Abstract
(−)-Epigallocatechin gallate (EGCG), a major tea polyphenol, elicits anti-cancer effects. However, the mechanism of action is not fully understood. Our laboratory previously showed that EGCG inhibits heat shock protein 90 (HSP90). We utilized non-tumorigenic (NT), tumorigenic, and metastatic cancer cells from a novel human prostate cancer (PRCA) progression model to test the hypotheses that certain stages are more or less sensitive to EGCG and that sensitivity is related to HSP90 inhibition. Treatment of cells with EGCG, novobiocin (NB), or 17-AAG resulted in more potent cytotoxic effects on tumorigenic and metastatic cells than NT cells. When tumorigenic or metastatic cells were grown in vivo, mice supplemented with 0.06% EGCG in drinking water developed significantly smaller tumors than untreated mice. Furthermore, EGCG prevented malignant transformation in vivo using the full PRCA model.
To elucidate the mechanism of EGCG action, we performed binding assays with EGCG-Sepharose, a C-terminal HSP90 antibody, and HSP90 mutants. These experiments revealed that EGCG-Sepharose bound more HSP90 from metastatic cells compared to NT cells and binding occurred through the HSP90 C-terminus. Additionally, EGCG bound HSP90 mutants that mimic both complexed and uncomplexed HSP90. Consistent with HSP90 inhibitory activity, EGCG, NB, and 17-AAG induced changes in HSP90-client proteins in NT cells and larger differences in metastatic cells. These data suggest that EGCG may be efficacious for the treatment of PRCA because it preferentially targets cancer cells and inhibits a molecular chaperone supportive of the malignant phenotype.
Keywords: Prostate cancer, green tea, EGCG, HSP90, chaperones
INTRODUCTION
A risk factor for prostate cancer (PRCA) is consumption of a Western diet (1). Asian populations have a significantly lower incidence of PRCA. Among the many dietary differences, greater amounts of green tea are consumed in Eastern nations (2). Several studies have shown the health benefits of green tea consumption, including inhibiting PRCA progression (3). The major constituents of green tea (GT) are polyphenols (GTPs), with EGCG making up about 60% (4). EGCG has shown efficacy in several animal models of cancer (5), including PRCA (6). Although EGCG has been shown to interact with several intracellular molecules and affect a number of signaling pathways (5), the exact mechanism of EGCG-elicited anti-cancer activity is not understood. While the anti-cancer effects of EGCG might be due to pleiotropic actions, it is also possible that many of these effects result from a common mechanism.
We (7, 8) and others (9, 10) showed that EGCG inhibits heat shock protein 90 (HSP90) by binding to its C-terminal region and inhibiting dimerization (7, 8). As a molecular chaperone, HSP90 facilitates protein homeostasis by preventing misfolding and aggregation and enhancing protein stability. Heat shock proteins are up-regulated in response to stressors including hypoxia, low nutrient availability, mutations, and over-expression of proteins/oncogenes (11, 12), characteristics important in cancer progression. HSP90 is also expressed at several-fold higher levels in many tumor cells (13), suggesting that tumor cell dependency on client proteins through mutations, aberrant expression, and improper cellular localization makes these cells critically reliant on HSP90 (14). HSP90 coordinates cancer-specific networks; its client proteins include signal effectors and transcription factors relevant to PRCA. These include AR, ErbB2, Akt, c-Src, VEGFR, and MMP2/9 (15, 16). Consistent with a role of HSP90 in supporting a malignant state through inappropriate stabilization of some client proteins, a significant fraction of HSP90 in cancer cells exists in multi-protein complexes, whereas in normal cells it remains predominantly free. HSP90 in tumor cells also appears to have a higher affinity for several HSP90 inhibitors (13). Taken together, these are likely reasons why normal cells are less sensitive to anti-HSP90 activity and why HSP90 is recognized as a critical factor in oncoprotein activity and support of the malignant phenotype. Thus, HSP90 may be a broad target for anti-cancer therapy (12).
Studies indicating the ability of EGCG to affect diverse cellular targets are consistent with its ability to act as an HSP90 inhibitor, since most of the proteins reported to be affected by EGCG are HSP90-clients (16). These data provide a compelling basis for the hypothesis that the reported anti-cancer activity of EGCG is due to its effect on HSP90 function. That the levels/activity and biochemistry of HSP90 change with cancer progression also suggests the possibility for cancer stage-specific effects of HSP90 inhibitors. Here, we utilized a humanized mouse model of PRCA (17) to examine the relative sensitivity of different stages of PRCA to EGCG and to test the hypothesis that these effects are related to HSP90 inhibition.
MATERIALS AND METHODS
Cell culture
BPH-1 (NT) prostatic epithelial cells were from Dr. Simon W. Hayward (Vanderbilt University). His lab authenticated these cells by full karyotyping, the presence of an active neomycin cassette (leading to G418 resistance), expression of the SV40 Large T-antigen, and PCR to ensure human specificity. BCaPT1 (early tumorigenic), BCaPT10 (late tumorigenic), and BCaPM-T10 (metastatic) human PRCA cells (see Supplemental Methods) were developed by Dr. William A. Ricke and derived from the BPH-1 line (17). His lab authenticated these cells by the same methods. These cells were cultured in RPMI-1640 (Cellgro) supplemented with 5% FBS, 25mM HEPES, and antibiotic/antimycotic (Cellgro). LNCaP cells (ATCC) were cultured in RPMI-1640 as above with 10% FBS. HEK293 (ATCC) were cultured in DMEM (Cellgro) supplemented with 10% FBS, 1mM sodium pyruvate, 2mM glutamine, and antibiotic/antimycotic. ATCC authenticated these cell lines by STR analysis. Cells were maintained in a 37°C and 5% CO2 humidified incubator, used at low passage number, and cultured for no more than 3 months.
Cell viability assay
Cells were seeded in 96-well plates at 3,000 cells/well. The following day, cells were treated with vehicle (DPBS), EGCG (Sigma) ± 30U/mL catalase and 30U/mL superoxide dismutase (SOD) (Sigma), novobiocin (NB) (Sigma), or 17-AAG (LC Laboratories) for 48 hours. After incubation, 10μL Alamar Blue (AB, Life Technologies) was added to each well, plates were incubated at 37°C for 3 hours, and percent AB reduced was quantified.
Cell proliferation assay
Cells were seeded and treated with EGCG as indicated above (no enzymes). After 96 hours, medium was removed and the plates frozen at −80°C. DNA content was measured using the FluoReporter Blue Fluorometric dsDNA Quantitation Kit (Molecular Probes).
Apoptosis assay
Cells were seeded in 4-well glass chamber slides. Once 60-70% confluent, EGCG (no enzymes) was added for 48 hours. Apoptotic cells were detected using the Fluorescein In Situ Cell Death Detection Kit (Roche Applied Biosciences).
Wound healing assay for motility
BCaPT10 cells were seeded at 300,000 cells/well in a 6-well plate. Once confluent, a plastic pipette tip was used to create a “wound” and cells were treated ± EGCG (no enzymes). Micrographs were taken at the time of wounding and 6 hours later at fixed locations. At these locations, distance traveled was quantified using a 500 micron reference ruler and ImageJ analysis.
SDS-PAGE & Western blotting (WB)
Cells were seeded at 500,000/100 mm dish. Twenty four hours later, cells were treated with vehicle, EGCG (+30U/mL catalase and SOD), NB, or 17-AAG for 24 hours. Some cells were dosed a second time with vehicle or 50μM EGCG for 24 more hours. Cell lysate was prepared in lysis buffer (50mM Tris [pH 7.5], 150mM NaCl, 2mM EDTA, 0.5% TritonX-100, protease/phosphatase inhibitors), centrifuged at 4°C, 20,000×g for 30 minutes, and protein concentration determined by Bradford assay. Protein (50μg) was separated by SDS-PAGE and transferred to a PVDF membrane. Membranes were blocked in TBS-T (50mM Tris [pH 7.5], 300mM NaCl, 0.5% Tween 20) containing 5% non-fat milk, cut horizontally to allow for probing of multiple proteins, and incubated with primary antibody in blocking buffer overnight at 4°C. Membranes were then washed in TBS-T and incubated with species-specific secondary antibody in blocking buffer at room temperature. Protein bands were visualized using the Li-Cor Odyssey or BioRad ChemiDocMP Imaging system. Densitometry was performed using ImageJ.
Binding assay
EGCG was conjugated to cyanogen bromide-activated Sepharose (Sigma) as described previously (8). Where applicable, cell lysate (50μg) was incubated with vehicle or excess EGCG for 1 hour, followed by incubation with EGCG-Sepharose (30μL) or unconjugated Sepharose for 1 hour with continuous rotation at 4°C. For immunoprecipitation, cell lysate (300μg) was incubated with vehicle or excess EGCG or NB for 1 hour before addition of C-terminal HSP90 primary antibody (sc-7947) for 3 hours and protein A/G PLUS-Agarose beads (Santa Cruz) overnight at 4°C. Beads were pelleted by microcentrifugation and washed with binding buffer (0.05M Tris [pH 7.5], 0.15M NaCl). Bound proteins were analyzed by WB.
Transfection
HEK293 cells were seeded at 250,000 cells/well in a 6-well plate. After 24 hours, cells were transfected with 2μg pcDNA3.1-FLAG-tagged HSP90 constructs (WT HSP90α, HSP90α-E47A, or HSP90α-D93A), kindly provided by Dr. Len Neckers (NCI), using TransFast (Promega) according to manufacturer’s instructions. Cell lysate was collected 36 hours post-transfection for binding assays and WB.
Chaperone function assay
Tumor xenograft assay
Animals were maintained and treated in accordance with the guidelines set by the University of Rochester Committee on Animal Resources and the American Association for Laboratory Animal Science. Six week old male athymic mice (Charles River) were allowed to acclimate for 1 week. Mice were then given sterile deionized water (n=8) or 0.06% EGCG in water (n=8) ad libitum using amber colored bottles for 1 week prior to surgery, with water changed every Monday, Wednesday, and Friday. BCaPT10 or BCaPM-T10 cells (100,000) were resuspended in 15μL rat tail collagen (BD) titrated to pH 7.4, and after polymerization, collagen grafts were placed subcutaneously into mice. Animals with BCaPT10 cells were euthanized after 2 months, while animals with BCaPM-T10 cells were euthanized after 1 month, and tumor mass was determined. Tumors were formalin fixed, paraffin embedded, cut in 8 micron sections, and stained with H&E.
Malignant Transformation (see Supplemental Methods)
Urogenital mesenchyme (UGM) isolation
Timed pregnant (E13) Sprague Dawley female rats (Charles River) were allowed to acclimate until E18. UGM was then isolated from rat embryos as described previously (18).
Preparation/implantation of grafts
Tissue recombinants/grafts were prepared by mixing 250,000 UGM cells with 100,000 BPH-1 cells in rat tail collagen as described above. Grafts were placed under the renal capsule of pre-acclimated 6-8 week old male athymic mice (1 graft/mouse) along with subcutaneous pellets containing testosterone (25mg) and 17β-estradiol (2.5mg). Mice were given water (n=5) or 0.06% EGCG (n=5) as above. After 3 months, grafts were dissected and processed for cell culture.
Isolation/purification of BPH-1 derived cells and re-grafting
A piece of graft from each animal was cut into small fragments, cultured, and the neomycin-resistant/large T-antigen/cytokeratin-positive (BPH-1 derived) cells were purified as described previously (17). Cells were expanded in culture and engrafted (1×106 cells of each line, prepared as above) subcutaneously in new untreated 6 week old male athymic mice (n=10) for 2 months. The number of isolated cell lines from previously untreated/treated animals that formed tumors was determined.
RESULTS
Metastatic cells are more sensitive than NT counterparts to EGCG and other HSP90 inhibitors
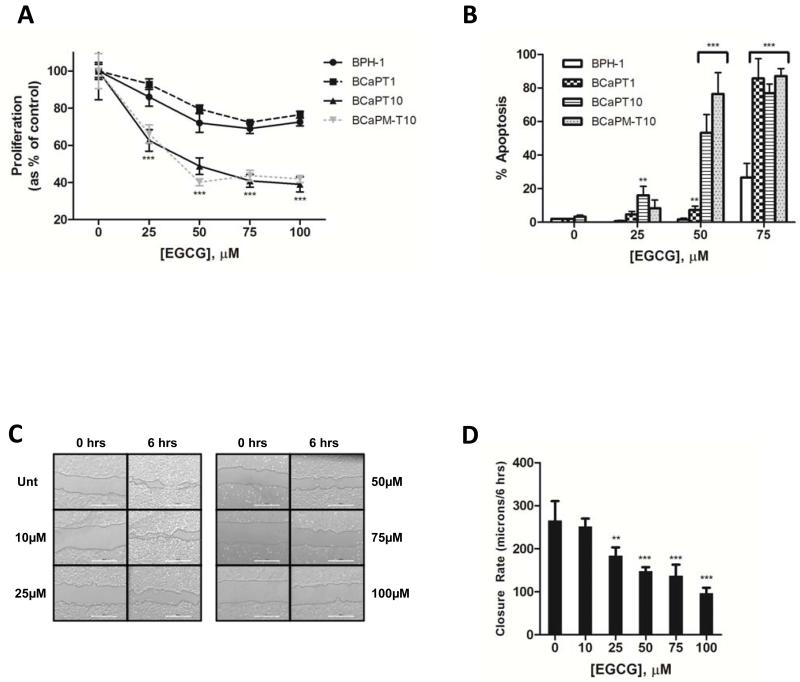
To test potential differences in sensitivity to HSP90 inhibitors, human NT (BPH-1), tumorigenic (BCaPT1, BCaPT10), and metastatic (BCaPM-T10) prostatic epithelial cells were treated with EGCG and cell viability assays were performed. NT cells were less sensitive to EGCG relative to tumorigenic and metastatic cells from this model. Concentrations up to 100μM EGCG had a relatively minor effect on the viability of NT cells, while the same concentrations led to significant decreases in viability of tumorigenic and metastatic cells (Fig. 1A). It has been suggested that hydrogen peroxide and superoxide are produced when EGCG is auto-oxidized in cell culture medium (19-21), which could affect the observed differences in sensitivity to EGCG. Therefore, using the least (BPH-1) and most (BCaPM-T10) sensitive cell lines from Fig. 1A, viability assays were repeated in the presence of catalase and SOD. In the presence of these enzymes, the difference in sensitivity between NT and metastatic cells to EGCG was still observed, albeit the effects were less potent than in the no-enzyme controls (Fig. 1B), indicating that free radicals do contribute to some toxicity under these culture conditions, but not to the stage sensitivity. To investigate if exposure to other HSP90 inhibitors results in similar stage sensitivity, viability assays were performed following treatment with another C-terminal inhibitor, NB, or an N-terminal inhibitor, 17-AAG. Both elicited dose-dependent effects on viability with greater potency in metastatic cells compared to NT cells (Figs. 1C, D). Collectively, these data suggest that EGCG mimics the anti-cancer effects of other HSP90 inhibitors on cell viability, with cancer cells being more sensitive than NT counterparts.
Figure 1.
Cell viability was analyzed by Alamar Blue assay after incubation with (A) EGCG, (B) EGCG+30U/mL catalase+30U/mL superoxide dismutase (SOD), (C) novobiocin (NB), or (D) 17-AAG. Experiments were repeated 3 times (representative results shown) and data represent a mean of 4-6 replicates ± SD. ***p<0.001 by one-way ANOVA or unpaired t-test comparing individual cell lines to BPH-1 at the same concentration. (B) ***p<0.001 for BPH-1 (catalase+SOD) vs. BCaPM-T10 (catalase+SOD).
The effect of EGCG on the viability of these cells could be the result of a decrease in proliferation and/or an induction of apoptosis. We found that EGCG (25-100μM) led to a significant decrease in the total DNA content of tumorigenic (BCaPT10) and metastatic cells compared to NT cells (Fig. 2A). A dose-dependent increase in TUNEL-positive cells was also observed in tumorigenic (BCaPT10) and metastatic cells treated with 25-75μM EGCG, whereas negligible effects were observed in NT cells treated with 25-50μM (Fig. 2B). Both of these assays, like the viability assay, indicated that cancer cells are more sensitive to EGCG than are the NT.
Figure 2.
(A) Cells were analyzed for DNA content after incubation with EGCG. Data represent a mean of 4-6 replicates ± SD. (B) Apoptotic cells were quantified by TUNEL staining after incubation with EGCG. Data represent mean percentage of positive TUNEL cells in 3 random fields of view ± SD. (C, D) Motility of BCaPT10 cells was determined ± EGCG exposure by scratch assay. Data represent mean distance traveled from 3 random fields of view ± SD. Experiments were repeated 3 times; representative results shown. **p<0.01, ***p<0.001 by one-way ANOVA or unpaired t-test by comparing to (A, B) BPH-1 at the same concentration or (D) untreated cells.
EGCG inhibits cancer cell motility
We examined whether EGCG affects cancer cell motility using tumorigenic (BCaPT10) cells since these originated from the site of a primary tumor and had yet to metastasize. Representative micrographs (Fig. 2C) and quantified data (Fig. 2D) show that untreated tumorigenic cells almost completely closed a pipette tip-created “wound” by 6 hours, whereas a significant inhibition of this effect was observed at concentrations of 25-100μM EGCG (Fig. 2D), with no significant effects on cell viability in response to EGCG over this time (data not shown). Furthermore, the addition of catalase and SOD did not affect the ability of EGCG to inhibit the motility of BCaPT10 cells (data not shown).
EGCG elicits anti-PRCA activity in vivo
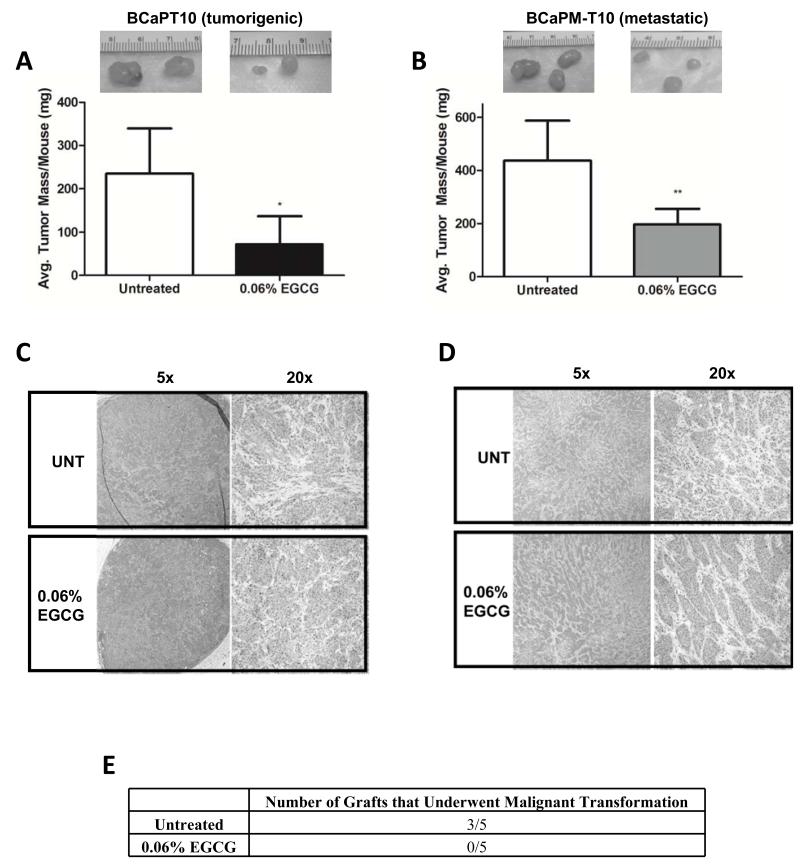
To test if EGCG consumed in drinking water affects the in vivo growth of human prostatic epithelial cells at different stages, mice were engrafted with cancer cells and given sterile deionized water with or without 0.06% EGCG. This concentration has shown efficacy in the TRAMP model of PRCA (22), and is based on other studies that used GTP concentrations of 0.1% (23). We observed an approximate 50% decrease in tumor size in mice engrafted with tumorigenic (Fig. 3A) or metastatic (Fig. 3B) cells after 2- and 1-month(s) EGCG treatment, respectively, relative to controls. No change in body weight was observed (data not shown), indicating this regimen was well tolerated. These results are the first to demonstrate that oral consumption of EGCG elicits anti-cancer activity in 2 different stages (primary tumor growth and metastatic tumor growth) of human PRCA from this model system. Microscopic examination revealed EGCG treated and untreated tumors from both cell types to be high-grade with equally identifiable mitotic figures and morphology (Figs. 3C, D). As such, it does not appear (at least visibly) that EGCG is selecting for a more aggressive cancer type.
Figure 3.
Tumor mass was determined after engrafting mice with (A) BCaPT10 or (B) BCaPM-T10 cells and continuing a regimen of sterile deionized water ± 0.06% EGCG for (A) 2 or (B) 1 month(s). Data represent average tumor mass/mouse ± SD with n=8 mice/group. *p<0.05, **p<0.01 by unpaired t-test. (C, D) H&E staining of (A) late tumorigenic and (B) metastatic tumors from untreated and EGCG treated mice. (E) Mice (n=5/group) were engrafted with one of the individually isolated/purified NT BPH-1 derived prostate epithelial populations from previously untreated or EGCG treated mice for 2 months. The extent of malignant transformation was then examined (# of tumors formed). Data represent number of mice in which tumors were observed for that group.
Some clinical data have suggested chemopreventive effects of GTPs given to patients with high-grade intraepithelial neoplasia (HGPIN), an early PRCA lesion (24). The NT BPH-1 cells described here are large T-antigen immortalized (representing an “initiated state”), comparable to early lesions in humans. To determine if EGCG would prevent malignant transformation of the NT BPH-1 cell line, mice were engrafted with UGM+BPH-1 tissue recombinants and T+E2 implants, and given sterile deionized water with or without 0.06% EGCG for 3 months (a time determined to produce small, localized tumors in untreated animals (17)). At this time, BPH-1-derived epithelial cells from these grafts were purified and grafted subcutaneously into new hosts without EGCG, T+E2, or stroma. After 2 months, the presence of tumors was determined. In all 5 treated mice, EGCG had prevented malignant transformation, since no tumors grew in secondary hosts. In contrast, tumors grew in 3/5 new hosts engrafted with cells from animals not treated with EGCG (Fig. 3E). These data suggest that EGCG prevents malignant transformation of a human cell type that mimics HGPIN.
EGCG-Sepharose binds more HSP90 from metastatic cells compared to NT cells
Most of the putative “targets” of EGCG are HSP90-client proteins, leading to a unifying hypothesis that the mechanism of EGCG’s anti-cancer activity is related to HSP90 inhibition. Since the above data suggest differences in sensitivity to EGCG, we sought to determine if there were also stage differences in HSP90 characteristics that may mediate this. We found that EGCG-Sepharose bound more HSP90 from metastatic cells than from NT cells, even though total amounts of HSP90 were similar (Fig. 4A). Excess EGCG was able to specifically compete with EGCG-Sepharose for binding to HSP90 in metastatic and LNCaP (another metastatic line) cell lysate. This was not reproduced in NT cells, suggesting the possibility that a specific subpopulation of HSP90 present in cancer cells may have a greater ability to bind EGCG-Sepharose, and/or the “affinity” of NT HSP90 binding to EGCG-Sepharose is low and cannot be further perturbed at the EGCG concentration used.
Figure 4.
(A, B) Binding assays were performed by incubating BPH-1, BCaPM-T10, or LNCaP lysate with (A) EGCG-Sepharose or (B) a C-terminal HSP90 antibody and Agarose beads after competition with excess ligand. (C) Lysate from HEK293 cells transfected with FLAG-tagged HSP90 constructs was used for EGCG-Sepharose binding assays. Membranes were cut horizontally to probe for different proteins and bound/total proteins were determined by WB. Experiments were repeated 3 times; representative results shown.
To verify, as suggested previously (7), that the C-terminal region of HSP90 is the target of EGCG, immunoprecipitation experiments were performed. In the presence of excess EGCG, the amount of HSP90 pulled down with a C-terminal antibody from NT and metastatic cell lysates was diminished (Fig. 4B). EGCG also closely mimicked the response generated by NB, another C-terminal HSP90 inhibitor (25), in a chaperone-mediated luciferase refolding assay (Supplemental Fig. 1). In contrast, excess NB had little effect on the ability to bind the C-terminal antibody (Fig. 4B), indicating that it has lower affinity for HSP90, it does not share the same binding site as EGCG or the sc-7947 HSP90 antibody, and/or NB binding elicits different conformational changes in HSP90 compared to EGCG.
HSP90 subpopulations exist that display different affinities for N-terminal HSP90 inhibitors and differentially interact with HSP90-clients (15, 26). Furthermore, a significant fraction of HSP90 in cancer cells exists in multi-protein complexes, whereas in normal cells it remains predominantly uncomplexed (13). To determine if EGCG prefers different conformations of HSP90 that might exist in NT versus metastatic cells, EGCG-Sepharose assays were performed using lysate from HEK cells transfected with FLAG-tagged WT HSP90α, HSP90α-E47A, or HSP90α-D93A. HSP90α-E47A binds but cannot hydrolyze ATP, and thus in cells, gets trapped in an N-domain dimerized, p23-binding (complexed) conformation not preferred by N-domain drugs. In contrast, HSP90α-D93A is thought to exist primarily in an apo, N-domain open (uncomplexed) conformation that does not bind ATP or N-terminal inhibitors (27). As shown in Fig. 4C, EGCG-Sepharose bound FLAG-WT HSP90α, HSP90α-E47A, and - D93A, suggesting that EGCG (like NB (28)) binding is not affected by either ATP-dependent alterations in N-domain conformation or by nucleotide occupancy of the N-domain binding pocket. Moreover, these data indicate that EGCG binds both complexed and uncomplexed HSP90 conformations.
EGCG elicits HSP90-client protein changes in NT and metastatic cells
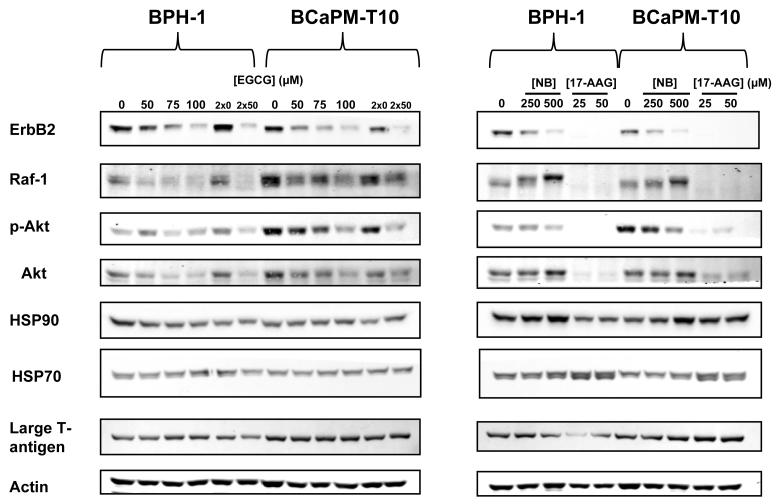
Since EGCG, NB, and 17-AAG demonstrate stage-specific cytotoxicity (Fig. 1), and EGCG binds more strongly to HSP90 in metastatic cells (Fig. 4A), we sought to determine how HSP90 function/activity differs between these two cell lines by examining changes in HSP90-client protein levels in response to HSP90 inhibition. Within 24 hours, EGCG treatment led to a dose-dependent loss of ErbB2, Raf-1, and Akt in both NT and metastatic cells. No significant or consistent changes were observed for total HSP90 levels in either cell line. Repeated treatment with 50μM EGCG (“2×50”, Fig. 5) led to a more pronounced decrease in HSP90-clients. However, certain clients appear to be more affected than others. Densitometric analyses suggested that p-Akt was more sensitive to EGCG in metastatic relative to NT cells (Fig. 5; Supplemental Table 1A). Moreover, metastatic cells have higher total basal levels of Akt, p-Akt, and Raf-1 (Supplemental Table 1B). Therefore, the total decrease in the levels of these client proteins with increasing dose of EGCG is much greater in metastatic than NT cells (Fig. 5), which could be associated with increased sensitivity.
Figure 5.
HSP90-client protein levels were examined in BPH-1 and BCaPM-T10 cells after treatment with EGCG (+30U/mL catalase, 30U/mL SOD), NB, or 17-AAG. Membranes were cut horizontally to probe for different proteins by WB. Experiments were repeated 3 times; representative results shown.
When comparing EGCG to NB and 17-AAG, loss of the same clients was observed, although the relative potencies of these inhibitors were different. 17-AAG at 25μM led to an almost complete loss of the clients tested (Fig. 5). In contrast to EGCG and NB, it also induced HSP70, consistent with its effects on the pro-survival heat shock response (HSR) (Fig. 5) (29). NB also led to HSP90-client protein changes, but at higher concentrations. Raf-1 levels also increased (with a band shift) in response to NB (Fig. 5), whereas this effect was not elicited by EGCG, suggesting, that although these compounds both bind to the HSP90 C-terminus, different binding sites within this region may lead to distinct changes in HSP90-regulated networks. Finally, the parental BPH-1 and its derived cell lines contain large T-antigen. Although this protein is an HSP90-client, its levels were largely unaffected by EGCG, NB, or 17-AAG (Fig. 5) at all concentrations tested, ensuring that the stage-specific sensitivity is unrelated to expression of this protein in these cell lines.
DISCUSSION
The efficacy of targeting HSP90 in human cancer has been demonstrated with various N-terminal HSP90 inhibitors using pre-clinical animal models of cancer where HSP90-client proteins have known roles in tumor progression and survival (12, 30, 31). Our data suggest a mechanism by which EGCG elicits anti-PRCA activity specifically in cancer cell subtypes by binding to HSP90 and affecting a number of client proteins critical to the malignant phenotype.
Previous studies have indicated PC-3 PRCA cells are more sensitive than primary and NT prostatic epithelial cells (RWPE-1) to the anti-cancer effects of EGCG (32-34). However, these cell models all have different genetic origins. An advantage of our model is that the cancer cells are derived from the clonal NT BPH-1 cell line, thus offering a means to identify and characterize changes in HSP90 that may be associated with differential sensitivity. Using this model, we have shown that NT cells are less sensitive than metastatic cells to EGCG and other HSP90 inhibitors. This may be a result of oncogenic mutation and/or over-expression of HSP90-client proteins in cancer cells, necessitating a greater reliance on HSP90 to stabilize client load and conformations of mutant proteins. In metastatic cells, compared to NT cells, Raf-1, p-Akt, and Akt are all present at higher levels, thus providing support to this hypothesis. Although the HSP90-clients examined appeared to change in both cell types, quantitative differences in the effects of EGCG on total levels of particular clients may be associated with differential sensitivity, given that metastatic cells may be uniquely dependent on these proteins. It is also possible there are other clients whose expression may be more dramatically affected by EGCG or other C-terminal inhibitors in metastatic cells compared with NT. Taken together, it is not unreasonable to suspect that normal/NT cells are less sensitive to HSP90 inhibitors because they are less dependent than cancer cells on one or more HSP90-clients, and/or client proteins other than those we examined are more sensitive to HSP90 inhibition in metastatic cells. Furthermore, given the evidence that structurally distinct N-terminal inhibitors trap different subpopulations of HSP90 (15, 26), it is possible that in metastatic cells, a critical stage-specific, pro-cancer subpopulation of HSP90 exists, leading to the observed binding by EGCG-Sepharose, and whose inhibition leads to anti-cancer effects.
Consistent with our in vitro data, these human tumorigenic/metastatic cells were also sensitive to EGCG in vivo (Figs. 3A, B). Published epidemiological and clinical of GTPs and PRCA chemoprevention/treatment have been inconclusive regarding the ability of GTPs to affect any particular stage of PRCA (35). It is possible that all stages of PRCA are affected by EGCG, but with varying degrees of sensitivity. Furthermore, in some cases, differences in stage sensitivity may be small or not observed due to other genetic and/or metabolic differences that influence HSP90 biochemistry or EGCG kinetics. Clearly, a variety of factors that influence the sensitivity of PRCA, or other tumor types, within a given individual need to be considered in any therapeutic approach.
Nevertheless, together, our results offer a unifying hypothesis to account for the numerous reported putative “targets” of EGCG in that most of these “targets” are HSP90-client proteins (7, 16). An advantage of the use of C-terminal HSP90 inhibitors such as EGCG is the lack of or low induction of the pro-survival HSR (36). This response, characterized by up-regulation of HSP70 (29), is induced by N-terminal inhibitors and is likely counterproductive for anti-cancer efficacy. Furthermore, the ability of EGCG-Sepharose to bind more HSP90 from metastatic cells, bind several HSP90 conformations, and display lower activity in normal/NT cells suggests that C-terminal inhibitors should be further pursued for treating PRCA. In addition, EGCG may have additive/synergistic effects when combined with specific and traditional anti-cancer agents (37), as has been observed with N-terminal HSP90 inhibitors (31). The use of EGCG in combination may maximize clinical benefit by sensitizing cancer cells to other anti-cancer therapies, potentially allowing for the use of lower drug doses. These clinical benefits may limit serious side effects and oncogene switching (12), which can result in cancer recurrence.
Overall, determining how HSP90 characteristics change with cancer progression and whether this affects binding to EGCG/other HSP90 inhibitors using this PRCA model system will provide critical details about specific and distinct changes in HSP90 networks and allow for more directed therapies to target HSP90 in human PRCA. The studies described here may lay the foundation to evaluate structure-activity relationships for analogs of EGCG that have increased bioavailability, while inhibiting HSP90 function to elicit stronger anti-cancer properties with limited side effects (38).
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Dr. Len Neckers for critical reading of this manuscript and Dr. Hiroshi Miyamoto (Johns Hopkins) for histology analysis.
Financial support: This work was supported by NIH grants AT006366 (T. A. Gasiewicz), ES01247 (T. A. Gasiewicz), ES07026 (T. A. Gasiewicz), CA123199 (W. A. Ricke), and the URMC Drug Development Pilot Program (T. A. Gasiewicz).
Footnotes
No conflicts of interest disclosed.
REFERENCES
- 1.Nelson WG, De Marzo AM, Isaacs WB. Prostate cancer. N Engl J Med. 2003;349:366–381. doi: 10.1056/NEJMra021562. [DOI] [PubMed] [Google Scholar]
- 2.Yuan JM, Sun C, Butler LM. Tea and cancer prevention: epidemiological studies. Pharmacol Res. 2011;64:123–135. doi: 10.1016/j.phrs.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khan N, Mukhtar H. Tea and health: studies in humans. Current Pharmaceutical Design. 2013;19:6141–6147. doi: 10.2174/1381612811319340008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balentine DA, Wiseman SA, Bouwens LC. The chemistry of tea flavonoids. Crit Rev Food Sci Nutr. 1997;37:693–704. doi: 10.1080/10408399709527797. [DOI] [PubMed] [Google Scholar]
- 5.Yang CS, Wang H, Li GX, Yang Z, Guan F, Jin H. Cancer prevention by tea: Evidence from laboratory studies. Pharmacol Res. 2011;64:113–122. doi: 10.1016/j.phrs.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Adhami VM, Siddiqui IA, Sarfaraz S, Khwaja SI, Hafeez BB, Ahmad N, et al. Effective prostate cancer chemopreventive intervention with green tea polyphenols in the TRAMP model depends on the stage of the disease. Clin Cancer Res. 2009;15:1947–1953. doi: 10.1158/1078-0432.CCR-08-2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yin Z, Henry EC, Gasiewicz TA. (−)-Epigallocatechin-3-gallate is a novel Hsp90 inhibitor. Biochemistry. 2009;48:336–345. doi: 10.1021/bi801637q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palermo CM, Westlake CA, Gasiewicz TA. Epigallocatechin gallate inhibits aryl hydrocarbon receptor gene transcription through an indirect mechanism involving binding to a 90 kDa heat shock protein. Biochemistry. 2005;44:5041–5052. doi: 10.1021/bi047433p. [DOI] [PubMed] [Google Scholar]
- 9.Li Y, Zhang T, Jiang Y, Lee HF, Schwartz SJ, Sun D. (−)-Epigallocatechin-3-gallate inhibits Hsp90 function by impairing Hsp90 association with cochaperones in pancreatic cancer cell line Mia Paca-2. Molecular Pharmaceutics. 2009;6:1152–1159. doi: 10.1021/mp900037p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tran PL, Kim SA, Choi HS, Yoon JH, Ahn SG. Epigallocatechin-3-gallate suppresses the expression of HSP70 and HSP90 and exhibits anti-tumor activity in vitro and in vivo. BMC Cancer. 2010;10:276. doi: 10.1186/1471-2407-10-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whitesell L, Lindquist SL. HSP90 and the chaperoning of cancer. Nat Rev Cancer. 2005;5:761–772. doi: 10.1038/nrc1716. [DOI] [PubMed] [Google Scholar]
- 12.Trepel J, Mollapour M, Giaccone G, Neckers L. Targeting the dynamic HSP90 complex in cancer. Nat Rev Cancer. 2010;10:537–549. doi: 10.1038/nrc2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamal A, Thao L, Sensintaffar J, Zhang L, Boehm MF, Fritz LC, et al. A high-affinity conformation of Hsp90 confers tumour selectivity on Hsp90 inhibitors. Nature. 2003;425:407–410. doi: 10.1038/nature01913. [DOI] [PubMed] [Google Scholar]
- 14.Workman P, Burrows F, Neckers L, Rosen N. Drugging the cancer chaperone HSP90: combinatorial therapeutic exploitation of oncogene addiction and tumor stress. Ann N Y Acad Sci. 2007;1113:202–216. doi: 10.1196/annals.1391.012. [DOI] [PubMed] [Google Scholar]
- 15.Moulick K, Ahn JH, Zong H, Rodina A, Cerchietti L, Gomes DaGama EM, et al. Affinity-based proteomics reveal cancer-specific networks coordinated by Hsp90. Nat Chem Biol. 2011;7:818–826. doi: 10.1038/nchembio.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Picard D. Hsp90 interactors. http://www.picard.ch/downloads/Hsp90interactors.pdf.
- 17.Ricke WA, Ishii K, Ricke EA, Simko J, Wang Y, Hayward SW, et al. Steroid hormones stimulate human prostate cancer progression and metastasis. Int J Cancer. 2006;118:2123–2131. doi: 10.1002/ijc.21614. [DOI] [PubMed] [Google Scholar]
- 18.Staack A, Donjacour AA, Brody J, Cunha GR, Carroll P. Mouse urogenital development: a practical approach. Differentiation. 2003;71:402–413. doi: 10.1046/j.1432-0436.2003.7107004.x. [DOI] [PubMed] [Google Scholar]
- 19.Elbling L, Weiss RM, Teufelhofer O, Uhl M, Knasmueller S, Schulte-Hermann R, et al. Green tea extract and (−)-epigallocatechin-3-gallate, the major tea catechin, exert oxidant but lack antioxidant activities. FASEB J. 2005;19:807–809. doi: 10.1096/fj.04-2915fje. [DOI] [PubMed] [Google Scholar]
- 20.Hong J, Lu H, Meng X, Ryu JH, Hara Y, Yang CS. Stability, cellular uptake, biotransformation, and efflux of tea polyphenol (−)-epigallocatechin-3-gallate in HT-29 human colon adenocarcinoma cells. Cancer Res. 2002;62:7241–7246. [PubMed] [Google Scholar]
- 21.Hou Z, Sang S, You H, Lee MJ, Hong J, Chin KV, et al. Mechanism of action of (−)-epigallocatechin-3-gallate: auto-oxidation-dependent inactivation of epidermal growth factor receptor and direct effects on growth inhibition in human esophageal cancer KYSE 150 cells. Cancer Res. 2005;65:8049–8056. doi: 10.1158/0008-5472.CAN-05-0480. [DOI] [PubMed] [Google Scholar]
- 22.Harper CE, Patel BB, Wang J, Eltoum IA, Lamartiniere CA. Epigallocatechin-3-Gallate suppresses early stage, but not late stage prostate cancer in TRAMP mice: mechanisms of action. Prostate. 2007;67:1576–1589. doi: 10.1002/pros.20643. [DOI] [PubMed] [Google Scholar]
- 23.Adhami VM, Siddiqui IA, Ahmad N, Gupta S, Mukhtar H. Oral consumption of green tea polyphenols inhibits insulin-like growth factor-I-induced signaling in an autochthonous mouse model of prostate cancer. Cancer Res. 2004;64:8715–8722. doi: 10.1158/0008-5472.CAN-04-2840. [DOI] [PubMed] [Google Scholar]
- 24.Bettuzzi S, Brausi M, Rizzi F, Castagnetti G, Peracchia G, Corti A. Chemoprevention of human prostate cancer by oral administration of green tea catechins in volunteers with high-grade prostate intraepithelial neoplasia: a preliminary report from a one-year proof-of-principle study. Cancer Res. 2006;66:1234–1240. doi: 10.1158/0008-5472.CAN-05-1145. [DOI] [PubMed] [Google Scholar]
- 25.Marcu MG, Schulte TW, Neckers L. Novobiocin and related coumarins and depletion of heat shock protein 90-dependent signaling proteins. J Natl Cancer Inst. 2000;92:242–248. doi: 10.1093/jnci/92.3.242. [DOI] [PubMed] [Google Scholar]
- 26.Beebe K, Mollapour M, Scroggins B, Prodromou C, Xu W, Tokita M, et al. Posttranslational modification and conformational state of heat shock protein 90 differentially affect binding of chemically diverse small molecule inhibitors. Oncotarget. 2013;4:1065–1074. doi: 10.18632/oncotarget.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neckers L. Personal communication. Oct 21, 2013. [Google Scholar]
- 28.Marcu MG, Chadli A, Bouhouche I, Catelli M, Neckers LM. The heat shock protein 90 antagonist novobiocin interacts with a previously unrecognized ATP-binding domain in the carboxyl terminus of the chaperone. J Biol Chem. 2000;275:37181–37186. doi: 10.1074/jbc.M003701200. [DOI] [PubMed] [Google Scholar]
- 29.Whitesell L, Bagatell R, Falsey R. The stress response: implications for the clinical development of hsp90 inhibitors. Curr Cancer Drug Targets. 2003;3:349–358. doi: 10.2174/1568009033481787. [DOI] [PubMed] [Google Scholar]
- 30.Travers J, Sharp S, Workman P. HSP90 inhibition: two-pronged exploitation of cancer dependencies. Drug Discov Today. 2012;17:242–252. doi: 10.1016/j.drudis.2011.12.021. [DOI] [PubMed] [Google Scholar]
- 31.Centenera MM, Fitzpatrick AK, Tilley WD, Butler LM. Hsp90: still a viable target in prostate cancer. Biochim Biophys Acta. 2013;1835:211–218. doi: 10.1016/j.bbcan.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 32.Albrecht DS, Clubbs EA, Ferruzzi M, Bomser JA. Epigallocatechin-3-gallate (EGCG) inhibits PC-3 prostate cancer cell proliferation via MEK-independent ERK1/2 activation. Chem Biol Interact. 2008;171:89–95. doi: 10.1016/j.cbi.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 33.Caporali A, Davalli P, Astancolle S, D’Arca D, Brausi M, Bettuzzi S, et al. The chemopreventive action of catechins in the TRAMP mouse model of prostate carcinogenesis is accompanied by clusterin over-expression. Carcinogenesis. 2004;25:2217–2224. doi: 10.1093/carcin/bgh235. [DOI] [PubMed] [Google Scholar]
- 34.Rizzi F, Naponelli V, Silva A, Modernelli A, Ramazzina I, Bonacini M, et al. Polyphenon E(R), a standardized green tea extract, induces endoplasmic reticulum stress, leading to death of immortalized PNT1a cells by anoikis and tumorigenic PC3 by necroptosis. Carcinogenesis. 2014;35:828–839. doi: 10.1093/carcin/bgt481. [DOI] [PubMed] [Google Scholar]
- 35.Johnson JJ, Bailey HH, Mukhtar H. Green tea polyphenols for prostate cancer chemoprevention: a translational perspective. Phytomedicine: International Journal of Phytotherapy and Phytopharmacology. 2010;17:3–13. doi: 10.1016/j.phymed.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eskew JD, Sadikot T, Morales P, Duren A, Dunwiddie I, Swink M, et al. Development and characterization of a novel C-terminal inhibitor of Hsp90 in androgen dependent and independent prostate cancer cells. BMC Cancer. 2011;11:468. doi: 10.1186/1471-2407-11-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suganuma M, Saha A, Fujiki H. New cancer treatment strategy using combination of green tea catechins and anticancer drugs. Cancer Sci. 2011;102:317–323. doi: 10.1111/j.1349-7006.2010.01805.x. [DOI] [PubMed] [Google Scholar]
- 38.Bhat R, Adam AT, Lee JJ, Gasiewicz TA, Henry EC, Rotella DP. Towards the discovery of drug-like epigallocatechin gallate analogs as Hsp90 inhibitors. Bioorganic & Medicinal Chemistry Letters. 2014;24:2263–2266. doi: 10.1016/j.bmcl.2014.03.088. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.