TO THE EDITOR:
Low-income and minority children suffer disproportionately high rates of asthma moribidity.1 An important contributing factor may be higher rates of low-health literacy in underserved populations,2 which is associated with poor adherence3 and health outcomes.2
To date, no tailored health literacy intervention has demonstrated objective improvement in adherence to inhaled corticosteroids (ICS) and asthma control in underserved African American adolescents. This treatment group only proof-of-concept study aimed to assess the feasibility and explore the efficacy of an intervention featuring an electronic medication monitor and companion smartphone asthma application to deliver a tailored intervention to improve ICS adherence and asthma control in this low health literacy population. This intervention integrates principles of behavior change based on social cognitive theory4 with recommended design strategies for effective communication with low literacy populations.5 Feasibility was measured in terms of acceptability (and use). Efficacy was measured in terms of ability to achieve participant ICS adherence to the clinically significant target, ≥50%,6 and 3 point improvement, (minimal clinically important difference = 3), on the Asthma Control Test (ACT) .7
Study participants met the following inclusion criteria: 11-16 years of age; African American; a diagnosis of persistent asthma;8 possession of an active prescription for ICS verified by pharmacy records; and completion of the baseline run-in protocol. Participants were recruited from the Rush Pediatric Primary Care Center, which serves a primarily urban minority patient population.
The Rush University Medical Center Institutional Review Board approved the study protocol. After each caregiver and child dyad provided written consent and assent, 12 participants entered a 3-week run-in period to determine baseline characteristics and ICS adherence. Adherence was measured using the Mobile Adolescents’ Disease Empowerment and Persistency Technology (M-ADEPT) electronic medication monitor (see Figure E1 in the Online Repository) that was fitted to participants’ ICS. All caregivers and adolescents were informed that this device would collect the date and time of each actuation of medication. Upon completion of the run-in period, the 12 adolescents entered the 8-week treatment phase.
For the duration of their participation in this research, all participants received: a smartphone (HTC One V, Virgin Mobile USA, Warren, New Jersey) with an unlimited texting, talking, and data plan (Virgin Mobile USA, Warren, New Jersey); ICS (fluticasone propionate HFA MDI 110 mcg inhalation aerosol) and short-acting beta2-agonist (SABA) (albuterol sulfate HFA MDI 90 mcg inhalation aerosol) medications provided by (GlaxoSmithKline, Research Triangle Park, NC) fitted with M-ADEPT electronic medication monitors; and the M-ADEPT asthma application loaded onto their study smartphones.
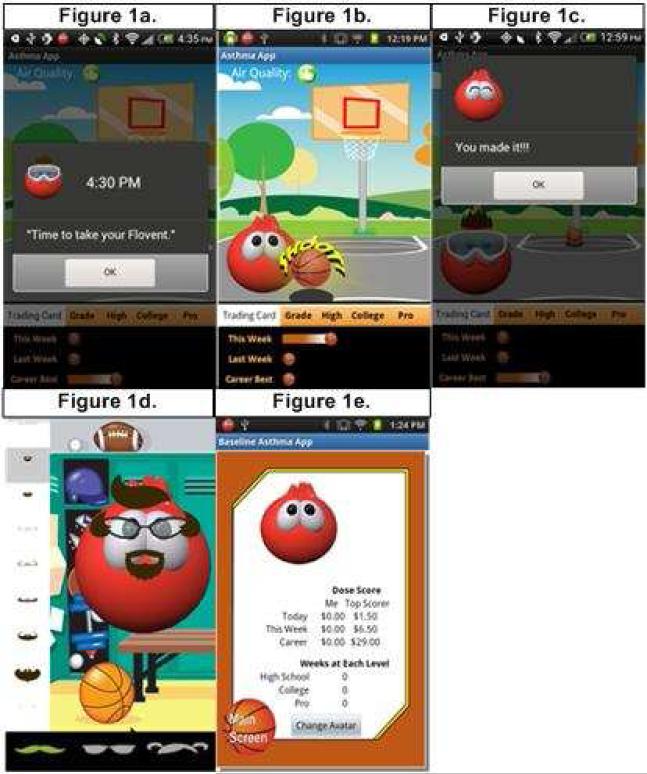
Figure E2 in the Online Repository illustrates the M-ADEPT System. The electronic medication monitors and smartphone application were developed and built by the investigators (Invention: NIH EIR #0577703-13-0020). The application (Figure 1) was programmed to deliver: stimulus control using twice daily visual reminders to take his/her ICS (Figure 1a); immediate reinforcement for taking ICS provided by the opportunity to shoot a basket (Figure 1b) and a positive text message for each ICS puff taken (Figure 1c); and immediate and long-term rewards each time he/she completed a full dose of ICS within the preselected morning and evening time windows. These rewards included immediate rewards of accessories to customize his/her game avatar (e.g. hairstyles, sunglasses, and animation) as illustrated in Figure 1d and the long-term reward of monetary incentives ($1.00/dose) to purchase age-appropriate (i.e. radio-edited) music, movies, applications, and games for download onto their study smartphones (Figure 1e).4-5 Participants met weekly with an asthma specialist (G.M.) for 30 minutes. The doctor provided standard asthma education as outlined in the asthma guidelines8 and tailored feedback (based on participant ICS and SABA use) to promote ICS adherence. To ensure treatment fidelity, participants’ met with a research assistant each week to complete questionnaires and testing for proper functioning of his/her M-ADEPT medication monitoring devices, smartphone, and application.
Figure 1. M-ADEPT Application.
Figure 1a-e describes the behavioral principle integrated into each screen shot of the M-ADEPT smartphone application. 1a) ICS Reminder: Stimulus Control; 1b) Basketball Game: Immediate Reinforcement; 1c) Text Messages: Immediate Reinforcement; 1d) Avatar Rewards: Immediate Reinforcement; and 1e) Smartphone Rewards: Long-term Reinforcement.
The primary efficacy outcome was percent daily ICS adherence measured using the electronic medication monitor and truncated at 100% of the prescribed dose. Average daily adherence over the prior 7 days was assessed at baseline and each week of the 8-week treatment phase.
Baseline assessments included demographics and asthma history. Asthma control (using the ACT),9 was assessed at baseline and week 8. Daily SABA use was collected for the duration of their participation in this research.
Descriptive statistics were used to summarize continuous and discrete variables. Discrete variables were summarized as the total number and percentage in each category. Continuous variables were summarized as the 25th percentile, median, and 75th percentile.
Of the twelve participants enrolled, 10 of 12 completed the 8-week treatment phase, all study data collection instruments, and ≥ 80% of study visits. For the two participants who did not complete the final assessment, the last observation carried forward was used for analyses.
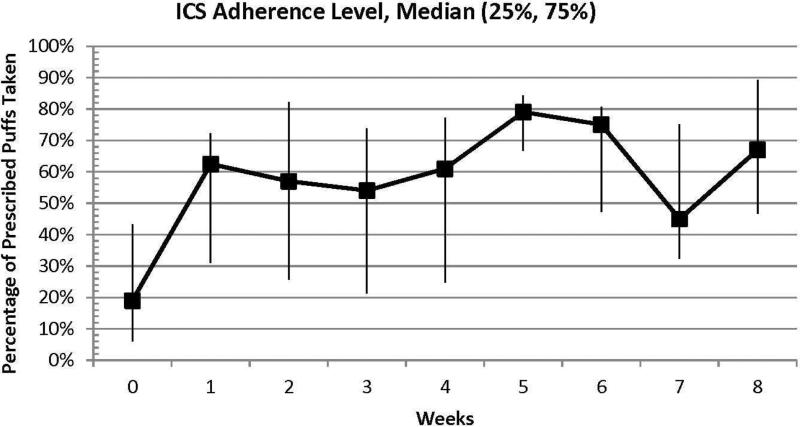
Baseline characteristics of the 12 Medicaid-insured participants who entered the treatment phase include: median age o f 14 years, 58% female, 33% reading ≥ 2 levels below their grade (measured using the Wide Range Achievement Test-4),10 and 75% with uncontrolled asthma (ACT ≤ 19).9 Median baseline and week 8 ICS adherence were 19% and 50% (intent-to-treat)/ 67% (observed), respectively (Figure 2). Eight percent and 58% of participants met target ICS adherence (≥50%) at baseline and week 8. ACT scores increased from a median of 18 at baseline to 23 at week 8. Fifty-eight percent of participants achieved the minimal clinically important difference (3 points) in ACT score from baseline to week 8.7 SABA use decreased from a median of 3 puffs per week at baseline to 0 puffs per week at 8 weeks.
Figure 2. Median Percent ICS Adherence at Baseline and During Treatment.
This graphically represents median ICS adherence at baseline and each week of the 8-week active treatment phase for the cohort of study participants.
Cognizant of important limitations (short time-frame, small sample size, inclusion of participants from only one racial/ethnic group, and lack of a control group), this M-ADEPT tailored low-health literacy intervention is the first to demonstrate objective improvement in ICS adherence5 and asthma control7 among underserved minority adolescents. Eighty-three percent of adolescents completed the 8-week treatment, indicating that the intervention was acceptable and feasible in our target population.
M-ADEPT integrates design strategies for communication with low-literacy populations5 and principles of behavior change6 delivered on a mobile phone to improve health outcomes. The quasi-experimental, treatment-only design of this study is consistent with recommendations for early preliminary testing of a new intervention.11 These promising results merit further testing of this intervention in a larger and more diverse sample using a rigorous randomized design. Studies are needed to test whether a longer duration of M-ADEPT intervention (12 months) may lead to long-lasting (i.e. 2-5 year) maintenance of clinically significant ICS adherence. It is hypothesized that a 12-month improvement in ICS adherence will translate into decreased impairment and risk over a sufficiently long period of time for adolescents to make the connection between medication taking behavior and asthma control. This, in turn, will intrinsically motivate them to continue their adherent behavior to maintain asthma control without reliance on the M-ADEPT intervention. Future studies should seek to understand the M-ADEPT hypothesized pathways for efficacy and impact on clinical asthma outcomes such as emergency room visits and hospitalizations, and include a cost-effectiveness analysis.
Supplementary Material
Clinical Implication.
A tailored intervention delivered on a mobile phone platform, integrating low literacy design strategies and basic principles of behavior change, may be a powerful way to promote increased adherence and asthma control among underserved minority adolescents.
Acknowledgments
Funding: This study was supported by the National Heart Lung and Blood Institute grants K23 HL092292 and R21 HL098812 and is part of the Rush Center for Urban Health Equity which is funded by the National Heart Lung and Blood grant 1P50HL105189. Support in the form of study drug was provided by a grant from GlaxoSmithKline (FLV117251).
Abbreviations
- ICS
Inhaled corticosteroids
- SABA
Short acting beta-2 agonists
- ACT
Asthma Control Test
- M-ADEPT
Mobile Adolescents’ Disease Empowerment and Persistency Technology
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Moorman JE, Zahran H, Truman B, Molla M. Current Asthma Prevalence – United States, 2006-2008. Centers for Disease Control & Prevention. MMWR. 2011;60(Suppl):84–6. [PubMed] [Google Scholar]
- 2.Berkman ND, De Walt DA, Pignone MP, Sheridan SL, Lohr KN, Lux L, Sutton SF, Swinson T, Bonito AJ. Literacy and health outcomes. Evid Rep Technol Assess (Summ) Jan. 2004;87:1–8. Review. [PMC free article] [PubMed] [Google Scholar]
- 3.Gazmararian JA, Kriplani S, Miller MJ, Echt KV, Ren J, Rask K. Factors associated with medication refill adherence in cardiovascular-related diseases: a focus on health literacy. J Gen Intern Med. 2006 Dec;21(12):1215–21. doi: 10.1111/j.1525-1497.2006.00591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bandura A. Social foundations of thought and action: a social cognitive theory. Prentice Hall; Englewood Cliffs, NJ: 1986. pp. 523–82. [Google Scholar]
- 5.Doak C, Doak L, Root J. Teaching patients with low literacy skills. 2nd Edition J. B. Lippincott Company; Philadelphia, PA: 1996. [Google Scholar]
- 6.Suissa S, Ernst P, Benayoun S, Baltzan M, Cai B. Low-dose inhaled corticosteroids and the prevention of death from asthma. N Engl J Med. Aug 3. 2000;343(5):332–6. doi: 10.1056/NEJM200008033430504. [DOI] [PubMed] [Google Scholar]
- 7.Schatz M, Kosinski M, Yarlas AS, Hanlon J, Watson ME, Jhingran P. The minimally important difference of the Asthma Control Test. J Allergy Clin Immunol. 2009;124(4):719–723. e1. doi: 10.1016/j.jaci.2009.06.053. Epub 2009 Sep 22. [DOI] [PubMed] [Google Scholar]
- 8.National Heart, Lung, and Blood Institute . National Asthma Education and Prevention Program Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. National Institutes of Health; Bethedsa, MD: 2007. pp. 1–440. Full Report. [Google Scholar]
- 9.Nathan RA, Sorkness CA, Kosinski M, Schatz M, Li JT, Marcus P, Murray JJ, Pendergraft TB. Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol. Jan. 2004;113(1):59–65. doi: 10.1016/j.jaci.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 10.Wilkinson GS, Robertson GJ. Wide Range Achievement Test 4: professional manual. Psychological Assessment Resources; Lutz, FL: 2006. [Google Scholar]
- 11.Czajkowski SM, Powell LH, Adler N, Naar-King S, Reynolds KD, Hunter CM, Laraia B, Olster Dh, Perna FM, Peterson JC, Epel E, Charlson M. From Ideas to Efficacy: The ORBIT Model for Developing Behavioral Treatments for Chronic Diseases. Health Psychology. doi: 10.1037/hea0000161. Accepted for Publication August 2014. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.