Abstract
Toll-like receptors (TLRs), important in rapid clearance of incident human papillomavirus (HPV) infections, may also be important in shaping the adaptive response to persistent infections. We examined here the association between TLR expression and clearance of HPV16 infections following periods of persistence, using longitudinal TLR measurements and a time-to-clearance analysis, as well as the interaction between TLRs and adaptive, cell-mediated responses involved in clearance. TLR2, TLR3, TLR7, TLR8, and TLR9 mRNA expression were measured in cervical cytobrush samples by quantitative PCR. Responses to the HPV16 E6 and E7 oncoproteins were measured by an interferon-γ immunospot assay. Bivariable and multivariable Cox proportional hazard models were used to estimate the effect of TLRs on HPV16 clearance. Higher expression of TLR3 or TLR7 at an HPV16-positive visit was a significant (p ≤ 0.05) predictor of clearance by the following visit, in both unadjusted and adjusted (for smoking and oral contraceptive use) models. In women with, but not those without, a positive response to E6, higher expression of TLR3 (hazard ratio: 1.2 [95% confidence interval: 1.04–1.39], p = 0.012), TLR7 (1.39 [1.14–1.7], p = 0.001), TLR8 (1.37 [1.11–1.69], p = 0.003), or TLR9 (1.53 [1.13–2.08], p = 0.006) was significantly associated with clearance, revealing an important link between innate and adaptive immunity in the control of HPV infections following periods of persistence.
Keywords: human papillomavirus infection, Toll-like receptors, cell-mediated immunity, cohort study, women
Human papillomavirus (HPV)16 is the causative agent in approximately 50–60% of cervical cancers worldwide and is also the most common HPV type detected in the cervix in women with normal cytology.1-3 The majority of these infections are rapidly cleared, with only 5–10% persisting after 3 years. Because persistence is a critical risk factor for the development of pre-cancerous lesions and invasive carcinoma,4 appreciation of the determinants of clearance as defined by loss of DNA detection is central to understanding the natural history of HPV-related cervical disease and informing treatment modalities. While in vitro and in vivo studies have highlighted the roles of both innate and adaptive immunity in cross-sectional design, few have examined host responses to this relatively slow clearing pathogen in longitudinal design.
One molecular component of the innate immune response thought to play a critical role in HPV clearance is the Toll-like receptor (TLR) family. These molecules are present on the surface (TLR1, TLR2, TLR4, TLR5, TLR6, and TLR10) and endosomal membranes (TLR3, TLR7, TLR8, and TLR9) of cells, where they respond to pathogen-associated molecular patterns indicating the presence of extra- and intracellular pathogens, respectively (reviewed by Takeuchi and Akira5). Ligation of TLRs by pathogen-associated molecular patterns leads to immune activation and expression of proinflammatory and immune-mediating cytokines; in the case of the intracellularly located TLRs, the ligands are generally bacterial and viral nucleic acids.
In a previous study, we examined the association between in vivo expression of TLR mRNA and clearance of incident HPV16 infection and demonstrated that incident HPV16 infections that clear on the first 4-month follow-up are significantly associated with higher expression of the four intracellular TLRs, as well as TLR2.6 Because of the short time frame involved in that study, examining clearance only at that first follow-up visit after acquisition, it is presumed that the innate immune response was critical in the clearance cases.
While most closely associated with the innate immune response, TLRs are also critical in shaping the adaptive immune response, through mechanisms that include dendritic cell-mediated control of T-cell activation and differentiation, and the induction of type I interferons that promote memory T-cell proliferation and prevent T-cell apoptosis.7 We’ve previously examined the role of adaptive responses in HPV clearance using an interferon (IFN)-γ enzyme-linked immunospot approach to examine cell-mediated responses to the early-expressed (E)6 and E7 oncoproteins of HPV16.8 Positive responses to E6 were seen in a majority of women with recent clearance, and, in many, could be detected months after clearance. Although detected less often, responses to E7 showed a similar pattern.
The aims of the present study were to extend our previous findings associating TLR expression with rapid clearance of incident infections, using longitudinal TLR measurements and a time-to-clearance analysis, and to examine the interaction between TLRs and cell-mediated, adaptive responses in HPV16 clearance. We hypothesized that, over longer time-frames when adaptive responses would be expected to be more important, TLR expression would be associated with shaping these responses.
Materials and Methods
Study population
Cervical samples for HPV genotyping and TLR testing and peripheral blood samples for examining cell-mediated immune responses to HPV16 antigens were collected from young women recruited between 2000 and 2002 for participation in a longitudinal study of the natural history of HPV infection. The recruitment and study design of that longitudinal study are described elsewhere.9 Briefly, women aged 13–21 years with less than 5 years of sexual experience were recruited at two family planning clinics and followed prospectively. Exclusion criteria were immunosuppression, history of ablative or surgical therapy of the cervix, or intention to move out of the study area within 3 years of enrollment. Informed consent was obtained from all participating women, following guidelines approved by the Committee for Human Research at the University of California, San Francisco. At each study visit, conducted at four- to six-month intervals, women were interviewed regarding changes in demographic and behavioral variables, following which a pelvic exam was conducted and samples collected for HPV genotyping, immune marker measurement, cytology, and sexually transmitted infection testing. Peripheral blood was collected for cell-mediated immune studies from a subset of women who were demonstrated to be positive for HPV16 and who consented to venipuncture.
HPV genotyping
HPV genotyping was performed using cervicovaginal lavage samples (obtained by washing the ectocervix with 5 ml of normal saline) and a polymerase chain reaction (PCR)-driven reverse line-blot method described elsewhere.8 Briefly, pooled, non-degenerate PGMY09/11 primers10 were used to amplify a 452-base pair segment of the L1 gene, followed by hybridization in a reverse line-blot assay11 (Roche Diagnostics, Inc., Alameda, California) that tested for HPV types 6, 11, 16, 18, 26, 31, 33, 35, 39, 40, 42, 45, 51, 52, 53, 54, 55, 56, 57, 58, 59, 66, 68, 73, 82, 83, and 84, plus beta-globin as a measure of sample sufficiency.
TLR measurement
Endocervical samples for TLR measurement were obtained prior to other cervical samples by rotating a cervical cytology brush counterclockwise in the cervical os through two full turns, then placing into RNAlater RNA stabilization solution (Ambion, Austin, Texas) for transport and storage. RNA was extracted using TRI Reagent (Molecular Research Center, Cincinnati, Ohio) following the manufacturer’s protocol, with several modifications as previously described,9 and stored at −80°C until testing.
DNase treatment (TURBO DNA-free, Ambion), reverse transcription (OmniScript RT, Qiagen, Germantown, Maryland), and TLR quantitative PCR were performed as previously described.6 Relative expression was determined by the efficiency-corrected method,12 with amplification efficiency determined from the linear slope of the standard curve included in each run and prepared from serial, fourfold dilutions from 80 to 0.078 ng per reaction of Stratagene QPCR Human Reference Total RNA (Stratagene, La Jolla, California), reverse transcribed identically to the unknowns. Duplicates were averaged and efficiency-corrected TLR expression normalized against the geometric mean13 of the reference genes GAPDH and RPLP0, previously validated for cervical samples in HPV studies.14
IFN-γ enzyme-linked immunospot assay
The measurement of responses to E6 and E7 by enzyme-linked immunospot was previously reported.8 Briefly, E6 or E7 antigen was presented to peripheral blood mononuclear cells isolated from freshly collected, heparinized, peripheral venous blood by autologous CD14+ monocytes infected with recombinant vaccinia virus expressing whole E6 or E7 protein, in plates coated with a monoclonal capture antibody to human IFN-γ. A second monoclonal detection antibody to human IFN-γ was then added and a diaminobenzidine read-out system used. Spots representing cytokine-producing cells were counted visually. The assay was considered positive if the average (of triplicates) number of spot-forming cells in the HPV-antigen wells was 3 standard deviations above background, determined by reactivity to wild-type vaccinia-infected cells.
Statistical analysis
Survival time in days was measured from first HPV16 incidence (defined as first HPV16 DNA detection) until HPV16 was first cleared (defined as the first of two consecutive visits with negative HPV16 genotyping results following the last HPV16-positive result) or the case was right-censored. Prevalent (left-censored) cases were excluded. Kaplan-Meier survival function was used to estimate median time to clearance. To estimate the effect of TLRs on hazard of HPV16 clearance, bivariable, time-dependent Cox proportional hazard models were used. Because the distributions of TLRs were skewed, we log-transformed them, using log2 for easier interpretation. Smoking status and oral contraceptive use, and, separately, the presence of multiple HPV types, were then included in the models to check whether they confounded the bivariable findings. To examine whether the relationships between TLRs and HPV16 clearance depended on cell-mediated immune responses to HPV16 E6 and E7, we further created multivariable Cox regression models including E6 and E7 responses and their interactions with TLRs. Because the first detection of these responses often lags behind initial acquisition, is highly intermittent after first detection, and can persist beyond the time of clearance,8 a woman was counted as having a positive response if she had a positive immunospot result at any point in her history. To ensure that the proportionality assumption was met, we also included the interaction terms between survival time and all the covariates in the models. None of these interaction terms with survival time were significant at 0.05 level, suggesting that the assumption was not violated. All the tests of statistical significance were two-tailed; a hazard-ratio confidence interval that excluded 1 was considered significant at 0.05 level.
Results
Study population and follow-up
We identified, from our longitudinal cohort study of the natural history of HPV infection, fifty-five women who had an incident HPV16 infection, were not immunocompromised, and had stored cervical samples for TLR analysis available from the incident and subsequent visits, for inclusion in this study. Demographic and behavioral characteristics of the study population are shown in Table 1. TLR mRNA expression was measured at the HPV16 incident visit and each visit thereafter, through HPV16 clearance or censoring. The mean number of visits was 4.2 ± 1.4 (standard deviation, SD) and the mean follow-up from incidence through the last TLR measurement was 338 ± 234 (SD) days. The time to 50% clearance as estimated by Kaplan-Meier survival analysis was 371 days (95% confidence interval: 253–391; inter-quartile range: 245–607, Fig. 1). There were no cases of cervical intraepithelial neoplasia 2 or 3 during the follow-up period.
Table 1.
Demographic and behavioral characteristics of study population
| Mean ±SD | |
|---|---|
| Age | 20.5 ±2.9 |
| Lifetime number of sexual partners | 8.6 ±9.7 |
| Age at first intercourse | 16.1 ±1.8 |
| Age of first menstruation | 12.6 ±1.1 |
|
| |
| Race and Ethnicity | Number (%) |
|
| |
| African American | 8 (14.5%) |
| Asian | 8 (14.5%) |
| White | 16 (29.1%) |
| Other/mixed race | 4 (7.3%) |
| Any plus Latina | 19 (34.6%) |
|
| |
| Behavioral Characteristics | Number (%) |
|
| |
| Current tobacco smoker | 14 (24.6%) |
| Current alcohol drinker | 19 (33.3%) |
| Current oral contraceptive user | 23 (40.3%) |
Abbreviation: SD, standard deviation.
Figure 1.
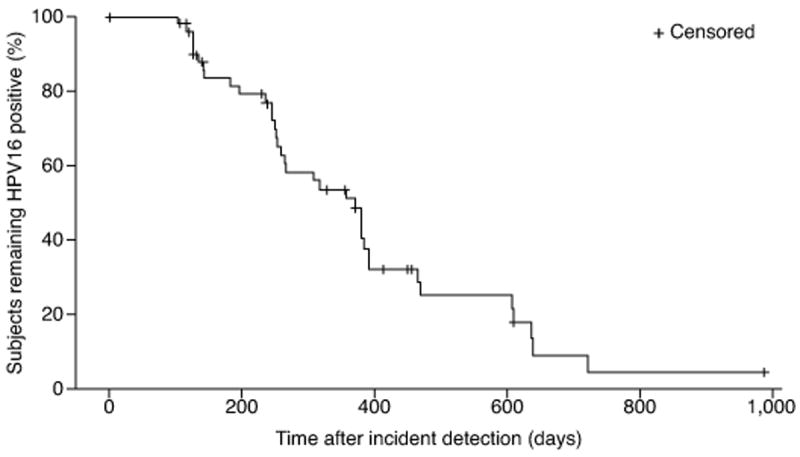
Time to HPV16 clearance after first detection of incident infection by Kaplan-Meier survival function. The median time to clearance was 371 days (95% confidence interval: 253–391; inter-quartile range: 245–607). Tick marks represent points at which women were right-censored.
TLR expression and clearance of HPV16
We first examined the association between the expression of five TLRs (TLR2, TLR3, TLR7, TLR8, and TLR9) and HPV16 clearance. These five TLRs were included in this study because they were significant predictors of HPV16 clearance in our previous, short-term study,6 and include the four intracellular TLRs (TLR3, TLR7, TLR8, and TLR9) that detect bacterial and viral nucleic acid patterns. We used a time-dependent Cox proportional hazard model to examine whether TLR expression was associated with HPV16 clearance by the following visit. Higher TLR expression at an HPV16-positive visit was a significant (p ≤ 0.05) predictor of clearance at the following visit for TLR3 and TLR7 in both unadjusted and adjusted models (Table 2). The latter were adjusted for current tobacco smoking and oral contraceptive use, as these have been shown to be important covariates of immune markers in cervical samples.9,15 Adjustment for the presence of multiple HPV types did not alter the results (data not shown).
Table 2.
TLR mRNA expression as predictor of HPV16 clearance
| TLR | Unadjusted HR1 | p value | Adjusted2 HR1 | p value |
|---|---|---|---|---|
| TLR2 | 1.010 (0.942–1.083) | 0.779 | 1.010 (0.942–1.084) | 0.776 |
| TLR3 | 1.057 (1.005–1.112) | 0.032 | 1.060 (1.007–1.117) | 0.026 |
| TLR7 | 1.105 (1.015–1.204) | 0.022 | 1.110 (1.017–1.212) | 0.020 |
| TLR8 | 1.109 (0.994–1.195) | 0.067 | 1.091 (0.994–1.197) | 0.065 |
| TLR9 | 1.060 (0.980–1.148) | 0.148 | 1.062 (0.980–1.150) | 0.142 |
Cox proportional hazard ratio (95% confidence interval) representing incremental likelihood of clearance for each additional 2-fold difference in relative TLR expression.
Adjusted for tobacco smoking and oral contraceptive pill use.
Abbreviation: HR, hazard ratio.
Interaction between TLR expression and E6- and E7-specific adaptive responses in predicting HPV16 clearance
We next examined the interaction between TLR expression and HPV16 E6- and E7-specific, cell-mediated immune responses in predicting clearance. Forty-six of the fifty-five women examined in this current study had enzyme-linked immunospot data from our earlier study of cell-mediated immunity to HPV16.8 We fitted a series of similar Cox proportional hazard models for the five TLRs with the addition of an interaction term for an IFN-γ immunospot response to E6 or E7 detected in that previous study. The interaction terms with a response to E6 were significant (p ≤ 0.05) for all five TLRs; in contrast, none of the interaction terms with a response to E7 were significant. We further investigated these interactions by examining the association between TLR expression and subsequent HPV16 clearance separately in women with and without an E6- or E7-specific response (Fig. 2). In women with, but not in those without, a positive E6 response, higher levels of TLR expression were significantly associated with subsequent HPV16 clearance for TLR3 (hazard ratio: 1.2 [95% confidence interval: 1.04–1.39], p = 0.012), TLR7 (1.39 [1.14–1.7], p = 0.001), TLR8 (1.37 [1.11–1.69], p = 0.003), and TLR9 (1.53 [1.13–2.08], p = 0.006); a similar trend was observed for TLR2 (1.34 [0.99–1.8], p = 0.057). No such associations were seen for women with E7-specific responses.
Figure 2.

Association between TLR expression and clearance by immunospot status. Cox proportional hazard ratios represent the incremental likelihood of HPV16 clearance associated with each additional 2-fold difference in expression of the indicated TLR, in absence (“−”) or presence (“+”) of a positive E6- or E7-directed response detected by an IFN-γ enzyme-linked immunospot assay at any point in a woman’s history after HPV16 detection. Error bars represent 95% confidence intervals. *p ≤ 0.05, **p ≤ 0.001.
Discussion
This longitudinal follow-up to our previous report6 demonstrates that higher TLR expression continues to be associated with HPV16 clearance even after longer periods of persistence than examined in that study. While this demonstrates the importance of TLRs as a primary and necessary component in the control of HPV, their association with clearance is strongly dependent on an E6-specific effector response: in women with a positive E6 IFN-γ response, all four intracellular TLRs were significant predictors of clearance. We believe this is the first in vivo study to demonstrate a link between TLR expression and the adaptive immune responses to HPV16 infections that are critical in clearance after periods of persistence.
The natural ligands for these four TLRs are varied: TLR3 recognizes double-stranded RNA, TLR7 and TLR8 recognize single-stranded RNA, and TLR9 recognizes unmethylated cytosine-phosphate-guanine sequences in DNA (reviewed by Arpaia and Barton16). Exactly how these TLRs distinguish HPV, or any viral, RNA from host RNA is not clear, although work is ongoing in this area.17 Compartmentalization likely plays an important role: viral RNA is protected inside viral particles from the rapid cytosolic degradation to which self RNA is subjected, and then released within phagosomes where it is then exposed for recognition by TLRs. Self nucleic acids, in contrast, appear not to be efficiently delivered to these compartments.17 Within the endosome, TLR9’s affinity for unmethylated cytosine-phosphate-guanine sequences may further help explain its preference for responding to pathogen-derived, rather than self, DNA, as unmethylated motifs are relatively uncommon in mammalian DNA.17,18 Among the nucleic acid-sensing TLRs, a critical role for TLR9 makes sense—HPV is a double-stranded DNA virus—as does a role for the two single-stranded RNA-sensing TLRs. These, TLR7 and TLR8, were initially identified for their response not to pathogens, but to synthetic single-stranded RNA analogs of the imidazoquinolone family. Imidazoquinolone compounds have long been known to activate immune responses and their use thus advocated for invoking host responses to viral infections. The TLR7 agonist Imiquimod is approved for the treatment of genital warts and may prove to be useful in management of other HPV-related diseases.19,20 It has also been shown to enhance the response to an HPV16 E7 therapeutic vaccine in an animal model.21
Although HPV is a DNA virus, a role for double-stranded RNA-sensing TLR3 in the host response to HPV is consistent with observations of its role in the host response to other double-stranded DNA viruses—mouse cytomegalovirus22 and herpes simplex virus-123,24—presumably as a result of either double-stranded RNA or double-stranded secondary structure in viral transcripts being produced at some point during the viral replicative life cycle. Whether the production of double-stranded RNA intermediates is involved in TLR3’s role in HPV16 clearance is not known, although double-stranded RNA has been shown to be generated during genome replication for a variety of DNA, as well as double-stranded RNA and positive-strand RNA, viruses,25 suggesting some universality to its presence during viral replication, and thus its likely role in immune activation in many viral infections.
Several studies have highlighted the ability of various oncoviruses, including HPV, to down-regulate transcription of TLRs.26-30 Hasan, et al., showed that E6 and E7 proteins from HPV16, but not those from non-oncogenic HPV type 6, are capable of down-regulating TLR9 transcription, pointing to an apparent immune evasion strategy by oncogenic HPV types.27 Further investigating this phenomenon, they recently demonstrated that HPV16 E7 activates an inhibitory nuclear factor-κB complex with estrogen receptor-α that suppresses TLR9 transcription and, through this mechanism, inhibits the interferon response.28 They conclude that TLR9 transcriptional agonists may provide a novel avenue for management of cervical cancer. Reiser, et al., demonstrated that HPV16, -18, and -31 E6 protein inhibits constitutive expression of IFN-κ, which induces transcription of a variety of genes involved in the innate response, including TLR3.29 Taken together with the longitudinal findings reported here, a model emerges in which TLR induction is critical to clearance of oncogenic HPV types, and those types, in turn, have evolved mechanisms to evade this process. It is not clear, however, whether the eventual clearance of established infections examined here represents an immune response that has broken through such viral immune evasion, or, possibly, another pathogen or factor not detected in our study contributed to TLR induction and fortuitous clearance of HPV.
The relationship between TLR expression and the cell-mediated effector response was striking. In contrast to our prior study examining rapid clearance of incident infections, here we examined clearance in women with HPV16 persistence, when adaptive responses would be likely to play an increasingly important role. Our observed association between TLR expression and clearance in women with, but not those without, a positive immunospot response to the E6 oncoprotein suggests strongly that, in women with HPV16 persistence, higher TLR expression alone doesn’t translate to clearance until such time as an effector response develops. These data add further support to our previous conclusion that, in the setting of HPV infection in women without cervical disease, E6-directed responses are more important than E7-directed responses.8 The absence of a similar association with E7 reactivity may be due to the lower frequency of those responses. Not surprisingly, localized, mucosal infection by HPV invokes systemic responses that are, at best, weak and difficult to measure.8,31 The intermittency and delayed timing of detection of immunospot responses to HPV antigens precluded further exploration of the temporal relationship between TLR expression and the onset of adaptive responses. Whether the higher TLR expression preceded or followed the induction of adaptive responses, therefore, remains an open question: TLRs are well understood to be involved in early, innate detection of pathogens, followed by both indirect and direct effects on adaptive responses, although that early role doesn’t preclude the possibility of positive feedback from those adaptive response contributing to TLR up-regulation.
The main downstream effect of TLR stimulation on cell-mediated immunity is likely indirect, through the well-described role of the innate response in the induction and shaping of the adaptive one.7,32 There is also interesting, if yet limited, evidence for a more direct role for TLRs in cell-mediated responses.33 Expression of all four nucleic-acid-sensing TLRs has been demonstrated in CD4+ T cells, while TLR3 and TLR9 have been shown to be expressed in CD8+ T cells. In human CD4+ T cells, costimulation with a TLR3-agonistic double-stranded RNA analog, polyinosinic:polycytidylic acid, induces anti-viral chemokine and granzyme B release,34 while the TLR7- and TLR8-agonist Resiquimod upregulates proliferation and IFN-γ production.35 In human CD8+ effector T cells, polyinosinic:polycytidylic acid costimulation promotes significantly increased IFN-γ production.36 Support for a direct role for TLR9 in T-cell responses is currently limited. In an animal model, cytosine-phosphate-guanine sequence-containing oligodeoxynucleotides have been shown to costimulate proliferation of both CD4+ and CD8+ T cells, with the latter showing the more pronounced response.37 The role of TLR9 in this process is uncertain, however, as studies in TLR9-knockout models have recently demonstrated that oligodeoxynucleotides with or without cytosine-phosphate-guanine sequences are both able to costimulate CD4+ T cells in a TLR9-independent fashion.38
As in our earlier study,8 the relative contributions of CD4+ and CD8+ T cells to the E6-directed response measured by the enzyme-linked immunospot method is unknown. The latter are presumed to be the predominant responders given our use of whole protein as the antigen source, but we’ve previously demonstrated contributions by both subsets.8,39 While the endosomal location of the nucleic acid-sensing TLRs would seem to suggest a stronger role in induction of CD4 responses, through MHC class II presentation, viral antigens inside the endosomal compartment may also be loaded onto MHC class I molecules following TLR activation, for cross presentation to CD8+ cytotoxic T cells.16,40
Similarly, the precise mix of cell-types expressing the TLR mRNA measured here is unknown, given our methodologic approach in which cells are lysed directly off a cytobrush in order to extract RNA for quantitative PCR analysis. Previous studies of the cervical epithelium, using immunohistochemical approaches, have shown, in addition to abundant squamous epithelial cells, a wide complement of immune cell types, including CD4+ and CD8+ T cells, CD19+ and CD20+ B cells, macrophages, dendritic cells, and others.41,42
Extending our earlier study, our present findings support a model in which higher expression of nucleic acid-sensing TLRs enhances HPV16 clearance in association with the downstream effector mechanisms of cell-mediated immunity, emphasizing an important synergistic interaction between innate and adaptive immunity. As interest grows in the use of TLR agonists for the prevention and treatment of cancer,43,44 an appreciation of these interactions is critical. Because persistent infection by oncogenic HPV types is an essential etiologic event in cervical cancer—one that precedes, by years, the development of frank disease—that infection itself becomes a feasible target for the use of TLR agonists in cancer prevention strategies, an approach supported by our findings. Furthermore, our data suggest that, in addition to the TLR7/8 agonists already in use for the treatment of HPV-related disease and the TLR4 agonist monophosphoryl lipid A, used as an adjuvant in an FDA-approved HPV vaccine, TLR3 and TLR9 agonists may also be prove beneficial in the management of persistent cervical HPV infection.
Novelty and Impact.
Toll-like receptors (TLRs) may be important in shaping the adaptive response to HPV infections. In a longitudinal design, we found that higher expression of nucleic acid-sensing TLRs enhances HPV16 clearance after periods of persistence, and that this association requires a cell-mediated adaptive response, demonstrating an important link between innate and adaptive immunity in the control of HPV infections following periods of persistence.
Acknowledgments
The authors thank Dorothy Thai, Dien Vo, and Ibrahim Daud for performing TLR assays, and Susanna Benningfield, Cheryl Godwin De Medina, Lynn Hanson, Julie Jay, and Janet Jonte for collecting the samples and behavioral data from the Natural History of HPV Cohort.
Grant sponsor: National Cancer Institute, National Institutes of Health; Grant number: R37 CA051323; Grant sponsor: National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through the University of California, San Francisco-Clinical and Translational Science Institute; Grant number: UL1 RR024131. The contents of this work are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- E
early-expressed
- HPV
human papillomavirus
- HR
hazard ratio
- IFN
interferon
- SD
standard deviation
- TLR
Toll-like receptor
Footnotes
Conflicts of interest: The authors declare no conflicts of interest.
References
- 1.Clifford G, Franceschi S, Diaz M, Muñoz N, Villa LL. Chapter 3: HPV type-distribution in women with and without cervical neoplastic diseases. Vaccine. 2006;24(Suppl 3):S26–34. doi: 10.1016/j.vaccine.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 2.Muñoz N, Bosch FX, de Sanjosé S, Herrero R, Castellsagué X, Shah KV, Snijders PJF, Meijer CJLM International Agency for Research on Cancer Multicenter Cervical Cancer Study Group. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348:518–27. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 3.Schiffman M, Herrero R, Desalle R, Hildesheim A, Wacholder S, Rodriguez AC, Bratti MC, Sherman ME, Morales J, Guillen D, Alfaro M, Hutchinson M, et al. The carcinogenicity of human papillomavirus types reflects viral evolution. Virology. 2005;337:76–84. doi: 10.1016/j.virol.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 4.Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet. 2007;370:890–907. doi: 10.1016/S0140-6736(07)61416-0. [DOI] [PubMed] [Google Scholar]
- 5.Takeuchi O, Akira S. Recognition of viruses by innate immunity. Immunol Rev. 2007;220:214–24. doi: 10.1111/j.1600-065X.2007.00562.x. [DOI] [PubMed] [Google Scholar]
- 6.Daud II, Scott ME, Ma Y, Shiboski S, Farhat S, Moscicki A-B. Association between toll-like receptor expression and human papillomavirus type 16 persistence. Int J Cancer. 2011;128:879–86. doi: 10.1002/ijc.25400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–95. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 8.Farhat S, Nakagawa M, Moscicki A-B. Cell-mediated immune responses to human papillomavirus 16 E6 and E7 antigens as measured by IFN gamma ELISpot in women with cleared or persistent HPV infection. Int J Gynecol Cancer. 2009;19:508–12. doi: 10.1111/IGC.0b013e3181a388c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scott ME, Ma Y, Farhat S, Shiboski S, Moscicki A-B. Covariates of cervical cytokine mRNA expression by real-time PCR in adolescents and young women: effects of Chlamydia trachomatis infection, hormonal contraception, and smoking. J Clin Immunol. 2006;26:222–32. doi: 10.1007/s10875-006-9010-x. [DOI] [PubMed] [Google Scholar]
- 10.Gravitt PE, Peyton CL, Alessi TQ, Wheeler CM, Coutlée F, Hildesheim A, Schiffman MH, Scott DR, Apple RJ. Improved amplification of genital human papillomaviruses. J Clin Microbiol. 2000;38:357–61. doi: 10.1128/jcm.38.1.357-361.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gravitt PE, Peyton CL, Apple RJ, Wheeler CM. Genotyping of 27 human papillomavirus types by using L1 consensus PCR products by a single-hybridization, reverse line blot detection method. J Clin Microbiol. 1998;36:3020–7. doi: 10.1128/jcm.36.10.3020-3027.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bookout AL, Cummins CL, Mangelsdorf DJ, Pesola JM, Kramer MF. High-throughput real-time quantitative reverse transcription PCR. In: Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K, editors. Current Protocols in Molecular Biology Hoboken. NJ: John Wiley & Sons, Inc.; 2006. pp. 15.8.1–15.8.28. [DOI] [PubMed] [Google Scholar]
- 13.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-7-research0034. research0034.1-0034.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daud II, Scott ME. Validation of reference genes in cervical cell samples from human papillomavirus-infected and -uninfected women for quantitative reverse transcription-PCR assays. Clin Vaccine Immunol. 2008;15:1369–73. doi: 10.1128/CVI.00074-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lieberman JA, Moscicki A-B, Sumerel JL, Ma Y, Scott ME. Determination of cytokine protein levels in cervical mucus samples from young women by a multiplex immunoassay method and assessment of correlates. Clin Vaccine Immunol. 2008;15:49–54. doi: 10.1128/CVI.00216-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arpaia N, Barton GM. Toll-like receptors: key players in antiviral immunity. Curr Opin Virol. 2011;1:447–54. doi: 10.1016/j.coviro.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barbalat R, Ewald SE, Mouchess ML, Barton GM. Nucleic acid recognition by the innate immune system. Annu Rev Immunol. 2011;29:185–214. doi: 10.1146/annurev-immunol-031210-101340. [DOI] [PubMed] [Google Scholar]
- 18.Krieg AM. CpG motifs in bacterial DNA and their immune effects. Annu Rev Immunol. 2002;20:709–60. doi: 10.1146/annurev.immunol.20.100301.064842. [DOI] [PubMed] [Google Scholar]
- 19.Stanley MA. Imiquimod and the imidazoquinolones: mechanism of action and therapeutic potential. Clin Exp Dermatol. 2002;27:571–7. doi: 10.1046/j.1365-2230.2002.01151.x. [DOI] [PubMed] [Google Scholar]
- 20.van Poelgeest MIE, van Seters M, van Beurden M, Kwappenberg KMC, Heijmans-Antonissen C, Drijfhout JW, Melief CJM, Kenter GG, Helmerhorst TJM, Offringa R, van der Burg SH. Detection of human papillomavirus (HPV) 16-specific CD4+ T-cell immunity in patients with persistent HPV16-induced vulvar intraepithelial neoplasia in relation to clinical impact of imiquimod treatment. Clin Cancer Res. 2005;11:5273–80. doi: 10.1158/1078-0432.CCR-05-0616. [DOI] [PubMed] [Google Scholar]
- 21.Chuang C-M, Monie A, Hung C-F, Wu T-C. Treatment with imiquimod enhances antitumor immunity induced by therapeutic HPV DNA vaccination. J Biomed Sci. 2010;17:32. doi: 10.1186/1423-0127-17-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tabeta K, Georgel P, Janssen E, Du X, Hoebe K, Crozat K, Mudd S, Shamel L, Sovath S, Goode J, Alexopoulou L, Flavell RA, et al. Toll-like receptors 9 and 3 as essential components of innate immune defense against mouse cytomegalovirus infection. Proc Natl Acad Sci U S A. 2004;101:3516–21. doi: 10.1073/pnas.0400525101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo Y, Audry M, Ciancanelli M, Alsina L, Azevedo J, Herman M, Anguiano E, Sancho-Shimizu V, Lorenzo L, Pauwels E, Philippe PB, Pérez de Diego R, et al. Herpes simplex virus encephalitis in a patient with complete TLR3 deficiency: TLR3 is otherwise redundant in protective immunity. J Exp Med. 2011;208:2083–98. doi: 10.1084/jem.20101568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang S-Y, Jouanguy E, Ugolini S, Smahi A, Elain G, Romero P, Segal D, Sancho-Shimizu V, Lorenzo L, Puel A, Picard C, Chapgier A, et al. TLR3 deficiency in patients with herpes simplex encephalitis. Science. 2007;317:1522–7. doi: 10.1126/science.1139522. [DOI] [PubMed] [Google Scholar]
- 25.Weber F, Wagner V, Rasmussen SB, Hartmann R, Paludan SR. Double-stranded RNA is produced by positive-strand RNA viruses and DNA viruses but not in detectable amounts by negative-strand RNA viruses. J Virol. 2006;80:5059–64. doi: 10.1128/JVI.80.10.5059-5064.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fathallah I, Parroche P, Gruffat H, Zannetti C, Johansson H, Yue J, Manet E, Tommasino M, Sylla BS, Hasan UA. EBV latent membrane protein 1 is a negative regulator of TLR9. J Immunol. 2010;185:6439–47. doi: 10.4049/jimmunol.0903459. [DOI] [PubMed] [Google Scholar]
- 27.Hasan UA, Bates E, Takeshita F, Biliato A, Accardi R, Bouvard V, Mansour M, Vincent I, Gissmann L, Iftner T, Sideri M, Stubenrauch F, et al. TLR9 expression and function is abolished by the cervical cancer-associated human papillomavirus type 16. J Immunol. 2007;178:3186–97. doi: 10.4049/jimmunol.178.5.3186. [DOI] [PubMed] [Google Scholar]
- 28.Hasan UA, Zannetti C, Parroche P, Goutagny N, Malfroy M, Roblot G, Carreira C, Hussain I, Müller M, Taylor-Papadimitriou J, Picard D, Sylla BS, et al. The human papillomavirus type 16 E7 oncoprotein induces a transcriptional repressor complex on the Toll-like receptor 9 promoter. J Exp Med. 2013;210:1369–87. doi: 10.1084/jem.20122394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reiser J, Hurst J, Voges M, Krauss P, Münch P, Iftner T, Stubenrauch F. High-risk human papillomaviruses repress constitutive kappa interferon transcription via E6 to prevent pathogen recognition receptor and antiviral-gene expression. J Virol. 2011;85:11372–80. doi: 10.1128/JVI.05279-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vincent IE, Zannetti C, Lucifora J, Norder H, Protzer U, Hainaut P, Zoulim F, Tommasino M, Trépo C, Hasan U, Chemin I. Hepatitis B virus impairs TLR9 expression and function in plasmacytoid dendritic cells. PLoS One. 2011;6:e26315. doi: 10.1371/journal.pone.0026315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maldonado L, Teague JE, Morrow MP, Jotova I, Wu TC, Wang C, Desmarais C, Boyer JD, Tycko B, Robins HS, Clark RA, Trimble CL. Intramuscular therapeutic vaccination targeting HPV16 induces T cell responses that localize in mucosal lesions. Sci Transl Med. 2014;6:221ra13. doi: 10.1126/scitranslmed.3007323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee HK, Iwasaki A. Innate control of adaptive immunity: dendritic cells and beyond. Semin Immunol. 2007;19:48–55. doi: 10.1016/j.smim.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 33.Reynolds JM, Dong C. Toll-like receptor regulation of effector T lymphocyte function. Trends Immunol. 2013;34:511–9. doi: 10.1016/j.it.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 34.Meyer T, Oberg H-H, Peters C, Martens I, Adam-Klages S, Kabelitz D, Wesch D. poly(I:C) costimulation induces a stronger antiviral chemokine and granzyme B release in human CD4 T cells than CD28 costimulation. J Leukoc Biol. 2012;92:765–74. doi: 10.1189/jlb.0811407. [DOI] [PubMed] [Google Scholar]
- 35.Caron G, Duluc D, Frémaux I, Jeannin P, David C, Gascan H, Delneste Y. Direct stimulation of human T cells via TLR5 and TLR7/8: flagellin and R-848 up-regulate proliferation and IFN-γ production by memory CD4+ T cells. J Immunol. 2005;175:1551–7. doi: 10.4049/jimmunol.175.3.1551. [DOI] [PubMed] [Google Scholar]
- 36.Tabiasco J, Devêvre E, Rufer N, Salaun B, Cerottini J-C, Speiser D, Romero P. Human effector CD8+ T lymphocytes express TLR3 as a functional coreceptor. J Immunol. 2006;177:8708–13. doi: 10.4049/jimmunol.177.12.8708. [DOI] [PubMed] [Google Scholar]
- 37.Bendigs S, Salzer U, Lipford GB, Wagner H, Heeg K. CpG-oligodeoxynucleotides co-stimulate primary T cells in the absence of antigen-presenting cells. Eur J Immunol. 1999;29:1209–18. doi: 10.1002/(SICI)1521-4141(199904)29:04<1209::AID-IMMU1209>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 38.Landrigan A, Wong MT, Utz PJ. CpG and non-CpG oligodeoxynucleotides directly costimulate mouse and human CD4+ T cells through a TLR9- and MyD88-independent mechanism. J Immunol. 2011;187:3033–43. doi: 10.4049/jimmunol.1003414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakagawa M, Stites DP, Palefsky JM, Kneass Z, Moscicki AB. CD4-positive and CD8-positive cytotoxic T lymphocytes contribute to human papillomavirus type 16 E6 and E7 responses. Clin Diagn Lab Immunol. 1999;6:494–8. doi: 10.1128/cdli.6.4.494-498.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schulz O, Diebold SS, Chen M, Näslund TI, Nolte MA, Alexopoulou L, Azuma Y-T, Flavell RA, Liljeström P, Reis e Sousa C. Toll-like receptor 3 promotes cross-priming to virus-infected cells. Nature. 2005;433:887–92. doi: 10.1038/nature03326. [DOI] [PubMed] [Google Scholar]
- 41.Johansson EL, Rudin A, Wassén L, Holmgren J. Distribution of lymphocytes and adhesion molecules in human cervix and vagina. Immunology. 1999;96:272–7. doi: 10.1046/j.1365-2567.1999.00675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pudney J, Quayle AJ, Anderson DJ. Immunological microenvironments in the human vagina and cervix: mediators of cellular immunity are concentrated in the cervical transformation zone. Biol Reprod. 2005;73:1253–63. doi: 10.1095/biolreprod.105.043133. [DOI] [PubMed] [Google Scholar]
- 43.Galluzzi L, Vacchelli E, Eggermont A, Fridman WH, Galon J, Sautès-Fridman C, Tartour E, Zitvogel L, Kroemer G. Trial Watch: Experimental Toll-like receptor agonists for cancer therapy. Oncoimmunology. 2012;1:699–716. doi: 10.4161/onci.20696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vacchelli E, Galluzzi L, Eggermont A, Fridman WH, Galon J, Sautès-Fridman C, Tartour E, Zitvogel L, Kroemer G. Trial watch: FDA-approved Toll-like receptor agonists for cancer therapy. Oncoimmunology. 2012;1:894–907. doi: 10.4161/onci.20931. [DOI] [PMC free article] [PubMed] [Google Scholar]


