Abstract
Introduction
Brain metastases (BM) are common in non-small-cell lung cancer (NSCLC). However, the baseline incidence and evolution of BM over time in oncogene-driven NSCLCs are seldom reported. In this study, we evaluated the frequency of BM in patients with epidermal growth factor receptor (EGFR)-mutated or anaplastic lymphoma kinase (ALK)-rearranged NSCLC.
Methods
The presence of BM, clinicopathologic data, and tumor genotype were retrospectively compiled and analyzed from a cohort of 381 patients.
Results
We identified 86 EGFR-mutated (90.7% with metastatic disease; 85.9% received an EGFR inhibitor) and 23 ALK-rearranged (91.3% with metastatic disease; 85.7% received an ALK inhibitor) NSCLCs. BM were present in 24.4% of EGFR-mutated and 23.8% of ALK-rearranged NSCLCs at the time of diagnosis of advanced disease. This study did not demonstrate a difference in the cumulative incidence of BM over time between the two cohorts (EGFR/ALK cohort competing risk regression [CRR] coefficient of 0.78 [95% CI 0.44–1.39], p=0.41). In still living patients with advanced EGFR-mutated NSCLC, 34.2% had BM at 1 year, 38.4% at 2 years, 46.7% at 3 years, 48.7% at 4 years, and 52.9% at 5 years. In still living patients with advanced ALK-rearranged NSCLC, 23.8% had BM at 1 year, 45.5% at 2 years, and 58.4% at 3 years.
Conclusions
BM are frequent in advanced EGFR-mutated or ALK-rearranged NSCLCs, with an estimated >45% of patients with CNS involvement by three years of survival with the use of targeted therapies. These data point toward the CNS as an important unmet clinical need in the evolving schema for personalized care in NSCLC.
Keywords: lung cancer, NSCLC, brain metastases, EGFR, ALK, mutation, rearrangement, central nervous system, CNS
INTRODUCTION
Non-small-cell lung cancer (NSCLC) is the leading cause of brain metastases (BM) (1). Amongst those with recurrent/advanced NSCLC, BM are a common culprit for cancer-related morbidity and mortality. The incidence of BM in NSCLC has been reported as >20% at diagnosis, with a significant proportion of patients developing BM over the course of their disease (2, 3). Recent reports also demonstrate an increase in the documented incidence of BM in NSCLC, likely as a consequence of prolonged survival with newer therapies coupled with improvements in neuroimaging modalities (4).
Far less is known about the baseline incidence and subsequent evolution of BM in the subset of patients with oncogene-driven tumors, i.e. epidermal growth factor (EGFR)-mutated or anaplastic lymphoma kinase (ALK)-rearranged NSCLC (5, 6). Given the growing emphasis on molecular profiling and use of targeted therapeutics (7), this is a critical population. An improved understanding of the incidence and evolution of BM in these cases is therefore an area of ongoing need.
Herein, we report the baseline and cumulative incidence of BM in patients with EGFR-mutated or ALK-rearranged advanced NSCLC.
MATERIALS AND METHODS
Cohort selection
Patients seen at Beth Israel Deaconess Medical Center (BIDMC) with a diagnosis of NSCLC and whose tumors were genotyped were identified through an ongoing Institutional Review Board-approved study (8,9), with a data cutoff of December 19, 2012 for patient inclusion and June 24, 2014 for outcomes.
Tumor genotype
EGFR mutation analysis (exons 18 to 21) was performed using standard sequencing techniques, and ALK rearrangement was analyzed using the Vysis ALK break-apart fluorescence in situ hybridization (FISH) probe (8).
Data collection and detection of BM
Data was collected by retrospective chart review and managed using REDCap electronic data capture tools hosted at BIDMC. All patients with advanced NSCLC (stage IV/recurrent disease) had baseline CNS evaluation with either computed tomography (CT) or magnetic resonance imaging (MRI). Subsequent CNS assessment was performed at the discretion of the treating physicians. BM were diagnosed either radiographically or pathologically (tumor resection/biopsy or malignant CSF cytology).
Statistical methods
Fisher’s exact test was used to compare categorical variables, and Wilcoxon rank test was used for continuous variables. All p-values reported are two-sided, and tests were conducted at the 0.05-level. Time to BM was defined as the time from diagnosis to date of detected BM, and cumulative incidence curves were fitted and compared using the methodologies of Fine and Gray (10), adjusting for death as a competing risk. Patients not experiencing BM or death by the time of data cutoff were censored at their last date of follow-up. Overall survival (OS) was analyzed using the Kaplan–Meier method. Statistical analyses and curves were performed with the cmprsk package in R Statistical Programming Language.
RESULTS
Patient and tumor characteristics
The complete cohort comprised 381 patients, with a median age at diagnosis of 65 years. Self-reported racial groups were 75.9% white, 13.1% Asian, 6.5% black, and 4.4% other. 27.8% of patients were never smokers, 54.9% were former smokers, and 17.3% current smokers. At the time of initial entry into the cohort, 73.8% had stage IV/recurrent disease, and 86.1% had adenocarcinoma histology. EGFR and ALK analysis was successful in 94.2% (359/381) and 91.6% (252/275) of tested samples, respectively (8). The overall frequency of EGFR mutations and ALK FISH positivity was 23.9% (86/359) and 9.1% (23/252), respectively. Abnormalities in EGFR and ALK were mutually exclusive in all genotyped tumors.
Characteristics of EGFR-mutated or ALK-rearranged NSCLCs
The EGFR-mutated cohort was comprised of more women; however, both cohorts predominantly involved patients with no tobacco history, advanced disease, and adenocarcinoma histology (Table 1). 85.9% (67/86) of patients with EGFR-mutated NSCLCs received an EGFR tyrosine kinase inhibitor (TKI) -gefitinib, erlotinib, or dacomitinib – at some point in their treatment course. 85.7% (18/23) of patients with ALK-rearranged NSCLCs received the ALK TKI, crizotinib (Table 1). The median follow-up for patients with EGFR-mutated and ALK-rearranged NSCLC was 45.2 and 36.4 months, respectively.
Table 1.
Baseline characteristics of patients and tumors with EGFR-mutated and ALK-rearranged NSCLC.
| Tumor genotype
|
|||
|---|---|---|---|
|
EGFR-mutated (n=86) |
ALK-rearranged (n=23) |
p-value | |
|
| |||
| Age at time of biopsy | |||
| Median (range) | 65 (33–90) | 56 (29–80) | <0.0001* |
|
| |||
| Women n (%) | 64 (74.4) | 11 (47.8) | 0.0218 |
|
| |||
| Race n (%) | |||
| White | 50 (58.1) | 15 (65.2) | 0.6357 |
| Asian | 31 (36.1) | 4 (17.4) | |
| Black | 4 (4.6) | 0 (0) | |
| Other | 1 (1.2) | 4 (17.4) | |
|
| |||
| Smoking status n (%) | |||
| Current smoker | 4 (4.65) | 1 (4.3) | 0.4909 |
| Former smoker | 37 (43.0) | 8 (34.8) | |
| Never smoker | 45 (52.3) | 14 (60.9) | |
|
| |||
| Stage n (%) | |||
| I–III | 8 (9.3) | 2 (8.7) | 1.0000 |
| IV/recurrent | 78 (90.7) | 21 (91.3) | |
|
| |||
| Histology n (%) | |||
| Adenocarcinoma | 84 (97.7) | 21 (91.3) | 0.1952 |
| Squamous cell carcinoma | 0 (0) | 1 (4.3) | |
| NSCLC (NOS) | 2 (2.3) | 1 (4.3) | |
|
| |||
| Use of precision TKI n (%) | |||
| When stage IV/recurrent | 67 (85.9) | 18 (85.7) | 1.0000 |
|
| |||
| Baseline brain metastases n (%) | |||
| When stage IV/recurrent | 19 (24.4) | 5 (23.8) | 1.0000 |
NSCLC = non-small-cell lung cancer; NOS = not otherwise specified; TKI = tyrosine kinase inhibitor.
Wilcoxon-Rank Sum test
BM in EGFR-mutated NSCLC
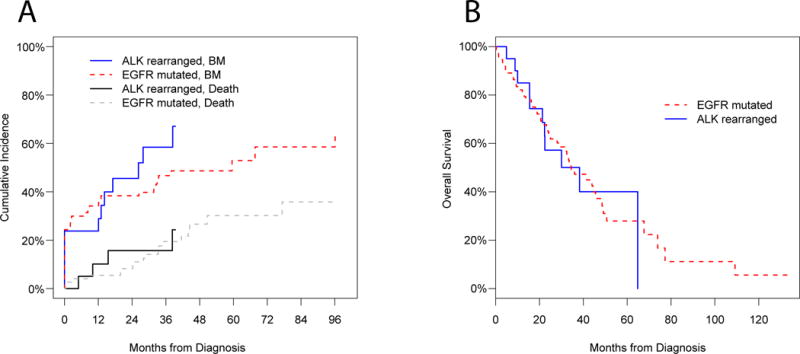
24.4% (19/78) of the patients with EGFR-mutated NSCLC had BM at initial evaluation. The cumulative incidence of post-diagnosis BM increased over time (Figure 1A): 34.2% at 1 year, 38.4% at 2 years, 46.7% at 3 years, 48.7% at 4 years, and 52.9% at 5 years.
Figure 1.
BM in ALK-rearranged NSCLC
23.8% (5/21) of the patients with ALK-rearranged NSCLC had BM at initial evaluation. The cumulative incidence of post-diagnosis BM increased over time (Figure 1A): 23.8% at 1 year, 45.5% at 2 years, and 58.4% at 3 years. At the time of data cutoff, none of the patients with ALK-rearranged NSCLC had reached beyond the 4-year survival mark.
Differences in BM between EGFR-mutated and ALK-rearranged NSCLC
No statistically significant difference was observed in the baseline and subsequent incidence of BM with EGFR-mutated versus ALK-rearranged tumors (Figure 1A). Additionally, this study did not demonstrate a difference in the cumulative incidence of BM over time between the two cohorts (EGFR/ALK cohort CRR coefficient of 0.78 [95% CI 0.44–1.39], p=0.41). However, since the ALK-rearranged cohort had fewer patients and less follow-up time than the EGFR-mutated group, it is unknown if differences may have evolved during subsequent follow-up.
Overall survival
Survival curves are depicted in Figure 1B. Amongst the 78 patients with an EGFR mutation, the median OS was 34.4 months (95% CI 28.1–49.1). Median OS amongst the 21 patients with an ALK rearrangement was 38.3 months (95% CI 22.3-NA).
DISCUSSION
We retrospectively evaluated a cohort of patients with EGFR-mutated or ALK-rearranged advanced NSCLC. BM were common, with nearly 25% of patients with BM at initial diagnosis and approximately half of patients with BM by 3 years in the setting of precision therapies. Limitations of this study include its retrospective nature and the lack of a standardized protocol for CNS assessment. Nevertheless, to our knowledge, this is the largest study to assess the baseline incidence and subsequent evolution of BM in these molecularly-defined groups.
The clinical course of patients with these oncogene-driven NSCLCs is an area of ongoing investigation. The University of Colorado evaluated patterns of metastatic spread in 209 patients with treatment-naïve EGFR-mutated, KRAS-mutated, ALK-rearranged, and EGFR/KRAS/ALK-unaffected advanced NSCLC (11). Similar to our findings, BM was present at diagnosis in 23% and 24% of EGFR-mutated and ALK-rearranged patients, respectively (11). In one retrospective study, BM were reported in 8% (5 of 62) EGFR-mutated patients at diagnosis, with an additional 24% diagnosed with BM at follow-up (12). The overall incidence of BM in this cohort was 32%, and time to BM and post-BM survival were 20.8 and 12.1 months, respectively (12). One other study of 93 patients with NSCLC and BM undergoing mutational analysis for EGFR found 49% (20 of 41) of EGFR-mutated NSCLCs with synchronous BM at the time of initial diagnosis (13). Time to progression in the brain was non-significantly longer in EGFR-mutated NSCLCs as compared to those with wild-type EGFR (13). The prevalence of BM in the published trials of TKIs in advanced NSCLC to-date is similarly impressive, with more than 10% of patients in the first line trials of EGFR TKIs (5) and 35% of patients in the second line trials of ALK TKIs (6) noted to have baseline asymptomatic BM.
As targeted therapies continue to improve outcomes for patients with molecularly-driven NSCLCs (5–7), the deterrence of BM has become an increasingly relevant therapeutic dilemma. Most available TKIs (gefitinib, erlotinib, and crizotinib) inefficiently cross the intact blood–brain barrier with cerebrospinal fluid-to-plasma ratios as low as 0.01 to 0.003 detected in patients (14). Even so, it seems that their use can still alter the natural history of BM. In one report of 155 EGFR-mutated NSCLCs, the 12- and 24-month cumulative risk of CNS progression was 6% and 21% in the group initially treated with an EGFR TKI, as compared to 19% and 32% in the group receiving first line chemotherapy; a hazard ratio of 0.56 (95% CI 0.34–0.94) was noted for CNS progression, favoring upfront EGFR TKI over chemotherapy (15). Although a similar comparison has not been performed for ALK-rearranged NSCLC, crizotinib and other ALK TKIs (such as ceritinib and alectinib) can also positively influence CNS disease (7). Ceritinib and alectinib have reported CNS activity in patients with ALK-rearranged NSCLC that are naïve or resistant to crizotinib therapy (7). Since our group’s cohort and those of others (5–7) predominantly studied patients that received TKIs, it is tempting to postulate that the cumulative incidence of BM may have been even higher in patients treated with chemotherapy alone. Contemporary clinical trials of novel EGFR and ALK TKIs now mandate baseline CNS imaging at study entry for all patients and have started to stratify patients based on presence/absence of BM. Such efforts will hopefully define prospectively whether CNS and systemic sites differ in patterns of response/progression in the setting of TKI use (7). Moreover, these studies will aid in delineating strategies for optimal surveillance for and detection of BM early in the course of CNS progression, for which there is currently no established standard.
Acquired resistance to targeted therapies remains a key limitation in achieving a durable benefit. Even as the major mechanisms of acquired systemic resistance are known to result from resistant tyrosine kinase mutations and activation of bypass pathways (7), the mechanisms that explain CNS progression remain largely unknown. As reported here and elsewhere (5–7, 10–12), the CNS is amongst the most frequent sites of disease progression in patients whose systemic disease is otherwise being successfully controlled with TKIs. The optimal management of BM in oncogene-driven NSCLCs remains uncertain, and the use of whole brain or stereotactic radiotherapy remains a cornerstone of care (16).
In conclusion, this retrospective study demonstrates the high initial incidence (nearly 25%) and subsequent prevalence of BM in patients with advanced EGFR-mutated or ALK-rearranged NSCLCs treated with TKIs. Given their frequency in tumors with these driver mutations, increased inclusion of patients with BM in innovative trials of targeted therapies will be critical in defining their full potential and facilitating their application in real-world practice. Developing optimal strategies for the prevention and management of CNS disease has become one of the foremost clinical questions in the care of these patients. Future studies should address the role of TKIs with improved CNS penetration, the use of less toxic radiotherapy techniques for BM, and the need to better understand the molecular underpinnings of malignant CNS disease.
HIGHLIGHTS.
-
-
Brain metastases are frequent in ALK rearranged lung cancers
-
-
Brain metastases are frequent in EGFR mutated lung cancers
-
-
~25% of patients at diagnosis and half at 3-years of survival have brain metastases
Acknowledgments
This work was funded in part through a fellowship from the American Society of Clinical Oncology Conquer Cancer Foundation (DBC), an American Cancer Society grant (RSG 11-186 to DBC), a Lung Cancer Foundation of America-International Association for the Study of Lung Cancer grant (to DBC), National Institutes of Health (NIH) grant CA090578 (to DBC), and Gallup Funds from the Thoracic Oncology Program at the Dana-Farber Cancer Institute (to SED).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: annexed separately.
References
- 1.Nayak L, Lee EQ, Wen PY. Epidemiology of brain metastases. Curr Oncol Rep. 2012;14:48–54. doi: 10.1007/s11912-011-0203-y. [DOI] [PubMed] [Google Scholar]
- 2.Shi AA, Digumarthy SR, Temel JS, Halpern EF, Kuester LB, Aquino SL. Does initial staging or tumor histology better identify asymptomatic brain metastases in patients with non-small cell lung cancer? J Thorac Oncol. 2006;1:205–10. doi: 10.1016/s1556-0864(15)31569-0. [DOI] [PubMed] [Google Scholar]
- 3.Heon S, Yeap BY, Britt GJ, Costa DB, Rabin MS, Jackman DM, et al. Development of central nervous system metastases in patients with advanced non-small cell lung cancer and somatic EGFR mutations treated with gefitinib or erlotinib. Clin Cancer Res. 2010;16:5873–82. doi: 10.1158/1078-0432.CCR-10-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnholtz-Sloan JS, Sloan AE, Davis FG, Vigneau FD, Lai P, Sawaya RE. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol. 2004;22:2865–72. doi: 10.1200/JCO.2004.12.149. [DOI] [PubMed] [Google Scholar]
- 5.Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–46. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 6.Shaw AT, Kim DW, Nakagawa K, Seto T, Crino L, Ahn MJ, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368:2385–94. doi: 10.1056/NEJMoa1214886. [DOI] [PubMed] [Google Scholar]
- 7.Gerber DE, Gandhi L, Costa DB. Management and future directions in non-small cell lung cancer with known activating mutations. Am Soc Clin Oncol Educ Book. 2014:e353–e365. doi: 10.14694/EdBook_AM.2014.34.e353. [DOI] [PubMed] [Google Scholar]
- 8.VanderLaan PA, Yamaguchi N, Folch E, Boucher DH, Kent MS, Gangadharan SP, et al. Success and failure rates of tumor genotyping techniques in routine pathological samples with non-small-cell lung cancer. Lung Cancer. 2014;84:39–44. doi: 10.1016/j.lungcan.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamaguchi N, VanderLaan PA, Folch E, Boucher DH, Canepa HM, Kent MS, et al. Smoking status and self-reported race affect the frequency of clinically relevant oncogenic alterations in non-small-cell lung cancers at a United States-based academic medical practice. Lung Cancer. 2013;82:31–7. doi: 10.1016/j.lungcan.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fine JP, Grayzel D. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association. 1999;94:496–509. [Google Scholar]
- 11.Doebele RC, Lu X, Sumey C, Maxson DA, Weickhardt AJ, Oton AB, et al. Oncogene status predicts patterns of metastatic spread in treatment-naive nonsmall cell lung cancer. Cancer. 2012;118:4502–11. doi: 10.1002/cncr.27409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hendriks LE, Smit EF, Vosse BA, Mellema WW, Heideman DA, Bootsma GP, et al. EGFR mutated non-small cell lung cancer patients: more prone to development of bone and brain metastases? Lung Cancer. 2014;84:86–91. doi: 10.1016/j.lungcan.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 13.Eichler AF, Kahle KT, Wang DL, Joshi VA, Willers H, Engelman JA, et al. EGFR mutation status and survival after diagnosis of brain metastasis in nonsmall cell lung cancer. Neuro Oncol. 2010;12:1193–9. doi: 10.1093/neuonc/noq076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Costa DB, Kobayashi S, Pandya SS, Yeo WL, Shen Z, Tan W, et al. CSF concentration of the anaplastic lymphoma kinase inhibitor crizotinib. J Clin Oncol. 2011;29:e443–e445. doi: 10.1200/JCO.2010.34.1313. [DOI] [PubMed] [Google Scholar]
- 15.Heon S, Yeap BY, Lindeman NI, Joshi VA, Butaney M, Britt GJ, et al. The impact of initial gefitinib or erlotinib versus chemotherapy on central nervous system progression in advanced non-small cell lung cancer with EGFR mutations. Clin Cancer Res. 2012;18:4406–14. doi: 10.1158/1078-0432.CCR-12-0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zimmermann S, Dziadziuszko R, Peters S. Indications and limitations of chemotherapy and targeted agents in non-small cell lung cancer brain metastases. Cancer Treat Rev. 2014;40:716–22. doi: 10.1016/j.ctrv.2014.03.005. [DOI] [PubMed] [Google Scholar]


