Abstract
Life-threatening fungal infections have risen sharply in recent years, owing to the advances and intensity of medical care that may blunt immunity in patients. This emerging crisis has created the growing need to clarify immune defense mechanisms against fungi with the ultimate goal of therapeutic intervention. We describe recent insights in understanding the mammalian immune defenses that are deployed against pathogenic fungi. We focus on adaptive immunity to the major medically important fungi and emphasize three elements that coordinate the response: (1) dendritic cells and subsets that are mobilized against fungi in various anatomical compartments; (2) fungal molecular patterns and their corresponding receptors that signal responses and shape the differentiation of T-cell subsets and B cells; and, ultimately (3) the effector and regulatory mechanisms that eliminate these invaders while constraining collateral damage to vital tissue. These insights create a foundation for the development of new, immune-based strategies for prevention or enhanced clearance of systemic fungal diseases.
Three key factors orchestrate the adaptive immune response to pathogenic fungi: dendritic cells, pattern-recognition receptors, and antigen-specific T and B cells.
Encounters with fungi require a coordinated host innate and adaptive immune response to successfully eradicate the fungus and promote long-lived immunological memory of the encounter. This review covers three key elements that orchestrate this coordinated response: dendritic cells (DCs), pattern-recognition receptors (PRR), and antigen-specific T and B cells. DCs lie at the intersection of innate and adaptive immunity. These cells are capable of taking up and processing antigen for display by major histocompatibility complex (MHC) class I or MHCII molecules to naïve T cells and of mediating fungicidal activity. Surface and intracellular PRRs enable DCs to sense fungi. On fungal recognition, DCs secrete cytokines and express costimulatory molecules that help drive naïve CD4+ T-cell differentiation into a T-helper (Th) phenotype. In immunocompetent hosts, CD4+ T-cell-mediated clearance of fungi with limited tissue damage requires a finely tuned balance among Th1, Th17, and Treg (regulatory T cell) subsets; in CD4-deficient hosts, CD8+ T cells may come into play. A calibrated balance of helper, regulatory, and effector T- and B-cell responses integrate optimal innate and adaptive immunity to fungi.
CHARACTERIZATION AND FUNCTION OF DC AND MONOCYTE SUBSETS
Steinman and Cohn first reported the identification of a cell with “continually elongating, retracting, and reorienting” long cytoplasmic processes in the spleen and lymph nodes of mice (Steinman and Cohn 1973). These cells, termed DCs, are hematopoietic cells that serve as professional antigen (Ag)-presenting cells (APCs) and initiate T-cell responses. When DCs encounter Ag at the boundary of immunological defense sites, such as the skin, airways of the lung, or draining nodes of the lymphatic system, DCs amplify the innate immune response by secreting cytokines that recruit and activate other leukocytes. After uptake, processing and presentation of Ag, DCs initiate and shape adaptive responses by promoting naïve T-cell differentiation into effector or regulatory T cells. Since the discovery of DCs, many subsets have been described based on anatomical location, function, and surface marker expression (Fig. 1).
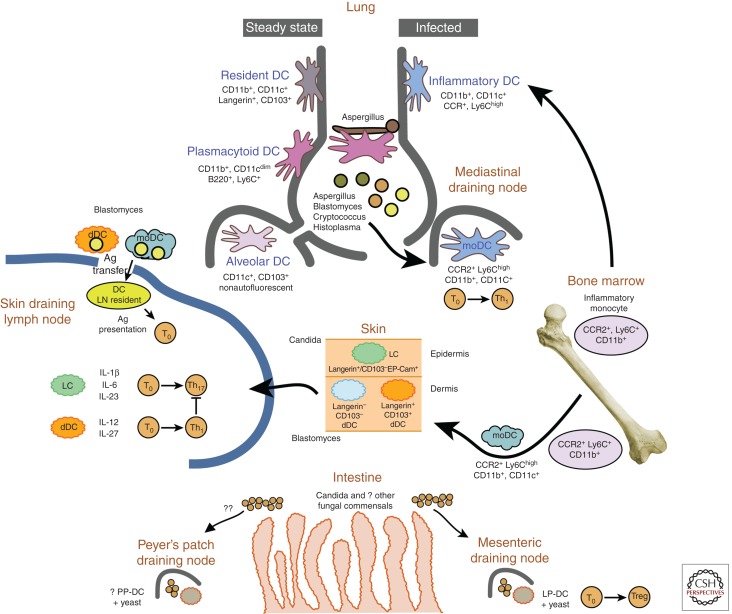
Figure 1.
Dendritic cells and priming of adaptive immunity to fungi. There are at least five subsets of DCs that participate in priming T cells during fungal infection. Lung DCs can be divided into CD11b+ and CD11b−. CD103+-resident classical (c)DCs are important in response to viruses, whereas inflammatory DCs participate in response to several fungal pathogens, and plasmacytoid DCs are vital in immunity to Aspergillus. Inflammatory monocyte-derived DCs (moDCs) are CD11b+ and Ly6Chigh. These cells express the chemokine receptor CCR2, which mediates egress from the marrow chiefly in response to the chemokines CCL2 and CCL7. In the absence of CCR2 (CCR2−/− mice), animals evince a skewed Th response in the lung, dominated by Th2 cytokines. Inflammatory monocyte-derived DCs also deliver subcutaneously injected vaccine yeast into draining lymph nodes, where they collaborate with migratory dermal and Langerhans DCs in priming CD4 T cells on antigen transfer into resident lymph node DCs. Dermal DCs elaborate IL-12 and IL-27 and efficiently prime Th1 cells, whereas Langerhans DCs elaborate IL-1β, IL-6, and IL-23 and skew the response toward Th17. Candida, a commensal of the intestinal tract, and other as-yet-unidentified fungi make up the “mycobiome” and modulate host physiology through interaction with C-type lectins, such as Dectin-1, likely displayed on intestinal DCs (Iliev et al. 2012). DCs in the lamina propria (LP-DC) influence the development of Treg, whereas those in the Pyer’s patch (PP-DC) have not been investigated with respect to fungi.
Plasmacytoid DCs
Plasmacytoid dendritic cells (pDCs) are typified by interferon-α (IFN-α) production in response to nucleic acids sensed by endosomal Toll-like receptors, and are characterized by surface expression of sialic acid binding immunoglobulin-like lectin H (Siglec H). pDCs induce IL-10-producing CD4+ Foxp3+ Treg cells, limit Th1 and Th17 cell polarization at mucosal sites, and activate CD8+ T cells (Takagi et al. 2011). pDCs control viral infection via the induction of CD8+ T cells, but may impair bacterial clearance and contribute to septic shock. Although pDCs mediate antiviral immunity, the role of pDCs in fungal infections is less clear. pDCs recognize Aspergillus fumigatus DNA via TLR9 (Ramirez-Ortiz et al. 2008) and inhibit Aspergillus growth in vitro. pDCs accumulate in the lungs in a murine model of Aspergillus pulmonary infection (Ramirez-Ortiz et al. 2011), and their elimination enhances progression of infection, suggesting that pDCs may recognize and combat fungi directly in vivo.
A subset of pDCs exists that develops in the context of elevated IFN-α and is similar to pDCs found in Peyer’s patches (Li et al. 2011). Uncharacteristically, this pDC subset fails to produce IFN-α after stimulation with TLR ligands, but secretes elevated levels of interleukin (IL)-6 and IL-23 and primes Ag-specific Th17 cells in vivo. This finding suggests a potential role for IFN-α-elicited pDCs in the polarization of antifungal Th17 cells. Combined with the recent findings that pDCs are critical mediators of Treg/Th17 balance at mucosal surfaces, recognition of fungi by pDCs or IFN-α-elicited pDCs at mucosal surfaces may tilt the balance toward tolerance or inflammation.
Conventional DCs
Conventional DCs or resident DCs exist in the lymphoid tissue and are comprised of two main subpopulations: CD8+ and CD4+CD8− resident DCs. The spleen contains a third, minor population of so-called double-negative DCs, which lack CD4 and CD8 expression and appear to be largely similar in function to CD4+CD8− DCs (Luber et al. 2010). CD8+ resident DCs are identified by the surface phenotype CD8+CD4−CD11b−CD11c+MHCII+DEC205+ and are located chiefly in the T-cell zone of the spleen and lymph nodes (Idoyaga et al. 2009). A major function of CD8+ DCs is to cross-present Ag via MHCI to CD8+ cytotoxic T lymphocytes (CTLs) (den Haan et al. 2000). CD8+ DCs obtain Ag by engulfment of live or apoptotic cells or Ag-containing apoptotic vesicles.
DCs acquire and cross-present Histoplasma capsulatum Ags to CTL by ingestion of live or killed yeasts or uptake of Histoplasma-containing apoptotic macrophages (Lin et al. 2005b). Subcutaneous injection of apoptotic phagocytes containing carboxyfluorescein succinimidyl ester (CFSE)-labeled, heat-killed Histoplasma results in the accumulation of CFSE in CD11c+ cells in skin-draining lymph nodes and CD11c+-dependent CTL-mediated protection against Histoplasma challenge (Hsieh et al. 2011). Although these studies show that fungal Ags can be acquired and presented by resident DCs, the resident DC subpopulation(s) involved in vivo remain undefined.
CD4+CD8− resident DCs also cross-present fungal Ags. In studies using an OVA-expressing strain of Saccharomyces cerevisiae, both CD8+ and CD4+CD8− DCs from mouse spleen that were primed with OVA–S. cerevisiae–primed robust OVA-specific CD4+ T-cell proliferation ex vivo; however, only CD4+CD8− DCs stimulated an OVA-specific CD8+ T-cell response (Backer et al. 2008). Further work will be needed to unravel the relative contributions of resident DC subpopulations to CD4+ T-cell and CTL activation and polarization in vivo.
During vaccine immunity to Blastomyces dermatitidis, lymph node–resident DCs primed antifungal T-cell responses after Ag transfer from other cells, although the phenotype of resident DC was not defined. DC acquisition of Ag required ferrying of yeast from the skin to the lymph node by migratory and monocyte-derived DCs, after which resident DCs in the skin-draining lymph nodes acquired and displayed Ag and primed Ag-specific CD4+ T cells (Ersland et al. 2010).
Migratory DCs
Migratory DCs (or tissue DCs) are immature DCs located mainly in peripheral tissues, such as the skin, lung, and gut. Following uptake of Ag, migratory DCs exit the tissue and mature as characterized by (1) enhanced Ag processing and presentation, (2) down-regulation of tissue homing receptors, (3) up-regulation of CCR7, and (4) increased surface display of costimulatory molecules. CCR7+ DCs migrate to the T-cell zone of lymphoid tissue where they can activate naïve T cells or transfer Ag to resident DCs (Banchereau and Steinman 1998; Allan et al. 2006). Migratory DCs have the capacity for division and self-renewal in situ, whereas monocyte subsets also contribute to replenishment of migratory DCs. Migratory DCs line the surfaces of the body exposed to the environment and thus encounter fungi and other pathogens and Ags. Although the migratory DC networks that line the skin, lung, and intestine share similarities, each site has functional differences that are important in antifungal immunity.
Skin
The skin contains a network of DCs that can be divided into the epidermis-associated Langerhans cells (LCs) and a collection of dermis-associated dermal DCs (Henri et al. 2010). In addition to their epidermal location, LCs are characterized by surface expression of langerin (CD207), CD11c, and MHCII. The dermis contains LCs that migrate to the draining lymph node, CD207+CD103+ dermal DCs, and a group of CD207−CD103− DCs (Henri et al. 2010). On subcutaneous injection of B. dermatitidis vaccine, DEC205+ skin-derived DCs migrated to the draining lymph nodes in a CCR7-dependent fashion, presented (or transferred) model Ag expressed by the yeast, and activated CD4+ T cells (Ersland et al. 2010).
LCs and CD8+ dermal DCs have been shown to specialize in Ag presentation and T-cell polarization functions in a cutaneous exposure model to Candida albicans (Igyarto et al. 2011). LCs are required for the generation of Ag-specific Th17 cells via the production of elevated levels of IL-6, IL-1β, and IL-23. Although LCs are not required for the generation of CTL, CD207+ dermal DCs are required for CTL and also Th1 polarization. Compared with LCs, CD207+ dermal DCs produce more IL-12 and IL-27, and less IL-1β and IL-6, and no IL-23, making them poor promoters of Th17. CD207+ dermal DCs also blunt the ability of LCs and CD207− dermal DCs to promote Th17 responses. Because IL-12 and IL-27, as well as IFN-γ from Th1 cells, inhibit Th17 differentiation and proliferation, CD207+ dermal DCs likely block Candida-specific Th17 cells by promoting Th1 differentiation. Thus, exposure of LCs and CD207+ dermal DCs to Candida can promote opposing effects via elaboration of polarizing cytokines that induce development of Th1 or Th17 responses (Igyarto et al. 2011).
Lung
DCs in the lung and airways confront constant exposure to inhaled spores and hyphal fragments. A network of DCs lines the airways, sampling inhaled Ags and shuttling them to the mediastinal lymph nodes. Besides pDCs, lung DC subsets include two broad subsets: CD103+ DCs and CD11b+ DCs. CD103+ lung DCs also express CD207, making them similar to CD207+CD103+ dermal DCs. CD103+ DCs extend dendrites into the airway lumen to sample Ag without disturbing the epithelium (Jahnsen et al. 2006; Sung et al. 2006). CD103+ DCs can acquire soluble and apoptotic-cell-associated Ags from the airway and migrate to mediastinal lymph nodes under steady state and inflammatory conditions. At the lymph node, CD103+ DCs cross-present Ag and activate CTL (Desch et al. 2011). CD11b+ DCs differ from monocyte-derived DCs and specialize in cytokine and chemokine production (Beaty et al. 2007) as well as presenting Ag to CD4+ T cells in the mediastinal lymph node after migration (del Rio et al. 2007). Rapid recruitment of Ly6C+ monocyte-derived DCs to the lung on inflammation has clouded the functional analysis of lung resident CD11b+ DCs, which lack Ly6C expression. On lung exposure to A. fumigatus conidia, CD103+ DCs failed to take up and transport conidia to the mediastinal lymph node, whereas CD11b+ DCs did transport conidia (Hohl et al. 2009). In this model, lung CD11b+ DCs were reduced in CCR2−/− mice, relative to wild-type mice, following A. fumigatus exposure. Conversely, naïve CCR2−/− mice had lung CD11b+ DC numbers similar to wild-type mice, suggesting that recruited monocyte-derived DCs and not lung resident CD11b+ DCs are responsible for conidial uptake and Ag presentation in the setting of A. fumigatus–induced inflammation.
Intestine
As in the lung, DCs in the intestine are situated on the basolateral side of the epithelium, and largely isolated from the gut microflora. DCs in the intestine localize to the lamina propria (LP-DCs) and Peyer’s patch (PP-DCs); both subsets in each region differentially regulate immune responses. The role of PP-DCs and LP-DCs in generating antifungal immunity in the gut is unclear and relatively unstudied. C. albicans, a gut commensal, can cause systemic infection if the gut epithelial/DC barrier is breached or in the setting of broad-spectrum antibiotic use, leading to Candida overgrowth. Strong induction of Treg cells by LP-DCs in the mesenteric lymph node highlights the critical role of limiting inflammation in the gut to maintain the epithelial barrier and prevent disseminated infection. Furthermore, heightened Th17 responses in the gut impair protective Th1 responses and worsen Candida infection (Zelante et al. 2007). Although bone marrow–derived DCs produce IL-23 in response to Candida in vitro and IL-23 neutralization promoted fungal clearance in vivo, the identity of the DC subset recognizing and responding to the fungus in this model was not determined. Nevertheless, DCs in the gut appear to tightly control tolerance and immunity to fungal organisms.
Monocytes, Monocyte-Derived DCs, and Inflammatory DCs
Monocytes are derived from a macrophage-DC progenitor and, in the absence of inflammation, are found in the bone marrow and circulating at low levels in the blood and spleen. Two classes of CD11b+CD115+ monocytes arise from the progenitor and circulate in the blood, Ly6C+CCR2+ and Ly6C−CX3CR1hi monocytes (Geissmann et al. 2003). Monocytes have broad developmental plasticity, replenish subsets of DCs and LCs in the setting of experimental depletion (Ginhoux et al. 2007), and represent an emergency store of DC precursors that can be rapidly deployed. Under inflammatory conditions, Ly6C+CCR2+ monocytes migrate to inflammatory sites and acquire expression of the DC markers CD11c and MHCII, while losing expression of Ly6C (Osterholzer et al. 2009), thus becoming “inflammatory DCs.” Whereas Ly6C−CX3CR1hi cells do not appear to be involved in innate immunity to fungi (Dominguez and Ardavin 2010), Ly6C+CCR2+ monocytes play a critical role in responding to many medically important fungi including A. fumigatus (Hohl et al. 2009), Cryptococcus neoformans (Osterholzer et al. 2009), H. capsulatum (Szymczak and Deepe 2009), and B. dermatitidis (Ersland et al. 2010).
Monocyte-derived DCs have an outsized role in antifungal immunity, particularly through the induction of Th1 cells. CCR2−/− mice show skewed Th2 responses and poorly controlled H. capsulatum infection compared to wild-type mice (Szymczak and Deepe 2009). Similar CCR2-dependent phenotypes are found in experimental infection with A. fumigatus or C. neoformans; that is, priming Th1 cells in response to fungi requires CCR2+ monocyte-derived inflammatory DCs (Traynor et al. 2000; Hohl et al. 2009; Osterholzer et al. 2009; Ersland et al. 2010). The tissue environment has a prominent role in inflammatory DC function, as the defect in CD4+ T-cell priming by these DCs during infection with A. fumigatus is restricted to the lung in CCR2−/− mice and not to other lymphoid organs, such as the spleen (Hohl et al. 2009). Similarly, although Ly6C+ CCR2+ monocytes play a major role in delivering B. dermatitidis into skin-draining lymph nodes after subcutaneous vaccination, this shuttling function can be compensated by other skin migratory DC subsets in CCR2−/− mice (Ersland et al. 2010). Conversely, CCR2+ monocytes and monocyte-derived inflammatory DCs are essential to prime Ag-specific CD4+ T cells in the lung during infection with Blastomyces or Histoplasma (Wuthrich et al. 2012b). Thus, in the lung, which is often the primary route of infection for fungi, but not in the skin or the spleen, monocyte-derived DCs play a critical and indispensable role in antifungal immunity.
PATTERN-RECOGNITION RECEPTORS
In mammals, fungi are detected by germline encoded PRRs that are expressed by innate cells (Medzhitov 2007). The three major pathogen-associated molecular patterns (PAMPs) that are unique to fungi and set them apart from the mammalian host are chitin, α- and β-glucans, and mannans. The innate recognition of these fungal PAMPs activates signaling cascades to induce the expression of MHC, costimulatory molecules and cytokines by APC that influence the development of adaptive immunity. Because Th17 and Th1 cells are the principal T-helper subsets that contribute to protective immunity to several pathogenic fungi, we highlight the most recent literature on the signaling pathways and other factors that influence the differentiation of these two T-helper subsets.
Among the best-characterized PRRs that recognize fungi are the Toll-like receptors (TLR) and C-type lectins (CLR) (Fig. 2). TLR1–4, 6, 7, and 9 recognize a variety of fungal species through mostly undefined ligands (Netea et al. 2004b; Biondo et al. 2005, 2011; Chai et al. 2009; Kasperkovitz et al. 2010; Bourgeois et al. 2011). The main TLRs involved in sensing fungal ligands are TLR2, TLR4, and TLR9 that recognize zymosan, phospholipomannan, O-linked mannans, glucoronoxylomannan, and fungal DNA (Shoham et al. 2001; Brown 2011; Romani 2011). Mice lacking the signaling adaptor myeloid differentiation primary response protein 88 (Myd88) are more susceptible to infection with C. neoformans, C. albicans, A. fumigatus, B. dermatitidis, and Paracoccidioides brasiliensis (Bellocchio et al. 2004; Biondo et al. 2005; Bretz et al. 2008; Calich et al. 2008; Wuthrich et al. 2011), emphasizing important roles for TLR signaling in antifungal immunity, but also reflecting the involvement of Myd88 in IL-1 signaling. However, in murine experimental models of infection, there are conflicting reports on individual contributions of multiple TLRs and fungal species (Calich et al. 2008; Netea et al. 2008). In humans, a similar controversy on the role of Myd88 in mediating antifungal immunity has been reported. Whereas fungal infections were not a problem for children with autosomal recessive Myd88 deficiency (von Bernuth et al. 2008), patients with TLR1 and TLR4 polymorphisms were more susceptible to candidemia (Plantinga et al. 2012) and invasive aspergillosis during stem cell transplantation (Bochud et al. 2008).
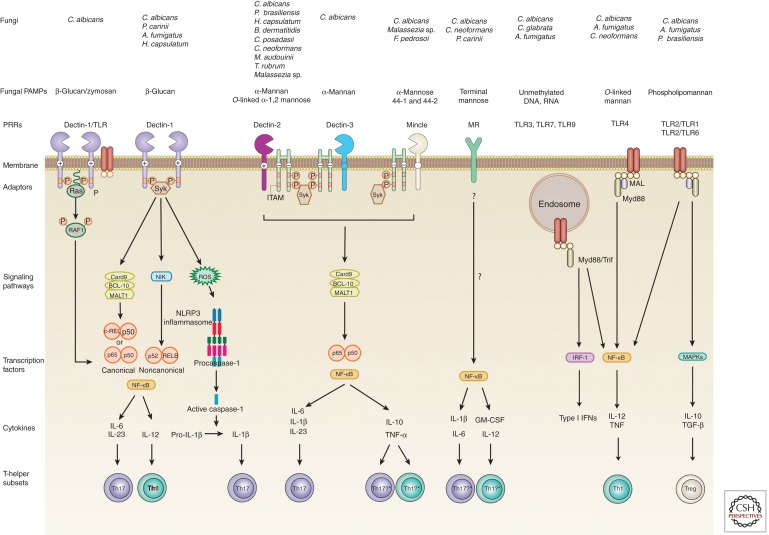
Figure 2.
PRR and signaling pathways that lead to differentiation of antifungal T-helper cells. Recognition of fungal pathogen–associated molecular patterns (PAMPs) is mediated by TLRs and CLRs. The binding of fungi or β-glucan to Dectin-1 recruits SYK to the two phosphorylated receptors, which leads to the formation of a complex involving Card9, BCL10, and MALT1 (BCM). This results in the release of NF-κB consisting of either p65–p50 or REL–p50 dimers into the nucleus (Geijtenbeek and Gringhuis 2009). Syk activation also induces the noncanonical NF-κB pathway mediated by NF-κB-inducing kinase (NIK) and the nuclear translocation of p52–RELB dimers. Dectin-1 enhances TLR2 and TLR4-induced cytokines in a Syk-independent manner through the serine/threonine protein kinase RAF1 by Ras proteins, which leads to the phosphorylation of p65 (Gringhuis et al. 2009). Among other cytokines, these pathways lead to the production of IL-6, IL-23, and IL-12 that induce Th17 and Th1 cells, respectively. Dectin-1 recognition of C. albicans can also activate the NLRP3 inflammasome through a mechanism that involves Syk, ROS, and potassium efflux (Poeck and Ruland 2010). Fungus-induced pro-IL-1β is cleaved by active caspase-1 to bioactive IL-1β to favor Th17 development. Dectin-2 activation leads to FcR-γ-dependent recruitment and phosphorylation of Syk and activated NF-κB and MAPKs (p38, JNK, and Erk) (Saijo et al. 2010). Card9 is required for the activation of NF-κB and production of cytokines that lead to Th17 cell differentiation. Recognition of α-mannose in Malassezia species by Mincle activates the FcR-γ-Syk-Card9 pathway and translocates NF-κB into the nucleus to induce the activation of proinflammatory cytokines (Yamasaki et al. 2009). *Although fungal PAMPs have yet to show the ability to induce a distinct T-helper subset by this pathway, the mycobacterial cord factor and its synthetic analog are potent adjuvants for the differentiation of Mincle-induced Th1 and Th17 cells (Schoenen et al. 2010). Dectin-3 (MCL, Clec4d, or Clecsf8) also recognizes α-mannan from C. albicans (Zhu et al. 2013) and TDM from M. tuberculosis (Miyake et al. 2013). Dectin-3 can dimerize with Dectin-2 (Zhu et al. 2013) and Mincle (Lobato-Pascual et al. 2013). It is unclear whether the induction of Th17 and Th1 cells requires recognition of fungal PAMPs by homo- versus heterodimers of Dectin-2. The MR lacks a classical signaling motif in its short cytoplasmic tail, but it induces proinflammatory cytokines that have been implicated in Th17 and Th1 differentiation (Willment and Brown 2008). Although MR-dependent triggering of human memory T cells produced IL-17, further studies with naïve T cells will be needed to establish the role of the MR in Th17 cell differentiation (van de Veerdonk et al. 2009). Myd88 is critical for the signaling of TLR2 and TLR4. Phospholipomannans and O-linked mannans are recognized by TLRs at the plasma membrane, whereas fungal nucleic acids are sensed by endosomal TLRs and induce NF-κB-, MAPK-, and IRF-dependent cytokine production. TLR2 signaling is thought to generate weaker proinflammatory signals, but induce strong stimulation of TGF-β and IL-10 that induces Treg cells (Netea et al. 2004a; Sutmuller et al. 2006).
Besides the TLRs, CLRs expressed by myeloid and mucosal epithelial cells are key PRRs for the recognition of fungi and induction of protective immunity (Netea et al. 2008; Kerrigan and Brown 2010, 2011; Brown 2011; Romani 2011). CLRs belong to a large family of proteins that recognize ligands in a calcium-dependent manner, which vary in microbes from endogenous to exogenous and are often conserved and carbohydrate based (Kerrigan and Brown 2010). Some CLR contain cytoplasmic signaling motifs that allow direct activation of intracellular signaling cascades; others lacking these motifs make use of adaptor molecules to initiate signal transduction (Kerrigan and Brown 2010). Below, we discuss recent progress on the cell-associated CLRs Dectin-1, Dectin-2, Dectin-3, Mincle, and the mannose receptor.
Dectin-1
Dectin-1 is the archetypical and best-studied non-TLR PRR shown to link innate and adaptive immunity and instruct differentiation of Th1 and Th17 cells (LeibundGut-Landmann et al. 2007; Rivera et al. 2011). Dectin-1 recognizes β-1,3-glucan from fungi, plants and some bacteria (Taylor et al. 2007), and unidentified T cell and mycobacterial ligands (Tsoni and Brown 2008). On ligand activation, Dectin-1 signals through an immunoreceptor tyrosine-based activation-like motif (ITAM-like or HemITAM) (Kerrigan and Brown 2010) to produce cytokines via multiple signaling pathways (reviewed in Vautier et al. 2012). The best characterized Dectin-1 signaling pathway is the Syk/CARD9 pathway leading to activation of the canonical NF-κB subunits p65 and c-Rel and subsequent production of pro-IL-1β, IL-6, IL-10, IL-23, and tumor necrosis factor (TNF)-α (Gringhuis et al. 2011). Cytokine production can be modulated via the formation of inactive RelB–p65 dimers through the noncanonical NF-κB subunit RelB in a NIK-dependent pathway (Gringhuis et al. 2009) and the Syk-independent activation of Raf-1. The former reduces IL-1β and IL-12p40 expression, whereas the latter increases the production of these pro Th1/Th17 cytokines. The balance toward a Th17 response is favored through the collaborative interaction of Dectin-1 with TLR-2 that leads to the production of prostaglandin E2, which will up-regulate the Th17 polarizing cytokines IL-6 and IL-23 (Smeekens et al. 2010).
In addition, recognition of β-glucan, A. fumigatus, and C. albicans by Dectin-1 and TLR2 activates the NLRP3 inflammasome leading to the production of bioactive IL-1β (Gross et al. 2009; Hise et al. 2009; Kankkunen et al. 2010; Poeck and Ruland 2010; Said-Sadier et al. 2010), thereby enhancing Th17 cell development and antifungal immunity. In a pulmonary model of A. fumigatus infection, Dectin-1 decreased the production of IL-12 and IFN-γ in innate cells, which decreased T-bet expression in A. fumigatus–specific CD4 T cells and enabled Th17 differentiation (Rivera et al. 2011). Thus, Dectin-1 signaling drives the production of Th17-cell-promoting cytokines that yield resistance to C. albicans, A. fumigatus, and Pneumocystis carinii infection (Saijo et al. 2007; Taylor et al. 2007; Werner et al. 2009).
Some fungi (e.g., A. fumigatus and C. albicans) have developed mechanisms to mask their β-glucan exposure and limit detection by immune cells (Hohl et al. 2005; Steele et al. 2005; Wheeler et al. 2008). β-glucan shielding can also vary among different strains within a fungal species. For example, H. capsulatum strain G186A shields its β-glucan by α-glucan so that yeast are not recognized by Dectin-1 (Rappleye et al. 2007), whereas strain G217B mostly lacks α-(1,3)-glucan on the yeast surface and is recognized by Dectin-1 (Wang et al. 2014). Thus, the induction of Th17 cells, the acquisition of vaccine-induced resistance and primary resistance to the latter strain is blunted in Dectin-1–/– versus wild-type mice (Wang et al. 2014).
Dectin-2 Cluster
The last few years have revealed exciting new insights into the function and role of the Dectin-2 cluster in mediating antifungal immunity. The Dectin-2 family is comprised of Dectin-2, Mincle, MCL, DCIR, DCAR, and BDCA-2. Aside from DCIR, all of the other receptors have short cytoplasmic tails that lack signaling motifs and associate with the FcR-γ chain, an adaptor containing an ITAM motif (Graham and Brown 2009). Dectin-2 recognizes C. albicans, S. cerevisiae, Microsporum audouinii, Trichophyton rubrum, A. fumigatus, H. capsulatum, P. brasiliensis, Malassezia sp., B. dermatitidis, and C. posadasii (Kerscher et al. 2013; Wang et al. 2014). Dectin-2 recognizes C. albicans and Malassezia via N- and O-linked α-mannan, respectively, on their surface (McGreal et al. 2006; Sato et al. 2006; Saijo et al. 2010; Ishikawa et al. 2013). It has been proposed that an active Dectin-2 ligand could be a multivalent terminal α-1,2-mannose attached to glycans, proteins, and presumably any kind of scaffold (Ishikawa et al. 2013). Dectin-2-deficient mice are more susceptible to primary C. albicans infection (Robinson et al. 2009; Saijo et al. 2010). The Dectin-2/FcR-γ/Syk/Card9 signaling axis is indispensable for the development of vaccine-induced, Ag-specific Th17 cells and immunity to the systemic, endemic, dimorphic fungi B. dermatitidis, H. capsulatum, and Coccidioides posadasii (Wang et al. 2014).
Mincle is another FcR-γ-coupled activating receptor that recognizes pathogenic fungi and Mycobacteria (Ishikawa et al. 2009, 2013; Yamasaki et al. 2009). Mincle binds glycolipids, such as trehalose-6,6-dimycolate (TDM) from Mycobacterium tuberculosis, and novel glyceroglycolipids from Malassezia (Ishikawa et al. 2013). Although not shown yet for fungi, Mincle induces Th1/Th17 adaptive immunity in response to TDM and its synthetic analogue trehalose-6,6-dibehenate (TDB) (Schoenen et al. 2010; Shenderov et al. 2013). Mincle-deficient mice are more susceptible to primary infection with C. albicans and Malassezia (Wells et al. 2008; Yamasaki et al. 2009). However, Mincle is dispensable for development vaccine-induced immunity and Th17 cells against the three major systemic dimorphic fungi: B. dermatitidis, H. capsulatum, and C. posadasii (Wang et al. 2014).
Similar to Mincle, macrophage C-type lectin (MCL, also called Dectin-3, Clecsf8, and Clec4d) is also an FcR-γ-coupled activating receptor that binds to TDM (Miyake et al. 2013). MCL is constitutively expressed in myeloid cells, whereas Mincle is barely expressed in resting cells, but inducible by TDM (Schoenen et al. 2010). In response to TDM, MCL and Mincle promote the development of Th1/Th17 cells by up-regulating the costimulatory molecules CD80, CD86, and CD40 (Miyake et al. 2013). MCL also contributes to Th17-cell-mediated EAE development. Whether MCL induces antifungal Th17 cell responses is unclear but MCL-deficient mice are highly susceptible to C. albicans infection (Zhu et al. 2013).
The multivalent ligands on the fungal surface likely induce multimerization of monomeric and dimeric CLRs. For example, Mincle and MCL form a disulfide-linked heterodimer associated with FcR-γ (Lobato-Pascual et al. 2013) and Dectin-2 forms a heterodimeric PRR with Dectin-3 for sensing and mediating host defense against C. albicans (Zhu et al. 2013). The heterodimer showed higher affinity to fungal α-mannan than their respective homodimers and responded effectively to fungal infection, indicating that dimerization may provide different sensitivity and diversity for host cells to detect fungal pathogens and induce adaptive immunity. Because individual and dimeric CLR of the Dectin-2 cluster discussed here signal through the FcR-γ/Syk/Card9 axis, it is noteworthy that Card9 affects the development of Th1/Th17 cells in vivo mostly at the stage of T-cell differentiation and not during the activation, expansion, and survival during the contraction phase (Wang et al. 2014).
Mannose Receptor (MR)
The MR has a short cytoplasmic tail that lacks classical signaling motifs, and its downstream signaling pathway is unknown (Willment and Brown 2008). The MR can induce NF-κB activation and the production of IL-12, GM-CSF, IL-8, IL-1β, and IL-6 (Zhang et al. 2004; Pietrella et al. 2005; Taylor et al. 2005; Tachado et al. 2007). One caveat of studies with MR-deficient mice is that MRC1 expression is coregulated with the macrophage activating miRNA (miR-511-3p) (Squadrito et al. 2012). Although the MR has been reported to induce Th17 cell differentiation of human T cells in response to C. albicans, memory and not naïve T cells were the major source of the IL-17 produced (Levitz 2009; van de Veerdonk et al. 2009). Because different sets of cytokines are required to prime naïve T cells and propagate already primed memory T cells, the role of the MR in inducing Th17-cell differentiation remains inconclusive. Because the TLR2/Dectin-1 pathway has a secondary amplification effect on MR-induced IL-17 production (Levitz 2009; van de Veerdonk et al. 2009), it is conceivable that C. albicans–derived β-glucan is more important for the differentiation of naïve cells into Th17 cells, whereas the MR might play a more prominent role in triggering IL-17 production by memory cells.
T- AND B-CELL IMMUNITY
It is generally acknowledged that activation of the adaptive arm of the immune system is critical for resolution of fungal infection in the host. The transition from innate to adaptive immunity is facilitated primarily by DCs, although macrophages contribute. These phagocytes process and present fungal Ag to naïve CD4+ T cells in the context of class II MHC. This interaction initiates the commitment to effector Th subsets. DCs also activate CD8+ T cells by Ag presentation via MHCI (Fig. 3). For Ags that enter through the exogenous pathway, engagement of CD8+ proceeds through a mechanism termed cross-presentation in which Ags are shuttled into the class I MHC pathway. In contrast to the requirement of Ag processing for activation of T cells, B cells directly react to fungal Ags and secrete immunoglobulins that may influence the outcome of infection.
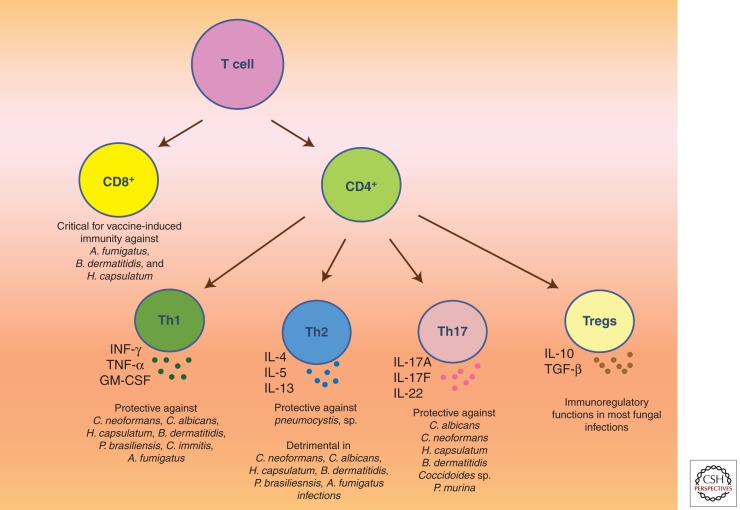
Figure 3.
Schematic illustration of different subsets of T lymphocytes and their roles in combating different fungal pathogens. T cells form an integral part of adaptive immunity in vertebrates. They are classified into two distinct lineages: CD4+ (also known as Th cells) and CD8+ cells. The former are further subdivided into Th1, Th2, Th17, and Treg cells based on their effector functions. Th1 cells are the primary source of IFN-γ and are critical for host defense against a majority of fungal pathogens. Similarly, Th17 cells orchestrate potent anticandidacidal activity by secreting IL-17A, IL-17F, and/or IL-22. In contradistinction, Th2-cell-derived cytokines are associated with exacerbation of most fungal infections, with the exception of pneumocystosis. Treg cells prevent excessive damage to the host during infection through various soluble mediators and contact-dependent mechanisms. CD8+ T cells represent an additional line of defense to combat fungal infections. Two distinct lineages namely Tc1 (IFN-γ-producing CD8+ T cells) and Tc17 (IL-17-producing CD8+ T cells) may exist to bolster Th1 and Th17 immunity, respectively.
Th1 Immunity
The Th1 immune response is instrumental in host defense against most fungal pathogens, and its importance is well established in experimental murine models and in human infections. Following exposure, APCs produce IL-12 that is critical for Th1 lineage commitment. Genetic mutation in the IL-12 signaling pathway is associated with predisposition to a wide variety of fungal diseases, such as cryptococcosis, candidiasis, paracoccidioidomycosis, and coccidioidomycosis (Moraes-Vasconcelos et al. 2005; Aytekin et al. 2011; Vinh et al. 2011; Jirapongsananuruk et al. 2012). Furthermore, increased susceptibility to histoplasmosis has been observed in mice lacking IFN-γ (Allendoerfer and Deepe 1997) and in a patient with a genetic deletion mutation in IFN-γ receptor-1 gene (Zerbe and Holland 2005). Conversely, patients receiving adjunctive IFN-γ immunotherapy display augmented protection against aspergillosis, cryptococcosis, and coccidioidomycosis (Kelleher et al. 2006; Duplessis et al. 2011; Jarvis et al. 2012).
Th1 cells orchestrate antifungal immune responses through the release of proinflammatory cytokines IFN-γ, TNF-α, and GM-CSF (Fig. 3). The signature Th1 cytokine, IFN-γ, manifests pleotropic effects on immune cells during infection. It induces classical activation of macrophages that is critical for arresting growth of intracellular fungal pathogens including H. capsulatum, B. dermatitidis, P. brasiliensis, and Coccidioides immitis (Beaman 1987; Brummer and Stevens 1995; Sugar et al. 1995; Calvi et al. 2003). These classically activated phagocytes are speculated to mediate their fungicidal activities through the release of nitric oxide and reactive oxygen intermediates (Wuthrich et al. 2012a). In addition to its effects on macrophages, IFN-γ prompts antibody class switching to IgG2a in B cells (associated with antifungal effects [Snapper and Paul 1987; Lin et al. 2009]) and enhances phagocytosis (Shalaby et al. 1985) and Ag processing/presentation in APCs (Schroder et al. 2004) to combat fungal infections.
TNF-α shares multiple redundant functions with IFN-γ, including classical activation of macrophages (Novak and Koh 2013). Animals deficient in TNF-α are highly vulnerable to a range of fungal infections (Nagai et al. 1995; Huffnagle et al. 1996; Allendoerfer and Deepe 1998; Opata et al. 2013). The protective effects of this cytokine during fungal diseases have also been corroborated in humans. Individuals homozygous for A/A at position -308 in the TNF-α promoter display elevated TNF-α activity and augmented resistance to aspergillosis (Sambatakou et al. 2006). In contrast, patients given TNF-α blockers to treat inflammatory diseases suffer from a myriad of fungal infections (Wallis et al. 2004). The importance of GM-CSF in fungal diseases has been elucidated in murine model of pulmonary histoplasmosis. Administration of anti-GM-CSF antibody reduced protective immunity to H. capsulatum (Deepe et al. 1999). A recent report has indicated that GM-CSF facilitates its antifungal responses through sequestration of zinc from intracellular yeasts and stimulation of reactive oxygen species in macrophages (Subramanian Vignesh et al. 2013).
Th2 Immunity
For the vast majority of fungal infections, Th2 immunity manifests a detrimental influence on the host. These Th2 responses are comprised of CD4+ T-cell-derived cytokines IL-4, IL-5, and IL-13, and B-cell-secreted IgE. Exaggerated synthesis of these soluble factors in most mycotic diseases interferes with pathogen clearance, and on rare occasions, failure to regulate them can result in a fatal outcome. For example, in mice lacking the chemokine receptor CCR2, infection with H. capsulatum or C. neoformans induces a dominant IL-4 response that is associated with impaired host resistance (Traynor et al. 2000; Szymczak and Deepe 2009). In pulmonary aspergillosis and cryptococcosis, undesired activation of Th2 response leads to nonprotective allergic inflammation in mice (Hogaboam et al. 2000; Muller et al. 2013). These animals show signs of eosinophilia, goblet-cell hyperplasia, and heightened susceptibility to the pathogen. In addition to these experimental findings, a single nucleotide polymorphism in the IL-4 promoter region that is linked with amplified IL-4 production occurs with increased frequency in women with recurrent vulvovaginal candidiasis (Babula et al. 2005). This genetic defect is hypothesized to predispose the individuals to C. albicans by suppressing the fungicidal activity of macrophages encountering C. albicans yeasts (Cenci et al. 1993).
The mechanisms by which Th2 cytokines dampen host immunity are multifactoral. Both IL-4 and IL-13 drive alternative activation of macrophages that is associated with uncontrolled fungal growth. C. neoformans and H. capsulatum proliferate robustly in macrophages primed with IL-4, as opposed to the ones that are classically activated (Voelz et al. 2009; Winters et al. 2010). These alternatively activated phagocytes display amplified levels of arginase-1, an enzyme that potentially diminishes the amount of nitric oxide required for fungicidal activity (Davis et al. 2013). Additionally, IL-4 modulates fungal access to specific micronutrients in macrophages. This cytokine up-regulates the transferrin receptor (Weiss et al. 1997) on the cell surface that results in enhanced iron acquisition. This phenomenon is believed to augment fungal growth within the cells. In addition, IL-4 alters intracellular survival by increasing zinc content that supports the growth of fungal pathogens (Winters et al. 2010).
In sharp contrast to these findings, Th2 responses bestow protection to the host in pneumocystosis. A recent report suggested that alternatively activated macrophages driven by IL-13 show increased fungicidal capacity in Pneumocystis murina infection, as opposed to classically activated cells (Nelson et al. 2011). Antibody class switching to a more protective IgG subclass induced by Th2 cytokines also might contribute to the heightened protection against P. murina because patients with this defect are vulnerable to Pneumocystis pneumonia (Tsai et al. 2012; Al-Saud et al. 2013). Our understanding of why Th2 cytokines are protective in pneumocystis but not other mycotic diseases is incomplete. A probable explanation could be that Pneumocystis behaves similarly to helminth parasites by tightly adhering to pulmonary epithelial cells to establish infection (Perez-Nazario et al. 2013). In such a setting, release of Th2 cytokines by effector cells is critical for minimizing the virulence of helminths. Although Th2 immunity significantly contributes to host defense against pneumocystosis, it cannot compensate for the loss of other Th subsets. Patients with autosomal dominant hyper IgE syndrome (HIES) show defects in generation of Th17 responses (but not Th2 responses) and are found to be progressively more susceptible to Pneumocystis pneumonia (Grimbacher et al. 2005). Thus, in summary, resolution of pneumocystosis depends on a coordinated action of the Th2 arm of the immune system and other Th subsets.
Th17 Immunity
Th17 cells are a subset of CD4+ T cells that are developmentally distinct from Th1 and Th2 cells and are identified by the expression of cytokines IL-17A, IL-17F, and IL-22. The differentiation of this T-cell lineage requires various cytokines and transcription factors. TGF-β and IL-6 prime the initial differentiation of naïve CD4+ T cells to Th17 cells and IL-23 is critical for maintenance and expansion of these cells (Zuniga et al. 2013). Activation of STAT3 by IL-6 is indispensable for this process because the former directly regulates the transcription of ROR-γt, the master transcription factor controlling Th17 lineage commitment. The influence of Th17 immunity in bolstering host defenses against fungal pathogens has been well substantiated. Indeed, humans with genetic defects in IL-17 signaling axis are severely compromised in their ability to counter mycoses. Individuals with HIES show dominant negative mutation in STAT3 (Minegishi and Saito 2011). Consequently, these individuals are vulnerable to a spectrum of fungal infections (Grimbacher et al. 2005). Another rare genetic disorder called autoimmune polyendocrinopathy with candidiasis and ectodermal dystrophy (APECED) is linked with chronic and recurrent mucocutaneous candidiasis (Vautier et al. 2012). This defect is characterized by mutations in the autoimmune regulator (AIRE) gene that result in generation of autoantibodies directed against Th17 cytokines. Furthermore, hereditary mutations in the Dectin-1 signaling that shapes Th17 immunity have been identified in patients with chronic mucocutaneous candidiasis (Ferwerda et al. 2009). The importance of IL-17 responses in antifungal immunity is best studied in diverse models of experimental candidiasis. Mice deficient in IL-17 receptor fail to generate functional Th17 cells and are vulnerable to systemic and oral candidiasis (Conti et al. 2009). The IL-23–IL-17 axis is necessary for development of optimal immunity against C. albicans. In models of dermal and oral infection, IL-23-deficient animals develop progressively worse disease (Conti et al. 2009; Kagami et al. 2010). Likewise, in vulvovaginal C. albicans infection, impairing Th17 cell differentiation leads to heightened fungal burden at the site of challenge (Pietrella et al. 2011).
Two disparate mechanisms have been described through which Th17 cells manifest antifungal responses. In systemic challenge models, these cells recruit neutrophils by prompting the release of CXC chemokines (Hernandez-Santos and Gaffen 2012). The neutrophils, in turn, show potent anticandidacidal activity and clear the pathogen. In mucosal infection models, IL-17 prompts keratinocytes and epithelial cells to release antimicrobial peptides (AMPs), such as S100A proteins, β-defensins, and histatins that show direct killing activity (Liang et al. 2006; Conti et al. 2011). To overcome the antifungal effects of IL-17 pathway, pathogens have evolved to subvert the activity of this cytokine. Live Candida secretes an uncharacterized soluble factor that diminishes the production of IL-17 in human peripheral blood mononuclear cells by inhibiting indoleamine 2,3-dioxygenase (IDO) expression (Cheng et al. 2010). IDO is an enzyme that catalyzes the breakdown of tryptophan, thereby depriving microbes of this essential amino acid (Loughman and Hunstad 2012). Furthermore, IL-17 prompts morphological changes in C. albicans and A. fumigatus. This cytokine directly binds to the outer surface of these two pathogens and induces transcriptional changes that are associated with augmented hyphal growth and enhanced resistance to host antifungal defenses (Zelante et al. 2012).
Other less-appreciated Th17 cytokines, IL-17F and IL-22, may be involved in anti-Candida responses. The latter is fundamental for antifungal resistance in gastric and systemic candidiasis. IL-22 facilitates its protective effects through the elicitation of AMPs and by maintaining the integrity of the mucosal barrier to prevent dissemination of the disease (De Luca et al. 2010; Eyerich et al. 2011). However, contradictory findings have emerged wherein IL-22 is dispensable in murine models of oral and dermal candidiasis (Conti et al. 2009; Kagami et al. 2010). Much less is known about IL-17F and its role in fungal infections. During systemic Candida infection, deficiency of this cytokine does not impact host resistance (Saijo et al. 2010). Despite the limited or conflicting literature on IL-17F and IL-22, there is clinical evidence to support their protective effects in mycoses. Self-reacting antibodies against IL-17F and IL-22 are detected in APECED patients, who are vulnerable to mucocutaneous candidiasis (Hernandez-Santos and Gaffen 2012). Moreover, patients with dominant negative mutations in IL-17F are at an increased risk of developing chronic mucocutaneous candidiasis (Puel et al. 2011).
In other experimental models of mycoses, Th17 cells offer protection against several fungal infections, but they are not requisite for host survival. Neutralization of IL-17 during C. neoformans, P. carinii, or H. capsulatum infection results in a delayed clearance of disease but does not impact the lifespan of animals (Rudner et al. 2007; Deepe and Gibbons 2009; Wozniak et al. 2011). In contrast, the Th17 arm is critical for vaccine-induced immunity against the dimorphic fungal pathogens B. dermatitidis, C. posadasii, and H. capsulatum (Wuthrich et al. 2011). In this setting, IL-17-producing cells compensate for the loss of Th1 cells to counter the disease. The proposed mechanism by which these cells orchestrate antifungal activity is through recruitment and activation of neutrophils and macrophages in the lungs.
Contradictory to the above findings, unfavorable aspects of Th17 cells in fungal diseases have been discovered. In mice with gastric candidiasis, IL-23 induces exaggerated activation of Th17 pathway that is linked with deterioration of the disease (Zelante et al. 2007). The mice show heightened tissue damage and defective antifungal activity in neutrophils. Animals deficient in the transmembrane protein Toll IL-1R8 mount amplified Th17 responses and are highly susceptible to candidiasis (Bozza et al. 2008). Parallel findings are reported in murine aspergillosis in which Th17 cells hamper the outcome of infection by impairing the effector functions of neutrophils and exacerbating inflammatory pathology (Zelante et al. 2007). In these scenarios, neutralization of Th17 cytokines enhances resistance to the pathogen. To date, no definite mechanism to clarify the antagonizing function of Th17 cells in different fungal infections has been described.
Regulatory T Cells
Appropriate regulation of proinflammatory immune responses generated against invading infectious agents is necessary to limit collateral damage to the host. Regulatory T cells (Treg cells) contribute significantly to this task. This subset of T cells dampens the immune responses through a multitude of suppressive mechanisms that include secretion of inhibitory cytokines IL-10, TGF-β, or IL-35, repression of IL-2 release, perforin/granzyme-dependent cytolysis of APCs, synthesis of immunosuppressive adenosine, and through contact-dependent down-modulation of APC functions via cytotoxic T-lymphocyte-associated protein 4 (CTLA4) and lymphocyte activation gene 3 (LAG3) (Goodman et al. 2012).
In murine models of fungal infections, accelerated clearance of disease is achieved by altering the Treg cell activity. In candidiasis and paracoccidioidomycosis, signaling through the Toll-like receptor TLR2 and its downstream molecule MyD88 is critical for prolonging survival of Treg cells (Netea et al. 2004a; Loures et al. 2009). TLR2–/– mice express fewer Treg cells under homeostasis and disease state. Moreover, the infected mutant mice show a concomitant increase in Th17 cells. As a consequence, TLR2–/– mice resolve C. albicans and P. brasiliensis more efficiently than wild-type controls. Similarly, the chemokine receptor CCR5 regulates the equilibrium between Th17 cells and Treg cells. In the absence of this chemokine receptor, mice display reduced influx of the latter to the site of infection and enhanced resistance to P. brasiliensis and H. capsulatum (Moreira et al. 2008; Kroetz and Deepe 2010).
A common approach to treat inflammatory diseases, such as rheumatoid arthritis, inflammatory bowel disease, and psoriasis, is administration of TNF-α antagonists. However, an undesirable consequence of this therapy is the enhanced frequency and suppressor activity of Treg cells that in turn have been linked with exacerbating fungal diseases (Wallis et al. 2004). These clinical findings are corroborated by experimental evidence. Animals given TNF-α-neutralizing antibody show elevated numbers of IL-10+ Treg cells and are highly susceptible to the disease when infected with H. capsulatum (Deepe and Gibbons 2008). Elimination of Treg cells enhances survival of TNF-α-neutralized mice, and, conversely, adoptive transfer of these cells exacerbates infection. Thus, the immunosuppressive functions of Treg cells are deleterious for host immunity in this scenario.
Despite their potent immunosuppressive functions, Treg cells impart a positive impact on host defense by promoting durable antifungal immunity. In murine gastric candidasis, these cells prevent excessive inflammation and allow the fungus to persist in the gastrointestinal tract, thereby generating an effective secondary immune response (Montagnoli et al. 2002). A similar mechanism is in effect in A. fumigatus infection wherein Treg cells shape immune responses to be adequate to confer protection against the pathogen, but not cause unwarranted damage to the host (Montagnoli et al. 2006). In addition to immunosuppressive functions, a remarkable component of Treg cell biology is their ability to promote Th17 cell differentiation and contribute to host defenses against C. albicans (Pandiyan et al. 2011). Treg cells manifest such an effect by sequestration of IL-2, a cytokine that inhibits Th17 cell differentiation. As a consequence, the robust Th17 response generated aids in resolution of oral candidiasis. Intriguingly, humans with a genetic defect in genesis of Treg cells are also predisposed to chronic mucocutaneous candidiasis (Kekalainen et al. 2007). Thus, Treg cells represent a double-edged sword; they are crucial for subduing inflammatory responses, but their suppressive functions may be undesirable in certain settings of mycoses.
CD8+ T Cells
CD8+ T cells are vital for protection against viral pathogens and tumors; however, their relative contribution in host immunity against fungal infections is not as comprehensively understood as CD4+ T cells. Multiple experimental models suggest that the former share redundant functions with the latter and confer protection in the setting of CD4+ T-cell deficiency (Wuthrich et al. 2003, 2006; McAllister et al. 2004; Lindell et al. 2005; Marquis et al. 2006; Chiarella et al. 2007; Nanjappa et al. 2012b). In mice deficient in MHC class II, CD8+ T cells suppress H. capsulatum infection by targeting macrophages laden with yeasts (Lin et al. 2005a). These cytotoxic CD8+ T cells are primed by DCs through the process of cross-presentation (presentation of exogenous Ags on MHC class I). Furthermore, this lineage of T cells is critical for vaccine-induced immunity against A. fumigatus, B. dermatitidis, and H. capsulatum (Wuthrich et al. 2003, 2006; Hsieh et al. 2011; Carvalho et al. 2012; Nanjappa et al. 2012a). The most likely mechanisms through which memory CD8+ T cells coordinate resolution of pathogen in these models is by the release of IFN-γ and IL-17, and cytotoxic effects on infected cells. Therefore, vaccines that elicit a robust CD8+ T-cell response can potentially be used as an alternative strategy to prevent fatal mycoses in immunodeficient patients.
Humoral Immunity
Although recent advances have been made in our understanding of the contribution of humoral immunity to host defense against fungal infections, contrasting opinions on its involvement still exist. Much of the confusion stems from earlier studies that concluded antibodies were dispensable for resolution of fungal infections (Hurd and Drake 1953; Goren 1967; Diamond et al. 1974; Monga et al. 1979; Carrow et al. 1984). However, the advent of monoclonal antibody technology made it possible to identify the protective effects of immunoglobulins against fungi. Since then, the impact of immunoglobulins and B cells secreting them has been well scrutinized in C. neoformans (Aguirre and Johnson 1997; Rivera et al. 2005; Szymczak et al. 2013), C. albicans (Wahab et al. 1995; Matthews and Burnie 2001; Saville et al. 2008), H. capsulatum (Nosanchuk et al. 2003), and Pneumocystis sp. infections (Rapaka et al. 2010) (Fig. 4). The clinical importance of immunoglobulins in mycoses is evident from reports that patients with B-cell defects including X-linked hyperIgM (Winkelstein et al. 2003; de Gorgolas et al. 2005), hypogammaglobulinemia (Gupta et al. 1987; Wahab et al. 1995; Neto et al. 2000), and IgG2 deficiency (Marr et al. 2012) are susceptible to cryptococcosis. Additionally, pneumocystosis was reported in a patient with hypergammaglobulinemia who displayed inability to class switch from IgM to a more specific IgG subclass (Matheson and Green 1987).
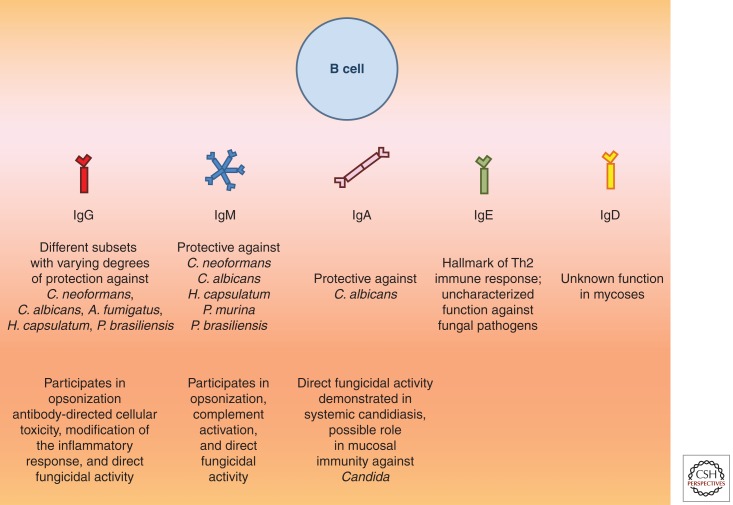
Figure 4.
Functions of different subclasses of immunoglobulins in mycoses. On encountering fungal pathogens, B cells undergo clonal expansion and antibody class switching. Depicted below are the five major classes of immunoglobulins secreted by B cells, namely, IgG, IgM, IgA, IgE, and IgD. Whereas specific roles of the former three have been reported in fungal diseases, the impact of IgE in this setting remains unclear. IgD has the ability to bind to outer surfaces of certain bacterial pathogens in the respiratory mucosa (Chen et al. 2009); however, its importance in fungal infections has not been studied.
Immunoglobulins elicit protective immune responses in the host by predominantly targeting Ags in the fungal cell wall, such as β-glucan (A. fumigatus, C. albicans, and C. neoformans), agglutinin like sequence 3 (C. albicans), glucuronoxylomannan (C. neoformans), and heat shock protein 60 (H. capsulatum) (Casadevall and Pirofski 2012). The mechanisms by which these antibodies mediate protection in the host are broadly classified into direct and indirect mechanisms. By definition, direct mechanisms are those that result in inhibition of growth or microbicidal activity when immunoglobulins bind to the pathogen. The fungicidal activity of certain monoclonal antibodies is well illustrated against C. albicans, C. neoformans, and A. fumigatus (Torosantucci et al. 2005, 2009; Rachini et al. 2007). Additionally, antibody binding to the outer fungal surface prompts alteration in gene expression and fungal metabolism that ultimately suppress virulence of the pathogen (McClelland et al. 2010; Brena et al. 2011).
Indirect mechanisms comprise immunoglobulin-mediated resolution of infection by enhancing the microbicidal potential of effector cells. Opsonization, activation of complement pathway, and antibody-directed cell toxicity (ADCC) are associated with indirect effects of antibodies during infection and form integral components of host defense against fungal pathogens, such as C. albicans, C. neoformans, and H. capsulatum (Nabavi and Murphy 1986; Han et al. 2001; Shi et al. 2008). ADCC induces lysis of cells decorated with antibodies, whereas the former two actions trigger innate defense mechanisms including phagocytosis. In murine cryptococcosis, administration of an anticapsular IgG1 monoclonal antibody is associated with heightened production of IL-10 in the lungs and diminished levels of IFN-γ (Feldmesser et al. 2002). In this setting, dampening of the proinflammatory response is speculated to contribute to accelerated resolution of infection and improved survival in mice. Our knowledge of why the IgG1 monoclonal antibody elicits an anti-inflammatory response is incomplete, but a probable mechanism could be the suppression of the complement pathway facilitated by highly galactosylated antibodies (Karsten al. 2012). N-glycan galactosylation of IgG1 engages the inhibitory IgG receptor FcγRIIB and Dectin-1, which in turn suppress C5a receptor functions. In sharp contrast, administration of monoclonal antibody to H. capsulatum histone-like protein, H2B improves the outcome of infection by inducing Th1 cytokines and by enhancing the fungicidal activity of macrophages (Nosanchuk et al. 2003). Thus, antibodies shape the inflammatory response in fungal infections.
Fungal infections represent a serious threat in immunocompromised patients, such as those suffering from AIDS (Brown et al. 2012). The majority of these affected individuals have severe defects in cell-mediated immunity. Thus, there is an urgent requirement for immunotherapy that bypasses the need for CD4+ T cells and combats fungal infections. One such strategy is DNA vaccination with Pneumocystis Ag, kexin linked to CD40 ligand (Zheng et al. 2005). This vaccination approach induces a robust antibody response in mice and confers protection during Pneumocystis pneumonia. Other fungal Ags that elicit a vigorous humoral response in experimental models have been reported (Devi 1996; Han et al. 1999; Fleuridor et al. 2001; Torosantucci et al. 2005; Sandini et al. 2011; Xin and Cutler 2011). Thus, immunoglobulin therapy represents a promising approach that could be used to treat mycoses in individuals with immunosuppression. However, an important consideration while administering these monoclonal antibodies to the host must be their dose. At a very high concentration, certain immunoglobulins show prozone-like effects and can be nonprotective or even deleterious during the course of the disease (Taborda and Casadevall 2001; Taborda et al. 2003).
CONCLUDING REMARKS
We have highlighted the major advances in knowledge concerning the development, maintenance, and function of the adaptive immune response to medically important fungi. We have tried to emphasize common pathways, mechanisms, and themes. The benefits of unearthing the pathways leading to adaptive immunity and the functions of this response are that (1) vaccines may be created that prevent or treat fungal diseases; (2) predictions may be made as to who will be at risk for serious fungal infections; and (3) biological agents may be developed that bolster immunity in the face of severe and life-threatening infections that are being diagnosed worldwide in increasingly large numbers of patients.
ACKNOWLEDGMENTS
This work is supported by U.S. Public Health Service Grants AI35681 and AI40996 to B.K., AI 093553 to M.W., AI83313 and AI106269 to G.D., and Merit Review BX000717 to G.D.
Footnotes
Editors: Arturo Casadevall, Aaron P. Mitchell, Judith Berman, Kyung J. Kwon-Chung, John R. Perfect, and Joseph Heitman
Additional Perspectives on Human Fungal Pathogens available at www.perspectivesinmedicine.org
REFERENCES
- Aguirre KM, Johnson LL 1997. A role for B cells in resistance to Cryptococcus neoformans in mice. Infect Immun 65: 525–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan RS, Waithman J, Bedoui S, Jones CM, Villadangos JA, Zhan Y, Lew AM, Shortman K, Heath WR, Carbone FR 2006. Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity 25: 153–162. [DOI] [PubMed] [Google Scholar]
- Allendoerfer R, Deepe GS Jr 1997. Intrapulmonary response to Histoplasma capsulatum in γ interferon knockout mice. Infect Immun 65: 2564–2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allendoerfer R, Deepe GS Jr 1998. Blockade of endogenous TNF-α exacerbates primary and secondary pulmonary histoplasmosis by differential mechanisms. J Immunol 160: 6072–6082. [PubMed] [Google Scholar]
- Al-Saud BK, Al-Sum Z, Alassiri H, Al-Ghonaium A, Al-Muhsen S, Al-Dhekri H, Arnaout R, Alsmadi O, Borrero E, Abu-Staiteh A, et al. 2013. Clinical, immunological, and molecular characterization of hyper-IgM syndrome due to CD40 deficiency in eleven patients. J Clin Immunol 33: 1325–1335. [DOI] [PubMed] [Google Scholar]
- Aytekin C, Dogu F, Tuygun N, Tanir G, Guloglu D, Boisson-Dupuis S, Bustamante J, Feinberg J, Casanova JL, Ikinciogullari A 2011. Bacille Calmette-Guérin lymphadenitis and recurrent oral candidiasis in an infant with a new mutation leading to interleukin-12 receptor β-1 deficiency. J Investig Allergol Clin Immunol 21: 401–404. [PMC free article] [PubMed] [Google Scholar]
- Babula O, Lazdane G, Kroica J, Linhares IM, Ledger WJ, Witkin SS 2005. Frequency of interleukin-4 (IL-4)-589 gene polymorphism and vaginal concentrations of IL-4, nitric oxide, and mannose-binding lectin in women with recurrent vulvovaginal candidiasis. Clin Infect Dis 40: 1258–1262. [DOI] [PubMed] [Google Scholar]
- Backer R, van Leeuwen F, Kraal G, den Haan JM 2008. CD8-dendritic cells preferentially cross-present Saccharomyces cerevisiae antigens. Eur J Immunol 38: 370–380. [DOI] [PubMed] [Google Scholar]
- Banchereau J, Steinman RM 1998. Dendritic cells and the control of immunity. Nature 392: 245–252. [DOI] [PubMed] [Google Scholar]
- Beaman L 1987. Fungicidal activation of murine macrophages by recombinant γ interferon. Infect Immun 55: 2951–2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaty SR, Rose CE Jr, Sung SS 2007. Diverse and potent chemokine production by lung CD11bhigh dendritic cells in homeostasis and in allergic lung inflammation. J Immunol 178: 1882–1895. [DOI] [PubMed] [Google Scholar]
- Bellocchio S, Montagnoli C, Bozza S, Gaziano R, Rossi G, Mambula SS, Vecchi A, Mantovani A, Levitz SM, Romani L 2004. The contribution of the Toll-like/IL-1 receptor superfamily to innate and adaptive immunity to fungal pathogens in vivo. J Immunol 172: 3059–3069. [DOI] [PubMed] [Google Scholar]
- Biondo C, Midiri A, Messina L, Tomasello F, Garufi G, Catania MR, Bombaci M, Beninati C, Teti G, Mancuso G 2005. MyD88 and TLR2, but not TLR4, are required for host defense against Cryptococcus neoformans. Eur J Immunol 35: 870–878. [DOI] [PubMed] [Google Scholar]
- Biondo C, Signorino G, Costa A, Midiri A, Gerace E, Galbo R, Bellantoni A, Malara A, Beninati C, Teti G, et al. 2011. Recognition of yeast nucleic acids triggers a host-protective type I interferon response. Eur J Immunol 41: 1969–1979. [DOI] [PubMed] [Google Scholar]
- Bochud PY, Chien JW, Marr KA, Leisenring WM, Upton A, Janer M, Rodrigues SD, Li S, Hansen JA, Zhao LP, et al. 2008. Toll-like receptor 4 polymorphisms and aspergillosis in stem-cell transplantation. N Engl J Med 359: 1766–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeois C, Majer O, Frohner IE, Lesiak-Markowicz I, Hildering KS, Glaser W, Stockinger S, Decker T, Akira S, Muller M, et al. 2011. Conventional dendritic cells mount a type I IFN response against Candida spp. requiring novel phagosomal TLR7-mediated IFN-β signaling. J Immunol 186: 3104–3112. [DOI] [PubMed] [Google Scholar]
- Bozza S, Zelante T, Moretti S, Bonifazi P, DeLuca A, D'Angelo C, Giovannini G, Garlanda C, Boon L, Bistoni F, et al. 2008. Lack of Toll IL-1R8 exacerbates Th17 cell responses in fungal infection. J Immunol 180: 4022–4031. [DOI] [PubMed] [Google Scholar]
- Brena S, Cabezas-Olcoz J, Moragues MD, Fernandez de Larrinoa I, Dominguez A, Quindos G, Ponton J 2011. Fungicidal monoclonal antibody C7 interferes with iron acquisition in Candida albicans. Antimicrob Agents Chemother 55: 3156–3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretz C, Gersuk G, Knoblaugh S, Chaudhary N, Randolph-Habecker J, Hackman RC, Staab J, Marr KA 2008. MyD88 signaling contributes to early pulmonary responses to Aspergillus fumigatus. Infect Immun 76: 952–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GD 2011. Innate antifungal immunity: The key role of phagocytes. Annu Rev Immunol 29: 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, White TC 2012. Hidden killers: Human fungal infections. Sci Transl Med 4: 165rv113. [DOI] [PubMed] [Google Scholar]
- Brummer E, Stevens DA 1995. Antifungal mechanisms of activated murine bronchoalveolar or peritoneal macrophages for Histoplasma capsulatum. Clin Exp Immunol 102: 65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calich VL, Pina A, Felonato M, Bernardino S, Costa TA, Loures FV 2008. Toll-like receptors and fungal infections: The role of TLR2, TLR4 and MyD88 in paracoccidioidomycosis. FEMS Immunol Med Microbiol 53: 1–7. [DOI] [PubMed] [Google Scholar]
- Calvi SA, Peracoli MT, Mendes RP, Marcondes-Machado J, Fecchio D, Marques SA, Soares AM 2003. Effect of cytokines on the in vitro fungicidal activity of monocytes from paracoccidioidomycosis patients. Microbes Infect 5: 107–113. [DOI] [PubMed] [Google Scholar]
- Carrow EW, Hector RF, Domer JE 1984. Immunodeficient CBA/N mice respond effectively to Candida albicans. Clin Immunol Immunopathol 33: 371–380. [DOI] [PubMed] [Google Scholar]
- Carvalho A, De Luca A, Bozza S, Cunha C, D’Angelo C, Moretti S, Perruccio K, Iannitti RG, Fallarino F, Pierini A, et al. 2012. TLR3 essentially promotes protective class I-restricted memory CD8+ T-cell responses to Aspergillus fumigatus in hematopoietic transplanted patients. Blood 119: 967–977. [DOI] [PubMed] [Google Scholar]
- Casadevall A, Pirofski LA 2012. Immunoglobulins in defense, pathogenesis, and therapy of fungal diseases. Cell Host Microbe 11: 447–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenci E, Romani L, Mencacci A, Spaccapelo R, Schiaffella E, Puccetti P, Bistoni F 1993. Interleukin-4 and interleukin-10 inhibit nitric oxide-dependent macrophage killing of Candida albicans. Eur J Immunol 23: 1034–1038. [DOI] [PubMed] [Google Scholar]
- Chai LY, Kullberg BJ, Vonk AG, Warris A, Cambi A, Latge JP, Joosten LA, van der Meer JW, Netea MG 2009. Modulation of Toll-like receptor 2 (TLR2) and TLR4 responses by Aspergillus fumigatus. Infect Immun 77: 2184–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Xu W, Wilson M, He B, Miller NW, Bengten E, Edholm ES, Santini PA, Rath P, Chiu A, et al. 2009. Immunoglobulin D enhances immune surveillance by activating antimicrobial, proinflammatory and B cell-stimulating programs in basophils. Nat Immunol 10: 889–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng SC, van de Veerdonk F, Smeekens S, Joosten LA, van der Meer JW, Kullberg BJ, Netea MG 2010. Candida albicans dampens host defense by downregulating IL-17 production. J Immunol 185: 2450–2457. [DOI] [PubMed] [Google Scholar]
- Chiarella AP, Arruda C, Pina A, Costa TA, Ferreira RC, Calich VL 2007. The relative importance of CD4+ and CD8+T cells in immunity to pulmonary paracoccidioidomycosis. Microbes Infect 9: 1078–1088. [DOI] [PubMed] [Google Scholar]
- Conti HR, Shen F, Nayyar N, Stocum E, Sun JN, Lindemann MJ, Ho AW, Hai JH, Yu JJ, Jung JW, et al. 2009. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med 206: 299–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti HR, Baker O, Freeman AF, Jang WS, Holland SM, Li RA, Edgerton M, Gaffen SL 2011. New mechanism of oral immunity to mucosal candidiasis in hyper-IgE syndrome. Mucosal Immunol 4: 448–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MJ, Tsang TM, Qiu Y, Dayrit JK, Freij JB, Huffnagle GB, Olszewski MA 2013. Macrophage M1/M2 polarization dynamically adapts to changes in cytokine microenvironments in Cryptococcus neoformans infection. MBio 4: e00264–00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deepe GS Jr, Gibbons RS 2008. TNF-α antagonism generates a population of antigen-specific CD4+CD25+ T cells that inhibit protective immunity in murine histoplasmosis. J Immunol 180: 1088–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deepe GS Jr, Gibbons RS 2009. Interleukins 17 and 23 influence the host response to Histoplasma capsulatum. J Infect Dis 200: 142–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deepe GS Jr, Gibbons R, Woodward E 1999. Neutralization of endogenous granulocyte-macrophage colony-stimulating factor subverts the protective immune response to Histoplasma capsulatum. J Immunol 163: 4985–4993. [PubMed] [Google Scholar]
- de Gorgolas M, Erice A, Gil A, Gutierrez J, Rivas P, Hernando C, Rodriguez MC 2005. Cryptococcal meningitis in a patient with X-linked hyper-IgM1 syndrome. Scand J Infect Dis 37: 526–528. [DOI] [PubMed] [Google Scholar]
- del Rio ML, Rodriguez-Barbosa JI, Kremmer E, Forster R 2007. CD103− and CD103+ bronchial lymph node dendritic cells are specialized in presenting and cross-presenting innocuous antigen to CD4+ and CD8+ T cells. J Immunol 178: 6861–6866. [DOI] [PubMed] [Google Scholar]
- De Luca A, Zelante T, D’Angelo C, Zagarella S, Fallarino F, Spreca A, Iannitti RG, Bonifazi P, Renauld JC, Bistoni F, et al. 2010. IL-22 defines a novel immune pathway of antifungal resistance. Mucosal Immunol 3: 361–373. [DOI] [PubMed] [Google Scholar]
- den Haan JM, Lehar SM, Bevan MJ 2000. CD8+ but not CD8− dendritic cells cross-prime cytotoxic T cells in vivo. J Exp Med 192: 1685–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desch AN, Randolph GJ, Murphy K, Gautier EL, Kedl RM, Lahoud MH, Caminschi I, Shortman K, Henson PM, Jakubzick CV 2011. CD103+ pulmonary dendritic cells preferentially acquire and present apoptotic cell-associated antigen. J Exp Med 208: 1789–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devi SJ 1996. Preclinical efficacy of a glucuronoxylomannan-tetanus toxoid conjugate vaccine of Cryptococcus neoformans in a murine model. Vaccine 14: 841–844. [DOI] [PubMed] [Google Scholar]
- Diamond RD, May JE, Kane MA, Frank MM, Bennett JE 1974. The role of the classical and alternate complement pathways in host defenses against Cryptococcus neoformans infection. J Immunol 112: 2260–2270. [PubMed] [Google Scholar]
- Dominguez PM, Ardavin C 2010. Differentiation and function of mouse monocyte-derived dendritic cells in steady state and inflammation. Immunol Rev 234: 90–104. [DOI] [PubMed] [Google Scholar]
- Duplessis CA, Tilley D, Bavaro M, Hale B, Holland SM 2011. Two cases illustrating successful adjunctive interferon-γ immunotherapy in refractory disseminated coccidioidomycosis. J Infect 63: 223–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersland K, Wuthrich M, Klein BS 2010. Dynamic interplay among monocyte-derived, dermal, and resident lymph node dendritic cells during the generation of vaccine immunity to fungi. Cell Host Microbe 7: 474–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyerich S, Wagener J, Wenzel V, Scarponi C, Pennino D, Albanesi C, Schaller M, Behrendt H, Ring J, Schmidt-Weber CB, et al. 2011. IL-22 and TNF-α represent a key cytokine combination for epidermal integrity during infection with Candida albicans. Eur J Immunol 41: 1894–1901. [DOI] [PubMed] [Google Scholar]
- Feldmesser M, Mednick A, Casadevall A 2002. Antibody-mediated protection in murine Cryptococcus neoformans infection is associated with pleotrophic effects on cytokine and leukocyte responses. Infect Immun 70: 1571–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferwerda B, Ferwerda G, Plantinga TS, Willment JA, van Spriel AB, Venselaar H, Elbers CC, Johnson MD, Cambi A, Huysamen C, et al. 2009. Human Dectin-1 deficiency and mucocutaneous fungal infections. N Engl J Med 361: 1760–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleuridor R, Lees A, Pirofski L 2001. A cryptococcal capsular polysaccharide mimotope prolongs the survival of mice with Cryptococcus neoformans infection. J Immunol 166: 1087–1096. [DOI] [PubMed] [Google Scholar]
- Geijtenbeek TB, Gringhuis SI 2009. Signalling through C-type lectin receptors: Shaping immune responses. Nat Rev Immunol 9: 465–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissmann F, Jung S, Littman DR 2003. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 19: 71–82. [DOI] [PubMed] [Google Scholar]
- Ginhoux F, Collin MP, Bogunovic M, Abel M, Leboeuf M, Helft J, Ochando J, Kissenpfennig A, Malissen B, Grisotto M, et al. 2007. Blood-derived dermal langerin+ dendritic cells survey the skin in the steady state. J Exp Med 204: 3133–3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman WA, Cooper KD, McCormick TS 2012. Regulation generation: The suppressive functions of human regulatory T cells. Crit Rev Immunol 32: 65–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goren MB 1967. Experimental murine cryptococcosis: Effect of hyperimmunization to capsular polysaccharide. J Immunol 98: 914–922. [PubMed] [Google Scholar]
- Graham LM, Brown GD 2009. The Dectin-2 family of C-type lectins in immunity and homeostasis. Cytokine 48: 148–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimbacher B, Holland SM, Puck JM 2005. Hyper-IgE syndromes. Immunol Rev 203: 244–250. [DOI] [PubMed] [Google Scholar]
- Gringhuis SI, den Dunnen J, Litjens M, van der Vlist M, Wevers B, Bruijns SC, Geijtenbeek TB 2009. Dectin-1 directs T helper cell differentiation by controlling noncanonical NF-κB activation through Raf-1 and Syk. Nat Immunol 10: 203–213. [DOI] [PubMed] [Google Scholar]
- Gringhuis SI, Wevers BA, Kaptein TM, van Capel TM, Theelen B, Boekhout T, de Jong EC, Geijtenbeek TB 2011. Selective C-Rel activation via Malt1 controls anti-fungal TH-17 immunity by Dectin-1 and Dectin-2. PLoS Pathog 7: e1001259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross O, Poeck H, Bscheider M, Dostert C, Hannesschlager N, Endres S, Hartmann G, Tardivel A, Schweighoffer E, Tybulewicz V, et al. 2009. Syk kinase signaling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature 459: 433–436. [DOI] [PubMed] [Google Scholar]
- Gupta S, Ellis M, Cesario T, Ruhling M, Vayuvegula B 1987. Disseminated cryptococcal infection in a patient with hypogammaglobulinemia and normal T cell functions. Am J Med 82: 129–131. [DOI] [PubMed] [Google Scholar]
- Han Y, Ulrich MA, Cutler JE 1999. Candida albicans mannan extract-protein conjugates induce a protective immune response against experimental candidiasis. J Infect Dis 179: 1477–1484. [DOI] [PubMed] [Google Scholar]
- Han Y, Kozel TR, Zhang MX, MacGill RS, Carroll MC, Cutler JE 2001. Complement is essential for protection by an IgM and an IgG3 monoclonal antibody against experimental, hematogenously disseminated candidiasis. J Immunol 167: 1550–1557. [DOI] [PubMed] [Google Scholar]
- Henri S, Poulin LF, Tamoutounour S, Ardouin L, Guilliams M, de Bovis B, Devilard E, Viret C, Azukizawa H, Kissenpfennig A, et al. 2010. CD207+ CD103+ dermal dendritic cells cross-present keratinocyte-derived antigens irrespective of the presence of Langerhans cells. J Exp Med 207: 189–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Santos N, Gaffen SL 2012. Th17 cells in immunity to Candida albicans. Cell Host Microbe 11: 425–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hise AG, Tomalka J, Ganesan S, Patel K, Hall BA, Brown GD, Fitzgerald KA 2009. An essential role for the NLRP3 inflammasome in host defense against the human fungal pathogen Candida albicans. Cell Host Microbe 5: 487–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogaboam CM, Blease K, Mehrad B, Steinhauser ML, Standiford TJ, Kunkel SL, Lukacs NW 2000. Chronic airway hyperreactivity, goblet cell hyperplasia, and peribronchial fibrosis during allergic airway disease induced by Aspergillus fumigatus. Am J Pathol 156: 723–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohl TM, Van Epps HL, Rivera A, Morgan LA, Chen PL, Feldmesser M, Pamer EG 2005. Aspergillus fumigatus triggers inflammatory responses by stage-specific β-glucan display. PLoS Pathog 1: e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohl TM, Rivera A, Lipuma L, Gallegos A, Shi C, Mack M, Pamer EG 2009. Inflammatory monocytes facilitate adaptive CD4 T cell responses during respiratory fungal infection. Cell Host Microbe 6: 470–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh SH, Lin JS, Huang JH, Wu SY, Chu CL, Kung JT, Wu-Hsieh BA 2011. Immunization with apoptotic phagocytes containing Histoplasma capsulatum activates functional CD8+ T cells to protect against histoplasmosis. Infect Immun 79: 4493–4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffnagle GB, Toews GB, Burdick MD, Boyd MB, McAllister KS, McDonald RA, Kunkel SL, Strieter RM 1996. Afferent phase production of TNF-α is required for the development of protective T cell immunity to Cryptococcus neoformans. J Immunol 157: 4529–4536. [PubMed] [Google Scholar]
- Hurd RC, Drake CH 1953. Candida albicans infections in actively and passively immunized animals. Mycopathol Mycol Appl 6: 290–297. [DOI] [PubMed] [Google Scholar]
- Idoyaga J, Suda N, Suda K, Park CG, Steinman RM 2009. Antibody to Langerin/CD207 localizes large numbers of CD8α+ dendritic cells to the marginal zone of mouse spleen. Proc Natl Acad Sci 106: 1524–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igyarto BZ, Haley K, Ortner D, Bobr A, Gerami-Nejad M, Edelson BT, Zurawski SM, Malissen B, Zurawski G, Berman J, et al. 2011. Skin-resident murine dendritic cell subsets promote distinct and opposing antigen-specific T helper cell responses. Immunity 35: 260–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliev ID, Funari VA, Taylor KD, Nguyen Q, Reyes CN, Strom SP, Brown J, Becker CA, Fleshner PR, Dubinsky M, et al. 2012. Interactions between commensal fungi and the C-type lectin receptor Dectin-1 influence colitis. Science 336: 1314–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa E, Ishikawa T, Morita YS, Toyonaga K, Yamada H, Takeuchi O, Kinoshita T, Akira S, Yoshikai Y, Yamasaki S 2009. Direct recognition of the mycobacterial glycolipid, trehalose dimycolate, by C-type lectin Mincle. J Exp Med 206: 2879–2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa T, Itoh F, Yoshida S, Saijo S, Matsuzawa T, Gonoi T, Saito T, Okawa Y, Shibata N, Miyamoto T, et al. 2013. Identification of distinct ligands for the C-type lectin receptors Mincle and Dectin-2 in the pathogenic fungus Malassezia. Cell Host Microbe 13: 477–488. [DOI] [PubMed] [Google Scholar]
- Jahnsen FL, Strickland DH, Thomas JA, Tobagus IT, Napoli S, Zosky GR, Turner DJ, Sly PD, Stumbles PA, Holt PG 2006. Accelerated antigen sampling and transport by airway mucosal dendritic cells following inhalation of a bacterial stimulus. J Immunol 177: 5861–5867. [DOI] [PubMed] [Google Scholar]
- Jarvis JN, Meintjes G, Rebe K, Williams GN, Bicanic T, Williams A, Schutz C, Bekker LG, Wood R, Harrison TS 2012. Adjunctive interferon-γ immunotherapy for the treatment of HIV-associated cryptococcal meningitis: A randomized controlled trial. AIDS 26: 1105–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirapongsananuruk O, Luangwedchakarn V, Niemela JE, Pacharn P, Visitsunthorn N, Thepthai C, Vichyanond P, Piboonpocanun S, Fleisher TA 2012. Cryptococcal osteomyelitis in a child with a novel compound mutation of the IL12RB1 gene. Asian Pac J Allergy Immunol 30: 79–82. [PubMed] [Google Scholar]
- Kagami S, Rizzo HL, Kurtz SE, Miller LS, Blauvelt A 2010. IL-23 and IL-17A, but not IL-12 and IL-22, are required for optimal skin host defense against Candida albicans. J Immunol 185: 5453–5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kankkunen P, Teirila L, Rintahaka J, Alenius H, Wolff H, Matikainen S 2010. (1,3)-β-glucans activate both Dectin-1 and NLRP3 inflammasome in human macrophages. J Immunol 184: 6335–6342. [DOI] [PubMed] [Google Scholar]
- Karsten CM, Pandey MK, Figge J, Kilchenstein R, Taylor PR, Rosas M, McDonald JU, Orr SJ, Berger M, Petzold D, et al. 2012. Anti-inflammatory activity of IgG1 mediated by Fc galactosylation and association of FcγRIIB and Dectin-1. Nat Med 18: 1401–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasperkovitz PV, Cardenas ML, Vyas JM 2010. TLR9 is actively recruited to Aspergillus fumigatus phagosomes and requires the N-terminal proteolytic cleavage domain for proper intracellular trafficking. J Immunol 185: 7614–7622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kekalainen E, Tuovinen H, Joensuu J, Gylling M, Franssila R, Pontynen N, Talvensaari K, Perheentupa J, Miettinen A, Arstila TP 2007. A defect of regulatory T cells in patients with autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy. J Immunol 178: 1208–1215. [DOI] [PubMed] [Google Scholar]
- Kelleher P, Goodsall A, Mulgirigama A, Kunst H, Henderson DC, Wilson R, Newman-Taylor A, Levin M 2006. Interferon-γ therapy in two patients with progressive chronic pulmonary aspergillosis. Eur Respir J 27: 1307–1310. [DOI] [PubMed] [Google Scholar]
- Kerrigan AM, Brown GD 2010. Syk-coupled C-type lectin receptors that mediate cellular activation via single tyrosine based activation motifs. Immunol Rev 234: 335–352. [DOI] [PubMed] [Google Scholar]
- Kerrigan AM, Brown GD 2011. Syk-coupled C-type lectins in immunity. Trends Immunol 32: 151–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerscher B, Willment JA, Brown GD 2013. The Dectin-2 family of C-type lectin-like receptors: An update. Int Immunol 25: 271–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroetz DN, Deepe GS Jr 2010. CCR5 dictates the equilibrium of proinflammatory IL-17+ and regulatory Foxp3+ T cells in fungal infection. J Immunol 184: 5224–5231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeibundGut-Landmann S, Gross O, Robinson MJ, Osorio F, Slack EC, Tsoni SV, Schweighoffer E, Tybulewicz V, Brown GD, Ruland J, et al. 2007. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat Immunol 8: 630–638. [DOI] [PubMed] [Google Scholar]
- Levitz SM 2009. Th17 cells bounce off the fungal wall. Cell Host Microbe 5: 311–313. [DOI] [PubMed] [Google Scholar]
- Li HS, Gelbard A, Martinez GJ, Esashi E, Zhang H, Nguyen-Jackson H, Liu YJ, Overwijk WW, Watowich SS 2011. Cell-intrinsic role for IFN-α-STAT1 signals in regulating murine Peyer patch plasmacytoid dendritic cells and conditioning an inflammatory response. Blood 118: 3879–3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, Fouser LA 2006. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med 203: 2271–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JS, Yang CW, Wang DW, Wu-Hsieh BA 2005a. Dendritic cells cross-present exogenous fungal antigens to stimulate a protective CD8 T cell response in infection by Histoplasma capsulatum. J Immunol 174: 6282–6291. [DOI] [PubMed] [Google Scholar]
- Lin JS, Yang CW, Wang DW, Wu-Hsieh BA 2005b. Dendritic cells cross-present exogenous fungal antigens to stimulate a protective CD8 T cell response in infection by Histoplasma capsulatum. J Immunol 174: 6282–6291. [DOI] [PubMed] [Google Scholar]
- Lin L, Ibrahim AS, Baquir B, Avanesian V, Fu Y, Spellberg B 2009. Immunological surrogate marker of rAls3p-N vaccine-induced protection against Staphylococcus aureus. FEMS Immunol Med Microbiol 55: 293–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindell DM, Moore TA, McDonald RA, Toews GB, Huffnagle GB 2005. Generation of antifungal effector CD8+ T cells in the absence of CD4+ T cells during Cryptococcus neoformans infection. J Immunol 174: 7920–7928. [DOI] [PubMed] [Google Scholar]
- Lobato-Pascual A, Saether PC, Fossum S, Dissen E, Daws MR 2013. Mincle, the receptor for mycobacterial cord factor, forms a functional receptor complex with MCL and FcεRI-γ. Eur J Immunol 43: 3167–3174. [DOI] [PubMed] [Google Scholar]
- Loughman JA, Hunstad DA 2012. Induction of indoleamine 2,3-dioxygenase by uropathogenic bacteria attenuates innate responses to epithelial infection. J Infect Dis 205: 1830–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loures FV, Pina A, Felonato M, Calich VL 2009. TLR2 is a negative regulator of Th17 cells and tissue pathology in a pulmonary model of fungal infection. J Immunol 183: 1279–1290. [DOI] [PubMed] [Google Scholar]
- Luber CA, Cox J, Lauterbach H, Fancke B, Selbach M, Tschopp J, Akira S, Wiegand M, Hochrein H, O’Keeffe M, et al. 2010. Quantitative proteomics reveals subset-specific viral recognition in dendritic cells. Immunity 32: 279–289. [DOI] [PubMed] [Google Scholar]
- Marquis M, Lewandowski D, Dugas V, Aumont F, Senechal S, Jolicoeur P, Hanna Z, de Repentigny L 2006. CD8+ T cells but not polymorphonuclear leukocytes are required to limit chronic oral carriage of Candida albicans in transgenic mice expressing human immunodeficiency virus type 1. Infect Immun 74: 2382–2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marr KA, Datta K, Pirofski LA, Barnes R 2012. Cryptococcus gattii infection in healthy hosts: A sentinel for subclinical immunodeficiency? Clin Infect Dis 54: 153–154. [DOI] [PubMed] [Google Scholar]
- Matheson DS, Green BJ 1987. Defect in production of B cell differentiation factor-like activity by mononuclear cells from a boy with hypogammaglobulinemia. J Immunol 138: 2469–2472. [PubMed] [Google Scholar]
- Matthews R, Burnie J 2001. Antifungal antibodies: A new approach to the treatment of systemic candidiasis. Curr Opin Investig Drugs 2: 472–476. [PubMed] [Google Scholar]
- McAllister F, Steele C, Zheng M, Young E, Shellito JE, Marrero L, Kolls JK 2004. T cytotoxic-1 CD8+ T cells are effector cells against pneumocystis in mice. J Immunol 172: 1132–1138. [DOI] [PubMed] [Google Scholar]
- McClelland EE, Nicola AM, Prados-Rosales R, Casadevall A 2010. Ab binding alters gene expression in Cryptococcus neoformans and directly modulates fungal metabolism. J Clin Invest 120: 1355–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGreal EP, Rosas M, Brown GD, Zamze S, Wong SY, Gordon S, Martinez-Pomares L, Taylor PR 2006. The carbohydrate-recognition domain of Dectin-2 is a C-type lectin with specificity for high mannose. Glycobiology 16: 422–430. [DOI] [PubMed] [Google Scholar]
- Medzhitov R 2007. Recognition of microorganisms and activation of the immune response. Nature 449: 819–826. [DOI] [PubMed] [Google Scholar]
- Minegishi Y, Saito M 2011. Molecular mechanisms of the immunological abnormalities in hyper-IgE syndrome. Ann NY Acad Sci 1246: 34–40. [DOI] [PubMed] [Google Scholar]
- Miyake Y, Toyonaga K, Mori D, Kakuta S, Hoshino Y, Oyamada A, Yamada H, Ono KI, Suyama M, Iwakura Y, et al. 2013. C-type lectin MCL is an FcRγ-coupled receptor that mediates the adjuvanticity of mycobacterial cord factor. Immunity 38: 1050–1062. [DOI] [PubMed] [Google Scholar]
- Monga DP, Kumar R, Mohapatra LN, Malaviya AN 1979. Experimental cryptococcosis in normal and B-cell-deficient mice. Infect Immun 26: 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagnoli C, Bacci A, Bozza S, Gaziano R, Mosci P, Sharpe AH, Romani L 2002. B7/CD28-dependent CD4+CD25+ regulatory T cells are essential components of the memory-protective immunity to Candida albicans. J Immunol 169: 6298–6308. [DOI] [PubMed] [Google Scholar]
- Montagnoli C, Fallarino F, Gaziano R, Bozza S, Bellocchio S, Zelante T, Kurup WP, Pitzurra L, Puccetti P, Romani L 2006. Immunity and tolerance to Aspergillus involve functionally distinct regulatory T cells and tryptophan catabolism. J Immunol 176: 1712–1723. [DOI] [PubMed] [Google Scholar]
- Moraes-Vasconcelos D, Grumach AS, Yamaguti A, Andrade ME, Fieschi C, de Beaucoudrey L, Casanova JL, Duarte AJ 2005. Paracoccidioides brasiliensis disseminated disease in a patient with inherited deficiency in the β1 subunit of the interleukin (IL)-12/IL-23 receptor. Clin Infect Dis 41: e31–37. [DOI] [PubMed] [Google Scholar]
- Moreira AP, Cavassani KA, Massafera Tristao FS, Campanelli AP, Martinez R, Rossi MA, Silva JS 2008. CCR5-dependent regulatory T cell migration mediates fungal survival and severe immunosuppression. J Immunol 180: 3049–3056. [DOI] [PubMed] [Google Scholar]
- Muller U, Stenzel W, Piehler D, Grahnert A, Protschka M, Kohler G, Frey O, Held J, Richter T, Eschke M, et al. 2013. Abrogation of IL-4 receptor-α-dependent alternatively activated macrophages is sufficient to confer resistance against pulmonary cryptococcosis despite an ongoing Th2 response. Int Immunol 25: 459–470. [DOI] [PubMed] [Google Scholar]
- Nabavi N, Murphy JW 1986. Antibody-dependent natural killer cell-mediated growth inhibition of Cryptococcus neoformans. Infect Immun 51: 556–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai H, Guo J, Choi H, Kurup V 1995. Interferon-γ and tumor necrosis factor-α protect mice from invasive aspergillosis. J Infect Dis 172: 1554–1560. [DOI] [PubMed] [Google Scholar]
- Nanjappa SG, Heninger E, Wuthrich M, Gasper DJ, Klein BS 2012a. Tc17 cells mediate vaccine immunity against lethal fungal pneumonia in immune deficient hosts lacking CD4+ T cells. PLoS Pathog 8: e1002771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanjappa SG, Heninger E, Wuthrich M, Sullivan T, Klein B 2012b. Protective antifungal memory CD8+ T cells are maintained in the absence of CD4+ T cell help and cognate antigen in mice. J Clin Invest 122: 987–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson MP, Christmann BS, Werner JL, Metz AE, Trevor JL, Lowell CA, Steele C 2011. IL-33 and M2a alveolar macrophages promote lung defense against the atypical fungal pathogen Pneumocystis murina. J Immunol 186: 2372–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netea MG, Sutmuller R, Hermann C, Van der Graaf CA, Van der Meer JW, van Krieken JH, Hartung T, Adema G, Kullberg BJ 2004a. Toll-like receptor 2 suppresses immunity against Candida albicans through induction of IL-10 and regulatory T cells. J Immunol 172: 3712–3718. [DOI] [PubMed] [Google Scholar]
- Netea MG, Van der Graaf C, Van der Meer JW, Kullberg BJ 2004b. Recognition of fungal pathogens by Toll-like receptors. Eur J Clin Microbiol Infect Dis 23: 672–676. [DOI] [PubMed] [Google Scholar]
- Netea MG, Brown GD, Kullberg BJ, Gow NA 2008. An integrated model of the recognition of Candida albicans by the innate immune system. Nat Rev Microbiol 6: 67–78. [DOI] [PubMed] [Google Scholar]
- Neto R, Guimaraes MC, Moya MJ, Oliveira FR, Louzada PL Jr, Martinez R 2000. Hypogammaglobulinemia as risk factor for Cryptococcus neoformans infection: Report of 2 cases. Rev Soc Bras Med Trop 33: 603–608. [PubMed] [Google Scholar]
- Nosanchuk JD, Steenbergen JN, Shi L, Deepe GS Jr, Casadevall A 2003. Antibodies to a cell surface histone-like protein protect against Histoplasma capsulatum. J Clin Invest 112: 1164–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak ML, Koh TJ 2013. Macrophage phenotypes during tissue repair. J Leukoc Biol 93: 875–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opata MM, Ye Z, Hollifield M, Garvy BA 2013. B cell production of tumor necrosis factor in response to Pneumocystis murina infection in mice. Infect Immun 81: 4252–4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterholzer JJ, Chen GH, Olszewski MA, Curtis JL, Huffnagle GB, Toews GB 2009. Accumulation of CD11b+ lung dendritic cells in response to fungal infection results from the CCR2-mediated recruitment and differentiation of Ly-6Chigh monocytes. J Immunol 183: 8044–8053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandiyan P, Conti HR, Zheng L, Peterson AC, Mathern DR, Hernandez-Santos N, Edgerton M, Gaffen SL, Lenardo MJ 2011. CD4+CD25+Foxp3+ regulatory T cells promote Th17 cells in vitro and enhance host resistance in mouse Candida albicans Th17 cell infection model. Immunity 34: 422–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Nazario N, Rangel-Moreno J, O’Reilly MA, Pasparakis M, Gigliotti F, Wright TW 2013. Selective ablation of lung epithelial IKK2 impairs pulmonary Th17 responses and delays the clearance of Pneumocystis. J Immunol 191: 4720–4730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrella D, Corbucci C, Perito S, Bistoni G, Vecchiarelli A 2005. Mannoproteins from Cryptococcus neoformans promote dendritic cell maturation and activation. Infect Immun 73: 820–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrella D, Rachini A, Pines M, Pandey N, Mosci P, Bistoni F, d’Enfert C, Vecchiarelli A 2011. Th17 cells and IL- in protective immunity to vaginal candidiasis. PLoS ONE 6: e22770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plantinga TS, Johnson MD, Scott WK, van de Vosse E, Velez Edwards DR, Smith PB, Alexander BD, Yang JC, Kremer D, Laird GM, et al. 2012. Toll-like receptor 1 polymorphisms increase susceptibility to candidemia. J Infect Dis 205: 934–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poeck H, Ruland J 2010. SYK kinase signaling and the NLRP3 inflammasome in antifungal immunity. J Mol Med 88: 745–752. [DOI] [PubMed] [Google Scholar]
- Puel A, Cypowyj S, Bustamante J, Wright JF, Liu L, Lim HK, Migaud M, Israel L, Chrabieh M, Audry M, et al. 2011. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science 332: 65–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachini A, Pietrella D, Lupo P, Torosantucci A, Chiani P, Bromuro C, Proietti C, Bistoni F, Cassone A, Vecchiarelli A 2007. An anti-β-glucan monoclonal antibody inhibits growth and capsule formation of Cryptococcus neoformans in vitro and exerts therapeutic, anticryptococcal activity in vivo. Infect Immun 75: 5085–5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Ortiz ZG, Specht CA, Wang JP, Lee CK, Bartholomeu DC, Gazzinelli RT, Levitz SM 2008. Toll-like receptor 9-dependent immune activation by unmethylated CpG motifs in Aspergillus fumigatus DNA. Infect Immun 76: 2123–2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Ortiz ZG, Lee CK, Wang JP, Boon L, Specht CA, Levitz SM 2011. A nonredundant role for plasmacytoid dendritic cells in host defense against the human fungal pathogen Aspergillus fumigatus. Cell Host Microbe 9: 415–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapaka RR, Ricks DM, Alcorn JF, Chen K, Khader SA, Zheng M, Plevy S, Bengten E, Kolls JK 2010. Conserved natural IgM antibodies mediate innate and adaptive immunity against the opportunistic fungus Pneumocystis murina. J Exp Med 207: 2907–2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappleye CA, Eissenberg LG, Goldman WE 2007. Histoplasma capsulatum α-(1,3)-glucan blocks innate immune recognition by the β-glucan receptor. Proc Natl Acad Sci 104: 1366–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera J, Zaragoza O, Casadevall A 2005. Antibody-mediated protection against Cryptococcus neoformans pulmonary infection is dependent on B cells. Infect Immun 73: 1141–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera A, Hohl TM, Collins N, Leiner I, Gallegos A, Saijo S, Coward JW, Iwakura Y, Pamer EG 2011. Dectin-1 diversifies Aspergillus fumigatus-specific T cell responses by inhibiting T helper type 1 CD4 T cell differentiation. J Exp Med 208: 369–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MJ, Osorio F, Rosas M, Freitas RP, Schweighoffer E, Gross O, Verbeek JS, Ruland J, Tybulewicz V, Brown GD, et al. 2009. Dectin-2 is a Syk-coupled pattern recognition receptor crucial for Th17 responses to fungal infection. J Exp Med 206: 2037–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani L 2011. Immunity to fungal infections. Nat Rev Immunol 11: 275–288. [DOI] [PubMed] [Google Scholar]
- Rudner XL, Happel KI, Young EA, Shellito JE 2007. Interleukin-23 (IL-23)-IL-17 cytokine axis in murine Pneumocystis carinii infection. Infect Immun 75: 3055–3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Said-Sadier N, Padilla E, Langsley G, Ojcius DM 2010. Aspergillus fumigatus stimulates the NLRP3 inflammasome through a pathway requiring ROS production and the Syk tyrosine kinase. PLoS ONE 5: e10008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saijo S, Fujikado N, Furuta T, Chung SH, Kotaki H, Seki K, Sudo K, Akira S, Adachi Y, Ohno N, et al. 2007. Dectin-1 is required for host defense against Pneumocystis carinii but not against Candida albicans. Nat Immunol 8: 39–46. [DOI] [PubMed] [Google Scholar]
- Saijo S, Ikeda S, Yamabe K, Kakuta S, Ishigame H, Akitsu A, Fujikado N, Kusaka T, Kubo S, Chung SH, et al. 2010. Dectin-2 recognition of α-mannans and induction of Th17 cell differentiation is essential for host defense against Candida albicans. Immunity 32: 681–691. [DOI] [PubMed] [Google Scholar]
- Sambatakou H, Pravica V, Hutchinson IV, Denning DW 2006. Cytokine profiling of pulmonary aspergillosis. Int J Immunogenet 33: 297–302. [DOI] [PubMed] [Google Scholar]
- Sandini S, La Valle R, Deaglio S, Malavasi F, Cassone A, De Bernardis F 2011. A highly immunogenic recombinant and truncated protein of the secreted aspartic proteases family (rSap2t) of Candida albicans as a mucosal anticandidal vaccine. FEMS Immunol Med Microbiol 62: 215–224. [DOI] [PubMed] [Google Scholar]
- Sato K, Yang XL, Yudate T, Chung JS, Wu J, Luby-Phelps K, Kimberly RP, Underhill D, Cruz PD Jr, Ariizumi K 2006. Dectin-2 is a pattern recognition receptor for fungi that couples with the Fc receptor γ chain to induce innate immune responses. J Biol Chem 281: 38854–38866. [DOI] [PubMed] [Google Scholar]
- Saville SP, Lazzell AL, Chaturvedi AK, Monteagudo C, Lopez-Ribot JL 2008. Use of a genetically engineered strain to evaluate the pathogenic potential of yeast cell and filamentous forms during Candida albicans systemic infection in immunodeficient mice. Infect Immun 76: 97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenen H, Bodendorfer B, Hitchens K, Manzanero S, Werninghaus K, Nimmerjahn F, Agger EM, Stenger S, Andersen P, Ruland J, et al. 2010. Cutting edge: Mincle is essential for recognition and adjuvanticity of the mycobacterial cord factor and its synthetic analog trehalose-dibehenate. J Immunol 184: 2756–2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder K, Hertzog PJ, Ravasi T, Hume DA 2004. Interferon-γ: An overview of signals, mechanisms and functions. J Leukoc Biol 75: 163–189. [DOI] [PubMed] [Google Scholar]
- Shalaby MR, Aggarwal BB, Rinderknecht E, Svedersky LP, Finkle BS, Palladino MA Jr 1985. Activation of human polymorphonuclear neutrophil functions by interferon-γ and tumor necrosis factors. J Immunol 135: 2069–2073. [PubMed] [Google Scholar]
- Shenderov K, Barber DL, Mayer-Barber KD, Gurcha SS, Jankovic D, Feng CG, Oland S, Hieny S, Caspar P, Yamasaki S, et al. 2013. Cord factor and peptidoglycan recapitulate the Th17-promoting adjuvant activity of mycobacteria through Mincle/CARD9 signaling and the inflammasome. J Immunol 190: 5722–5730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Albuquerque PC, Lazar-Molnar E, Wang X, Santambrogio L, Gacser A, Nosanchuk JD 2008. A monoclonal antibody to Histoplasma capsulatum alters the intracellular fate of the fungus in murine macrophages. Eukaryot Cell 7: 1109–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoham S, Huang C, Chen JM, Golenbock DT, Levitz SM 2001. Toll-like receptor 4 mediates intracellular signaling without TNF-α release in response to Cryptococcus neoformans polysaccharide capsule. J Immunol 166: 4620–4626. [DOI] [PubMed] [Google Scholar]
- Smeekens SP, van de Veerdonk FL, van der Meer JW, Kullberg BJ, Joosten LA, Netea MG 2010. The Candida Th17 response is dependent on mannan- and β-glucan-induced prostaglandin E2. Int Immunol 22: 889–895. [DOI] [PubMed] [Google Scholar]
- Snapper CM, Paul WE 1987. Interferon-γ and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science 236: 944–947. [DOI] [PubMed] [Google Scholar]
- Squadrito ML, Pucci F, Magri L, Moi D, Gilfillan GD, Ranghetti A, Casazza A, Mazzone M, Lyle R, Naldini L, et al. 2012. miR-511–3p modulates genetic programs of tumor-associated macrophages. Cell Rep 1: 141–154. [DOI] [PubMed] [Google Scholar]
- Steele C, Rapaka RR, Metz A, Pop SM, Williams DL, Gordon S, Kolls JK, Brown GD 2005. The β-glucan receptor Dectin-1 recognizes specific morphologies of Aspergillus fumigatus. PLoS Pathog 1: e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman RM, Cohn ZA 1973. Identification of a novel cell type in peripheral lymphoid organs of mice: I. Morphology, quantitation, tissue distribution. J Exp Med 137: 1142–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian Vignesh K, Landero Figueroa JA, Porollo A, Caruso JA, Deepe GS Jr 2013. Granulocyte macrophage-colony stimulating factor induced Zn sequestration enhances macrophage superoxide and limits intracellular pathogen survival. Immunity 39: 697–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugar AM, Picard M, Wagner R, Kornfeld H 1995. Interactions between human bronchoalveolar macrophages and Blastomyces dermatitidis conidia: Demonstration of fungicidal and fungistatic effects. J Infect Dis 171: 1559–1562. [DOI] [PubMed] [Google Scholar]
- Sung SS, Fu SM, Rose CE Jr, Gaskin F, Ju ST, Beaty SR 2006. A major lung CD103 (αE)-β7 integrin-positive epithelial dendritic cell population expressing Langerin and tight junction proteins. J Immunol 176: 2161–2172. [DOI] [PubMed] [Google Scholar]
- Sutmuller RP, den Brok MH, Kramer M, Bennink EJ, Toonen LW, Kullberg BJ, Joosten LA, Akira S, Netea MG, Adema GJ 2006. Toll-like receptor 2 controls expansion and function of regulatory T cells. J Clin Invest 116: 485–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szymczak WA, Deepe GS Jr 2009. The CCL7-CCL2-CCR2 axis regulates IL-4 production in lungs and fungal immunity. J Immunol 183: 1964–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szymczak WA, Davis MJ, Lundy SK, Dufaud C, Olszewski M, Pirofski LA 2013. X-linked immunodeficient mice exhibit enhanced susceptibility to Cryptococcus neoformans infection. MBio 4: e00265–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taborda CP, Casadevall A 2001. Immunoglobulin M efficacy against Cryptococcus neoformans: Mechanism, dose dependence, and prozone-like effects in passive protection experiments. J Immunol 166: 2100–2107. [DOI] [PubMed] [Google Scholar]
- Taborda CP, Rivera J, Zaragoza O, Casadevall A 2003. More is not necessarily better: Prozone-like effects in passive immunization with IgG. J Immunol 170: 3621–3630. [DOI] [PubMed] [Google Scholar]
- Tachado SD, Zhang J, Zhu J, Patel N, Cushion M, Koziel H 2007. Pneumocystis-mediated IL-8 release by macrophages requires coexpression of mannose receptors and TLR2. J Leukoc Biol 81: 205–211. [DOI] [PubMed] [Google Scholar]
- Takagi H, Fukaya T, Eizumi K, Sato Y, Sato K, Shibazaki A, Otsuka H, Hijikata A, Watanabe T, Ohara O, et al. 2011. Plasmacytoid dendritic cells are crucial for the initiation of inflammation and T cell immunity in vivo. Immunity 35: 958–971. [DOI] [PubMed] [Google Scholar]
- Taylor PR, Gordon S, Martinez-Pomares L 2005. The mannose receptor: Linking homeostasis and immunity through sugar recognition. Trends Immunol 26: 104–110. [DOI] [PubMed] [Google Scholar]
- Taylor PR, Tsoni SV, Willment JA, Dennehy KM, Rosas M, Findon H, Haynes K, Steele C, Botto M, Gordon S, et al. 2007. Dectin-1 is required for β-glucan recognition and control of fungal infection. Nat Immunol 8: 31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torosantucci A, Bromuro C, Chiani P, De Bernardis F, Berti F, Galli C, Norelli F, Bellucci C, Polonelli L, Costantino P, et al. 2005. A novel glyco-conjugate vaccine against fungal pathogens. J Exp Med 202: 597–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torosantucci A, Chiani P, Bromuro C, De Bernardis F, Palma AS, Liu Y, Mignogna G, Maras B, Colone M, Stringaro A, et al. 2009. Protection by anti-β-glucan antibodies is associated with restricted β-1,3 glucan binding specificity and inhibition of fungal growth and adherence. PLoS ONE 4: e5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traynor TR, Kuziel WA, Toews GB, Huffnagle GB 2000. CCR2 expression determines T1 versus T2 polarization during pulmonary Cryptococcus neoformans infection. J Immunol 164: 2021–2027. [DOI] [PubMed] [Google Scholar]
- Tsai HY, Yu HH, Chien YH, Chu KH, Lau YL, Lee JH, Wang LC, Chiang BL, Yang YH 2012. X-linked hyper-IgM syndrome with CD40LG mutation: Two case reports and literature review in Taiwanese patients. J Microbiol Immunol Infect 10.1016/j.jmii.2012.07.004. [DOI] [PubMed] [Google Scholar]
- Tsoni SV, Brown GD 2008. β-Glucans and Dectin-1. Ann NY Acad Sci 1143: 45–60. [DOI] [PubMed] [Google Scholar]
- van de Veerdonk FL, Marijnissen RJ, Kullberg BJ, Koenen HJ, Cheng SC, Joosten I, van den Berg WB, Williams DL, van der Meer JW, Joosten LA, et al. 2009. The macrophage mannose receptor induces IL-17 in response to Candida albicans. Cell Host Microbe 5: 329–340. [DOI] [PubMed] [Google Scholar]
- Vautier S, MacCallum DM, Brown GD 2012. C-type lectin receptors and cytokines in fungal immunity. Cytokine 58: 89–99. [DOI] [PubMed] [Google Scholar]
- Vinh DC, Schwartz B, Hsu AP, Miranda DJ, Valdez PA, Fink D, Lau KP, Long-Priel D, Kuhns DB, Uzel G, et al. 2011. Interleukin-12 receptor β1 deficiency predisposing to disseminated coccidioidomycosis. Clin Infect Dis 52: e99–e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voelz K, Lammas DA, May RC 2009. Cytokine signaling regulates the outcome of intracellular macrophage parasitism by Cryptococcus neoformans. Infect Immun 77: 3450–3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bernuth H, Picard C, Jin Z, Pankla R, Xiao H, Ku CL, Chrabieh M, Mustapha IB, Ghandil P, Camcioglu Y, et al. 2008. Pyogenic bacterial infections in humans with MyD88 deficiency. Science 321: 691–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahab JA, Hanifah MJ, Choo KE 1995. Bruton’s agammaglobulinaemia in a child presenting with cryptococcal empyema thoracis and periauricular pyogenic abscess. Singapore Med J 36: 686–689. [PubMed] [Google Scholar]
- Wallis RS, Broder MS, Wong JY, Hanson ME, Beenhouwer DO 2004. Granulomatous infectious diseases associated with tumor necrosis factor antagonists. Clin Infect Dis 38: 1261–1265. [DOI] [PubMed] [Google Scholar]
- Wang H, LeBert V, Hung CY, Galles K, Saijo S, Lin X, Cole GT, Klein BS, Wuthrich M 2014. C-type lectin receptors differentially induce Th17 cells and vaccine immunity to the endemic mycosis of North America. J Immunol 192: 1107–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss G, Bogdan C, Hentze MW 1997. Pathways for the regulation of macrophage iron metabolism by the anti-inflammatory cytokines IL-4 and IL-13. J Immunol 158: 420–425. [PubMed] [Google Scholar]
- Wells CA, Salvage-Jones JA, Li X, Hitchens K, Butcher S, Murray RZ, Beckhouse AG, Lo YL, Manzanero S, Cobbold C, et al. 2008. The macrophage-inducible C-type lectin, mincle, is an essential component of the innate immune response to Candida albicans. J Immunol 180: 7404–7413. [DOI] [PubMed] [Google Scholar]
- Werner JL, Metz AE, Horn D, Schoeb TR, Hewitt MM, Schwiebert LM, Faro-Trindade I, Brown GD, Steele C 2009. Requisite role for the Dectin-1 β-glucan receptor in pulmonary defense against Aspergillus fumigatus. J Immunol 182: 4938–4946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler RT, Kombe D, Agarwala SD, Fink GR 2008. Dynamic, morphotype-specific Candida albicans β-glucan exposure during infection and drug treatment. PLoS Pathog 4: e1000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willment JA, Brown GD 2008. C-type lectin receptors in antifungal immunity. Trends Microbiol 16: 27–32. [DOI] [PubMed] [Google Scholar]
- Winkelstein JA, Marino MC, Ochs H, Fuleihan R, Scholl PR, Geha R, Stiehm ER, Conley ME 2003. The X-linked hyper-IgM syndrome: Clinical and immunologic features of 79 patients. Medicine (Baltimore) 82: 373–384. [DOI] [PubMed] [Google Scholar]
- Winters MS, Chan Q, Caruso JA, Deepe GS Jr 2010. Metallomic analysis of macrophages infected with Histoplasma capsulatum reveals a fundamental role for zinc in host defenses. J Infect Dis 202: 1136–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak KL, Hardison SE, Kolls JK, Wormley FL 2011. Role of IL-17A on resolution of pulmonary C. neoformans infection. PLoS ONE 6: e17204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuthrich M, Filutowicz HI, Warner T, Deepe GS Jr, Klein BS 2003. Vaccine immunity to pathogenic fungi overcomes the requirement for CD4 help in exogenous antigen presentation to CD8+ T cells: Implications for vaccine development in immune-deficient hosts. J Exp Med 197: 1405–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuthrich M, Fisette PL, Filutowicz HI, Klein BS 2006. Differential requirements of T cell subsets for CD40 costimulation in immunity to Blastomyces dermatitidis. J Immunol 176: 5538–5547. [DOI] [PubMed] [Google Scholar]
- Wuthrich M, Gern B, Hung CY, Ersland K, Rocco N, Pick-Jacobs J, Galles K, Filutowicz H, Warner T, Evans M, et al. 2011. Vaccine-induced protection against 3 systemic mycoses endemic to North America requires Th17 cells in mice. J Clin Invest 121: 554–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuthrich M, Deepe GS Jr, Klein B 2012a. Adaptive immunity to fungi. Annu Rev Immunol 30: 115–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuthrich M, Ersland K, Sullivan T, Galles K, Klein B 2012b. Fungi subvert vaccine T-cell priming at the respiratory mucosa by inducing MMP2 and retarding CCL7-mediated influx of inflammatory monocytes. Immunity 36: 680–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin H, Cutler JE 2011. Vaccine and monoclonal antibody that enhance mouse resistance to candidiasis. Clin Vaccine Immunol 18: 1656–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki S, Matsumoto M, Takeuchi O, Matsuzawa T, Ishikawa E, Sakuma M, Tateno H, Uno J, Hirabayashi J, Mikami Y, et al. 2009. C-type lectin Mincle is an activating receptor for pathogenic fungus, Malassezia. Proc Natl Acad Sci 106: 1897–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelante T, De Luca A, Bonifazi P, Montagnoli C, Bozza S, Moretti S, Belladonna ML, Vacca C, Conte C, Mosci P, et al. 2007. IL-23 and the Th17 pathway promote inflammation and impair antifungal immune resistance. Eur J Immunol 37: 2695–2706. [DOI] [PubMed] [Google Scholar]
- Zelante T, Iannitti RG, De Luca A, Arroyo J, Blanco N, Servillo G, Sanglard D, Reichard U, Palmer GE, Latge JP, et al. 2012. Sensing of mammalian IL-17A regulates fungal adaptation and virulence. Nat Commun 3: 683. [DOI] [PubMed] [Google Scholar]
- Zerbe CS, Holland SM 2005. Disseminated histoplasmosis in persons with interferon-γ receptor 1deficiency. Clin Infect Dis 41: e38–e41. [DOI] [PubMed] [Google Scholar]
- Zhang J, Zhu J, Imrich A, Cushion M, Kinane TB, Koziel H 2004. Pneumocystis activates human alveolar macrophage NF-κB signaling through mannose receptors. Infect Immun 72: 3147–3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng M, Ramsay AJ, Robichaux MB, Norris KA, Kliment C, Crowe C, Rapaka RR, Steele C, McAllister F, Shellito JE, et al. 2005. CD4+ T cell-independent DNA vaccination against opportunistic infections. J Clin Invest 115: 3536–3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu LL, Zhao XQ, Jiang C, You Y, Chen XP, Jiang YY, Jia XM, Lin X 2013. C-type lectin receptors Dectin-3 and Dectin-2 form a heterodimeric pattern-recognition receptor for host defense against fungal infection. Immunity. [DOI] [PubMed] [Google Scholar]
- Zuniga LA, Jain R, Haines C, Cua DJ 2013. Th17 cell development: From the cradle to the grave. Immunol Rev 252: 78–88. [DOI] [PubMed] [Google Scholar]