Abstract
Background
Injectable hormonal contraception may increase women’s risk of HIV acquisition, and can affect biological risk factors in animal models of HIV. We established, for the first time, a model to investigate whether combined oral contraceptives (COC) alter SHIV susceptibility in macaques.
Methods
Seven pigtail macaques were administered a monophasic levonorgestrel (LNG)/ethinyl estradiol (EE) COC at 33% or 66% of the human dose for 60 days. Menstrual cycling, vaginal epithelial thickness and other SHIV susceptibility factors were monitored for a mean of 18 weeks.
Results
Mean vaginal epithelial thicknesses was 290.8 μm at baseline and 186.2 μm during COC (p=0.0141, Mann Whitney test). Vaginal pH decreased from 8.5 during to 6.5 post- treatment (0.0176 two-tailed t-test). Measured microflora was unchanged.
Conclusions
COC caused thinning of the vaginal epithelium and vaginal pH changes, which may increase SHIV susceptibility. 0.033 mg LNG + 0.0066 mg EE appeared effective in suppressing ovulation.
Keywords: contraception, SHIV, susceptibility, transmission risk, Non-Human Primate (NHP)
Introduction
Hormonal contraception (HC) with high doses of synthetic progesterone may increase HIV (Human Immunodeficiency Virus) risk in women, a concern raised by several studies [1-3]. Most concerns have focused on Depot-medroxyprogesterone acetate (DMPA). In women, DMPA, often referred to by the trade name Depo-Provera® (Pfizer; New York, New York), is injected intramuscularly every three months at a bolus dose of 150 mg. It is a widely used contraceptive in Africa, where HIV transmission rates are high. Progestin administration mimics the high progesterone levels that occur naturally during pregnancy and during the luteal phase of the menstrual cycle, situations that are associated with increased HIV risk in some human studies, and increased SIV risk in animal studies [4-7]. Biological mechanisms for increased HIV risk during periods of high progesterone levels or with progestin administration are multifactorial, and include: vaginal epithelial thinning, bacterial dysbiosis, altered pH, and likely also direct effects on genital immunity. The vaginal epithelium serves as a barrier for HIV infection. Marx et al and others documented severe thinning of the vaginal epithelium in rhesus and pigtail macaques after DMPA use [8-13]. The stratum corneum, the superficial epithelial layer, maintains the vaginal microbiome [14], which in turn regulates the vaginal pH. Imbalance of the bacterial microbiome as seen during bacterial vaginosis is a documented risk factor for HIV infection [15]. Progesterone and synthetic progestins likely have additional effects on immunity to genital pathogens like HIV [16, 17]. Many of these biological mechanisms are being investigated in animal models, which allow more frequent and invasive access to genital tissues than is feasible in human studies.
In the US, oral birth control products are more popular than DMPA injections [18]. Seventeen percent of reproductive age women in the US used oral contraceptives from 2006 – 2010, compared to 2.3% using DMPA. These products are often referred to as combined oral contraceptives (COC) and deliver a combination of synthetic estrogen and progesterone, induce lower systemic levels of synthetic hormones, and have fewer side effects than DMPA. To date, COCs have not been associated with significantly increased HIV risk [1-3], although the issue warrants further investigation for all age groups and sub-groups with co-sexually transmitted infections (STI). Regardless, careful analysis of biological HIV risk factors after COC use has not been undertaken. To begin addressing these issues, we set out to analyze mucosal HIV susceptibility factors in an animal model.
We used a pigtail macaque model to determine the effect of oral contraceptives on mucosal SHIV (Simian Human Immunodeficiency Virus) susceptibility factors. This species was chosen because it is a suitable model for female-specific, HIV- related research. These animals have year-round menstrual cycles, perineal sex skin swellings that are a visible indicator of the menstrual cycle sex hormone levels [19], and are easier to infect vaginally than rhesus macaques, which have more varying cycles [20, 21]. We have previously shown that pigtail macaques have higher susceptibility to SHIV infection around the time of menstruation, during and after periods of high progesterone [6, 7]. This model has previously been used to document the effects of DMPA on HIV susceptibility factors [12].
We now hypothesized that a commercially available COC, a synthetic levonorgestrel (LNG)/ethinyl estradiol (EE) combination [22, 23] used in women at a dose of 0.10 mg LNG and 0.02 mg EE, would effectively suppress ovulation [24]. We tested two doses for this purpose. Furthermore, we hypothesized that the progestin-dominated product would affect known HIV susceptibility factors by leading to a thinning of the vaginal epithelium, and possibly also to changes in pH, as has been observed for DMPA (K Butler, E Kersh et al, in preparation).
Materials and Methods
Humane Care Guidelines
Seven adult female pigtail macaques (Macaca nemestrina) were single housed in enrichment cages at the Centers for Disease Control and Prevention (CDC; Atlanta, GA). This is an AAALAC-accredited facility. Husbandry, housing, and all procedures were done in accordance with the Guide for the Care and Use of Laboratory Animals [25]. The study protocol was approved by the CDC Institutional Animal Care and Use Committee (IACUC).
Oral contraceptive treatment
The human product is marketed under the trade name, Alesse® (Wyeth; Philadelphia, PA) and contains a dosage of 0.1 mg LNG and 0.02 mg EE. This COC prevents pregnancy by suppressing ovulation, thickening cervical mucus, and suppressing proliferation of the endometrium [24]. Animals were randomly assigned to a low dose (0.033 mg LNG + 0.0066 mg EE) group (n=3) or a high dose (0.066 mg LNG + 0.0132 mg EE) group (n=4). The low dose group ranged in weights from 5.59 kg -7.18 kg and ages were from 6 yrs-9.5 yrs. of age. The animals in the high dose group ranged in weights from 5.56 kg-7.54 kg and ages were from 4 yrs-8.5 yrs. of age. The two doses given were based on previous studies that administered oral contraceptives to rhesus, cynomolgus, and pigtail macaques. Only one previous study [26] had used oral contraceptives in pigtail macaques; therefore, we extrapolated dosages from studies that used cynomolgus and rhesus macaques. The weight of the animal [27], species, metabolic rate [28-31], and success with inhibiting ovulation [26, 32] were all considered when selecting dosages. The dosing was aimed at modeling similar effects in humans, such as inhibiting ovulation and halting menstruation [24, 33]
Study Design
Procedures
Animals were anesthetized with 10mg/kg intramuscular (IM) ketamine hydrochloride prior to weekly sampling procedures. When vaginal biopsies were performed, 5mg/kg IM Tiletamine/Zolazepam (Telazol) was used as the anesthetic and animals were given meloxicam 0.2 mg/kg IM for pain relief. Blood was collected weekly from the femoral veins in vacutainer tubes (CPT: BD, Franklin Lakes, NJ, USA) for hormone and drug concentration analysis. Progesterone content was determined by EIA (Wisconsin National Primate Center, Madison; WI, USA). LNG/EE levels were analyzed by the Assay Services Unit & ICTR Lab Core (Wisconsin National Primate Center; Madison, WI, USA). While anesthetized, animals were placed in sternal recumbency, and a non-lubricated pediatric speculum was placed in the vaginal vault. Visual observation of sex skin swelling, menstruation, tissue integrity, and presence/consistency of cervical and vaginal discharge were recorded. At each vaginal biopsy appointment, three punch biopsies were collected per animal using a rigid biopsy punch (EuroMed®, Tuttlingen, Germany). Each sample location (proximal, mid, and distal) was recorded based on relative position in the vaginal vault (right lateral, ventral, left lateral and dorsal) in order to prevent future re-biopsy of scar tissue at previously biopsied sites. Gel foam was placed in the vaginal vault after biopsy to control bleeding. Animals were monitored for bleeding and discomfort after biopsy procedures.
Baseline
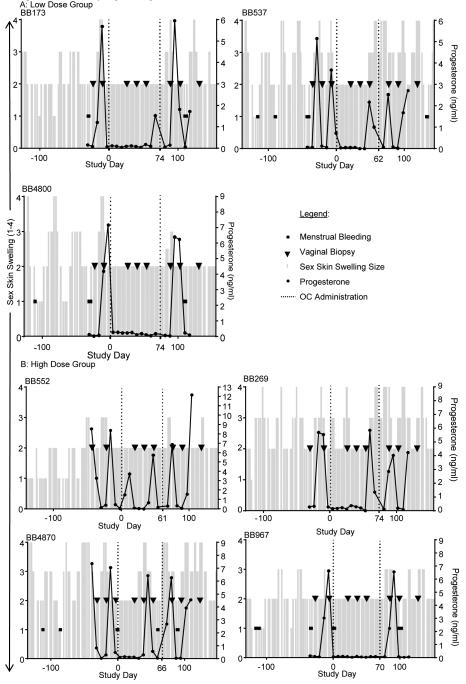
Animals were observed at least three times weekly for four months to evaluate the extent of perineal sex skin swelling and to visualize menstrual bleeding. Sex skin swellings were graded on a 1-4 scale, with one being no swelling and four being the most swollen. Plasma progesterone and vaginal pH were measured weekly for at least one month. Vaginal biopsies were obtained for baseline follicular and luteal phases, based on progesterone levels (Figure 1). Two animals (BB537 and BB4870) required a third biopsy in order to obtain an accurate baseline for both desired phases since the initial biopsy taken was in a transitional phase. Two bacterial cultures were done two weeks apart. LNG/EE was measured the week prior to starting the COC.
Figure 1. Menstrual cycling following COC use.
For 96 days prior to baseline sampling, animals were observed for menstrual bleeding and their perineal sex skin swellings were graded. The sex skin swellings were graded on a 1-4 scale, with 1 being no swelling up to 4 being the most swollen. The sex skin swellings are denoted as the columns on the x-axis. Menstrual bleeding is recorded as black dots. Baseline samples included progesterone levels and vaginal biopsies. Biopsies are shown as black triangles and progesterone levels are the solid black line. Progesterone levels were continued for four weeks after COC’s were discontinued. 2-3 biopsies were taken as baselines, three were taken during COC administration and three were taken after COC’s were discontinued. Study day zero is the first day of COC administration. COC’s were given for at least 62 days and no longer than 74 days. The first and last doses are shown on the time line as a dotted line extending from the x-axis. The weights for the animals were: BB173 (7.18kg), BB537 (6.79 kg), BB4800 (5.59kg), BB552 (7.21 kg), BB 269 (6.65 kg), BB4870 (7.54 kg), and BB967 (5.56 kg).
Treatment
During COC administration, LNG/EE, progesterone, vaginal pH, menstrual bleeding, and sex skin swellings were monitored weekly. Vaginal biopsies were taken at weeks three, five and seven. Bacterial cultures were done at two week intervals for a total of four samples.
Follow up
After COC withdrawal, weekly measurements included LNG/EE for one week, progesterone levels for four weeks, vaginal pH for six weeks, and sex skin swelling/menstrual bleeding for eight weeks. Vaginal biopsies were taken at weeks two, four and eight.
Drug administration
The COC was compounded by a pharmacist into sweet cherry flavored syrup (Humco Flavor Sweet; Texarkana, TX USA). Animals were given the syrup on raisin bread once daily and received either one cc of the compounded medication for the low dose or two ccs for the high dose. COC administration was started the Monday after a drop in their progesterone level was detected. This hormonal change coincided with a detumescence of the sex skin swelling. Animals received the COC for a minimum of 60 days. Since animals were started on the medication based on their individual cycles and all animals were withdrawn from COC on the same date, duration of drug administration varied from 62 days to 74 days (Figure 1).
Menstrual cycle determination
The menstrual cycle was determined based on the changes in sex skin swelling, menstrual bleeding, and progesterone levels (Figure 1). Since pigtails have a median 32 day menstrual cycle, we used the following phases, as defined previously (in days): follicular: 1–16, luteal: 17–32. The first day of the menstrual cycle was defined as the first day after the steepest decline in progesterone [6].
Vaginal pH and vaginal microbiome
Vaginal pH was determined weekly with colorphast pH test strips (EMD Millipore, Billerica, MA). Microflora from the vaginal compartment were collected at six of the weekly appointments in six animals with a polyester-tipped swab and placed in a Port-A-Cul tube (Department of Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh and the Magee-Women’s Research Institute Microbiology Research Laboratory). We cultured three animals in each group to exact a closer comparison of animals with menstrual cycles and therefore drug administration that started on approximately the same day.
Vaginal epithelial thickness
Vaginal epithelial thickness was measured using the HALO image analysis software’s epithelial thickness algorithm (Indica Labs, Corrales, NM) on whole slide images of H&E stained biopsy tissue sections captured with the ScanScope system (Aperio, Vista, CA). Measurements were taken at 50 μm intervals along the entire length of appropriately oriented tissue biopsies. Thicknesses of nucleated and non-nucleated (stratum corneum) cell layers were measured separately at each point, and when both layers were present, total thickness was calculated by addition of these two measurements.
LNG/EE Liquid Chromatography (LC)/ Mass Spectrometry (MS)/MS Method
Sample preparation
Unknowns, high, and low quality control pools were vortex-mixed and 350 μL of sample were transferred into glass tubes with screw caps. Standards were prepared by adding 100 μL of the working solution for each standard to 350 μL of rhesus monkey serum. Water was added to all the samples; 1 mL to the standards, and 1.1 mL to the unknowns and pools, and then 50 μL of the working internal standard solution (100 ng/mL LNG-d6, 10 ng/mL EE-d4) was added to all the samples, standards and pools. The tubes were vortexed and then centrifuged. The organic phase was pipetted into a clean test tube and evaporated. Ethanol was added to the test tube followed by the addition of water and dichloromethane. The tubes were vortexed and centrifuged. The dichloromethane layer was extracted into a clean test tube and evaporated in a water bath. The samples were then derivitized with dansyl chloride, dried and reconstituted in 75 μL 50% acetonitrile and transferred to minivials.
LC/MS/MS analysis
Aliquots of the derivitized extracts (30 μL) were separated on a Shimadzu Prominence (Addison, IL, USA) integrated HPLC interfaced with an AB SCIEX (Foster City, CA) QTRAP 5500 Quadrupole - Linear Ion Trap Mass Spectrometer (Framingham, MA, USA) operating in positive TurboIonSpray mode. The analytical column was a Phenomenex (Torrance, CA, USA) Kinetex (2.4 μm, 2.1 × 100 mm). Samples were eluted at a flow rate of 0.20 mL/min using a binary reversed phase gradient (Channel A = 0.1% formic acid in 18 MΩ/cm water, Channel B =0.1% formic acid in acetonitrile). The transitions monitored were: LNG 312.917-225.1 (quantitation), LNG 312.917-171.1 (qualifying), LNG-d6 318-973-129.1 and for EE: 530.092-128.8 (quantitation), 530.092 (qualifying), EE-d4 534.102-129.1. The limit of detection was 10pg/mL for EE and 500 pg/mL for LNG. Calibration curves were constructed by plotting the peak area ratio of the analyte against its deuterated version versus the corresponding concentration ratios and fitting the data using linear regression with 1/X weighting. Intra-assay variation was less than 6%, and inter-assay variation was less than 14% for both analytes.
Results
Effect of COC on menstrual cycling patterns in pigtail macaques
The impact of COC on menstrual cycling was documented because susceptibility to SHIV infection fluctuates during the menstrual cycle in this species [6, 7]. All seven macaques had evidence of cycling during the 4-6 weeks of baseline monitoring before administration and after cessation of the COC (Figure 1). In the low dose group, COC suppressed cycling completely in one animal (BB4800). For the two other animals (BB173 and BB537), it took seven weeks for the first progesterone peak to return, while still on medication. In the high dose COC group, menstruation was also completely suppressed in one animal (BB967). Two animals (BB4870 and BB269) had progesterone peaks after COC administration at seven and nine weeks, respectively. BB552 did not have progesterone suppression and had peaks at weeks two and seven.
Synthetic hormone levels
The hormonal components of the COC, levonorgestrel (LNG) and ethinyl estradiol (EE), were measured in each animal once at baseline, weekly during administration, and once after COC withdrawal. No animals had any LNG or EE detected in their baseline sample (Data not shown). The median concentration of LNG measured for the low dose and high dose groups was 0.3763ng/ml and 0.5919ng/ml, respectively. (Figure 2) EE levels were not detectable in both groups. Limit of detection was 0.1ng/ml for LNG and 0.006ng/ml for EE. All animals except one (BB4800) had LNG detectable one week after drug withdrawal. All animals had at least one sample with a non-detectable level of LNG during treatment.
Figure 2. Drug Levels.
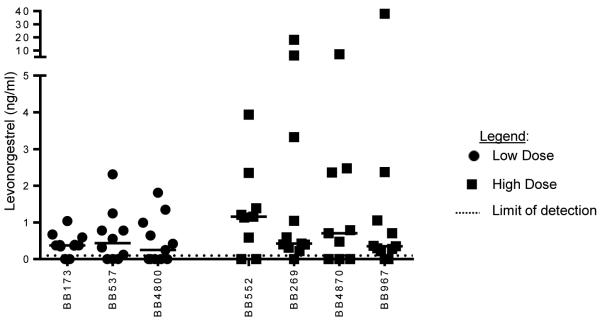
LNG and EE were measured one week prior to COC, during COC administration and one week after withdrawal. The low dose group is denoted with black dots and the high dose group is black squares. Ethinyl Estradiol levels in both groups were not detectable. Limit of detection was 0.1 (ng/ml) for LNG and (0.006 ng/ml) for EE. No synthetic hormones were detected in the baseline samples. The median amount of LNG measured for the low dose and high dose groups were 0.3763ng/ml LNG + 0.5919ng/ml respectively.
Evaluation of vaginal epithelial thickness as SHIV susceptibility factor
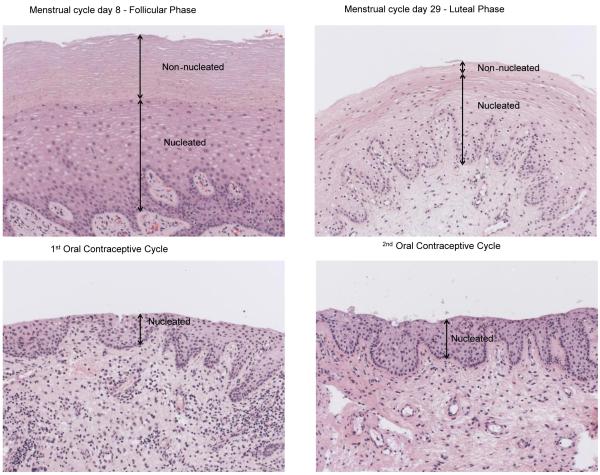
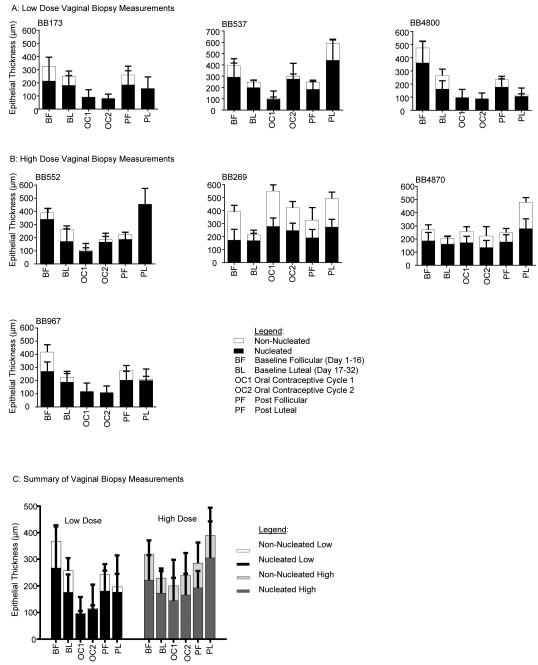
DMPA thins the vaginal epithelium [8-13], and this likely facilitates HIV entry to target cells residing below the epithelial cell layers [14].We evaluated whether COC with Alesse® (Wyeth, Philadelphia, PA) also thins the vaginal epithelium. Vaginal biopsies were obtained throughout the entire study to determine the effects of COC on the thickness and composition of the vaginal epithelium[9, 34, 35]. Non-nucleated and nucleated layers are illustrated in Figure 3, which shows the histology of BB967 as an example of the vaginal epithelial changes seen throughout the study. This animal displayed the typical baseline histologic features expected for the follicular and luteal phases; follicular phase epithelium has a thicker non-nucleated layer compared to the luteal phase. During administration of the COC, the non-nucleated layer was completely absent. The non-nucleated and nucleated layers were measured throughout the study and compared to the baseline follicular and luteal phase biopsies for each animal. Figures 4A and 4B show a quantitative evaluation of epithelial thickness for each animal, where we analyzed biopsies from the following time points: baseline follicular and luteal phase samples, samples during each 30 day COC administration cycle, and post-COC follicular and luteal phase samples. In 5 of the 7 animals (all except BB269 and BB4870), the epithelium was thinner three weeks after start of COC than it was in the two samples taken during undisturbed cycling (Fig 4B). The mean epithelial thicknesses of the two study groups is summarized in figure 4C. Three weeks after onset of COC, there was a mean thickness of 96.6 μm after the low dose COC, and 200.2 μm after the high dose. When all COC-treated animals were analyzed together, the mean epithelial thickness was 186.2 μm. In the follicular phase, mean thickness was 341.1μm, while it was 240.5μm in the luteal phase. This is a statistically significant thinning between phases (p=0.0379, Mann Whitney test). When baseline measurements were evaluated together, mean thickness was 290.8μm. Epithelium was thinned after COC treatment, with a mean thickness of 186.2μm (measured during both 30 day drug administration cycles). This thinning effect of COC is statistically significant (p=0.0141, Mann Whitney test).
Figure 3. Vaginal epithelial changes following COC administration.
Hematoxylin and eosin stained vaginal biopsies from BB967 during follicular (day 8), luteal (day 29), and first and second medicated phases. The follicular phase biopsy shows an overall thicker vaginal epithelial thickness, with a markedly thickened non-nucleated layer compared to the luteal phase biopsy. Both biosies taken during COC administration show a nucleated layer that is thinner than both follicular and luteal phase specimens, and complete absence of a non-nucleated layer.
Figure 4. Quantitative evaluation of vaginal epithelial thickness changes following COC use.
Mean epithelial thickness measurements for each animal in the low dose (A) and high dose (B) groups and summary of the mean measurements for both groups (C). The low dose group showed more consistent thinning of the vaginal epithelium during drug administration, with almost complete elimination of the non-nucleated layer, as compared to the high dose group.
Evaluation of Vaginal pH
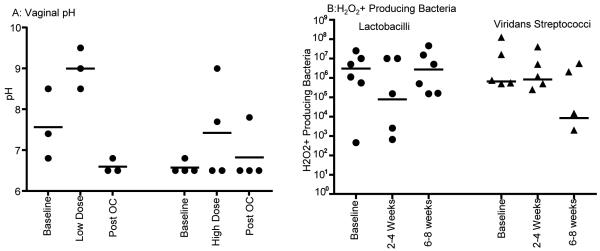
Throughout the entire study, pH measurements of the vaginal vault were obtained for all animals. (Figure 5A) The median pH at baseline, during COC treatment, and after COC treatment was 6.8, 8.5, and 6.5, respectively. The change in pH from baseline to during COC treatment was not significant (0.0644 two-tailed t test). The change in pH during COC treatment to post COC treatment was stastistically significant (0.0176 two-tailed t-test). Six animals (BB173, BB537, BB4800, BB552, BB269, and BB4870) had 6 bacterial cultures taken at two week intervals prior to and during COC administration to evaluate the types of bacteria present and potentially affecting the vaginal pH. Figure 5B shows the number of peroxide producing lactobacilli and viridans streptococci cultured during COC treatment. The median number of lactobacilli dropped from a median baseline of 3.055 × 106 to 7.65 × 104 during the first cycle of COC, and recovered during the second cycle of COC treatment to median 2.755 × 106 lactobacilli. The viridans streptococci started at a baseline of 6.5 × 105, rose slightly to 8.3 × 105, and then dropped to 8500 during the second cycle of COC. These differences were not statistically significant (Mann-Whitney test).
Figure 5. Vaginal pH and H202+ producing microflora following COC use.
A. Vaginal pH
The vaginal pH was taken on each animal weekly during the all three phases of the study. The median pH of each animal is shown as black dots. They are displayed separately in high and low dose groups.
B. Peroxide producing bacteria
Colonies of lactobacilli and viridans streptococci H2O2 producing bacteria are shown from baseline samples and both cycles of COC administration. The lactobacilli are shown as black dots and the viridans streptococci are shown as black triangles. Since the axis is logarithmic, only values greater than zero can be plotted. For this graph, five values were zero or negative, so are not included on the graph.
Discussion
This study is the first to introduce pigtail macaques as a model to study the effect of COCs on HIV susceptibility factors. We validated our hypothesis that a LNG/ EE combination, of which LNG is a widely used progestin found in various contraceptive products, can thin the vaginal epithelium and can also affect vaginal pH in this model. This animal model will be useful for further studies on hormonal contraception (HC) and HIV risk, which will improve our understanding of the relative safety of different HC products with regards to HIV transmission.
Overall, the HIV susceptibility factor of epithelial thickness was less affected by COC than has previously been reported for DMPA in this model [12], and was also found in unpublished studies by our group. In the current model, mean epithelial thickness after COC use was 186.2 μm, while after DMPA dosing in our laboratory, it was 53, 45, and 85 μm for 2.5, 1.5, or 0.5 mg/kg of DMPA, respectively (K Butler, E Kersh et al, in preparation). Similarly, pH rose to 7.7 after COC, while it rose to 8.2 after DMPA (K Butler, E Kersh et al, in preparation). This finding is consistent with oral contraceptives having a lesser effect on HIV risk than DMPA in human observational studies, although most studies suffered from small sample sizes and other problems that preclude sound comparisons between these HC using groups [1-3].
We tested two hormone doses with the hypothesis that the higher dose would result in more suppression of ovulation and stronger impact on SHIV susceptibility factors than the lower dose. Due to the small study groups, we refrained from a comparison of the two groups; however, our observations of effects on ovulation suppression, vaginal epithelial thinning, and pH increases in the small groups did not support our hypothesis, and the lower dose appeared more efficient in induction of expected effects. Future work with additional animals and greater statistical power could clarify the effects of different COC doses on these biomarkers.
A study limitation was the small study groups, prompting us to perform combined statistical analyses for COC-treated animals compared to untreated macaques. Furthermore, our analyses of pH and accompanying bacterial microflora changes remained somewhat inconclusive and deserve further evaluation. While COC discontinuation appeared to affect pH with statistical significance, COC initiation did not. Likewise, H2O2 producing bacteria did not change in accordance with pH changes. The regulation of vaginal pH in macaques is only incompletely understood, and unknown additional bacterial species or other factors may contribute. Nevertheless, recent work in women clearly demonstrates that HC impacts vaginal microflora [10, 11]. Additional studies with larger animal groups and additional methodological approaches such as genetic identification of microbial species will improve our understanding of these HIV risk factors. Analyses of additional HIV risk factors that could also be informative include mucus changes, which we have not yet studied in macaques, and mucosal cytokine and lymphocyte infiltration into the vaginal epithelium[36-38].
In summary, we here developed a COC macaque model to enable future studies into HIV risk in women after COC use, and show that COC can impact vaginal epithelial thickness in pigtail macaques.
Acknowledgements
We would like to thank Brianna Skinner, Yvonne Reed, David Garber, James Mitchell, Shannon Ellis, Julian Jolly, and the Animal Resources Branch care staff for all of their help and support with this research project. An additional thank you goes to Toni Zeigler and Amita Kapoor at the Wisconsin National Primate Center for providing the LNG/EE assays.
This work was funded by CDC and an interagency agreement (Y1-A1-0681-02) between CDC and NIH.
Footnotes
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
References
- 1.Morrison CS, et al. Hormonal contraception and HIV acquisition: reanalysis using marginal structural modeling. AIDS. 2010;24(11):1778–81. doi: 10.1097/QAD.0b013e32833a2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heffron R, et al. Use of hormonal contraceptives and risk of HIV-1 transmission: a prospective cohort study. Lancet Infect Dis. 2012;12(1):19–26. doi: 10.1016/S1473-3099(11)70247-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polis CB, Curtis KM. Use of hormonal contraceptives and HIV acquisition in women: a systematic review of the epidemiological evidence. Lancet Infect Dis. 2013;13(9):797–808. doi: 10.1016/S1473-3099(13)70155-5. [DOI] [PubMed] [Google Scholar]
- 4.Mugo NR, et al. Increased risk of HIV-1 transmission in pregnancy: a prospective study among African HIV-1-serodiscordant couples. AIDS. 2011;25(15):1887–95. doi: 10.1097/QAD.0b013e32834a9338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sodora DL, et al. Vaginal transmission of SIV: assessing infectivity and hormonal influences in macaques inoculated with cell-free and cell-associated viral stocks. AIDS Res Hum Retroviruses. 1998;14(Suppl 1):S119–23. [PubMed] [Google Scholar]
- 6.Vishwanathan SA, et al. High susceptibility to repeated, low-dose, vaginal SHIV exposure late in the luteal phase of the menstrual cycle of pigtail macaques. J Acquir Immune Defic Syndr. 2011;57(4):261–4. doi: 10.1097/QAI.0b013e318220ebd3. [DOI] [PubMed] [Google Scholar]
- 7.Kersh EN, et al. SHIV susceptibility changes during the menstrual cycle of pigtail macaques. J Med Primatol. 2014 doi: 10.1111/jmp.12124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marx PA, et al. Progesterone implants enhance SIV vaginal transmission and early virus load. Nat Med. 1996;2(10):1084–9. doi: 10.1038/nm1096-1084. [DOI] [PubMed] [Google Scholar]
- 9.Hild-Petito S, et al. Effects of two progestin-only contraceptives, Depo-Provera and Norplant-II, on the vaginal epithelium of rhesus monkeys. AIDS Res Hum Retroviruses. 1998;14(Suppl 1):S125–30. [PubMed] [Google Scholar]
- 10.Miller L, et al. Depomedroxyprogesterone-induced hypoestrogenism and changes in vaginal flora and epithelium. Obstet Gynecol. 2000;96(3):431–9. doi: 10.1016/s0029-7844(00)00906-6. [DOI] [PubMed] [Google Scholar]
- 11.Mitchell CM, et al. Long-term Effect of Depot Medroxyprogesterone Acetate on Vaginal Microbiota, Epithelial Thickness and HIV Target Cells. J Infect Dis. 2014;210(4):651–5. doi: 10.1093/infdis/jiu176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Radzio J, et al. Physiologic doses of depot-medroxyprogesterone acetate do not increase acute plasma simian HIV viremia or mucosal virus shedding in pigtail macaques. AIDS. 2014;28(10):1431–9. doi: 10.1097/QAD.0000000000000294. [DOI] [PubMed] [Google Scholar]
- 13.Hadzic SV, et al. Comparison of the vaginal environment of Macaca mulatta and Macaca nemestrina throughout the menstrual cycle. Am J Reprod Immunol. 2014;71(4):322–9. doi: 10.1111/aji.12201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson DJ, Marathe J, Pudney J. The Structure of the Human Vaginal Stratum Corneum and its Role in Immune Defense. Am J Reprod Immunol. 2014 doi: 10.1111/aji.12230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Atashili J, et al. Bacterial vaginosis and HIV acquisition: a meta-analysis of published studies. AIDS. 2008;22(12):1493–501. doi: 10.1097/QAD.0b013e3283021a37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ochiel DO, et al. Innate Immunity in the Female Reproductive Tract: Role of Sex Hormones in Regulating Uterine Epithelial Cell Protection Against Pathogens. Curr Womens Health Rev. 2008;4(2):102–117. doi: 10.2174/157340408784246395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wira CR, Fahey JV. A new strategy to understand how HIV infects women: identification of a window of vulnerability during the menstrual cycle. AIDS. 2008;22(15):1909–17. doi: 10.1097/QAD.0b013e3283060ea4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones J, Mosher W, Daniels K. Current contraceptive use in the United States, 2006-2010, and changes in patterns of use since 1995. Natl Health Stat Report. 2012;60:1–25. [PubMed] [Google Scholar]
- 19.Steiner RA, et al. Sex hormones correlated with sex skin swelling and rectal temperature during the menstrual cycle of the pigtail macaque (Macaca nemestrina) Lab Anim Sci. 1977;27(2):217–21. [PubMed] [Google Scholar]
- 20.McNicholl JM, et al. Non-human primate models of hormonal contraception and HIV. Am J Reprod Immunol. 2014;71(6):513–22. doi: 10.1111/aji.12246. [DOI] [PubMed] [Google Scholar]
- 21.Veazey RS, Marx PA. The Symposium on Nonhuman Primate Models for AIDS. Introduction. J Med Primatol. 2013;42(5):229. doi: 10.1111/jmp.12067. [DOI] [PubMed] [Google Scholar]
- 22.Creinin MD. Types of combined oral contraceptives used by US women. Contraception. 2013;88(1):192–3. doi: 10.1016/j.contraception.2012.12.015. [DOI] [PubMed] [Google Scholar]
- 23.Hall KS, Trussell J, Schwarz EB. Progestin-only contraceptive pill use among women in the United States. Contraception. 2012;86(6):653–8. doi: 10.1016/j.contraception.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dando TM, Curran MP. Low-dose ethinylestradiol/levonorgestrel. Drugs. 2005;65(16):2299–306. doi: 10.2165/00003495-200565160-00007. discusion 2307-8. [DOI] [PubMed] [Google Scholar]
- 25.National Research Council (U.S.) Institute for Laboratory Animal Research (U.S.), and National Academies Press (U.S.), Guide for the care and use of laboratory animals. 2011, National Academies Press; Washington, D.C.: Committee for the Update of the Guide for the Care and Use of Laboratory Animals; pp. xxv–220. [Google Scholar]
- 26.Livingston L, et al. Hormonal synchronization of the menstrual cycles of pigtail macaques to facilitate biomedical research including modeling HIV susceptibility. J Med Primatol. 2011;40(3):164–70. doi: 10.1111/j.1600-0684.2010.00465.x. [DOI] [PubMed] [Google Scholar]
- 27.Bellinger DA, et al. Oral contraceptives and hormone replacement therapy do not increase the incidence of arterial thrombosis in a nonhuman primate model. Arterioscler Thromb Vasc Biol. 1998;18(1):92–9. doi: 10.1161/01.atv.18.1.92. [DOI] [PubMed] [Google Scholar]
- 28.Manning JM, et al. Effects of contraceptive estrogen and progestin on the atherogenic potential of plasma LDLs in cynomolgus monkeys. Arterioscler Thromb Vasc Biol. 1997;17(7):1216–23. doi: 10.1161/01.atv.17.7.1216. [DOI] [PubMed] [Google Scholar]
- 29.Register TC, Jayo MJ, Jerome CP. Oral contraceptive treatment inhibits the normal acquisition of bone mineral in skeletally immature young adult female monkeys. Osteoporos Int. 1997;7(4):348–53. doi: 10.1007/BF01623776. [DOI] [PubMed] [Google Scholar]
- 30.Henderson JA, Shively CA. Triphasic oral contraceptive treatment alters the behavior and neurobiology of female cynomolgus monkeys. Psychoneuroendocrinology. 2004;29(1):21–34. doi: 10.1016/s0306-4530(02)00132-4. [DOI] [PubMed] [Google Scholar]
- 31.Kaplan JR, et al. Dominant social status and contraceptive hormone treatment inhibit atherogenesis in premenopausal monkeys. Arterioscler Thromb Vasc Biol. 1995;15(12):2094–100. doi: 10.1161/01.atv.15.12.2094. [DOI] [PubMed] [Google Scholar]
- 32.Dutta GP, et al. Interactions between oral contraceptives and malaria infections in rhesus monkeys. Bull World Health Organ. 1984;62(6):931–9. [PMC free article] [PubMed] [Google Scholar]
- 33.Wagstaff AJ. Continuous-use ethinylestradiol/levonorgestrel 20microg/90microg: as an oral contraceptive. Drugs. 2007;67(16):2473–7. doi: 10.2165/00003495-200767160-00010. discussion 2478-9. [DOI] [PubMed] [Google Scholar]
- 34.Eschenbach DA, et al. Effects of oral contraceptive pill use on vaginal flora and vaginal epithelium. Contraception. 2000;62(3):107–12. doi: 10.1016/s0010-7824(00)00155-4. [DOI] [PubMed] [Google Scholar]
- 35.Ildgruben AK, Sjoberg IM, Hammarstrom ML. Influence of hormonal contraceptives on the immune cells and thickness of human vaginal epithelium. Obstet Gynecol. 2003;102(3):571–82. doi: 10.1016/s0029-7844(03)00618-5. [DOI] [PubMed] [Google Scholar]
- 36.Chandra N, et al. Depot medroxyprogesterone acetate increases immune cell numbers and activation markers in human vaginal mucosal tissues. AIDS Res Hum Retroviruses. 2013;29(3):592–601. doi: 10.1089/aid.2012.0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goode D, et al. Sex hormones selectively impact the endocervical mucosal microenvironment: implications for HIV transmission. PLoS One. 2014;9(5):e97767. doi: 10.1371/journal.pone.0097767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morrison C, et al. Cervical inflammation and immunity associated with hormonal contraception, pregnancy, and HIV-1 seroconversion. J Acquir Immune Defic Syndr. 2014;66(2):109–17. doi: 10.1097/QAI.0000000000000103. [DOI] [PubMed] [Google Scholar]