMed] [Google Scholar]
- 25.Nag S, Orton C, Young D, Erickson B. The American brachytherapy society survey of brachytherapy practice for carcinoma of the cervix in the United States. Gynecol Oncol. 1999;73:111–8. doi: 10.1006/gyno.1998.5334. [DOI] [PubMed] [Google Scholar]
- 26.Eifel PJ, Moughan J, Erickson B, Iarocci T, Grant D, Owen J. Patterns of radiotherapy practice for patients with carcinoma of the uterine cervix: a patterns of care study. Int J Radiat Oncol Biol Phys. 2004;60:1144–53. doi: 10.1016/j.ijrobp.2004.04.063. [DOI] [PubMed] [Google Scholar]
- 27.Eifel PJ, Ho A, Khalid N, Erickson B, Owen J. Patterns of radiation therapy practice for patients treated for intact cervical cancer in 2005 to 2007: a quality research in radiation oncology study. Int J Radiat Oncol Biol Phys. 2014;89:249–56. doi: 10.1016/j.ijrobp.2013.11.228. [DOI] [PubMed] [Google Scholar]
- 28.Wright J, Jones G, Whelan T, Lukka H. Patient preference for high or low dose rate brachytherapy in carcinoma of the cervix. Radiother Oncol. 1994;33:187–94. doi: 10.1016/0167-8140(94)90353-0. [DOI] [PubMed] [Google Scholar]
- 29.Petereit DG, Fowler JF, Kinsella TJ. Optimizing the dose rate and technique in cervical carcinoma--balancing cases versus complications. Int J Radiat Oncol Biol Phys. 1994;29:1195–7. doi: 10.1016/0360-3016(94)90420-0. [DOI] [PubMed] [Google Scholar]
- 30.Smith GL, Eifel PJ. Trends in the utilization of brachytherapy in cervical cancer in the United States. In regard to Han et al. Int J Radiat Oncol Biol Phys. 2014;88:459–60. doi: 10.1016/j.ijrobp.2013.10.032. [DOI] [PubMed] [Google Scholar]
- 31.Han K, Milosevic M, Fyles A, Viswanathan AN. In reply to Smith and Eifel. Int J Radiat Oncol Biol Phys. 2014;88:460–1. doi: 10.1016/j.ijrobp.2013.10.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.