Abstract
In 1968, Dr. James Cleaver reported defective DNA repair in cultured cells from patients with xeroderma pigmentosum. This link between clinical disease and molecular pathophysiology has sparked interest in understanding not only the clinical characteristics of sun sensitivity, damage and cancer that occurred in XP patients but also the mechanisms underlying the damage and repair. While affected patients are rare, their exaggerated UV damage provides a window into the workings of DNA repair. These studies have clarified the importance of a functioning DNA repair system to the maintenance of skin and neurologic health in the general population. Understanding the role of damage in causing cancer, neurologic degeneration, hearing loss and internal cancers provides an opportunity for prevention and treatment. Characterizing complementation groups pointed to the importance of different underlying genes. Studying differences in cancer age of onset and underlying molecular signatures in cancers occurring either in XP patients or the general population has led to insights into differences in carcinogenic mechanisms. The accelerated development of cancers in XP has been used as a model to discover new cancer chemopreventive agents. An astute insight can be a “tipping point” triggering decades of productive inquiry.
Prelude
In 1968, Dr. James Cleaver published a seminal paper demonstrating defective DNA repair in cultured cells from patients with xeroderma pigmentosum (XP)(1). Cleaver measured DNA repair by assessment of autoradiographic incorporation of tritiated thymidine (3HTdR) into nuclei of cultured fibroblasts following ultraviolet radiation (UV) exposure.. The few normally dividing cells in S phase were heavily labeled while the remainder of the cell population was lightly labeled. This “unscheduled DNA synthesis” (UDS) was a measure of DNA repair and indicated that repair was proceeding in most of the cell population. Post-UV UDS was greatly reduced in cultured skin fibroblasts from XP patients. In addition, he used a version of the 3H-bromodeoxyuridine (3H-BUdR) density gradient method of Petttijohn and Hanawalt (2) and reported reduced post-UV repair replication in XP cells. The XP patients had sun sensitivity, skin pigmentation in sun exposed sites and a markedly increased risk of development of sunlight induced cancers. This paper thus was an important link between UV-induced DNA damage, faulty DNA repair and human cancer. These cell culture studies were soon confirmed by in vivo studies of UV irradiated skin of the same XP patients (3). Further, they found that the XP patients with neurological degeneration had an even lower rate of post-UV DNA repair than the patient who had no neurological involvement (3,4).
XP Studies Begin at NIH
Before its publication in 1968, Dr. Cleaver presented this work at the National Institutes of Health (NIH) in Bethesda, MD. Dr. Jay Robbins, a Senior Investigator in the Dermatology Branch of the National Cancer Institute (NCI), NIH listened to this talk and decided to begin performing clinical and DNA repair laboratory studies of XP patients. NIH is the primary medical research agency of the United States Federal Government with a mission to seek fundamental knowledge about the nature of living things, to enhance health and to reduce illness and disability. The 300 acre campus in Bethesda, MD contains more than 75 research buildings for 17 NIH Institutes and is home of the NIH Clinical Center, the largest hospital in the world devoted entirely to clinical research. There currently are about 1,500 clinical research studies in progress at the Clinical Center. About half are studies of the natural pathogenesis of disease, especially rare diseases such as XP, which are infrequently investigated elsewhere. For each study, patients are examined in accordance with a clinical research protocol that is approved by an institutional review board (IRB). There is no charge to the patients for any of the medical testing performed and much of the transportation, food and lodging costs are supported by NIH. Patients who are approved for entry into the protocols come to NIH from all over the US and elsewhere in the world.
Dr. Robbins began studying XP patients with Dr. Peter Burk, a Clinical Associate in the Dermatology Branch, and investigating their clinical features and DNA repair levels. They developed a rapid in vitro method to study DNA repair in peripheral blood lymphocytes treated with UV. The first 3 NIH XP patients had multiple skin cancers and reduced post-UV DNA repair (5), like the study of Cleaver. Interestingly, these cells continued to incorporate radioactive thymidine for a longer time than the normal donors’ lymphocytes and eventually incorporated as much as the normal cells. For those patients that had measurable repair, this demonstrated that the defect was in the rate of repair.
XP Variants
The fourth XP patient at NIH (XP4BE1) had severe clinical disease and eventually died of metastatic melanoma (6,7). However, the post-UV DNA repair in his lymphocytes and cultured skin fibroblasts had a normal rate and duration (5). Thus, this clinically severe XP patient had normal DNA repair, raising the theoretical possibility that the DNA repair abnormality in the other 3 XP patients was not the critical defect that resulted in their disease. The following year, James Cleaver reported 3 more XP patients with severe clinical disease but normal DNA repair and gave them the name “XP variants” (8). Cleaver subsequently remarked that he was fortunate to not have found these XP variant patients in his initial investigation because he might have concluded that all XP patients had normal DNA repair. Subsequent studies over several decades revealed that the XP patients with reduced UDS had defects in nucleotide excision repair (NER) while the XP variants had defects in “post-replication repair” resulting from defects in polymerase eta [reviewed in (9)].
Clinical and Laboratory Studies of XP
Dr. Kenneth Kraemer, one of the authors, arrived at NIH in 1971 as a Clinical Associate in the Dermatology Branch, NCI with an appointment as a Commissioned Officer in the United States Public Health Service. After training in internal medicine at Harlem Hospital, a large inner city hospital in New York City, he began working with XP patients under the supervision of Dr. Robbins. His role would be to take care of their medical problems and begin a study of their DNA repair defects. Patients were selected to examine who had different clinical features from each other. This was a collaborative effort with a dermatologist, Dr. Marvin Lutzner, Chief, Dermatology Branch, NCI, a neurologist, Dr. Barry W. Festoff, Clinical Associate, Medical Neurology Branch, National Institute of Neurological Diseases and Stroke (NINDS), and a cell biologist, Dr. Hayden G. Coon, Senior Investigator, Laboratory of Cell Biology, NCI. This multidisciplinary approach to investigation of XP patients was found to be very effective and has continued to the present day.
Data on the first 15 patients studied at NIH (XP1BE though XP15BE) was presented in a paper published in the Annals of Internal Medicine in 1974 (6), a citation classic. There was a high frequency of skin cancer at an early age. Skin cancer was present in 13 of the patients, with first neoplasm between ages 3 and 22 years and 3 of the patients each had more than 50 primary skin cancers. The type of skin cancers were the same as in the general population (basal cell carcinoma (BCC), squamous cell carcinoma (SCC) and melanomas). Neurological degeneration was present in 6 of the patients. One of these patients (XP11BE) had features of both XP and Cockayne Syndrome (CS), another rare neurodegenerative disorder, thus she was the first patient with the XP/CS complex. The DNA repair rate was reduced in 12 of the patients and normal in one patient (XP4BE), the first XP variant. Detailed tables of cutaneous abnormalities, ocular manifestations (demonstrating the effects of UV damage on the anterior portions of the eye – the lids, conjunctiva, cornea and iris but not the retina), and neurological abnormalities were presented showing the importance of normal DNA repair in maintenance and protection of these organ systems.
XP Complementation Groups
Fusion of pairs of cells from XP patients with different clinical features and low post-UV UDS was performed in the laboratory of Dr. Dirk Bootsma in Rotterdam, the Netherlands. They reported that fusion of certain pairs of cells corrected the DNA repair defect in both cells (10). Thus each supplied what the other was missing and they were said to “complement” each other, and thereby form different “complementation groups.” Dr. Kraemer’s initial laboratory project, under the guidance of Dr. Coon, was to perform cell fusions of XP fibroblasts using inactivated Sendai virus (grown in chicken eggs in his laboratory) and perform post-UV UDS on the fused hybrid cells. Four complementation groups were soon found from among the 12 XP cell lines studied (6,11). Surprisingly, unfused XP cells in each complementation group had a characteristic rate of post-UV UDS. This led to a collaboration with the Bootsma laboratory, which by this time had reported a third XP complementation group (12), and the labs exchanged cell lines representative of each complementation group. The joint publication found 5 complementation groups which they chose to name XP-A through XP-E in order of increasing post-UV UDS (13). This early work set the stage for international collaborations and sharing of cell lines among XP researchers which has continued to the present. To this end, Dr. Robbins established a policy of submitting XP cell lines established at NIH to Cell Banks (initially the American Type Culture Collection in Rockville, MD and then the Human Genetic Mutant Cell Repository, Camden, NJ) and making them available to all researchers. This helped to stimulate the large volume of publications on this rare disorder.
Host Cell Reactivation of Viruses and Plasmids in XP Cells
Dr. Rufus S. Day III, a senior investigator in the Laboratory of Molecular Carcinogenesis (LMC), NCI in 1975 reported a “host cell reactivation” assay that was able to measure the DNA repair function in undamaged living cells. He treated adenovirus 2 with UV and then infected unirradiated cells. The virus cannot repair the UV-induced DNA damage on its own but relies on the repair capacity of the cells. Virus survival is assessed as plaque-forming ability on inactivated Sendai virus fused fibroblast monolayers. This assay revealed lower repair in XP cells than in wild type cells. However, when tested with fused XP cells from different complementation groups a second component with a shallower slope was present on the UV dose response curve. This second component was not seen when cells from the same complementation group were fused. This indicates that the complementation resulted in correction of the DNA repair defect that was sufficient to result in functional correction of the damaged adenovirus (14).
Dr. Kraemer left NIH in 1974 for residency training in Dermatology at the University of Miami where he diagnosed a new XP patient who was very well sun protected (15). They subsequently documented that cultured melanocytes and nevus cells from her skin had the same low level of repair as her fibroblasts (15). He returned to NCI in 1976 and joined the LMC as a Senior Investigator.
At NIH, Dr. Kraemer decided to attempt to develop another functional assay to measure the extent of the repair defect in XP cells. Dr. Bruce Howard, a Senior Investigator in NCI, had constructed a new series of plasmids that express the bacterial enzyme chloramphenicol acetyl transferase (CAT) in mammalian cells (16). CAT activity was measured by thin-layer chromatography of acetylated chloramphenicol reaction products. They realized that they could precisely measure the extent of UV damage in this supercoiled plasmid by treating with T4 endonuclease which nicks the plasmid at sites of CPD and thereby relaxes the supercoil and changes migration on a gel. They found that CAT activity of UV treated transfected plasmids was reduced to a greater extent in XP than normal cells. Comparison of the extent of inhibition of CAT expression with the amount of damage produced indicated that one CPD inactivated the expression in the XPA cells (17). This was similar to the finding by Richard Setlow in 1963 that one dimer blocked DNA synthesis in UV sensitive bacteria (18).
Dr. Michael Seidman in LMC, NCI constructed a “shuttle vector” plasmid that could be used for mutation assessment. The plasmid, pZ189, replicates in mammalian cells in addition to bacteria. pZ189 contains a marker gene, the 150-base-pair suppressor transfer RNA, supF, as well as a gene for antibiotic resistance. pZ189 was treated with UV and transfected into the untreated XP or normal cells where the plasmid was repaired or mutated and replicated. The replicated plasmid was extracted and used to transfect E. coli with a suppressible amber mutation in the beta-galactosidase gene and then plated on antibiotic containing plates. The number of bacterial colonies reflects plasmid survival and the color of the colonies (blue or white) reflect the mutation frequency. Plasmid survival was greatly reduced after passing through cells from an XP-A patient compared normal cells (19). Sequencing of supF in mutated plasmids from the XP-A cells showed a restricted mutational spectrum compared with normal cells with a higher frequency of G:C to A:T transitions (19).
They also used plasmid host cell reactivation assays to investigate the repair of different types of UV induced photoproducts in cells from patients with Cockayne syndrome (CS) or XP. CS is a rare autosomal recessive disorder with defective DNA transcription coupled repair, sun sensitivity, short stature, progressive neurological and retinal degeneration without an increase in skin cancer (reviewed in (9)). The CAT-containing plasmid was exposed to UV, which forms CPD and 6-4 photoproducts, and then treated with photo-reactivating enzyme (PR) that selectively removes the CPD. The plasmids were transfected into CS, XP and normal cells, incubated to permit repair, and then CAT activity was measured. The CAT activity was abnormally decreased in the CS and XP cells after the plasmid UV treatments. However, removal of CPD by PR treatment resulted in normal CAT expression in the CS cells. This indicated that repair of non-dimer photoproducts in the plasmid, but not CPD, was normal in CS cells (20). They performed similar experiments using the pZ189 plasmid and assessing the influence of different photoproducts in mutagenesis. They found that in XP cells plasmid mutagenesis was related to faulty repair of all types of photoproducts while in the CS cells there was proficient repair of non-dimer photoproducts. This finding suggests that prevention of UV-induced skin cancers in CS is associated with proficient repair of non-dimer photoproducts in actively transcribing genes (21).
Assessment of Cancer Risk in XP
XP is a very rare disease but individual patients had different clinical features. In order to obtain an idea of the frequency of different abnormalities in XP patients, we realized that the medical library would contain articles on many patients. With a talented undergraduate and then medical student, Myung Moo Lee who spent several summers in my lab, we developed a standard abstracting form and obtained articles from the NIH Library and the National Library of Medicine (that is on the same campus in Bethesda). We found information on 830 individual XP patients in reports published from the first description by Moriz Kaposi in 1874 to 1982 (22). The information was entered into a mainframe computer and analyzed with a data retrieval system. While Cleaver realized that XP patients had a high risk of cancer, we found that the median age of first non-melanoma skin cancer was 8 years, more than 50 years younger than patients with skin cancers in the general population. Neurologic abnormalities were reported in 18% of the cases. This was the first quantitative documentation of the dramatically increased cancer risk in XP.
Over the years we made several estimates of the magnitude of the increased cancer risk in XP. The XP literature review reported that in comparison to the US general population, XP patients under 29 years of age had a more than 2000-fold increase in basal cell and squamous cell carcinoma of the skin, of cutaneous melanoma, of cancer of the anterior eye and cancer of the anterior tongue, all UV exposed sites (23). Ten years later our study of 135 XP patients reported to the XP Registry (in collaboration with Drs. Alan Andrews of Columbia University and W. Clark Lambert of NJ College of Medicine and Dentistry) indicated a more than 1000-fold increase in skin cancer in patients under age 20 years. There was a different anatomic site distribution for melanomas than for basal cell and squamous cell carcinomas indicating different mechanisms of induction of these skin cancers (24). These studies provide a quantitative indication of the essential role of DNA repair in protection from skin cancer and neurodegeneration. A more detailed review of the first 20 years of research on xeroderma pigmentosum at the NIH can found at (25).
Chemoprevention of Skin Cancer in XP
Drs. John DiGiovanna, Gary Peck of the Dermatology Branch, NCI, Robert Tarone of NCI, Alan Moshell of the National Institute of Arthritis, Musculoskeletal, and Skin Diseases (NIAMS) and Dr. Kraemer embarked on a study to attempt to prevent new skin cancers in XP patients using vitamin A derivatives. These agents are associated with promotion of normal differentiation. They selected 5 XP patients with a high frequency of new primary skin cancers, cataloged and removed all of their cancers surgically for a period of 2 years and then treated for 2 years with high dose oral 13-cis retinoic acid (isotretinoin). During the treatment interval there was a 63% reduction in frequency of new skin cancers. Although there were numerous side effects, this treatment was the first demonstration of effective chemoprevention of cancer in humans (26).
DNA Repair Interest Group
Drs. Vilhelm Bohr of NCI and Kraemer began a monthly DNA Repair in-house lecture series in 1985 which was attended by scientists at the Bethesda, MD campus of NIH. In 1992 the Bohr group moved to the National Institute of Aging (NIA) in Baltimore and, with support of the NIH Deputy Director for Intramural Research, they initiated a series of monthly videoconferences that continues to the present. A speaker would deliver a lecture at one site and then be questioned by participants viewing from the other sites. In addition to the Bethesda and Baltimore campuses of NIH, the videoconferences initially included Dr. Thomas Kunkel and colleagues in the National Institute of Environmental Health Sciences (NIEHS) in Research Triangle Park, NC and the late Dr. Anthony Dipple at NCI in Frederick, MD. Over the years the number of additional sites has fluctuated and included groups at different locations: Dr. Richard Setlow, Brookhaven National Laboratory in Upton, NY; Dr. Larry Thompson, Lawrence Livermore National Laboratory, Livermore, CA; Dr. Arthur Grollman, State University of NY, Stony Brook, NY; Dr. Mats Ljungman, University of Michigan; Drs. Rodney Nairn, Richard Wood and David Mitchell, M.D. Anderson Hospital, Smithville, TX; Dr. David Orren, University of Kentucky, Lexington, KY; Drs. William Kaufmann and Marila Cordeiro-Stone, University of North Carolina, Chapel Hill, NC; Dr. Stephen Lloyd, Oregon Health and Science University, Portland, OR; and Drs. Bennet Van Houten and Robert Sobel, University of Pittsburgh, PA. Additional details are presented in “The DNA Repair Interest Group: a Global Village” (27). More than 150 of these lectures have been archived and can be viewed at http://videocast.nih.gov. Each year one lecture was reserved for a “History of DNA repair”. In 2001, Dr. James Cleaver described his early work in discovering defective DNA repair in XP in a lecture “Mending human genes” http://videocast.nih.gov/launch.asp?10561.
Long-term Multidisciplinary Studies of XP
NIH is a referral center and we became known for our interest in XP. Typically a clinician would suspect the diagnosis and then send skin cells for testing. For many years Dr. James Cleaver’s lab in San Francisco performed this service in addition to his research studies. Once this testing was performed the patient might be referred to NIH for detailed clinical evaluation and additional research laboratory testing. In this manner we collaborated with Cleaver in studying an unusual 4 year old boy who had XP with multiple skin cancers including melanomas, autism and hypoglycinemia associated with a splice site mutation in the XPC DNA repair gene. He responded well to sun protection and oral glycine (28). The NIH group also collaborated with Dr. Cleaver on studies of a 14 year old girl with mild clinical symptoms who had a missense mutation in the XPG DNA repair gene, and another XP-G patient who had the XP/CS complex (29).
Dr. John J. DiGiovanna, NCI, a clinical and research dermatologist joined the NIH group in 1980, to study XP patients in the clinic. Sikandar Khan, Ph.D., a Staff Scientist began working in our laboratory in 1995. He supervised all of the laboratory investigations including testing of patient cells for complementation groups and mutations. We had a group of excellent post-doctoral fellows who remained in the lab for 2 to 5 years, medical students who stayed for 1 year and multiple undergraduate or medical students who spent 8 weeks in the lab during the summers. In 2003, Deborah Tamura, R.N. who is trained in genetic counseling and had experience in obstetrics and gynecology joined us as a research nurse. Over the years we developed collaborations with an ophthalmologist, Dr. Brian Brooks of the National Eye Institute; a neuro-radiologist, Dr. Nicholas Patronas of the Clinical Center; epidemiologists, Dr. Margaret Tucker and Alisa Goldstein, NCI; an audiologist, Dr. Carmen Brewer; a psychologist, Dr. Edythe Wiggs; neurologists, Drs. Raphael Schiffmann and Tyler Pierson of NINDS, pathologists, Drs. Jere Stern, Chyi-Chia Richard Lee, Mark Raffeld, Martha Quezado, and others.
Debby Tamura reviews the medical records of XP patients referred from outside doctors and arranges for a 3 to 5 day visit at the Clinical Center. During this time they have detailed examinations of their skin, eyes, hearing tests, neurological exam, psychometric testing, total body photography with images of individual lesions, and imaging of their brain if neurological abnormalities are present as well as standard laboratory blood and urine tests. Skin biopsies are performed and blood samples taken for establishment of cell cultures.
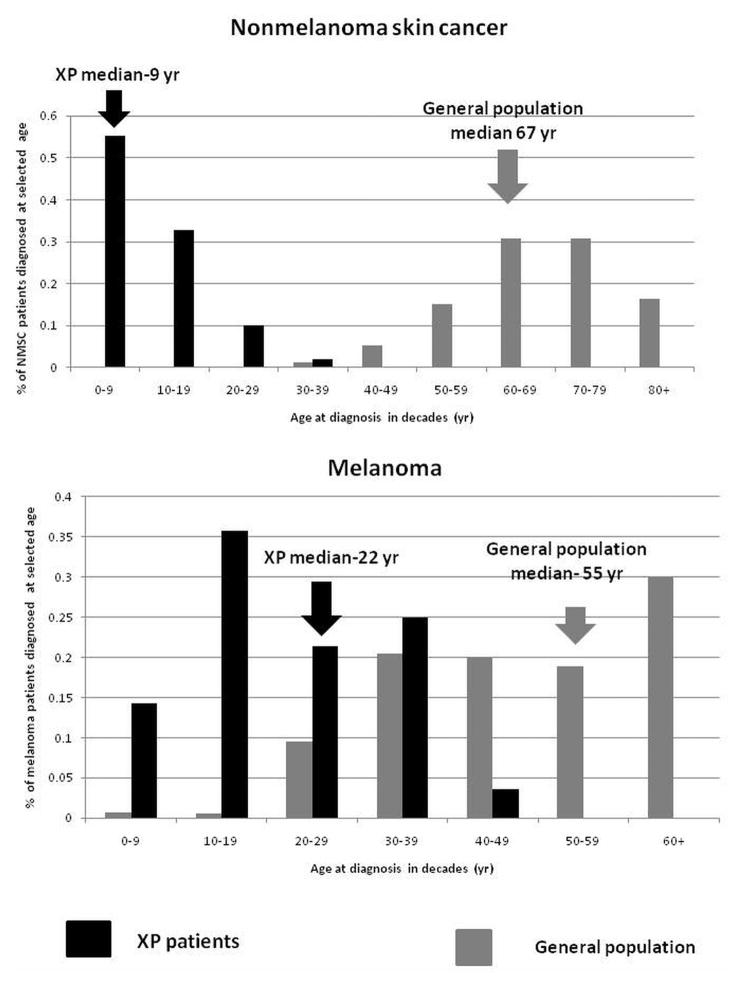
A 40 year follow-up of 106 XP patients admitted to NIH between 1971 and 2009, performed in collaboration with Dr. Porcia Bradford, an Epidemiology Fellow, and Drs. Tucker and Goldstein found that XP patients under age 20 years had a more than 10,000-fold increase in non-melanoma skin cancer and a more than 2000-fold increase in melanoma of the skin (30). In the general population, the average age of onset of melanoma occurs at a younger age than non-melanoma skin cancer (NMSC). However, this is reversed in XP, where we found that the median age of onset of first NMSC (9 years) was significantly younger than the median age of first melanoma (22 years) (Figure 1). This relative age reversal from the general population suggested different mechanisms of carcinogenesis between NMSC and melanoma. About half of the XP patients reported a history of acute burning on minimal sun exposure (Figure 2A). The others typically did not burn but developed freckle-like lesions on the face before age 2 years (Figure 2B). The burning patients had a lower rate of skin cancer than those that did not burn, this may be related to their extreme sun protection at an early age.
Figure 1.
XP skin cancer by age at first skin cancer diagnosis and skin cancer type compared to US general population. Upper panel: Proportion of non-melanoma skin cancer (NMSC) patients diagnosed at selected ages. Lower panel: Proportion of melanoma patients diagnosed at selected ages. Individuals with both NMSC and melanoma were used for both analyses. General population data was taken from (37) (Figure from (30)).
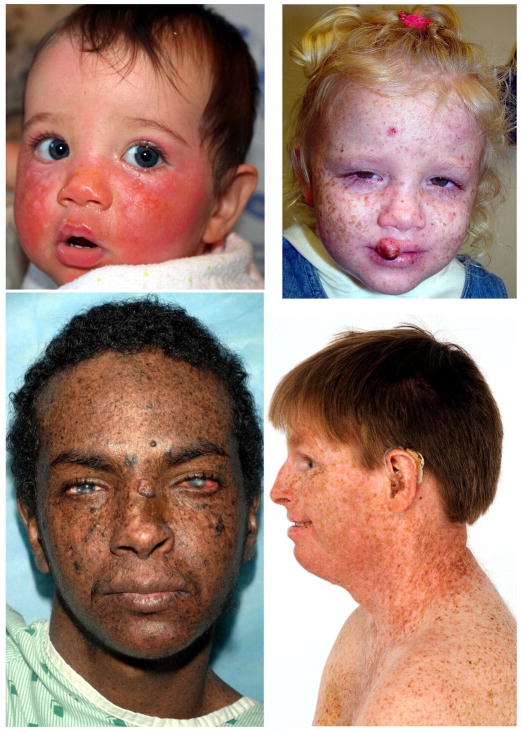
Figure 2.
XP patients in study. (A) Patient XP420BE complementation group XP-D at 9 months of age with severe blistering erythema of the malar area following minimal sun exposure. Note sparing of her forehead and eyes that were protected by a hat. (B) Patient XP358BE (XP-C) at age 2 years did not sunburn easily but developed multiple hyperpigmented macules on her face. A rapidly growing squamous cell carcinoma (SCC) or keratoacanthomas grew on her upper lip and a pre-cancerous lesion appeared on her forehead. (C) Northern African patient XP393BE (XP-C)(38) at age 23 years with numerous hyperpigmented macules on his face. Nodular basal cell cancer is present on his left nasal root. Pigmented basal cell cancer is present on his left cheek. His eyes show cornea scarring from unprotected sun exposure. (D) Patient XP19BE (XP-A)(39) at age 35 years with neurological degeneration. He has numerous hyperpigmented macules on sun exposed areas of his face and neck. Progressive sensorineural deafness requires the use of a hearing aid (Figure from (30)).
XP is present in dark skinned African patients (Figure 2C). In addition to the skin cancers, they frequently have involvement of less pigmented sites such as the eyes and tip of the tongue. Progressive neurological degeneration was present in 24% with most in complementation group XP-D (Figure 2D). The median age at death of 29 years in XP patients with neurodegeneration was significantly younger than the patients without neurodegeneration (37 years) (30).
Long term follow-up by Dr. Brooks of the eye abnormalities in 83 XP and 3 XP/CS patients examined at NIH from 1964 to 2011 was reported (31). More than 90% of the patients had at least one ocular abnormality marking the eye as a major target for UV damage. These abnormalities included conjunctivitis (51%) to corneal scarring (26%) and cataracts (14%). Ocular surface cancer was reported in 10% of the patients. XP patients with an acute burning phenotype were more likely to develop neoplastic ocular lesions than non-burning patients. These studies provide evidence that DNA repair plays a major role in protection of the eye from sunlight induced damage.
Mariam Totonchy, a medical student in the lab for a year, worked with Dr. Brewer and they evaluated audiograms from 77 XP and 2 XP/CS patients who were examined at NIH from 1971 to 2012 (32). Clinically significant hearing loss was present in 29% of the patients especially those in complementation groups XP-A and XP-D who had acute burning on minimal sun exposure. The severity of hearing loss paralleled the neurological decline and thus audiograms could be used to assess the rate of XP neurological degeneration. These results provide evidence that DNA repair is critical in maintaining neurological integrity of the auditory system (32).
Autopsies were performed on 4 adult XP patients by the NCI Laboratory of Pathology (33). The XP-A and XP-D patients had severe progressive neurological degeneration and at age 44 and 45 years, respectively. Both had infantile sized brains indicative of profound atrophy. One XP-C patient with hundreds of skin cancers died at age 35 years of a grade IV glioblastoma of her brain. Another XP-C patient had hundreds of skin cancers, and bilateral eye cancers requiring enucleations, died at age 49 years from widespread metastatic adenocarcinoma of her uterine endocervix. Thus, despite many skin cancers these 4 XP patients died of internal tumors and neurodegeneration (33). This demonstrates that the mortality from skin cancers can be managed and prevented, and also points to the importance of surveillance for internal malignancies.
Recent NIH studies
In order to look for molecular evidence of UV damage in melanomas from XP patients, Yun Wang, M.D., Ph.D., a dermatologist post-doctoral fellow from China, performed laser capture microdissection on paraffin fixed tissue blocks from 59 melanomas from 8 XP patients. He sequenced the PTEN tumor suppressor gene and found mutations in 56% of the melanomas. Further, 91% of the melanomas with mutations had UV type mutations occurring at adjacent pyrimidines, indicating an important role for sun exposure in induction of these melanomas (34). Coming nearly full circle, Dr. Cleaver edited this manuscript for the Proceedings of the National Academy of Sciences.
Taro Masaki, M.D., Ph.D., a dermatologist post-doctoral fellow from Japan, continued these studies by investigating pre-cancerous pigmented lesions (nevi) in the XP patients (35). There was a similar high frequency of UV type PTEN mutations in the nevi as in the melanomas. Surprisingly, unlike the mutations previously described in nevi in the general population, the frequency of BRAF, NRAS and KIT mutations was lower than for PTEN. The mTOR pathway appeared to be activated in these XP lesions. This may provide an approach to controlling melanomas in XP patients by use of topical mTOR inhibitors.
In determining the molecular defects in XP patients we found that about 15% of the XP-C patients had premature termination codons (PTC) in their germline DNA. Christiane Kuschal, Ph.D., a post-doctoral fellow from Germany, studied the ability of aminoglycoside antibiotics to readthrough PTC in cells from XP-C patients (36). She developed a sensitive assay measuring the appearance of XPC protein in localized areas of UV damage. She found that the response to Geneticin and gentamycin depended on the PTC sequence and it location within the gene. Characterizing parameters governing effective PTC readthrough may provide a new prophylactic therapy for skin cancer prevention in selected XP-C patients.
Acknowledgments
This research was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, NIH. We want to thank the many postdoctoral fellows, medical students, and undergraduate summer students who worked in the laboratory. We are most grateful to the patients and their families who participated in these studies over the past 4 decades.
Footnotes
This paper is part of the Special Issue honoring James Cleaver
Cell lines are named for the disease (Xeroderma Pigmentosum), sequential number followed by city of origination (Bethesda)
References
- 1.Cleaver JE. Defective repair replication of DNA in xeroderma pigmentosum. Nature. 1968;218:652–656. doi: 10.1038/218652a0. [DOI] [PubMed] [Google Scholar]
- 2.PETTIJOHN D, Hanawalt P. Evidence for repair-replication of ultraviolet damaged DNA in bacteria. J Mol Biol. 1964;9:395–410. doi: 10.1016/s0022-2836(64)80216-3. [DOI] [PubMed] [Google Scholar]
- 3.Epstein JH, Fukuyama K, Reed WB, Epstein WL. Defect in DNA synthesis in skin of patients with xeroderma pigmentosum demonstrated in vivo. Science. 1970;168:1477–1478. doi: 10.1126/science.168.3938.1477. [DOI] [PubMed] [Google Scholar]
- 4.Reed WB, Landing B, Sugarman G, Cleaver JE, Melnyk J. Xeroderma pigmentosum. Clinical and laboratory investigation of its basic defect. JAMA. 1969;207:2073–2079. doi: 10.1001/jama.207.11.2073. [DOI] [PubMed] [Google Scholar]
- 5.Burk PG, Yuspa SH, Lutzner MA, Robbins JH. Xeroderma pigmentosum and D. N. A. repair. Lancet. 1971;1:601. doi: 10.1016/s0140-6736(71)91203-7. [DOI] [PubMed] [Google Scholar]
- 6.Robbins JH, Kraemer KH, Lutzner MA, Festoff BW, Coon HG. Xeroderma pigmentosum. An inherited diseases with sun sensitivity, multiple cutaneous neoplasms, and abnormal DNA repair. Ann Intern Med. 1974;80:221–248. doi: 10.7326/0003-4819-80-2-221. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y, Tan XH, DiGiovanna JJ, Lee CC, Stern JB, Raffeld M, Jaffe ES, Kraemer KH. Genetic diversity in melanoma metastases from a patient with xeroderma pigmentosum. J Invest Dermatol. 2010;130:1188–1191. doi: 10.1038/jid.2009.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cleaver JE. Xeroderma pigmentosum: variants with normal DNA repair and normal sensitivity to ultraviolet light. J Invest Dermatol. 1972;58:124–128. doi: 10.1111/1523-1747.ep12538913. [DOI] [PubMed] [Google Scholar]
- 9.DiGiovanna JJ, Kraemer KH. Shining a light on xeroderma pigmentosum. J Invest Dermatol. 2012;132:785–796. doi: 10.1038/jid.2011.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Weerd-Kastelein EA, Keijzer W, Bootsma D. Genetic heterogeneity of xeroderma pigmentosum demonstrated by somatic cell hybridization. Nat New Biol. 1972;238:80–83. doi: 10.1038/newbio238080a0. [DOI] [PubMed] [Google Scholar]
- 11.Kraemer KH, Coon HG, Petinga RA, Barrett SF, Rahe AE, Robbins JH. Genetic heterogeneity in xeroderma pigmentosum: complementation groups and their relationship to DNA repair rates. Proc Natl Acad Sci U S A. 1975;72:59–63. doi: 10.1073/pnas.72.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Weerd-Kastelein EA, Keijzer W, Bootsma D. A third complementation group in xeroderma pigmentosum. Mutat Res. 1974;22:87–91. doi: 10.1016/0027-5107(74)90013-x. [DOI] [PubMed] [Google Scholar]
- 13.Kraemer KH, De Weerd-Kastelein EA, Robbins JH, Keijzer W, Barrett SF, Petinga RA, Bootsma D. Five complementation groups in xeroderma pigmentosum. Mutat Res. 1975;33:327–340. doi: 10.1016/0027-5107(75)90208-0. [DOI] [PubMed] [Google Scholar]
- 14.Day RS, Kraemer KH, Robbins JH. Complementing xeroderma pigmentosum fibroblasts restore biological activity to UV-damaged DNA. Mutat Res. 1975;28:251–255. doi: 10.1016/0027-5107(75)90103-7. [DOI] [PubMed] [Google Scholar]
- 15.Kraemer KH, Herlyn M, Yuspa SH, Clark WH, Jr, Townsend GK, Neises GR, Hearing VJ. Reduced DNA repair in cultured melanocytes and nevus cells from a patient with xeroderma pigmentosum. Arch Dermatol. 1989;125:263–268. [PubMed] [Google Scholar]
- 16.Gorman CM, Moffat LF, Howard BH. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982;2:1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Protic-Sabljic M, Kraemer KH. One pyrimidine dimer inactivates expression of a transfected gene in xeroderma pigmentosum cells. Proc Natl Acad Sci U S A. 1985;82:6622–6626. doi: 10.1073/pnas.82.19.6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Setlow RB, Swenson PA, Carrier WL. Thymine dimers and inhibition of DNA synthesis by ultraviolet irradiation of cells. Science. 1963;142:1464–1466. doi: 10.1126/science.142.3598.1464. [DOI] [PubMed] [Google Scholar]
- 19.Bredberg A, Kraemer KH, Seidman MM. Restricted ultraviolet mutational spectrum in a shuttle vector propagated in xeroderma pigmentosum cells. Proc Natl Acad Sci U S A. 1986;83:8273–8277. doi: 10.1073/pnas.83.21.8273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barrett SF, Robbins JH, Tarone RE, Kraemer KH. Evidence for defective repair of cyclobutane pyrimidine dimers with normal repair of other DNA photoproducts in a transcriptionally active gene transfected into Cockayne syndrome cells. Mutat Res. 1991;255:281–291. doi: 10.1016/0921-8777(91)90032-k. [DOI] [PubMed] [Google Scholar]
- 21.Parris CN, Kraemer KH. Ultraviolet-induced mutations in Cockayne syndrome cells are primarily caused by cyclobutane dimer photoproducts while repair of other photoproducts is normal. Proc Natl Acad Sci U S A. 1993;90:7260–7264. doi: 10.1073/pnas.90.15.7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kraemer KH, Lee MM, Scotto J. Xeroderma pigmentosum. Cutaneous, ocular, and neurologic abnormalities in 830 published cases. Arch Dermatol. 1987;123:241–250. doi: 10.1001/archderm.123.2.241. [DOI] [PubMed] [Google Scholar]
- 23.Kraemer KH, Lee MM, Scotto J. DNA repair protects against cutaneous and internal neoplasia: evidence from xeroderma pigmentosum. Carcinogenesis. 1984;5:511–514. doi: 10.1093/carcin/5.4.511. [DOI] [PubMed] [Google Scholar]
- 24.Kraemer KH, Lee MM, Andrews AD, Lambert WC. The role of sunlight and DNA repair in melanoma and nonmelanoma skin cancer. The xeroderma pigmentosum paradigm. Arch Dermatol. 1994;130:1018–1021. [PubMed] [Google Scholar]
- 25.Kraemer KH. Twenty years of research on xeroderma pigmentosum at the National Institutes of Health. In: Riklis E, editor. Photobiology. Plenum Press; New York: 1991. pp. 211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kraemer KH, DiGiovanna JJ, Moshell AN, Tarone RE, Peck GL. Prevention of skin cancer in xeroderma pigmentosum with the use of oral isotretinoin. N Engl J Med. 1988;318:1633–1637. doi: 10.1056/NEJM198806233182501. [DOI] [PubMed] [Google Scholar]
- 27.Kraemer KH, Bohr VA. The DNA Repair Interest Group: a global village. DNA Repair (Amst) 2005;4:405–406. doi: 10.1016/j.dnarep.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 28.Khan SG, Levy HL, Legerski R, Quackenbush E, Reardon JT, Emmert S, Sancar A, Li L, Schneider TD, Cleaver JE, Kraemer KH. Xeroderma pigmentosum group C splice mutation associated with autism and hypoglycinemia. J Invest Dermatol. 1998;111:791–796. doi: 10.1046/j.1523-1747.1998.00391.x. [DOI] [PubMed] [Google Scholar]
- 29.Emmert S, Slor H, Busch DB, Batko S, Albert RB, Coleman D, Khan SG, bu-Libdeh B, DiGiovanna JJ, Cunningham BB, Lee MM, Crollick J, Inui H, Ueda T, Hedayati M, Grossman L, Shahlavi T, Cleaver JE, Kraemer KH. Relationship of neurologic degeneration to genotype in three xeroderma pigmentosum group G patients. J Invest Dermatol. 2002;118:972–982. doi: 10.1046/j.1523-1747.2002.01782.x. [DOI] [PubMed] [Google Scholar]
- 30.Bradford PT, Goldstein AM, Tamura D, Khan SG, Ueda T, Boyle J, Oh KS, Imoto K, Inui H, Moriwaki S, Emmert S, Pike KM, Raziuddin A, Plona TM, DiGiovanna JJ, Tucker MA, Kraemer KH. Cancer and neurologic degeneration in xeroderma pigmentosum: long term follow-up characterises the role of DNA repair. J Med Genet. 2011;48:168–176. doi: 10.1136/jmg.2010.083022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brooks BP, Thompson AH, Bishop RJ, Clayton JA, Chan CC, Tsilou ET, Zein WM, Tamura D, Khan SG, Ueda T, Boyle J, Oh KS, Imoto K, Inui H, Moriwaki S, Emmert S, Iliff NT, Bradford P, DiGiovanna JJ, Kraemer KH. Ocular manifestations of xeroderma pigmentosum: long-term follow-up highlights the role of DNA repair in protection from sun damage. Ophthalmology. 2013;120:1324–1336. doi: 10.1016/j.ophtha.2012.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Totonchy MB, Tamura D, Pantell MS, Zalewski C, Bradford PT, Merchant SN, Nadol J, Khan SG, Schiffmann R, Pierson TM, Wiggs E, Griffith AJ, DiGiovanna JJ, Kraemer KH, Brewer CC. Auditory analysis of xeroderma pigmentosum 1971–2012: hearing function, sun sensitivity and DNA repair predict neurological degeneration. Brain. 2013;136:194–208. doi: 10.1093/brain/aws317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lai JP, Liu YC, Alimchandani M, Liu Q, Aung PP, Matsuda K, Lee CC, Tsokos M, Hewitt S, Rushing EJ, Tamura D, Levens DL, DiGiovanna JJ, Fine HA, Patronas N, Khan SG, Kleiner DE, Oberholtzer JC, Quezado MM, Kraemer KH. The influence of DNA repair on neurological degeneration, cachexia, skin cancer and internal neoplasms: autopsy report of four xeroderma pigmentosum patients (XP-A, XP-C and XP-D) Acta Neuropathol Commun. 2013;1:4. doi: 10.1186/2051-5960-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Y, DiGiovanna JJ, Stern JB, Hornyak TJ, Raffeld M, Khan SG, Oh KS, Hollander MC, Dennis PA, Kraemer KH. Evidence of ultraviolet type mutations in xeroderma pigmentosum melanomas. Proc Natl Acad Sci U S A. 2009;106:6279–6284. doi: 10.1073/pnas.0812401106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Masaki T, Wang Y, DiGiovanna JJ, Khan SG, Raffeld M, Beltaifa S, Hornyak TJ, Darling TN, Lee CC, Kraemer KH. High frequency of PTEN mutations in nevi and melanomas from xeroderma pigmentosum patients. Pigment Cell Melanoma Res. 2014;27:454–464. doi: 10.1111/pcmr.12226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuschal C, DiGiovanna JJ, Khan SG, Gatti RA, Kraemer KH. Repair of UV photolesions in xeroderma pigmentosum group C cells induced by translational readthrough of premature termination codons. Proc Natl Acad Sci U S A. 2013;110:19483–19488. doi: 10.1073/pnas.1312088110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Glass AG, Hoover RN. The emerging epidemic of melanoma and squamous cell skin cancer. JAMA. 1989;262:2097–2100. [PubMed] [Google Scholar]
- 38.Mahindra P, DiGiovanna JJ, Tamura D, Brahim JS, Hornyak TJ, Stern JB, Lee CC, Khan SG, Brooks BP, Smith JA, Driscoll BP, Montemarano AD, Sugarman K, Kraemer KH. Skin cancers, blindness, and anterior tongue mass in African brothers. J Am Acad Dermatol. 2008;59:881–886. doi: 10.1016/j.jaad.2008.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robbins JH, Brumback RA, Mendiones M, Barrett SF, Carl JR, Cho S, Denckla MB, Ganges MB, Gerber LH, Guthrie RA. Neurological disease in xeroderma pigmentosum. Documentation of a late onset type of the juvenile onset form. Brain. 1991;114(Pt 3):1335–1361. doi: 10.1093/brain/114.3.1335. [DOI] [PubMed] [Google Scholar]