Abstract
Heart failure (HF) is a syndrome characterized by a complex pathophysiology which involves multiple organ systems, with the kidney playing a major role. HF can present with reduced ejection fraction (EF), HFrEF, or with preserved EF (HFpEF). The interplay between diverse organ systems contributing to HF is mediated by the activation of counteracting neurohormonal pathways focused to re-establishing hemodynamic homeostasis. During early stages of HF, these biochemical signals, consisting mostly of hormones and neurotransmitters secreted by a variety of cell types, are compensatory and the patient is asymptomatic. However, with disease progression, the attempt to reverse or delay cardiac dysfunction is deleterious, leading to multi-organ congestion, fibrosis and decompensation and finally symptomatic HF. In conclusion, these neurohormonal pathways mediate the evolution of HF and have become a way to monitor HF. Specifically, these mediators have become important in the diagnosis and prognosis of this highly fatal cardiovascular disease. Finally, while these multiple neurohumoral factors serve as important HF biomarkers, they can also be targeted for more effective and curative HF treatments.
Keywords: biomarkers, mediators, heart failure, natriuretic peptides, cardiorenal axis, RAAS
1. HEART FAILURE
1.1 Definition
Human heart failure (HF) is a condition in which the cardiac pump is not able to provide the appropriate blood supply to diverse organ systems and tissues, and remove deleterious waste products. Thus HF, with its mosaic of signs and symptoms is defined as a syndrome. In this regard, two pathognomonic symptoms are dyspnea and fatigue, with congestion secondary to renal sodium and water retention and elevated venous pressure which favors transudation of intravascular fluid into the interstitium. Most often, it is the elderly who present with HF and have many risk factors which contribute to the development of this syndrome, such as hypertension, diabetes mellitus, renal disease, obesity, sleep apnea and depression [1]. The severity of the clinical manifestations of HF is variable; nevertheless, a progressive condition which may have recurrent exacerbations and requires constant therapeutic interventions is defined as chronic heart failure (CHF), whereas a gradual or sudden onset which requires urgent treatment is acute HF syndrome (AHF) [2].
Relevant to this review is a more contemporary and emerging picture of HF which goes beyond the concept of sodium and water retention and congestion. What is emerging is a concept of a multi-organ syndrome in which multiple deleterious cellular pathways are activated by known and unknown humoral and mechanical mediators. This picture of cascading mechanisms results in tissue remodeling in the heart and kidney and likely in other organ systems, leading to organ fibrosis and end-stage HF. Indeed, it is in such a context that circulating biomarkers may be valuable diagnostic and prognostic entities in addition to serving as protective biochemical factors or deleterious potentiators of HF.
1.2 HFrEF and HFpEF
Although every cell type and chamber of the heart can potentially be involved in the beginning of HF, often there is initially left ventricular (LV) dysfunction linked to an elevation of LV filling pressures. Such a maladaptation secondary to loss of muscle due to myocardial infarction, reduced contractility due to idiopathic cardiomyopathy or increased afterload with hypertension results in a reduced cardiac output and/or increased LV wall tension with a reduced compliance to inflow. When the ejection fraction (EF) of the LV is reduced (≤40%), a condition called “systolic dysfunction”, HF is defined as “reduced ejection fraction” (HFrEF). When the EF is ≥ 50%, but there is concomitant impaired relaxation of the left ventricle, a condition called “diastolic dysfunction”, along with the presence of pathognomonic signs and symptoms of HF, is classified as “HF with preserved EF” (HFpEF). Subjects with HF and an EF between 40% and 50% are considered as part of an intermediate group [3]. Diastolic dysfunction can also be present in HFrEF [4]. Interestingly, HFrEF and HFpEF could be additionally distinguished according to a patient’s phenotype. Subjects with HFpEF, compared to HFrEF, are more often older women, with a higher body-mass index, greater prevalence of diabetes, atrial fibrillation and a long history of arterial hypertension [5]. The estimated prevalence of HFpEF among subjects with HF is approximately 50% [6, 7]. Mortality rate may be higher in patients with HFrEF, however, the high prevalence of HFpEF in the elderly lead to the absolute number of deaths being higher in HFpEF [6]. HFpEF patients principally die from cardiovascular deaths; nevertheless, they also have a higher incidence of non-cardiovascular mortality compared to HFrEF. Subjects with HFrEF are more likely to have cardiovascular related deaths compared to HFpEF patients [5, 8].
Structural characteristics of HFrEF and HFpEF are markedly different. In HFrEF the LV is dilated with hypertrophic walls [9]. Histologically, fibrosis is present, cardiac myocytes are elongated and have a smaller diameter than in HFpEF, and their inner myofibrillar density is also reduced. In addition, myocytes are less stiff compared to HFpEF. In HFpEF, the LV cavity has typically a normal volume and the walls are hypertrophic. Histological examination shows collagen deposition and larger, more rigid cardiomyocytes than in HFrEF [4]. Despite diverse cardiac structure and function, the hemodynamic patterns of HFrEF and HFpEF share similarities as well as differences. Clinical symptoms, renal dysfunction, neurohormonal activation, response to exercise, and outcomes may overlap [10]. Nevertheless, increased ventricular and vascular stiffening may play a more important role than an actual volume overload, in acute HFpEF compared to HFrEF. Thus, these two forms of HF are two well distinguished entities, with different pathophysiology and consequently therapeutic approaches. It is possible that HFpEF may evolve into HFrEF and, thus, the two conditions might be considered as “extremes of a single disease” [11].
Treatment and prognosis of cardiovascular disease have dramatically improved over the last decades. Nevertheless, increased mortality and re-hospitalization rates remain high in patients with HF [5]. The American Heart Association Guidelines for the management of HF [3] are clear and straightforward for HFrEF; however, there is lack of consensus in the management of HFpEF [8]. Importantly, therapies such as beta-blockers, angiotensin receptor blockers (ARBs), angiotensin converting enzyme inhibitors (ACE-I), diuretics and mineralocorticoid receptor antagonists (MRAs) which are widely used in HFrEF, do not always have the same beneficial outcome in HFpEF. Most recently, PARAMOUNT [12] was a phase II clinical trial testing the efficacy of a novel compound created by the combination of an ARB, valsartan, with a neprilysin inhibitor (AHU377). Neprilysin is one of the most important enzymes responsible for the degradation of the natriuretic peptides (NPs). The name of this new first-in-class Angiotensin Receptor Neprilysin Inhibitor (ARNI) is LCZ696. The strategy behind this complex molecule is based on the effect targeting two different pathways, both important in the pathogenesis of HFrEF and HFpEF: the renin-angiotensin-aldosterone-system (RAAS) and the NP system [8]. This new drug was added to baseline therapy in n=301 HFpEF patients and compared to HFpEF subjects with baseline treatment plus valsartan alone. The results of this study showed a greater reduction in NT-proBNP levels in the LCZ696-treated group compared to controls; however, this difference was no longer present after 36 weeks of observation. Further, this reduction in NT-proBNP levels remains to be translated into improved clinical outcomes. LCZ696 has been recently added to standard therapy in chronic symptomatic HFrEF patients: the PARADIGM-HF trial [13, 14]. This trial was stopped early (March 2014 instead of October 2014) due to mortality benefit in subjects taking LCZ696 compared to subjects on standard therapy with added ACE-I (enalapril) alone. LCZ696 compared to enalapril also reduced the risk of hospitalization for HF and significantly improved the symptoms of HF. These impressive results have been obtained using a drug that targets at the same time the RAAS and the NP system, and it supports a favorable and enhancing effect of the combination of the two molecules together. LCZ696 may change the therapeutic strategy and the long-term survival of HFrEF patients [15]; however, subjects selected for this landmark trial had a cardiac EF ≤ 35% and had to tolerate a dose of 10 mg twice a day of enalapril before being considered for taking LCZ696. Translating it into the clinical practice may require careful considerations. Another important trial is TOPCAT that tested the efficacy of spironolactone, an MRA, in HFpEF patients [16]. In this case the investigators selected patients with an EF ≥ 45%, from the Americas as well as from Russia and Georgia, and it reported that treatment with spironolactone did not reduce the primary composite outcome of death from cardiovascular causes, aborted cardiac arrest, or hospitalization for the management of heart failure. However, in a post-hoc analysis spironolactone seemed to benefit patients from the Americas but not those in Russia or Georgia as to reflect a possible diverse approach to the conduct of clinical trials in clinical practice in different countries [17].
In conclusion, the treatment for HF and, particularly, HFpEF remains a challenge that certainly warrants new alternative and novel therapeutic approaches, in the acute setting as well as in CHF.
2. NEUROHORMONAL ACTIVATION IN HF
2.1 From Asymptomatic to Symptomatic HF
Despite different pathophysiologies, HFrEF and HFpEF share activation of three major neurohormonal systems: the NP system, sympathetic nervous system (SNS) and the RAAS. The neurohormonal activation has laid the foundation of the field of HF biomarkers. The initial phase of HF syndrome is usually asymptomatic. The stretched cardiomyocytes of the failing heart secrete NPs primarily from the atria [18] to reduce the hemodynamic impairment secondary to vasoconstriction and sodium retention due to the SNS and RAAS [1]. More specifically, the SNS augments inotropic function and peripheral vasoconstriction [19], whereas the RAAS maintains and expands intravascular volume and renal perfusion through vasoconstriction in the kidney and active tubular sodium reabsorption.
The human NPs system consists of three structurally similar but genetically distinct hormones: atrial natriuretic peptide (ANP), B-type natriuretic peptide (BNP) and C-type natriuretic peptide (CNP). ANP and BNP are primarily synthetized in the heart whereas CNP is produced mainly by the endothelium and kidney. ANP and BNP act through the membrane-associated particulate guanylate cyclase A (GC-A) receptor. CNP preferentially binds to the particulate guanylate cyclase B receptor (GC-B). There is a third receptor for the clearance of NPs, NP receptor type C (NPR-C), which may also have proliferative actions in cardiomyocytes and anti-fibrotic actions in cardiac fibroblasts [20, 21]. Only GC-A and GC-B, after binding their specific peptides, produce the second messenger cyclic guanosine 3′,5′-monophosphate (cGMP). Importantly, cGMP mediates diverse cardiovascular actions which involve suppression of cellular proliferation, inhibition of inflammation [8], reduced platelet activation [22] and preservation of myocardial function and structure [23]. The elevation of plasma NPs and, subsequently, cGMP levels may be viewed as a compensatory response to reduce the initial cardiovascular maladaptation present in HF. NPs have numerous and remarkable actions including natriuresis [24], inhibition of aldosterone synthesis [25] and enhancement of vasodilation [26]. NPs are not the only contributors to the increase of cGMP levels. Nitric oxide (NO) acting through soluble guanylate cyclase, the other guanyate cyclase receptor, through cGMP production may modulate inflammation [27], myocardial contractility[28] and endothelial dysfunction[29]. However, NO bioavailability may be reduced in HF [30], contributing to a state of relative cGMP deficiency. NPs, together with NO, attempt to compensate for hemodynamic dysfunction characterizing the initial stage of HF, through cGMP activation. However, the SNS, which releases catecholamines, induces opposing effects and, additionally, can directly activate the RAAS [31]. The peripheral vasoconstriction, including vasoconstriction of the renal arteries, induced by SNS can also lead to glomerular hypoperfusion followed by renin release from the kidney, and consequently augmented RAAS activation. Directly or indirectly, there is a major renal involvement, with an increase in angiotensin II (Ang II) and eventually aldosterone levels. Importantly, the kidney plays a major role during HF, finally leading to sodium and water retention through the activation of the mineralocorticoid receptors in the distal nephron [32]. In addition, Ang II can stimulate norepinephrine release, sustaining a vicious cycle between SNS and RAAS [33]. During disease progression, the increased intravascular volume secondary to renal retention of salt and water and the systemic vasoconstriction are deleterious to cardiorenal function but also structure. The cardiorenal actions of the RAAS and SNS overwhelm the beneficial effects of NPs-NO/cGMP leading to symptomatic HF, increased re-hospitalization and death.
It is important to underscore that these counteracting and complex interactions are present in both AHF and CHF, although with different severities. Moreover, after myocardial infarction aldosterone is also at its highest plasmatic concentration [34]. Indeed, EPHESUS is a landmark clinical trial in which the investigators tested the efficacy of a mineralocorticoid receptor blocker, eplerenone, given after acute myocardial infarction complicated by left ventricular dysfunction and HF. This trial showed that the addition of eplerenone to optimal medical therapy reduced morbidity and mortality [35]. Also, eplerenone showed early survival benefit. Specifically, it significantly reduced all-cause mortality 30 days after randomization in patients with HFrEF [36]. Therefore, the role that aldosterone may play in the pathogenesis of cardiovascular diseases clearly goes beyond HF and hypertension.
2.2 Cardiorenal Syndrome
As originally stated by Braunwald, the hallmark symptoms of CHF are secondary to the kidney and its retention of salt and water [5]. Therefore, the interaction between the heart and kidney remains a crucial component of CHF. In physiological conditions as well as during HF these two organs interact to maintain hemodynamic homeostasis and electrolyte equilibrium, especially in the maintenance of sodium. Eventually, HF becomes a true cardiorenal syndrome either under acute conditions or in chronic state in which glomerular filtration rate is inadequate and sodium and water retention prevail with refractoriness to diuretics and endogenous natriuretic peptides. When the heart begins to fail, the juxtaglomerular cells of the kidney secrete renin. Renin cleaves angiotensinogen, produced and released in the circulation by the liver, to angiotensin I. ACE, broadly present throughout the vasculature and in renal tubules, cleaves angiotensin I to Ang II. Ang II, also a potent vasoconstrictor, activates aldosterone synthesis and release from the zona glomerulosa of the adrenal cortex, with secondary increase in circulating aldosterone [32]. Importantly, aldosterone binds to the mineralocorticoid receptor (MR) present in the epithelial cells of the collecting duct in the nephron, inducing sodium retention and potassium excretion. This action is counter-regulated by the natriuretic effect of the cardiac ANP and BNP, and at least at the beginning, in an effective manner. However, with the evolution of HF, the protective actions of NPs/cGMP are lost, leading to disease progression. The volume overload further induces cardiomyocyte stress and cell loss through apoptosis. Also, inflammatory and pro-fibrotic processes are initiated with extracellular matrix accumulation. Remodeling, hypertrophy, fibrosis and cell death with reduced myocardial regeneration [37] finally mediate irreversible cardiac organ damage and end-stage HF. In parallel, the kidney undergoes maladaptation. Renal blood flow is reduced, and/or the reduced venous return can result in renal congestion [38–40] with secondary increase in renal interstitial pressure [41]. HFrEF and HFpEF are strongly associated with renal dysfunction [39, 42]. As in the heart, long-term impaired renal perfusion induces local inflammatory pathways and fibrosis, and consequent reduction in glomerular and tubular function and eventually parenchymal damage (chronic kidney disease, CKD). This unique cardiac and renal damage is reciprocally maintained and perpetuated. Both organs no longer optimally respond to the compensatory and cardiorenal protective neurohormonal systems and decompensation progresses. This is the picture of cardiorenal syndrome with high risk for death and re-hospitalization.
This strong and complex intercommunication between heart and kidney is bidirectional. Therefore, the kidney can be the first organ to initiate HF. This concept is supported by previous clinical studies that have shown even mild renal impairment to contribute to increased cardiovascular risk [43, 44]. In a recent paper from our group, Martin et al. [45] showed that mild CKD, produced by uninephrectomy (UNX), resulted in early cardiac fibrosis with mild diastolic impairment and preserved systolic function in rats. These findings were independent of blood pressure, sodium, water retention, or aldosterone activity. Further, this kidney-heart connection in mild early CKD could involve at least two gene pathways in the heart: TGF-β and apoptosis pathways. These important results support the hypothesis that impaired kidney function is associated with release of renal humoral and/or cellular factors that contribute to changes in myocardial function and structure.
In conclusion, regardless of the organ of origin, when treating either HF or renal disease it is indispensable to focus on both organs as this synergistic interaction plays a key role in HF and CKD progression.
3. HF: BIOMARKERS OR MEDIATORS?
Neurohormonal activation is a hallmark of HF (Table 1). In this review, we have focused on the roles played by the NP system, the SNS and the RAAS. Indeed, BNP, norepinephrine/epinephrine, Ang II and aldosterone are robust markers of HF [46, 47]. Arginine vasopressin (AVP), also known as antidiuretic hormone (ADH), is synthetized by the hypothalamus and stored in the neurohypophysis. This hormone induces water reabsorption by the nephron, finally increasing the intravascular volume [48]. AVP is also part of neurohormonal activation during HF. Adrenomedullin (ADM) is a vasodilator peptide first found in pheochromocytoma cells, but it has been subsequently found synthetized by different organs such as heart, kidney, lung, smooth muscle cells and endothelium [5, 49, 50]. ADM levels are increased during HF to reduce preload and afterload. Cortisol is produced in the zona fasciculata of the adrenal cortex. This glucocorticoid hormone, if not completely locally inactivated by the enzyme 11β-hydroxysteroid dehydrogenase type 2 (11 βHSD2), can induce sodium and water retention during HF, through binding to the MR in the kidney [51, 52]. In HF subjects, cortisol levels are higher than in subjects without HF [53].
Table 1.
Biomarkers and Mediators in HF.
| Neurohormonal Factors | Cell of Origin | Principal Biological Actions |
|---|---|---|
| Natriuretic peptides | Cardiomyocyte |
|
| Adrenomedullin | Cardiomyocyte |
|
| ST2 | Cardiomyocyte |
|
| Troponins | Cardiomyocyte |
|
| Renin | Juxtaglomerular cell |
|
| Angiotensin II | Pulmonary endothelial cell and renal epithelial cell |
|
| Aldosterone | Zona glomerulosa cell (adrenal cortex) |
|
| Cortisol | Zona fasciculata cell (adrenal cortex) |
|
| Epinephrine/Norepinephrine | Adrenal medulla cell/SNS |
|
| NO | Endothelial cell |
|
| CNP | Endothelial cell and renal tubular cell |
|
| Endothelins | Endothelial cell |
|
| PTH | Parathyroid chief cell |
|
| AVP | Hypothalamus |
|
| MMPs | Fibroblast, Monocyte/Macrophage |
|
| Cytokines (IL-1, IL-6, TNF α..) | Lymphocyte, Monocyte/Macrophage, Fibroblast |
|
| Galectin-3 | Macrophage |
|
| FGF 23 | Osteocyte |
|
SNS, sympathetic nervous system; NO, nitric oxide; CNP, C-type natriuretic peptide; PTH, parathyroid hormone; AVP, arginine vasopressin; MMPs, matrix metalloproteinases; IL, interleukin; TNFα, tumor necrosis factor alpha; FGF 23, fibroblast grow factor 23; Ang, angiotensin; MR, mineralocorticoid receptor; ECM, extracellular matrix.
The activation of the endothelium, the extracellular matrix, immune system cells and the oxidative stress reaction also contribute to the deleterious progression of HF from a compensated to a decompensated and symptomatic stage. Specifically, endothelins, released by endothelial cells, induce vasoconstriction and aldosterone release [54, 55]. Cytokines such as TNF-α, IL-1, IL-6, and other inflammatory proteins and molecules are produced by lymphocytes, macrophages, endothelial cells and many other tissues under stress-related conditions. The injured myocardium can also secrete inflammatory cytokines. All these factors culminate in progression of CHF [56].
Most recently, in the systemic circulation in HF, it is also possible to detect matrix proteinases, enzymes involved in collagen metabolism. Their presence indicates extracellular matrix remodeling and active fibrotic processes [5]. Indeed, Iraqi and coworkers showed changes in biomarkers of collagen synthesis and degradation in patients with congestive HF and left ventricular systolic dysfunction after acute myocardial infarction. This extracellular matrix remodeling was attenuated with aldosterone antagonist [57]. Further, soluble ST2, interleukin receptor released in response to myocardial strain [58–60], and galectin-3, macrophage-derived mediator inductor of collagen synthesis [61], are also measured to evaluate the severity/prognosis of HF. Soluble ST2 plays a role in the cardio-protective stress-response, while galectin-3 is involved in the cardiac fibrotic process. Therefore, both soluble ST2 and galectin-3 along with the collagen peptidases, currently widely used, extend the important concept of monitoring the extracellular collagen turnover during HF.
Importantly, ischemic injury might characterize HF progression, provoking release of specific cardiac peptides, such as troponins, in the bloodstream [47]. In HF it has also been reported that secondary hyperparathyroidism may exist. Specifically, parathyroid hormone (PTH) is released from the parathyroid glands when hypocalcemia is present. Thus, calcium homeostasis may be impaired in HF as well as in CKD. In addition, PTH has been shown to have direct pleiotropic effects on cardiomyocytes [62]. Another connection between altered bone metabolism and HF is the release of the fibroblast growth factor 23 (FGF 23) from the osteocyte [63]. FGF 23, in addition to modulating serum phosphorus, has direct myocardial hypertrophic actions.
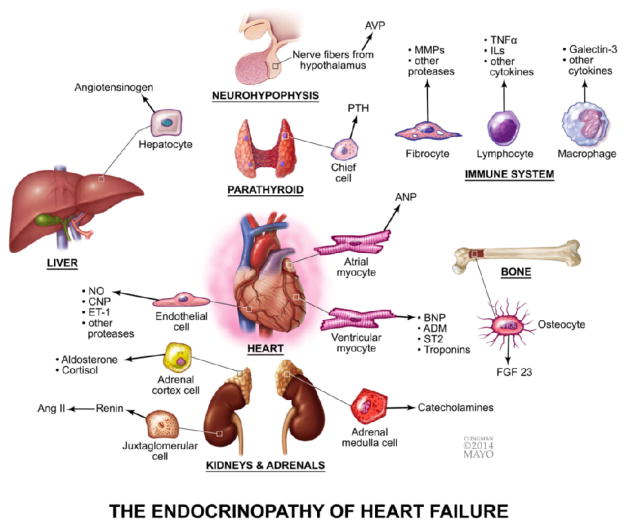
In summary, all these factors are considered valid biomarkers of HF. However, to date, BNP and the N-terminus of the pro-hormone (NT-proBNP) are the only ones recommended by the American Heart Association Guidelines for the diagnosis of HF. In addition to BNP, biomarkers of myocardial injury (tropinins) and cardiac fibrosis (soluble ST2 and galectin-3) may be considered for additive risk stratification in AHF [3]. An illustration of the multiple and complex interconnections activated during HF is shown in Figure 1.
Figure 1.
The complex neurohormonal activation in HF: an Endocrinopathy. The roles played by endothelium, kidney, adrenal, hypothalamus and hypophysis, parathyroid, liver, immune system and bone in response to the failing heart. All these organ systems secrete mediators to compensate the cardiac dysfunction. AVP, arginine vasopressin; MMPs, matrix metalloproteinases, TNFα, tumor necrosis factor alpha; ILs, interleukins; PTH, parathormone; ANP, atrial natriuretic peptide; BNP, B-type natriuretic peptide; ADM, adrenomedullin; FGF 23, fibroblast grow factor; NO, nitric oxide; CNP, C-type natriuretic peptide; ET-1, endothelin-1; Ang II, angiotensin II.
An ideal biomarker should allow for the diagnosis, treatment and prognosis of a specific disease, in addition to being highly sensitive, specific, reliable and standardized, regardless of age, sex and anthropometric parameters of the subject [64]. In HF, despite the continued research and identification of new biomarkers, challenges and questions remain. Importantly, biomarkers, while diagnostic and prognostic, clearly have a role in mediating disease. Biomarkers are part of important pathways of disease and provide a powerful rational for us to monitor specific pathological conditions, such as HF. One could then speculate that using biomarkers just to track disease might not be completely an optimal strategy. Targeting biomarkers with specific therapeutic agents together with their diagnostic and prognostic potential could block or promote a selected pathway involved in the pathogenesis of disease, leading to better outcomes [65]. In HF, the use of ACE-I, ARBs, β-blockers and, recently, the new class of drugs ARNIs are examples of therapeutic strategies targeting biomarkers. The efficacy of these drugs, particularly in HFrEF is unquestionable.
4. PERSPECTIVES
Despite progress in the science and medicine of cardiovascular disease, HF remains an enormous public health burden. The need for new effective drugs should involve the re-studying of the pathophysiology of HFrEF and HFpEF and the re-defining new and diverse pathways and biomarkers to target with novel therapies. Recognizing the activation of numerous hormonal systems in HF and the imbalance between the NPs and RAAS, the use of novel natriuretic peptide receptor agonists, to increase the beneficial effects of NPs/cGMP pathway and counteracting the negative actions of Ang II and mineralocorticoid receptor activation, could be warranted. Nonetheless, as for any disease in which the majority of risk factors are modifiable, HF prevention is the first step. In this regard, the data reported by Eschalier et al. are very interesting. These authors highlight the importance of monitoring blood pressure, procollagen-III-N-terminal peptide, and central obesity to identify early structural and functional changes in the heart, before overt HF [66]. From the same group, a reduced ratio between the low amino terminal pro-peptide of type III procollagen and the type I collagen telopeptide was predictive of ventricular remodeling as well as cardiovascular deaths and hospitalizations for HF, in addition to BNP and cardiac EF [67]. Both these studies underscore the importance of the biomarkers in preventing adverse cardiac remodeling and progression of HF. In conclusion, strict follow-up of identified high-risk subjects, with the use of biomarkers and the modulation of their targets, could help in early diagnosis and therefore in a more personalized strategy to reduce the burden of this syndrome.
HIGHLIGHTS.
Heart failure (HF) is a complex multi-organ syndrome.
The kidney plays a pivotal role in the pathophysiology of HF.
In HF there is simultaneous activation of diverse neurohormonal mediators.
The neurohormonal mediators are biomarkers as they help to monitor disease evolution.
Biomarkers can be targeted for tailored treatment.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jessup M, Brozena S. Heart failure. N Engl J Med. 2003;348:2007–18. doi: 10.1056/NEJMra021498. [DOI] [PubMed] [Google Scholar]
- 2.Gheorghiade M, Zannad F, Sopko G, Klein L, Pina IL, Konstam MA, et al. Acute heart failure syndromes: current state and framework for future research. Circulation. 2005;112:3958–68. doi: 10.1161/CIRCULATIONAHA.105.590091. [DOI] [PubMed] [Google Scholar]
- 3.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240–327. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- 4.van Heerebeek L, Borbely A, Niessen HW, Bronzwaer JG, van der Velden J, Stienen GJ, et al. Myocardial structure and function differ in systolic and diastolic heart failure. Circulation. 2006;113:1966–73. doi: 10.1161/CIRCULATIONAHA.105.587519. [DOI] [PubMed] [Google Scholar]
- 5.Braunwald E. Heart failure. JACC Heart Fail. 2013;1:1–20. doi: 10.1016/j.jchf.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Lam CS, Donal E, Kraigher-Krainer E, Vasan RS. Epidemiology and clinical course of heart failure with preserved ejection fraction. Eur J Heart Fail. 2011;13:18–28. doi: 10.1093/eurjhf/hfq121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–9. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 8.Komajda M, Lam CS. Heart failure with preserved ejection fraction: a clinical dilemma. Eur Heart J. 2014;35:1022–32. doi: 10.1093/eurheartj/ehu067. [DOI] [PubMed] [Google Scholar]
- 9.Redfield MM. Understanding “diastolic” heart failure. N Engl J Med. 2004;350:1930–1. doi: 10.1056/NEJMp048064. [DOI] [PubMed] [Google Scholar]
- 10.Borlaug BA, Redfield MM. Diastolic and systolic heart failure are distinct phenotypes within the heart failure spectrum. Circulation. 2011;123:2006–13. doi: 10.1161/CIRCULATIONAHA.110.954388. discussion 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Little WC, Zile MR. HFpEF: cardiovascular abnormalities not just comorbidities. Circ Heart Fail. 2012;5:669–71. doi: 10.1161/CIRCHEARTFAILURE.112.972265. [DOI] [PubMed] [Google Scholar]
- 12.Solomon SD, Zile M, Pieske B, Voors A, Shah A, Kraigher-Krainer E, et al. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double-blind randomised controlled trial. The Lancet. 2012;380:1387–95. doi: 10.1016/S0140-6736(12)61227-6. [DOI] [PubMed] [Google Scholar]
- 13.McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, et al. Dual angiotensin receptor and neprilysin inhibition as an alternative to angiotensin-converting enzyme inhibition in patients with chronic systolic heart failure: rationale for and design of the Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure trial (PARADIGM-HF) Eur J Heart Fail. 2013;15:1062–73. doi: 10.1093/eurjhf/hft052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 15.Jessup M. Neprilysin inhibition--a novel therapy for heart failure. N Engl J Med. 2014;371:1062–4. doi: 10.1056/NEJMe1409898. [DOI] [PubMed] [Google Scholar]
- 16.Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370:1383–92. doi: 10.1056/NEJMoa1313731. [DOI] [PubMed] [Google Scholar]
- 17.McMurray JJ, O’Connor C. Lessons from the TOPCAT trial. N Engl J Med. 2014;370:1453–4. doi: 10.1056/NEJMe1401231. [DOI] [PubMed] [Google Scholar]
- 18.Burnett JC, Jr, Kao PC, Hu DC, Heser DW, Heublein D, Granger JP, et al. Atrial natriuretic peptide elevation in congestive heart failure in the human. Science. 1986;231:1145–7. doi: 10.1126/science.2935937. [DOI] [PubMed] [Google Scholar]
- 19.Triposkiadis F, Karayannis G, Giamouzis G, Skoularigis J, Louridas G, Butler J. The sympathetic nervous system in heart failure physiology, pathophysiology, and clinical implications. J Am Coll Cardiol. 2009;54:1747–62. doi: 10.1016/j.jacc.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 20.Becker JR, Chatterjee S, Robinson TY, Bennett JS, Panakova D, Galindo CL, et al. Differential activation of natriuretic peptide receptors modulates cardiomyocyte proliferation during development. Development. 2014;141:335–45. doi: 10.1242/dev.100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sangaralingham SJ, Huntley BK, Martin FL, McKie PM, Bellavia D, Ichiki T, et al. The aging heart, myocardial fibrosis, and its relationship to circulating C-type natriuretic Peptide. Hypertension. 2011;57:201–7. doi: 10.1161/HYPERTENSIONAHA.110.160796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moro MA, Russel RJ, Cellek S, Lizasoain I, Su Y, Darley-Usmar VM, et al. cGMP mediates the vascular and platelet actions of nitric oxide: confirmation using an inhibitor of the soluble guanylyl cyclase. Proc Natl Acad Sci U S A. 1996;93:1480–5. doi: 10.1073/pnas.93.4.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greene SJ, Gheorghiade M, Borlaug BA, Pieske B, Vaduganathan M, Burnett JC, Jr, et al. The cGMP signaling pathway as a therapeutic target in heart failure with preserved ejection fraction. J Am Heart Assoc. 2013;2:e000536. doi: 10.1161/JAHA.113.000536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burnett JC, Jr, Opgenorth TJ, Granger JP. The renal action of atrial natriuretic peptide during control of glomerular filtration. Kidney Int. 1986;30:16–9. doi: 10.1038/ki.1986.144. [DOI] [PubMed] [Google Scholar]
- 25.Richards AM. The renin-angiotensin-aldosterone system and the cardiac natriuretic peptides. Heart. 1996;76:36–44. doi: 10.1136/hrt.76.3_suppl_3.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Melo LG, Veress AT, Ackermann U, Sonnenberg H. Chronic regulation of arterial blood pressure by ANP: role of endogenous vasoactive endothelial factors. Am J Physiol. 1998;275:H1826–33. doi: 10.1152/ajpheart.1998.275.5.H1826. [DOI] [PubMed] [Google Scholar]
- 27.Eiserich JP, Hristova M, Cross CE, Jones AD, Freeman BA, Halliwell B, et al. Formation of nitric oxide-derived inflammatory oxidants by myeloperoxidase in neutrophils. Nature. 1998;391:393–7. doi: 10.1038/34923. [DOI] [PubMed] [Google Scholar]
- 28.Liu VW, Huang PL. Cardiovascular roles of nitric oxide: a review of insights from nitric oxide synthase gene disrupted mice. Cardiovasc Res. 2008;77:19–29. doi: 10.1016/j.cardiores.2007.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilkinson IB, Franklin SS, Cockcroft JR. Nitric oxide and the regulation of large artery stiffness: from physiology to pharmacology. Hypertension. 2004;44:112–6. doi: 10.1161/01.HYP.0000138068.03893.40. [DOI] [PubMed] [Google Scholar]
- 30.Bhushan S, Kondo K, Polhemus DJ, Otsuka H, Nicholson CK, Tao YX, et al. Nitrite therapy improves left ventricular function during heart failure via restoration of nitric oxide-mediated cytoprotective signaling. Circ Res. 2014;114:1281–91. doi: 10.1161/CIRCRESAHA.114.301475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jackson G, Gibbs CR, Davies MK, Lip GY. ABC of heart failure. Pathophysiology. Bmj. 2000;320:167–70. doi: 10.1136/bmj.320.7228.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weber KT. Aldosterone in congestive heart failure. N Engl J Med. 2001;345:1689–97. doi: 10.1056/NEJMra000050. [DOI] [PubMed] [Google Scholar]
- 33.von Lueder TG, Sangaralingham SJ, Wang BH, Kompa AR, Atar D, Burnett JC, Jr, et al. Renin-angiotensin blockade combined with natriuretic peptide system augmentation: novel therapeutic concepts to combat heart failure. Circ Heart Fail. 2013;6:594–605. doi: 10.1161/CIRCHEARTFAILURE.112.000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beygui F, Vicaut E, Ecollan P, Machecourt J, Van Belle E, Zannad F, et al. Rationale for an early aldosterone blockade in acute myocardial infarction and design of the ALBATROSS trial. Am Heart J. 2010;160:642–8. doi: 10.1016/j.ahj.2010.06.049. [DOI] [PubMed] [Google Scholar]
- 35.Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309–21. doi: 10.1056/NEJMoa030207. [DOI] [PubMed] [Google Scholar]
- 36.Pitt B, White H, Nicolau J, Martinez F, Gheorghiade M, Aschermann M, et al. Eplerenone reduces mortality 30 days after randomization following acute myocardial infarction in patients with left ventricular systolic dysfunction and heart failure. J Am Coll Cardiol. 2005;46:425–31. doi: 10.1016/j.jacc.2005.04.038. [DOI] [PubMed] [Google Scholar]
- 37.Vigliano CA, Cabeza Meckert PM, Diez M, Favaloro LE, Cortes C, Fazzi L, et al. Cardiomyocyte hypertrophy, oncosis, and autophagic vacuolization predict mortality in idiopathic dilated cardiomyopathy with advanced heart failure. J Am Coll Cardiol. 2011;57:1523–31. doi: 10.1016/j.jacc.2010.09.080. [DOI] [PubMed] [Google Scholar]
- 38.Ronco C, Haapio M, House AA, Anavekar N, Bellomo R. Cardiorenal syndrome. J Am Coll Cardiol. 2008;52:1527–39. doi: 10.1016/j.jacc.2008.07.051. [DOI] [PubMed] [Google Scholar]
- 39.Metra M, Cotter G, Gheorghiade M, Dei Cas L, Voors AA. The role of the kidney in heart failure. Eur Heart J. 2012;33:2135–42. doi: 10.1093/eurheartj/ehs205. [DOI] [PubMed] [Google Scholar]
- 40.Bock JS, Gottlieb SS. Cardiorenal syndrome: new perspectives. Circulation. 2010;121:2592–600. doi: 10.1161/CIRCULATIONAHA.109.886473. [DOI] [PubMed] [Google Scholar]
- 41.Burnett JC, Jr, Knox FG. Renal interstitial pressure and sodium excretion during renal vein constriction. Am J Physiol. 1980;238:F279–82. doi: 10.1152/ajprenal.1980.238.4.F279. [DOI] [PubMed] [Google Scholar]
- 42.Gori M, Senni M, Gupta DK, Charytan DM, Kraigher-Krainer E, Pieske B, et al. Association between renal function and cardiovascular structure and function in heart failure with preserved ejection fraction. Eur Heart J. 2014 doi: 10.1093/eurheartj/ehu254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dries DL, Exner DV, Domanski MJ, Greenberg B, Stevenson LW. The prognostic implications of renal insufficiency in asymptomatic and symptomatic patients with left ventricular systolic dysfunction. J Am Coll Cardiol. 2000;35:681–9. doi: 10.1016/s0735-1097(99)00608-7. [DOI] [PubMed] [Google Scholar]
- 44.Fried LF, Shlipak MG, Crump C, Kronmal RA, Bleyer AJ, Gottdiener JS, et al. Renal insufficiency as a predictor of cardiovascular outcomes and mortality in elderly individuals. J Am Coll Cardiol. 2003;41:1364–72. doi: 10.1016/s0735-1097(03)00163-3. [DOI] [PubMed] [Google Scholar]
- 45.Martin FL, McKie PM, Cataliotti A, Sangaralingham SJ, Korinek J, Huntley BK, et al. Experimental mild renal insufficiency mediates early cardiac apoptosis, fibrosis, and diastolic dysfunction: a kidney-heart connection. Am J Physiol Regul Integr Comp Physiol. 2012;302:R292–9. doi: 10.1152/ajpregu.00194.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anand IS. Changes in Brain Natriuretic Peptide and Norepinephrine Over Time and Mortality and Morbidity in the Valsartan Heart Failure Trial (Val-HeFT) Circulation. 2003;107:1278–83. doi: 10.1161/01.cir.0000054164.99881.00. [DOI] [PubMed] [Google Scholar]
- 47.Braunwald E. Biomarkers in heart failure. N Engl J Med. 2008;358:2148–59. doi: 10.1056/NEJMra0800239. [DOI] [PubMed] [Google Scholar]
- 48.Finley JJt, Konstam MA, Udelson JE. Arginine vasopressin antagonists for the treatment of heart failure and hyponatremia. Circulation. 2008;118:410–21. doi: 10.1161/CIRCULATIONAHA.108.765289. [DOI] [PubMed] [Google Scholar]
- 49.Kitamura K, Kangawa K, Kawamoto M, Ichiki Y, Nakamura S, Matsuo H, et al. Adrenomedullin: a novel hypotensive peptide isolated from human pheochromocytoma. Biochem Biophys Res Commun. 1993;192:553–60. doi: 10.1006/bbrc.1993.1451. [DOI] [PubMed] [Google Scholar]
- 50.Yu CM, Cheung BM, Leung R, Wang Q, Lai WH, Lau CP. Increase in plasma adrenomedullin in patients with heart failure characterised by diastolic dysfunction. Heart. 2001;86:155–60. doi: 10.1136/heart.86.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Whitworth JA, Mangos GJ, Kelly JJ. Cushing, Cortisol, and Cardiovascular Disease. Hypertension. 2000;36:912–6. doi: 10.1161/01.hyp.36.5.912. [DOI] [PubMed] [Google Scholar]
- 52.Funder JW. Minireview: Aldosterone and mineralocorticoid receptors: past, present, and future. Endocrinology. 2010;151:5098–102. doi: 10.1210/en.2010-0465. [DOI] [PubMed] [Google Scholar]
- 53.Anand IS, Ferrari R, Kalra GS, Wahi PL, Poole-Wilson PA, Harris PC. Edema of cardiac origin. Studies of body water and sodium, renal function, hemodynamic indexes, and plasma hormones in untreated congestive cardiac failure. Circulation. 1989;80:299–305. doi: 10.1161/01.cir.80.2.299. [DOI] [PubMed] [Google Scholar]
- 54.Lerman A, Sandok EK, Hildebrand FL, Jr, Burnett JC., Jr Inhibition of endothelium-derived relaxing factor enhances endothelin-mediated vasoconstriction. Circulation. 1992;85:1894–8. doi: 10.1161/01.cir.85.5.1894. [DOI] [PubMed] [Google Scholar]
- 55.Kiowski W, Sütsch G, Hunziker P, Müller P, Jonghun K, Oechslin E, et al. Evidence for endothelin-1-mediated vasoconstriction in severe chronic heart failure. Lancet. 1995;346:732–36. doi: 10.1016/s0140-6736(95)91504-4. [DOI] [PubMed] [Google Scholar]
- 56.Anker SD. Inflammatory mediators in chronic heart failure: an overview. Heart. 2004;90:464–70. doi: 10.1136/hrt.2002.007005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Iraqi W, Rossignol P, Angioi M, Fay R, Nuee J, Ketelslegers JM, et al. Extracellular cardiac matrix biomarkers in patients with acute myocardial infarction complicated by left ventricular dysfunction and heart failure: insights from the Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS) study. Circulation. 2009;119:2471–9. doi: 10.1161/CIRCULATIONAHA.108.809194. [DOI] [PubMed] [Google Scholar]
- 58.Ky B, French B, McCloskey K, Rame JE, McIntosh E, Shahi P, et al. High-sensitivity ST2 for prediction of adverse outcomes in chronic heart failure. Circ Heart Fail. 2011;4:180–7. doi: 10.1161/CIRCHEARTFAILURE.110.958223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen WC, Tran KD, Maisel AS. Biomarkers in heart failure. Heart. 2010;96:314–20. doi: 10.1136/hrt.2008.151639. [DOI] [PubMed] [Google Scholar]
- 60.Felker GM, Fiuzat M, Thompson V, Shaw LK, Neely ML, Adams KF, et al. Soluble ST2 in ambulatory patients with heart failure: Association with functional capacity and long-term outcomes. Circ Heart Fail. 2013;6:1172–9. doi: 10.1161/CIRCHEARTFAILURE.113.000207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sharma UC, Pokharel S, van Brakel TJ, van Berlo JH, Cleutjens JP, Schroen B, et al. Galectin-3 marks activated macrophages in failure-prone hypertrophied hearts and contributes to cardiac dysfunction. Circulation. 2004;110:3121–8. doi: 10.1161/01.CIR.0000147181.65298.4D. [DOI] [PubMed] [Google Scholar]
- 62.Gruson D, Buglioni A, Burnett JC., Jr PTH: Potential role in management of heart failure. Clin Chim Acta. 2014;433:290–6. doi: 10.1016/j.cca.2014.03.029. [DOI] [PubMed] [Google Scholar]
- 63.Gruson D, Lepoutre T, Ketelslegers JM, Cumps J, Ahn SA, Rousseau MF. C-terminal FGF23 is a strong predictor of survival in systolic heart failure. Peptides. 2012;37:258–62. doi: 10.1016/j.peptides.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 64.Bozkurt B. Use of Biomarkers in the Management of Heart Failure: Are We There Yet? Circulation. 2003;107:1231–3. doi: 10.1161/01.cir.0000057608.97285.20. [DOI] [PubMed] [Google Scholar]
- 65.Kakkar R, Lee RT. The IL-33/ST2 pathway: therapeutic target and novel biomarker. Nat Rev Drug Discov. 2008;7:827–40. doi: 10.1038/nrd2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Eschalier R, Rossignol P, Kearney-Schwartz A, Adamopoulos C, Karatzidou K, Fay R, et al. Features of cardiac remodeling, associated with blood pressure and fibrosis biomarkers, are frequent in subjects with abdominal obesity. Hypertension. 2014;63:740–6. doi: 10.1161/HYPERTENSIONAHA.113.02419. [DOI] [PubMed] [Google Scholar]
- 67.Eschalier R, Fertin M, Fay R, Bauters C, Zannad F, Pinet F, et al. Extracellular matrix turnover biomarkers predict long-term left ventricular remodeling after myocardial infarction: insights from the REVE-2 study. Circ Heart Fail. 2013;6:1199–205. doi: 10.1161/CIRCHEARTFAILURE.113.000403. [DOI] [PubMed] [Google Scholar]