Abstract
Bone marrow fat may serve a metabolic role distinct from other fat depots, and it may be altered by metabolic conditions including diabetes. Caloric restriction paradoxically increases marrow fat in mice, and women with anorexia nervosa have high marrow fat. The longitudinal effect of weight loss on marrow fat in humans is unknown. We hypothesized that marrow fat increases after Roux-en-Y gastric bypass (RYGB) surgery, as total body fat decreases. In a pilot study of 11 morbidly obese women (6 diabetic, 5 nondiabetic), we measured vertebral marrow fat content (percentage fat fraction) before and 6 months after RYGB using magnetic resonance spectroscopy. Total body fat mass declined in all participants (mean ±SD decline 19.1 ±6.1 kg or 36.5 ±10.9%, p<0.001). Areal bone mineral density (BMD) decreased by 5.2 ±3.5% and 4.1 ±2.6% at the femoral neck and total hip, respectively, and volumetric BMD decreased at the spine by 7.4 ±2.8% (p<0.001 for all). Effects of RYGB on marrow fat differed by diabetes status (adjusted p=0.04). There was little mean change in marrow fat in nondiabetic women (mean +0.9%, 95% CI -10.0 to +11.7%, p=0.84). In contrast, marrow fat decreased in diabetic women (−7.5%, 95% CI -15.2 to +0.1%, p=0.05). Changes in total body fat mass and marrow fat were inversely correlated among nondiabetic (r=−0.96, p=0.01) but not diabetic (r=0.52, p=0.29) participants. In conclusion, among those without diabetes, marrow fat is maintained on average after RYGB, despite dramatic declines in overall fat mass. Among those with diabetes, RYGB may reduce marrow fat. Thus, future studies of marrow fat should take diabetes status into account. Marrow fat may have unique metabolic behavior compared with other fat depots.
Keywords: bone marrow fat, bariatric surgery, gastric bypass surgery, diabetes
1. INTRODUCTION
Bone marrow is well recognized as a depot for adipose tissue, but the physiological significance of bone marrow fat remains undefined. Because adipocytes and osteoblasts share a common mesenchymal stem cell precursor within the marrow microenvironment, bone marrow fat has gained increasing attention as a potential biomarker or regulator of the interaction between fat and bone metabolism [1, 2]. Greater bone marrow fat is associated with lower bone mineral density (BMD) [3-7] as well as more rapid bone loss [8] and vertebral fracture [9]. In addition, marrow fat, which can now be quantified non-invasively with proton magnetic resonance spectroscopy (1H-MRS), has been studied recently as an endocrine organ with systemic effects [10].
Increasing evidence suggests that bone marrow fat is regulated differently from visceral fat and subcutaneous fat. In young mice, caloric restriction results in high bone marrow fat compared to mice on a normal diet, despite lower percentage body fat [11]. In humans, women with anorexia nervosa have higher marrow fat than controls, despite having much lower total body fat [12]. These findings have led to the proposal that marrow fat may serve as a depot for energy stores in the setting of starvation or relative starvation [13, 14]. Further, an increase or relative preservation of bone marrow fat may play a role in the decline in bone mass seen with weight loss in humans [15-18]. However, no published studies have examined the longitudinal effects of weight loss on marrow fat in humans.
Other metabolic conditions potentially linked to marrow fat include diabetes. In mouse models of type 1 or type 2 diabetes, marrow fat content is high [19, 20]. In women with type 2 diabetes, higher hemoglobin A1c (HbA1c) levels are associated with greater marrow fat, suggesting that marrow fat may influence or be influenced by glucose metabolism and glycemic control [21]. No published studies have assessed change in marrow fat in the setting of improving or declining glycemic control.
Weight loss surgery, including the Roux-en-Y gastric bypass (RYGB), produces dramatic weight loss and substantial improvements in diabetes [22, 23]. These striking metabolic changes provide an ideal opportunity for the longitudinal study of marrow fat in humans. In a pilot study of morbidly obese diabetic and nondiabetic women undergoing RYGB, we examined the effects of RYGB on vertebral bone marrow fat content. We hypothesized that marrow fat content increases after RYGB while total body fat decreases markedly.
2. MATERIAL AND METHODS
2.1 Study population
Pilot study participants were enrolled from a larger study in progress examining body composition and skeletal changes after RYGB. Funding from a pilot study grant allowed for a sample of 11 from the larger cohort.
We recruited women ≥25 years of age from two academic bariatric surgery centers (the University of California, San Francisco and the San Francisco Veterans Affairs Medical Center), between October 2012 and July 2013. Women were eligible if they were scheduled for an upcoming RYGB procedure. Participants were enrolled without regard to diabetes status. We excluded women who were perimenopausal (defined as last menses >3 months but <5 years ago), in order to minimize changes in sex hormones unrelated to surgical weight loss. Premenopausal women on stable hormonal contraception and postmenopausal women on stable estrogen/progestin therapy were eligible. We also excluded women who used medications known to impact bone metabolism or marrow fat, including thiazolidinediones, bisphosphonates or teriparatide (in the last year or for >12 months ever), and oral glucocorticoids (>5 mg prednisone equivalent daily for >10 days in the last 3 months). Other exclusion criteria included prior bariatric surgery, estimated glomerular filtration rate <30 mL/min, and weight >300 pounds (due to concerns about MR scanner capacity).
Participants took a chewable calcium citrate supplement at a dose determined by the study investigators to achieve a total daily calcium intake of approximately 1200 mg, with reassessment of dietary calcium intake and adjustment of the supplement dose during the study period. Low 25-hydroxyvitamin D levels were repleted following enrollment with a target level ≥30 ng/mL, and vitamin D supplements were dosed to maintain that target level.
The institutional review board approved the study protocol. All participants provided written informed consent.
2.2 Vertebral bone marrow fat: acquisition and analysis
Pre-operatively and 6 months post-operatively, vertebral marrow fat content (percentage fat fraction) was measured using a GE MR750 wide bore scanner (GE Healthcare, Milwaukee, WI) with embedded posterior phased array coils (GEM suite, GE Healthcare). The imaging protocol included a standard clinical sagittal T2-weighted Fast Spin Echo (FSE) sequence (Repetition [TR]/Echo Time [TE]=5000/87 msec, echo train length=32, field of view=22 cm, slice thickness=6 mm), which was used for visual assessment of lumbar vertebrae and for prescription of the spectral acquisition box. Single voxel MRS was acquired in the L3 and L4 vertebrae using the Point Resolved Spectroscopy (PRESS) sequence with the following parameters: TF/TE=3000/37 msec, 64 averages without water suppression, sweep width=5000 Hz, data points=4096, voxel size=15 × 15 × 20 mm3=4.5 cm3. The PRESS box was positioned in the middle of the vertebral body. The PRESS box size was kept the same for all subjects. Outer volume saturation bands were used to eliminate potential contamination of outside signals.
Spectral data were analyzed using jMRUI software [24]. After phase, baseline, and frequency shift correction, 2 peaks were fitted using Marquardt Fit: water peak at 4.67 ppm and fat peak at 1.3 ppm (the bulk CH2 methylene protons). Using this technique, bone does not contribute to the signal. The area under each peak was calculated, and marrow fat content was determined as fat/(fat+water) x 100%. Study team members responsible for MRS analysis were blinded to patient characteristics including diabetes status.
2.3 Vertebral bone marrow fat: reproducibility
In a previous same-day reproducibility study (two MR scans on the same day with repositioning between scans), the coefficient of variation (CV) for vertebral marrow fat content was 1.7% [25].
As a part of this pilot study, we determined the 6-month longitudinal reproducibility of the 1H-MRS technique in healthy adult women. Six healthy, normal weight women were scanned twice, with the two scans 6 months apart. Women did not have osteoporosis or diabetes and did not use medications known to impact bone metabolism. Mean 6-month CV for L3-L4 marrow fat content was 3.8% (range 0.2% to 5.6%). At L3, mean CV was 4.4%, and at L4, mean CV was 3.2%.
2.4 Other measures
Pre-operatively and 6 months post-operatively, body mass index (BMI) was calculated as weight/height2 (kg/m2). Waist circumference was measured in the midaxillary line at the level of the lowest rib, and hip circumference at the maximum extension of the buttocks, viewed from the side.
Whole body fat (grams) and areal BMD (aBMD, g/cm2) were measured pre-operatively and 6 months post-operatively by DXA (Hologic Discovery W densitometer, Bedford, MA, USA). Modified half-body scans were employed if a participant's body dimensions exceeded the width of the scanning area [26]. Spinal volumetric BMD (vBMD, g/cm3) at the L3 and L4 vertebrae was assessed by quantitative computed tomography (QCT). Findings on QCT were evaluated according to methods described previously (Mindways Software, Austin, TX, USA) [27, 28]. Visceral adipose tissue area (cm2) was measured by computed tomography (CT) (General Electrics VCT64 scanner, Milwaukee, WI) using a single axial slice at the mid-L4 vertebra. The fascial borders of the internal abdominal wall were traced manually, using specialized software developed at the University of California, San Francisco [29]. Visceral adipose area was calculated by multiplying the number of pixels within the adipose attenuation threshold by the pixel area.
HbA1c and basic chemistries were measured. Diabetes was defined as HbA1c level ≥6.5% or a prior physician's diagnosis of diabetes plus use of an antidiabetic medication.
Physical activity was assessed pre-operatively and 6 months post-operatively using the International Physical Activity Questionnaire short form [30], with data reported as Metabolic Equivalent of Task (MET, or metabolic equivalent) minutes per week (MET-min/wk).
2.5 Statistical analysis
Means and medians were calculated for baseline characteristics. Because of evidence in murine and human studies that diabetes may influence marrow fat content, we considered diabetic and nondiabetic women separately in addition to examining the cohort as a whole. Differences in baseline characteristics between diabetic and nondiabetic participants were assessed using χ2, Mann-Whitney, and Student's t-tests. For all participants and for each diabetes stratum, we used Wilcoxon signed-rank tests or paired t-tests as appropriate to determine whether study outcomes changed between pre-operative and 6 month post-operative time points. Similarly, we used Mann-Whitney and t-tests to assess between-group differences in these changes. Next, we used linear models to estimate minimally adjusted associations between diabetes status and 6-month change in marrow fat. We included as covariates those variables which, when added to the unadjusted base model, changed the point estimate for diabetes status by ≥10%. Finally, we used Spearman's rank and Pearson's correlation tests to characterize the unadjusted relationships between change in marrow fat and changes in other study parameters. Data were analyzed using Stata 12 software (StataCorp, College Station, TX).
3. RESULTS
3.1 Baseline participant characteristics and correlations
Participants were 44.6 ± 13.2 (mean ± SD) years old (Table 1). Mean pre-operative weight was 111.1 ± 16.0 kg, and mean BMI was 40.4 ± 4.6 kg/m2. Six women had diabetes, while 5 did not. Participants were premenopausal with the exception of two women with diabetes who were postmenopausal; one took no estrogen replacement therapy and the other took daily oral estradiol, which was stable throughout follow-up. Mean pre-operative HbA1c level was 7.2% among those with diabetes and 5.5% among those without diabetes (p<0.01). Mean pre-operative marrow fat content was 49.2% (95% CI 38.8 to 59.5%); marrow fat content was 54.2% (95% CI 41.1 to 67.2%) and 43.1% (95% CI 20.9 to 65.2%) in diabetic and nondiabetic participants, respectively. At baseline, there was a correlation between age and marrow fat content among nondiabetic participants (r=0.93, p=0.02), such that older participants had higher marrow fat content. Among the nondiabetic participants, there was an inverse correlation between baseline total body fat and marrow fat content, such that those with the greater total body adiposity had lower marrow fat content (r=-0.62, p=0.04); a similar correlation existed for baseline body weight and marrow fat. In contrast, there was a trend towards a positive correlation between baseline visceral fat and marrow fat content, such that those with greater visceral fat had higher marrow fat content (r=0.58, p=0.06).
Table 1.
Baseline characteristics of study participants
| All Participants (n=11) | Diabetic Participants (n=6) | Nondiabetic Participants (n=5) | p-value (between groups) | |
|---|---|---|---|---|
| Age - yr | 44.6 ± 13.2 | 49.3 ± 13.8 | 39.0 ± 11.2 | 0.21 |
| Menopause status - no. (%) | 0.15 | |||
| Premenopausal | 9 (82%) | 4 (67%) | 5 (100%) | |
| Postmenopausal | 2 (18%) | 2 (33%) | 0 (0%) | |
| Race - no. (%) | 0.45 | |||
| White | 6 (55%) | 4 (67%) | 2 (40%) | |
| Black | 4 (36%) | 2 (33%) | 2 (40%) | |
| Asian | 1 (9%) | 0 (0%) | 1 (20%) | |
| Weight (kg) | 111.1 ± 16.0 | 105.3 ± 12.8 | 118.0 ± 17.9 | 0.20 |
| Body mass index (kg/m2) | 40.4 ± 4.6 | 38.8 ± 3.8 | 42.3 ± 5.2 | 0.23 |
| Total body fat (kg) | 52.5 ± 9.5 | 47.8 ± 7.5 | 58.1 ± 9.2 | 0.07 |
| Visceral fat (cm2) | 153.8 ± 77.3 | 187.9 ± 83.0 | 112.8 ± 50.0 | 0.11 |
| Waist circumference (cm) | 111.1 ± 7.5 | 111.1 ± 8.5 | 111.0 ± 7.2 | 0.99 |
| Percentage body fat (%) | 47.8 ± 4.4 | 45.8 ± 4.3 | 50.1 ± 3.6 | 0.11 |
| Hip circumference (cm) | 130.8 ± 10.9 | 127.0 ± 9.1 | 135.5 ± 12.1 | 0.22 |
| Waist-hip ratio | 0.85 ± 0.06 | 0.88 ± 0.06 | 0.82 ± 0.06 | 0.15 |
| Hemoglobin A1c (%) | 6.4 ± 1.2 | 7.2 ± 1.0 | 5.5 ± 0.3 | <0.01 |
| Physical activity (MET-min/wk) | 495 (0, 3066) | 1634 (33, 3066) | 198 (0, 2439) | 0.58 |
| Femoral neck aBMD (g/cm2) | 0.906 ± 0.151 | 0.875 ± 0.174 | 0.942 ± 0.128 | 0.50 |
| Total hip aBMD (g/cm2) | 1.064 ± 0.120 | 1.066 ± 0.153 | 1.061 ± 0.083 | 0.95 |
| Lumbar spine aBMD (g/cm2) | 1.133 ± 0.105 | 1.137 ± 0.127 | 1.129 ± 0.087 | 0.92 |
| Spinal vBMD (g/cm3) | 0.162 ± 0.036 | 0.156 ± 0.039 | 0.169 ± 0.035 | 0.56 |
| Marrow fat content (%) | 49.2 ± 15.4 | 54.2 ± 12.4 | 43.1 ± 17.8 | 0.25 |
Values are means ± SDs, counts (percentages), or medians (interquartile ranges).
At baseline, marrow fat content was inversely correlated with spinal vBMD overall (r=−0.76, p<0.01), such that those with higher marrow fat content had lower vBMD; this relationship was not different between diabetic and nondiabetic women. Correlations between marrow fat content and aBMD were r=−0.59, p=0.06 at the femoral neck; r=−0.12, p=0.73 at the total hip; and r=−0.14, p=0.68 at the lumbar spine.
3.2 Changes in body composition, metabolic parameters, and BMD after RYGB
All participants lost weight after RYGB, with a mean 26.7 ± 9.6 kg decrease (p<0.001, Table 2). Total body fat mass declined in all participants (by a mean 19.1 ± 6.1 kg, or by 36.5% ± 10.9%, p<0.001). HbA1c levels decreased to a greater extent among participants with diabetes than among those without diabetes (p<0.01 for difference between groups). Six months postoperatively, 5 of 6 diabetic women had fasting glucose levels <100 mg/dL and HbA1c levels <6.5% without the use of antidiabetic medications. BMD decreased over the 6-month period, with overall aBMD declines of 5.2% ± 3.5% and 4.1% ± 2.6% at the femoral neck and total hip, respectively, and a spinal vBMD decline of 7.4% ± 2.8% (p<0.001 for all). Change in aBMD at the lumbar spine was −1.6% ± 2.6% (p=0.08). There were no consistent or statistically significant differences in BMD change between diabetic and nondiabetic participants.
Table 2.
Changes in body composition, metabolic parameters, and vertebral marrow fat content 6 months after RYGB
| Parameter | Change from baseline | ||||||
|---|---|---|---|---|---|---|---|
| All participants | p-value (within group) | Diabetic participants | p-value (within group) | Nondiabetic participants | p-value (within group) | p-value (between groups) | |
| Weight (absolute change, kg) | −26.7 ± 9.6 (95% CI −33.1, −20.3) | <0.0001 | −27.8 ± 10.6 (95% CI −39.0, −16.6) | <0.01 | −25.3 ± 9.1 (95% CI −36.7, −14.0) | <0.01 | 0.69 |
| Weight (% change) | −23.8 ± 7.4 (95% CI −28.8, −18.9) | <0.0001 | −26.2 ± 8.6 (95% CI −35.3, −17.2) | <0.001 | −20.9 ± 4.8 (95% CI −26.9, −14.9) | <0.001 | 0.25 |
| Total body fat (absolute change, kg) | −19.1 ± 6.1 (95% CI −23.2, −15.0) | <0.0001 | −19.5 ± 6.9 (95% CI −26.7, −12.3) | <0.001 | −18.6 ± 5.9 (95% CI −25.9, −11.3) | <0.01 | 0.83 |
| Total body fat (% change) | −36.5 ± 10.9 (95% CI −43.7, −29.2) | <0.0001 | −40.7 ± 12.6 (95% CI −53.9, −27.4) | <0.001 | −31.4 ± 6.0 (95% CI −38.8, −24.0) | <0.001 | 0.17 |
| Visceral fat (absolute change, cm2) | −77.9 ± 49.8 (95% CI −111.4, −44.5) | <0.001 | −105.2 ± 52.9 (95% CI −160.7, −49.7) | <0.01 | −45.2 ± 16.0 (95% CI −65.1, −25.3) | <0.01 | 0.04 |
| Visceral fat (% change) | −50.4 ± 16.5 (95% CI −61.5, −39.3) | <0.0001 | −57.4 ± 18.8 (95% CI −77.1, −37.8) | <0.001 | −41.9 ± 8.9 (95% CI −53.0, −30.9) | <0.001 | 0.13 |
| Hemoglobin A1c (absolute change, %) | −1.0 ± 0.9 (95% CI −1.6, −0.4) | <0.01 | −1.6 ± 0.7 (95% CI −2.3, −0.8) | <0.01 | −0.2 ± 0.3 (95% CI −0.5, +0.3) | 0.18 | <0.01 |
| Physical activity (abs chg, MET-min/wk) | +186 (−2094, +1899) (95% CI −2507, +4675) | 0.93 | −207 (−2094, +1007) (95% CI −2043, +1438) | 0.60 | +297 (−973, +3192) (95% CI −7001, +12,498) | 0.69 | 0.58 |
| Femoral neck aBMD (% change) | −5.2 ± 3.5 (95% CI −7.6, −2.8) | <0.001 | −4.4 ± 4.3 (95% CI −8.9, +0.1) | 0.05 | −6.2 ± 2.5 (95% CI −9.3, −3.1) | <0.01 | 0.43 |
| Total hip aBMD (% change) | −4.1 ± 2.6 (95% CI −5.8, −2.3) | <0.001 | −5.3 ± 2.8 (95% CI −8.2, −2.4) | <0.01 | −2.6 ± 1.3 (95% CI −4.2, −1.0) | 0.01 | 0.08 |
| Lumbar spine aBMD (% change) | −1.6 ± 2.6 (95% CI −3.3, +0.2) | 0.08 | −0.7 ± 2.5 (95% CI −3.4, +1.9) | 0.50 | −2.5 ± 2.7 (95% CI −5.8, +0.8) | 0.10 | 0.29 |
| Spinal vBMD (% change) | −7.4 ± 2.8 (95% CI −9.3, −5.5) | <0.0001 | −8.7 ± 3.1 (95% CI −11.9, −5.4) | 0.001 | −5.9 ± 1.7 (95% CI −8.0, −3.8) | 0.002 | 0.11 |
| Marrow fat content (absolute change, %) | −3.7 ± 8.7 (95% CI −9.6, +2.1) | 0.19 | −7.5 ± 7.3 (95% CI −15.2, +0.1) | 0.05 | +0.9 ± 8.8 (95% CI −10.0, +11.7) | 0.84 | 0.12 |
Values are means ± SDs or medians (interquartile ranges) and 95% confidence intervals.
3.3 Changes in marrow fat after RYGB
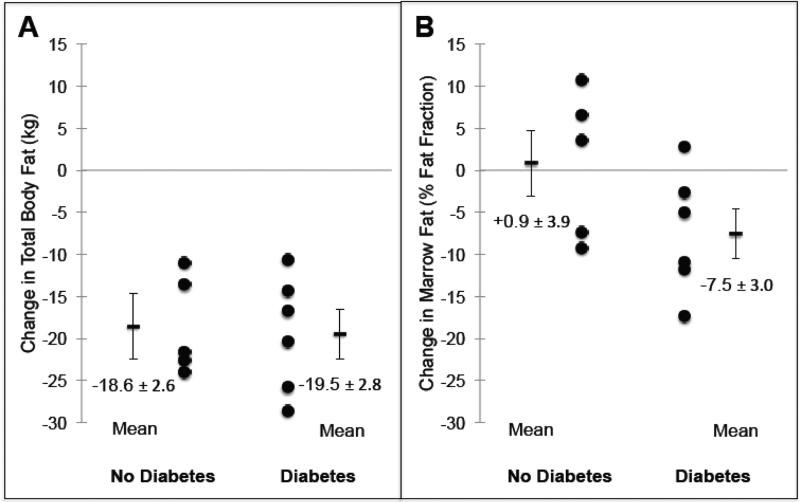
Overall, marrow fat did not change significantly after RYGB, with mean change −3.7% (95% CI −9.6 to +2.1%, p=0.19, Table 2). Among nondiabetic women, marrow fat showed very little change (mean change +0.9%, 95% CI −10.0 to +11.7%, p=0.84, Figure 1). In contrast, among diabetic women, marrow fat decreased, with a mean decline of −7.5% (95% CI −15.2 to +0.1%, p=0.05). In univariate analysis, the mean difference in marrow fat change between those with and without diabetes was 8.4% (95% CI −2.5 to 19.3%, p=0.12). After adjustment for menopausal status and race (covariates meeting criteria for inclusion in a multivariable model), diabetes status was associated with change in marrow fat, such that those with diabetes were more likely to experience a decline in marrow fat post-operatively (p=0.04). Adjusting for age instead of menopausal status yielded a similar result.
Figure 1.
Changes in total body fat (A) and vertebral bone marrow fat content (B) 6 months after Roux-en-Y gastric bypass surgery, stratified by diabetes status. Mean values are ± SEM.
3.4 Changes in marrow fat, body composition, metabolic parameters, and BMD
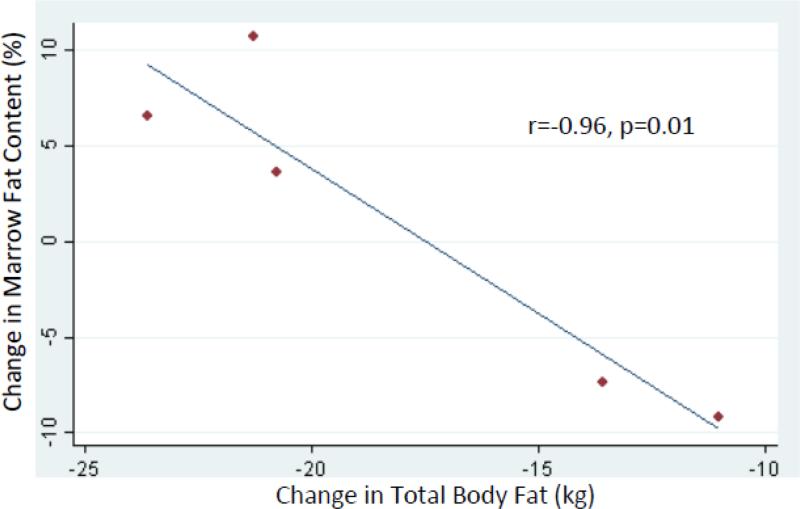
Among nondiabetic women, changes in total body fat and marrow fat content were inversely correlated (r=−0.96, p=0.01), such that those with greater decreases in total body fat mass were more likely to have increases in marrow fat (Table 3, Figure 2). No statistically significant correlation was observed between changes in visceral fat and marrow fat content (r=−0.33, p=0.59). Among diabetic women, no inverse correlations between changes in total body fat or visceral fat and marrow fat content were apparent (r=0.52, p=0.29, and r=−0.00, p=0.99, respectively).
Table 3.
Unadjusted correlations between 6-month changes in body composition and hemoglobin A1c, and 6-month change in vertebral marrow fat content, stratified by diabetes status
| Weight (% change) | Total body fat (absolute change) | Visceral fat (absolute change) | HbA1c (absolute change) | ||
|---|---|---|---|---|---|
| Marrow fat content (absolute change) | All participants | 0.15 (p=0.65) | −0.09 (p=0.79) | 0.28 (p=0.42) | 0.44 (p=0.17) |
| Diabetes | 0.44 (p=0.38) | 0.52 (p=0.29) | −0.00 (p=0.99) | 0.25 (p=0.63) | |
| No diabetes | −0.96 (p=0.01) | −0.96 (p=0.01) | −0.33 (p=0.59) | −0.36 (p=0.55) |
Values are Pearson's coefficients of correlation and corresponding p-values.
Figure 2.
Correlation between 6-month changes in vertebral bone marrow fat content and total body fat among nondiabetic women.
Despite the post-operative decline in HbA1c level, there was no statistically significant correlation between change in HbA1c and change in marrow fat. However, the direction of correlation was opposite for women with and without diabetes.
Changes in physical activity and marrow fat were correlated among those with diabetes (r=0.86, p=0.03) but not among those without diabetes (r=0.08, p=0.89).
No statistically significant correlations were observed between changes in marrow fat and BMD, among the pilot study participants as a group or with stratification by diabetes status (data not shown).
4. DISCUSSION
To our knowledge, this is the first study to evaluate the longitudinal effects of weight loss on bone marrow fat in humans. We found that these effects differed by diabetes status, with a reduction in marrow fat content after RYGB surgery in diabetic women but no significant mean change in those without diabetes.
Based on published observations that caloric restriction paradoxically increases marrow fat in mice [11] and that women with anorexia nervosa have high marrow fat [12, 31], we had hypothesized that marrow fat content would increase after RYGB. In fact, we had hypothesized that marrow fat content would increase markedly, given the consistent and dramatic declines in total body fat induced by RYGB. However, our findings did not support this hypothesis. Instead, our diabetic participants experienced a decrease in marrow fat content, concurrent with an impressive decline in total body fat. We propose now that this may have been because RYGB improved diabetes control for these women, by means of the resulting weight loss and the additional neurohormonal changes known to accompany RYGB [32]. Indeed, 5 of the 6 diabetic women had fasting glucose and HbA1c values at nondiabetic levels post-operatively, without the use of antidiabetic medications.
Animal models and some clinical data indicate that marrow fat may influence or be influenced by glucose metabolism and glycemic control. Mouse models of type 1 and type 2 diabetes have shown higher marrow fat content than nondiabetic controls [19, 20]. In a cohort of older men, those with diabetes had higher marrow fat content than those without diabetes [33], and in a study of postmenopausal women, there was a strong positive correlation between HbA1c and marrow fat content among those with diabetes [21]. In our small pilot study, mean baseline marrow fat was 54% in diabetic women vs. 43% in nondiabetic women, although this difference was not statistically significant and could have reflected the nonsignificant older mean age of the diabetic women. Marrow fat content decreased after RYGB among diabetic women, making this the first study (to our knowledge) to assess marrow fat longitudinally during changes in glucose metabolism.
In contrast, among nondiabetic women, mean marrow fat content did not change after RYGB (mean change +0.9%, p=0.84), despite impressive declines in weight, total body fat, and visceral fat. These data are inconsistent with the findings from murine models of caloric restriction and women with anorexia nervosa, which would predict an increase in marrow fat content. A potential explanation is that caloric restriction causing an obese person to move closer to normal weight differs fundamentally from caloric restriction causing a normal weight person to become underweight (as in the starvation of anorexia nervosa). However, the maintenance of marrow fat content during RYGB-induced weight loss, despite the dramatic decline of other adipose depots, supports the view that the behavior of marrow fat may oppose the behavior of total body fat. In support of this interpretation is our finding of a strong inverse correlation between change in weight or total body fat and change in marrow fat content, such that those with greater decreases in weight or total body fat were more likely to have increases in marrow fat. This view allows for the possibility that marrow fat is a distinct endocrine organ with unique systemic effects, which is the premise of active basic and clinical investigation [10].
Increasing evidence indicates that RYGB negatively impacts bone metabolism, with declines in BMD and detrimental microstructural effects [34, 35]. Although greater bone marrow fat has been associated with lower bone mineral density (BMD) [3-7], our longitudinal findings do not support the hypothesis that change in marrow fat plays an important causal role in the bone loss after RYGB. Our findings also suggest that observed decreases in BMD are not fully explained as artifactual decreases in measured BMD resulting from increases in marrow fat [36, 37]. However, given our study's small sample size, these conclusions are necessarily speculative.
The principal limitation of this pilot study is its small sample size. For nondiabetic participants in particular, the 95% CI around the mean +0.9% change in marrow fat content was broad (-10.0% to +11.7%), and therefore we cannot rule out the possibility of a change (increase or decrease). However, even if that is the case, the preponderance of evidence is that change in marrow fat content is not uniform after RYGB, making marrow fat distinct from total body fat and visceral fat in its behavior. Our power to detect significant correlations was likewise limited. For example, we did not observe a significant correlation between changes in HbA1c and marrow fat content among diabetic women, although we speculate that a positive correlation might be demonstrable in a larger study or a study standardizing the pre-operative use and post-operative discontinuation of antidiabetic medications. In addition, a limitation of this study is its short duration of follow-up. While the 6-month duration of our study captured the period of most dramatic weight loss after RYGB, changes in marrow fat content over this period might not reflect changes in marrow fat content over a longer period of weight loss and stabilization (i.e., upon achievement of a new steady state).
In conclusion, the effect of RYGB on marrow fat may vary depending on diabetes status. Our data suggest that among those without diabetes, marrow fat content is maintained after RYGB despite dramatic declines in overall fat mass. In contrast, among those with diabetes, RYGB may reduce marrow fat content, possibly by improving glycemic control. While the conclusions to be drawn from this pilot study are limited by its small sample size and short duration, we propose that future studies of marrow fat during weight loss in humans should take into account participants’ diabetes status. Not only are larger and longer studies required for the definitive examination of marrow fat after RYGB, but also future research is needed to understand the physiological significance and regulation of marrow fat, including mechanisms for possible regulation by nutritional status or glucose metabolism. Our findings provide further evidence that marrow fat may have unique metabolic behavior compared with other fat depots.
HIGHLIGHTS.
Caloric restriction paradoxically increases marrow fat in mice, and women with anorexia nervosa have high marrow fat.
We hypothesized that marrow fat increases after Roux-en-Y gastric bypass (RYGB) surgery, as total body fat decreases.
In 11 obese women, vertebral marrow fat content was measured before and 6 months after RYGB, using magnetic resonance spectroscopy.
Among the 6 diabetic women, RYGB decreased marrow fat. Among the 5 nondiabetic women, there was no mean change.
Future studies of marrow fat should take diabetes status into account and explore marrow fat and bone mass relationships further.
ACKNOWLEDGMENTS
The authors thank Barbara Arnold for her work in study coordination and data collection; Aisia Azus for MRS processing; Viva Tai, RD, MPH for DXA scan acquisition; Lisa Palermo, MA for her role in data management; Dimitry Petrenko and Thomas Lang, PhD for CT image analysis; Saunak Sen, PhD for biostatistical support; and Melissa Guan, Gina Szefc, MSN, Lucy Wu, Hanling Chang, and Nooshin Yashar, MD for their roles in study support and data coordination.
Grant support: The research described here was supported by the Department of Veterans Affairs, VHA, CSR&D Service (CDA-2 5 IK2 CX000549-04 to ALS, SFVAMC) and by a pilot and feasibility grant with funds provided by the UCSF Diabetes Center. Additional support was provided by the National Center for Advancing Translational Sciences of the National Institutes of Health through UCSF-CTSI Grant UL1 TR000004, and by NIH grant 5 K12 HD052163-15. Manuscript contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. Calcium citrate supplements were supplied by Bariatric Advantage.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement:
The authors have nothing to disclose.
ClinicalTrials.gov identifier: NCT01330914
REFERENCES
- 1.Rosen CJ, Bouxsein ML. Mechanisms of disease: is osteoporosis the obesity of bone? Nat Clin Pract Rheumatol. 2006;2:35–43. doi: 10.1038/ncprheum0070. [DOI] [PubMed] [Google Scholar]
- 2.Fazeli PK, Horowitz MC, MacDougald OA, Scheller EL, Rodeheffer MS, Rosen CJ, Klibanski A. Marrow fat and bone--new perspectives. J Clin Endocrinol Metab. 2013;98:935–45. doi: 10.1210/jc.2012-3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sheu Y, Cauley JA. The role of bone marrow and visceral fat on bone metabolism. Curr Osteoporos Rep. 2011;9:67–75. doi: 10.1007/s11914-011-0051-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen A, Dempster DW, Stein EM, Nickolas TL, Zhou H, McMahon DJ, Muller R, Kohler T, Zwahlen A, Lappe JM, Young P, Recker RR, Shane E. Increased marrow adiposity in premenopausal women with idiopathic osteoporosis. J Clin Endocrinol Metab. 2012;97:2782–91. doi: 10.1210/jc.2012-1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meunier P, Aaron J, Edouard C, Vignon G. Osteoporosis and the replacement of cell populations of the marrow by adipose tissue. A quantitative study of 84 iliac bone biopsies. Clin Orthop Relat Res. 1971;80:147–54. doi: 10.1097/00003086-197110000-00021. [DOI] [PubMed] [Google Scholar]
- 6.Schellinger D, Lin CS, Hatipoglu HG, Fertikh D. Potential value of vertebral proton MR spectroscopy in determining bone weakness. AJNR Am J Neuroradiol. 2001;22:1620–7. [PMC free article] [PubMed] [Google Scholar]
- 7.Shen W, Chen J, Punyanitya M, Shapses S, Heshka S, Heymsfield SB. MRI-measured bone marrow adipose tissue is inversely related to DXA-measured bone mineral in Caucasian women. Osteoporos Int. 2007;18:641–7. doi: 10.1007/s00198-006-0285-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Griffith JF, Yeung DK, Leung JC, Kwok TC, Leung PC. Prediction of bone loss in elderly female subjects by MR perfusion imaging and spectroscopy. Eur Radiol. 2011;21:1160–9. doi: 10.1007/s00330-010-2054-6. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz AV, Sigurdsson S, Hue TF, Lang TF, Harris TB, Rosen CJ, Vittinghoff E, Siggeirsdottir K, Sigurdsson G, Oskarsdottir D, Shet K, Palermo L, Gudnason V, Li X. Vertebral bone marrow fat associated with lower trabecular BMD and prevalent vertebral fracture in older adults. J Clin Endocrinol Metab. 2013;98:2294–300. doi: 10.1210/jc.2012-3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cawthorn WP, Scheller EL, Learman BS, Parlee SD, Simon BR, Mori H, Ning X, Bree AJ, Schell B, Broome DT, Soliman SS, DelProposto JL, Lumeng CN, Mitra A, Pandit SV, Gallagher KA, Miller JD, Krishnan V, Hui SK, Bredella MA, Fazeli PK, Klibanski A, Horowitz MC, Rosen CJ, MacDougald OA. Bone marrow adipose tissue is an endocrine organ that contributes to increased circulating adiponectin during caloric restriction. Cell Metab. 2014;20:368–75. doi: 10.1016/j.cmet.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Devlin MJ, Cloutier AM, Thomas NA, Panus DA, Lotinun S, Pinz I, Baron R, Rosen CJ, Bouxsein ML. Caloric restriction leads to high marrow adiposity and low bone mass in growing mice. J Bone Miner Res. 2010;25:2078–88. doi: 10.1002/jbmr.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bredella MA, Fazeli PK, Miller KK, Misra M, Torriani M, Thomas BJ, Ghomi RH, Rosen CJ, Klibanski A. Increased bone marrow fat in anorexia nervosa. J Clin Endocrinol Metab. 2009;94:2129–36. doi: 10.1210/jc.2008-2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Devlin MJ. Why does starvation make bones fat? Am J Hum Biol. 2011;23:577–85. doi: 10.1002/ajhb.21202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scheller EL, Rosen CJ. What's the matter with MAT? Marrow adipose tissue, metabolism, and skeletal health. Ann N Y Acad Sci. 2014;1311:14–30. doi: 10.1111/nyas.12327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chao D, Espeland MA, Farmer D, Register TC, Lenchik L, Applegate WB, Ettinger WH., Jr Effect of voluntary weight loss on bone mineral density in older overweight women. J Am Geriatr Soc. 2000;48:753–9. doi: 10.1111/j.1532-5415.2000.tb04749.x. [DOI] [PubMed] [Google Scholar]
- 16.Ensrud KE, Ewing SK, Stone KL, Cauley JA, Bowman PJ, Cummings SR. Intentional and unintentional weight loss increase bone loss and hip fracture risk in older women. J Am Geriatr Soc. 2003;51:1740–7. doi: 10.1046/j.1532-5415.2003.51558.x. [DOI] [PubMed] [Google Scholar]
- 17.Villareal DT, Shah K, Banks MR, Sinacore DR, Klein S. Effect of weight loss and exercise therapy on bone metabolism and mass in obese older adults: a one-year randomized controlled trial. J Clin Endocrinol Metab. 2008;93:2181–7. doi: 10.1210/jc.2007-1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ensrud KE, Fullman RL, Barrett-Connor E, Cauley JA, Stefanick ML, Fink HA, Lewis CE, Orwoll E. Voluntary weight reduction in older men increases hip bone loss: the osteoporotic fractures in men study. J Clin Endocrinol Metab. 2005;90:1998–2004. doi: 10.1210/jc.2004-1805. [DOI] [PubMed] [Google Scholar]
- 19.Botolin S, McCabe LR. Bone loss and increased bone adiposity in spontaneous and pharmacologically induced diabetic mice. Endocrinology. 2007;148:198–205. doi: 10.1210/en.2006-1006. [DOI] [PubMed] [Google Scholar]
- 20.Devlin M, Vliet MV, Motyl K, Karim L, Brooks D, Louis L, Conlon C, Rosen C, Bouxsein M. Early onset type 2 diabetes impairs skeletal acquisition in the male TALLYHO/JngJ mouse. Endocrinology. 2014;155:3806–3816. doi: 10.1210/en.2014-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baum T, Yap SP, Karampinos DC, Nardo L, Kuo D, Burghardt AJ, Masharani UB, Schwartz AV, Li X, Link TM. Does vertebral bone marrow fat content correlate with abdominal adipose tissue, lumbar spine bone mineral density, and blood biomarkers in women with type 2 diabetes mellitus? J Magn Reson Imaging. 2012;35:117–24. doi: 10.1002/jmri.22757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sjostrom L, Narbro K, Sjostrom CD, Karason K, Larsson B, Wedel H, Lystig T, Sullivan M, Bouchard C, Carlsson B, Bengtsson C, Dahlgren S, Gummesson A, Jacobson P, Karlsson J, Lindroos AK, Lonroth H, Naslund I, Olbers T, Stenlof K, Torgerson J, Agren G, Carlsson LM. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357:741–52. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 23.Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, Schoelles K. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–37. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 24.Naressi A, Couturier C, Castang I, de Beer R, Graveron-Demilly D. Java-based graphical user interface for MRUI, a software package for quantitation of in vivo/medical magnetic resonance spectroscopy signals. Comput Biol Med. 2001;31:269–86. doi: 10.1016/s0010-4825(01)00006-3. [DOI] [PubMed] [Google Scholar]
- 25.Li X, Kuo D, Schafer AL, Porzig A, Link TM, Black D, Schwartz AV. Quantification of vertebral bone marrow fat content using 3 Tesla MR spectroscopy: reproducibility, vertebral variation, and applications in osteoporosis. J Magn Reson Imaging. 2011;33:974–9. doi: 10.1002/jmri.22489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tataranni PA, Ravussin E. Use of dual-energy X-ray absorptiometry in obese individuals. Am J Clin Nutr. 1995;62:730–4. doi: 10.1093/ajcn/62.4.730. [DOI] [PubMed] [Google Scholar]
- 27.Lang TF, Li J, Harris ST, Genant HK. Assessment of vertebral bone mineral density using volumetric quantitative CT. J Comput Assist Tomogr. 1999;23:130–7. doi: 10.1097/00004728-199901000-00027. [DOI] [PubMed] [Google Scholar]
- 28.Khoo BC, Brown K, Cann C, Zhu K, Henzell S, Low V, Gustafsson S, Price RI, Prince RL. Comparison of QCT-derived and DXA-derived areal bone mineral density and T scores. Osteoporos Int. 2009;20:1539–45. doi: 10.1007/s00198-008-0820-y. [DOI] [PubMed] [Google Scholar]
- 29.Harris TB, Launer LJ, Eiriksdottir G, Kjartansson O, Jonsson PV, Sigurdsson G, Thorgeirsson G, Aspelund T, Garcia ME, Cotch MF, Hoffman HJ, Gudnason V. Age, Gene/Environment Susceptibility-Reykjavik Study: multidisciplinary applied phenomics. Am J Epidemiol. 2007;165:1076–87. doi: 10.1093/aje/kwk115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF, Oja P. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–95. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 31.Fazeli PK, Bredella MA, Freedman L, Thomas BJ, Breggia A, Meenaghan E, Rosen CJ, Klibanski A. Marrow fat and preadipocyte factor-1 levels decrease with recovery in women with anorexia nervosa. J Bone Miner Res. 2012;27:1864–71. doi: 10.1002/jbmr.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thaler JP, Cummings DE. Minireview: hormonal and metabolic mechanisms of diabetes remission after gastrointestinal surgery. Endocrinology. 2009;150:2518–25. doi: 10.1210/en.2009-0367. [DOI] [PubMed] [Google Scholar]
- 33.Sheu Y, Schwartz AV, Armati F, Goodpaster B, Li X, Bauer D, Cauley JA. Bone marrow adiposity is elevated in older men with type 2 diabetes.. Program of the 72nd Scientific Sessions of the American Diabetes Association; Philadelphia, PA. 2012; (Abstract 1412-P) [Google Scholar]
- 34.Yu EW. Bone metabolism after bariatric surgery. J Bone Miner Res. 2014;29:1507–18. doi: 10.1002/jbmr.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stein EM, Silverberg SJ. Bone loss after bariatric surgery: causes, consequences, and management. Lancet Diabetes Endocrinol. 2014;2:165–74. doi: 10.1016/S2213-8587(13)70183-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bolotin HH. DXA in vivo BMD methodology: an erroneous and misleading research and clinical gauge of bone mineral status, bone fragility, and bone remodelling. Bone. 2007;41:138–54. doi: 10.1016/j.bone.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 37.Blake GM, Griffith JF, Yeung DK, Leung PC, Fogelman I. Effect of increasing vertebral marrow fat content on BMD measurement, T-Score status and fracture risk prediction by DXA. Bone. 2009;44:495–501. doi: 10.1016/j.bone.2008.11.003. [DOI] [PubMed] [Google Scholar]