Abstract
Objective
To determine the lifetime rate and distribution of supportive academic and educational services provided to children with new/recent onset epilepsy and typically-developing controls, the relationship of this history to objective academic test performance, and the course of performance over serial evaluations (baseline, 2 and 5 years later).
Methods
Research participants were 91 children aged 8-18 at study entry, including 50 youth with recent-onset epilepsy (28 Focal [FE] and 22 Generalized [GE] epilepsy) and healthy first-degree cousin controls (n=41). Epilepsy sample included children with uncomplicated epilepsy, normal imaging and development. Lifetime history of a diversity of supportive educational services was determined via structured interview with parents at the baseline study visit. Associations were examined between these support services and participants' academic performance in reading, spelling, and arithmetic (Wide Range Achievement Test Rev. 3 [WRAT3][1]) during three serial study visits including baseline, 2 and 5-years later.
Results
Children with epilepsy had a higher lifetime rate of provision of diverse academic supportive services compared to controls at the baseline visit (52% vs. 18%). These services antedated epilepsy diagnosis in the majority (80.8%) of the children with epilepsy. Among children with epilepsy, children who presented with academic services had significantly lower WRAT3 reading, spelling, and arithmetic performance at baseline, 2 and 5-year follow-up.
Conclusion
A brief structured clinical interview conducted with parents identifies children with epilepsy who are at academic risk at the time of diagnosis with that risk persisting up to 5-years later.
1. Introduction
Academic struggles and clinically significant academic underachievement are known complications of the childhood epilepsies [2-4]. These are critical issues as they may contribute to subsequent adverse impacts on career trajectories, income, and socioeconomic status—long term complications of childhood onset epilepsy that have been reported by many investigators [5]. These issues are not an unanticipated association with severe and intractable epilepsies, but in children with “epilepsy only” without comorbid neurological disease, who have average intelligence and are attending regular classes, ongoing academic difficulties are often unrecognized [6].
There are differing views of the natural history of academic problems in childhood epilepsy. Some authors suggest that significant academic problems are not evident at or near the time of diagnosis, but tend to worsen over time [2, 7], while others contend that the academic careers of children with new onset epilepsy are already at risk at the time of diagnosis [8]. These differing views may be attributable to the varying nature of the populations studied, and the methods used to assess and define academic performance.
From a practical standpoint, it is difficult to determine how best to screen efficiently for potential cognitive and academic problems in the clinic setting. In the current United States healthcare environment, there are limitations regarding referral for cognitive and academic assessments and when and how often testing may be repeated. A quick, efficient, informed and validated system that could be used in the clinic to identify those children most in need of and likely to benefit from assessment would be useful. In this study we performed a brief structured interview with parents that inquired about their concerns regarding their child's academic performance focusing on the concrete steps that they, or the school, had taken to address the academic concerns. In order to characterize the prospective academic trajectories of the children, this history was examined in the context of traditional objective measures of word reading, spelling and arithmetic computation at the time of the baseline parent interview, and also longitudinally at 2 and 5-year follow-up visits. By examining children with new and recent onset epilepsy, we were able to address the natural history of these relationships and the specific contribution of epilepsy related factors. We hypothesize that children with epilepsy and a history of parent reported academic problems and services for academic struggles at baseline will have significantly lower objective academic performance over time when compared to children with epilepsy without academic problems or services. Further, children with epilepsy and no history of academic problems and services will be comparable to controls in academic performance at baseline, 2 and 5-year follow-up assessments.
2. Methods
2.1 Participants
Research participants consisted of 91 youth aged 8-18 at baseline, including 50 with new and recent-onset epilepsy and 41 healthy first-degree cousin controls (Table 1). All participants attended regular schools at baseline.
Table 1. Sample Demographics.
| Group | |||
|---|---|---|---|
|
|
|||
| Control | Epi_AP- | Epi_AP+ | |
| Group N | 41 | 24 | 26 |
| Age in years | 12.51 (3.0) | 12.6 (3.0) | 11.87 (3.36) |
| Gender (Male/Female) | 17 (41.5%)/24 (58.5%) | 10 (41.7%)/14 (58.3%) | 16 (61.5%)/10 (38.5%) |
| Grade | 6.42 (2.92) | 6.5 (3.2) | 5.73 (3.38) |
| Full Scale IQ* | 112.32 (8.79) | 110.92 (11.64) | 97.42 (10.24) |
| Epilepsy Syndrome (FE/GE)** | ---- | 13/11 | 15/11 |
| Age of onset in years | ---- | 11.8 (3.04) | 10.89 (3.58) |
| AED (0 / ≥ 1) | |||
| Baseline | ---- | 6/18 | 3/23 |
| 2-year follow-up | ---- | 12/12 | 7/19 |
| 5-year follow-up | ---- | 14/10 | 14/12 |
| Epilepsy Remission (No, Yes) | |||
| 2-Year Follow-up | 12, 12 | 24, 2 | |
| 5-Year Follow-up | 10, 14 | 13, 11*** | |
Note.
p<0.001
Epilepsy Syndromes: Epi_AP-: FE (7-CECTS, 4-TLE, 1-FE NOS, 1-FLE), GE (4-Absence, 7-JME); Epi_AP+: FE (6-CECTS, 1-COE, 3-TLE, 2-FE NOS, 3-FLE), GE (4-Absence, 7-JME)
Epilepsy remission status could not be determined for 2 participants
All participants completed three waves of assessment including baseline, 2 and 5-year follow-ups. At baseline, all participants attended regular schools. Children with epilepsy were recruited from pediatric neurology clinics at three Midwestern medical centers (University of Wisconsin-Madison, Marshfield Clinic, Dean Clinic) and met the following inclusion criteria: (i) diagnosis of epilepsy within the past 12 months; (ii) no other developmental disabilities (e.g. intellectual impairment, autism); (iii) no other neurological disorder, and (iv) normal clinical MRI. Children entered the study with active epilepsy diagnosed by their treating pediatric neurologists and confirmed by medical record review of the research study pediatric neurologist. We did not exclude children on the basis of psychiatric comorbidities (including ADHD) or learning disabilities. We did however exclude children with intellectual disability (IQ <70), autism, and/or other neurological disorders. Specifics regarding the participant selection process have been described in detail in previous publications [9]. In general, we tried to stay true to the concept of “epilepsy only” as defined broadly in the literature: normal neurological exams, average intelligence, and attendance at regular schools.
Each child's epilepsy syndrome was defined in a research consensus meeting by the research pediatric neurologist who reviewed all available clinical data (e.g., seizure description and phenomenology, EEG, clinical imaging, neurodevelopmental history) while blinded to all research cognitive, behavioral, and neuroimaging data. Two levels of epilepsy syndrome classification were undertaken and confirmed by a board-certified pediatric neurologist who was blinded to all research data. Children with epilepsy were first classified into broad syndrome groups including generalized (GE) and focal (FE) epilepsy, followed by classification into specific GE (juvenile myoclonic epilepsy [JME], childhood and juvenile absence [Absence], and GE not otherwise specified [GE NOS]) and FE (childhood epilepsy with centrotemporal spikes [CECTS], temporal lobe epilepsy [TLE], childhood occipital epilepsy [COE], frontal lobe epilepsy [FLE], and FE not otherwise specified [FE NOS]). Syndrome data and a summary of antiepileptic treatment for all epilepsy participants are provided in Table 1.
First-degree cousins were used as controls and exclusion criteria were as follows: (i) history of any initial precipitating insult (e.g. simple or complex febrile seizures, cerebral infections, perinatal stroke); (ii) any seizure or seizure-like episode; (iii) diagnosed neurological disease; (iv) loss of consciousness greater than 5 min; (v) other family history of a first-degree relative with epilepsy or febrile convulsions. For the current analysis we also excluded control participants who received special services in school (n=9). We used cousin controls rather than siblings or other potential control groups for the following reasons: (i) first-degree cousins are more genetically distant from the participants with epilepsy and thus less pre-disposed than siblings to shared genetic factors that may contribute to anomalies in brain structure and cognition; (ii) a greater number of first-degree cousins are available than siblings in the target age range and (iii) the family link was anticipated to facilitate participant recruitment and especially retention over time (which is our intent) compared to more general control populations (e.g. unrelated school mates).
This study was reviewed and approved by the Institutional Review Boards of all participating institutions. On the day of study participation, families and children gave informed consent and assent. All procedures were consistent with the Declaration of Helsinki [10].
2.2 Procedures
Participating children completed three study visits: baseline, 2 and 5-years following the baseline visit. Each participant completed a comprehensive battery of neuropsychological tests, questionnaires, clinical interview, structured psychiatric interview (Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children [K-SADS] [11]), and MRI. At the baseline visit each participating child was accompanied by a parent who underwent a clinical interview, completed a brief intelligence test (Wechsler Abbreviated Scale of Intelligence [WASI] [12]) and completed questionnaires characterizing the child's gestation, delivery, neurodevelopment, academics, and seizure history. At subsequent follow-up visits, only participants who were under the age of 21 years were accompanied by a parent. All pertinent medical records were obtained after signed release of information was obtained from the parent or the participant (if over the age 18 years).
Information regarding each child's academic course was obtained at the baseline, 2, and 5-year follow-up visits through a structured interview with the participating parent. During the baseline interview, to date lifetime academic service history was obtained, while at 2 and 5-year follow-up visits information was obtained only regarding “new” school related services (Baseline interview in Supplemental File 1). Of particular interest were any steps taken to date by the parent or school to address perceived concerns regarding academic problems exhibited by the child. These included history of participation in any of the following: formal Individualized Education Plan [IEP], birth-to-age 3 services, early childhood programs, grade retention, remedial summer school attendance, parent arranged or school-based tutors, and learning centers (i.e. Sylvan Learning). Provision of any of these services was considered to reflect the presence of academic problems (AP). Participating children with epilepsy were grouped based on their history of special services at baseline: children with epilepsy who had previously received and(or) were currently receiving special services for academic problems (Epi_AP+), and those who had never received any special services (Epi_AP-). Due to the limited sample size of control participants with a history of academic services at baseline (n=9), these participants were not included in the current sample. We also excluded children who did not present with services at baseline, but added services at either 2 or 5-year follow-up (2 controls, 5 children with epilepsy). Each child was administered the Wide Range Achievement Test–Revision 3 (WRAT3) [1] at the baseline, 2 and 5-year follow-up visits. Children completed the blue form version of WRAT3 reading (pronouncing out of context words), spelling (writing words to dictation), and arithmetic (performing written computations). The time from epilepsy diagnosis to baseline WRAT3 administration was 8 months (Epi_AP-, 8.4 months; Epi_AP+, 7.6 months).
2.3 Data Analyses
Analyses were conducted using IBM SPSS Statistics Software 22.0. The initial focus of the analyses was to examine history of support services across three groups (Epi_AP+, Epi_AP-, and controls) using MANOVA and follow-up ANOVA with post-hoc Tukey's Tests to adjust for multiple pair-wise comparisons. Using this analytic approach a total of 3 MANOVAs were computed (for reading, spelling, and arithmetic performance) with an adjusted overall p-value (.05/8 or 0.006) for each test.
3. Results
All groups had IQ scores (WASI) in the average range (Table 1). Academic problems (AP) were more frequent in children with epilepsy (52%) compared to controls (18%), χ2(1, N=100)=12.7, p<0.001. Majority of children with epilepsy who had academic problems were receiving services prior to their diagnosis with epilepsy (80.8%). Differences between groups (Control, Epi_AP-, and Epi_AP+) on academic achievement in reading, spelling, and arithmetic were evaluated by examining WRAT3 scores at baseline, 2 and 5-year follow-up visits. A summary of type of services received by children with epilepsy is provided in Table 2.
Table 2. Education Service Summary in Children with Epilepsy.
| Education Services | N=26* |
|---|---|
| IEP | 13 (50.0%) |
| Birth-Age 3 | 7 (26.9%) |
| Early Childhood | 8 (30.8%) |
| Special School Services | 14 (53.8%) |
| Tutor | 12 (46.2%) |
| Required Summer School | 10 (38.5%) |
| Repeat Grade | 1 (3.8%) |
Note.
65.4% of children with academic services had more than 1 service.
For more information on type of services see Supplemental File I: Academic Problems Screening Interview.
3.1 Primary MANOVA Analyses
WRAT3 Reading
MANOVA for scaled reading scores at baseline, 2 and 5-year follow-up visits by academic service groups yielded a significant overall main effect, F(6,170) = 6.3, p<0.001, partial η2 = 0.18. Significant group differences were found at baseline, F(2,87) = 14.96, p<0.001, 2-year follow-up visit, F(2,87) = 18.1, p<0.001, and 5-year follow-up visit, F(2,87) = 14.94, p<0.001. Group means are illustrated in Figure 1. Post-hoc analyses revealed that Epi_AP+ group had significantly lower scaled reading scores compared to Epi_AP- and controls at all three study visits, p<0.001. No significant group differences were found between Epi_AP- and the control group.
Figure 1.
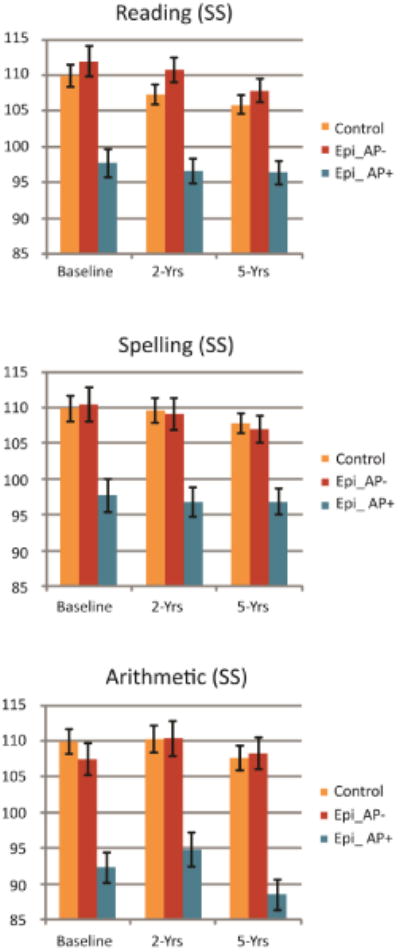
WRAT3 standard score means and SE by academic service groups for reading, spelling, and arithmetic at baseline, 2 and 5-year follow-up.
WRAT3 Spelling
MANOVA for scaled spelling scores at baseline, 2 and 5-year follow-up visits by academic service groups yielded a significant overall main effect, F(6,170) = 4.38, p<0.001, partial η2 = 0.13. Significant group differences were found at baseline, F(2,87) = 10.43, p<0.001, 2-year follow-up visit, F(2,87) = 12.58, p<0.001, and 5-year follow-up visit, F(2,87) = 12.57, p<0.001. Group means are illustrated in Figure 1. Post-hoc analyses revealed that Epi_AP+ group had significantly lower scaled spelling scores compared to Epi_AP- and controls at all three study visits, p<0.001. No significant group differences were found between Epi_AP- and the control group.
WRAT3 Arithmetic
MANOVA for scaled arithmetic scores at baseline, 2 and 5-year follow-up visits by academic service groups yielded a significant overall main effect, F(6,172) = 9.7, p<0.001, partial η2 = 0.25. Significant group differences were found at baseline, F(2,88) = 22.22, p<0.001, 2-year follow-up visit, F(2,88) = 15.51, p<0.001, and 5-year follow-up visit, F(2,88) = 29.1, p<0.001. Group means are illustrated in Figure 1. Post-hoc analyses revealed that Epi_AP+ group had significantly lower scaled arithmetic scores compared to Epi_AP- and controls at all three study visits, p<0.001. No significant group differences were found between Epi_AP- and the control group.
3.2 Secondary Analyses
Academic Service Groups by IEP (Individualized Education Program)
To rule out the possibility that children with an established school-based Individual Education Program Plans (IEP) were driving the reported findings, we conducted a secondary set of analyses in which the epilepsy AP+ group was divided into those with and without a formal IEP provided by the child's school system. The results showed that Epi_AP+ children with an IEP (n =13) did not differ significantly from those children without an IEP (n = 13) at any time point across measures of reading, spelling, or arithmetic (details of analyses, summary tables, and figure provided in Supplemental File 2).
Academic Service Groups and Epilepsy Syndrome and Remission Status
A Chi Square analysis was used to assess differences in the rates of academic services between epilepsy syndromes (GE, FE). Results yield no significant group differences: χ2 (1, N=50) = 0.063, p = 0.8. To assess whether the academic service groups had different epilepsy trajectories, we examined the rate of epilepsy remission at the 5-year follow-up across the academic service groups. A patient was considered remitted if he/she had been seizure free for a minimum of 12 months and was not taking AEDs. A Chi Square analysis yield no significant differences in the rate of remission across the academic service groups, χ2 (1, N=48) = 0.75, p = 0.39.
4. Discussion
Academic problems and academic underachievement are known complications of the childhood epilepsies and likely have a long term impact on educational, career, and socio-economic outcomes [13]. There are differing views of the natural history of these problems as some suggest that academic problems are not evident at diagnosis but develop over time [2], while others argue that the academic careers of children with new onset epilepsy are at risk at the time of diagnosis [8]. Developing clinically useful approaches to screen for the risk of academic problems at epilepsy onset is critical in order to identify children with epilepsy at risk to continue to struggle with academic achievement long term. Five core findings emerged from this examination of the academic histories and performance of children with new and recent onset epilepsy and controls.
First, the academic careers of children with new onset epilepsy appear to be already at risk at the time of diagnosis [8]. Our brief structured interview with parents inquired into their level of concern, but more importantly, identified any concrete steps taken to help the child academically—either through the school or independently. 52% of the children with “epilepsy only” compared to 18% of the controls presented with a lifetime history of academic struggles resulting in the provision of a diversity of supportive services. Among children with epilepsy whose parents endorsed academic problems at the baseline evaluation, the vast majority (80.8%) identified these problems antecedent to the recognition, diagnosis, and treatment of epilepsy.
Second, parent provided history of lifetime academic problems was significantly associated with performance on objective tests of reading, spelling and arithmetic. Furthermore, the remaining 48% of children with epilepsy who had no such history demonstrated academic performance virtually identical to controls, findings consistent with those reported by Berg et. al. [14]. Thus, it is possible to use a brief and efficient clinic screen to identify those children at risk to continue to have significantly poorer reading, spelling and arithmetic performance. These children would likely benefit from more detailed neuropsychological assessment in order to elucidate the cognitive difficulties underlying these academic problems to help address the specific learning needs of the child. In fact, we have previously shown that a history of AP in children with epilepsy is associated with significantly more impaired neuropsychological test performance [15] suggesting that there is important yield from formal assessment for children presenting with academic problem histories.
Third, a history of AP at baseline was related to objective academic screening at baseline, and both 2 and 5-years later indicating persisting difficulty. Academic performance neither improved nor worsened in the children with AP, but remained significantly below children with epilepsy with no academic problems and controls up to 5-years. Therefore, simple screening can identify a cohort of children at risk for persisting academic problems.
Fifth, the relationship between parent report of AP and the child's objective academic achievement screening performance held regardless of the definition of AP (e.g., those with vs without an IEP). Further, there was no relationship between AP and overall epilepsy syndrome (FE, GE), thus AP appears to be a phenotype that is independent of epilepsy syndrome. Since the majority of children with epilepsy exhibited academic problems antecedent to their diagnosis of epilepsy and initiation of treatment, it is unlikely that medication or social consequences of seizures were solely responsible for these problems. It is possible that a number of other factors may contribute to the development of AP including “epilepsy related” considerations (e.g., process of epileptogenesis including interictal EEG abnormalities), early unrecognized neurodevelopmental complications, or family aggregation reflecting shared environmental and/or genetic causes, or other factors[16].
Finally, it is important to recognize that despite the fact that children had been provided with services of varying types and varying durations by their schools, academic performance remained abnormal and did not improve over time. Thus, “usual care” had little measureable impact. Demonstration projects designed to improve this situation are needed, and have occasionally been reported in the literature [17].
4.1 Limitations
First, the objective measures of reading, spelling and arithmetic administered here are relatively uncomplicated and likely underestimate of the degree of academic deficiency compared to more comprehensive academic achievement tests (e.g., Wechsler Individual Achievement Test-3rd edition [WIAT-III] [18], Woodcock Johnson Test of Achievement – 4th edition, [WJIV]) [19], and it is possible that such measures might identify abnormalities in the Epi_AP- group, or a greater degree of abnormality in the Epi_AP+ group.
Second, as parents were asked to retrospectively report the child's services starting with early childhood it is possible some parents underreported the incidents of such services being received. Third, formal neuropsychological profiles of AP+ and AP- groups, not provided in the current work, would have provided a better understanding of the corresponding cognitive correlates. We have previously examined full baseline neuropsychological profiles of children with epilepsy and academic services and found that AP+ children with epilepsy have significant neurocognitive impairment when compared to children without services (AP-) [15].
Fourth, Attention Deficit Hyperactivity Disorder (ADHD) is a known to be overrepresented in the pediatric epilepsy population, associated with academic difficulties, and can occur antecedent to epilepsy onset and diagnosis [20, 21]. A significant proportion of the children with epilepsy who had services (Epi_AP+) were diagnosed with ADHD (34.6%). However, due to the limited sample size of ADHD+ in the control (n=1) and the Epi_AP- (n=1) groups we were not able to control for the presence of co-morbid ADHD as part of the current analyses.
Finally, while our study found no differences in baseline academic service rates and epilepsy remission status at 2 and 5-year follow-ups, our sample was not substantial enough to examine differences in academic performance scores and epilepsy specific factors such as changes in AEDs, remission, or seizure frequency.
5. Conclusion
This investigation demonstrates that a brief parent interview regarding a history of supportive actions or services taken by parents or school for concerns regarding academic performance is associated with the child's performance on objective measures of core academic skills both at baseline and 5-years later. This brief clinical screening appears useful in identifying those children with epilepsy who may be especially likely to benefit from comprehensive neuropsychological assessment [15], facilitating a defendable decision to refer the child for formal assessment.
Supplementary Material
Highlights.
Children with epilepsy have higher rate of academic services (47.3% vs. 17.3%)
Academic services antedated epilepsy diagnosis in 80.8% of children with epilepsy
Academic performance and parent reports are associated long-term (up to 5 years)
Brief interview with parents identifies children with epilepsy and academic risk
Children with epilepsy without academic services perform similarly to controls
Acknowledgments
Supported by NIH 3RO1-44351 and the Clinical and Translational Science Award (CTSA) program, previously through the National Center for Research Resources (NCRR) grant 1UL1RR025011, and now by the National Center for Advancing Translational Sciences (NCATS), grant 9U54TR000021. The funding sources had no role in study design; in collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication. We thank Raj Sheth MD, Carl Stafstrom MD, Lucyna Zawadzki, MD, and Monica Koehn MD for study participation and subject recruitment. Also greatly appreciated are Melissa Hanson and Kate Young for overall study coordination, participant recruitment, cognitive assessment, and data management.
Footnotes
Conflict of Interest: We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Disclosure Statement: We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us.
We confirm that we have given due consideration to the protection of intellectual property associated with this work and that there are no impediments to publication, including the timing of publication, with respect to intellectual property. In so doing we confirm that we have followed the regulations of our institutions concerning intellectual property.
We further confirm that any aspect of the work covered in this manuscript that has involved human participants has been conducted with the ethical approval of all relevant bodies and that such approvals are acknowledged within the manuscript.
We understand that the Corresponding Author is the sole contact for the Editorial process (including Editorial Manager and direct communications with the office). Dace Almane, MS is responsible for communicating with the other authors about progress, submissions of revisions and final approval of proofs. We confirm that we have provided a current, correct email address which is accessible by the Corresponding Author.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wilkinson GS. Wide Range Achievement Test–Revision 3. Wilmington, DE: Jastak Association; 1993. [Google Scholar]
- 2.Dunn DW, Johnson CS, Perkins SM, Fastenau PS, Byars AW, Degrauw TJ, Austin JK. Academic problems in children with seizures: Relationships with neuropsychological functioning and family variables during the 3 years after onset. Epilepsy Behav. 2010 doi: 10.1016/j.yebeh.2010.08.023. [DOI] [PubMed] [Google Scholar]
- 3.Melbourne Chambers R, Morrison-Levy N, Chang S, Tapper J, Walker S, Tulloch-Reid M. Cognition, academic achievement, and epilepsy in school-age children: a case-control study in a developing country. Epilepsy Behav. 2014;33:39–44. doi: 10.1016/j.yebeh.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 4.Russ SA, Larson K, Halfon N. A national profile of childhood epilepsy and seizure disorder. Pediatrics. 2012;129:256–64. doi: 10.1542/peds.2010-1371. [DOI] [PubMed] [Google Scholar]
- 5.Lin JJ, Mula M, Hermann BP. Uncovering the neurobehavioural comorbidities of epilepsy over the lifespan. Lancet. 2012;380:1180–92. doi: 10.1016/S0140-6736(12)61455-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sillanpaa M, Jalava M, Kaleva O, Shinnar S. Long-term prognosis of seizures with onset in childhood. N Engl J Med. 1998;338:1715–22. doi: 10.1056/NEJM199806113382402. [DOI] [PubMed] [Google Scholar]
- 7.McNelis AM, Dunn DW, Johnson CS, Austin JK, Perkins SM. Academic performance in children with new-onset seizures and asthma: a prospective study. Epilepsy & Behavior. 2007;10:311–8. doi: 10.1016/j.yebeh.2006.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oostrom KJ, Smeets-Schouten A, Kruitwagen CL, Peters AC, Jennekens-Schinkel A. Not only a matter of epilepsy: early problems of cognition and behavior in children with “epilepsy only”—a prospective, longitudinal, controlled study starting at diagnosis. Pediatrics. 2003;112:1338–44. doi: 10.1542/peds.112.6.1338. [DOI] [PubMed] [Google Scholar]
- 9.Hermann B, Jones J, Sheth R, Dow C, Koehn M, Seidenberg M. Children with new-onset epilepsy: neuropsychological status and brain structure. Brain. 2006;129:2609–19. doi: 10.1093/brain/awl196. [DOI] [PubMed] [Google Scholar]
- 10.World Medical Association Declaration of Helsinki. The Journal of Law, Medicine & Ethics. 1991;19:264–265. [PubMed] [Google Scholar]
- 11.Kaufman J, Birmaher B, Brent D, Rao U, Ryan N. The Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children. Pittsburgh, PA: University of Pittsburgh Medical Center; 1996. [Google Scholar]
- 12.Wechsler D. Wechsler Abbreviated Scale of Intelligence. New York, NY: The Psychological Corporation: Harcourt Brace & Company; 1999. [Google Scholar]
- 13.Moschetta S, Valente KD. Impulsivity and seizure frequency, but not cognitive deficits, impact social adjustment in patients with juvenile myoclonic epilepsy. Epilepsia. 2013;54:866–70. doi: 10.1111/epi.12116. [DOI] [PubMed] [Google Scholar]
- 14.Berg AT, Hesdorffer DC, Zelko FA. Special education participation in children with epilepsy: what does it reflect? Epilepsy Behav. 2011;22:336–41. doi: 10.1016/j.yebeh.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jackson DC, Dabbs K, Walker NM, Jones JE, Hsu DA, Stafstrom CE, Seidenberg M, Hermann BP. The neuropsychological and academic substrate of new/recent-onset epilepsies. J Pediatr. 2013;162:1047–53 e1. doi: 10.1016/j.jpeds.2012.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eom S, Eun SH, Kang HC, Eun BL, Nam SO, Kim SJ, Chung HJ, Kwon SH, Lee YM, Lee JS, Kim DW, Oh KJ, Kim HD. Epilepsy-related clinical factors and psychosocial functions in pediatric epilepsy. Epilepsy Behav. 2014;37:43–8. doi: 10.1016/j.yebeh.2014.05.019. [DOI] [PubMed] [Google Scholar]
- 17.Freeman JM, Jacobs H, Vining E, Rabin CE. Epilepsy and the inner city schools: a school-based program that makes a difference. Epilepsia. 1984;25:438–42. doi: 10.1111/j.1528-1157.1984.tb03440.x. [DOI] [PubMed] [Google Scholar]
- 18.Wechsler D. Wechsler Individual Achievement Test-Third Edition. San Antonion, TX: Pearson; 2009. [Google Scholar]
- 19.Schrank F, McGrew K, Mather N. Woodcock-Johnson. Fourth. Houghton Mifflin Harcourt Publishing Company; 2014. [Google Scholar]
- 20.Dunn DW, Kronenberger WG. Childhood epilepsy, attention problems, and ADHD: review and practical considerations. Semin Pediatr Neurol. 2005;12:222–8. doi: 10.1016/j.spen.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 21.Jones JE, Watson R, Sheth R, Caplan R, Koehn M, Seidenberg M, Hermann B. Psychiatric comorbidity in children with new onset epilepsy. Dev Med Child Neurol. 2007;49:493–7. doi: 10.1111/j.1469-8749.2007.00493.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


