Abstract
Chaperone-mediated autophagy (CMA) is a multistep process that involves selective degradation and digestion of a pool of soluble cytosolic proteins in lysosomes. Cytosolic substrates are selectively identified and targeted by chaperones to lysosomes where they are subsequently translocated into the organelle lumen through a dedicated CMA-associated lysosomal membrane receptor/translocation complex. CMA contributes to maintaining a functional proteome, through elimination of altered proteins, and participates in the cellular energetic balance through amino acid recycling. Defective or dysfunctional CMA has been associated with human pathologies such as neurodegeneration, cancer, immunodeficiency or diabetes, increasing the overall interest in methods to monitor this selective autophagic pathway. Here, we describe approaches used to study CMA in different experimental models.
Keywords: autophagy, fluorescent reporters, lysosomes, organelle isolation, proteolysis
1. INTRODUCTION
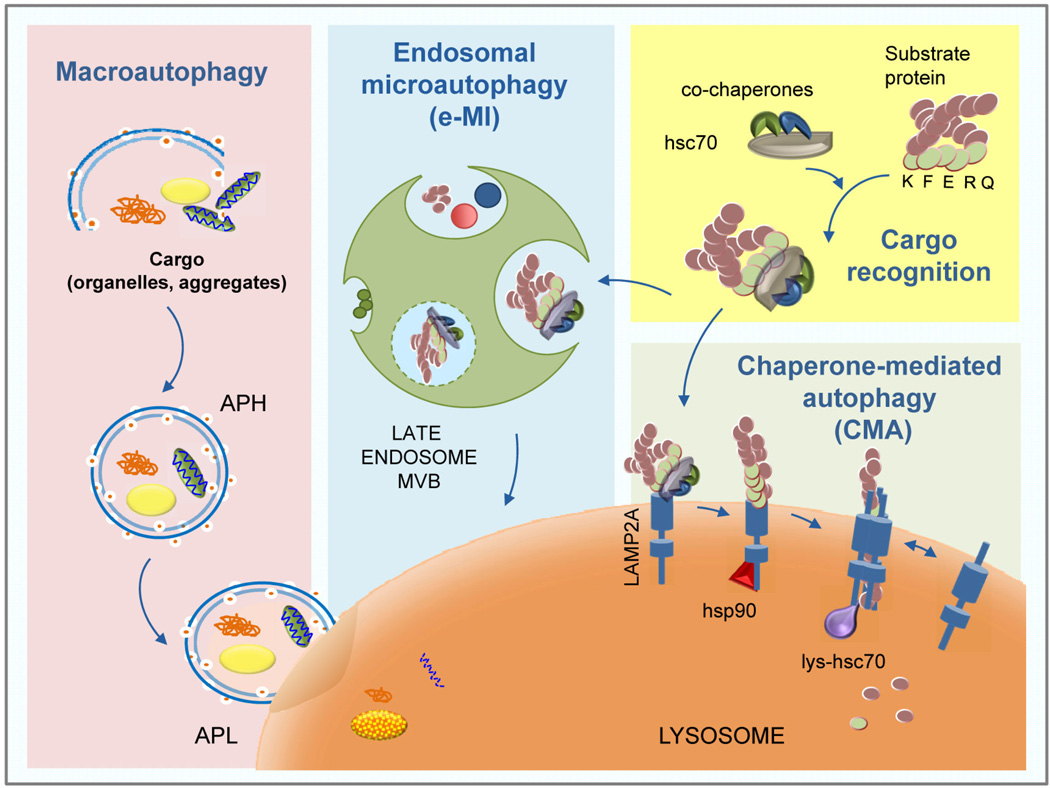
Chaperone-mediated autophagy (CMA) refers to a very selective form of autophagy whereby a specific pool of intracellular proteins is targeted to the lysosome for degradation [1]. Selectivity is determined by the presence on the substrate proteins of a CMA targeting motif – a penta-peptide amino-acid sequence biochemically similar to KFERQ [2]. This motif is recognized by the constitutively expressed intracellular heat shock-cognate chaperone of 70kDa (hsc70) [3]. Upon binding of hsc70, substrates are targeted to the lysosomal membrane for docking at the cytosolic tail of the monomeric lysosome-associated membrane protein type-2A (LAMP2A) [4]. Substrate binding initiates multimerization of the LAMP2A monomers at the lysosomal membrane, which constitutes the basis of the CMA translocation complex [5]. A lysosomal resident form of hsp90 assures LAMP2A stability during multimerization [5]. Unfolding of the substrate proteins is followed by their translocation into lysosomes assisted by a lysosome resident hsc70 (lys-hsc70) [6], and leads to the complete degradation of the substrate into its constitutive amino acids by the luminal proteases known as cathepsins (Fig. 1).
Figure 1. Types of mammalian autophagy.
Scheme of the basic steps of three different types of mammalian autophagy: Macroautophagy: cargo such as protein aggregates, lipid droplets, organelles, are sequestered into double membrane vesicles (autophagosomes; APH), which then fuse with lysosomes (autophagolysosomes; APL) where cargo degradation occurs. Endosomal microautophagy (e-MI): cytosolic content is delivered “in bulk” or upon interaction with the hsc70 chaperone to the membrane of late endosomes where multivesicular bodies (MVB) form trapping and internalizing the cargo; degradation occurs upon disruption of the vesicles in the endosomal lumen or after fusion of endosomes with lysosomes. Chaperone-Mediated Autophagy (CMA): substrate proteins recognized by hsc70 are targeted to the lysosomes and translocated across the lysosomal membrane assisted by CMA receptor LAMP2A to be degraded by lysosomal hydrolases.
In contrast with other types of autophagy, such as macroautophagy or microautophagy that require vesicles for sequestration of the substrates degraded by these pathways, in CMA, substrate proteins are recognized individually without interfering with adjacent proteins and they are translocated into the lysosomal lumen independently of vesicular trafficking [1]. Recently, selective targeting of individual proteins has also been reported in a more specific type of microautophagy, known as endosomal-microautophagy (e-MI) (Fig. 1). In this process, the chaperone hsc70 also recognizes the KFERQ-like motif in the substrate proteins but delivers them to late endosomes where cargo is internalized into small vesicles that form on the surface of this organelle [7]. An additional difference between e-MI and CMA, is that substrate unfolding is a pre-requisite for internalization in CMA [8], whereas folded proteins and even small oligomeric protein complexes can be internalized in the e-MI vesicles [7]. The similarities between CMA and e-MI in the selective recognition of substrates by hsc70, has made developing methods that discriminate between CMA and e-MI necessary.
Although most cells display some basal level of CMA activity, several types of cellular stressors upregulate CMA, such as nutrient-deprivation [9], oxidative stress [10], proteotoxicity [11] and lipotoxicity [12]. Under these conditions, the selective removal of proteins by CMA contributes to the elimination of altered proteins, recycling of amino acids resulting from proteolysis and selective adjustment of the proteome composition. CMA activity decreases with age in almost all tissues analyzed, and this probably aggravates the course of age-related disorders related to proteotoxicity [13–15]. In fact, reduced CMA activity has been described in neurodegenerative diseases such as Parkinson’s disease [16] and tauopathies [17], and in metabolic disorders, such as diabetes [18] and diet-induced obesity [12]. Contrarily, abnormal upregulation of CMA activity is common in many types of cancer [19] and has been proposed to contribute to the pathogenesis of immune disorders such as lupus [20], whereas reduced CMA underlies the basis of defective T-cell function with age and of immunosenescence [15]. This connection between CMA and different human diseases has motivated a growing interest in understanding this fundamental cellular process and manipulating it for therapeutic purposes.
Although the lysosomal steps of CMA are now well understood, the signaling cascades responsible for CMA activation are not clearly delineated yet. Treatment with chemical atypical antagonists of the nuclear retinoic-acid receptor α induces CMA, revealing this signaling pathway as an endogenous inhibitor of CMA [21]. In the case of T-cells, CMA activation is mainly attained through calcineurin/NFAT signaling in a ROS-dependent manner [15]. However, additional signaling cascades responsible for CMA activation/inhibition remain unknown, making modulation of this pathway for therapeutic purposes still challenging.
In this review, we summarize two types of CMA-related methods: 1) methods that assess CMA activity in a specific pathological or physiological condition or in response to possible therapeutic interventions and, 2) methods that determine if a protein of interest is a CMA substrate, which has become important for understanding the functional consequences of malfunctioning of this autophagic pathway.
2. METHODS TO ASSAY CMA ACTIVITY
We describe in this section the experimental models more commonly used to analyze CMA activity along with a battery of assays and their use either in cultured cells or in tissues (Fig. 2).
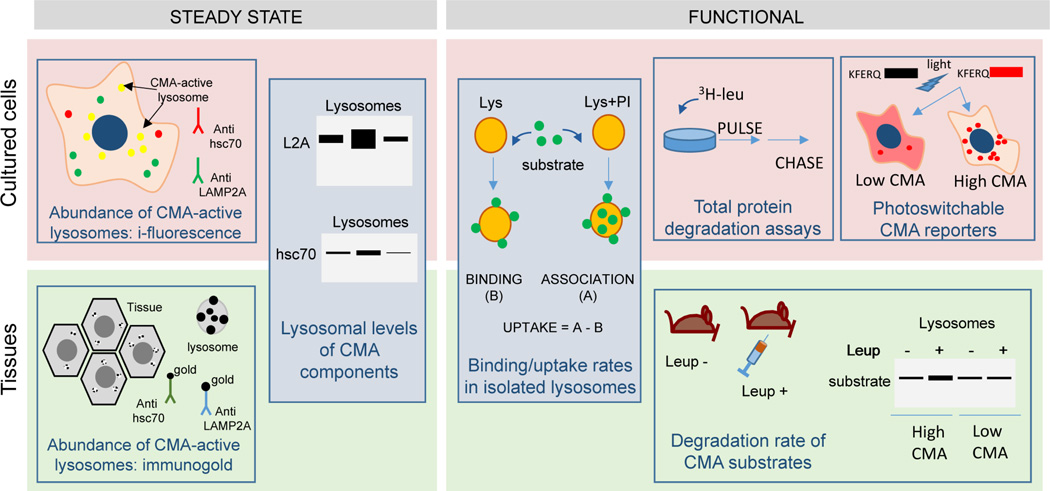
Figure 2. Assays to measure CMA activity.
Scheme of the most common assays to measure CMA activity grouped on whether they can be used on cells in culture (top), animal tissues (bottom) or in both (middle). Steady-state assays provide a snap-shot of the status of CMA and can be used for comparative purposes in between conditions. However, complete assessment of CMA requires the use of functional assays as depicted in this scheme.
2.1. Experimental Models
Autophagy is conserved in all eukaryotes [22], however CMA has only been described in mammals, and in fact, the limiting component for this autophagic pathway, LAMP2A, is a spliced variant that appears in birds and mammals [23], but it is not present in yeast, worms, flies or even fish. In the case of mammalian cells, some level of CMA activity has been detected in almost all of the cell types studied. Consequently, all the methods currently available for the study of CMA have been developed in mammals.
CMA activity has been detected in many different mammalian cell types, including fibroblasts [24], hepatocytes [25], astrocytes [26], primary neurons [27], macrophages [28], dendritic cells [28], T-cells [15], retinal cells [29] and a large array of cancer cells of different origins [19]. Moreover, CMA studies have been performed with lysosomes isolated from liver [30], spleen [31], different brain regions [27] and kidney [32]. However, by far, liver remains the most studied and characterized organ where most of the molecular dissection of CMA has been performed and hence, methods to measure CMA activity in this organ are the best characterized.
2.2. Steady-state read outs of CMA
Changes in the abundance of key CMA components and in the amount and distribution of CMA-active lysosomes can be used as an indirect way to assess CMA. However, because this type of analysis only offers a snapshot of the CMA status, when possible, these should be complemented with functional assays described in Section 2.3.
Immunoblotting and imaging are the most commonly used methods to analyze changes in key CMA components because, in most conditions, changes in these components are posttranslational. Lysosomal levels of LAMP2A are limiting for CMA [4], hence, changes in the abundance of this protein in lysosomes usually correlates with CMA activity (Fig. 2). However, the presence of LAMP2A is not sufficient to conclude that a lysosome is active for CMA because not all LAMP2A-positive lysosomes are capable of performing CMA. In fact, while most lysosomal subpopulations contain LAMP2A, only those that in addition contain lys-hsc70 in their lumen are capable to perform CMA, which in most cells is not more than 30% of total lysosomes under basal conditions [33]. Because LAMP2A is one of the three splice variants of a single gene, lamp2, with all of them having identical luminal regions, it is very important to use antibodies that can specifically recognize the 12 amino acid residues that constitute the cytosolic tail of LAMP2A and that are different from those in LAMP2B and C tails [34]. Antibodies against the luminal region of LAMP2, the most commonly offered commercially, do not provide information specifically about CMA since the other variants function in macroautophagy, vesicular trafficking, lysosomal maturation and even nucleotide transport into lysosomes [34]. In addition, the relation of LAMP2A levels and CMA activity applies only to LAMP2A present at the lysosomal membrane [35]. Consequently, immunoblot for LAMP2A in lysosome-enriched fractions or at least a membranous cell fraction is more informative than in total whole cell lysates. Although CMA activation often occurs without de novo synthesis of LAMP2A (i.e. during starvation), transcriptional upregulation of LAMP2A has been described in other conditions such as oxidative stress that also activate CMA [36]. Consequently, measurement of LAMP2A mRNA levels could correlate with CMA activity, but absence of mRNA changes does not rule out changes in CMA activity.
Levels of lysosomal-hsc70 are also proportional to CMA activity [6, 33], but because hsc70 is one of the most abundant cellular chaperones, and the fraction located in lysosomes is a small amount, immunoblot for hsc70 in total cellular lysates is not informative for CMA (Fig. 2). Similarly, measurement of mRNA levels of this constitutive chaperone does not have any predictive value for CMA activity. When performing immunoblot of lysosome-enriched fractions or immunofluorescence to look for lysosomal association of the chaperone, it is important to utilize antibodies that recognize only hsc70 and not those that recognize both the constitutive (hsc70) and the inducible (hsp70) chaperones that only differ in a very small number of amino acid residues [6].
Colocalization by immunofluorescence of hsc70 with lysosomal markers (LAMP2, LAMP1, etc.) can be used to identify the subset of cellular lysosomes active for CMA [33]. The amount of these lysosomes in proportion to the whole lysosomal pool increases when CMA is activated [36]. Short methanol fixation is necessary in this analysis to extract the diffused cytosolic hsc70 and retain only the vesicle-associated hsc70 [37]. When using tissues where methanol extraction is not possible, immunogold staining for hsc70 and electron microscopy can also give information about the pool of CMA-active lysosomes (Fig. 2) [33]. Interestingly, in many cell types, subcellular mobilization of hsc70-positive lysosomes toward the perinuclear region can be used as an indirect indication of CMA activation, although, the reason behind this preferred perinuclear lysosomal accumulation is still not well understood [37].
Additional proteins have been shown to participate in CMA and to reside in the lysosomal compartment, such as glial fibrillary acidic protein, elongation factor 1α, lysosomal hsp90, cathepsin A (for details please see [1]). However, they are not used commonly to assess CMA activity because they also participate in other cellular functions and/or they do not change in abundance or location during CMA activation. Furthermore, for the standard CMA markers described here (LAMP2A and lys-hsc70), the magnitude of changes in their lysosomal levels is cell type dependent, as different cells have different basal CMA activity, and consequently, studies using these markers should be done comparatively rather than in absolute values.
Lastly, when working with isolated lysosomes from cells or tissues, an increase in the levels of well-known CMA substrates is also a good indication of CMA. Because degradation of CMA substrates occurs rapidly after translocation, comparison of lysosomal levels of CMA substrates in cells or animals treated with inhibitors of lysosomal proteases (i.e. leupeptin) with those untreated allows measuring flux trough CMA [25].
2.3. Functional Assays
Several functional assays allow tracking CMA activity over time in cells, tissues and isolated organelles.
2.3.1. Intracellular protein degradation assessment
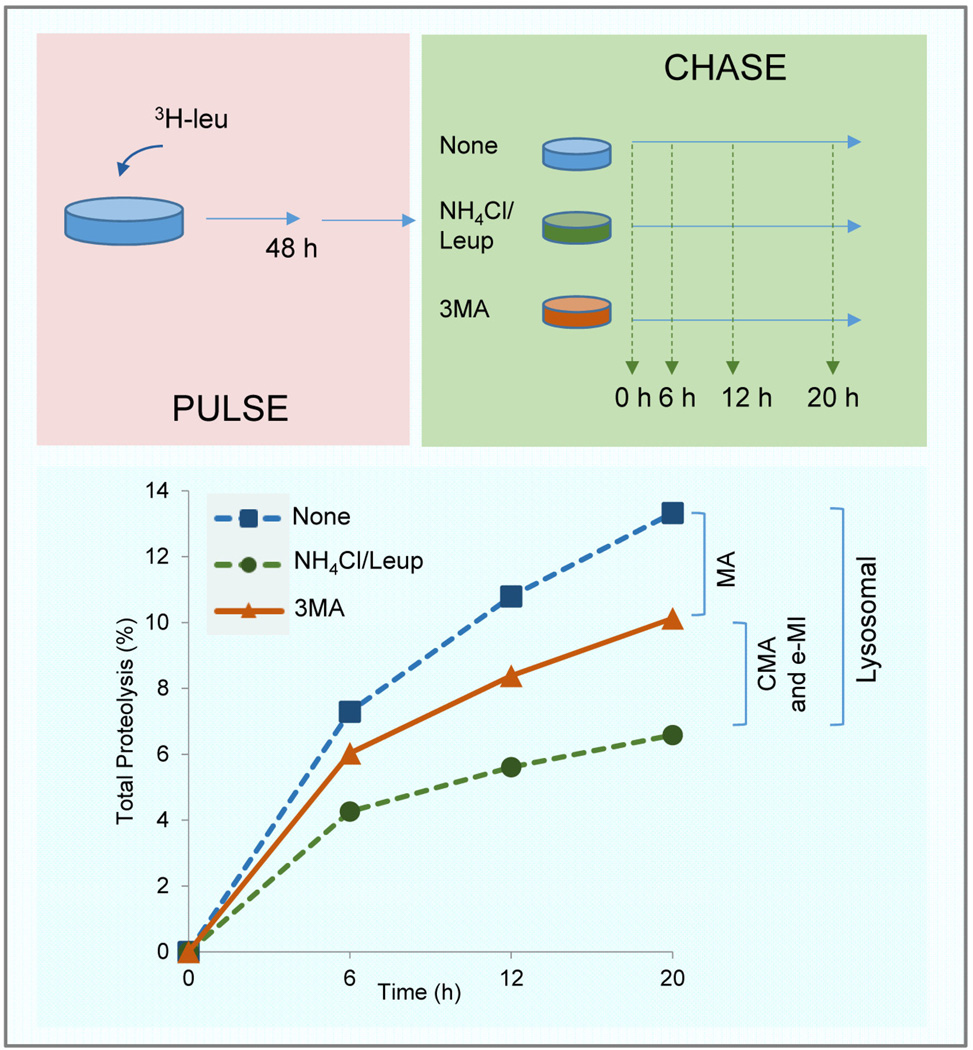
Protein sequence analysis indicates that about 30% of total cytosolic proteins have the potential to undergo degradation by CMA, although the actual fraction degraded at a given time varies depending on the cell type and cellular conditions. Consequently, measurement of the pool of cellular proteins that undergo degradation through CMA is a common way to determine overall activity of this pathway. It is advisable to narrow the study to rates of long-lived protein degradation because the majority of the proteins degraded by lysosomes through any autophagic pathway have long half-lives (>6h) [38]. This could be attained through pulse and chase experiments by using a radiolabeled amino acid and inhibitors of either lysosomal proteases or other autophagic pathways to discriminate those proteins undergoing degradation in lysosomes through CMA [37]. Cells are first pulsed by radiolabeling of newly synthesized proteins as they incorporate the radiolabeled amino acid 3H-leucine (Fig. 3). 3H-valine is also used, especially in cells where leucine could have inhibitory effect on macroautophagy. Ideal pulse duration is 48 hours in order to preferentially label long-lived proteins. During the chase, break down of the radiolabeled proteins is monitored as the release of free radiolabeled amino acid into the culture media, which is supplemented with an excess of the non-labeled amino acid (e.g. leucine or valine, as appropriate) to prevent reutilization of the radiolabeled one [39]. Right after the labeling, cells can be incubated for an additional 2h in medium containing an excess of the non-labeled amino acid to allow for the degradation of short-lived proteins and eliminate them from the proteolysis measurements. To determine the fraction of long-lived proteins degraded in lysosomes, values in untreated cells are compared with those in cells treated with general inhibitors of lysosomal proteolysis. A commonly used combination is that of leupeptin, a selective inhibitor of cysteine and serine peptidases, with ammonium chloride (NH4Cl), a weak base that neutralizes the lysosomal acidic pH necessary for lysosomal protease activity [40]. 3-methyladenine (3MA), an inhibitor of phosphatidylinositol-3-kinases (PI3K) and widely used for macroautophagy inhibition [41], can be used to estimate lysosomal degradation through macroautophagy (short treatment times are advisable to avoid compensatory upregulation of CMA). According to this combination CMA-dependent degradation would be calculated as the degradation sensitive to lysosomal inhibitors but insensitive to 3MA-inhibition (Fig. 3). However, this assay alone cannot be used to conclude that there are changes in CMA activity, since degradation by e-MI can still be part of the fraction of lysosomal proteolysis insensitive to 3MA. In addition, because prolonged blockage of macroautophagy often leads to upregulation of CMA, this method could underestimate the fraction of cellular proteins degraded through CMA [24].
Figure 3. Discrimination of pathways responsible for intracellular proteolysis.
Experimental set up of measurement of total rates of intracellular protein degradation modified to discriminate lysosomal degradation (fraction inhibited by treatment with lysosomal protease inhibitors) from non-lysosomal degradation (remaining upon lysosomal proteolysis blockage). Addition of inhibitors of macroautophagy (i.e. 3-methyladenine) also allows to separate degradation by this pathway (sensitive to 3MA) from degradation by CMA and e-MI (insensitive to 3MA).
2.3.2. Photoconvertible CMA reporters
Another approach to follow substrate delivery and degradation through CMA is to monitor the lysosomal association of artificial fluorescent CMA reporters [42]. Although addition of the penta-peptide motif KFERQ to any given protein, including artificial fluorescent proteins, leads to its degradation through CMA, often the abundance of these proteins in the cytosol mask the lysosomal association. This limitation is mostly due to the fact that in order to translocate across the lysosomal membrane, substrate proteins need to fully unfold [8] and consequently they no longer fluoresce in the lysosomal lumen. Thus, the fraction visible is the fluorescent protein bound to the lysosomal membrane at a given time. This lack of “cumulative” effect makes it difficult to discriminate lysosomes against the diffuse fluorescence of the cytosolic protein. To overcome this limitation, we now utilize photoconvertible fluorescent reporters, either photoswitchable (KFERQ-ps-CFP; fluorescence switches from CFP to GFP upon exposure to 405nm light) or photoactivable (KFERQ-PA-mCherry1; becomes fluorescent upon exposure to 405nm light) [42]. By inducing photoconversion at a given time, it is possible to track the association of the photoconverted protein with lysosomes in a different fluorescence channel than the newly synthesized protein (that fluoresces in the original wavelength because it is not photoconverted) (Fig. 2). Changes in the number of fluorescent puncta per cell - read out of CMA activation - vary depending on the cell type and their basal CMA activity, however, in general, an increase of 2–4 folds in puncta/cell is a good indication of CMA activation. Although these reporters are useful to get a general idea of possible changes in CMA, these reporters have limitations and need to be complemented with other methods to measure CMA activity. Thus, because they only monitor lysosomal binding, changes in degradation once inside the lysosomal compartment cannot be detected. Furthermore, if translocation is compromised, there will be a higher amount of reporter bound to lysosomes giving a false impression of enhanced CMA. Once differences are detected with these reporters, changes in uptake and degradation of CMA substrates need to be directly analyzed using other methods, such as the analysis of flux of endogenous CMA substrates described in Section 2.1 or by reconstituting CMA in vitro with lysosomes isolated from those cells or tissues (see Section 2.3.2).
2.3.3. In vitro reconstitution of CMA with isolated lysosomes
The limitations of the assays described in Sections 2.3.1 and 2.3.2 and the fact that manipulation of CMA in whole cells could induce changes in the other autophagic pathways and vice versa, make necessary the use of other methods that take endogenous substrates into consideration, and that allow to separately analyze all the functional steps involved in this dynamic degradation process. Since CMA takes place at the lysosomal compartment, functional assays with isolated lysosomes are the ultimate way to measure functional CMA differences. In this respect, and because not all lysosomes are hsc70-positive and competent for CMA [33], protocols for isolation of lysosomes exclusively contributing to CMA are preferred to the most standard methods that only isolate a pool of mixed lysosomes. A second requirement is the need to preserve the integrity of the lysosomal membrane, making unsuitable protocols in which cell disruption requires detergents or other physical methods, such as sonication, that will disrupt the lysosomal membranes. Nitrogen cavitation in the case of cultured cells [43], and motorized Teflon/glass homogenization in the case of tissues are the most efficient ways of cell disruption (for specific details please consult [37]). Lysosomal isolation is attained through ultra-high speed centrifugation and flotation in a discontinuous metrizamide gradient [44]. The main advantage of this method is that CMA-active lysosomes can be separated based on their different density and size from lysosomes more active for other autophagic processes or for endocytosis [33]. Strict analysis of purity and enrichment of lysosomal enzymes in the isolated fractions, as described before [43], are also required to be able to compare activity in fractions isolated from different cells and conditions.
One limitation of this procedure is the large amount of cultured cells or starting tissue required to isolate a sufficient amount of CMA-active lysosomes. When such amounts of starting material are not available, isolation of a more “crude” lysosomal fraction, prepared by differential centrifugation is still preferred to analysis of the whole cellular lysate. A caveat of this enriched fraction is the presence of considerable amount of mitochondria, which requires normalization of any observed change in CMA substrate levels to the amount of mitochondrial content (using mitochondrial markers) across fractions.
Isolation of the pool of lysosomes active for CMA permits analysis of their content of endogenous CMA substrates at the moment of isolation. Information of flux of endogenous substrates through CMA can be obtained if isolation is done after blockage of lysosomal proteolysis (ammonium chloride/leupeptin in cultured cells or injection of animals with leupeptin, for in vivo studies) (Fig. 2) [25, 27]. The other advantage of isolated lysosomes is that they allow reconstituting CMA in vitro and separately tracking the steps involved in this process - substrate binding, lysosomal uptake and lysosomal degradation [37]. To that purpose, [14C]-radiolabeled CMA substrates (i.e. GAPDH) or even a pool of long-lived radiolabeled cytosolic proteins (common CMA substrates) can be incubated with isolated lysosomes, and their association and degradation in this compartment can be monitored by free [14C]-radiolabeled amino acids released in the incubation media as proteolysis occurs [45]. By comparing degradation of these substrates when incubated with intact or disrupted lysosomes, it is possible to distinguish substrate uptake from degradation [33].
The above-described procedure does not separate lysosomal binding and uptake of CMA substrates, making necessary the use of another immunoblot-based assay based on incubation of isolated lysosomes with purified CMA substrates [46]. Taking advantage of the fact that the internalized substrate will only be visible if lysosomal proteolysis is prevented, because otherwise it will be rapidly degraded when it reaches the lysosomal lumen, it is possible to separately quantify binding and uptake. Substrate binding can be quantified as the amount of substrate recovered in the lysosomal fraction after incubation of the CMA substrate with intact lysosomes not treated with protease inhibitors. Previous lysosomal treatment with lysosomal protease inhibitors followed by incubation with the CMA substrate will allow measuring substrate bound and translocated into lysosomes (binding+uptake) and, by discounting the amount bound to lysosomes in which proteolysis has not be prevented, it is possible to calculate uptake (Fig. 2) [8]. A variation of this method was previously utilized where the bound protein still on the cytosolic side of the lysosomal membrane was removed by addition of exogenous proteases (i.e. proteinase K or trypsin) to detect only the protein internalized (protected from these exogenous proteases by the lysosomal membrane) [33]. However, calculating the amount of exogenous proteases required to remove all bound substrate without affecting the integral proteins of the lysosomal membrane was always a major challenge and this method is now used less frequently.
Lastly, the isolated lysosomal fractions also allow directly comparing changes in the content, posttranslational modification and organization of CMA components at the lysosomal membrane. Decrease in lysosomal LAMP2A or lys-hsc70 levels in isolated lysosomes is a good indication for decreased CMA activity [13, 14]. Increase in LAMP2A levels would indicate possible upregulation of CMA activity [35]. However, in certain instances, higher levels of LAMP2A can be observed without a subsequent increase in CMA activity. For example, although LAMP2A is initially limiting for CMA, if the levels of lys-hsc70 in the lumen are not sufficient to accommodate increased binding to LAMP2A, uptake does not increase proportionally because lys-hsc70 becomes limiting [9]. In addition, efficient substrate uptake depends on the ability of LAMP2A to organize into the high molecular weight CMA translocation complex [47]. Lysosomal LAMP2A multimerization is driven by presence of CMA substrates and, although it is a dynamic process, it is possible to determine the ratio of lysosomal LAMP2A assembled into multimeric complex at a given time using blue native electrophoresis of isolated lysosomes and immunoblot for LAMP2A [48].
3. METHODS TO IDENTIFY IF A PROTEIN IS A CMA SUBSTRATE
When studying specific proteins and their fate in different cellular conditions or pathologies, it is important to consider their possible clearance through CMA, since 30% of total cytosolic proteins are known to bear KFERQ-like CMA targeting motifs [2]. The presence of the targeting motif denotes the potential of the protein to undergo degradation by CMA, but it is not an indication that the protein bearing the motif is continuously degraded through this pathway. In many cases, CMA targeting motifs are inaccessible to the chaperone when the protein is properly folded or if the motif is masked by interacting proteins or posttranslational modifications. Consequently, more direct proof is needed to validate if a protein is a CMA substrate. Several readouts can be utilized to identify if a protein is a CMA substrate based on changes in i) cellular location and levels of the protein and ii) the degradation rate/half-life of the protein upon modulation of CMA (Table 1) [37].
Table 1.
Characteristics of a CMA substrate
| Characteristic of a CMA substrate |
Assay to confirm | Limitations |
|---|---|---|
| KFERQ-like motif in its sequence |
|
|
| Long half-life |
|
|
| Detectable in lysosomes |
|
|
| Changes in cellular levels or lysosomal association upon CMA modulation |
|
|
| Interacts with hsc70 in cytosol |
|
|
| Interacts with LAMP2A at the lysosomes |
|
|
| Translocates into isolated lysosomes in an ATP/hsc70 dependent manner |
|
|
Chaperone-mediated autophagy;
endosomal microautophagy,
lysosome-associate membrane protein type 2A;
retinoic acid receptor;
heat shock cognate protein;
3.1. Association with the lysosomal compartment
Colocalization of the potential CMA substrate with lysosomes by immunofluorescence is a commonly used first approach because authentic CMA substrates will be delivered to lysosomes for their degradation. Common lysosomal markers used for this colocalization are LAMPs (LAMP1, LAMP2) or cathepsins (D or B). However, because these proteins are also present in endosomes (the compartment utilized for e-MI), it is necessary to exclude colocalization of the substrate with late endosomal/MVB markers, such as rab9 or ESCRT proteins [7]. Although immunostaining can be performed in cellular models, this approach becomes more challenging when working with intact tissues. In this case, immunoblot analysis with isolated lysosomes from the tissues of interest can be performed. Enrichment of a specific protein in CMA-active lysosomes can be a good indication of a possible CMA substrate [25, 27, 31]. However, it is also advisable to confirm degradation in lysosomes, to eliminate the possibility that the protein of interest associates with lysosomes for purposes other than degradation. Degradation can be confirmed as an increase in lysosomal association in presence of lysosomal inhibitors [27]. Complete proteomics of CMA-active lysosomes isolated from untreated and leupeptin treated mice has proven to be a very efficient method to determine the proteins undergoing degradation by CMA at a given time or condition [25].
3.2. Changes in levels/rate of degradation upon CMA activation
Increase in cellular levels of a CMA substrate is usually observed in response to inhibition of lysosomal degradation by protease inhibitors; however, support for CMA degradation requires further confirmation, because blockage of lysosomal degradation also results in increase in cellular levels of proteins degraded by other autophagic pathways and by endocytosis [37]. Since CMA is upregulated in response to cellular stress such as nutrient-deprivation, oxidative stress and proteo- and lipo-toxicity, increases in the rate of lysosomal degradation of a protein under those conditions are a possible indirect indication of CMA degradation. However, direct modulation of CMA activity through genetic or chemical procedures is required as an ultimate proof (Table 1).
3.2.1. Methods to activate CMA
Upregulation of CMA can be achieved genetically by overexpression of LAMP2A [4, 30]. Early screening studies measuring overall lysosomal protein degradation rates, proposed a CMA activating effect for the glucose-6-phosphate dehydrogenase inhibitor, 6-aminonicotinamide, and for the hsp90 inhibitor geldanamycin [49]. However, use of both molecules as CMA activators is discouraged due to the fact that 6-aminonicotinamide affects other forms of lysosomal degradation and has an almost negligible effect on CMA when using more direct assays to measure this pathway, and the effect of geldanamycin on CMA seems to be highly cell-type dependent and biphasic (early activation followed by inhibition of CMA) [5]. More recent studies using an array of methods to measure CMA have identified that signaling through the retinoic-acid receptor alpha is inhibitory for CMA [21]. Using a structure activity relationship approach, novel atypical antagonists of this receptor have been developed, which can potently and selectively activate CMA without affecting other degradation pathways [21]. If a protein is a CMA substrate, a decrease in its cellular levels is expected upon treatment with these novel CMA activators. However, because of the long half-life of CMA substrates, if the protein of interest is abundant in the cell it may not be possible to notice a decrease in overall cellular levels by immunoblot, because the degraded protein is replaced by newly synthesized protein. In this case, labeling of intracellular proteins with a radiolabeled amino acid, as described before, and immunoprecipitation of the protein of interest at different times, can provide more accurate evidence of its accelerated degradation upon CMA activation [16].
3.2.2. Methods to inhibit CMA
Currently, there are no suitable chemical methods to selectively block CMA. Early studies using protein degradation measurements demonstrated inhibition of CMA protein degradation by inhibitors of protein synthesis [49]; however because of the many cellular changes associated with protein synthesis inhibition, their use as CMA inhibitors is discouraged. Currently, the only selective and efficient way to inhibit CMA is by knocking down or knocking out LAMP2A, the CMA receptor as this will prevent substrate binding/translocation into lysosomes [24, 25]. This approach is preferred to blockage of other CMA components, such as hsc70 that have other important cellular functions. Blockage of hsc70 will affect not only CMA, but also e-MI, macroautophagy and endocytosis (by inhibiting clathrin disassembly), as well as processes beyond protein degradation, such as protein folding or aggregation.
Although LAMP2A can also have other functions in lysosomes, LAMP2B and LAMP2C can compensate for these other functions upon reduction of LAMP2A levels, as they have identical luminal regions than LAMP2A. This limits the effect of LAMP2A reduction to decreased CMA only, since this function relies on the distinctive cytosolic tail of LAMP2A and cannot be performed by the other LAMP2 protein variants [24, 25]. Genetic reduction of LAMP2A levels does not affect lysosomal stability and function [15, 24, 25] and it is particularly suitable for discrimination between CMA and e-MI degradation, as both pathways require hsc70, whereas only CMA depends on LAMP2A and consequently, knock-down of LAMP2A does not reduce degradation by e-MI [7]. One limitation of the genetic approaches is that the long half-life of LAMP2A makes necessary a waiting period of at least 5 days before cellular levels of pre-existing LAMP2A decrease significantly. This long waiting time precludes the study of the effect of acute inhibition of CMA and henceforth, the search for specific chemical inhibitors of CMA is ongoing.
An additional consideration when manipulating degradation through CMA is that other forms of autophagy are known to be upregulated when CMA is compromised [24], therefore accumulation of a CMA substrate upon CMA blockage could be missed. In that case, it becomes important to verify a possible switch towards degradation through macroautophagy and/or e-MI. Contribution of these pathways to the degradation of the protein of interest can be estimated by knocking down essential components for macroautophagy (i.e. ATG7 or ATG5) or e-MI (i.e. Vps4).
3.3. Interaction with CMA components
For a substrate to be degraded by CMA, it has to interact with CMA components (Table 1). Substrate interaction with cytosolic hsc70 chaperone is the initial step of CMA [3]. Co-immunoprecipitation of hsc70 chaperone with the protein of interest indicates possible CMA degradation. However, it is important to note that this interaction does not correlate with changes in uptake rate, since hsc70 binding will occur, and even be higher, if CMA binding/uptake are blocked [25]. In addition, protein binding to hsc70 can also occur as part of its degradation via e-MI [7], making necessary the use of additional studies to support CMA degradation, such as proof of interaction with LAMP2A.
3.4. Elimination of the targeting motif
Since substrate proteins are targeted to the lysosomal membrane by hsc70 upon recognition of the KFERQ-like targeting motif in their sequences, alterations in this motif are commonly utilized to support involvement of this chaperone in their degradation. The motif consists of a glutamine residue (Q) flanked on either side by 4 amino acid residues consisting of only one acidic residue (D, E), and one or two basic (K, R) or bulky hydrophobic residues (K, R, F, I, L, V) [2]. Mutation of Q and the residue next to it in the motif is usually sufficient to disrupt hsc70-substrate interaction [15, 16, 27]. Because the interaction of hsc70 with the motif is based on the physical properties of the amino acids rather than the sequence per se, in some instances the negative charge in the motif can be contributed by phosphorylation of S, T or Y. Furthermore, Q has been shown to be replaced by N in some substrates such as glyceraldehyde-3-phosphate dehydrogenase, but this Q by N substitution seems very context dependent and does not work in most of the known substrate proteins. Binding of hsc70 through this motif will not discriminate between degradation through CMA and e-MI, and consequently an additional method should be used to determine the fate of the protein (Table 2). Elimination or alteration of the targeting motif will lead to decreased association of the substrate with lysosomes in the case of CMA or with late endosomes/MVB in the case of e-MI.
Table 2.
Criteria to differentiate between a CMA substrate and an e-MI substrate
| Criteria | CMA | e-MI |
|---|---|---|
| Half-Life of the substrate | Long Half-life | Long Half-life |
| Substrate association with organelle |
CMA active lysosomes | Late endosomes/MVB a |
| Interacts with hsc70 through KFERQ-like motif |
Yes | Yes |
| Interacts with LAMP2A | Yes | No |
| Requires protein substrate unfolding |
Yes | No |
| Lumenal delivery |
|
|
Multivesicular bodies;
knock-down,
endosomal sorting complexes required for transport
Furthermore, in some instances, interaction with hsc70 persists even in the absence of the CMA targeting motif. Hsc70 binding in these cases often has switched to another region in the protein, due to the ability of hsc70 to bind hydrophobic protein patches. However, that type of interaction does not target the protein for CMA degradation, and can be easily identified by demonstrating lack of competition for hsc70 binding with other proteins bearing the CMA targeting motif such as ribonuclease A [27].
4. CONCLUDING REMARKS
The array of methods developed through the years to study CMA now makes it possible to quantify changes in CMA activity under different conditions using a variety of experimental models and to differentiate degradation profiles through CMA from other forms of autophagy, such as macroautophagy and e-MI. Steady-state readouts provide preliminary information on the state of CMA in some instances such as primary cultured cells or tissues, when the available material is limited. As for other forms of degradation, functional assays are the only conclusive way to infer changes in CMA activity. These approaches have been successfully utilized to identify CMA malfunctioning in experimental models of disease such as neurological disorders, metabolic diseases or cancer and in physiological states such as aging.
Since degradation through CMA is a dynamic process, evaluation of each of the steps involved in CMA is necessary when investigating the basis for CMA malfunctioning. Moreover, since translocation of substrate into lysosomes is the rate-limiting step in CMA and the one that differentiates it from other forms of autophagy, in vitro experiments using isolated lysosomes yield the most precise analysis of CMA.
Although overall changes in CMA activity occur in different pathological conditions, often, it is the inability to degrade a specific protein through CMA that causes disease. In these instances, it becomes necessary to identify the faulty substrate using the set of specific criteria that a protein needs to fulfill to be confirmed as a bona fide CMA substrate as described in this review.
Future challenges in the study of CMA include the need for efficient chemical approaches that provide fast and selective inhibition of this process, which will help not only to further identify new physiological functions of CMA, but also could be utilized with therapeutic purposes in conditions with abnormally enhanced CMA activity such as cancer. An additional remaining challenge is the development of efficient methods for analysis of CMA function in vivo in a whole organism. Transgenic mice expressing the novel CMA reporters are being generated and they could become a valuable tool for tracking CMA in multiple tissues simultaneously as well as in primary cell cultures.
Highlights.
Specific methods distinguish lysosomal degradation by different autophagic pathways
Changes in levels of CMA components are indirect estimation of CMA activity
Direct assessment of flux through CMA is the best method to measure CMA
Modulation of CMA could be used for therapeutic purposes
Acknowledgements
Work in our laboratory is supported by grants from the NIA, NIDDK and NINDS, from the Glenn and Rainwater foundations and by the generous support of R&R Belfer. We thank Dr. Susmita Kaushik for critically reviewing this manuscript.
Abbreviations
- 3MA
3-methyladenine
- ATG
autophagy-related protein
- CMA
chaperone-mediated autophagy
- e-MI
endosomal microautophagy
- hsc
heat shock cognate protein
- LAMP
lysosome-associated membrane protein
- MVB
multivesicular body
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kaushik S, Cuervo AM. Trends Cell Biol. 2012;22:407–417. doi: 10.1016/j.tcb.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dice JF. Trends Biochem Sci. 1990;15:305–309. doi: 10.1016/0968-0004(90)90019-8. [DOI] [PubMed] [Google Scholar]
- 3.Chiang H, Terlecky S, Plant C, Dice JF. Science. 1989;246:382–385. doi: 10.1126/science.2799391. [DOI] [PubMed] [Google Scholar]
- 4.Cuervo AM, Dice JF. Science. 1996;273:501–503. doi: 10.1126/science.273.5274.501. [DOI] [PubMed] [Google Scholar]
- 5.Bandyopadhyay U, Kaushik S, Varticovski L, Cuervo AM. Mol Cell Biol. 2008;28:5747–5763. doi: 10.1128/MCB.02070-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agarraberes F, Terlecky S, Dice J. J Cell Biol. 1997;137:825–834. doi: 10.1083/jcb.137.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sahu R, Kaushik S, Cannizzo E, Scharf B, Follenzi A, Clement C, Potolicchio I, Nieves E, Cuervo A, Santambrogio L. Develop. Cell. 2011;20:131–139. doi: 10.1016/j.devcel.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salvador N, Aguado C, Horst M, Knecht E. J Biol Chem. 2000;275:27447–27456. doi: 10.1074/jbc.M001394200. [DOI] [PubMed] [Google Scholar]
- 9.Cuervo AM, Knecht E, Terlecky SR, Dice JF. Am J Physiol. 1995;269:C1200–C1208. doi: 10.1152/ajpcell.1995.269.5.C1200. [DOI] [PubMed] [Google Scholar]
- 10.Kiffin R, Bandyopadhyay U, Cuervo A. Antioxid Redox Signal. 2006;8:152–162. doi: 10.1089/ars.2006.8.152. [DOI] [PubMed] [Google Scholar]
- 11.Koga H, Martinez-Vicente M, Arias E, Kaushik S, Sulzer D, Cuervo AM. J Neurosci. 2011;31:18492–18505. doi: 10.1523/JNEUROSCI.3219-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodriguez-Navarro JA, Kaushik S, Koga H, Dall'armi C, Shui G, Wenk MR, Di Paolo G, Cuervo AM. Proc Natl Acad Sci U S A. 2012;109:E705–E714. doi: 10.1073/pnas.1113036109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cuervo AM, Dice JF. J Biol Chem. 2000;275:31505–31513. doi: 10.1074/jbc.M002102200. [DOI] [PubMed] [Google Scholar]
- 14.Kiffin R, Kaushik S, Zeng M, Bandyopadhyay U, Zhang C, Massey AC, Martinez-Vicente M, Cuervo AM. J Cell Sci. 2007;120:782–791. doi: 10.1242/jcs.001073. [DOI] [PubMed] [Google Scholar]
- 15.Valdor R, Mocholi E, Botbol Y, Guerrero-Ros I, Chandra D, Koga H, Gravekamp C, Cuervo AM, Macian F. Nat Immunol. 2014;15:1046–1054. doi: 10.1038/ni.3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cuervo AM, Stefanis L, Fredenburg R, Lansbury PT, Sulzer D. Science. 2004;305:1292–1295. doi: 10.1126/science.1101738. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Martinez-Vicente M, Kruger U, Kaushik S, Wong E, Mandelkow EM, Cuervo AM, Mandelkow E. Hum Mol Genet. 2009;18:4153–4170. doi: 10.1093/hmg/ddp367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sooparb S, Price SR, Shaoguang J, Franch HA. Kidney Int. 2004;65:2135–2144. doi: 10.1111/j.1523-1755.2004.00639.x. [DOI] [PubMed] [Google Scholar]
- 19.Kon M, Kiffin R, Koga H, Chapochnick J, Macian F, Varticovski L, Cuervo AM. Sci Transl Med. 2011;3 doi: 10.1126/scitranslmed.3003182. 109ra117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Macri C, Wang F, Tasset I, Schall1 N, Page N, Briand J-P, Cuervo A, Muller S. Autophagy. 2014 doi: 10.1080/15548627.2015.1017179. E-pub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anguiano J, Garner TP, Mahalingam M, Das BC, Gavathiotis E, Cuervo AM. Nat Chem Biol. 2013;9:374–382. doi: 10.1038/nchembio.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang Z, Klionsky DJ. Nat Cell Biol. 2010;12:814–822. doi: 10.1038/ncb0910-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gough NR, Hatem CL, Fambrough DM. DNA Cell Biol. 1995;14:863–867. doi: 10.1089/dna.1995.14.863. [DOI] [PubMed] [Google Scholar]
- 24.Massey AC, Kaushik S, Sovak G, Kiffin R, Cuervo AM. Proc Nat Acad Sci USA. 2006;103:5905–5910. doi: 10.1073/pnas.0507436103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schneider JL, Suh Y, Cuervo AM. Cell Metab. 2014;20:417–432. doi: 10.1016/j.cmet.2014.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin A, Joseph JA, Cuervo AM. J Neurochem. 2002;82:538–549. doi: 10.1046/j.1471-4159.2002.00978.x. [DOI] [PubMed] [Google Scholar]
- 27.Orenstein SJ, Kuo SH, Tasset I, Arias E, Koga H, Fernandez-Carasa I, Cortes E, Honig LS, Dauer W, Consiglio A, Raya A, Sulzer D, Cuervo AM. Nat Neurosci. 2013;16:394–406. doi: 10.1038/nn.3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cannizzo ES, Clement CC, Morozova K, Valdor R, Kaushik S, Almeida LN, Follo C, Sahu R, Cuervo AM, Macian F, Santambrogio L. Cell Rep. 2012;2:136–149. doi: 10.1016/j.celrep.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodriguez-Muela N, Koga H, Garcia-Ledo L, de la Villa P, de la Rosa EJ, Cuervo AM, Boya P. Aging Cell. 2013;12:478–488. doi: 10.1111/acel.12072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang C, Cuervo AM. Nat Med. 2008;14:959–965. doi: 10.1038/nm.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cuervo AM, Hu W, Lim B, Dice JF. Mol Biol Cell. 1998;9:1995–2010. doi: 10.1091/mbc.9.8.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cuervo AM, Hildebrand H, Bomhard EM, Dice JF. Kidney Int. 1999;55:529–545. doi: 10.1046/j.1523-1755.1999.00268.x. [DOI] [PubMed] [Google Scholar]
- 33.Cuervo AM, Dice JF, Knecht E. J Biol Chem. 1997;272:5606–5615. doi: 10.1074/jbc.272.9.5606. [DOI] [PubMed] [Google Scholar]
- 34.Eskelinen EL, Cuervo AM, Taylor MR, Nishino I, Blum JS, Dice JF, Sandoval IV, Lippincott-Schwartz J, August JT, Saftig P. Traffic. 2005;6:1058–1061. doi: 10.1111/j.1600-0854.2005.00337.x. [DOI] [PubMed] [Google Scholar]
- 35.Cuervo AM, Dice JF. Traffic. 2000;1:570–583. doi: 10.1034/j.1600-0854.2000.010707.x. [DOI] [PubMed] [Google Scholar]
- 36.Kiffin R, Christian C, Knecht E, Cuervo AM. Mol Biol Cell. 2004;15:4829–4840. doi: 10.1091/mbc.E04-06-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaushik S, Cuervo AM. Methods Enzymol. 2009;452:297–324. doi: 10.1016/S0076-6879(08)03619-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dice JF. FASEB J. 1987;1:349–356. doi: 10.1096/fasebj.1.5.2824267. [DOI] [PubMed] [Google Scholar]
- 39.Auteri JS, Okada A, Bochaki V, Dice JF. J Cell Physiol. 1983;115:159–166. doi: 10.1002/jcp.1041150210. [DOI] [PubMed] [Google Scholar]
- 40.Fuertes G, Martin De Llano J, Villarroya A, Rivett A, Knecht E. Biochem J. 2003;375:75–86. doi: 10.1042/BJ20030282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seglen P, Gordon P. Proc Nat Acad Sci USA. 1982;79:1889–1892. doi: 10.1073/pnas.79.6.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koga H, Martinez-Vicente M, Macian F, Verkhusha VV, Cuervo AM. Nat Commun. 2011;2:386. doi: 10.1038/ncomms1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Storrie B, Madden E. Methods Enzymol. 1990;182:203–225. doi: 10.1016/0076-6879(90)82018-w. [DOI] [PubMed] [Google Scholar]
- 44.Wattiaux R, Wattiaux-De Coninck S, Ronveaux-Dupal M, Dubois F. J Cell Biol. 1978;78:349–368. doi: 10.1083/jcb.78.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Terlecky SR, Chiang H-L, Olson TS, Dice JF. J Biol Chem. 1992;267:9202–9209. [PubMed] [Google Scholar]
- 46.Aniento F, Roche E, Cuervo AM, Knecht E. J Biol Chem. 1993;268:10463–10470. [PubMed] [Google Scholar]
- 47.Orenstein SJ, Cuervo AM. Semin Cell Dev Biol. 2010;21:719–726. doi: 10.1016/j.semcdb.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bandyopadhyay U, Cuervo AM. Autophagy. 2008;4:1101–1103. doi: 10.4161/auto.7150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Finn P, Mesires N, Vine M, Dice JF. Autophagy. 2005;1:141–145. doi: 10.4161/auto.1.3.2000. [DOI] [PubMed] [Google Scholar]