Abstract
Objectives
Targeting high TB transmission sites may offer a novel approach to TB prevention in sub-Saharan Africa. We sought to characterize TB transmission sites in a rural Ugandan township.
Methods
We recruited adults starting TB treatment in Tororo, Uganda over one year. 54 TB cases provided names of frequent contacts, sites of residence, health care, work and social activities, and two sputum samples. Mycobacterium tuberculosis (MTB) culture-positive specimens underwent spoligotyping to identify strains with shared genotypes. We visualized TB case social networks, and obtained, mapped and geo-coded global positioning system measures for every location that cases reported frequenting one month before treatment. Locations of spatial overlap among genotype-clustered cases were considered potential transmission sites.
Results
Six distinct genotypic clusters were identified involving 21/33(64%) MTB culture-positive, genotyped cases; none shared a home. Although 18/54(33%) TB cases shared social network ties, none of the genotype-clustered cases shared social ties. Using spatial analysis, we identified potential sites of within-cluster TB transmission for five of six genotypic clusters. All sites but one were health care and social venues, including sites of drinking, worship and marketplaces. Cases reported spending the largest proportion of pre-treatment person-time (22.4%) at drinking venues.
Conclusions
Using molecular epidemiology, geospatial and social network data from adult TB cases identified at clinics, we quantified person-time spent at high-risk locations across a rural Ugandan community, and determined the most likely sites of recent TB transmission to be health care and social venues. These sites may not have been identified using contact investigation alone.
Keywords: tuberculosis, transmission dynamics, rural Africa, molecular epidemiology
Introduction
Tuberculosis (TB) is the leading cause of death among HIV-infected people in sub-Saharan Africa and a global threat to public health advancement, including the gains associated with antiretroviral scale-up.[1,2,3,4,5] Interventions to prevent TB transmission have the potential to reduce mortality and provide broad public health benefits to both HIV-infected and uninfected people. However, our incomplete understanding of TB transmission dynamics in African settings with a high prevalence of HIV represents a major gap in knowledge and an obstacle to developing effective TB prevention strategies. Studies of TB prevention interventions, including isoniazid preventive therapy (IPT) and active TB case finding, have had variable efficacy on community TB outcomes, with differing TB transmission dynamics in the communities proposed as possible explanations for the differing outcomes.[6,7,8,9]
One consequence of the relative lack of data on TB transmission dynamics in rural Africa, compared to data from low TB/HIV prevalence settings, is limited understanding of where TB transmission is occurring. Understanding what proportion of incident TB disease results from reactivation versus recent transmission, and what proportion of transmission occurs in household versus non-household settings such as clinics or other community venues, can potentially serve as a foundation for developing TB prevention strategies appropriate to a given epidemiologic context. The importance of non-household-based TB transmission in sub-Saharan Africa has been demonstrated in several settings.[10,11,12] However, epidemiologic links between TB cases that do not share a household have proven very difficult to identify.[13,14,15] Molecular epidemiologic studies in urban and sub-urban African communities have identified drinking venues and health care settings as high-risk locations for TB transmission.[16,17,18] Similarly, in the United States and Europe, the role of shared locations in promoting TB transmission between seemingly “casual” acquaintances has been shown.[19,20,21,22] However, there are few data on location of non-household transmission from rural African settings.
To fill this knowledge gap, we sought to characterize potential TB transmission sites in a rural township in Uganda, using a combination of molecular epidemiology markers, social network mapping and geographic data.
Methods
Over one year from July 2012 to June 2013, study staff sought to recruit all adult cases (≥18 years old) of active TB disease initiating anti-tuberculous treatment in Tororo Municipality, a rural town in eastern Uganda with an estimated population of 44,800 living in a geographic area of 38 km2.[23] In Tororo, TB treatment is only available at six government-run or associated clinics. Prior to initiating recruitment, study staff visited each of the six TB clinics to introduce and explain the study, and encourage clinic staff to contact the study team each time an adult resident of Tororo initiated TB treatment. Mobile phones were provided to the clinic staff at the clinic with the highest volume of TB patients to facilitate recruitment. Adult residents of Tororo (defined as ≥18 years of age, and living in Tororo for at least the one month prior to the date of TB treatment initiation), who had been diagnosed with either pulmonary or extrapulmonary TB, were eligible for enrollment. Enrollment was restricted to adults as children make up a small minority of TB cases diagnosed in this community (based on local clinical registries; data not shown) and face unique challenges in diagnosis and sputum collection.[24] Incident TB cases were defined as new cases, prior defaulters and recurrent cases. Study staff also routinely examined TB clinic government registries to look for eligible patients missed by clinic staff.
After providing written, informed consent, eligible TB cases were enrolled and interviewed. Study staff inquired about demographics, occupation, TB symptoms and duration of illness, and clinical risk factors for TB disease. Staff also obtained names and demographic information of all household members (defined as persons living under the same roof as the TB case), and of all frequent non-household contacts with which the participant had spent time (at least 12 hours in total) either in or out of the home over the two weeks prior to enrollment. Participants provided information on locations visited for work and health care (not including the visit to initiate TB treatment when enrollment occurred), as well as social activities (places of worship, commerce, and entertainment, or any other activity), where they spent at least 12 hours in total, over the one-month prior to enrollment. Additional clinical data, including HIV antibody test results (free HIV voluntary counseling and testing is offered to all TB cases), were obtained from the TB clinic provider and clinic registries. Lastly, study staff collected two sputum specimens from each participant, either as spot specimens, or early morning specimens, based on participant preference. Participants who were unable to spontaneously expectorate sputum underwent induction with hypertonic saline.
After completing the study interview and sputum collection, study staff accompanied participants to their homes in order to measure global position system (GPS) coordinates of the home and to conduct a household contact investigation. Consenting adult household members underwent TB symptom screening, a brief medical questionnaire regarding TB and HIV, and provided names and demographic information of non-household contacts with whom they spent time (at least 12 hours total) over the two weeks prior to enrollment. Child household contacts (<18 years) were not interviewed, but study staff provided information regarding TB signs and symptoms in children, and encouraged the adults to bring any symptomatic children to Tororo District Hospital for evaluation. Any adult household contact reporting current cough, or any recent hemoptysis, fever, weight loss, or night sweats, were advised to seek evaluation for TB at Tororo District Hospital and provided a transport voucher reimbursable at the hospital. Study staff recorded the results of the hospital-based TB evaluation for each household contact, and recruited any household contacts newly diagnosed with active TB disease and starting TB treatment.
Sputum samples from TB cases were kept at 4 °C and transported to the Makerere University Mycobacteriology laboratory in Kampala, Uganda for direct sputum examination using fluorescence microscopy and Mycobacterium tuberculosis (MTB) culture using Lowenstein-Jensen and BACTEC MGIT. MTB DNA isolated from all culture-positive specimens was sent to the San Francisco General Hospital MTB Research Laboratory for genotyping. Spoligotyping was used to identify MTB lineage and the spoligotype. Strains that share the same spoligotype were considered to belong to the same genotypic cluster and assumed to belong to a recent or ongoing TB transmission network.
Social networks were analyzed using Gephi software (Gephi.org, version 0.8) to identify epidemiologic ties between TB cases, their household members and non-household contacts, as well as ties between TB cases and locations within the study community. Name matching was used to identify persons named by more than one study participant, and confirmed by comparing age and village of named contacts whenever possible. Study staff obtained GPS coordinates for all locations named by TB cases, and the locations were mapped and geo-coded by site type (home, work, clinic or social site) using ArcGIS (Esri, Redlands, CA). Locations of spatial overlap among genotype-clustered cases were identified to determine probable site of within-cluster TB transmission. All locations frequented by TB cases were also examined independent of genotype to describe where and for how long TB cases spent time in the community in the one month prior to TB treatment.
The Makerere University School of Medicine Research and Ethics Committee, the Ugandan National Council on Science and Technology, and the UCSF Committee on Human Research approved the study.
Results
Over one year, 68 adults initiated TB treatment in Tororo, for an adult TB incidence rate of approximately 304 per 100,000 person-years; 54/68 (79%) cases were enrolled. All 14 cases that were not enrolled had left the TB clinic prior to meeting with study staff and could not be located despite attempts to contact them in the community; none declined study enrollment. Demographic and clinical characteristics of the 54 enrolled TB cases are shown in Table 1. Fifty participants underwent HIV testing, and 30 (60%) were HIV-infected; four participants declined HIV testing. Only 4/30 (13%) HIV/TB co-infected participants reported antiretroviral therapy (ART) use at the time of TB treatment start, although 24 (80%) reported engagement in HIV clinical care. Sputum was obtained from 47 (87%) TB cases. In seven cases, sputum could not be obtained despite induction; six of these cases were sputum acid-fast bacilli (AFB) smear negative at the time of pulmonary TB diagnosis, according to the TB registry, prior to referral for TB treatment and study enrollment. One was a case of extra-pulmonary TB (Pott’s disease) without evidence of pulmonary TB. Of 36 culture-positive cases, 33 were genotyped: 21/33 (64%) belonged to six distinct MTB genotypic clusters (see Table 2), and 12 (36%) were unique MTB isolates. Of the 33 genotyped cases, MTB lineage was Euro-American (Lineage 4) in 25 (76%) cases, East African-Indian (Lineage 3) in 6 (18%) cases, and Indo-Oceanic (Lineage 1) in 2 (6%) cases.
Table 1.
Demographic and clinical characteristics of TB cases enrolled over one year in Tororo, Uganda.
| TB Cases (N=54) | N | % |
|---|---|---|
|
Demographic Characteristics |
||
| Age, median (IQR) | 32 (27–42) | |
| Female | 23 | 43 |
| Hospitalized at enrollment | 9 | 17 |
| Single | 13 | 24 |
| Live alone | 11 | 20 |
| Residence in Tororo ≥ 6 months prior to TB diagnosis | 46 | 85 |
|
Clinical Characteristics |
||
| AFB smear positive (N=47 with sputum obtained) | 41 | 87 |
| MTB culture positive | 36 | 77 |
| HIV-infected (N=50 accepted HIV testing) | 30 | 60 |
| In HIV care | 24/30 | 80 |
| On antiretroviral therapy at enrollment | 4/30 | 13 |
| New TB Diagnosis | 50 | 93 |
| Prior TB Diagnosis | 4 | 7 |
| Defaulted during past treatment | 3 | |
| Completed prior treatment (recurrent TB) | 1 | |
| Site of TB Involvement | ||
| Pulmonary TB | 48 | 89 |
| Pulmonary & Extrapulmonary TB | 5 | 9 |
| Extrapulmonary TB | 1 | 2 |
| Cough duration, median | 6 weeks | |
| Smoke tobacco | 16 | 30 |
| Any alcohol use | 26 | 48 |
| Diabetes, self-reported | 0 | 0 |
Abbreviations: IQR = interquartile range. AFB = acid-fast bacilli. MTB = Mycobacterium tuberculosis.
Table 2.
Potential locations of TB transmission for each Mycobacterium tuberculosis genotypic cluster, categorized by location type.
| Genotypic Cluster |
Number of TB cases |
HIV+ Cas- es |
Location Type | |||
|---|---|---|---|---|---|---|
| Household | Clinical Site | Social Venue | Work place | |||
| 1 | Six | 3/6 | Cases 4 & 5* Neighbors | Cases 3, 5* & 6 attend co-located clinical sites. | Case 2* & 3 share a place of worship | - |
| 2 | Three | 1/3 | - | Case 2 & 3 share a clinical site (pharmacy) | - | - |
| 3 | Two | 1/2 | No Geographic Overlap Found | |||
| 4 | Three | 2/3 | - | Mixed: Case 1 attends a clinic geographically adjacent to social locations frequented by cases 2* & 3* | - | |
| 5 | Four | 3/4 | - | Cases 1*, 2* & 3* share a clinical site | a) Cases 1* & 2* frequent geographically adjacent bars b) Cases 2* & 3* share a bar site c) Case 2*, 3* & 4 share a marketplace (all as buyers) |
- |
| 6 | Three | 1/3 | - | Cases 2 & 3 share a clinic | a) Cases 1* & 3 share a place of worship b) Cases 2 & 3 share a bar site c) Cases 2 & 3 share a marketplace (buyers) |
- |
= HIV-infected TB case.
Participants reported a total of 131 household contacts; 11/54 (20%) cases reported living alone. Median household size was 3 persons (interquartile range [IQR]: 2–4), among 43 participants who did not live alone. Of 55 adult household contacts enumerated, 51 (93%) were enrolled (four contacts declined), and 13/51 (25%) had a positive TB symptom screen at the time of enrollment. Weight loss was the most commonly reported symptom (9/13), followed by cough (8/13), night sweats (8/13), and fever (7/13), among symptomatic contacts. Eleven symptomatic household contacts were further evaluated at the TB clinic, and one contact was diagnosed with active TB, started on TB treatment and enrolled as a TB case; this secondary case was AFB smear and MTB culture negative. The total yield of adult household contact investigation in identifying secondary active TB cases was 2% (1 of 51 contacts enrolled). Two (4%) of 51 household contacts reported a diagnosis of TB in the past (one in 1991 and one in 2009); both reported completing TB treatment. Of 38 adult household contacts with a history of ever HIV testing, nine (24%) reported testing HIV positive, and 29 (76%) reported negative results on their last HIV test.
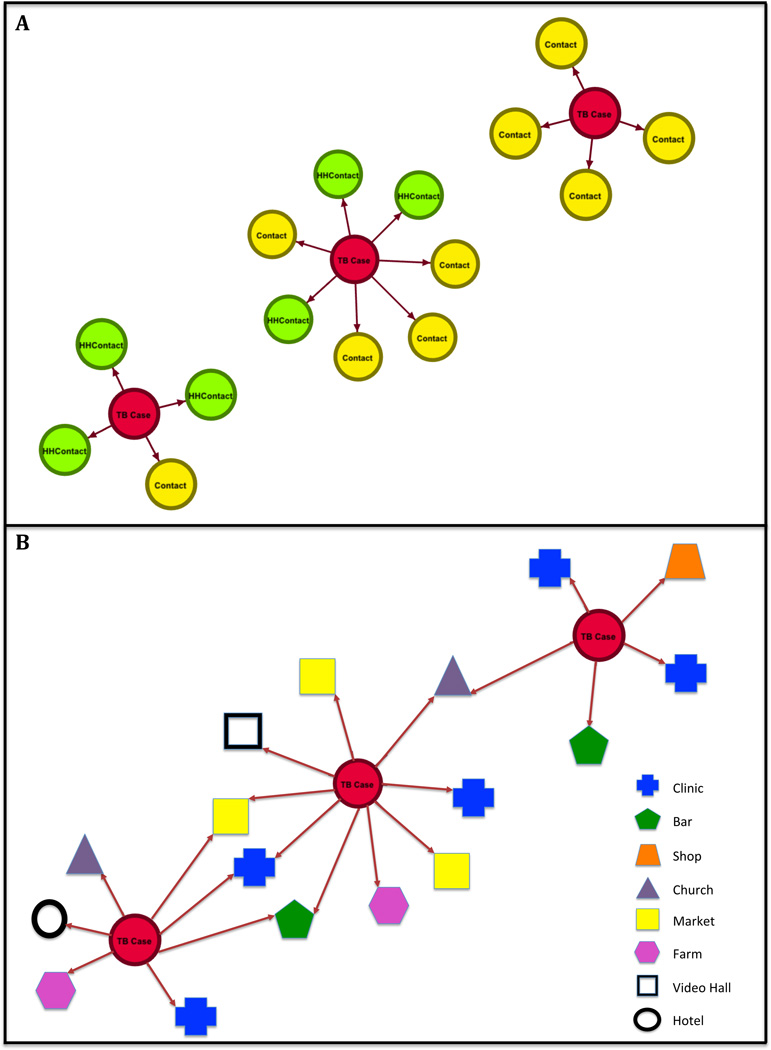
On examination of social networks of all TB cases, household members, and non-household contacts, 18/54 (33%) of TB cases could be linked to one another through reported social links; all but one of the social ties between TB cases involved non-household contacts. No social network links were found between TB cases within each of the six MTB genotypic clusters. When specific locations were included as nodes within genotype-clustered transmission networks, potential ties between genotypic clusters become apparent (see Figure 1). With analysis of location data (place names and overlapping GPS coordinates), potential sites of within-cluster TB transmission could be identified for five of the six MTB genotypic clusters, with the results shown in Table 2. One location, the Tororo district level hospital (a large referral center) was named as a clinic visited by all but three TB cases enrolled, as it is the site where the majority of TB cases started treatment and were enrolled in the study. On this basis, this specific location was excluded from the analysis of potential locations for within-cluster transmission. For one cluster (cluster 3: composed of two cases), no potential site of TB transmission could be identified. None of the genotype-matched TB cases shared a home. In cluster 1, two cases were next-door neighbors of one another, as determined by GPS mapping, though neither case identified the other as a social contact. Overall, seven of the TB cases belonging to genotypic clusters spent time in commuter taxis, including all TB cases in cluster 6, two TB cases in cluster 2, and one case each in clusters 4 and 5. The remaining sites of likely intra-cluster TB transmission were isolated to health care and social venues.
Figure 1.
Social Network Graphic of Mycobacterium tuberculosis Genotype Cluster 6, shown without location information (Panel A), and with location information (Panel B) included as network nodes in Tororo, Uganda. Each circle (node) represents a person (TB case or contact), and shapes indicated in the Panel B legend represent locations.
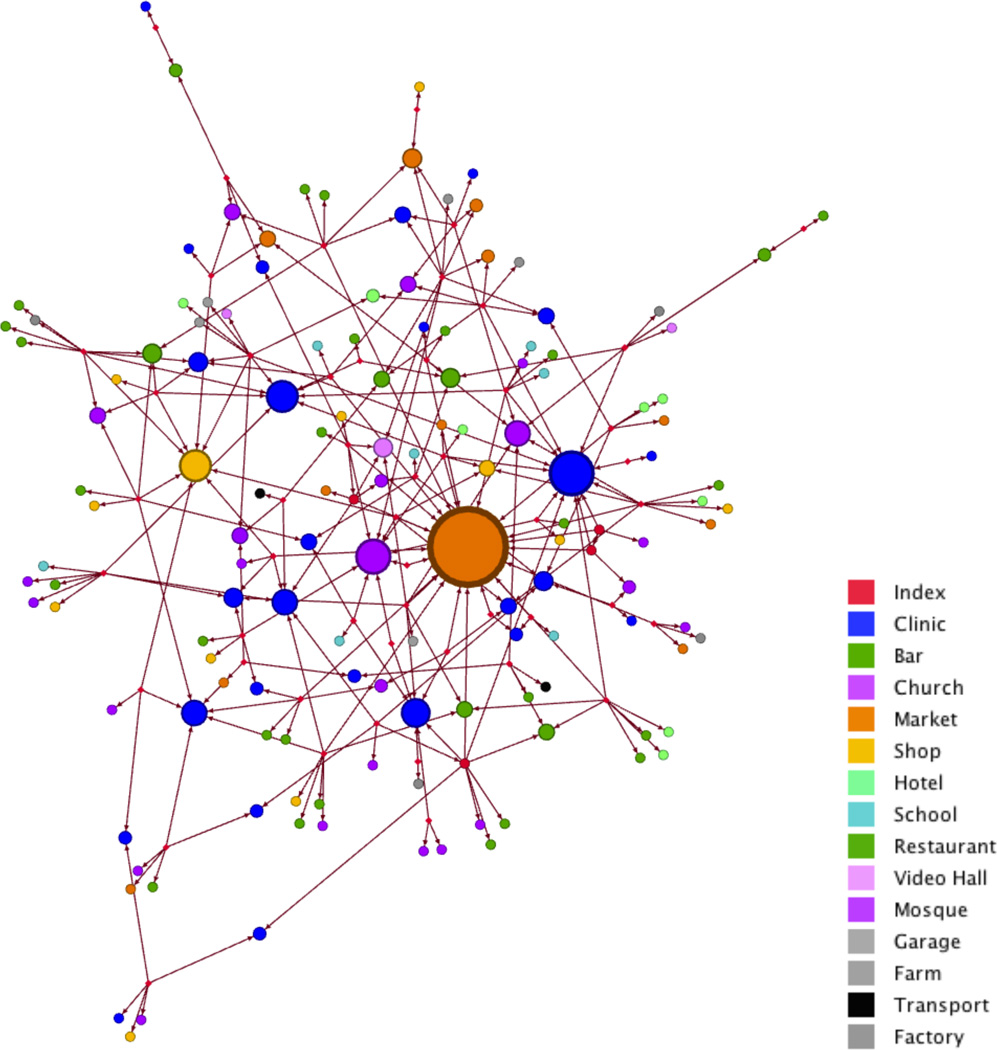
In the month prior to TB treatment, enrolled TB cases reported spending time outside of the home at a total of 135 distinct locations within the community. Of the potential 19,440 person-hours (assuming 12 daytime hours out of the household per day for a 30 day period of recall) spent by 54 TB cases in the month prior to starting TB treatment, cases reported a total of 14,432 person-hours, accounting for 74% of TB case person-time. The distribution of location types and the amount of time spent at each location is shown in Table 3. The locations most commonly named by TB cases were clinics, shops, markets, places of worship, and drinking venues (bars/local breweries). Figure 2 shows a graphical representation of the relative network “degree” (i.e. the number of TB cases reporting visiting each specific location) for each of the 134 locations named by cases (excluding the district level hospital, named by 51/54 cases). The greatest mean duration of person-time reported was spent at drinking venues, workplaces, shops and marketplaces. The largest proportion (22.4%) of the total person-time reported was spent at drinking venues.
Table 3.
Distribution of time spent at various community locations in the one-month prior to TB treatment start, stratified by location type, among 54 TB cases in Tororo, Uganda.
| Location Type | Total number TB cases (N=54) reporting time spent at location type |
Mean hours/month spent at location by all TB cases |
Median hours/month spent at location among those TB cases reporting a visit to the location type |
% of total person-hours reported spent at loca- tion |
|---|---|---|---|---|
| Clinic | 54 | 19.1 | 4 (2–12) | 7.2% |
| Workplace | 8 | 52.3 | 420 (300–420) | 19.6% |
| Market | 36 | 46.0 | 16 (8–40) | 17.2% |
| Shop | 12 | 51.4 | 300 (60–300) | 19.2% |
| Church/Mosque | 44 | 13.4 | 8 (8–24) | 5.0% |
| Bar/Local Brewery | 26 | 59.9 | 14 (4–60) | 22.4% |
| Restaurant | 5 | 3.0 | 40 (8–48) | 1.1% |
| Hotel | 6 | 6.0 | 16 (4–24) | 2.3% |
| Movie Theater (Video Hall) | 6 | 3.0 | 24 (8–40) | 1.1% |
| School | 7 | 13.0 | 14 (6–180) | 5.0% |
Figure 2.
Graphical representation of the network of TB cases (red circles) and specific locations (non-red circles) that TB cases reported spending time in the one month prior to initiation of TB treatment in Tororo, Uganda. For location nodes, each node (circle) represents a specific location named by a TB case, colored by location type (see legend), with node size proportional to the network degree (i.e. as the number of TB cases reporting visiting that location increases, the node size increases).
Discussion
Using a combination of molecular epidemiology tools, and geospatial and social network data obtained from TB cases identified in clinics, we were able to identify potential sites of recent TB transmission across a rural Ugandan community. The majority of active TB cases genotyped suggested recent TB transmission linked to specific community locations. Connections identified between genotype-clustered TB cases using this multi-domain approach would not have been found using traditional household contact investigation alone. Improved understanding of where, and for how long, TB cases spend their time prior to TB diagnosis and treatment, may provide a foundation for developing targeted strategies for community-based, TB prevention interventions.
Our study found a high proportion of incident TB cases that shared the same MTB genotype, despite a relatively short enrollment period (one year); a finding consistent with other studies in sub-Saharan Africa.[14,25] TB disease attributable to recent transmission is likely influenced by the high prevalence of HIV and the large burden of undiagnosed active TB disease in east Africa.[26] Antiretroviral therapy (ART) reduces the risk of developing TB disease in HIV-infected persons,[27,28] and the large proportion of HIV-infected adults engaged in HIV clinical care but not on ART (20/24; 83%) among the TB cases enrolled in our study highlights the missed opportunity for earlier ART initiation as a TB prevention strategy in settings with a high prevalence of TB and HIV. Although the burden of undiagnosed TB disease in our study community is not known, a community prevalence survey in rural western Kenya (directly across the Uganda-Kenya border from Tororo) found the prevalence of TB disease to be substantially higher than national estimates for the region; 64% of prevalent cases in this survey were not on treatment and had not yet sought care.[26] In this context, combined efforts to offer ART at higher CD4 cell counts and to identify and treat TB cases earlier in the course of disease using HIV-clinic based intensified case-finding, and community-based active case finding (ACF), may have a relatively large impact on TB transmission. The latter approach has been shown to result in a reduction in TB prevalence in a mobile van-based intervention in Malawi,[8] though questions regarding the efficacy of community-based ACF in reducing TB incidence remain.[29]
Our study also suggests that targeting active TB case finding to clinics and high TB-risk social sites may be higher yield for identifying TB cases than household contact investigation. None of the genotype-clustered cases identified in our study shared a household, a finding consistent with other studies from sub-Saharan Africa in which the majority of TB transmission events occur outside of the household.[10,11,12] However, to our knowledge few attempts have been made to identify specific locations of TB transmission between genotype-clustered TB cases in rural sub-Saharan Africa, despite the clear importance of non-household based TB transmission in this setting. Classen, et al. identified frequent non-household transmission at social drinking venues in suburban South Africa.[16] Drinking venues as sites of TB transmission have also been observed in urban settings in South Africa and Benin.[30,31] In our study, despite finding non-household social ties between one-third of TB cases, only by including locations frequented by cases were potential sites of TB transmission identified. This suggests that TB transmission events frequently occur between relatively “casual” acquaintances at shared locations in this community and would not have been identified through contact investigation alone. TB outbreak investigations involving HIV-infected persons in the United States and Europe have observed this phenomenon as well.[20,21,32] On this basis, location-based active TB case finding and infection control interventions merit further investigation as tools to interrupt TB transmission in rural east Africa. Notably, many of the locations frequented by TB cases are indoor environments such as movie theaters (“video halls”), churches, clinics, shops and bars, likely to have poor ventilation and frequent crowding.
As a starting point for developing location-based, TB prevention approaches, we sought to understand where, and for how long, TB cases spent time in their community prior to initiating TB treatment - the peak period of infectiousness. Through this process, we found that although clinics, churches/mosques and open-air marketplaces were the location types named most frequently by TB cases (as shown in Figure 2), only a small number of location types (drinking venues, workplaces, shops and marketplaces) accounted for the majority of TB case person-time (Table 3). Our findings are similar to the findings of a qualitative approach used to identify high-risk TB transmission sites employed by Murray et al. in Cape Town, in which the highest theoretical transmission risk scores were assigned to drinking places, clinics, churches and shops.[33]
This study has several limitations. TB molecular epidemiology tools can only confirm transmission events that result in active disease and that occur between culture positive TB cases. The “true” frequency of TB transmission, including transmission resulting in latent TB infection cannot be definitively determined with existing methods. We asked for named social contacts over the two weeks prior to enrollment to facilitate participant recall and based on the assumption that social contacts in this rural setting are relatively stable over time, however this may have resulted in missing important contacts within the social networks of TB cases that contributed to TB spread. Misclassification of cases as unique that are truly genotype-clustered is more likely to occur with a short duration of enrollment, such as our one-year study period.[34] Misclassification of genotype-clustered cases that are truly unique also occurs more frequently with spoligotyping compared to more discriminating genotyping methods, such as whole genome sequencing or restriction fragment polymorphism (RFLP) analysis.[35] However, misclassification by spoligotyping is less likely with the TB lineages found in this community, compared to other lineages, such as the Beijing strain.[36] In addition, incomplete sampling due to under-diagnosis of TB in the community can result in either under or over-estimation of the proportion of genotype clustering;[34] not all TB cases identified in our study could be cultured or genotyped, and the cases included may not be representative of all TB cases in this community. For example, under-sampling of HIV-uninfected TB cases in our study could have resulted in over-estimation of the proportion of genotype clustering, as HIV infection is associated with a higher risk of TB disease attributable to recent TB transmission.[25] Furthermore, restriction of study enrollment to adults likely resulted in underestimation of household TB transmission, which may disproportionately involve children.[12] Finally, we do not have baseline estimates of the number of community members without active TB who spend time at the various community locations; some locations identified as potential TB transmission sites (such as marketplaces) may simply reflect locations at which residents spend a large proportion of their time. Despite these limitations, our developmental piloting of these methods in this study suggests that a multidisciplinary approach incorporating microbiologic, geographic and social network data can identify likely sites of TB transmission in a rural African setting.
Conclusion
In summary, using a combination of molecular epidemiologic tools, and social network and GIS analyses, we found that the majority of TB cases identified in this rural Ugandan community likely developed TB disease following recent TB transmission that could be linked to specific community locations. Location-based strategies for TB prevention and early diagnosis, including infection control and active TB case-finding interventions, may offer a high yield, community-based approach to reduce TB incidence in settings with a high prevalence of HIV and TB.
Acknowledgements
We thank the study participants for their generous participation in our study. We also thank Olive Mugagga and Onau Joshua Okada, in Tororo Uganda.
References
- 1.Ansari NA, Kombe AH, Kenyon TA, Hone NM, Tappero JW, et al. Pathology and causes of death in a group of 128 predominantly HIV-positive patients in Botswana, 1997–1998. Int J Tuberc Lung Dis. 2002;6:55–63. [PubMed] [Google Scholar]
- 2.Corbett EL, Marston B, Churchyard GJ, De Cock KM. Tuberculosis in sub-Saharan Africa: opportunities, challenges, and change in the era of antiretroviral treatment. Lancet. 2006;367:926–937. doi: 10.1016/S0140-6736(06)68383-9. [DOI] [PubMed] [Google Scholar]
- 3.Rana FS, Hawken MP, Mwachari C, Bhatt SM, Abdullah F, et al. Autopsy study of HIV-1-positive and HIV-1-negative adult medical patients in Nairobi, Kenya. J Acquir Immune Defic Syndr. 2000;24:23–29. doi: 10.1097/00126334-200005010-00004. [DOI] [PubMed] [Google Scholar]
- 4.Wong EB, Omar T, Setlhako GJ, Osih R, Feldman C, et al. Causes of death on antiretroviral therapy: a post-mortem study from South Africa. PLoS ONE. 2012;7:e47542. doi: 10.1371/journal.pone.0047542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cox JA, Lukande RL, Lucas S, Nelson AM, Van Marck E, et al. Autopsy causes of death in HIV-positive individuals in sub-Saharan Africa and correlation with clinical diagnoses. AIDS reviews. 2010;12:183–194. [PubMed] [Google Scholar]
- 6.Ayles H, Muyoyeta M, Du Toit E, Schaap A, Floyd S, et al. Effect of household and community interventions on the burden of tuberculosis in southern Africa: the ZAMSTAR community-randomised trial. Lancet. 2013;382:1183–1194. doi: 10.1016/S0140-6736(13)61131-9. [DOI] [PubMed] [Google Scholar]
- 7.Churchyard GJ, Fielding KL, Lewis JJ, Coetzee L, Corbett EL, et al. A trial of mass isoniazid preventive therapy for tuberculosis control. N Engl J Med. 2014;370:301–310. doi: 10.1056/NEJMoa1214289. [DOI] [PubMed] [Google Scholar]
- 8.Corbett EL, Bandason T, Duong T, Dauya E, Makamure B, et al. Comparison of two active case-finding strategies for community-based diagnosis of symptomatic smear-positive tuberculosis and control of infectious tuberculosis in Harare, Zimbabwe (DETECTB): a cluster-randomised trial. Lancet. 2010 doi: 10.1016/S0140-6736(10)61425-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Durovni B, Saraceni V, Moulton LH, Pacheco AG, Cavalcante SC, et al. Effect of improved tuberculosis screening and isoniazid preventive therapy on incidence of tuberculosis and death in patients with HIV in clinics in Rio de Janeiro, Brazil: a stepped wedge, cluster-randomised trial. Lancet Infect Dis. 2013;13:852–858. doi: 10.1016/S1473-3099(13)70187-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verver S, Warren RM, Munch Z, Richardson M, van der Spuy GD, et al. Proportion of tuberculosis transmission that takes place in households in a high-incidence area. Lancet. 2004;363:212–214. doi: 10.1016/S0140-6736(03)15332-9. [DOI] [PubMed] [Google Scholar]
- 11.Crampin AC, Glynn JR, Traore H, Yates MD, Mwaungulu L, et al. Tuberculosis transmission attributable to close contacts and HIV status, Malawi. Emerg Infect Dis. 2006;12:729–735. doi: 10.3201/eid1205.050789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Middelkoop K, Bekker LG, Morrow C, Lee N, Wood R. Decreasing household contribution to TB transmission with age: a retrospective geographic analysis of young people in a South African township. BMC Infect Dis. 2014;14:221. doi: 10.1186/1471-2334-14-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crampin AC, Glynn JR, Fine PE. What has Karonga taught us? Tuberculosis studied over three decades. Int J Tuberc Lung Dis. 2009;13:153–164. [PMC free article] [PubMed] [Google Scholar]
- 14.Middelkoop K, Bekker LG, Mathema B, Shashkina E, Kurepina N, et al. Molecular epidemiology of Mycobacterium tuberculosis in a South African community with high HIV prevalence. J Infect Dis. 2009;200:1207–1211. doi: 10.1086/605930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilkinson D, Pillay M, Crump J, Lombard C, Davies GR, et al. Molecular epidemiology and transmission dynamics of Mycobacterium tuberculosis in rural Africa. Tropical medicine & international health : TM & IH. 1997;2:747–753. doi: 10.1046/j.1365-3156.1997.d01-386.x. [DOI] [PubMed] [Google Scholar]
- 16.Classen CN, Warren R, Richardson M, Hauman JH, Gie RP, et al. Impact of social interactions in the community on the transmission of tuberculosis in a high incidence area. Thorax. 1999;54:136–140. doi: 10.1136/thx.54.2.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andrews JR, Gandhi NR, Moodley P, Shah NS, Bohlken L, et al. Exogenous reinfection as a cause of multidrug-resistant and extensively drug-resistant tuberculosis in rural South Africa. J Infect Dis. 2008;198:1582–1589. doi: 10.1086/592991. [DOI] [PubMed] [Google Scholar]
- 18.Gandhi NR, Weissman D, Moodley P, Ramathal M, Elson I, et al. Nosocomial transmission of extensively drug-resistant tuberculosis in a rural hospital in South Africa. J Infect Dis. 2013;207:9–17. doi: 10.1093/infdis/jis631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jackson AD, Seagar AL, Reid ME, Doig C, Forbes KJ, et al. Characterising transmission of a tuberculosis genotype in Scotland: a qualitative approach to social network enquiry. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 2009;13:486–493. [PubMed] [Google Scholar]
- 20.Klovdahl AS, Graviss EA, Yaganehdoost A, Ross MW, Wanger A, et al. Networks and tuberculosis: an undetected community outbreak involving public places. Soc Sci Med. 2001;52:681–694. doi: 10.1016/s0277-9536(00)00170-2. [DOI] [PubMed] [Google Scholar]
- 21.Sterling TR, Thompson D, Stanley RL, McElroy PD, Madison A, et al. A multi-state outbreak of tuberculosis among members of a highly mobile social network: implications for tuberculosis elimination. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 2000;4:1066–1073. [PubMed] [Google Scholar]
- 22.Yaganehdoost A, Graviss EA, Ross MW, Adams GJ, Ramaswamy S, et al. Complex transmission dynamics of clonally related virulent Mycobacterium tuberculosis associated with barhopping by predominantly human immunodeficiency virus-positive gay men. J Infect Dis. 1999;180:1245–1251. doi: 10.1086/314991. [DOI] [PubMed] [Google Scholar]
- 23.Uganda Bureau of Statistics: Population Projections 2008–2012. 2008. Dec, [Google Scholar]
- 24.Newton SM, Brent AJ, Anderson S, Whittaker E, Kampmann B. Paediatric tuberculosis. Lancet Infect Dis. 2008;8:498–510. doi: 10.1016/S1473-3099(08)70182-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glynn JR, Crampin AC, Yates MD, Traore H, Mwaungulu FD, et al. The importance of recent infection with Mycobacterium tuberculosis in an area with high HIV prevalence: a long-term molecular epidemiological study in Northern Malawi. J Infect Dis. 2005;192:480–487. doi: 10.1086/431517. [DOI] [PubMed] [Google Scholar]
- 26.van't Hoog AH, Laserson KF, Githui WA, Meme HK, Agaya JA, et al. High prevalence of pulmonary tuberculosis and inadequate case finding in rural western Kenya. Am J Respir Crit Care Med. 2011;183:1245–1253. doi: 10.1164/rccm.201008-1269OC. [DOI] [PubMed] [Google Scholar]
- 27.Badri M, Wilson D, Wood R. Effect of highly active antiretroviral therapy on incidence of tuberculosis in South Africa: a cohort study. Lancet. 2002;359:2059–2064. doi: 10.1016/S0140-6736(02)08904-3. [DOI] [PubMed] [Google Scholar]
- 28.Lawn SD, Badri M, Wood R. Tuberculosis among HIV-infected patients receiving HAART: long term incidence and risk factors in a South African cohort. Aids. 2005;19:2109–2116. doi: 10.1097/01.aids.0000194808.20035.c1. [DOI] [PubMed] [Google Scholar]
- 29.Kranzer K, Afnan-Holmes H, Tomlin K, Golub JE, Shapiro AE, et al. The benefits to communities and individuals of screening for active tuberculosis disease: a systematic review. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 2013;17:432–446. doi: 10.5588/ijtld.12.0743. [DOI] [PubMed] [Google Scholar]
- 30.Affolabi D, Faihun F, Sanoussi N, Anyo G, Shamputa IC, et al. Possible outbreak of streptomycin-resistant Mycobacterium tuberculosis Beijing in Benin. Emerg Infect Dis. 2009;15:1123–1125. doi: 10.3201/eid1507.080697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Munch Z, Van Lill SW, Booysen CN, Zietsman HL, Enarson DA, et al. Tuberculosis transmission patterns in a high-incidence area: a spatial analysis. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 2003;7:271–277. [PubMed] [Google Scholar]
- 32.Cook VJ, Sun SJ, Tapia J, Muth SQ, Arguello DF, et al. Transmission network analysis in tuberculosis contact investigations. J Infect Dis. 2007;196:1517–1527. doi: 10.1086/523109. [DOI] [PubMed] [Google Scholar]
- 33.Murray EJ, Marais BJ, Mans G, Beyers N, Ayles H, et al. A multidisciplinary method to map potential tuberculosis transmission 'hot spots' in high-burden communities. Int J Tuberc Lung Dis. 2009;13:767–774. [PubMed] [Google Scholar]
- 34.Murray M. Sampling bias in the molecular epidemiology of tuberculosis. Emerg Infect Dis. 2002;8:363–369. doi: 10.3201/eid0804.000444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kremer K, van Soolingen D, Frothingham R, Haas WH, Hermans PW, et al. Comparison of methods based on different molecular epidemiological markers for typing of Mycobacterium tuberculosis complex strains: interlaboratory study of discriminatory power and reproducibility. J Clin Microbiol. 1999;37:2607–2618. doi: 10.1128/jcm.37.8.2607-2618.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hanekom M, van der Spuy GD, Gey van Pittius NC, McEvoy CR, Hoek KG, et al. Discordance between mycobacterial interspersed repetitive-unit-variable-number tandem-repeat typing and IS6110 restriction fragment length polymorphism genotyping for analysis of Mycobacterium tuberculosis Beijing strains in a setting of high incidence of tuberculosis. J Clin Microbiol. 2008;46:3338–3345. doi: 10.1128/JCM.00770-08. [DOI] [PMC free article] [PubMed] [Google Scholar]