Abstract
Purpose
Examine whether risk factors, including prepregnancy body mass index (BMI), differ between recurrent and incident preeclampsia.
Methods
Data included electronic medical records of nulliparas (n=26,613) delivering ≥2 times in Utah (2002–2010). Modified Poisson regression models were used to examine: 1) adjusted relative risks (RR) of preeclampsia and 95% confidence intervals (CI) associated with prepregnancy BMI; 2) maternal risk factor differences between incident and recurrent preeclampsia among primiparous women.
Results
In the 1st pregnancy, compared to normal weight women [BMI:18.5–24.9], preeclampsia risks for overweight [BMI:25–29.9], obese class I [BMI:30–34.9], and obese class II/III [BMI: ≥35] women were 1.82 (95% CI=1.60–2.06), 2.10 (95% CI=1.76–2.50), and 2.84 (95% CI=2.32–3.47), respectively while 2nd pregnancy incident preeclampsia risks were 1.66 (95% CI=1.27–2.16), 2.31 (95% CI=1.67–3.20), and 4.29 (95% CI=3.16–5.82), respectively. Recurrent preeclampsia risks associated with BMI were highest among obese class I women (RR=1.60; 95% CI=1.06–2.42) without increasing in a dose-response manner. Non-white women had higher recurrence risk than white women (RR=1.70; 95% CI=1.16–2.50) while 2nd pregnancy incident preeclampsia risk did not differ by race.
Conclusion
Prepregnancy BMI appeared to have stronger associations with risk of incident preeclampsia either in the 1st or 2nd pregnancy, than with recurrence risk. Non-white women had higher recurrence risk.
Keywords: preeclampsia, recurrent, incident, obesity, body mass index
INTRODUCTION
Preeclampsia, defined as new onset hypertension and proteinuria after 20 weeks of gestation, complicates 5–8% of all pregnancies in the United States and contributes significantly to maternal and neonatal morbidity and mortality (1–4). Although preeclampsia pathogenesis remains elusive, maternal prepregnancy body mass index (BMI) has been identified as an important independent risk factor for preeclampsia development (5–6). In a recent meta-analysis of nulliparous women and multiparas, overweight or obese women (BMI ≥25 kg/m2) had approximately 2–4 fold increased preeclampsia risk compared to normal weight women (BMI 20–24.9 kg/m2) (7). Women with preeclampsia in a previous pregnancy also have increased recurrence risk in subsequent pregnancies (8–11). While prepregnancy BMI is an established risk factor for incident preeclampsia, less is known about this relationship in recurrent preeclampsia.
Apart from prepregnancy BMI, other factors may differ by whether preeclampsia is incident or recurrent among primiparous women (9,10,12–14). However, studies evaluating how these factors differ are limited. We used data from the National Institute of Child Health and Human Development (NICHD) Consecutive Pregnancies Study which captured data from women with ≥2 consecutive pregnancies to: 1) assess the association between prepregnancy BMI and incident preeclampsia in the 1st pregnancy, incident preeclampsia in the 2nd pregnancy, and recurrent preeclampsia after adjusting for confounders; and 2) examine the association between demographic and clinical factors (in addition to prepregnancy BMI) and preeclampsia risk in the 2nd pregnancy, both incident and recurrent.
METHODS
Study Population
The NICHD Consecutive Pregnancies Study is a longitudinal retrospective study of women with ≥2 consecutive pregnancies delivered during 2002–2010 at 20 hospitals in Utah (15). Data sources included both electronic medical records (EMRs) and International Classification of Diseases, 9th revision (ICD-9) discharge codes. Hospitals extracted detailed information from the EMRs for each delivery on maternal demographics, obstetric and medical history, as well as neonatal outcomes. ICD-9 discharge codes from maternal and newborn discharge summaries were linked to each delivery. The dataset included 114,679 pregnancies (livebirths or stillbirths ≥20 weeks’ gestation) from 51,086 women who gave birth at least twice and up to 6 times during the study period. All study sites obtained approval from their individual institutional review boards.
As multifetal pregnancies increase preeclampsia risk (11), we limited this study to women (n=49,868) who delivered singletons in their first 2 pregnancies during the study period. We excluded 202 women with data inconsistencies such as women on hypertensive medications or with hypertension recorded as the reason for labor induction without having any hypertensive disorder diagnosis resulting in 49,666 women with 99,332 singleton deliveries.
Since nulliparous women have a higher preeclampsia risk than multiparous women (11) and to control for absence of past medical history among parous women in the dataset, in the primary analysis we restricted our sample to nulliparous women at study entry who had their first 2 singleton pregnancies observed during the study period (n=26,963). Due to a small number, women with eclampsia (n=20) were combined with the preeclampsia group. Women with superimposed preeclampsia or chronic hypertension during either pregnancy were excluded (n=350) resulting in 26,613 women for the primary analysis (Supplemental Table S1). We also conducted secondary analysis including the singleton pregnancies irrespective of parity at study entry and similarly excluding women with superimposed preeclampsia or chronic hypertension resulting in 48,941 women with 109,837 singleton deliveries.
Study variables
During the study period, preeclampsia defined as blood pressure ≥140 mm Hg systolic or ≥90 mm Hg diastolic occurring >20 weeks’ gestation among previously normotensive women plus proteinuria, urinary excretion ≥0.3 g protein in 24-h urine specimen, was widely adopted in US clinical practice (16). Hypertensive disorders were ascertained from both EMRs and ICD-9 discharge codes. Women were categorized as having the condition during pregnancy if a diagnosis was coded in either source (Supplemental Table S2 lists ICD-9 codes). Once diagnosed with a chronic condition, women were considered to have the condition during all subsequent pregnancies. In instances where the two sources disagreed (e.g. preeclampsia vs. gestational hypertension), ICD-9 codes took precedence over the EMRs. In the primary analysis (nulliparous women with their first two singleton pregnancies during the study period), 266 (14.5%) pregnancies had a preeclampsia diagnosis in only one source (263 in the medical record and 3 in the ICD-9 discharge codes), and 34 (1.9%) pregnancies had discrepant diagnoses between the two sources.
For incident preeclampsia in the 1st pregnancy, all demographic and clinical factors were drawn from the 1st pregnancy. When examining preeclampsia risk in the 2nd pregnancy, demographic and clinical factors were drawn from the 2nd pregnancy while obstetric characteristics including small for gestational age (SGA) birth (< 5th percentile of sex specific birthweight for gestational age) (17), and early preterm birth (<34 weeks’ gestation) were drawn from the 1st (previous) pregnancy. Interpregnancy interval was defined as time elapsed between the woman’s 1st delivery date and her 2nd pregnancy last menstrual period date (18), and prepregnancy weight difference, weight difference between the first two births, was examined as: lost >2 kg, maintained within 2 kg, and gained >2 kg (19).
Statistical Analysis
Poisson regression models with robust variance estimation (20) examined the unadjusted and adjusted associations between prepregnancy BMI (categorical variable) accounting for confounders and risk of: 1) preeclampsia in the 1st pregnancy, 2) preeclampsia in the 2nd pregnancy without prior preeclampsia, and 3) recurrent preeclampsia. Confounding variables (noted in table footnotes) were included based on previous literature. Additionally, we examined the unadjusted association between preeclampsia risk (incident in the 1st or 2nd pregnancy or recurrent) and a continuous prepregnancy BMI using generalized additive models where cross validation was used to choose the smoothing degree in the cubic spline function. These models make no assumptions about the shape of the association between the exposure of interest and the outcome other than to assume additive effects of the adjustment variable.
To examine the significance of other factors (in addition to BMI) on 2nd pregnancy incidence and recurrence risk, we used longitudinal transition models (modified Poisson regression with an interaction between preeclampsia occurrence in the 1st pregnancy and each of the risk factors of interest to distinguish incident and recurrent preeclampsia risk). In these models, we tested for a significant multiplicative interaction between 1st pregnancy preeclampsia and each risk factor. A significant interaction denotes that for the examined factor, the risk associated with preeclampsia in the 2nd pregnancy differs based on having a prior pregnancy with preeclampsia (i.e. differs between incident versus recurrent preeclampsia).
In secondary analysis utilizing the whole dataset irrespective of parity at study entry, we examined if there were changes in the risk estimates. Analyses were conducted using SAS (version 9.3; SAS Institute, Cary, NC) with a p-value <0.05 considered for statistical significance for main effects and interactions. R (version 3.0.1) was used to fit the generalized additive models and to plot the risk estimates.
RESULTS
A total of 23,913 women were normotensive in their 1st pregnancy and nearly all remained normotensive in their 2nd pregnancy (n=23,301, 97.4%). Preeclampsia/eclampsia in the 1st pregnancy occurred among 5.0% of women. A total of 11.6% (155/1,336) of women experienced preeclampsia/eclampsia recurrence while 1.3% (341/25,451) experienced incident preeclampsia/eclampsia in their 2nd pregnancy. There were no differences in maternal age and marital status between the examined groups (Table 1). Women with preeclampsia in either pregnancy were more likely to have a higher prepregnancy BMI and preterm birth <34 weeks in their 1st pregnancy compared to women with preeclampsia during their first two pregnancies.
Table 1.
Maternal characteristics of the 2nd pregnancy and obstetric outcomes of the 1st pregnancy by preeclampsia status among nulliparous women in the NICHD Consecutive Pregnancy Study, 2002–2010
| No preeclampsia in 1st or 2nd pregnancyc N=24,980 |
Preeclampsia in 1st pregnancy onlyd N=1,137 |
Preeclampsia in 2nd pregnancy onlye N=341 |
Recurrent preeclampsia N=155 |
|
|---|---|---|---|---|
|
|
||||
| Maternal characteristics of the 2nd pregnancy/Obstetric outcomes of the 1st pregnancy
|
||||
| Maternal age (y)a | 26.2 (4.1) | 26.3 (4.3) | 26.5 (4.1) | 26.2 (4.7) |
| Non-Hispanic Whiteb | 21802 (87) | 1007 (89) | 298 (88) | 125 (81) |
| Married | 22278 (89) | 1010 (89) | 299 (88) | 138 (89) |
| Private insurance | 18187 (73) | 833 (73) | 238 (70) | 100 (65) |
| Alcohol during pregnancy | 405 (2) | 9 (1) | 8 (2) | 3 (2) |
| Smoke during pregnancy | 710 (3) | 32 (3) | 12 (4) | 6 (4) |
| Interpregnancy interval (months) | ||||
| 0–5 | 1423 (6) | 68 (6) | 24 (7) | 18 (12) |
| 6–11 | 4602 (18) | 196 (17) | 49 (14) | 24 (16) |
| 12–17 | 6583 (26) | 304 (27) | 88 (26) | 31 (20) |
| 18–23 | 5477 (22) | 238 (21) | 55 (16) | 31 (20) |
| ≥24 | 6895 (28) | 331 (29) | 125 (37) | 51 (33) |
| 1st & 2nd prepregnancy weight difference (kg) | ||||
| <−2 kg | 4876 (20) | 235 (21) | 51 (15) | 31 (21) |
| −2 to 2 kg | 8571 (35) | 325 (29) | 86 (26) | 42 (28) |
| >2 kg | 10813 (45) | 543 (49) | 195 (59) | 77 (51) |
| Pre-pregnancy BMI (kg/m2)a | 24.5 (5) | 26.9 (6) | 27.9 (7) | 27.3 (6) |
| Pre-pregnancy BMI (kg/m2) | ||||
| Underweight [<18.5] | 1378 (6) | 25 (2) | 5 (1) | 1 (1) |
| Normal [18.5–24.9] | 14435 (59) | 514 (46) | 139 (41) | 55 (36) |
| Overweight [25.0–29.9] | 5361 (22) | 302 (27) | 88 (26) | 53 (35) |
| Obese class I [30.0–34.9] | 2138 (9) | 147 (13) | 49 (14) | 28 (19) |
| Obese class II & III [>35.0] | 1321 (5) | 136 (12) | 58 (17) | 14 (9) |
| Gestational weight gain (kg)a | 13.4 (6) | 13.8 (6) | 13.9 (7) | 14.3 (7) |
| Thyroid disease | 1253 (5) | 68 (6) | 17 (5) | 9 (6) |
| Asthma | 2078 (8) | 105 (9) | 24 (7) | 15 (10) |
| Depression | 2496 (10) | 141 (12) | 38 (11) | 20 (13) |
| Gestational diabetes | 683 (3) | 58 (5) | 11 (3) | 15 (10) |
| Preexisting diabetes | 152 (1) | 10 (1) | 3 (1) | 2 (1) |
| Antihypertensive medication | 15 (0) | 3 (0) | 25 (7) | 19 (12) |
| Induction of labor | 9157 (37) | 437 (38) | 235 (69) | 72 (46) |
| Cesarean delivery | 4408 (18) | 369 (32) | 92 (27) | 76 (49) |
| Cesarean for failing to progress | 518 (2) | 37 (3) | 8 (2) | 11 (7) |
| SGA in 1st pregnancy | 1017 (4) | 92 (8) | 19 (6) | 8 (5) |
| Preterm <34 week in 1st pregnancy | 393 (2) | 80 (7) | 15 (4) | 25 (16) |
Abbreviations: BMI: body mass index; SGA: small for gestational age.
Data were missing for: race/ethnicity: 30; married: 2; alcohol: 67; smoke: 18; prepregnancy weight difference: 768; prepregnancy BMI: 366; gestational weight gain: 397; gestational diabetes/preexisting diabetes: 13; cesarean delivery: 1; SGA in previous pregnancy: 26.
All figures are N (%) unless otherwise stated.
Mean (SD).
Non-whites included: non-Hispanic Black (n=110, 0.4%); Hispanic (n=2554, 10%); Asian/Pacific Islander (n=592, 2%); Other race (n=95, 0.4%).
Includes normotensive women and women with gestational hypertension during the 1st and/or 2nd pregnancy.
Includes women with preeclampsia/eclampsia in their 1st pregnancy who were either normotensive or had gestational hypertension in their 2nd pregnancy.
Includes women with preeclampsia/eclampsia in their 2nd pregnancy who were either normotensive or had gestational hypertension in their 1st pregnancy.
Incident preeclampsia risk whether in the 1st or 2nd pregnancy increased continuously with BMI (Figure 1). Recurrent preeclampsia risk also increased in a dose response manner up to a BMI of about 31 kg/m2. Although the risk slightly decreased or leveled off with increasing BMI thereafter, only 14 women with recurrent preeclampsia had a BMI >35 kg/m2 which limits our ability to make definitive conclusions for higher BMI. Worth noting is that the absolute risk of preeclampsia is highest in the recurrent preeclampsia group at every BMI level up to about 37 kg/m2.
Figure 1.
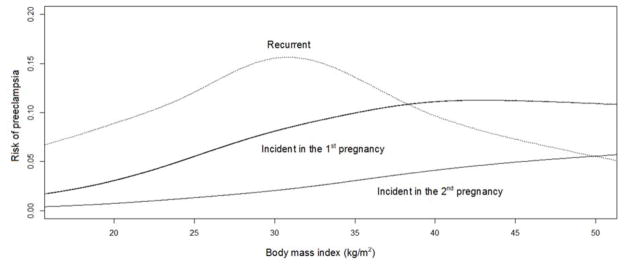
Unadjusted association between prepregnancy body mass index (BMI, kg/m2) of the index pregnancy and the risk of incident preeclampsia in the 1st pregnancy (solid black line), incident preeclampsia in the 2nd pregnancy (solid grey line), and recurrent preeclampsia (dotted grey line) using generalized additive models with a spline smoother.
For incident preeclampsia in the 1st pregnancy, prepregnancy BMI of the 1st pregnancy was used.
For incident preeclampsia in the 2nd pregnancy and recurrent preeclampsia, prepregnancy BMI of the 2nd pregnancy was used.
Risk estimates by BMI categories reflected the strong dose-response relationship between prepregnancy BMI and incident preeclampsia in the 1st or 2nd pregnancy (Table 2). For example, compared to normal weight women, incident preeclampsia risk was 1.7–1.8 fold higher among overweight women and 2.1–2.3 fold higher among class I obese women. Among class II/III obese women however, incident preeclampsia risk in the 2nd pregnancy (RR=4.29; 95% CI=3.16–5.82) was more pronounced than incident risk in the 1st pregnancy (RR=2.84; 95% CI=2.32–3.47). This dose-response relationship was not as apparent among women with recurrent preeclampsia. For instance, among overweight women recurrence risk was 1.47 (95% CI=1.04–2.09) similar to the observed 1.60 (95% CI=1.06–2.42) risk estimate among class I obese women and there was no significantly increased risk among class II/III obese women compared to normal weight women.
Table 2.
Association between prepregnancy body mass index categories and the risk of preeclampsia among nulliparous women at study entry in the NICHD Consecutive Pregnancy Study, 2002–2010
| Incident preeclampsia in 1st pregnancya N=26,613 |
Incident preeclampsia in 2nd pregnancyb N=25,321 |
Recurrent preeclampsiac N=1,292 |
||||
|---|---|---|---|---|---|---|
|
|
||||||
| Body mass index kg/m2 | Unadjusted RR (95% CI) | Adjusted RR (95% CI) | Unadjusted RR (95% CI) | Adjusted RR (95% CI) | Unadjusted RR (95% CI) | Adjusted RR (95% CI) |
| Underweight [<18.5] | 0.54 (0.39, 0.76) | 0.54 (0.38, 0.76) | 0.38 (0.16, 0.92) | 0.38 (0.15, 0.92) | 0.40 (0.06, 2.76) | 0.38 (0.05, 2.72) |
| Normal [18.5–24.9] | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) |
| Overweight [25.0–29.9] | 1.82 (1.61, 2.07) | 1.82 (1.60, 2.06) | 1.69 (1.30, 2.21) | 1.66 (1.27, 2.16) | 1.54 (1.08, 2.20) | 1.47 (1.04, 2.09) |
| Obese I [30.0–34.9] | 2.09 (1.76, 2.49) | 2.10 (1.76, 2.50) | 2.35 (1.70, 3.24) | 2.31 (1.67, 3.20) | 1.66 (1.09, 2.53) | 1.60 (1.06, 2.42) |
| Obese II & III [>35.0] | 2.85 (2.34, 3.49) | 2.84 (2.32, 3.47) | 4.41 (3.26, 5.96) | 4.29 (3.16, 5.82) | 0.97 (0.55, 1.69) | 0.91 (0.52, 1.61) |
RRs and CIs from a modified Poisson regression model. Models adjusted for maternal race/ethnicity (non-Hispanic white, other), age (<29, 30–34, ≥35years), marital status (married, not married), insurance (public, private), and smoking status (yes, no).
Association examined with prepregnancy BMI of the 1st pregnancy. Final sample size after excluding missing values: unadjusted model N=26,160 (1,270 preeclampsia cases), adjusted model N=26,095 (1,268 preeclampsia cases). BMI distribution: underweight n=1635, normal n=16763, overweight n=5093, class I n=1762, class II/III n=907.
Association examined with prepregnancy BMI of the 2nd pregnancy. Final sample size after excluding missing values: unadjusted model N=24,972 (339 preeclampsia cases), adjusted model N=24,926 (338 preeclampsia cases). BMI distribution: underweight n=1383, normal n=14574, overweight n=5449, class I n=2187, class II/III n=1379.
Association examined with prepregnancy BMI of the 2nd pregnancy. Final sample size after excluding missing values: unadjusted model N=1,275 (151 preeclampsia cases), adjusted model N=1,273 (151 preeclampsia cases). BMI distribution: underweight n=26, normal n=569, overweight n=355, class I n=175, class II/III n=150.
The differences observed in the associations between BMI and incident preeclampsia risk in the 2nd pregnancy and recurrent preeclampsia risk, were further substantiated by our test for interaction between preeclampsia in the 1st pregnancy and BMI (Table 3). While 2nd pregnancy incident preeclampsia risk increased from 0.42 (95% CI=0.17–1.03) for underweight women to 2.97 (95% CI=2.10–4.20) for obese class II/III women, this increase was not observed among women with recurrent preeclampsia. Race/ethnicity also differed in its associations between incident and recurrent preeclampsia (Table 3). Non-white women had an increased recurrence risk (RR=1.70; 95% CI=1.16–2.50) compared to Non-Hispanic white women, while no association was observed when preeclampsia was incident in the 2nd pregnancy. Preterm birth <34 weeks in the first pregnancy increased both recurrent (RR=1.96; 95% CI=1.34–2.88) and 2nd pregnancy incident preeclampsia risk (RR=3.29; 95% CI=1.99–5.45). Longer inter-pregnancy interval (≥24 months) compared to 18–23 month interval was only associated with an increased incident preeclampsia risk (RR=1.63; 95% CI=1.18–2.25). The other examined risk factors including maternal age, marital status, insurance type, smoking, alcohol, diabetes, other maternal chronic conditions, and 1st pregnancy SGA were not significantly associated with either 2nd pregnancy incident preeclampsia or recurrent preeclampsia.
Table 3.
Adjusted risk of recurrent and incident preeclampsia in the 2nd pregnancy according to maternal characteristics of the 2nd pregnancy and neonatal outcomes of the 1st pregnancy among nulliparous women at study entry in the NICHD Consecutive Pregnancy Study, 2002–2010
| Recurrent Preeclampsia
|
Incident Preeclampsia in the 2nd pregnancy
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Maternal characteristics of the 2nd pregnancy/Obstetric outcomes of the 1st pregnancy | Unadjusted | Adjusted | Unadjusted | Adjusted | |||||||
|
|
|
|
|
|
|||||||
| RR | 95% CI | RR | 95% CI | Pa | RR | 95% CI | RR | 95% CI | Pa | Pb | |
| Maternal age, years | 0.33 | 0.45 | 0.19 | ||||||||
| 13–29 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | |||
| 30–34 | 0.89 | 0.56, 1.40 | 0.82 | 0.51, 1.32 | 1.31 | 0.99, 1.73 | 1.22 | 0.91, 1.63 | |||
| ≥35 | 1.46 | 0.83, 2.57 | 1.47 | 0.83, 2.61 | 1.12 | 0.67, 1.88 | 1.00 | 0.58, 1.72 | |||
| Race/ethnicity | 0.02 | 0.54 | 0.02 | ||||||||
| White | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | |||
| Other | 1.72 | 1.20, 2.47 | 1.70 | 1.16, 2.50 | 1.07 | 0.77, 1.47 | 0.90 | 0.63, 1.27 | |||
| Marital status | 0.14 | 0.92 | 0.23 | ||||||||
| Married | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | |||
| Non-married | 0.98 | 0.61, 1.57 | 0.69 | 0.40, 1.17 | 1.20 | 0.87, 1.65 | 1.02 | 0.70, 1.49 | |||
| Insurance | 0.12 | 0.29 | 0.59 | ||||||||
| Private | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | |||
| Public | 1.43 | 1.05, 1.94 | 1.30 | 0.94, 1.80 | 1.21 | 0.97, 1.52 | 1.16 | 0.89, 1.52 | |||
| SGA in 1st pregnancyc | 0.18 | 0.21 | 0.07 | ||||||||
| No | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | |||
| Yes | 0.65 | 0.33, 1.29 | 0.69 | 0.36, 1.30 | 1.35 | 0.85, 2.14 | 1.41 | 0.89, 2.22 | |||
| Preterm <34 weeks in 1st pregnancy | 0.009 | 0.006 | 0.15 | ||||||||
| No | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | |||
| Yes | 2.17 | 1.49, 3.17 | 1.96 | 1.34, 2.88 | 3.15 | 1.88, 5.26 | 3.29 | 1.99, 5.45 | |||
| Interpregnancy interval, months | 0.23 | 0.01 | 0.29 | ||||||||
| 0–5 | 1.82 | 1.07, 3.08 | 1.33 | 0.76, 2.34 | 1.71 | 1.06, 2.74 | 1.43 | 0.88, 2.33 | |||
| 6–11 | 0.95 | 0.58, 1.57 | 0.87 | 0.53, 1.44 | 1.06 | 0.72, 1.55 | 1.01 | 0.69, 1.49 | |||
| 12–17 | 0.83 | 0.50, 1.29 | 0.75 | 0.46, 1.20 | 1.33 | 0.95, 1.86 | 1.34 | 0.96, 1.88 | |||
| 18–23 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | |||
| ≥24 | 1.16 | 0.76, 1.76 | 1.12 | 0.73, 1.72 | 1.74 | 1.27, 2.38 | 1.63 | 1.18, 2.25 | |||
| Prepregnancy body mass index | 0.08 | <0.001 | 0.005 | ||||||||
| Underweight [<18.5] | 0.40 | 0.06, 2.76 | 0.40 | 0.06, 2.85 | 0.40 | 0.17, 0.98 | 0.42 | 0.17, 1.03 | |||
| Normal weight [18.5–24.9] | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | |||
| Overweight [25.0–29.9] | 1.55 | 1.09, 2.20 | 1.40 | 0.99, 1.98 | 1.53 | 1.17, 2.00 | 1.43 | 1.07, 1.89 | |||
| Obese Class I [30.0–34.9] | 1.66 | 1.09, 2.53 | 1.47 | 0.94, 2.28 | 1.96 | 1.41, 2.72 | 1.76 | 1.23, 2.53 | |||
| Obese Class II & III [>35.0] | 0.97 | 0.55, 1.69 | 0.92 | 0.51, 1.66 | 3.32 | 2.43, 4.55 | 2.97 | 2.10, 4.20 | |||
| 1st & 2nd prepregnancy weight difference kg | 0.82 | 0.33 | 0.37 | ||||||||
| <−2 kg | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | |||
| −2 to 2 kg | 0.98 | 0.63, 1.52 | 1.03 | 0.67, 1.58 | 0.97 | 0.69, 1.37 | 0.98 | 0.70, 1.39 | |||
| >2 kg | 1.07 | 0.72, 1.58 | 0.92 | 0.63, 1.36 | 1.60 | 1.18, 2.18 | 1.19 | 0.87, 1.64 | |||
| Smoking during pregnancy | 0.46 | 0.59 | 0.73 | ||||||||
| No | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | |||
| Yes | 1.33 | 0.63, 2.81 | 1.43 | 0.63, 3.23 | 1.34 | 0.76, 2.36 | 1.19 | 0.67, 2.12 | |||
| Alcohol during pregnancy | 0.53 | 0.56 | 0.78 | ||||||||
| No | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | |||
| Yes | 2.10 | 0.78, 5.66 | 1.50 | 0.52, 4.33 | 1.45 | 0.72, 2.90 | 1.25 | 0.64, 2.45 | |||
| Gestational diabetes in 1st pregnancy | 0.43 | 0.53 | 0.85 | ||||||||
| No | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | |||
| Yes | 1.51 | 0.79, 2.88 | 1.36 | 0.69, 2.68 | 1.39 | 0.76, 2.56 | 1.24 | 0.67, 2.30 | |||
| Preexisting diabetes mellitus | 0.18 | 0.83 | 0.21 | ||||||||
| No | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | |||
| Yes | 2.33 | 1.37, 3.95 | 1.73 | 0.92, 3.26 | 1.26 | 0.63, 2.50 | 0.93 | 0.46, 1.87 | |||
| Asthma/depression/thyroid disease | 0.74 | 0.07 | 0.44 | ||||||||
| No | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent | |||
| Yes | 0.97 | 0.69, 1.37 | 0.94 | 0.66, 1.34 | 0.93 | 0.71, 1.20 | 0.79 | 0.60, 1.03 | |||
RRs and CIs from a modified Poisson regression with preeclampsia occurrence in the 1st pregnancy treated as a covariate. Model mutually adjusted for all factors in the table. Final analytic sample was 25,688 after excluding women who were missing any of the above examined factors in the model.
p-value, only presented for the adjusted RR, represents the global significance of each of the examined predictive factor within the recurrent or incident preeclampsia group using the score test derived from generalized estimating equations.
p-value, only presented for the adjusted RR, represents the interaction of each of the examined predictive factor between recurrent and incident preeclampsia.
SGA defined as <5th percentile of sex specific birthweight for gestational age based on a Canadian reference population.
As BMI was the strongest risk factor for preeclampsia, weight change between the two pregnancies was of interest. In analyses adjusting for 2nd prepregnancy BMI, no significant associations were identified for either incident or recurrent preeclampsia with a 2 kg weight change (Table 3) or a 4.5 kg weight change (equivalent to a 2 unit BMI change for an overweight woman of average height) (data not shown). In a different model adjusting for prepregnancy BMI at 1st pregnancy rather than in the 2nd pregnancy (as most previous studies have done), we noted an increase in only incident and not recurrent preeclampsia risk among women who gained >2 BMI units between pregnancies (RR=1.44; 95% CI=1.13–1.83) (data not shown).
When we examined the whole dataset of singleton pairs irrespective of parity at study entry, there were no meaningful qualitative changes in the associations between prepregnancy BMI and incident or recurrent preeclampsia risk (Supplemental Table S3). While the impact of prepregnancy BMI, preterm birth in the previous pregnancy, and interpregnancy interval on the risk of incident preeclampsia in the 2nd pregnancy and recurrent preeclampsia using the whole dataset was similar to what we reported using the nulliparous dataset, we observed other significant factors (Supplemental Table S4).
DISCUSSION
In this large longitudinal dataset of women with at least two consecutive pregnancies, we show that prepregnancy BMI was more strongly associated with incident preeclampsia risk, whether incident in the 1st pregnancy or 2nd pregnancy than with recurrent preeclampsia risk. Our findings build on previous studies that have shown the importance of prepregnancy BMI on incident preeclampsia risk (7). We expand on these findings by examining the association between prepregnancy BMI, using both a continuous and a categorical measure, and risk of incident and recurrent preeclampsia among primiparous women.
To our knowledge, studies examining incident preeclampsia risk among nulliparous women in association with a continuous prepregnancy BMI measure are limited. Bodnar et al., found that preeclampsia risk increases steeply from BMI values of 15 to 30, plateaus between BMI values of 30 to 35, and finally declines beyond a BMI of 35 (5). The authors however, noted that the sample size of women with a BMI ≥30 was limited, constituting only 15% of the overall sample (obese n=178) (5). We did not observe the decline in incident preeclampsia risk at higher BMI levels, whether incident in the 1st or 2nd pregnancy, but we demonstrate a continuous increase in risk even within the conventional BMI groupings. The plateau and declining effects in preeclampsia risk among obese women however, were observed when we examined recurrent preeclampsia risk. While we hypothesize that the plateauing might be a ceiling effect where very high BMI levels no longer have additional impact due to the high underlying risk among women with a previous preeclampsia history, a limited sample size precludes a definitive conclusion. Women with a BMI ≥30 constituted 25% of the sample of those with recurrent preeclampsia (obese n=325) while those with a BMI≥35 constituted 12% of the sample (n=150).
Our reported estimates of preeclampsia recurrence (11.6%) and preeclampsia incidence in the 2nd pregnancy (1.3%) are fairly similar to what has been previously published (approximately 15% and 0.5%–1.8%, respectively) (13,14,21,22). While recurrence estimates have been examined previously in several studies, data assessing prepregnancy BMI impact on the risk of incident and recurrent preeclampsia among primiparous women are very limited. In an Icelandic study of women with preeclampsia in their 1st pregnancy (n=151), being overweight (BMI ≥25 kg/m2) was not associated with preeclampsia recurrence (OR=1.34; 95% CI=0.40–4.46) (23). In another population based study using maternally-linked birth certificate data in Missouri, prepregnancy BMI had a more pronounced impact on 2nd pregnancy incident preeclampsia risk than recurrence. Compared to normal weight women, incident preeclampsia risk in the 2nd pregnancy increased from 2.1 (95% CI=1.8–2.4) among overweight women to 4.5 (95% CI=3.9–5.3) among class II/III obese women while recurrent preeclampsia risk only increased from 1.3 (95% CI=1.1–1.5) among overweight women to 1.8 (95% CI=1.5–2.2) among obese class II/III women (13). While we similarly noted the pronounced increase in incident preeclampsia risk in the 2nd pregnancy with increasing prepregnancy BMI, we did not observe this increased risk with recurrent preeclampsia. Differences in data sources, sample size, and study populations might explain the observed discrepancies.
How obesity impacts preeclampsia risk is not well understood but several features characterize both conditions including oxidative stress, circulating markers of inflammation, dyslipidemia, hyperinsulinemia, insulin resistance, and impaired vascular function (24–26). As biomarkers become delineated (27), more will be understood about the association.
Our findings indicate that after accounting for 1st prepregnancy BMI, women who gained >2 BMI units between pregnancies had increased incident preeclampsia risk in the 2nd pregnancy. Similarly, in a nationwide Swedish study, women who gained >3 BMI units had increased preeclampsia risk (OR=1.8; 95% CI=1.5–2.1) compared to those whose BMI remained relatively unchanged (i.e. ±1 unit) between pregnancies (28). Our null findings for weight change as a risk factor for recurrent preeclampsia risk are in contrast to another study which found that women who increased their BMI by >2 units between pregnancies increased their recurrence risk (RR=1.3; 95% CI=1.2–1.4) compared to those who remained relatively unchanged (i.e. ±2 units) (9).
Other than prepregnancy BMI, very few factors differed between 2nd pregnancy incident and recurrent preeclampsia. Non-white women (12%) had increased recurrent but not incident preeclampsia risk. Given the sample size, we cannot make firm conclusions about recurrence risk among non-White women. However, Hispanics who predominantly comprise this group (76%) have been previously shown to have a higher preeclampsia risk than non-Hispanic Caucasian women (29). And while some evidence also suggests that black women have a higher preeclampsia risk, (30,31) we were limited in examining this further given the low number of black women in our study (n=110).
The impact of prior preterm birth on subsequent preeclampsia risk was similar whether preeclampsia was incident or recurrent. It is well established that preeclampsia recurrence risk is higher with an earlier gestational age at delivery in the prior pregnancy complicated by preeclampsia (13,32). Recurrence risk estimates increased from approximately 13% when gestational age at 1st birth was ≥37 weeks to 39% when gestational age was ≤28 weeks (13). Preterm birth as a risk factor for future preeclampsia among women without prior preeclampsia is postulated to be due to the two conditions sharing a common pathway or that these women were destined to develop preeclampsia but their preterm delivery occurred prior to its clinical manifestation (13).
Strengths of our study include its large size and diagnostic data availability from EMRs and ICD9 discharge codes. As our study design requires women to have ≥2 pregnancies to be in the dataset, we are only able to provide risk estimates for 1st pregnancy incident preeclampsia based on those who successfully achieved a subsequent pregnancy within the study period. We lacked data on preeclampsia severity. We also did not have data on paternity and were unable to examine the association between change in paternity and preeclampsia risk. Whether partner change or longer interpregnancy interval confounds or modifies the paternal effect on preeclampsia risk is debated (22,33). In our study population however, we expect that the majority of the women are in stable relationships. Married women constituted 89% of our sample compared to the 41.5% national estimate (34). As Caucasians comprised the majority of our study population (87%), we were unable to examine whether the impact of prepregnancy BMI on preeclampsia risk differed by race/ethnicity. A previous study has reported a significant interaction between BMI and race/ethnicity; lean black women had higher preeclampsia risk than lean white women while this trend reversed at high BMI values (6). Finally, the lack of associations between smoking and alcohol and preeclampsia risk in our study might have been due to the low prevalence of gestational smoking (2.9%) and alcohol drinking (1.6%).
In conclusion, we show that prepregnancy BMI is more strongly related to incident than recurrent preeclampsia while women of non-white race may have a higher recurrence risk. Further studies are needed to confirm our findings and to explore the different underlying mechanisms that differ between incident versus recurrent preeclampsia.
Supplementary Material
Acknowledgments
Funding: This research was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (Contracts numbers: HHSN275200800002I, HHSN27500004).
Abbreviations
- BMI
body mass index
- EMR
electronic medical record
- ICD9
International Classification of Diseases ninth revision
- SGA
small for gestational age
Footnotes
Disclosure of interests: None.
Contribution to authorship: NSB designed the study, completed the analysis, and wrote the manuscript. PSA aided in the design of the study’s analytic strategy and aided in the interpretation of the data and provided critical revision of the manuscript for important intellectual content. EY aided in study concept and design, and directed its implementation, including quality assurance and control, and provided critical revision of the manuscript for important intellectual content. PM, SNH, SKL, and CZ aided in the design of the study, interpretation of the data, and provided critical revision of the manuscript for important intellectual content and all authors approved the final version.
Details of ethics approval: Not required.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Sibai BM. Diagnosis and management of gestational hypertension and preeclampsia. Obstet Gynecol. 2003;102:181–192. doi: 10.1016/s0029-7844(03)00475-7. [DOI] [PubMed] [Google Scholar]
- 2.ACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002. Obstet Gynecol. 2002;99:159–67. doi: 10.1016/s0029-7844(01)01747-1. [DOI] [PubMed] [Google Scholar]
- 3.Barton JR, Sibai BM. Prediction and prevention of recurrent preeclampsia. Obstet Gynecol. 2008;112:359–372. doi: 10.1097/AOG.0b013e3181801d56. [DOI] [PubMed] [Google Scholar]
- 4.Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol. 2000;183:S1–S22. [PubMed] [Google Scholar]
- 5.Bodnar LM, Ness RB, Markovic N, Roberts JM. The risk of preeclampsia rises with increasing prepregnancy body mass index. Ann Epidemiol. 2005;15:475–482. doi: 10.1016/j.annepidem.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 6.Bodnar LM, Catov JM, Klebanoff MA, Ness RB, Roberts JM. Prepregnancy body mass index and the occurrence of severe hypertensive disorders of pregnancy. Epidemiology. 2007;18:234–239. doi: 10.1097/01.ede.0000254119.99660.e7. [DOI] [PubMed] [Google Scholar]
- 7.Wang Z, Wang P, Liu H, He X, Zhang J, Yan H, et al. Maternal adiposity as an independent risk factor for pre-eclampsia: a meta-analysis of prospective cohort studies. Obes Rev. 2013;14:508–521. doi: 10.1111/obr.12025. [DOI] [PubMed] [Google Scholar]
- 8.Zhang J, Troendle JF, Levine RJ. Risks of hypertensive disorders in the second pregnancy. Paediatr Perinat Epidemiol. 2001;15:226–231. doi: 10.1046/j.1365-3016.2001.00347.x. [DOI] [PubMed] [Google Scholar]
- 9.Mostello D, Jen CJ, Allen J, Luehr L, Shyken J, Leet T. Recurrent preeclampsia: the effect of weight change between pregnancies. Obstet Gynecol. 2010;116:667–672. doi: 10.1097/AOG.0b013e3181ed74ea. [DOI] [PubMed] [Google Scholar]
- 10.McDonald SD, Best C, Lam K. The recurrence risk of severe de novo pre-eclampsia in singleton pregnancies: a population-based cohort. BJOG. 2009;116:1578–1584. doi: 10.1111/j.1471-0528.2009.02317.x. [DOI] [PubMed] [Google Scholar]
- 11.Duckitt K, Harrington D. Risk factors for pre-eclampsia at antenatal booking: systematic review of controlled studies. BMJ. 2005;330:565. doi: 10.1136/bmj.38380.674340.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Getahun D, Ananth CV, Oyelese Y, Chavez MR, Kirby RS, Smulian JC. Primary preeclampsia in the second pregnancy: effects of changes in prepregnancy body mass index between pregnancies. Obstet Gynecol. 2007;110:1319–1325. doi: 10.1097/01.AOG.0000292090.40351.30. [DOI] [PubMed] [Google Scholar]
- 13.Mostello D, Kallogjeri D, Tungsiripat R, Leet T. Recurrence of preeclampsia: effects of gestational age at delivery of the first pregnancy, body mass index, paternity, and interval between births. Am J Obstet Gynecol. 2008;199:55–57. doi: 10.1016/j.ajog.2007.11.058. [DOI] [PubMed] [Google Scholar]
- 14.Trogstad LI, Eskild A, Magnus P, Samuelsen SO, Nesheim BI. Changing paternity and time since last pregnancy; the impact on pre-eclampsia risk. A study of 547 238 women with and without previous pre-eclampsia. Int J Epidemiol. 2001;30:1317–1322. doi: 10.1093/ije/30.6.1317. [DOI] [PubMed] [Google Scholar]
- 15.Laughon SK, Albert PS, Leishear K, Mendola P. The NICHD Consecoutive Pregnancies Study: recurrent preterm delivery by subtype. Am J Obstet Gynecol. 2014;210(2):131–138. doi: 10.1016/j.ajog.2013.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sibai BM. Evaluation and management of severe preeclampsia before 34 weeks’ gestation. Am J Obstet Gynecol. 2011;205(3):191–198. doi: 10.1016/j.ajog.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 17.Kramer MS, Platt RW, Wen SW, Joseph KS, Allen A, Abrahamowicz M, et al. A new and improved population-based Canadian reference for birth weight for gestational age. Pediatrics. 2001;108:E35. doi: 10.1542/peds.108.2.e35. [DOI] [PubMed] [Google Scholar]
- 18.Zhu BP. Effect of interpregnancy interval on birth outcomes: findings from three recent US studies. Int J Gynaecol Obstet. 2005;89 (Suppl 1):S25–S33. doi: 10.1016/j.ijgo.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 19.Michels KB, Terry KL, Eliassen AH, Hankinson SE, Willett WC. Adult weight change and incidence of premenopausal breast cancer. Int J Cancer. 2012;130(4):902–909. doi: 10.1002/ijc.26069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 21.Brown MA, Mackenzie C, Dunsmuir W, Roberts L, Ikin K, Matthews J, et al. Can we predict recurrence of pre-eclampsia or gestational hypertension? BJOG. 2007;114:984–993. doi: 10.1111/j.1471-0528.2007.01376.x. [DOI] [PubMed] [Google Scholar]
- 22.Basso O, Christensen K, Olsen J. Higher risk of pre-eclampsia after change of partner. An effect of longer interpregnancy intervals? Epidemiology. 2001;12:624–629. doi: 10.1097/00001648-200111000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Hjartardottir S, Leifsson BG, Geirsson RT, Steinthorsdottir V. Recurrence of hypertensive disorder in second pregnancy. Am J Obstet Gynecol. 2006;194:916–920. doi: 10.1016/j.ajog.2005.10.819. [DOI] [PubMed] [Google Scholar]
- 24.Bodnar LM, Ness RB, Harger GF, Roberts JM. Inflammation and triglycerides partially mediate the effect of prepregnancy body mass index on the risk of preeclampsia. Am J Epidemiol. 2005;162:1198–1206. doi: 10.1093/aje/kwi334. [DOI] [PubMed] [Google Scholar]
- 25.Roberts JM, Bodnar LM, Patrick TE, Powers RW. The Role of Obesity in Preeclampsia. Pregnancy Hypertens. 2011;1:6–16. doi: 10.1016/j.preghy.2010.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walsh SW. Obesity: a risk factor for preeclampsia. Trends Endocrinol Metab. 2007;18:365–370. doi: 10.1016/j.tem.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 27.Levine RJ, Karumanchi SA. Circulating angiogenic factors in preeclampsia. Clin Obstet Gynecol. 2005;48:372–386. doi: 10.1097/01.grf.0000160313.82606.d7. [DOI] [PubMed] [Google Scholar]
- 28.Villamor E, Cnattingius S. Interpregnancy weight change and risk of adverse pregnancy outcomes: a population-based study. Lancet. 2006;368:1164–1170. doi: 10.1016/S0140-6736(06)69473-7. [DOI] [PubMed] [Google Scholar]
- 29.Wolf M, Shah A, Jimenez-Kimble R, Sauk J, Ecker JL, Thadhani R. Differential risk of hypertensive disorders of pregnancy among Hispanic women. J Am Soc Nephrol. 2004;15:1330–1338. doi: 10.1097/01.asn.0000125615.35046.59. [DOI] [PubMed] [Google Scholar]
- 30.Eskenazi B, Fenster L, Sidney S. A multivariate analysis of risk factors for preeclampsia. JAMA. 1991;266(2):237–241. [PubMed] [Google Scholar]
- 31.Ghosh G, Grewal J, Mannisto T, Mendola P, Chen Z, Xie Y, et al. Racial/ethnic differences in pregnancy-related hypertensive disease in nulliparous women. Ethn Dis. 2014;24(3):283–289. [PMC free article] [PubMed] [Google Scholar]
- 32.Mostello D, Catlin TK, Roman L, Holcomb WL, Jr, Leet T. Preeclampsia in the parous woman: who is at risk? Am J Obstet Gynecol. 2002;187:425–429. doi: 10.1067/mob.2002.123608. [DOI] [PubMed] [Google Scholar]
- 33.Li DK, Wi S. Changing paternity and the risk of preeclampsia/eclampsia in the subsequent pregnancy. Am J Epidemiol. 2000;151(1):57–62. doi: 10.1093/oxfordjournals.aje.a010122. [DOI] [PubMed] [Google Scholar]
- 34.First marriages in the United States: Data from the 2006–2010 National Survey of Family Growth. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics; (Report) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


