Summary
Intermediate filament proteins form filaments, fibers and networks both in the cytoplasm and the nucleus of metazoan cells. Their general structural building plan accommodates highly varying amino acid sequences to yield extended dimeric α-helical coiled coils of highly conserved design. These “rod” particles are the basic building blocks of intrinsically flexible, filamentous structures that are able to resist high mechanical stresses, i.e. bending and stretching to a considerable degree, both in vitro and in the cell. Biophysical and computer modeling studies are beginning to unfold detailed structural and mechanical insights into these major supramolecular assemblies of cell architecture, not only in the “test tube” but also in the cellular and tissue context.
“Nanofilaments”: Fibrous protein assemblies that comprise a major cytoskeletal moiety and the nuclear lamina
The fibrous intermediate filament (IF) proteins constitute the nuclear lamina network as well as a 10-nm-diameter filament system in the cytoplasm of metazoan cells1. Supposedly, they all originate from a common precursor, most probably a kind of a “primordial nuclear lamin”2. All IF proteins follow a common structural principle including a central α-helical “rod” of conserved size that is flanked by non-α-helical N-terminal (“head”) and C-terminal (“tail”) domains both of highly variable size3. The central α-helical rod domain is comprised of three segments separated by two linkers: coil 1A; linker L1; coil 1B; linker L12; and coil 2 (Figure 1A). All three segments exhibit a distinct pattern of charged amino acid clusters (Figure 1B) that are important for a given IF protein to assemble into higher order structures. In addition, a heptad repeat pattern of hydrophobic amino acids yields a “hydrophobic seam” along the α-helical segments that mediates the formation of an unstaggered parallel coiled-coil dimer. This rod dimer is the basic building block of all IF-protein assemblies with an approximate length of 46 nm for the vertebrate cytoplasmic IF proteins and 52 nm for the nuclear lamins and the invertebrate cytoplasmic IF proteins (Figure 1C)3.
Figure 1. IF protein organization.
A. Domain organization of lamin A and vimentin as representatives for nuclear and cytoplasmic IF proteins: A central α-helical “rod” is flanked by non-α-helical “head” and “tail” domains. Boxes represent amino acid sequence segments engaged in coiled-coil (green) or “paired bundle” (yellow) formation; the IF-consensus motifs are indicated in blue. The “linker” segments between coil 1A and coil 1B as well as those between coil 1B and coil 2 may be α-helical in the case of lamins, but are probably of unique fold in cytoplasmic IF proteins. The circle in the lamin tail represents an Ig fold. B. Charge distribution of vimentin demonstrating the basic nature of the rod and the rather dense pattern of alternating basic and acidic charges along the rod. C. Model of a vimentin coiled-coil dimer. Vimentin-like IF proteins exhibit pre-coil domains probably adopting an α-helical fold (PCD) not seen in lamins and keratins (redrawn from Ref. 6). Paired bundles are in orange, the “linker” domains are designated L1 and L12. The numbers in brackets indicate the number of amino acids in the “heads” and “tails”, respectively. D. Potential hetero-coiled-coil formation exhibited by two coiled-coil dimers in the ~3 nm head-to-tail overlap region directly observed for lamins (for details see Ref. 13).
IF proteins form filaments, fibers and networks
The dynamic nature of IF proteins reflected in the assembly process is accompanied by extreme stability; IF filaments are notoriously insoluble under physiological conditions and therefore have to be solubilized with chaotropic agents (e.g., 8M urea or 6M guanidine-HCl) to employ them for in vitro assembly 4. In cells, IF structures retain this unparalleled resilience, and contribute considerably to mechanical stability. Generally, individual IF proteins can be renatured without the help of chaperones into soluble complexes (e.g., dimers, tetramers, octamers) by dialysis into low ionic strength buffers. In fact, assembly already starts during reconstitution of the urea-denatured molecules in the course of lowering the urea concentration. For example, monomeric vimentin denatured in 8M urea forms a coiled-coil dimer in 6 M urea, a tetramer in 5 M urea. Further dialysis into low ionic strength buffers preserves the tetrameric state5. In these tetramers, two dimers associate laterally by their coil 1 domains in an anti-parallel orientation, thereby yielding apolar, approximately 65-nm long rod-shaped particles with tapered ends. These so-called A11 tetramers have been clearly visualized by electron microscopy of rotary metal shadowed specimens5, and more recently by modeling the three-dimensional structure of a tetramer using the atomic structure of the vimentin coiled-coil dimer6.
In a subsequent assembly step, lateral association of tetramers leads to unit-length filaments (ULFs), or “mini-filaments”, of approximately 65 nm length5. These ULFs then further engage in an elongation reaction by longitudinal annealing of ULFs with one another and with already elongated filaments. In the center of the molecular mechanism is the “head-to-tail” association of the end domains of individual coiled coils (Figure 1D).
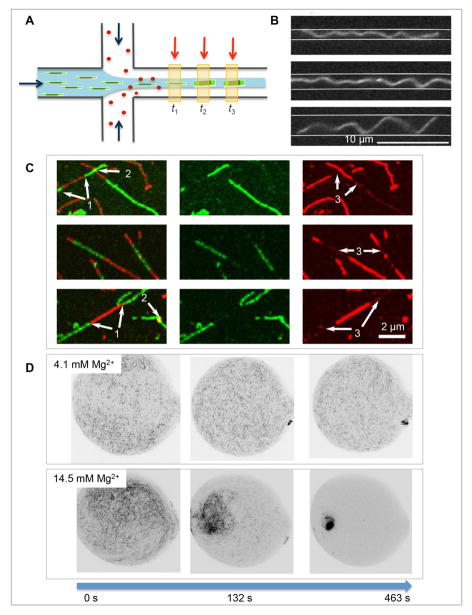
According to mass determination of individual ULFs and mature IFs by scanning transmission electron microscopy (STEM), IFs can be highly “polymorphic” with their mass-per-length (MPL) ranging between 20 and 60 kDa/nm along one and the same filament5. Indeed, this heterogeneity could potentially be of importance for the cell by providing a means to locally adjust the mechanical properties. This potential MPL heterogeneity of the ULFs has to be kept in mind when performing biophysical measurements, in particular when assembly is done in a “kick-start” mode rather than by a “slow” process such as dialysis that generally yields more uniform filaments5. In addition to electron microscopy (EM) and atomic force microscopy (AFM), more recently the very rapid association of tetramers to ULFs has also been monitored “in situ” using microfluidic methods, i.e. by employing a “protein-jet”7. Here, one takes advantage of the fact that on a small length scale diffusive mixing is fast and the continuous flow maps the temporal progression of the assembly reaction on a spatial axis. Such an experimental setup is depicted in Figure 2A, with the red arrows denoting different positions for measurements of the resulting protein complexes by x-ray scattering or fluorescence spectroscopy. After extended assembly times, long IFs are obtained that are about one order of magnitude more flexible than actin filaments and much more flexible than microtubules (Figure 2B).
Figure 2. Vimentin in vitro assembly.
A. Vimentin assembly in a diffusive mixing device. Assembly buffer injected from the two side channels are mixed diffusively with vimentin tetramers (green bars) injected from the main channel. As a result of the increased ionic strength, higher order complexes and eventually unit-length filaments form (green blocks at t2 and t3). The red arrows indicate examples for measurement positions, by e.g. x-ray scattering or fluorescence spectroscopy, which correspond to different time points in the assembly process (adapted from Ref. 7). B. Individual vimentin IF confined in microchannels. The width of the channels was 1.2 μm, 1.6 μm and 2.7 μm, top to bottom (adapted from Ref. 18). C. Subunit exchange along mature filaments. The overlay of the red and the green channel shows different modes of interaction between differently labeled subunits: 1 – end-to-end annealing; 2 – overlapping of filaments; 3 – exchanged subunits (adapted from Ref. 22). D. Impact of divalent ions on vimentin filament networks. Pre-assembled vimentin IFs were challenged with different concentrations of magnesium in microfluidic drops: above ~ 10 mM Mg2+ the networks aggregate strongly (adapted from Ref. 32).
In contrast to the cytoplasmic IF proteins, much less is known about the hierarchy of assembly steps that occur during the formation of nuclear lamin filaments, fibers and networks both in vitro and in vivo. For lamins, dimeric and not tetrameric complexes are obtained after renaturation both in low salt8 and high salt9,10 buffers. Starting from these two rather extreme in vitro assembly conditions, different structures are formed depending on the further assembly regime. In one scenario, several dimers first associate head-to-tail to a dimeric fiber of variable length8; in the next step, two such head-to-tail units associate laterally in a half-staggered, antiparallel manner into apolar tetrameric protofilaments. Subsequently, they further laterally associate into IF-like structures of generally heterogeneous diameter. Under most conditions lateral association does not come to a halt at this stage but continues. At the endpoint of assembly, large needles exhibiting regular banding patterns with an axial repeat of 48 to 49 nm and also referred to as paracrystalline fibers, are formed8,9,10. Lamin paracrystals were originally considered to be artificial structures, because relatively high concentrations of divalent cations were used to generate them in a very regular organizational state for high-resolution structural analyses10. However, paracrystals are the major assembly products when lamin A dimers are dialyzed from high salt conditions directly into physiological buffers11. These results correlate well with in vivo experiments employing the overexpression of A-type lamins in cultured cell systems, where at high cellular lamin expression large paracrystalline arrays were observed by electron microscopy both in the cytoplasm and the nucleus, probably because of lack in cellular chaperones and other organizing factors12. Hence, for lamins the formation of single filaments and the further association of filamentous precursors to paracrystalline fiber arrays are intimately coupled processes. In a somehow analogous manner, keratin IFs also further laterally associate into extensive fiber bundles under certain ionic conditions (see below).
Dissecting the molecular mechanism of the “head-to-tail” interaction
According to the assembly scenarios presented above, for both cytoplasmic and nuclear IFs the principal interaction for elongation is the head-to-tail association of dimers as shown by the use of “half-minilamins”13. These represent N- and C-terminal fragments of lamins with a truncated central α-helical rod domain. Accordingly, the IF consensus motifs residing at either end of the α-helical rod domain (see Figure 1A in blue), including conserved sequence motifs of the flanking non-α-helical end domains, mediate the basic elongation reaction of two coiled-coil dimers. As illustrated in Figure 1D, this interaction may engage the de novo formation of parallel coiled coils between the C-terminal end segments of one coiled coil (in cyan) with the N-terminal end segments of the connecting coiled coil (in magenta). Alternatively, a lateral type of interaction of these two structural modules may occur. In both cases, adjacent short segments of the head and tail domain are important, as in the case of lamins both of them harbor phosphorylation sites for the protein kinase CDK1. Phosphorylation of these sites at the onset of mitosis leads to an instantaneous disassembly of the lamina to the level of dimers. Since exactly the same amino acid rod end segments are conserved in all cytoplasmic IF proteins (see Fig. 1A), the same type of longitudinal association of dimeric coiled coils might mediate the head-to-tail interaction of the cytoplasmic IF as well (see also ref. 1). It appears indeed to be this head-to-tail interaction that specifies what makes a structural protein an IF protein, and the occurrence of these two motifs in newly found coiled-coil proteins, as revealed by genome sequencing of new species, may qualify the respective candidate as a potential IF protein14. Indeed, this criterion may serve as a suitable “test” for inclusion of proteins into the IF family, especially with respect to organisms outside the vertebrates.
Excitingly, it has recently been shown using single-molecule force spectroscopy by optical tweezers that the C-terminal end of a parallel dimer made from two coil 2 segments considerably resists pulling forces. This observation argues that this highly conserved part of all IF proteins provides a stable interaction module with a rather distinct structural design to allow proper longitudinal assembly by interacting with the amino-terminal end of coil 1A of a second dimer15. In stark contrast, the amino-terminal end domains of the coil 1A dimer have been demonstrated to be rather flexible indicating that their structural dynamics contribute to the “locking-in” reaction of the head-to-tail interaction16.
Mechanical properties and molecular architecture of single IFs
The persistence length of IF is in the range of a few hundred nm to a few μm17,18 classifying them - in a physical sense - as semiflexible biopolymers. Thus, eukaryotes are equipped with three filaments systems spanning nm to mm persistence lengths. Confinement in microchannels has been used to measure the equilibrium persistence length of freely fluctuating vimentin IFs in solution, i.e. unadsorbed (Figure 2B). Whereas the resulting bending rigidity is only one order of magnitude smaller than for actin filaments, the specific construction of IFs from laterally oriented fibrous molecules that strongly interact with each other results in the extreme extensibility of IFs, i.e. they are highly extensible, though possibly not in a reversible manner, which is not found for the other cytoskeletal filaments. Hence, experimentally, single IFs can be stretched by about 240%19.
Molecular dynamics simulations have shed some light on the mechanism enabling this extensibility, some of which has its origin in an α-helix-to-β-sheet transition20 accounting for about 100% extension. Hence, another and even larger contribution must come from “subunit gliding”, i.e. relative longitudinal movements of dimeric or tetrameric subunits against each other along the filament axis. As, the lateral association of tetramers provides a multitude of single molecular interactions, both ionic and hydrophobic, the resulting complex system of binding activities enables IFs to withstand strong forces, and, upon being stretched along the filament axis, to re-associate laterally when pulled apart because of the periodic type of longitudinal structural design of IF. As demonstrated by an AFM-based nanomechanical approach, laterally stretched desmin IFs exhibit a complex force-displacement curve showing a robust, strain stiffening-type behavior when extended above 50%21. Eventually, the “head-to-tail” associations of the individual continuous dimeric coiled-coil strands within an IF are physically broken at increasing force and the filaments are irreversibly torn apart.
Despite the notorious insolubility of IF subunits, IFs do exchange subunits over time, although at a rather slow rate, amounting to about 1 tetramers per hour along a 1-μm-long filament stretch corresponding to 1 in approximately 200 tetramers 22. Interestingly, the polymorphism of IF diameters impacts subunit exchange: evidently, polymorphic filaments are prone to a higher subunit exchange rate than filaments with a uniform diameter (Figure 2C). A possible reason for this difference is the fact that filaments with a non-uniform diameter harbor not just two but “many ends” (i.e. binding sites for subunits), thereby allowing subunit exchange all along the filament. However, the exchange scenario of IF in vivo differs significantly from the one encountered in vitro: in a cell disassembly is accelerated by phosphorylation reactions such that an entire IF network may disappear within minutes23.
In vitro IF “superstructures”
IF form bundles and networks
Like other filamentous biopolymers, IFs exhibit polymeric and polyelectrolyte properties24,25. The interplay between their intrinsic mechanical bending rigidity and interactions between the filaments leads to the formation of superstructures. Distinct bundling and cross-bridging proteins such as plectin link IFs to one-another and to actin filaments and microtubules26,27. Hence, within a cell the three principal cytoskeletal filament systems - actin filaments, intermediate filaments and microtubules - do not exist in “splendid isolation”. Rather, they form cell type-specific “composite filament networks” thereby combining different persistence lengths, extensibilities and surface charge densities. Hence, these composite filament networks are a major determinant of the mechanical properties of a cell and even more so of cell layers and tissues. Last but not least, as a consequence of the distinct surface charge patterns exhibited by IF protein monomers and polymers, even small cations such as Ca2+, Mg2+, Mn2+, Gd3+, mediate filament-filament interactions thereby mediating the formation of IF bundles and networks.
Structure of IF networks
Despite of their sequence homologies and similar monomer structures, different IF types yield distinct structures. For example, vimentin and desmin IFs form networks of small mesh size and high connectivity, with the deformation of these networks being affine. In contrast, keratin IFs form bundles or fibers, and eventually these bundles produce sparsely connected networks28,29. Rather than forming filament bundles, nuclear lamins assemble into filaments and paracrystalline fibers8,9,10. Moreover, IF proteins from these three groups do not form “hybrid” assemblies, i.e. vimentin-lamin, vimentin-keratin or lamin-keratin IFs, and hence have been grouped into three separate assembly groups30.
Direct imaging of filaments and higher-order structures is facilitated by fixing them in place. However, surface properties such as their charge may impact on the state of the filaments significantly. Hence, an alternative is, for example, encapsulation of the networks in microfluidic water-in-oil drops thereby taking advantage of the 3D-confinement, similar to the 3D confinement encountered in a cell. Furthermore, such a picoliter-sized environment can be tuned individually in terms of buffer, salt concentration and pH31,32, parameters that play a vital role in the cell. In Figure 2D examples of vimentin networks in the presence of different Mg2+ concentrations are shown: accordingly, high divalent salt concentrations lead to fast condensation of the filaments into dense aggregates.
Physical properties of the networks
Rheology experiments have yielded much valuable insight into the mechanical properties of IF networks, in particular concerning the response of vimentin IFs to increasing mechanical strain: they are less rigid at low strain but “harden” at high strain, a property called “strain-hardening” (or strain-stiffening33). Following up these pioneering experiments of Janmey and colleagues, divalent ions like Mg2+ and Ca2+ have been studied in particular in combination with vimentin34,35,36,32 keratin28,29,37,38, as well as neurofilaments39. Under these conditions the IFs become cross-linked thereby yielding filament networks that display rheological properties similar to those found for actin filaments cross-linked by actin binding proteins (ABPs). A recent study on keratin IFs demonstrated that interactions via charge patterns on their rod domains, which are even stronger than those mediated by hydrophobic patches on their tail domains, give rise to distinct filament networks38. Notably, the addition of low concentrations of non-ionic detergents to assembling keratin K8 and K18 impaired strain-stiffening indicating that hydrophobic interactions of the tail domains, which are supposed to reach out of the filament body, mediate strain-stiffening of the networks at low shear. Accordingly, tail-less variants of IF proteins such as desmin do not exhibit strain-stiffening neither in the presence nor in the absence of detergent40. The importance of the tail domains in filament cross-linking has also been shown for vimentin very impressively with variants of progressive deletion into the tail domain34. Moreover, strain-stiffening of vimentin IFs depends strongly on the ionic strength as at medium ionic strength (50 mM NaCl) no strain-stiffening is observed whereas at higher ionic strength (160 mM NaCl) a robust response is recorded, even though under both assembly conditions the same storage modulus is reached. This behavior is in sharp contrast to that of desmin IFs, which exhibit a nearly identical strain-stiffening response under both conditions (see Figure 6 in Ref. 17).
Cell mechanics
Whole cell experiments
In the cell, the situation is much more complex than in the test tube due to a largely unknown environment, which also makes it much more difficult to unambiguously dissect whole cell scenarios. Despite these challenges, it has been found important to approach the problem “bottom-up”, as described above, and “top-down” looking at cell experiments, and to eventually combine both complementary approaches.
To test the contribution of individual proteins to the deformability of cell components such as the nucleus or of whole cells, various methods have been employed: magnetic beads bound to surface receptors to pull on the cytoplasm, AFM cantilevers for indentation experiments, microaspiration with glass capillaries for nuclei, “cell stretching” of adherent cells on silicone membranes as well as of suspended cells by lasers, and microfluidic channels to “squeeze” whole cells41,42,43,44,45.
Keratinocyte mutants with the keratin network removed show a much higher deformability than the corresponding wild type cells, as consistently shown using optical stretchers46 (Figure 3A) and AFM47 (Figure 3B). Vimentin, as well, contributes to the cortical stiffness and therefore deformability of cells as shown by microrheology48.
Figure 3. In vivo IF mechanics.
A. Data from optical stretcher experiments show that wild type keratinocytes are less deformable than keratin knockout cells (J(t) shows creep deformation; from Ref. 46). B. Atomic force measurements; shown are stiffness maps of a live wild type keratinocyte (left) and a keratin knock-out cell (right); scale bar 10 μm (from Ref. 47). C. Pressurization of lobopodia by the “nuclear piston”. (Inset) Migration of primary human fibroblasts in a 3D extracellular matrix. (Main figure) Nesprin 3 connects the nucleus via plectin to intermediate filaments and actomyosin contractility. These connections help pull the nucleus forward to pressurize the forward cytoplasmic compartment and sustain high-pressure lobopodia-based 3D motility; from Ref. 52. D. Buckling event in a network of keratin bundles in a SW13 cell stably transfected with CFP-K8 and YFP-K18. (Left) two time frames, 2 s apart, showing the buckling event. (Right) marked ROI (region of interest) as shown on the left hand side55.
Cells on the move
Cultured fibroblasts are a classical object of cell biology to study cell locomotion. They constantly alter their shapes as they form both leading edge lamellipodia and trailing edge “tails” during locomotion on flat substrates. These shape transitions reflect changes in the viscoelastic and mechanical properties of the cells. Recent studies of resting versus moving fibroblasts have shown that upon stimulation of the cells by serum, vimentin filament arrays are decomposed in regions where lamellipodia form, yielding large numbers of IF “particles” as defined by fluorescence microsopy, i.e. probably both short IFs and ULFs. These latter structures are evident within the active ruffling membranes that are the hallmark of the advancing leading edge powered by the actin cytoskeleton49. These organizational changes in vimentin IFs appear to be caused by site-specific phosphorylation of vimentin. Furthermore, the microinjection of a vimentin coil 2 peptide, shown to depolymerize mature IFs into non-IF structures in vitro50, also induces the rapid disassembly of vimentin IFs in live cells and the formation of lamellipodia. Hence, vimentin IFs and cell locomotion are coupled in an interdependent regulatory network. Accordingly, vimentin IFs play an important role during the epithelial to mesenchymal transition (EMT). Epithelial cells typically exhibit complex IF networks of keratins but no vimentin. The expression of vimentin in epithelial cells results in their rapid transition to a mesenchymal shape combined with a significant increase in cell motility and focal adhesion dynamics51.
In this context, a new twist in the concept of how cells move confined in three-dimensional matrices has revealed that vimentin filaments cooperate with the actomyosin-system to establish a pressure gradient within the moving cell. In this process, the IF-stabilized nucleus serves as a piston to push cells through the matrix via lobopodium formation (Figure 3C). Here, already single IFs and not their networks may indeed serve as mechanical stress-absorbers52.
Networks and bundles within cells
Interestingly, the absence of vimentin in fibroblasts does not influence the generation of intracellular forces as determined by force spectrum microscopy 53. These novel results suggest that vimentin IFs have little influence on intracellular force generation, despite their –at the molecular level still only vaguely defined - role in cell mechanics. Different from vimentin, keratins form thick bundles both in vitro and in cells. These bundles display considerable dynamics, which are myosin motor dependent, confirming the strong linkage to the actin cytoskeleton. When the cells are exposed to external shear forces, the dynamics decrease, hinting at a “protection mechanism” of the cells against potentially harmful forces54. Looking more carefully at the bundles, which are on a higher hierarchical level organized in a network of bundles, more carefully, they show frequent short wavelength buckling events and much stronger bending than the high persistence length of the bundles would predict55 (Figure 3D, E). One interpretation of this phenomenon is a cellular response to compression, another possibility is that the actomyosin network pulls locally on keratin bundles via plectin linkages.
Conclusions
In most metazoan cells, the three cytoskeletal filament systems form networks that are linked to one another by so-called cytolinker proteins, thereby allowing “cross-talk”. This cross-talk, in turn, provides the link that is necessary to couple cell mechanics, in particular cell stiffness, deformation and stability, to intracellular transport and cell locomotion. Thus, we would like to stress the nanocomposite nature of the cytoskeleton as a whole. This sophisticated architecture allows the cell to fine tune its mechanical properties in a dynamic and local way to support, movement, cell division, organelle placement and cellular integration into tissues. Since IFs are apolar structures, they are probably not directly involved in intracellular transport and cell locomotion. However, for both cells and tissues they are a primary determinant of stiffness and deformation, and these properties together with cell motility are very important for basic processes such as cell invasion and hence metastasis. Moreover, the IF network provides intracellular stabilization connecting cell adhesion structures with organelles such as mitochondria and the nucleus. IFs can withstand rather large strains, thereby helping cells to respond locally to mechanical insults that otherwise would lead to significant deformations. Last but not least, their contribution to manage mechanical stress at the tissue and organ level is obvious from the deleterious effects of human IF disease mutations, resulting in one of the many so-called IF-pathies56.
Acknowledgments
S.K. thanks the German Research Foundation (DFG, KO/3752/5-1 and SFB 755 B07 and C10). H.H. received support from the German Research Foundation (DFG, HE/1853, FOR1228 and 1853/11-1) and from COST. R.D.G. was supported by NIH PO1GM096971 and Hannah’s Hope Fund. D.A.W. acknowledges support from the NIH (PO1GM096971), the Harvard Materials Research Science and Engineering Center (DMR-0820484), and the NSF (DMR-1310266).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Parry DAD, Steinert M. Intermediate filaments: molecular architecture, assembly, dynamics and polymorphism. Quart Rev Biophys. 1999;32:99–187. doi: 10.1017/s0033583500003516. [DOI] [PubMed] [Google Scholar]
- 2.Erber A, Riemer D, Hofemeister H, Bovenschulte M, Stick R, Panopoulou G, Lehrach H, Weber K. Characterization of the Hydra lamin and its gene: A molecular phylogeny of metazoan lamins. J Mol Evol. 1999;49:260–271. doi: 10.1007/pl00006548. [DOI] [PubMed] [Google Scholar]
- 3.Herrmann H, Aebi U. Intermediate Filaments: Molecular Structure, Assembly Mechanism, and Integration Into Functionally Distinct Intracellular Scaffolds. Ann Rev Biochem. 2004;73:749–789. doi: 10.1146/annurev.biochem.73.011303.073823. [DOI] [PubMed] [Google Scholar]
- 4.Herrmann H, Kreplak L, Aebi U. Isolation, characterization, and in vitro assembly of intermediate filaments. Methods Cell Biol. 2004;78:3–24. doi: 10.1016/s0091-679x(04)78001-2. [DOI] [PubMed] [Google Scholar]
- 5.Herrmann H, Häner M, Brettel M, Müller SA, Goldie KN, Fedtke B, Lustig A, Franke WW, Aebi U. Structure and Assembly Properties of the Intermediate Filament Protein Vimentin: The Role of its Head, Rod and Tail Domains. J Mol Biol. 1996;264:933–953. doi: 10.1006/jmbi.1996.0688. [DOI] [PubMed] [Google Scholar]
- 6.Chernyatina AA, Nicolet S, Aebi U, Herrmann H, Strelkov SV. Atomic structure of the vimentin central α-helical domain and its implications for intermediate filament assembly. Proc Natl Acad Sci USA. 2012;109:13620–13625. doi: 10.1073/pnas.1206836109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brennich ME, Nolting JF, Dammann C, Nöding B, Bauch S, Herrmann H, Pfohl T, Köster S. Dynamics of intermediate filament assembly followed in micro-flow by small angle X-ray scattering. Lab Chip. 2011;11:708–716. doi: 10.1039/c0lc00319k. [DOI] [PubMed] [Google Scholar]
- 8.Aebi U, Cohn J, Buhle L, Gerace L. The nuclear lamina is a meshwork of intermediate-type filaments. Nature. 1986;323:560–564. doi: 10.1038/323560a0. [DOI] [PubMed] [Google Scholar]
- 9.Heitlinger E, Peter M, Häner M, Lustig A, Aebi U, Nigg EA. Expression of chicken lamin B2 in Escherichia coli: characterization of its structure, assembly, and molecular interactions. J Cell Biol. 1991;113:485–495. doi: 10.1083/jcb.113.3.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heitlinger E, Peter M, Lustig A, Villiger W, Nigg EA, Aebi U. The role of the head and tail domain in lamin structure and assembly: analysis of bacterially expressed chicken lamin A and truncated B2 lamins. J Struct Biol. 1992;108:74–89. doi: 10.1016/1047-8477(92)90009-y. [DOI] [PubMed] [Google Scholar]
- 11.Zwerger M, Jaalouk DE, Lombardi ML, Isermann P, Mauermann M, Dialynas G, Herrmann H, Wallrath LL, Lammerding J. Myopathic lamin mutations impair nuclear stability in cells and tissue and disrupt nucleo-cytoskeletal coupling. Hum Mol Genet. 2013;22:2335–2349. doi: 10.1093/hmg/ddt079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klapper M, Exner K, Kempf A, Gehrig C, Stuurman N, Fisher PA, Krohne G. Assembly of A- and B-type lamins studied in vivo with the baculovirus system. J Cell Sci. 1997;110:2519–2532. doi: 10.1242/jcs.110.20.2519. [DOI] [PubMed] [Google Scholar]
- 13.Kapinos LE, Schumacher J, Mücke N, Machaidze G, Burkhard P, Aebi U, Strelkov SV, Herrmann H. Characterization of the Head-to-Tail Overlap Complexes Formed by Human Lamin A, B1 and B2 “Half-minilamin” Dimers. J Mol Biol. 2010;3:719–731. doi: 10.1016/j.jmb.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 14.Herrmann H, Strelkov S. History and phylogeny of intermediate filaments: Now in insects. BMC Biology. 2011;9:16–20. doi: 10.1186/1741-7007-9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramm B, Stigler J, Hinczewski M, Thirumalai D, Herrmann H, Woehlke G, Rief M. Sequence-resolved free energy profiles of stress-bearing vimentin intermediate filaments. Proc Natl Acad Sci USA. 2014;111:11359–11364. doi: 10.1073/pnas.1403122111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meier M, Padilla GP, Herrmann H, Wedig T, Hergt M, Patel TR, Stetefeld J, Aebi U, Burkhard P. Vimentin Coil 1A—A Molecular Switch Involved in the Initiation of Filament Elongation. J Mol Biol. 2009;390:245–261. doi: 10.1016/j.jmb.2009.04.067. [DOI] [PubMed] [Google Scholar]
- 17.Schopferer M, Bär H, Hochstein B, Sharma S, Mücke N, Herrmann H, Willenbacher N. Desmin and Vimentin Intermediate Filament Networks: Their Viscoelastic Properties Investigated by Mechanical Rheometry. J Mol Biol. 2009;388:133–143. doi: 10.1016/j.jmb.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 18.Nöding B, Köster S. Intermediate Filaments in Small Configuration Spaces. Phys Rev Lett. 2012;108:088101. doi: 10.1103/PhysRevLett.108.088101. [DOI] [PubMed] [Google Scholar]
- 19.Kreplak L, Bär H, Leterrier JF, Herrmann H, Aebi U. Exploring the Mechanical Behavior of Single Intermediate Filaments. J Mol Biol. 2005;354:569–577. doi: 10.1016/j.jmb.2005.09.092. [DOI] [PubMed] [Google Scholar]
- 20.Qin Z, Kreplak L, Buehler MJ. Nanomechanical properties of vimentin intermediate filament dimers. Nanotechnology. 2009;20:425101. doi: 10.1088/0957-4484/20/42/425101. [DOI] [PubMed] [Google Scholar]
- 21.Kreplak L, Herrmann H, Aebi U. Tensile Properties of Single Desmin Intermediate Filaments. Biophys J. 2008;94:2790–2799. doi: 10.1529/biophysj.107.119826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nöding B, Herrmann H, Köster S. Direct Observation of Subunit Exchange Along Mature Vimentin Intermediate Filaments. Biophys J. 2014 doi: 10.1016/j.bpj.2014.09.050. accepted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ram KS, Inagaki M, Yamaguchi T, Shea TB, Pant HC. Role of phosphorylation on the structural dynamics and function of types III and IV intermediate filaments. Exp Cell Res. 2007;313:2089–2109. doi: 10.1016/j.yexcr.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••24.Janmey P, Slochower DR, Wang YH, Wen Q, Cebers A. Polyelectrolyte properties of filamentous biopolymers and their consequences in biological fluids. Soft Matter. 2014;10:1439–1339. doi: 10.1039/c3sm50854d. The authors review polyelectrolyte properties of different biological polymers, including intermediate filaments. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••25.Wen Q, Janmey P. Polymer Physics of the Cytoskeleton. Curr Opin Solid State Mater Sci. 2011;15:177–182. doi: 10.1016/j.cossms.2011.05.002. Concepts from polymer physics are applied to cytoskeletal filaments to describe the mechanical properties of these “active materials”. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herrmann H, Bär H, Kreplak L, Strelkov SV, Aebi U. Intermediate filaments: from cell architecture to nanomechanics. Nat Rev Mol Cell Biol. 2007;8:562–573. doi: 10.1038/nrm2197. [DOI] [PubMed] [Google Scholar]
- 27.Wiche G, Osmanagic-Myers S, Castañón MJ. Networking and anchoring through plectin: a key to IF functionality and mechanotransduction. Curr Opin Cell Biol. 32:21–29. doi: 10.1016/j.ceb.2014.10.002. [DOI] [PubMed] [Google Scholar]
- ••28.Kayer J, Grabmayr H, Harasim M, Herrmann H, Bausch AR. Assembly kinetics determine the structure of keratin networks. Soft Matter. 2012;8:8873–8879. The authors show that the interplay between keratin filament elongation and and bundeling determine the large variety of emerging network and bundle structures. [Google Scholar]
- ••29.Kayser J, Haslbeck M, Dempfle L, Krause M, Grashoff C, Buchner J, Herrmann H, Bausch AR. The Small Heat Shock Protein Hsp27 Affects Assembly Dynamics and Structure of Keratin Intermediate Filament Networks. Biophy J. 2013;105:1778–1785. doi: 10.1016/j.bpj.2013.09.007. The authors demonstrate that small heat-shock proteins prominently determine keratin network properties. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herrmann H, Aebi U. Intermediate filaments and their associates: multi-talented structural elements specifying cytoarchitecture and cytodynamics. Curr Opi Cell Biol. 2000;12:79–90. doi: 10.1016/s0955-0674(99)00060-5. [DOI] [PubMed] [Google Scholar]
- 31.Dammann C, Nöding B, Köster S. Vimentin networks at tunable ion-concentration in microfluidic drops. Biomicrofluidics. 2013;6:02009. doi: 10.1063/1.4705103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dammann C, Köster S. Dynamics of counterion-induced attraction between vimentin filaments followed in microfluidic drops. Lab Chip. 2014;14:2681–2687. doi: 10.1039/c3lc51418h. [DOI] [PubMed] [Google Scholar]
- 33.Janmey PA, Euteneuer U, Traub P, Schliwa M. Viscoelastic properties of vimentin compared with other filamentous biopolymer networks. J Cell Biol. 1991;113:155–160. doi: 10.1083/jcb.113.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin YC, Broedersz CP, Rowat AC, Wedig T, Herrmann H, MacKintish FC, Weitz DA. Divalent Cations Crosslink Vimentin Intermediate Filament Tail Domains to Regulate Network Mechanics. J Mol Biol. 2010;4:673–644. doi: 10.1016/j.jmb.2010.04.054. [DOI] [PubMed] [Google Scholar]
- 35.Lin YC, Yao NY, Briederesz CP, Herrmann H, MacKintosh FC, Weitz DA. Origins of Elasticity in Intermediate Filament Networks. Phys Rev Lett. 2010;104:058101. doi: 10.1103/PhysRevLett.104.058101. [DOI] [PubMed] [Google Scholar]
- 36.Köster S, Lin YC, Herrmann H, Weitz DA. Origins of Elasticity in Intermediate Filament Networks. Soft Matter. 2010;9:1910–1914. doi: 10.1103/PhysRevLett.104.058101. [DOI] [PubMed] [Google Scholar]
- ••37.Pawelzyk P, Herrmann H, Willenbacher N. Mechanics of intermediate filament networks assembled from keratins K8 and K18. Soft Matter. 2013;9:8871–8880. [Google Scholar]
- ••38.Pawelzyk P, Mücke N, Herrmann H, Willenbacher N. Attractive Interactions among Intermediate Filaments Determine Network Mechanics In Vitro. PlosONE. 2014 doi: 10.1371/journal.pone.0093194. The auhors use rheology to show that the tails of keratins, and in particular their hydrophobic properties, determine the strain-stiffening behavior. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yao NY, Broederesz CO, Lin YC, Kasza KE, MacIntosh FC, Weitz DA. Elasticity in Ionically Cross-Linked Neurofilament Networks. Biophys J. 2010;98:2147–2153. doi: 10.1016/j.bpj.2010.01.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bär H, Schopferer M, Sharma S, Hochstein B, Mücke N, Herrmann H, Willenbacher N. Mutations in Desmin’s Carboxy-Terminal “Tail” Domain Severely Modify Filament and Network Mechanics. J Mol Biol. 2010;5:1188–1198. doi: 10.1016/j.jmb.2010.02.024. [DOI] [PubMed] [Google Scholar]
- 41.Rowat A, Jaalouk DE, Zwerger M, Ung WL, Eydelnant IA, Olins DE, Olins AL, Herrmann H, Weitz DA, Lammerding J. Nuclear Envelope Composition Determines the Ability of Neutrophil-type Cells to Passage through Micron-scale Constrictions. J Biol Chem. 2013;288:8610–8618. doi: 10.1074/jbc.M112.441535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••42.Swift J, Ivanovska IL, Buxboim A, Harada T, Dingal PCDP, Pinter J, Pajerowski JD, Spinler KR, Shin J-W, Tewari M, Rehfeldt F, Speicher DW, Discher DE. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science. 2013;341:1240104. doi: 10.1126/science.1240104. http://dx.doi.org/10.1126/science.1240104. The authors demonstrate by quantitative mass spectrometry that the expression of lamin A relative to lamin B in cells scales linearly with the elastic modulus of the substrate. High relative lamin A levels protect the nucleus from external mechanical impact thereby limiting DNA breaks. This work convincingly demonstrates the interconnection between mechanosensing and genetics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••43.Harada T, Swift J, Irianto J, Shin J-W, Spinler KR, Athirasala A, Diegmiller R, Dingal PCDP, Ivanovska IL, Discher DE. Nuclear lamin stiffness is a barrier to 3D migration, but softness can limit survival. J Cell Biol. 2014;204:669–682. doi: 10.1083/jcb.201308029. The authors show how the nuclear laminar hinders migration of cells through 3D tissues and protects the DNA in the nucleus. The limiting factor is the lamin A content in the nuclear lamina which varies between cell types. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lu YB, Iandiev I, Hollborn M, Körber N, Ulbricht E, Hirrlinger PG, Pannicke T, Wei EQ, Bringmann A, Wolburg H, Wilhelmsson U, Pekny M, Wiedemann P, Reichenbach A, Käs JA. Reactive glial cells: increased stiffness correlates with increased intermediate filament expression. FASEB J. 2011;25:624–631. doi: 10.1096/fj.10-163790. [DOI] [PubMed] [Google Scholar]
- 45.Plodinec M, Loparic M, Suetterlin R, Herrmann H, Aebi U, Schoenenberger CA. Interfering with the vimentin intermediate filament system modulates the nanomechanical properties of rat fibroblasts. J Struct Biol. 2011;174:476–484. doi: 10.1016/j.jsb.2011.03.011. [DOI] [PubMed] [Google Scholar]
- ••46.Seltmann K, Fritsch A, Käs J, Magin TM. Keratins significantly contribute to cell stiffness and impact invasive behavior. Proc Natl Acad Sci USA. 2013;110:18507–18512. doi: 10.1073/pnas.1310493110. Using an automated microfluidic optical stretcher, the authors compare the mechanical propeties of wild type keratinocytes and the corresponding keratin knockouts cells demonstrating a much higher deformability in the gene-targeted cells. Moreover, keratin-free cells exhibit a significantly higher invasiveness and grow much faster and in a kind of “disordered” manner in Matrigel. Hence, the authors hypothesize that loss of the keratin system promotes the establishment of an EMT-like condition in murine keratinocytes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••47.Ramms L, Fabris G, Windoffer R, Schwarz N, Springer R, Zhou C, Lazar J, Stiefel S, Hersch, Schnakenberg U, Magin TM, Leube RE, Merkel R, Hoffmann B. Keratins as the main component for the mechanical integrity of keratinocytes. Proc Natl Acad Sci USA. 2013;110:18513–18518. doi: 10.1073/pnas.1313491110. Keratinocytes with and without keratins are compared by AFM indentation and magnetic tweezer experiments and the authors show a softening of the keratin-free cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••48.Guo M, Ehrlicher AJ, Mahammad S, Fabich H, Jensen MH, Moore JR, Fredberg JJ, Goldman RD, Weitz DA. The Role of Vimentin Intermediate Filaments in Cortical and Cytoplasmic Mechanics. Biophys J. 2013;105:1562–1568. doi: 10.1016/j.bpj.2013.08.037. The contribution of vimentin IFs to cell mechanics is studied on wild type fibroblasts and corresponding vimentin knockouts. The author demonstrate that whereas the cortical stiffness is only little affected, the cytoplasmic mechanics are strongly influenced. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Helfand BT, Mendez MG, Murthy SNP, Shumakera DK, Grin B, Mahammad S, Aebi U, Wedig T, Wue YI, Hahn KM, Inagaki M, Herrmann H, Goldman RD. Vimentin organization modulates the formation of lamellipodia. Mol Biol Cell. 2011;15:1274–1289. doi: 10.1091/mbc.E10-08-0699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Strelkov SV, Herrmann H, Geisler N, Wedig T, Zimbelmann R, Aebi U, Burkhard P. Conserved segments 1A and 2B of the intermediate filament dimer: their atomic structures and role in filament assembly. EMBO. 2002;21:1255–1266. doi: 10.1093/emboj/21.6.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mendez M, Kojima SI, Goldman RD. Vimentin induces changes in cell shape, motility, and adhesion during the epithelial to mesenchymal transition. FASEB. 2010;24:1838–1851. doi: 10.1096/fj.09-151639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••52.Petrie RJ, Koo H, Yamada KM. Generation of compartmentalized pressure by a nuclear piston governs cell motility in a 3D matrix. Science. 2014;345:1062–1065. doi: 10.1126/science.1256965. With the help of nesprin-3, the cell is compartementalized into a front and a rear part and high pressures can be built up, which drive lamellipodia-independent 3D cell migration. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••53.Guo M, Ehrlicher AJ, Jensen MH, Renz M, Moore JR, Goldman RD, Lippincott-Schwartz J, MacKintosh FC, Weitz DA. Probing the stochastic, motor-driven properties of the cytoplasm using force spectrum microscopy. Cell. 2014;158:822–832. doi: 10.1016/j.cell.2014.06.051. Using force spectrum microscopy (FSM), these authors demonstrate that vimentin IFs contribute mainly to the internal structural mechanics of the cell. They suggest that a major function of vimentin IFs may rely in the anchorage of organelles against fluctuating forces in the cytoplasm as generated by motor protein activities. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nolting JF, Köster S. Influence of microfluidic shear on keratin networks in living cells. New J Phys. 2013;15 :045025. [Google Scholar]
- 55.Nolting JF, Möbius W, Köster S. Mechanics of Individual Keratin Bundles in Living Cells. Biophys J. 2014;107:2693–2699. doi: 10.1016/j.bpj.2014.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Omary MB. “IF-pathies”: a broad spectrum of intermediate filament–associated diseases. J Clin Invest. 2009;119:1756–1762. doi: 10.1172/JCI39894. [DOI] [PMC free article] [PubMed] [Google Scholar]