Abstract
Store-operated Ca2+ entry is the major route of replenishment of intracellular Ca2+ in animal cells in response to depletion of Ca2+ stores in the endoplasmic reticulum. It is primarily mediated by the Ca2+ selective release-activated Ca2+ (CRAC) channel which consists of the pore-forming subunits ORAI1–3 and the Ca2+ sensors, STIM1 and STIM2. Recessive loss-of-function mutations in STIM1 or ORAI1 result in immune deficiency and nonprogressive myopathy. Heterozygous gain-of-function mutations in STIM1 cause non-syndromic myopathies as well as syndromic forms of miosis and myopathy with tubular aggregates and Stormorken syndrome; some of these syndromic forms are associated with thrombocytopenia. Increased concentration of Ca2+ as a result of store-operated Ca2+ entry is essential for platelet activation. York Platelet syndrome (YPS) is characterized by thrombocytopenia, striking ultrastructural platelet abnormalities including giant electron opaque organelles and massive, multi-layered target bodies and deficiency of platelet Ca2+ storage in delta granules. We present clinical and molecular findings in 7 YPS patients from 4 families, demonstrating that YPS patients have a chronic myopathy associated with rimmed vacuoles and heterozygous gain-of-function STIM1 mutations. These findings expand the phenotypic spectrum of STIM1-related human disorders and define the molecular basis of YPS.
Keywords: STIM1, York Platelet syndrome
INTRODUCTION
When Ca2+ enters cells in response to depletion of intracellular stores, it is called store-operated Ca2+ entry, and serves as the major route of Ca2+ entry into animal cells (1). Store-operated Ca2+ entry is mediated primarily by the Ca2+ selective release-activated Ca2+ (CRAC) channel (2), which consists of the pore-forming subunits ORAI1–3 and the Ca2+ sensors, STIM1 and STIM2. STIM1 binds Ca2+ through two EF hand-containing domains located in the lumen of the endoplasmic reticulum (ER). Depletion of Ca2+ in the ER induces a series of events in the conformation and localization of STIM1, enabling it to interact with and open ORAI1, a CRAC channel located in the plasma membrane, restoring cytosolic and ER Ca2+ concentrations.
Platelets of Orai1−/− mice display defective store-operated Ca2+ entry and impaired activation and thrombus formation under flow in vitro (3). Similarly, Stim1−/− mice platelets show marked defect in agonist-induced Ca2+ responses, and impaired activation and thrombus formation (4). Stim1Sax mice, created by randon mutagenesis displaying dominant inheritance of elevated mean platelet volume and bleeding disorder, express an activating EF hand mutant of Stim1(5). Basal [Ca2+]i levels of Stim1Sax platelets are increased, resulting in a preactivation state.
Several human diseases are associated with mutations in CRAC channel components (Supplemental Table 1). Recessive loss-of-function mutations in STIM1 or ORAI1 result in immune deficiency and nonprogressive myopathy (6-8). Heterozygous gain-of-function mutations in STIM1 cause isolated or non-syndromic myopathies (9)(10), as well as syndromic forms of miosis and myopathy with tubular aggregates (11) and Stormorken syndrome (12). These latter disorders include thrombocytopenia in addition to muscle involvement.
In the cytosol of platelets, an increased concentration of Ca2+ is essential for platelet activation and occurs as a result of store-operated Ca2+ entry. Moreover, there exists a disorder of thrombocytopenia and deficiency of platelet Ca2+ storage in delta granules, i.e., York Platelet syndrome (YPS)(13-18). In YPS, megakaryocytes and platelets exhibit striking ultrastructural abnormalities including giant electron opaque organelles and massive, multi-layered target bodies. Here we document that YPS patients also have a myopathy characterized by the presence of rimmed vocuoles within muscle fibers. Based upon exome sequence analysis, we report that YPS results from specific STIM1 mutations.
METHODS
Patients
The patients were enrolled in protocols approved by the NHGRI Institutional Review Board and informed consent was obtained. Patients A-II-2, A-III-1 and B-II-2 were evaluated at the NIH Clinical Center through the NIH Undiagnosed Diseases Program (UDP) (19), (20).
Platelet studies
Details of methods of platelet aggregation (performed using a Chronolog 700 lumi-aggregometer from CHRONO-LOG Corporation, Havertwon, PA), flow cytometry, electron microscopy and energy dispersive spectra analysis are in Supplemental Methods. For platelet flow cytometry, platelets from patient A-II-2, A-III-1, and B-II-2 and 3 healthy volunteers were analyzed in parallel. Platelet-rich plasma (PRP) containing10,000 platelets/μL was analyzed at resting state, and after activation using multiparameter flow cytometry and the following antibodies: CD62p, CD63, CD41a, CD42b, CD9, CD31, CD36, CD61. Electron microscopy was carried out in a Joel 1200 ExII Transmission Electron microsope (JEOL Ltd., Tokyo, Japan) as previously described (21). Elemental composition of platelet dense granules, abnormal opaque objects and background were determined by analysis of energy dispersive spectra collected by an EDAX xray microanalysis system (EDAX, Mahwah, NJ) with 100-seconds per acquisition.
Sequencing
For whole exome sequencing, solution hybridization exome capture was carried out using the Ilumina TruSeqV1 capture kits. Illumina paired end sequencing was performed per manufacture's protocol. Flowcell preparation and 101-bp paired end read sequencing were carried out as per protocol for the HiSeq2000 GAIIx sequencer (Illumina Inc, San Diego CA) (22). Details of exome sequencing, variant analysis, and Sanger sequencing are in Supplemental Methods. The primer pairs listed in Supplementary Table 1 were used to amplify the genomic DNA in the coding exons and flanking nucleotides of each exon of STIM1. PCR amplification was performed using Qiagen HotStarTaq Plus master mix (Qiagen, Valencia, CA).
RESULTS
Patients
We describe 7 patients from 4 independent families with YPS (Figure 1). Family A had 3 affected individuals from 3 generations. Families B and C each contained a single YPS patient with a de novo mutation. Family D had 2 generations each containing a single affected individual. Patients A-II-2 and A-III-1 are the original YPS patients described by Dr. James White (15-18). Platelet EM findings of patient B-II-2 have been previously reported (14).
Figure 1. Pedigrees (A, C, E, G) and mutation chromatograms (B, D, F, H) for YPS families.
Conservation of the amino acid residues corresponding to the c.343A>T and c.910C>T mutations are shown in I and J, respectively.
Patient A-II-2
(UDP 3483) is a 46-year old female. During her first 2 years of life, she displayed weakness, tripped frequently, and had difficulty jumping. The remainder of her growth and development were unremarkable. Bruising and epistaxis appeared in early childhood, and menstrual periods were heavy. During the third month of her pregnancy at age 18, she developed a deep vein thrombosis treated with heparin throughout the pregnancy. She delivered 6 weeks prematurely and had postpartum hemorrhage requiring transfusion of red blood cells. A low platelet count was attributed to heparin injections. At age 20, while her son was being evaluated for thrombocytopenia, she was found to have persistent thrombocytopenia. At age 23, she had a second episode of deep vein thrombosis for which she required warfarin for 3 months. At age 46, a uterine ablation was performed for heavy menstrual bleeding.
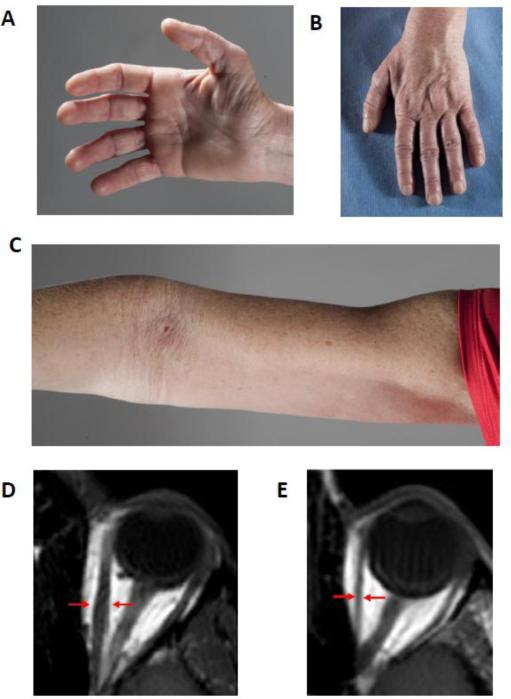
At NIH Clinical Center, at age 46, she complained of longstanding proximal leg and arm muscle weakness. She had difficulty rising from a chair. Recently, her feet also became weak, requiring the use of a cane, and hand weakness reduced her grip. She had multiple bruises. Her pupils were small and fixed at 2mm in diameter and did not react to one minute of dark adaptation or to light. Lateral eye movements were restricted and she had external up gaze ophthalmoplegia but no ptosis. She had bilateral facial weakness and weakness of the arm muscles, proximal worse than distal (Supplementary video). She had distal muscle atrophy including interossei and intrinsic hand muscles (Figure 2 A-C). Axial MRI images of the orbits indicated thin/atrophic extra ocular muscles (Figure 2 D and E).
Figure 2. Clinical photographs of hands (A and B) and arm (C) and orbital MRI of patient A-II-2.
Note atrophied hand muscles as well as proximal arm muscles. There is appreciable reduction in the bulk of all extra ocular muscles in A-II-2 (E) in comparison to control (D). The arrows indicate the cross-sectional diameter of the left medial rectus muscle.
The platelet count was 139, 000 /μL. Hemoglobin, hematocrit and white blood cell counts were normal at 14.9 gr/dL 44.8 % and 6.51 K/uL, respectively. Blood smear showed Howell-Jolly bodies. Platelets ranged in size from very small to very large and some were hypogranular and pale. Creatine kinase (CK) was 450 U/L (normal, 38-252). Abdominal USG showed a poorly visualized spleen spanning < 8 cm. MRI of thighs and pelvis showed extensive muscle atrophy involving all muscle groups (Figure 3A).
Figure 3. Muscle MRI and biopsy findings of YPS patients.
(A) Patient A-II-2 MRI and light microscopy.
(left) Thigh MRI showing marked fatty infiltration and muscle atrophy involving the adductors, hamstrings and quadriceps; the sartorius and gracilis muscles are slightly less affected. There is relative preservation of the psoas and gluteus maximus muscles.
(top right) Hematoxylin and eosin stained section showing increased variation in myofiber size and shape; rounded and angular atrophic myofibers, and hypertrophied fibers interspersed among fibers of normal diameter. Myopathic changes include degenerating fibers, regenerating fibers and increased numbers of myofibers with internalized nuclei. (20x)
(bottom right) Masson trichrome stained section showing moderately increased endomysial connective tissue accompanied by fat infiltration (20x)
(B) Patient A-III-1 MRI and light microscopy.
(top left) Thigh MRI showing the same findings as in Fig. 3A.
(bottom left) Hematoxylin and eosin stained section showing degenerating fibers, regenerating fibers, and increased numbers of myofibers with internalized nuclei. Inflammation is not seen. (20x)
(top right) Masson trichrome stained section showing moderate endomysial fibrosis and fat infiltration. There is increased variation in myofiber size, rounded and angular atrophic myofibers and hypertrophied fibers interspersed among fibers of normal diameter. (20x)
(bottom right) Gomori trichrome stain showing sarcoplasmic vacuoles. Fibers are green-blue; membranous whorls or rimmed vacuoles are red. (40x)
(C) Patient A-III-1 electron microscopy. Overall, the skeletal muscle fibers have well-preserved cytoarchitecture including Z bands and fibrils. There are abundant vacuoles limited by a distinct membrane and some loosely scattered electron dense granules (inset). Collections of the vacuoles are located between the myofibers or in the subsarcolemmal areas.
Patient A-III-1
(UDP 2542) is the 26-year old son of patient 1. At birth he had thrombocytopenia initially attributed to maternal heparin injections. Easy bruising was noted within the first year of life. At 12 months, muscle weakness was noted. He could not jump and he ran slower than other children. His muscle weakness, particularly proximal, progressed slowly.
At NIH at age 26 years, he reported that he was able to perform activities of daily living and to walk but not run. He had difficulty climbing stairs and rising from a chair. Examination showed multiple bruises. He had miosis and his pupils did not accommodate to darkness or react to light (supplementary video). On eye examination his pupils dilated poorly; after 3 sets of eye drops, the pupils dilated from 3mm to 5mm. He had external upgaze palsy. The platelet count was 60,000 /μL, WBC 6.2 K/μL, hemoglobin 13.5 g/dL, and hematocrit 39.4%. Blood smear showed platelets with different sizes ranging from very small to very large; some were hypogranular. Creatine kinase was 2541 U/L (normal, 52-386). MRI of hips and thighs showed bilateral symmetrical, moderate-to-severe fatty infiltration (Figure 3B). Abdominal USG showed severe fatty infiltration of the liver and borderline enlarged spleen.
Patient A-IV-1
(UDP 7935), age 1 year, was the son of patient 2. He had congenital thrombocytopenia; platelet count at birth was 28,000/μL and subsequently ranged between 30,000 and 69,000. His growth was normal and he met all his developmental milestones. Neurological examination was within normal. CK was elevated at 751 U/L (normal <160).
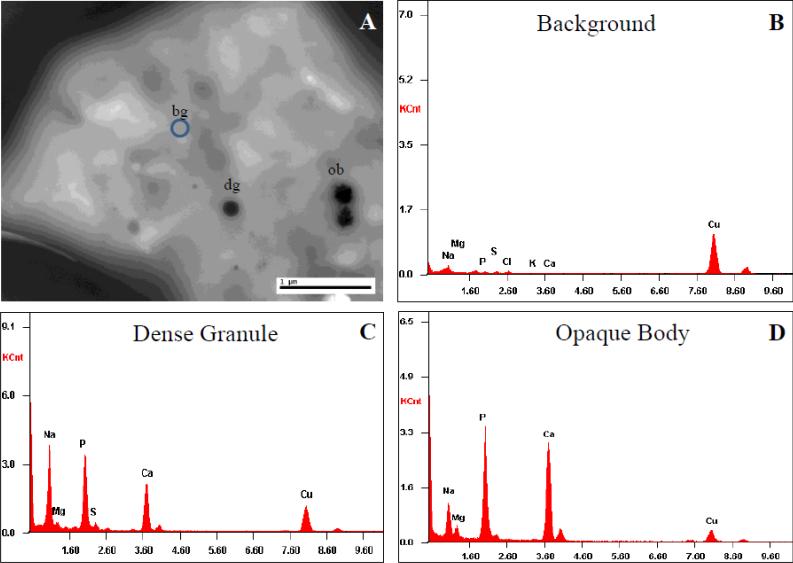
Platelet EM was consistent with YPS (Figure 4). Whole mount EM study demonstrated moderately reduced average dense bodies/platelet (0.6 dense bodies/platelet; normal range is >1.5 dense bodies/platelet). In addition, there were various irregular-shaped opaque objects. Platelet thin section EM study demonstrated normal-sized platelets with occasional large forms. Platelets contained moderately decreased alpha granules, and increased vacuoles and dense tubular network. There was no overt increase in Golgi complexes. There were rare large opaque objects and clusters, and rare targetoid inclusions. Abnormal platelet-platelet adhesion was also noted. Several complex smooth endoplasmic reticulums were seen. EM study of the buffy coat showed normal-appearing neutrophils, monocytes and lymphocytes. There were no identifiable cytoplasmic inclusions. X-ray diffraction studies revealed a large amount of calcium within the opaque bodies (Figure 5).
Figure 4. Platelet electron microscopy of patient A-IV-1A.
(A) Thin section transmission electron microscopy studies (TEM). Left panel shows normal platelet ultrastructure and alpha granules. Middle panel shows abnormal electron dense inclusion in a platelet of patient A-IV-1. Clusters (*) are in close association with an opaque body (O) and an area of fusion with clusters can be seen between them. Right panel shows abnormal target-like organelles (T).
(B) Whole mount TEM. Left panel shows normal dense bodies (DB) and chains (Ch). Middle panel shows abnormal opaque bodies (O) and clusters (*). Right panel shows target-like organelles (T).
(C) Thin section TEM of platelet. Opaque (O) and target-like (T) abnormal organelles are apparent.
(D) Whole mount TEM of platelet. Opaque bodies (O) and clusters (*) are evident.
Figure 5. Electron microscopy energy dispersive spectra of the opaque bodies.
(A) Whole mount transmission electron microscopy of the platelet was subjected to X-ray energy dispersive spectrum analysis. Areas studied included background (bg, B), a normal-appearing dense granule (dg, C), and an opaque body (ob, D) of patient A-IV-1.
(B) Background spectrum.
(C) Spectra of the normal-appearing dense granule, with prominent calcium and phosphorus peaks.
(D) Spectra of the opaque body show similar prominent calcium and phosphorus peaks.
Patient B-II-2
(UDP 2543), a 4 year-7 month-old female, was born at term. At 1 year of age, she had easy bruising and frequent epistaxis; the platelet count was low at 18 months and gray platelet syndrome was considered at 33 months. Motor, speech, and language development were normal.
At NIH, at 4 years 7 months, she had several bruises. She had difficulty climbing stairs, and walking long distances. Platelet count was 120, 000/μL. Hemoglobin, hematocrit and white blood cell counts were normal at 13.4 gr/dL, 39.8 % and 5.92 K/uL, respectively. CK was 668 U/L (normal, 0-143).
Patient C-II-1
Patient C-II-1 (UDP 10008) is a 6 year-old boy who was born at full term with an anoxic CNS insult from a precipitous vaginal delivery. He had seizures in the second week of life. He had diffuse petechiae at birth, with a platelet count of 19,000/μL. Computerized tomography of the head did not show intracranial hemorrhage, but he had periventricular leukomalacia. His most recent brain MRI confirmed stable periventricular leukomalacia. He experienced balance and coordination difficulties, tripping and falling frequently. CK was elevated at 412 U/L (normal, <160). His platelet counts ranged from 25,000-50,000/μL and he had easy bruising, recurrent epistaxis and blood in his stool. He had platelet transfusions at ages 2 and 4 for a torn frenulum and a perforated ear drum, respectively, both with bleeding, and had an adequate response to transfusion with normal platelet survival. Peripheral blood smear revealed many large agranular and hypogranular platelets along with normal platelets. Bone marrow biopsy showed preserved megakaryocytes that were morphologically unremarkable, and no reticulin fibrosis. Differential diagnostic considerations included gray platelet syndrome, but the platelet electron microscopy was consistent with YPS.
Patient D-1-1
(UDP 10023) was a male in his early 40s, diagnosed with “tubular aggregate myopathy” by muscle biopsy 6 years prior when he presented with progressive proximal muscle weakness, early fatigue, recurrent falls, and serum CK levels of 3000-4000 U/L. In retrospect, he had mild muscle weakness since childhood. He had no recognized bleeding tendencies, and underwent surgeries without excessive bleeding. Platelet electron microscopy showed features identical to those of reported cases of YPS.
Patient D-II-1
(UDP 10024) was the 7 month-old son of patient 7. He had thrombocytopenia; platelet counts ranged from 90,000 to 110,000/μL. At age 13 months, his thrombocytopenia was stable and his platelets exhibited morphologic findings similar to those of his father on light and electron microscopy, i.e., consistent with YPS. The infant did not have unusual bruising or bleeding, but his CK was persistently mildly elevated at 350-500 U/L.
Platelet Studies
Aggregation
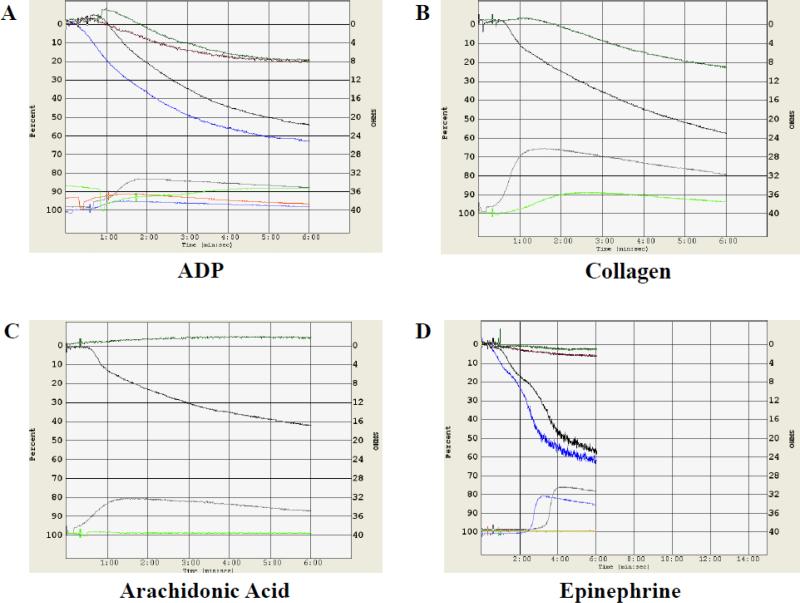
Whole blood aggregation studies, performed on patients A-III-1 and B-II-2, were abnormal. Aggregation to low dose (10 mM) or high dose (20 mM) ADP was only slightly present (Figure 6A). ATP was not released from dense granules in low dose ADP, but was released in high dose ADP. Low dose and high dose (5 mg/mL) collagen aggregation was present. For patient A-III-1, release of ATP from dense granules with low and high dose collagen was absent; for patient BII-2, it was absent with low dose collagen but present with high dose collagen (Figure 6B). For both patients, release of ATP from dense granules was present with thrombin. Arachidonic acid aggregation was absent (Figure 6C). The platelet aggregation response to both low dose and high dose ristocetin was normal. Both low and high dose epinephrine failed to cause platelet rich plasma aggregation or ATP release from dense granules (Figure 6D).
Figure 6. Platelet aggregation studies.
(A) Whole blood aggregation measured by impedance shows decreased aggregation in patient BII-2 at low dose (10 mM, red tracing) or high dose (20 mM, green tracing) ADP as compared to normal control in black (low dose) and blue (high dose). At bottom of the panel, ATP-induced chemiluminescence is reduced in patient B-II-2 compared to the control.
(B) Whole blood aggregation showed decreased aggregation in patient B-II-2 at high dose (5 mg/mL, green tracing) collagen compared to normal control in black (high dose). At bottom of the panel, ATP-induced chemiluminescence is reduced in patient B-II-2 compared to the control.
(C) Whole blood aggregation in arachidonic acid for patient B-II-2 is absent for arachidonic acid (green tracing) and present for the normal control (black tracing). At bottom of the panel, ATP-induced chemiluminescence is absent in patient B-II-2 but present in the control.
(D) Aggregation is absent for patient B-II-2 in platelet rich plasma stimulated with low dose (7.5 mM, red tracing) or high dose (21.5 mM, green tracing) epinephrine, while primary and secondary waves of aggregation are seen in the normal control stimulated with low dose (7.5 mM, black tracing) or high dose (21.5 mM, blue tracing) epinephrine. At bottom of panel, ATP-induced chemiluminescence is absent patient B-II-2 but present in the normal control, concomitant with the second wave of aggregation at high and low doses of epinephrine.
Flow cytometry
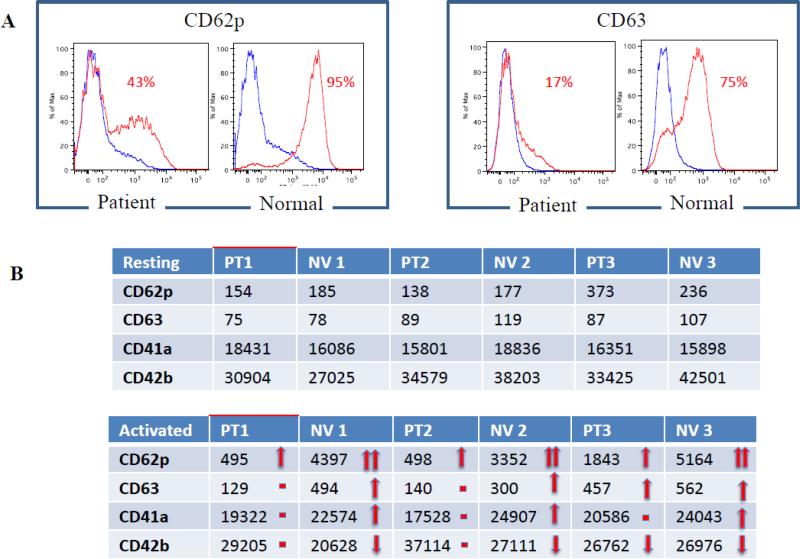
Flow studies were performed on patients A-II-2, A-III-1, and B-II-2. Using forward scatter (FCS), the patients’ platelets were 1.3 times smaller than normal controls (p=0.069). There was no significant difference in platelet granularity between patients and healthy controls using side scatter (SSC) analysis (not shown). No major differences were noted in any tested marker expression in the resting stage, but there were large differences in the rates of platelet activation between patients and normal controls. All 3 patients had much weaker responses to activation by CD62p, CD63, CD41a and CD42b compared with healthy controls (Figure 7). CD9, CD31, CD36 and CD61 showed no significant differences in expression between patients and healthy controls during the resting stage or after activation (not shown).
Figure 7. Flow cytometric analysis of platelet marker expression during resting stage and after activation.
Platelets from all three patients that underwent flow cytometric analysis had much weaker responses to activation by CD62p, CD63, CD41a and CD42b compared to normal controls processed in parallel. The expression of platelet surface markers was evaluated as a percentage of positive platelets (A) and by comparing geometric means of fluorescence (B).
Muscle biopsies
Patients A-II-2 and A-III-1 had muscle biopsies performed at NIH. Microscopic sections of skeletal muscle of patient A-II-2 (Figure 3A) showed marked variation in myofiber size due to angular and rounded atrophic myofibers and hypertrophied fibers interspersed among fibers of normal diameter. The Masson trichrome stain showed marked endomysial fibrosis accompanied by extensive fat infiltration (Figure 3A). Scattered pyknotic nuclear clumps and increased numbers of myofibers with internalized nuclei were seen. Myopathic changes including occasional myofibers with myophagocytosis, split fibers and increased numbers of myofibers with internalized nuclei were present. There was no inflammation, vasculitis, perivascular alkaline phosphatase staining (inflammatory product spillage) or increased expression of major histocompatibility complex I antigen. There was no evidence of mitochondrial disorder.
Microscopic sections of the skeletal muscle from patient A-III-1 (Figure 3B) showed marked variation in myofiber size due to rounded and angular atrophic myofibers and hypertrophied fibers interspersed among fibers of normal diameter. Scattered esterase positive myofibers were present. A Masson trichrome stain showed moderate endomysial fibrosis accompanied by fat infiltration (Figure 3B). There were myopathic changes, including degenerating fibers, regenerating fibers and increased numbers of myofibers with internalized nuclei. There was no definite evidence of inflammation, vasculitis or perivascular alkaline phosphatase staining. Focal patchy increased expression of major histocompatibility complex I antigen was noted. The Gomori trichrome stained section showed vacuoles lined by granular fuchsinophilic material consistent with rimmed vacuoles (Figure 3B). There was increased acid phosphatase staining. No pathological deficiencies in dystrophy-associated proteins were identified using an extended dystrophy panel. Electron microscopy showed skeletal muscle fibers with overall well-preserved cytoarchitecture including Z bands and myofibrils, vacuoles limited by a distinct membrane and some loosely scattered electron dense granules (Figure 3C). Abundant square and polygonal vacuoles limited by a distinct membrane, and some loosely scattered electron dense granules, were seen; these may represent ill-preserved glycogen. They were often associated with mitochondria. Paracrystalline inclusions were not identified. Focal areas of myelin figures and autophagic vacuoles were identified; definite tubulofilamentous inclusions were not seen.
Other findings
None of the patients had muscle cramps or pain. Nerve conduction studies and electromyelography, performed on patients A-II-2, A-III-1, and B-II-2 suggested a primary muscle disorder with membrane irritability. Echocardiogram in all patients was normal. Kidney and liver function tests in all patients were normal. Patients A-II-2, A-III-1, and B-II-2 had metabolic screening tests including plasma total and free carnitine, plasma acylcarnitine profile, urine organic acids, lactic acid, pyruvic acid and plasma coenzyme Q10 levels and were all normal. Electron microscopy of skin biopsy performed in patient A-III-1 and patient B-II-2 showed unremarkable epidermis and dermis with normal small capillaries, fibroblasts, and unmyelinated nerve fibers. Vitamin B12 was elevated in patients B-II-2 and C-II-1 at 1555 pg/mL (normal, 256-1320), and 1658 pg/mL (normal, <894), respectively but was normal in patients A-II-2 and A-III-1 at 1019 and 625 pg/mL (normal, 256-1320).
DNA Sequencing Findings
DNA sequencing from patients in families A and B was performed to look for de novo events occurring between two generations of family B(23); a single de novo dominant variant in STIM1 (c.343A>T) was found in both families. This was verified by Sanger sequencing, which also showed a disease-segregating mutation, c.910C>T, in YPS patients I-1 and II-1 in family C, and II-1 in family D. The c.343A>T mutation encodes p.Ile115Phe, predicted deleterious by SIFT and MutationTaster and conserved across species to C. elegans (Figure 1I). The c.910C>T mutation encodes p.Arg304Trp, predicted deleterious by SIFT and MutationTaster and conserved across species to C. elegans (Figure 1J).
DISCUSSION
We report 7 individuals in 4 families with classic presentations of autosomal dominant York Platelet Syndrome, including thrombocytopenia, delta storage pool deficiency, typical platelet morphological abnormalities, and a myopathy characterized by rimmed vacuoles in muscle fibers. All 7 of our YPS patients had a monoallelic mutation in STIM1, i.e., either c.343A>T (p.Ile115Phe) or c.910C>T (p.Arg304Trp). STIM1 mutations were previously reported in association with myopathy and immune deficiencies when they were biallelic and caused haploinsufficiency, or reduced activity of STIM1/ ORAI1 (24-27) (Supplementary table 1). STIM1 mutations were recently linked to myopathy, miosis, thrombocytopenia, and asplenia when they were monoallelic and caused gain of function (11)(12).
One of the two YPS mutations, c.910C>T, is the same STIM1 mutation reported in patients with Stormorken syndrome, a tubular aggregate myopathy with thrombocytopenia, miosis and functional or anatomical asplenia (12), (28, 29). The second YPS mutation, c.343A>T was previously reported as a de novo mutation in a female child with apparently isolated tubular aggregate myopathy who, similar to our patients, presented at age 4 years with limb girdle weakness with a positive Gower's sign (10). This missense mutation replaces the highly conserved isoleucine at position 115 of STIM1 with a phenylalanine. Residue 115 is located in the EF2-hand motif. The “hidden” EF2-hand function is important to stabilize the canonical EF1-hand through hydrogen bonding between the respective loop regions of each motif (C(O) Val83, Ile115: N(H) Val83, Ile115), forming a small antiparallel beta-sheet. Therefore, amino-acid substitution of STIM1 EF2-hand induces abnormal calcium responses in vitro (30). This supports the idea that the Ile115Phe mutation in the EF2-hand induces a conformational change in STIM1 and strongly alters EF-hand calcium binding. The physiological consequence of this could be that Ile115Phe mutant STIM1 associates with the Orai1 channel without depletion of calcium store and induces “leaky” store-operated Ca2+ entry in resting platelets, as similarly observed in Stim1Sax murine platelets(5). Interestingly, calcium accumulated within the opaque bodies of YPS platelets in the resting state supporting this idea (Figure 5). The EF2-hand is responsible for sensing changes in store-operated and basal cytoplasmic Ca2+ levels and initiates oligomerization. The EF hands sense and bind Ca2+ and in the bound state, the EF hands and the SAM domain are in steric proximity. When Ca2+ is unbound as a result of depletion, STIM1 unfolds and oligomerizes through its SAM domains (30). Other gain of function mutations in the EF hand domain of STIM1 are known to cause tubular aggregate myopathy (9). Further support for the pathogenic nature of c.343 A>T lies in the fact that more than 6000 normal controls were homozygous for reference at this location.
YPS and Stormorken syndrome patients display other similarities. Both Stormorken syndrome (31), and YPS are associated with myopathy. Stormorken patients have miosis (32), and two of our patients (A-II-2 and A-III-1) have small, fixed pupils that did not accommodate to darkness or respond to light or medications. Atrophy of the extraocular muscles on imaging and the clinical observation of restricted vertical and horizontal eye movements suggest a myopathy of the oculomotor muscles. Stormorken patients have splenic aplasia/functional asplenia and our YPS patient A-II-2 had Howell-Jolly bodies and a small spleen that was difficult to visualize on ultrasonography.
Finally, although myopathy is the primary manifestation in gain of function STIM1 mutations, platelet abnormalities have also been reported. Specifically, platelets in those individuals were in a pre-activated state, with high exposure of aminophospholipids on the outer surface of the plasma membrane (12). In addition, resting Ca2+ levels were elevated and store-operated Ca2+ entry was markedly attenuated. In our YPS patients, platelet flow cytometry results were consistent with this finding. Moreover, Misceo et al. found an increased percentage of microparticles in a patient with gain of function STIM1 mutations, indicating platelet activation similar to that observed during activated coagulation and fibrinolysis (12). Our YPS patients exhibited a similar accumulation of microvesicles.
In the process of determining the molecular basis of the largest series of YPS patients reported to date, we acquired new understanding of this disorder. First, patient A-II-2 had two thrombotic events, a clinically important feature not previously reported in YPS. However, mice with an EF hand mutation in Stim1 displayed premature platelet activation (5). Second, our x-ray diffraction studies of platelets revealed a large amount of calcium within the opaque bodies of YPS (Figure 4D), explaining their electron-opaque appearance. Third, flow cytometry, performed for the first time in YPS, showed weak responses to activation by CD62p, CD63, CD41a and CD42b. Finally, we characterized the muscle pathology of YPS as a myopathy with vacuoles that could represent glycogen vacuoles or sarcotubular vacuoles.
In summary, YPS occurs due to heterozygous mutations in STIM1, including c.343A>T and c.910C>T. Moreover, YPS and Stormorken syndrome, both caused monoallelic gain of function mutations in STIM1, belong to the same spectrum of disorders caused by constitutionally open CRAC channels (“CRAC channelopathies”). The emphasis on signs and symptoms represented by different organ systems (myopathy for Stormorken, thrombocytopenia for YPS) reflect the interests and the methods of ascertainment by the specialists who first described these disorders. Indeed, platelet electron microscopy was not performed in patients with syndromic and non syndromic forms of myopathy due to STIM1 mutations. We predict that, if performed, it would reveal features typical of YPS. Certainly, the role of store-operated Ca++ entry in platelet function can now be profitably investigated using YPS platelets. Perhaps more important is the potential therapeutic compounds that target the CRAC channel that may be useful for alleviating the myopathy, platelet dysfunction and other associated features for all patients with gain of function STIM1 mutations.
Supplementary Material
Extra Table
| What is known on this topic | What this paper adds |
|---|---|
| • YPS patients have abnormal platelets and an unspecified type of myopathy. | • YPS causes aplenia/hyposplenia and a progressive myopathy associated with miosis and rimmed vacuoles on electrone microscopy; YPS and Stormorken syndromes belong to the same spectrum of disorders caused by constitutionally open CRAC channels. |
| • The molecular cause of YPS is unknown | • YPS occurs due to specific, heterozygous gain-of-function mutations in STIM1 |
Acknowledgments
This study was supported by the Intramural Research Programs of the National Human Genome Research Institute and the Office of the Director's Common Fund in support of the NIH Undiagnosed Diseases Program. Dr. Hans Goeble reviewed the muscle biopsies of patients A-II-2 and A-III-1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Parekh AB, Putney JW., Jr. Store-operated calcium channels. Physiol Rev. 2005 Apr;85(2):757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- 2.Hoth M, Penner R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature. 1992 Jan 23;355(6358):353–6. doi: 10.1038/355353a0. [DOI] [PubMed] [Google Scholar]
- 3.Braun A, Varga-Szabo D, Kleinschnitz C, Pleines I, Bender M, Austinat M, et al. Orai1 (CRACM1) is the platelet SOC channel and essential for pathological thrombus formation. Blood. 2009 Feb 26;113(9):2056–63. doi: 10.1182/blood-2008-07-171611. [DOI] [PubMed] [Google Scholar]
- 4.Varga-Szabo D, Braun A, Kleinschnitz C, Bender M, Pleines I, Pham M, et al. The calcium sensor STIM1 is an essential mediator of arterial thrombosis and ischemic brain infarction. J Exp Med. 2008 Jul 7;205(7):1583–91. doi: 10.1084/jem.20080302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grosse J, Braun A, Varga-Szabo D, Beyersdorf N, Schneider B, Zeitlmann L, et al. An EF hand mutation in Stim1 causes premature platelet activation and bleeding in mice. J Clin Invest. 2007 Nov;117(11):3540–50. doi: 10.1172/JCI32312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, et al. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006 May 11;441(7090):179–85. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- 7.Byun M, Abhyankar A, Lelarge V, Plancoulaine S, Palanduz A, Telhan L, et al. Whole-exome sequencing-based discovery of STIM1 deficiency in a child with fatal classic kaposi sarcoma. J Exp Med. 2010 Oct 25;207(11):2307–12. doi: 10.1084/jem.20101597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Picard C, McCarl CA, Papolos A, Khalil S, Luthy K, Hivroz C, et al. STIM1 mutation associated with a syndrome of immunodeficiency and autoimmunity. N Engl J Med. 2009 May 7;360(19):1971–80. doi: 10.1056/NEJMoa0900082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bohm J, Chevessier F, Maues De Paula A, Koch C, Attarian S, Feger C, et al. Constitutive activation of the calcium sensor STIM1 causes tubular-aggregate myopathy. Am J Hum Genet. 2013 Feb 7;92(2):271–8. doi: 10.1016/j.ajhg.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hedberg C, Niceta M, Fattori F, Lindvall B, Ciolfi A, D'Amico A, et al. Childhood onset tubular aggregate myopathy associated with de novo STIM1 mutations. J Neurol. 2014 May;261(5):870–6. doi: 10.1007/s00415-014-7287-x. [DOI] [PubMed] [Google Scholar]
- 11.Nesin V, Wiley G, Kousi M, Ong EC, Lehmann T, Nicholl DJ, et al. Activating mutations in STIM1 and ORAI1 cause overlapping syndromes of tubular myopathy and congenital miosis. Proc Natl Acad Sci U S A. 2014 Mar 18;111(11):4197–202. doi: 10.1073/pnas.1312520111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Misceo D, Holmgren A, Louch WE, Holme PA, Mizobuchi M, Morales RJ, et al. A dominant STIM1 mutation causes stormorken syndrome. Hum Mutat. 2014 May;35(5):556–64. doi: 10.1002/humu.22544. [DOI] [PubMed] [Google Scholar]
- 13.White JG, Pakzad K, Meister L. The york platelet syndrome: A fourth case with unusual pathologic features. Platelets. 2013;24(1):44–50. doi: 10.3109/09537104.2012.658527. [DOI] [PubMed] [Google Scholar]
- 14.White JG, Gunay-Aygun M. The york platelet syndrome: A third case. Platelets. 2011;22(2):117–34. doi: 10.3109/09537104.2010.524323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.White JG, Ahlstrand GG. Giant electron dense chains, clusters and granules in megakaryocytes and platelets with normal dense bodies: An inherited thrombocytopenic disorder III. platelet analytical electron microscopy. Platelets. 2003 Aug;14(5):305–12. doi: 10.1080/0953710031000137046. [DOI] [PubMed] [Google Scholar]
- 16.White JG, Ahlstrand GG. Giant electron dense chains, clusters and granules in megakaryocytes and platelets with normal dense bodies: An inherited thrombocytopenic disorder IV. ultrastructural cytochemistry and analytical electron microscopy. Platelets. 2003 Aug;14(5):313–24. doi: 10.1080/09537100310001594534. [DOI] [PubMed] [Google Scholar]
- 17.White JG. Giant electron-dense chains, clusters and granules in megakaryocytes and platelets with normal dense bodies: An inherited thrombocytopenic disorder I. megakaryocytes. Platelets. 2003 Feb;14(1):53–60. doi: 10.1080/095371002100057550. [DOI] [PubMed] [Google Scholar]
- 18.White JG. Giant electron-dense chains, clusters and granules in megakaryocytes and platelets with normal dense bodies: An inherited thrombocytopenic disorder. Platelets. 2003 Mar;14(2):109–21. doi: 10.1080/0953710031000080044. [DOI] [PubMed] [Google Scholar]
- 19.Gahl WA, Boerkoel CF, Boehm M. The NIH undiagnosed diseases program: Bonding scientists and clinicians. Dis Model Mech. 2012 Jan;5(1):3–5. doi: 10.1242/dmm.009258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gahl WA, Tifft CJ. The NIH undiagnosed diseases program: Lessons learned. JAMA. 2011 May 11;305(18):1904–5. doi: 10.1001/jama.2011.613. [DOI] [PubMed] [Google Scholar]
- 21.White JG. Giant electron-dense chains, clusters and granules in megakaryocytes and platelets with normal dense bodies: An inherited thrombocytopenic disorder. Platelets. 2003 Mar;14(2):109–21. doi: 10.1080/0953710031000080044. [DOI] [PubMed] [Google Scholar]
- 22.Wang L, Si Y, Dedow LK, Shao Y, Liu P, Brutnell TP. A low-cost library construction protocol and data analysis pipeline for illumina-based strand-specific multiplex RNA-seq. PLoS One. 2011;6(10):e26426. doi: 10.1371/journal.pone.0026426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roach JC, Glusman G, Smit AF, Huff CD, Hubley R, Shannon PT, et al. Analysis of genetic inheritance in a family quartet by whole-genome sequencing. Science. 2010 Apr 30;328(5978):636–9. doi: 10.1126/science.1186802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feske S. ORAI1 and STIM1 deficiency in human and mice: Roles of store-operated Ca2+ entry in the immune system and beyond. Immunol Rev. 2009 Sep;231(1):189–209. doi: 10.1111/j.1600-065X.2009.00818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feske S, Picard C, Fischer A. Immunodeficiency due to mutations in ORAI1 and STIM1. Clin Immunol. 2010 May;135(2):169–82. doi: 10.1016/j.clim.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feske S, Skolnik EY, Prakriya M. Ion channels and transporters in lymphocyte function and immunity. Nat Rev Immunol. 2012 Jun 15;12(7):532–47. doi: 10.1038/nri3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shaw PJ, Feske S. Regulation of lymphocyte function by ORAI and STIM proteins in infection and autoimmunity. J Physiol. 2012 Sep 1;590(Pt 17):4157–67. doi: 10.1113/jphysiol.2012.233221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stormorken H, Sjaastad O, Langslet A, Sulg I, Egge K, Diderichsen J. A new syndrome: Thrombocytopathia, muscle fatigue, asplenia, miosis, migraine, dyslexia and ichthyosis. Clin Genet. 1985 Nov;28(5):367–74. doi: 10.1111/j.1399-0004.1985.tb02209.x. [DOI] [PubMed] [Google Scholar]
- 29.Stormorken H, Holmsen H, Sund R, Sakariassen KS, Hovig T, Jellum E, et al. Studies on the haemostatic defect in a complicated syndrome. an inverse scott syndrome platelet membrane abnormality? Thromb Haemost. 1995 Nov;74(5):1244–51. [PubMed] [Google Scholar]
- 30.Stathopulos PB, Zheng L, Li GY, Plevin MJ, Ikura M. Structural and mechanistic insights into STIM1-mediated initiation of store-operated calcium entry. Cell. 2008 Oct 3;135(1):110–22. doi: 10.1016/j.cell.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 31.Mizobuchi M, Tanaka C, Sako K, Murakami N, Nihira A, Abe T, et al. Muscle involvement of stormorken's syndrome. Rinsho Shinkeigaku. 2000 Sep;40(9):915–20. [PubMed] [Google Scholar]
- 32.Sjaastad O, Aasly J, Stormorken H, Wysocka-Bakowska MM, Horven I, Fredriksen TA. A new hereditary syndrome with a bleeding tendency, extreme miosis, spasms, dyslexia, thrombocytopathia etc. pupillometric, evaporimetric, and ophthalmological observations. Acta Ophthalmol (Copenh) 1992 Dec;70(6):713–20. doi: 10.1111/j.1755-3768.1992.tb04874.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.