Abstract
Dysregulated processing of natural rewards may be a central pathogenic process in the etiology and maintenance of prescription opioid misuse and addiction among chronic pain patients. This study examined whether a Mindfulness-Oriented Recovery Enhancement (MORE) intervention could enhance natural reward processing through training in savoring as indicated by event-related brain potentials (ERPs). Participants were chronic pain patients at risk for opioid misuse who were randomized to eight weeks of MORE (n=11) or a support group control condition (n=18). ERPs to images representing naturally rewarding stimuli (e.g., beautiful landscapes, intimate couples) and neutral images were measured before and after 8 weeks of treatment. Analyses focused on the late positive potential (LPP) - an ERP response in the 400 – 1000 ms time window thought to index allocation of attention to emotional information. Treatment with MORE was associated with significant increases in LPP response to natural reward stimuli relative to neutral stimuli which were correlated with enhanced positive affective cue-responses and reductions in opioid craving from pre- to post-treatment. Findings suggest that cognitive training regimens centered on strengthening attention to natural rewards may remediate reward processing deficits underpinning addictive behavior.
Keywords: Mindfulness, chronic pain, opioid misuse, reward, ERP, positive emotion, savoring, hedonic, allostasis
Dysregulated processing of natural rewards is a key mechanism subserving the maintenance of drug addiction. Preclinical and clinical studies indicate that drug addiction results in attenuated dopaminergic neurotransmission in response to natural rewards; this phenomenon has been observed among individuals with cocaine, methamphetamine, nicotine, alcohol, and opiate dependencies (Heinz et al. 2004; Kalivas and Volkow 2005; Lee et al. 2009; Lintas et al. 2012; Gipson et al. 2013). Substance dependent persons exhibit diminished subjective reward responsiveness and reduced brain activation to natural rewards compared to healthy control subjects (Volkow et al. 2010). Such reward processing deficits may have serious consequences for persons with substance use disorders. Indeed, reward valuation of salutary objects and events, encoded in dopaminergic activations of mesocorticolimbic brain circuits, is fundamental to flexible goal selection and the conservation of physical and psychological well-being. However, neurocognitive resources required for processing natural rewards may be hijacked by chronic exposure to psychoactive drugs, which, by eliciting changes to brain structure and function, reorganize basic learning mechanisms around pursuit of drug-induced reward and the alleviation of withdrawal-induced dysphoria and other aversive states (e.g., stress and pain) (Alcaro and Panksepp 2011). Putatively, this process is underpinned by allostatic changes to neural circuits subserving stress (e.g., amygdala) and reward (e.g., ventral striatum), through which the addict becomes increasingly insensitive to reward from healthful and socially affiliative stimuli, while becoming increasingly dependent on drug use to preserve hedonic homeostasis (Koob and Le Moal 2008).
Reward processing may be disrupted by illegal substances and prescription drugs alike. Indeed, emerging neuropsychopharmacologic models (Garland, Froeliger, Zeidan, Partin, & Howard, 2013) suggest that dyregulated reward processing is a central pathogenic process in the etiology of prescription opioid misuse and addiction among chronic pain patients. Chronic pain, coupled with opioid dose escalation, results in allostatic load on limbic-striatal neurocircuitry, resulting in decreased reward derived from healthful objects and events (Shurman, Koob, & Gutstein, 2010). The drive to alleviate dysphoria resulting from this reward deficit is manifested as craving, and may progress to the compulsive pattern of opioid use characteristic of opioid addiction (Koob & Le Moal, 2008). Evidence for dysregulation of natural reward processing in opioid addiction has been generated by studies showing reduced cue-elicited event-related brain potentials (ERPs) to images depicting natural rewards among opioid dependent individuals (Lubman et al. 2007, 2008) which predict future drug use (Lubman et al., 2009).
If dysregulated processing of natural rewards fuels the cycle of behavioral escalation from prescription opioid use to misuse and addiction, then enhancing natural reward processing may be a means of ameliorating or even reversing this pathogenic process. Plausibly, adaptive reward processing may be bolstered by therapeutic approaches which provide training in attending to and savoring naturally rewarding objects and events. Mindfulness training, which promotes emotion regulation through present-oriented attention (Holzel et al., 2011), may be especially efficacious in this regard. Though not the explicit goal of most mindfulness-based interventions, mindfulness may nonetheless amplify pleasure from perceptual and sensorimotor experiences in a fashion similar to sensate-focus techniques (Masters & Johnson, 1970) and promote positive emotion regulation by amplifying selective attentional processes (Wadlinger & Isaacowitz, 2010). We hypothesize that mindful savoring increases attentional capacity to focus more intensely on both the pleasurable features of objects, persons, and events, as well as the positive emotions that arise from experiencing them.
Increased attention to sensory experience has been shown to elevate pleasure in eating and sex (Heiman & Meston, 1997; LeBel & Dubé, 2001), and attending to present-moment experience robustly predicts happiness in large-scale, time-lagged analyses (Killingsworth & Gilbert, 2010). More directly supportive of the “mindful savoring” hypothesis, mindful eating was found to increase ratings of subsequent food liking and enjoyment (Hong, Lishner, Han, & Huss, 2011; Hong, Lishner, & Han, 2014). Even stronger support may be derived from a randomized controlled trial (RCT) of Mindfulness-Based Cognitive Therapy (MBCT) with adults with residual depressive symptoms, which found that mindfulness training increased the experience of reward and positive emotion from pleasant daily life activities (Geschwind, Peeters, Drukker, van Os, &Wichers, 2011).
We recently conducted an early-stage RCT of Mindfulness-Oriented Recovery Enhancement (MORE) (Garland, Gaylord, Boettiger, & Howard, 2010; Garland, Manusov, Froeliger, Kelly, Williams, & Howard, 2014), a new multimodal intervention designed to address chronic pain, craving, and opioid misuse behaviors, which integrates training in savoring natural rewards with training in mindfulness and positive reappraisal techniques. Results of this RCT demonstrated that relative to a support group (SG) control condition, MORE significantly decreased pain severity and functional impairment, as well as opioid craving and opioid misuse (Garland et al., 2014). A subsequent analysis of data from a subset of individuals in this trial revealed that MORE led to enhanced cardiac-autonomic responses to natural reward cues which statistically mediated the effect of the intervention on reductions in opioid craving (Garland, Froeliger, & Howard, 2014). These findings are perhaps the first in the scientific literature to support the notion that a behavioral intervention can restore natural reward processing in addiction. Despite the compelling nature of the findings, autonomic psychophysiological indices measured in blocks of trials, as in the previous study, may not capture the time course of neural mechanisms involved in natural reward processing. Hence, in the present study, we examined a neurophysiological marker of reward processing using ERPs. ERPs, which have a temporal resolution on the order of milliseconds, can allow us to determine how MORE might impact both the temporal dynamics and magnitude of natural reward processing. In this investigation, we focused on an ERP waveform that has been linked to reward processing in studies of persons with substance use disorders and healthy controls: the late positive potential (LPP).
The LPP is a positive deflection of the EEG waveform that tends to reach maximum amplitude 400–800 ms at parietal sites (Pz) after onset of a motivationally salient stimulus. LPP amplitude is robustly correlated with subjective ratings of arousal in response to viewing emotional pictures (Cuthbert, Schupp, Bradley, Birbaumer, & Lang, 2000). Studies indicate that earlier time windows (< 1000 ms) of the LPP can be modulated by attentional manipulations (e.g., Olofsson, Nordin, Sequeira, & Polich, 2008), suggesting that this stage of the LPP indexes allocation of attention to emotional information. LPP activation is strongly correlated with activity in orbitofrontal and medial prefrontal cortex, amygdala, and insula as measured by fMRI (Liu, Huang, McGinnis-Deweese, Keil, & Ding, 2012; Sabatinelli, Keil, Frank, & Lang, 2013). Of central importance to the current study, the LPP is also sensitive to top-down attentional control (Dunning & Hajcak, 2009; Scharmüller, Leutgeb, Schäfer, Köchel, & Schienle, 2011). In that regard, conscious down-regulation of negative emotional responses via reappraisal and reorienting of attention can reliably reduce the early and late time windows of the LPP (Hajcak & Nieuwenhuis, 2006; Moser, Hartwig, Moran, Jendrusina, & Kross, 2014; Schönfelder, Kanske, Heissler, & Wessa, 2013). Moreover, mindfulness training and related forms of meditation appear to reduce the LPP to negative affective stimuli (Gootjes, Franken, & Van Strien, 2011; Sobolewski, Holt, Kublik, & Wróbel, 2011). However, top-down enhancement of the LPP to positive emotional stimuli has proved to be an elusive target. Krompinger, Moser, and Simons (2008) found no effects on LPP magnitude when subjects were instructed to enhance positive emotions in response to pleasant photographs representing naturally rewarding stimuli.
If brain reward circuits instantiate a fundamental logic for goal selection (Shizgal and Hyman 2013), training in selective attention to natural rewards over drug rewards may reduce addictive propensities by shifting reward processing back to valuation of healthful and socially affiliative objects and behaviors. Hence, this study tested whether MORE could increase the LPP to natural reward cues in a sample of chronic pain patients at risk for prescription opioid misuse. We hypothesized that MORE would increase the parietal LPP to natural reward cues relative to neutral cues in the early time window where attention allocation predominates, and that this increase in LPP would be correlated with increased positive affective response to reward stimuli in individual difference analyses. Furthermore, given the clinical implications of the cardiac-autonomic findings in Garland, Froeliger, and Howard (2014), we hypothesized that increased LPP response to reward cues would be correlated with decreased opioid craving.
Method
Participants
This study examined data from a subset of participants (12 men and 17 women, mean age = 47.1, SD = 15.2) in a previously published RCT of MORE vs. a support group (SG) for chronic pain and prescription opioid misuse (Garland et al., 2014). Individuals with complete pre-post treatment EEG data (MORE, n = 11; SG, n = 18) from a psychophysiological assessment conducted one week before, and one week after, the study treatments were selected for the present investigation. Participants were recruited from primary care, pain, and neurology clinics, and met study inclusion criteria if they reported recurrent pain on more days than not stemming from chronic non-cancer-related pain conditions (participants had been in chronic pain for an average of 12.7 years) and had taken opioid analgesics daily or nearly every day for at least the past 90 days (Chou et al., 2009). At each assessment point, participants completed self-report measures of opioid craving and then participated in a lab protocol which involved passively viewing visual images of naturally rewarding stimuli and neutral stimuli while EEG was recorded. The protocol was approved by the Florida State University IRB, and all procedures complied with standards set forth in the Helsinki Declaration of 1975. Participants were assessed for comorbid psychiatric disorders with the Mini-International Neuropsychiatric Interview 6.0 (MINI) (Sheehan et al. 1998) and excluded if they were suicidal or psychotic. There were no significant pre-treatment between-groups differences in clinical characteristics (see Garland et al., 2014). Although all participants reported symptoms of physiological dependence (i.e., tolerance and withdrawal) on the MINI resulting from regular and prolonged opioid use , a smaller percentage (34.5%) met DSM-IV criteria for prescription opioid dependence (which includes psychological and behavioral as well as physiological signs and symptoms). However, most (86.2%) participants reported opioid analgesic misuse as defined by a validated cut-point on the Current Opioid Misuse Measure (COMM; Butler et al., 2007). Common comorbid psychiatric diagnoses included major depressive disorder (70.4%) and generalized anxiety disorder (33.3%); two participants also met criteria for post-traumatic stress disorder and alcohol abuse. Participants were paid $200 for the study. Following the pre-treatment assessment, participants were randomly assigned to an 8-week MORE group or SG.
MORE Group
The manualized 8-session MORE intervention (Garland, 2013) involved group training in mindfulness, reappraisal, and savoring skills designed to address mechanisms implicated in chronic pain and prescription opioid misuse. Group sessions were 2 hours long and led by a Masters-level social worker with over a decade of experience delivering mindfulness-based interventions to patients with psychiatric disorders. This clinician was supervised by the developer of MORE (the first author and an experienced, licensed psychotherapist). The first author reviewed video/audio-recordings of the sessions to monitor adherence to the MORE treatment manual and maintain treatment fidelity. MORE participants were asked to engage in daily 15 minute mindfulness practice sessions at home guided by a CD. Participants were instructed in savoring techniques which involved the use of mindfulness meditation to intentionally orient and sustain attention on the sensory features (i.e., visual, auditory, olfactory, gustatory, or tactile) of a pleasant experience or object (e.g., a beautiful nature scene like a sunset) while metacognitively reflecting on and absorbing any positive emotions arising in response to the pleasant event. For instance, participants were taught to mindfully focus their attention on the pleasant colors, textures, and scents of a bouquet of fresh flowers over a 20-minute long meditation session, and to attend to emotions of contentment and joy arising from this savoring practice. Comparable savoring techniques using an array of sensory targets were discussed across multiple sessions and prescribed as homework practice.
Support Group
The active control condition in this study consisted of 8 weekly, 2-hour support group sessions, in which a Master's-level clinical social worker facilitated emotional expression and discussion of topics pertinent to chronic pain and opioid use/misuse. This Rogerian, client-centered (Rogers, 2003) support group format was based on the evidence-based Matrix Model intensive outpatient treatment manual (Rawson & McCann, 2006). The first author reviewed video/audio-recordings of the sessions to monitor therapist adherence to the support group treatment manual and maintain treatment fidelity. Support group participants were asked to engage in 15 minutes of journaling a day on chronic pain-related themes at home.
Measures
Procedures and Stimuli
Participants were first given a general description of the experiment, and then electroencephalogram/electro-oculogram (EEG/EOG) sensor electrodes were attached. Participants were seated approximately 0.5 m directly in front of a 17-in computer monitor. Participants performed a randomized, event-related affective picture viewing task (Cuthbert et al., 2000) administered on computer, using Eprime 2.0 software to control the presentation and timing of all stimuli. During the task, full-screen pictures from the International Affective Picture System (IAPS; Lang, Bradley, & Cuthbert, 1997) were displayed in random order. Participants viewed 18 pleasant and 18 neutral IAPS pictures and were instructed to simply view the pictures as they were presented and allow themselves to respond naturally. A fixation cross (+) was presented for 500 ms at the beginning of each trial to orient participants to the center of the screen, and IAPS images were presented for 6 seconds following offset of the fixation cross. Responses were recorded for 6 seconds to measure effects on slower autonomic responses (not reported here), but in the present study, data from only the first second were analyzed to determine effects of MORE on LPP maxima. The inter-trial interval was randomly jittered from 2000 – 4000 ms. Following offset of the image, participants rated the valence of their affective response to the photograph (“How pleasant or unpleasant did you feel while viewing this photograph?”) on a 9-point scale ranging from “extremely unpleasant” to “extremely pleasant” and their degree of arousal (“How aroused or calm did you feel while viewing this photograph?”) from “extremely calm” to “extremely aroused.”
The natural reward picture set include images of endearing children and animals, athletic triumphs, appealing foods, beautiful landscapes, persons with happy facial expressions, and intimate/erotic couples. The neutral picture set included images of household items, furniture, and persons with neutral facial expressions. Natural reward and neutral images differed significantly on IAPS normative valence ratings (M = 7.09 and 5.05, respectively) and arousal ratings (M = 5.03 and 2.89, respectively).
ERPs
Continuous encephalographic (EEG) activity was recorded during the affective picture viewing paradigm described above using an ECI electrocap and a BIOPAC MP150 system (BIOPAC, Goleta, CA). During the task, data was recorded from Pz, as well as Cz, Fz, F3, and F4 (although data from these sites are not reported in the present manuscript because our a prior hypotheses were focused on Pz --- see below), and reference electrodes were placed on the earlobes. Also using the BIOPAC, vertical and horizontal electro-oculograms (EOG) recorded eye blinks and eye movements with miniature electrodes placed approximately 1 cm above and below the participant's right eye. All electrophysiological signals were digitized and analyzed on a computer with Acqknowledge 4.3 software (BIOPAC, Goleta, CA). The EEG was sampled at 1000 Hz, and signals were filtered online with a 35 Hz low pass filter. Off-line, the EEG for each trial was re-referenced to the mean of the ears, band-pass filtered between 0.01 and 30 Hz and artifact corrected for vertical and horizontal EOG movements using Gratton, Coles, and Donchin's (1983) classical approach. Trials were rejected for subsequent analyses if they were contaminated by excessive physiological artifacts. A semi-automated procedure identified and rejected physiological artifacts according to the following validated criteria: a voltage step > 50.0 μV between sample points, a voltage difference of > 300.0 μV within a trial, and a maximum voltage difference < 0.50 μV within any 100-ms interval (Dunning & Hajcak, 2009; Hajcak, Dunning, & Foti, 2009). EEG signals were smoothed digitally off-line for visualization purposes but analyses were conducted on the pre-smoothed data.
For each participant, an average ERP waveform was generated separately for natural reward and neutral trials. To depict overall effects and identify the time window for establishing the largest LPP to be used in analyses of the effects of MORE on reward processing, grand average waveforms were generated by averaging individual participant waveforms across the MORE group (# of reward trials retained for analysis: M = 12.6±2.7; # of neutral trials: M = 12.8±2.9) and SG (# of reward trials retained for analysis: M = 12.4±2.7; # of neutral trials: M = 13.1±3.2). Statistical analyses for the LPP component (defined below) were conducted on averages from each participant. Given ample evidence in the literature that the LPP is maximized at parietal sites (e.g., Hajcak & Nieuwenhuis, 2006; Krompinger et al., 2008; Olofsson et al., 2008), as well as after visually inspecting our own data for maximal LPP effects, we focused our LPP analyses on parietal site Pz. The LPP was quantified with the following procedure. First, a baseline equal to the average activity in the 150 ms window prior to image onset was subtracted from data points subsequent to image onset. Based on visual inspection and following conventions from previous research (Cuthbert et al., 2000), we defined the parietal LPP maxima as the average voltage in the time window from 400–1000 ms. Because age and gender are known to significantly influence the LPP to affective picture viewing (cf., Olofsson et al., 2008), we controlled for these variables in all ERP analyses.
Opioid craving
A single item “How much do you want your opioids right now?” anchored on a 10-point scale (1 = not at all, 10 = extremely) assessed current opioid craving once during the assessment session in the week prior to intervention and once during the assessment session in the week following intervention. Opioid craving scores as measured by this item have been significantly positively correlated with scores on the COMM, a validated measure of opioid misuse (Garland et al., 2014). Similar single-item measures of craving have been validated and shown to distinguish high- from low-risk opioid-using chronic pain patients and predict opioid misuse (Wasan et al., 2009).
Results
Pre-treatment LPP Response
Figure 1 presents the ERP waveforms associated with natural reward and neutral stimuli during passive viewing. In this sample, there was a small positive deflection in the EEG consistent with the LPP which began approximately 400 ms after stimulus onset and continued for several hundred ms. To determine if natural reward cues elicited a significantly greater LPP than neutral cues prior to treatment, a one-way (Cue Type: Natural Reward vs. Neutral) ANOVA was conducted, F(1,25) = 2.04, p = .16, indicating that, on average, natural reward cues did not elicit significantly greater LPP activation than neutral cues. There were also no significant between-groups differences in pre-treatment LPP activation to natural reward or neutral cues.
Figure 1.
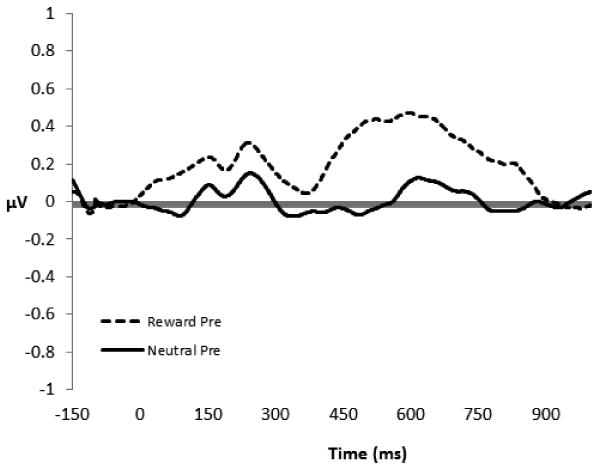
Grand average picture-locked event-related potentials at site Pz during viewing of natural reward and neutral cues by chronic pain patients (N = 29) prior to participation in study treatments. The y-axis is in microvolts; the x-axis is time in milliseconds.
Effects of Treatment on LPP to Natural Reward Cues
Figure 2a and 2b depict changes in LPP response to reward cues from pre- to post-treatment in the MORE group and SG, respectively. Following convention (Cuthbert et al., 2000; Moser et al., 2009; 2014), and because visual inspection of our data indicated that the parietal LPP began around 400 ms and ended around 1000 ms, effects of treatment on the LPP were examined from 400 – 1000 ms with repeated measures ANOVA. Emerging from this analysis was a significant Treatment Group (MORE vs. SG) × Time (Pre-Post Treatment) × Cue Type (Natural Reward vs. Neutral) interaction, F(1,25) = 4.99, p = .035, ηpartial2 = .17. Compared to the SG, the MORE group evidenced pre-post treatment increases in LPP activation to natural reward cues across the 400 – 1000 ms window, indicating that MORE enhanced electrocortical indices of reward processing. The Treatment Group × Time × Cue Type interaction remained significant in a sensitivity analysis controlling for pre-treatment opioid dependence status, F(1,24) = 4.80, p = .038, ηpartial2 = .17.
Figure 2.
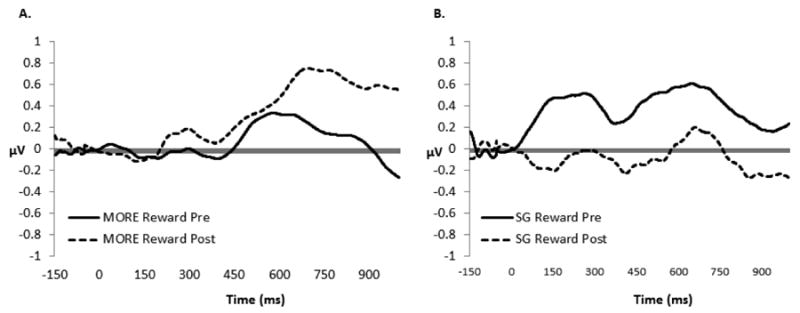
Figure 2a & 2b. Pre-post treatment changes in LPP (400 – 1000 ms) response at Pz during viewing of natural reward cues for A) Mindfulness-Oriented Recovery Enhancement (MORE) participants (n = 11) and B) Support Group (SG) participants (n = 18). The y-axis is in microvolts; the x-axis is time in milliseconds.
Individual Differences in Psychological Correlates of Treatment-Related Change in Natural Reward Processing
Linear regression analyses were used to examine individual differences in psychological correlates of natural reward cue-specific changes in LPP response over time. For these analyses, we created difference scores subtracting LPP activation to neutral cues from LPP activation to natural reward cues. These scores represent LPP activation that is specific to natural reward processing. In light of the phenotypic variability in the sample, we used regression models to assess increased LPP reward response as a predictor of residualized change in affect and craving, covarying pre-treatment levels of these psychological variables as well as pre-treatment opioid dependence status to control for subject heterogeneity.
We found that pre-post treatment increases in LPP activation during natural reward processing predicted residualized change in affective response elicited by natural reward cues (β = .66, p < .001, model R2 = .52). Individuals who exhibited greater LPP responses to natural reward cues reported greater increases in pleasant (positive) affect in response to those cues from pre- to post-treatment. In contrast, pre-post treatment increases in LPP activation did not significantly predict residualized change in subjective arousal response elicited by natural reward cues (p > .10).
In an additional regression model, we found that pre-post treatment increases in LPP activation during natural reward processing predicted residualized change in self-reported opioid craving (β = -.42, p = .03, model R2 = .29). Individuals who exhibited greater LPP responses to natural reward cues reported greater decreases in opioid craving from pre- to post-treatment.
Discussion
The present study provides direct neurophysiological evidence in support of the hypothesis that MORE modulates natural reward processing in opioid (mis)using chronic pain patients by enhancing the LPP at the stage of attentional allocation. After completing 8 weeks of treatment and in comparison to the SG, participants in the MORE group exhibited a heightened LPP response to natural reward stimuli relative to neutral stimuli. In individual difference analyses, increases in electrocortical activity during reward processing predicted enhanced positive affective responses to the photographs and reductions in opioid craving from pre- to post-treatment.
Study findings have implications for clinical and basic science. From a clinical standpoint, enhancement of natural reward processing in a sample of chronic pain patients at risk for opioid misuse by a multimodal, mindfulness-oriented intervention suggests that allostatic processes in addiction might be countered by cognitive training regimens centered on strengthening selective attention to natural rewards. MORE provides training in mindful savoring techniques, which involve intentional deployment and maintenance of attention on the pleasant features of healthful and socially affiliative objects and events, coupled with cultivation of reflexive awareness of positive emotional states without clinging. This latter point is of critical importance, given the impermanent nature of positive emotions and the ubiquity of pain, suffering, and stressful events in life (and particularly so in the patient population under investigation in the present study). MORE does not promote attachment to positive experience, but rather aims to foster a deep appreciation of moment-by-moment positive experience no matter how fleeting. Further, in the MORE treatment approach, mindful savoring techniques are tempered by other mindfulness techniques designed to promote attention to and acceptance of pain, craving, and negative emotions (Garland, 2013). Through such an approach, it is plausible that learning to mindfully attend to and savor positive events may offset the negative affect and anhedonia characteristic of persons suffering from chronic pain and opioid use disorders.
From a basic affective neuroscience perspective, current study findings suggest that repeated practice of conscious, top-down modulation of attentional allocation onto the positive features of a stimulus context can in fact amplify ERPs during motivated attention to natural reward stimuli. That these effects were observed for the LPP when it reached its maximum amplitude between 400 – 1000 ms suggests that mindful savoring may be an “antecedent” emotion regulation strategy (Gross, 1998) that can “boost the gain” on downstream emotional experience. In that regard, individuals who experienced the largest increases in LPP from pre- to post-treatment also reported the greatest increases in positive affective response during picture viewing. Hypothetically, this process may involve strengthening functional connectivity between a predominately left-lateralized metacognitive attentional control network (comprised of dorsolateral PFC, dorsal anterior cingulate cortex, and parietal cortex), the amgydala and insula, and the striatum, which, when operating in concert, may remediate impaired dopaminergic responses to hedonic stimuli and help to regulate craving (Garland, Froeliger, & Howard, 2014). This speculation is consistent with neuroimaging data indicating that volitional up-regulation of positive emotion is subserved by increased activation in PFC, caudate, and putamen (Kim & Hamann, 2007), and studies demonstrating that a prefrontal-striatal pathway is involved in the regulation of substance craving (Kober et al., 2011). Several prior reports suggest that meditation practice can enhance dopaminergic tone (Hagerty et al., 2013; Kjaer et al., 2002) and increase left-lateralized activation in prefrontal cortex (Davidson et al., 2003).
That said, LPP responses during the affective picture viewing task are at best indirect proxies of potential neural correlates of clinical outcome. Thus, it is unknown whether the observed modulations in LPP responses reflect changes in therapeutic mechanism or outcomes. To probe this question, future neuroimaging studies are needed to assess the neural correlates of outcome, including possible changes in underlying reward circuitry (e.g., corticostriatal) function. For example, resting-state functional connectivity analyses would allow for investigation of MORE's effects on the strength of basal reward circuitry function, whereas explicit emotion regulation paradigms would enable examination of MORE's effects on restoration of proactive, prefrontally mediated control over reward processes.
As a plausible alternative interpretation of the current study findings, it is possible that the observed modulations of LPP function were due to reductions in opioid intake over the course of the study. Reduction in opioid intake might improve dopaminergic pathway functioning, in light of the known effects of opioids on dopamine signaling in the nucleus accumbens (for a review, see Garland et al., 2013). However, we were unable to test this hypothesis due to the lack of a quantitative measure of opioid dosing. Although we attempted to administer a self-report measure of daily opioid dosing, we were unable to obtain accurate opioid dosing data for the entire sample due to non-responses and ambiguous responses (e.g., reporting the opioid dose without reporting number of pills taken per day). Consequently, we do not know to what extent MORE increased reward processing by reducing opioid use, or vice versa.
This study was limited in several other respects. First, MORE is a multimodal intervention that integrates mindfulness training with reappraisal and savoring techniques, and it is possible that any of these techniques separately, or in synergy, might enhance reward processing. Thus, we cannot disentangle the unique contribution that each of these techniques may have had on LPP response to natural reward cues. Future studies could employ dismantling designs and/or instruct participants to actively up-regulate positive emotion while viewing positive affective stimuli to parse the effects of mindful savoring from other intervention components. Further, the sample was small and heterogeneous, comprised of opioid misusers and those who met full DSM criteria for opioid dependence; the distinct nature of these phenotypes may have contributed to the degree of variability in the present study findings. Also, our LPP results might have been attenuated because the athletic photos from the IAPS we used tend to elicit weaker LPPs (Weinberg & Hajcak, 2010). To overcome these limitations, future studies should employ larger, more homogenous samples, as well as evaluate opioid dosing usage via a multi-pronged approach including toxicology screens, prescription history, pill count, and self-report.
Although the findings should be considered preliminary and heuristically informative given the limitations outlined above, the present exploratory study suggests that MORE may remediate deficits in natural reward processing among chronic pain patients at risk for opioid misuse by enhancing the allocation of attention to salutary objects and events. Restoration of the ability to extract a sense of reward, fulfillment, and meaning out of everyday pleasures may be crucial to the ability to self-generate positive emotions and to resilience itself (Garland et al., 2010). Research demonstrates that pleasant events actually outnumber unpleasant events by a 3-to-1 margin in everyday life (Oishi, Diener, Choi, Kim-Prieto, & Choi, 2007). Thus, naturally rewarding experiences are abundant, if people notice and appreciate them. Teaching clients to mindfully attend to positive aspects of their life experience (e.g., the sight of a beautiful sunset, the touch of a cherished loved one, or the taste of a nourishing meal) may increase the perceived value of natural rewards, and thereby counter the insensitivity to pleasurable objects, events, and experiences that can result from chronic pain and addiction. Future behavioral medicine research using neurophysiological measures may reveal that focusing one's attentional lens to more richly process the pleasurable, interesting, and meaningful experiences in life may make the painful and dissatisfying ones insignificant by comparison.
Figure 3.
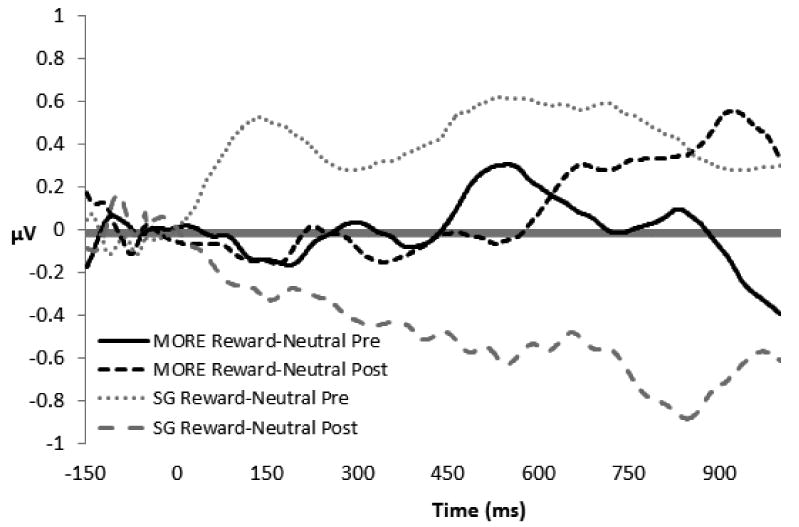
Difference waves at Pz (mean activation to natural reward cues – mean activation to neutral cues) for the Mindfulness-Oriented Recovery Enhancement (MORE, n = 11) and Support Groups (SG, n = 18). The y-axis is in microvolts; the x-axis is time in milliseconds.
Acknowledgments
We wish to thank Zhenghui Yu and Elizabeth McCoy for their assistance in ERP data processing. We also wish to thank two anonymous reviewers for their insightful suggestions with regard to the analysis and interpretation of ERP findings.
This work was supported by grant numbers DA032517 and DA037005 from the National Institutes of Health awarded to E.L.G.; and a grant from the Fahs Beck Fund for Research and Experimentation, also awarded to E.L.G. The authors would like to acknowledge Liz McCoy, Amy Kenney, and Zhenghui Yu for their assistance with data processing.
Footnotes
Conflicts of Interest: ELG, BF, & MOH declare that they have no conflict of interest.
Contributor Information
Eric L. Garland, University of Utah.
Brett Froeliger, Medical University of South Carolina.
Matthew O. Howard, University of North Carolina at Chapel Hill.
References
- Alcaro A, Panksepp J. The SEEKING mind: Primal neuro-affective substrates for appetitive incentive states and their pathological dynamics in addictions and depression. Neuroscience & Biobehavioral Reviews. 2011;35:1805–1820. doi: 10.1016/j.neubiorev.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Butler SF, Budman SH, Fernandez KC, Houle B, Benoit C, Katz N, Jamison RN. Development and validation of the Current Opioid Misuse Measure. Pain. 2007;130:144–56. doi: 10.1016/j.pain.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou R, Fanciullo GJ, Fine PG, Adler JA, Ballantyne JC, Davies P, et al. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. The Journal of Pain. 2009;10(2):113–130. doi: 10.1016/j.jpain.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert BN, Schupp HT, Bradley MM, Birbaumer N, Lang PJ. Brain potentials in affective picture processing: covariation with autonomic arousal and affective report. Biological Psychology. 2000;52:95–111. doi: 10.1016/s0301-0511(99)00044-7. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Kabat-Zinn J, Schumacher J, Rosenkranz M, Muller D, Santorelli SF, Sheridan JF. Alterations in brain and immune function produced by mindfulness meditation. Psychosomatic Medicine. 2003;65:564–70. doi: 10.1097/01.psy.0000077505.67574.e3. [DOI] [PubMed] [Google Scholar]
- Dunning JP, Hajcak G. See no evil: Directing visual attention within unpleasant images modulates the electrocortical response. Psychophysiology. 2009;46(1):28–33. doi: 10.1111/j.1469-8986.2008.00723.x. [DOI] [PubMed] [Google Scholar]
- Garland EL. Mindfulness-Oriented Recovery Enhancement for Addiction, Stress, and Pain. Washington, D.C.: NASW Press; 2013. [Google Scholar]
- Garland EL, Fredrickson BL, Kring AM, Johnson DP, Meyer PS, Penn DL. Upward spirals of positive emotions counter downward spirals of negativity: Insights from the broaden-and-build theory and affective neuroscience on the treatment of emotion dysfunctions and deficits in psychopathology. Clinical Psychology Review. 2010;30:849–864. doi: 10.1016/j.cpr.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Froeliger B, Zeidan F, Partin K, Howard MO. The downward spiral of chronic pain, prescription opioid misuse, and addiction: Cognitive, affective, and neuropsychopharmacologic pathways. Neuroscience & Biobehavioral Reviews. 2013;37:2597–2607. doi: 10.1016/j.neubiorev.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Froeliger B, Howard M. Mindfulness training targets neurocognitive mechanisms of addiction at the attention-appraisal-emotion interface. Frontiers in Psychiatry. 2014;4:173. doi: 10.3389/fpsyt.2013.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Froeliger B, Howard MO. Effects of Mindfulness-Oriented Recovery Enhancement on reward responsiveness and opioid cue-reactivity. Psychopharmacology. 2014 doi: 10.1007/s00213-014-3504-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Gaylord SA, Boettiger CA, Howard MO. Mindfulness training modifies cognitive, affective, and physiological mechanisms implicated in alcohol dependence: Results from a randomized controlled pilot trial. Journal of Psychoactive Drugs. 2010;42(2):177–192. doi: 10.1080/02791072.2010.10400690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Manusov EG, Froeliger B, Kelly A, Williams J, Howard MO. Mindfulness-Oriented Recovery Enhancement for chronic pain and prescription opioid misuse: Results from an early stage randomized controlled trial. Journal of Consulting and Clinical Psychology. 2014 doi: 10.1037/a0035798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind N, Peeters F, Drukker M, vas Os J, Wichers M. Mindfulness training increases momentary positive emotions and reward experience in adults vulnerable to depression: a randomized control trial. Journal of Consulting and Clinical Psychology. 2011;79(5):618–28. doi: 10.1037/a0024595. [DOI] [PubMed] [Google Scholar]
- Gipson CD, Reissner KJ, Kupchik YM, Smith AC, Stankeviciute N, Hensley-Simon ME, Kalivas PW. Reinstatement of nicotine seeking is mediated by glutamatergic plasticity. Proceedings of the National Academy of the Sciences USA. 2013;110:9124–9129. doi: 10.1073/pnas.1220591110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gootjes L, Franken IHA, Van Strien JW. Cognitive Emotion Regulation in Yogic Meditative Practitioners. Journal of Psychophysiology. 2011;25(2):87–94. doi: 10.1027/0269-8803/a000043. [DOI] [Google Scholar]
- Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalography and Clinical Neurophysiology. 1983;55(4):468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Gross JJ. Antecedent- and response-focused emotion regulation: divergent consequences for experience, expression, and physiology. Journal of Personality and Social Psychology. 1998;74:224–37. doi: 10.1037//0022-3514.74.1.224. [DOI] [PubMed] [Google Scholar]
- Hagerty MR, Isaacs J, Brasington L, Shupe L, Fetz EE, Cramer SC. Case Study of Ecstatic Meditation: fMRI and EEG Evidence of Self-Stimulating a Reward System. Neural Plasticity. 2013;2013 doi: 10.1155/2013/653572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajcak G, Nieuwenhuis S. Reappraisal modulates the electrocortical response to unpleasant pictures. Cognitive, Affective, & Behavioral Neuroscience. 2006;6(4):291–297. doi: 10.3758/cabn.6.4.291. [DOI] [PubMed] [Google Scholar]
- Heiman JR, Meston CM. Empirically validated treatments for sexual dysfunction. In: Rosen RC, Davis CM, Ruppel HJ, editors. Annual review of sex research: An integrative and interdisciplinary review 1997. Vol. 8. Mason City, IA: The Society for the Scientific Study of Sexuality; 1998. pp. 148–194. [PubMed] [Google Scholar]
- Heinz A, Siessmeier T, Wrase J, Hermann D, Klein S, Grusser SM, Flor H, Braus DF, Buchholz HG, Grunder G, Schreckenberger M, Smolka MN, Rosch F, Mann K, Bartenstein P. Correlation between dopamine D(2) receptors in the ventral striatum and central processing of alcohol cues and craving. American Journal of Psychiatry. 2004;161:1783–1789. doi: 10.1176/appi.ajp.161.10.1783. [DOI] [PubMed] [Google Scholar]
- Hölzel BK, Lazar SW, Gard T, Schuman-Olivier Z, Vago D, Ott U. How does mindfulness meditation work? Proposing mechanisms of action from a conceptual and neural perspective. Perspectives on Psychological Science. 2011;6(6):537–559. doi: 10.1177/1745691611419671. [DOI] [PubMed] [Google Scholar]
- Hong PY, Lishner DA, Han KH, Huss EA. The positive impact of mindful eating on expectations of food liking. Mindfulness. 2011;2:103–113. [Google Scholar]
- Hong PY, Lishner DA, Han KH. Mindfulness and eating: An experiment examining the effect of mindful raisin eating on the enjoyment of sampled food. Mindfulness. 2014;5:80–87. [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. American Journal of Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Killingsworth MA, Gilbert DT. A wandering mind is an unhappy mind. Science. 2010;330(6006):932. doi: 10.1126/science.1192439. [DOI] [PubMed] [Google Scholar]
- Kim SH, Hamann S. Neural correlates of positive and negative emotion regulation. Journal of Cognitive Neuroscience. 2007;19(5):776–798. doi: 10.1162/jocn.2007.19.5.776. [DOI] [PubMed] [Google Scholar]
- Kjaer TW, Bertelsen C, Piccini P, Brooks D, Alving J, Lou HC. Increased dopamine tone during meditation-induced change of consciousness. Cognitive Brain Research. 2002;13(2):255–259. doi: 10.1016/s0926-6410(01)00106-9. [DOI] [PubMed] [Google Scholar]
- Kober H, Mende-Siedlecki P, Kross EF, Weber J, Mischel W, Hart CL, Ochsner KN. Prefrontal-striatal pathway underlies cognitive regulation of craving. Proceedings of the National Academy of Sciences U S A. 2011;107:14811–6. doi: 10.1073/pnas.1007779107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Neurobiological mechanisms for opponent motivational processes in addiction. Philosophical Transactions of the Royal Society B: Biological Sciences. 2008;363:3113–3123. doi: 10.1098/rstb.2008.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GD, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- Krompinger JW, Moser JS, Simons RF. Modulations of the electrophysiological response to pleasant stimuli by cognitive reappraisal. Emotion. 2008;8(1):132. doi: 10.1037/1528-3542.8.1.132. [DOI] [PubMed] [Google Scholar]
- Lang P, Bradley M, Cuthbert B. International affective picture system (IAPS): Technical manual and affective ratings. NIMH Center for the Study of Emotion and Attention 1997 [Google Scholar]
- LeBel JL, Dubé L. The impact of sensory knowledge and attentional focus on pleasure and on behavioral responses to hedonic stimuli. 13th annual American Psychological Society Convention; Toronto, Ontario. 2001. [Google Scholar]
- Lee B, London ED, Poldrack RA, Farahi J, Nacca A, Monterosso JR, Mumford JA, Bokarius AV, Dahlbom M, Mukherjee J. Striatal dopamine d2/d3 receptor availability is reduced in methamphetamine dependence and is linked to impulsivity. Journal of Neuroscience. 2009;29:14734–14740. doi: 10.1523/JNEUROSCI.3765-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lintas A, Chi N, Lauzon NM, Bishop SF, Sun N, Tan H, Laviolette SR. Inputs from the basolateral amygdala to the nucleus accumbens shell control opiate reward magnitude via differential dopamine D1 or D2 receptor transmission. European Journal of Neuroscience. 2012;35:279–290. doi: 10.1111/j.1460-9568.2011.07943.x. [DOI] [PubMed] [Google Scholar]
- Liu Y, Huang H, McGinnis-Deweese M, Keil A, Ding M. Neural substrate of the late positive potential in emotional processing. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2012;32(42):14563–14572. doi: 10.1523/JNEUROSCI.3109-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubman DI, Allen NB, Peters LA, Deakin JF. Electrophysiological evidence of the motivational salience of drug cues in opiate addiction. Psychological Medicine. 2007;37:1203–1209. doi: 10.1017/S0033291707009932. [DOI] [PubMed] [Google Scholar]
- Lubman DI, Allen NB, Peters LA, Deakin JF. Electrophysiological evidence that drug cues have greater salience than other affective stimuli in opiate addiction. Journal of Psychopharmacology. 2008;22:836–842. doi: 10.1177/0269881107083846. [DOI] [PubMed] [Google Scholar]
- Lubman DI, Yucel M, Kettle JW, Scaffidi A, Mackenzie T, Simmons JG, Allen NB. Responsiveness to drug cues and natural rewards in opiate addiction: associations with later heroin use. Archives of General Psychiatry. 2009;66:205–212. doi: 10.1001/archgenpsychiatry.2008.522. [DOI] [PubMed] [Google Scholar]
- Moser JS, Hartwig R, Moran TP, Jendrusina AA, Kross E. Neural markers of positive reappraisal and their associations with trait reappraisal and worry. Journal of Abnormal Psychology. 2014;123(1):91. doi: 10.1037/a0035817. [DOI] [PubMed] [Google Scholar]
- Oishi S, Diener E, Choi DW, Kim-Prieto C, Choi I. The dynamics of daily events and well-being across cultures: when less is more. Journal of Personality and Social Psychology. 2007;93(4):685. doi: 10.1037/0022-3514.93.4.685. [DOI] [PubMed] [Google Scholar]
- Olofsson JK, Nordin S, Sequeira H, Polich J. Affective picture processing: An integrative review of ERP findings. Biological Psychology. 2008;77(3):247–265. doi: 10.1016/j.biopsycho.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawson R, McCann MJ. Counselor's Treatment Manual: Matrix Intensive Outpatient Treatment for People With Stimulant Use Disorders. DHHS Publication No (SMA) 2006 [Google Scholar]
- Rogers C. Client-centered therapy: Its current practice, implications, and theory. New York: Constable; 2003. [Google Scholar]
- Sabatinelli D, Keil A, Frank DW, Lang PJ. Emotional perception: correspondence of early and late event-related potentials with cortical and subcortical functional MRI. Biological Psychology. 2013;92(3):513–519. doi: 10.1016/j.biopsycho.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharmüller W, Leutgeb V, Schäfer A, Köchel A, Schienle A. Source localization of late electrocortical positivity during symptom provocation in spider phobia: An sLORETA study. Brain Research. 2011;1397:10–18. doi: 10.1016/j.brainres.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönfelder S, Kanske P, Heissler J, Wessa M. Time course of emotion-related responding during distraction and reappraisal. Social Cognitive and Affective Neuroscience. 2013 doi: 10.1093/scan/nst116. nst116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry. 1998;59(Suppl 20):22–33. [PubMed] [Google Scholar]
- Shurman J, Koob GF, Gutstein HB. Opioids, pain, the brain, and hyperkatifeia: a framework for the rational use of opioids for pain. Pain Medicine (Malden, Mass) 2010;11(7):1092–1098. doi: 10.1111/j.1526-4637.2010.00881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobolewski A, Holt E, Kublik E, Wróbel A. Impact of meditation on emotional processing—a visual ERP study. Neuroscience Research. 2011;71(1):44–48. doi: 10.1016/j.neures.2011.06.002. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D, Telang F, Baler R. Addiction: decreased reward sensitivity and increased expectation sensitivity conspire to overwhelm the brain's control circuit. BioEssays. 2010;32:748–755. doi: 10.1002/bies.201000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasan AD, Butler SF, Budman SH, Fernandez K, Weiss RD, Greenfield SF, Jamison RN. Does report of craving opioid medication predict aberrant drug behavior among chronic pain patients? Clinical Journal of Pain. 2009;25:193–8. doi: 10.1097/AJP.0b013e318193a6c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadlinger HA, Isaacowitz DM. Fixing our focus: training attention to regulate emotion. Personality and Social Psychology Review. 2010;15(1):75–102. doi: 10.1177/1088868310365565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg A, Hajcak G. Beyond good and evil: Implications of examining electrocortical activity elicited by specific picture content. Emotion. 2010;10(6):767–782. doi: 10.1037/a0020242. [DOI] [PubMed] [Google Scholar]


