Abstract
Allergic inflammation is associated closely with parasite infection but also asthma and other common allergic diseases. Despite the engagement of similar immunologic pathways, parasitized individuals often show no outward manifestations of allergic disease. In this perspective, we present the thesis that allergic inflammatory responses play a primary role in regulating circadian and environmental inputs involved with tissue homeostasis and metabolic needs. Parasites feed into these pathways and thus engage allergic inflammation to sustain aspects of the parasitic life cycle. In response to parasite infection, an adaptive and regulated immune response is layered on the host effector response, but in the setting of allergy, the effector response remains unregulated, thus leading to the cardinal features of disease. Further understanding of the homeostatic pressures driving allergic inflammation holds promise to further our understanding of human health and the treatment of these common afflictions.
Allergic inflammatory responses are engaged during both parasite infection and allergic disease, but parasite-infected individuals do not show signs of allergy. Allergic responses may thus play key roles in homeostasis.
Buoyed by the successes of prophylactic immunization against toxins at the turn of the 20th century, Portier and Richet began studies with hypnotoxin from the cnidarian, Physalia physalis, commonly known as the Portuguese man o’ war, and a related toxin from the sea anemones, Actinia equina and Anemonia sulcata. These investigations led to the paradoxical discovery of immediate hypersensitivity reactions and even death among some immunized animals by a process termed “anaphylaxis” (Richet 1913). Recognized by a Nobel Prize, these seminal findings underpinned the modern field of allergy and led to the eventual identification of the transferable nature of the activating agent in serum, first noted by Richet, as immunoglobulin E (IgE) (Ishizaka et al. 1966). The rapid advances in molecular biology, genetics, and genomics from 1970 to 2000 elucidated the central role for cytokines, particularly the duplicated genes for interleukin (IL)-4, IL-13, IL-5, and IL-9, in mediating the effector functions of allergic immunity. Although initial studies fueled by the discoveries of helper T-cell subsets focused on T cells, designated Th2 cells, as sources of these cytokines, recent findings have increasingly highlighted the role of innate cells in allergic immunity. These discoveries have raised hopes that insights regarding the initiation and/or maintenance of tissue pathology mediated by interactions between innate and adaptive cells might translate to new therapeutic modalities for diseases underpinned by type 2 immunity.
PREVALENCE OF TYPE 2 IMMUNE MANIFESTATIONS AND THE PARADOX OF ALLERGY
Clinical manifestations of type 2 immune responses are commonplace worldwide in association with parasite infections and allergic diseases. It is estimated that 2–4 billion people worldwide harbor parasitic infections with the vast majority concentrated in developing nations (Chan 1997). Despite the paucity of parasitic infections in developed countries, type 2 immunity significantly impacts human health in the form of allergic diseases, including IgE-mediated anaphylaxis, allergic rhinitis, asthma, atopic dermatitis, eosinophilic gastrointestinal diseases, and food allergies. Worldwide, it is estimated that 300 million people have asthma and 400 million have allergic rhinitis (WHO 2007). In the United States, annual asthma costs approximate 56 billion dollars (CDC 2011).
In humans, “normal” values for IgE and eosinophils are defined using populations from developed countries where elevated levels are associated with pathologic states. In less developed countries, however, parasitic infestation is more widespread, and IgE levels and eosinophils are high. Indeed, hypereosinophilia was the commonest criteria underlying exclusion of healthy Uganda volunteers for vaccine trials (Eller et al. 2008). Although data collection is imperfect, the consensus view is that allergic diseases such as asthma are less prevalent in underdeveloped countries (Godfrey 1975; ISAAC 1998; Eller et al. 2008). In considering nonhuman vertebrates, domestic dogs have IgE levels 100 times greater than that in humans, and populations of Scandinavian wolves and a variety of horses show even higher levels (Ledin et al. 2006, 2008; Wagner 2009). As in humans from less developed countries, elevated IgE and eosinophils in feral vertebrates are associated with widespread parasitism, particularly intestinal helminths (usually multiple species) and ectoparasites, such as mites and ticks. Crocodiles, Antarctic petrels, Icelandic minke whales, penguins, and arctic mammals including bears, wolves, and cervids all show evidence of ecto- and endoparasitism (Jones 1988; Frenot et al. 2001; Lavikainen et al. 2011; La Grange et al. 2013; Olafsdottir and Shinn 2013). Taken together, these data suggest that manifestations of allergic inflammation are universal in nonhuman vertebrate populations in association with high levels of parasitism but there is little evidence for pathology associated with human allergic disease.
By extrapolation, it is likely that human evolution was marked by a higher “set point” for the cells and effector molecules, such as eosinophils and IgE, which are now associated with allergic manifestations that remain unusual or infrequent in wild and indigenous vertebrates. A number of possibilities have been considered to explain this apparent paradox. First, as a variant of the hygiene hypothesis, exposure to pathogens during critical developmental periods may be necessary to entrain the immune system to focus on exogenous organisms rather than innocuous allergens. Mechanisms proposed to underlie such “training” include induction of regulatory T cells or blocking antibodies that function to establish tolerance to antigens acquired later through food or inhalation. The data underlying such explanations have been reviewed elsewhere (Soyer et al. 2013). Such a mechanism may also underlie the dysregulated inflammatory responses that accompany many diseases of developed countries, including atherosclerosis, dementia, and obesity. A variant of this possibility, based on increasing information regarding immune cells that develop during fetal but not adult hematopoiesis (e.g., Langerhans cells and microglia) (Ginhoux et al. 2010; Mold et al. 2010; Hoeffel et al. 2012), is that certain cells in tissues may function in “anticipatory” roles, awaiting terminal differentiation by developmental or environmental signals, such as microbes and food, that the organism encounters postbirth. Alterations in these environmental signals during early developmental periods may bypass windows of differentiation that leave the organism more prone to inflammatory states in later life, whether or not accompanied by excesses of Th1- or Th2-associated pathology (Mold et al. 2010). A final possibility considered here is that intestinal helminths and ectoparasites elicit immune responses that mimic homeostatic responses used by the vertebrate host, but to facilitate aspects of the differentiation or development of the parasite. By this scenario, parasites have evolved to elicit tissue reactions that promote their own parasitism. Although the advantages of such evolution are not always readily apparent, the consideration is warranted owing to the extreme penetrance of parasitic infestation on the vertebrate immune system and the relatively small impact of these infections on survival through the reproductive age.
COMPONENTS OF THE ALLERGIC MODULE IN HOST DEFENSE AND ALLERGIC DISEASE
Studies in humans as well as model organisms have detailed the immunologic constituents of allergic inflammation. Here, we summarize recent insights regarding the functions of these various components (Fig. 1).
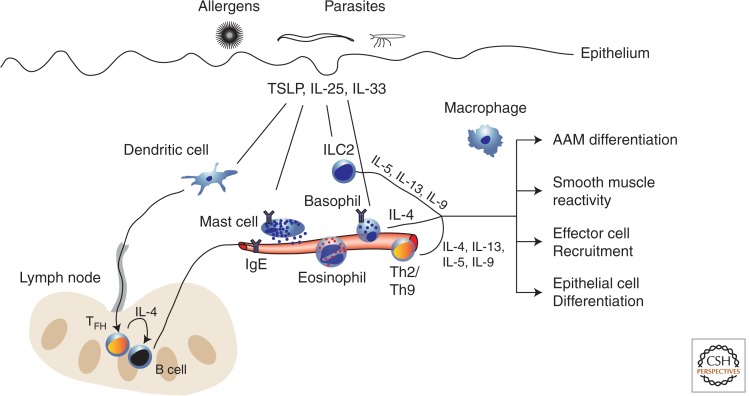
Figure 1.
Constituents of allergic inflammation. Allergic inflammatory responses involve the coordinated response of (1) tissue-resident cells (dendritic cells, innate lymphoid cell [ILC]2, mast cells, and macrophages), (2) expansion of antigen-specific T cells and B cells in the draining lymph node, and (3) recruited cells from the blood (Th2/Th9 cells, basophils, and eosinophils). On allergen exposure or parasite infection, epithelial sensors evoke the release/production of epithelial cytokines (TSLP, IL-25, and IL-33), which in turn drive dendritic cells to migrate to lymph nodes and facilitate IgE production by B cells. Mast cells can be activated through IgE–antigen interactions as well as epithelial cytokines and modulate vascular tone through the release of granules. ILC2s, recruited basophils, and Th2/Th9 are activated by antigen and/or epithelial cytokines, and all three are significant producers of various type 2 cytokines. The sum total of this response includes alternatively activated macrophage (AAM) differentiation, modulation of smooth muscle and epithelial cell function, and further recruitment of effector cells.
IgE, Mast Cells, and Basophils
IgE is the least represented serum immunoglobulin, consistent with a short serum half-life and distribution within peripheral tissues (Gould and Sutton 2008). Isotype switching of B cells to IgE requires IL-4-producing T follicular helper (TFH) cells (Reinhardt et al. 2009; Crotty 2011). T-cell–B-cell collaboration is likely short-lived because IgE-switched B cells egress rapidly from germinal centers to become plasma cells resident in extrafollicular regions of the lymph node (Talay et al. 2012; Yang et al. 2012). Clarification is needed to explain how allergen-specific IgE antibodies develop extensive hypermutation consistent with their high-affinity states (Davies et al. 2013), whether arising from previously mutated IgG1 germinal center B cells or through some unknown process driving extensively hypermutated antibodies during chronic antigen exposure, as revealed by studies of neutralizing HIV antibodies (Zhou et al. 2010). IgE disseminates through the bloodstream, where free IgE is stabilized on the surface of cells bearing the high-affinity IgE receptor, FcεRI. Unlike other immunoglobulin isotypes, IgE effector function is related almost entirely to its capacity to bind to FcεRI before antigen recognition. In mice, mast cells and basophils constitutively express FcεRI, and is inducible on some dendritic cell populations (Grayson et al. 2007). In humans, FcεRI is also constitutively expressed on mast cells and basophils, but is more widely expressed, including on dendritic cell populations (Gould and Sutton 2008).
Mast cells are tissue-resident cells found near blood vessels in a perivascular distribution. This positioning allows mast cells to extend cellular processes across the endothelial barrier to acquire circulating IgE as well as to control vascular tone and permeability (Galli and Tsai 2010; Cheng et al. 2013). On activation, mast cells release preformed and synthesized mediators, including histamine, lipid mediators, and cytokines. In the skin, the clinical manifestations of this reaction include the “wheal and flare” seen in urticaria. The evolutionary benefit of these rapidly activated responses may be to mitigate threats from insects or noxious substances by promoting resolution of the threat or alerting the host to establish avoidance behavior (Palm et al. 2012). In the gut, these mediators also lead to neural stimulation and intestinal smooth muscle hypermotility, which might function to clear ingested toxins via emesis and diarrhea.
Although commonly associated with allergic conditions, mast cells also participate in host defense to bacterial pathogens (Malaviya et al. 1996; McLachlan et al. 2003). As during allergy, these nonallergic stimulants promote changes in vascular permeability, activate antigen-presenting cells to mature and migrate to draining lymph nodes, and engage components of adaptive immunity (Shelburne et al. 2009). Because mast cells are tissue-resident cells capable of sensing a variety of inputs associated with disparate inflammatory modules, these cells may have evolved the capacity to acquire IgE to broaden functional capacity and respond to environmental insults.
Basophils are closely related to mast cells as revealed by developmental similarities driven by common transcription factor modules (Qi et al. 2013). Basophils also bind IgE but, unlike mast cells, circulate in blood with a half-life similar to other circulating myeloid cells (Voehringer 2013). The scarcest of all circulating leukocytes, basophils degranulate after FcεRI-mediated activation, but unlike mast cells, contribute little to anaphylaxis (Ohnmacht et al. 2010). During migratory helminth infection, basophils enter involved tissues and interact closely with CD4 T cells, which mediate contact-dependent and IL-3-dependent IL-4 release from basophils; antigen-specific IgE enhances basophil IL-4 production (Sullivan et al. 2011). Basophils also promote eosinophil entry into skin models of chronic allergic inflammation and induce the differentiation of recruited monocytes to alternatively activated macrophages in tissues (see below).
CD4 T Cells: Th2 Cells, Th9 Cells, and T Regulatory Cells (Tregs)
Activation of helper T cells in allergy is dependent on dendritic cells (DCs), although the precise signals and surface phenotype of type 2–inducing DCs may differ in response to different stimuli or within different tissues (Pulendran et al. 2010). A gradient defined by integration of T-cell receptor (TCR) signal strength, T-cell precursor frequency, and antigen abundance underlies the proclivity of some cells to enter follicles and become IL-4-expressing TFH cells (typically higher-affinity TCRs) or activate more extensive patterns of type 2 cytokine expression and leave lymph nodes to enter peripheral tissues as Th2 cells (Tubo et al. 2013) . Tissue Th2 cells show a range of cytokine patterns, including single and multiple combinations of IL-4, IL-5, IL-9, and IL-13. As shown most convincingly using knockout mice in models of helminth and allergic immune challenges, these cytokines have both unique and redundant functions in type 2 immunity (Fallon et al. 2002; Nath et al. 2007); importantly, absence of all of these attenuates most of the manifestations of allergy, in part owing to the inevitable outgrowth of effector T cells expressing inflammatory cytokines. Further information is needed to understand fully the distribution and life span of allergic memory cells to ascertain whether long-term tissue reservoirs exist for these cells as shown for other memory-effector T cells (Shin and Iwasaki 2013).
A second area of much importance and in need of clarification is the relationship of allergic immunity with Treg induction. Infants born with FOXP3 mutations, and thus lacking Treg, suffer from a multisystem inflammatory disorder termed immunodysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX). Although patients suffer from a wide range of inflammatory disease, allergic manifestations with severe eczema, elevated IgE, and eosinophilia are prominent (Ozcan et al. 2008). Other T-cell immunodeficiencies, including Wiskott-Aldrich and Omenn’s syndromes, show inflammatory disorders that are characterized by unrestrained allergic inflammation with clinical features including increased IgE and eosinophils and eczematous skin conditions. Both disorders are accompanied by Treg deficiency and/or dysfunction to which the allergic manifestations have been attributed (Ozcan et al. 2008). Mice with defects in extrathymically derived Treg also show systemic allergic inflammation (Josefowicz et al. 2012). Ablation of GATA3, a transcription factor required for Th2 function, in Foxp3-expressing cells leads to uncontrolled allergic inflammation mimicking total Foxp3 gene ablation, thus emphasizing a primary role for these cells in the control of allergic inflammation to environmental antigens (Rudra et al. 2012). Helminth infections have been linked with Treg induction, consistent with resistance to allergy induction in infected mice (Maizels and Smith 2011), but why environmental allergens fail to induce similarly restraining Tregs in susceptible individuals remains unknown.
Innate Lymphoid Cell (ILC)2
The identification of innate lymphoid cells, now designated ILC2, as the major source of innate type 2 cytokines has kindled much interest in these cells in studies of allergic immunity (Spits and Cupedo 2012). ILC2 are dispersed throughout the body in most organs, and particularly in barrier tissues like the lung, gastrointestinal tract, and skin (Moro et al. 2010; Neill et al. 2010; Price et al. 2010; Nussbaum et al. 2013). Recent studies show that these cells accumulate in organs in the perinatal period and constitute a relatively long-lived subset of tissue lymphoid cells. As shown using cytokine reporter mice, small numbers of ILC2 in tissues constitutively produce IL-5, thus accounting for basal eosinophilopoiesis (Nussbaum et al. 2013). In response to migratory helminths, ILC2 respond to tissue alarmins, such as IL-33, IL-25, and thymic stromal lymphopoietin (TSLP) (Walker and McKenzie 2013), to express increased amounts and range of type 2 cytokines, including IL-13 and IL-9 but also growth factors, such as amphiregulin, that may contribute to local epithelial repair (Monticelli et al. 2011). As such, activated ILC2 are required to mediate early recruitment of tissue eosinophils from blood and the differentiation of tissue macrophages and recruited monocytes to an alternatively activated macrophage (AAM) phenotype (Molofsky et al. 2013). Although less well studied, activated ILC2 may also play a role in the early attraction of Th2 effectors to tissues, where these cells amplify the cytokine milieu, but also provide growth signals that stimulate the proliferation and/or recruitment of the tissue ILC2 population; some studies suggest a role for IL-9, both from Th2 cells and, in an autocrine fashion, from ILC2 themselves (Wilhelm et al. 2011). Although Th2 cells migrate to involved sites deeper in tissues to mediate local immunity, it remains unclear whether ILC2 migrate from their position near vascular tissues; further study is needed. The ultimate fate of expanded ILC2 populations in tissue and their ultimate interactions with Th2 memory cells and Treg remain important areas for investigation.
AAMs
Macrophages are constitutive in all tissues and increase in number and change their phenotype during inflammation (Galli et al. 2011; Van Dyken and Locksley 2013). In the setting of type 2 immunity, IL-4 and/or IL-13 promote the coordinate expression of a set of genes that characterize alternatively activated (or M2) macrophages. Under some conditions, AAM differentiation is driven by in situ proliferation of resident tissue macrophages (Jenkins et al. 2011), whereas differentiation of both resident and recruited blood monocytes into AAMs occurs in other situations (Reese et al. 2007). Recent findings that resident tissue macrophages, such as Langerhans cells and microglia, accumulate in tissues from fetal blood precursors emphasize the potential that certain populations of hematopoietic cells may be intimately involved during discrete developmental windows, with implications for tissue integrity based on the capacity to fully renew these populations in response to injury or senescence (Schulz et al. 2012). Along these lines, AAM-like cells are found dispersed throughout the body during embryonic development and peak during periods of growth, remodeling, and organization of developing tissues, such as the kidney (Rae et al. 2007).
The role of AAMs in type 2 immunity has been explored in various systems involving deletion of key elements involved in their differentiation, such as the IL-4Rα component of the type 1 and 2 IL-4 receptors. After infection with Schistosoma mansoni, mice lacking the capacity to generate IL-4/IL-13-mediated AAM differentiation fail to control intestinal epithelial integrity around trapped eggs and die from sepsis owing to enhanced translocation of intestinal bacteria and their products into the systemic circulation (Herbert et al. 2004). Thus, part of the function of AAMs may involve control of barrier integrity in response to antigens that elicit granulomatous type 2 immunity.
Eosinophils
Elevations of blood and tissue eosinophils are hallmarks of virtually all disorders of type 2 immunity (Rosenberg et al. 2013). Although decades of work highlighted the capacity of eosinophils to mediate parasite damage in various in vitro systems, data supporting a direct role for eosinophils in limiting the initiation or duration of adapted intestinal helminth infection in vivo is modest at best. The widespread prevalence of helminths in feral vertebrates, together with the relatively long life span of adult worms, despite prolonged eosinophilia, is difficult to reconcile with a primary role for these cells in limiting established parasitism. During secondary infections, however, the combination of memory-effector Th2 cells and expanded tissue ILC2 may accelerate and focus eosinophils at sites of larval migration, thus limiting further infection by a process termed “concomitant immunity” (a situation in which immunity against larval forms limits infections despite the presence of living adult parasites in the body that cannot be rejected). An emerging concept that requires further exploration is the inevitable coaccumulation of eosinophils with AAMs, reflecting the stereotyped response of tissues to the presence of activated ILC2 and Th2 that produce type 2 cytokines.
As discussed above, another possibility is that helminths evoke eosinophilia to elicit a tissue response that favors parasitism. After infection with Trichinella spiralis, newborn larvae migrate from the intestines to skeletal muscle to complete their development. Larval maturation involves the dedifferentiation of muscle cells to nurse cells, which provide the necessary environment. Although not entirely understood, eosinophils accumulate around nurse cells and are themselves required for continued larval development, presumably owing to their role in sustaining the nurse cell (Gebreselassie et al. 2012). As noted below, aspects of this response resemble that seen during muscle injury, suggesting that Trichinella elicits a gene program used by the host for one purpose but co-opts it to establish a developmental niche. Evidence that bacteria can also subvert cellular gene programs to redirect fundamental pathways of differentiation suggests that further understanding of such highly involved interactions between microbes and hosts may yield fundamental insights into cellular reprogramming that might be applicable to many disease states (Masaki et al. 2013). Such examples offer the possibility that uncovering the basic roles for type 2 immunity in vertebrate homeostasis may lead to enhanced understanding of its stereotyped elicitation by helminths and allergens.
HOMEOSTATIC ROLES FOR COMPONENTS OF ALLERGIC MODULE
As noted above, the majority of studies of type 2 immunity have focused on pathologic associations with parasite infection and allergic diseases. Recent investigations have called attention to potential roles for type 2 immunity in metabolism and tissue homeostasis in response to injury that might suggest pathways by which these responses come to be linked with these two pathologic states (Fig. 2).
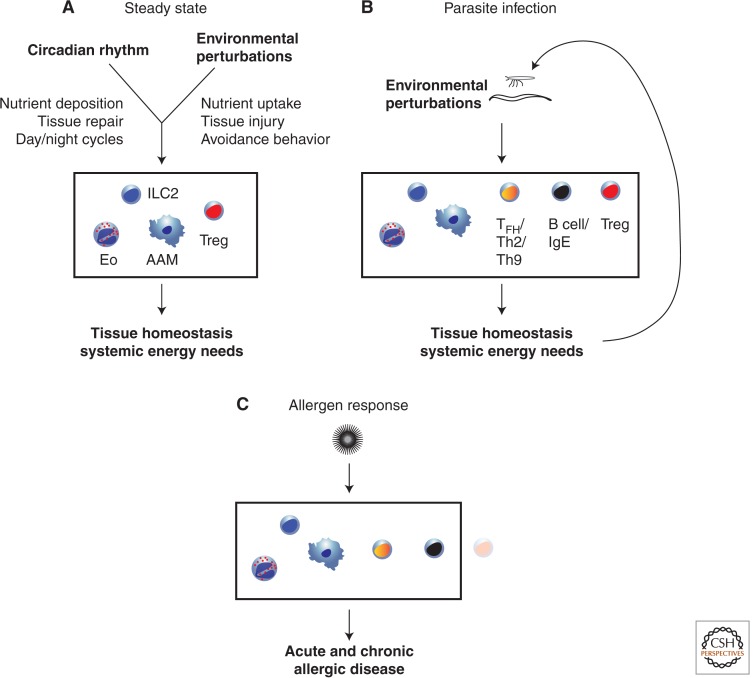
Figure 2.
An integrated view of allergic inflammation in infection and disease. (A) Innate components of allergic inflammation (ILC2, eosinophils, and AAMs) along with Tregs integrate broad inputs related to circadian patterns and environmental perturbations to drive tissue homeostasis and meet systemic energy needs. (B) During parasite infection, the additional stress on tissue homeostasis and energy needs engages a broader but balanced adaptive immune response, which promotes both host and parasite fitness, and is characterized by a regulatory component. (C) Allergen-specific responses fail to establish this balance, with a lack of Treg activation, resulting in tissue injury and disease.
Metabolism
A number of studies over the last decade have provided evidence that obesity is accompanied by an inflammatory state, marked by infiltration of activated macrophages and other immune cells into visceral adipose with adverse effects on systemic insulin resistance and lipid metabolism (Mathis 2013). Conversely, lean animals and perhaps humans contain AAMs in white adipose tissue, and deleting these cells in animals results in proclivity to obesity and insulin resistance (Odegaard et al. 2007). AAMs may also play a role in brown adipose tissue by facilitating early production of norepinephrine and the transfer of fat from reservoirs in white to metabolizing brown fat tissue during adaptive thermogenesis (Nguyen et al. 2011). As alluded to above, AAMs in tissue are accompanied by eosinophils and ILC2s, and, indeed, both of these cells are not only present in resting visceral adipose, but their presence is necessary to restrain metabolic abnormalities incurred by challenge with a high-fat diet (Wu et al. 2011; Molofsky et al. 2013). Further evidence for the linkage between these core elements of innate type 2 immunity and Treg cells is the finding that the latter cells are also present in fat and required for the maintenance of metabolic homeostasis (Feuerer et al. 2009). Like ILC2, adipose Treg express GATA-3 and the IL-33 receptor; conversely, ILC2 express CD25, the high-affinity IL-2 receptor. Thus, ILC2 and Treg in adipose tissues share these and additional surface receptors and transcription factors, a finding that may underlie the capacity of these cells to respond to common environmental signals in specialized tissues undergoing cyclic nutrient exchange.
Eosinophils display prominent circadian cycling that was shown to be entrained by food intake more than 35 years ago (Pauly et al. 1975). A small percentage of tissue-dispersed ILC2s constitutively secrete IL-5 necessary for reactive eosinophilopoiesis (Nussbaum et al. 2013). Unexpectedly, IL-5 secretion was circadian, thus accounting for the cyclic nature of blood eosinophils, but secretion was enhanced by feeding and depressed by fasting (Nussbaum et al. 2013). Eosinophils, like Treg, are present constitutively in the intestines, particularly the small bowel, and ILC2 in the intestine activated IL-13 secretion in response to nutrient intake. ILC2 also express vasoactive intestinal polypeptide receptor (VPAC)2, the type 2 receptor for the neuronal peptide, vasoactive intestinal peptide (VIP), and activate IL-5 secretion rapidly after activation by VIP or VPAC2 analogs in vitro (Nussbaum et al. 2013). VIP is itself secreted from intestinal neurons in response to feeding, and helps to coordinate pancreatic secretions, smooth muscle contractility, and hepatic metabolic cycles linked with nutrient absorption and disposition (Lelievre et al. 2007). Mice deficient in VIP or VPAC2, both of which are highly expressed in neurons of the suprachiasmatic nucleus (Maywood et al. 2013), lose the ability to coordinate environmental signals with the circadian cycle, and show metabolic dysregulation (Harmar et al. 2002; Colwell et al. 2003). Thus, ILC2 respond to VIP, integrating their activation with central circadian rhythms, although precisely what role recruited eosinophils might play in nutrient acquisition or other aspects of metabolism remains unknown. Type 2 cytokines and Stat6-mediated signaling have been shown to affect primary hepatic metabolism directly as well (Sajic et al. 2013; Stanya et al. 2013).
An additional layer of complexity in metabolic homeostasis is the relationship of intestinal commensal organisms with epithelial and immune cell populations. In mice, key innate lymphoid cells are established after birth during exposure to dietary nutrients via signals sustained through the aryl hydrocarbon receptor (Kiss et al. 2011; Li et al. 2011; Lee et al. 2012). Development of these immune cell populations is constrained to a defined window of time postbirth, suggesting that alterations of this window might have adverse effects on gut homeostasis. Intriguingly, antibiotics, which have long been used to increase body size and fat content in animals farmed for food, have been shown to affect body size and intestinal microbial communities in adult mice, even when given in subtherapeutic levels during the perinatal period (Cho et al. 2012). Antibiotic use in early life has been linked to patterns of obesity at the population level (Trasande et al. 2013). Although causality has yet to be established, the similarities in a number of the systemic consequences of dysbiotic microbial communities and the absence of AAMs, eosinophils, ILC2s, and/or Tregs raise the possibility that some or each of the components of this type 2 immune module are linked deeply with basal metabolic homeostasis (Fig. 2A).
Wound Healing
Maintenance of barrier integrity is critical for vertebrate survival, particularly at mucosal sites involved with gas exchange and nutrient acquisition in the lungs and bowel, respectively. Animals rendered unable to respond to IL-4/IL-13 signals from immune cells have been shown to suffer adverse epithelial injury against migratory and intestinal helminths in model systems (Gause et al. 2013). Recovery after toxin-mediated muscle injury was also enhanced by eosinophils and an intact type 2 cytokine system by a process linked to proliferation and differentiation of resident pluripotent fibro-/adipogenic precursor cells (FAPs) at the injury site (Heredia et al. 2013). FAPs were implicated in necrotic cell clearance and muscle regeneration; in the absence of IL-4/-13, the FAPs differentiated into adipocytes resulting in fatty degeneration. A similar role for eosinophils and IL-4/-13 was found in liver regeneration, but, in this case, IL-4 promoted hepatocyte proliferation and liver regeneration (Goh et al. 2013). In various skin models involving epicutaneous sensitization, a variety of type 2 immune cells, including basophils, eosinophils, ILC2, and AAMs, have been implicated along with IgE in mediating the manifestations of allergy (Mukai et al. 2005; Egawa et al. 2013). Thus, multiple components of the type 2 immune response have been implicated in barrier injury responses, although the precise molecular mechanisms by which these responses are mediated remain incompletely defined.
CONCLUDING REMARKS: SPECULATIONS AND FUTURE NEEDS
The deep penetrance of allergy prevalence into humans living in developed countries suggests that environmental alterations have impacted an evolved pathway that is deeply embedded in normal biology. Here, we have summarized recent support for involvement of type 2 immune cells in metabolism and tissue integrity, particularly at barriers. Although much more work is needed to flesh out the molecular details and mechanisms, the finding that multiple cell types, including Treg associated with type 2 immunity, localize to metabolically active tissues like adipose and small intestines is consistent with a role for these cells in more fundamental homeostatic processes of importance to vertebrate biology, such as tolerance to food or self-neoantigens exposed during normal tissue turnover. Chronic intestinal parasitism induces similar activation of type 2 immune cells, but these responses are not associated with pathologic responses associated with allergy, perhaps related to the capacity to induce a more balanced immune response, which includes Treg or additional suppressive mechanisms (Fig. 2B). This balanced response contrasts with allergen-specific responses in which suppressive mechanisms are lacking, and pathologic consequences emerge (Fig. 2C). Alternatively, the age of acquisition of these highly adapted parasites may have effects on immune system development or intestinal microbiota that protect against the subsequent dysregulated type 2 immune responses to innocuous environmental antigens. We propose that parasitic helminths, rather than being attacked by the components of type 2 immunity, have evolved to elicit these localized tissue responses to facilitate their own differentiation and maintenance within the vertebrate niche. Indeed, some of the properties of type 2 immunity we have outlined, including mobilization of nutrients and preservation of tissue integrity, may be appropriated by parasites for their own purposes. Numerous examples of bacterial commensals eliciting immune reactivity from the host that facilitates their colonization have been shown (Nussbaum and Locksley 2012), and the possibility that more complex parasites do the same thing would not be unexpected.
Despite evidence for this thesis, the mechanisms underlying these processes remain woefully undefined. In this way, study of parasite infections may yield information regarding the precise pathways that elicit the type 2 immune response, whether through induction of canonical alarmins or other mediators (Doherty et al. 2013; Gause et al. 2013). Conversely, more information regarding the mechanisms that control the tissue localization, organization, and activation of type 2 immune cells under homeostatic conditions will reveal the terrain that establishes this network within the greater context of vertebrate development and life span. Taken together, the study of type 2 immunity has evolved greatly in recent years and will be sure to contain additional surprises in the near future.
ACKNOWLEDGMENTS
We thank members of the Locksley Laboratory for insights, comments, and discussions, and we regret being unable to cite all of the relevant literature. Research support was provided from the National Institutes of Health, Howard Hughes Medical Institute, and the Sandler Asthma Basic Research Center at the University of California, San Francisco.
Footnotes
Editors: Ruslan M. Medzhitov
Additional Perspectives on Innate Immunity and Inflammation available at www.cshperspectives.org
REFERENCES
- CDC. 2011. Vital Signs, May 2011. [Google Scholar]
- Chan MS 1997. The global burden of intestinal nematode infections—Fifty years on. Parasitol Today 13: 438–443. [DOI] [PubMed] [Google Scholar]
- Cheng LE, Hartmann K, Roers A, Krummel MF, Locksley RM 2013. Perivascular mast cells dynamically probe cutaneous blood vessels to capture immunoglobulin E. Immunity 38: 166–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho I, Yamanishi S, Cox L, Methe BA, Zavadil J, Li K, Gao Z, Mahana D, Raju K, Teitler I, et al. 2012. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature 488: 621–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colwell CS, Michel S, Itri J, Rodriguez W, Tam J, Lelievre V, Hu Z, Liu X, Waschek JA 2003. Disrupted circadian rhythms in VIP- and PHI-deficient mice. Am J Physiol Regul Integr Comp Physiol 285: R939–R949. [DOI] [PubMed] [Google Scholar]
- Crotty S 2011. Follicular helper CD4 T cells (TFH). Annu Rev Immunol 29: 621–663. [DOI] [PubMed] [Google Scholar]
- Davies JM, Platts-Mills TA, Aalberse RC 2013. The enigma of IgE+ B-cell memory in human subjects. J Allergy Clin Immunol 131: 972–976. [DOI] [PubMed] [Google Scholar]
- Doherty TA, Khorram N, Lund S, Mehta AK, Croft M, Broide DH 2013. Lung type 2 innate lymphoid cells express cysteinyl leukotriene receptor 1, which regulates TH2 cytokine production. J Allergy Clin Immunol 132: 205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egawa M, Mukai K, Yoshikawa S, Iki M, Mukaida N, Kawano Y, Minegishi Y, Karasuyama H 2013. Inflammatory monocytes recruited to allergic skin acquire an anti-inflammatory M2 phenotype via basophil-derived interleukin-4. Immunity 38: 570–580. [DOI] [PubMed] [Google Scholar]
- Eller LA, Eller MA, Ouma B, Kataaha P, Kyabaggu D, Tumusiime R, Wandege J, Sanya R, Sateren WB, Wabwire-Mangen F, et al. 2008. Reference intervals in healthy adult Ugandan blood donors and their impact on conducting international vaccine trials. PLoS ONE 3: e3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon PG, Jolin HE, Smith P, Emson CL, Townsend MJ, Fallon R, McKenzie AN 2002. IL-4 induces characteristic Th2 responses even in the combined absence of IL-5, IL-9, and IL-13. Immunity 17: 7–17. [DOI] [PubMed] [Google Scholar]
- Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, Lee J, Goldfine AB, Benoist C, Shoelson S, et al. 2009. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med 15: 930–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenot Y, de Oliveira E, Gauthier-Clerc M, Deunff J, Bellido A, Vernon P 2001. Life cycle of the tick Ixodesuriae in penguin colonies: Relationships with host breeding activity. Int J Parasitol 31: 1040–1047. [DOI] [PubMed] [Google Scholar]
- Galli SJ, Tsai M 2010. Mast cells in allergy and infection: Versatile effector and regulatory cells in innate and adaptive immunity. Eur J Immunol 40: 1843–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli SJ, Borregaard N, Wynn TA 2011. Phenotypic and functional plasticity of cells of innate immunity: Macrophages, mast cells and neutrophils. Nat Immunol 12: 1035–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gause WC, Wynn TA, Allen JE 2013. Type 2 immunity and wound healing: Evolutionary refinement of adaptive immunity by helminths. Nat Rev Immunol 13: 607–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebreselassie NG, Moorhead AR, Fabre V, Gagliardo LF, Lee NA, Lee JJ, Appleton JA 2012. Eosinophils preserve parasitic nematode larvae by regulating local immunity. J Immunol 188: 417–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, et al. 2010. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330: 841–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey RC 1975. Asthma and IgE levels in rural and urban communities of The Gambia. Clin Allergy 5: 201–207. [DOI] [PubMed] [Google Scholar]
- Goh YP, Henderson NC, Heredia JE, Red Eagle A, Odegaard JI, Lehwald N, Nguyen KD, Sheppard D, Mukundan L, Locksley RM, et al. 2013. Eosinophils secrete IL-4 to facilitate liver regeneration. Proc Natl Acad Sci 110: 9914–9919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould HJ, Sutton BJ 2008. IgE in allergy and asthma today. Nat Rev Immunol 8: 205–217. [DOI] [PubMed] [Google Scholar]
- Grayson MH, Cheung D, Rohlfing MM, Kitchens R, Spiegel DE, Tucker J, Battaile JT, Alevy Y, Yan L, Agapov E, et al. 2007. Induction of high-affinity IgE receptor on lung dendritic cells during viral infection leads to mucous cell metaplasia. J Exp Med 204: 2759–2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmar AJ, Marston HM, Shen S, Spratt C, West KM, Sheward WJ, Morrison CF, Dorin JR, Piggins HD, Reubi JC, et al. 2002. The VPAC(2) receptor is essential for circadian function in the mouse suprachiasmatic nuclei. Cell 109: 497–508. [DOI] [PubMed] [Google Scholar]
- Herbert DR, Holscher C, Mohrs M, Arendse B, Schwegmann A, Radwanska M, Leeto M, Kirsch R, Hall P, Mossmann H, et al. 2004. Alternative macrophage activation is essential for survival during schistosomiasis and downmodulates T helper 1 responses and immunopathology. Immunity 20: 623–635. [DOI] [PubMed] [Google Scholar]
- Heredia JE, Mukundan L, Chen FM, Mueller AA, Deo RC, Locksley RM, Rando TA, Chawla A 2013. Type 2 innate signals stimulate fibro/adipogenic progenitors to facilitate muscle regeneration. Cell 153: 376–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeffel G, Wang Y, Greter M, See P, Teo P, Malleret B, Leboeuf M, Low D, Oller G, Almeida F, et al. 2012. Adult Langerhans cells derive predominantly from embryonic fetal liver monocytes with a minor contribution of yolk sac-derived macrophages. J Exp Med 209: 1167–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISAAC. 1998. Worldwide variation in prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and atopic eczema: ISAAC. The International Study of Asthma and Allergies in Childhood (ISAAC) Steering Committee. Lancet 351: 1225–1232. [PubMed] [Google Scholar]
- Ishizaka K, Ishizaka T, Hornbrook MM 1966. Physico-chemical properties of human reaginic antibody: IV. Presence of a unique immunoglobulin as a carrier of reaginic activity. J Immunol 97: 75–85. [PubMed] [Google Scholar]
- Jenkins SJ, Ruckerl D, Cook PC, Jones LH, Finkelman FD, van Rooijen N, MacDonald AS, Allen JE 2011. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science 332: 1284–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones HI 1988. Notes on parasites in penguins (Spheniscidae) and petrels (Procellariidae) in the Antarctic and Sub-Antarctic. J Wildl Dis 24: 166–167. [DOI] [PubMed] [Google Scholar]
- Josefowicz SZ, Niec RE, Kim HY, Treuting P, Chinen T, Zheng Y, Umetsu DT, Rudensky AY 2012. Extrathymically generated regulatory T cells control mucosal TH2 inflammation. Nature 482: 395–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss EA, Vonarbourg C, Kopfmann S, Hobeika E, Finke D, Esser C, Diefenbach A 2011. Natural aryl hydrocarbon receptor ligands control organogenesis of intestinal lymphoid follicles. Science 334: 1561–1565. [DOI] [PubMed] [Google Scholar]
- La Grange LJ, Govender D, Mukaratirwa S 2013. The occurrence of Trichinella zimbabwensis in naturally infected wild crocodiles (Crocodylus niloticus) from the Kruger National Park, South Africa. J Helminthol 87: 91–96. [DOI] [PubMed] [Google Scholar]
- Lavikainen A, Laaksonen S, Beckmen K, Oksanen A, Isomursu M, Meri S 2011. Molecular identification of Taenia spp. in wolves (Canis lupus), brown bears (Ursus arctos) and cervids from North Europe and Alaska. Parasitol Int 60: 289–295. [DOI] [PubMed] [Google Scholar]
- Ledin A, Bergvall K, Hillbertz NS, Hansson H, Andersson G, Hedhammar A, Hellman L 2006. Generation of therapeutic antibody responses against IgE in dogs, an animal species with exceptionally high plasma IgE levels. Vaccine 24: 66–74. [DOI] [PubMed] [Google Scholar]
- Ledin A, Arnemo JM, Liberg O, Hellman L 2008. High plasma IgE levels within the Scandinavian wolf population, and its implications for mammalian IgE homeostasis. Mol Immunol 45: 1976–1980. [DOI] [PubMed] [Google Scholar]
- Lee JS, Cella M, McDonald KG, Garlanda C, Kennedy GD, Nukaya M, Mantovani A, Kopan R, Bradfield CA, Newberry RD, et al. 2012. AHR drives the development of gut ILC22 cells and postnatal lymphoid tissues via pathways dependent on and independent of Notch. Nat Immunol 13: 144–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelievre V, Favrais G, Abad C, Adle-Biassette H, Lu Y, Germano PM, Cheung-Lau G, Pisegna JR, Gressens P, Lawson G, et al. 2007. Gastrointestinal dysfunction in mice with a targeted mutation in the gene encoding vasoactive intestinal polypeptide: A model for the study of intestinal ileus and Hirschsprung’s disease. Peptides 28: 1688–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Innocentin S, Withers DR, Roberts NA, Gallagher AR, Grigorieva EF, Wilhelm C, Veldhoen M 2011. Exogenous stimuli maintain intraepithelial lymphocytes via aryl hydrocarbon receptor activation. Cell 147: 629–640. [DOI] [PubMed] [Google Scholar]
- Maizels RM, Smith KA 2011. Regulatory T cells in infection. Adv Immunol 112: 73–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaviya R, Ikeda T, Ross E, Abraham SN 1996. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-α. Nature 381: 77–80. [DOI] [PubMed] [Google Scholar]
- Masaki T, Qu J, Cholewa-Waclaw J, Burr K, Raaum R, Rambukkana A 2013. Reprogramming adult Schwann cells to stem cell-like cells by leprosy bacilli promotes dissemination of infection. Cell 152: 51–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis D 2013. Immunological goings-on in visceral adipose tissue. Cell Metab 17: 851–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maywood ES, Drynan L, Chesham JE, Edwards MD, Dardente H, Fustin JM, Hazlerigg DG, O’Neill JS, Codner GF, Smyllie NJ, et al. 2013. Analysis of core circadian feedback loop in suprachiasmatic nucleus of mCry1-luc transgenic reporter mouse. Proc Natl Acad Sci 110: 9547–9552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan JB, Hart JP, Pizzo SV, Shelburne CP, Staats HF, Gunn MD, Abraham SN 2003. Mast cell-derived tumor necrosis factor induces hypertrophy of draining lymph nodes during infection. Nat Immunol 4: 1199–1205. [DOI] [PubMed] [Google Scholar]
- Mold JE, Venkatasubrahmanyam S, Burt TD, Michaelsson J, Rivera JM, Galkina SA, Weinberg K, Stoddart CA, McCune JM 2010. Fetal and adult hematopoietic stem cells give rise to distinct T cell lineages in humans. Science 330: 1695–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molofsky AB, Nussbaum JC, Liang HE, Van Dyken SJ, Cheng LE, Mohapatra A, Chawla A, Locksley RM 2013. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J Exp Med 210: 535–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monticelli LA, Sonnenberg GF, Abt MC, Alenghat T, Ziegler CG, Doering TA, Angelosanto JM, Laidlaw BJ, Yang CY, Sathaliyawala T, et al. 2011. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat Immunol 12: 1045–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, Furusawa J, Ohtani M, Fujii H, Koyasu S 2010. Innate production of TH2 cytokines by adipose tissue-associated c-Kit+Sca-1+ lymphoid cells. Nature 463: 540–544. [DOI] [PubMed] [Google Scholar]
- Mukai K, Matsuoka K, Taya C, Suzuki H, Yokozeki H, Nishioka K, Hirokawa K, Etori M, Yamashita M, Kubota T, et al. 2005. Basophils play a critical role in the development of IgE-mediated chronic allergic inflammation independently of T cells and mast cells. Immunity 23: 191–202. [DOI] [PubMed] [Google Scholar]
- Nath P, Leung SY, Williams AS, Noble A, Xie S, McKenzie AN, Chung KF 2007. Complete inhibition of allergic airway inflammation and remodelling in quadruple IL-4/5/9/13−/− mice. Clin Exp Allergy 37: 1427–1435. [DOI] [PubMed] [Google Scholar]
- Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, Bucks C, Kane CM, Fallon PG, Pannell R, et al. 2010. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature 464: 1367–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen KD, Qiu Y, Cui X, Goh YP, Mwangi J, David T, Mukundan L, Brombacher F, Locksley RM, Chawla A 2011. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature 480: 104–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussbaum JC, Locksley RM 2012. Infectious (non)tolerance—Frustrated commensalism gone awry? Cold Spring Harb Perspect Biol 4: a007328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussbaum JC, Van Dyken SJ, von Moltke J, Cheng LE, Mohapatra A, Molofsky AB, Thornton EE, Krummel MF, Chawla A, Liang HE, et al. 2013. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature 502: 248–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L, Red Eagle A, Vats D, Brombacher F, Ferrante AW, et al. 2007. Macrophage-specific PPARγ controls alternative activation and improves insulin resistance. Nature 447: 1116–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnmacht C, Schwartz C, Panzer M, Schiedewitz I, Naumann R, Voehringer D 2010. Basophils orchestrate chronic allergic dermatitis and protective immunity against helminths. Immunity 33: 364–374. [DOI] [PubMed] [Google Scholar]
- Olafsdottir D, Shinn AP 2013. Epibiotic macrofauna on common minke whales, Balaenoptera acutorostrata Lacépède, 1804, in Icelandic waters. Parasit Vectors 6: 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozcan E, Notarangelo LD, Geha RS 2008. Primary immune deficiencies with aberrant IgE production. J Allergy Clin Immunol 122: 1063–1054. [DOI] [PubMed] [Google Scholar]
- Palm NW, Rosenstein RK, Medzhitov R 2012. Allergic host defences. Nature 484: 465–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauly JE, Burns ER, Halberg F, Tsai S, Betterton HO, Scheving LE 1975. Meal timing dominates the lighting regimen as a synchronizer of the eosinophil rhythm in mice. Acta Anat (Basel) 93: 60–68. [DOI] [PubMed] [Google Scholar]
- Price AE, Liang HE, Sullivan BM, Reinhardt RL, Eisley CJ, Erle DJ, Locksley RM 2010. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc Natl Acad Sci 107: 11489–11494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulendran B, Tang H, Manicassamy S 2010. Programming dendritic cells to induce TH2 and tolerogenic responses. Nat Immunol 11: 647–655. [DOI] [PubMed] [Google Scholar]
- Qi X, Hong J, Chaves L, Zhuang Y, Chen Y, Wang D, Chabon J, Graham B, Ohmori K, Li Y, et al. 2013. Antagonistic regulation by the transcription factors C/EBPα and MITF specifies basophil and mast cell fates. Immunity 39: 97–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae F, Woods K, Sasmono T, Campanale N, Taylor D, Ovchinnikov DA, Grimmond SM, Hume DA, Ricardo SD, Little MH 2007. Characterisation and trophic functions of murine embryonic macrophages based upon the use of a Csf1r-EGFP transgene reporter. Dev Biol 308: 232–246. [DOI] [PubMed] [Google Scholar]
- Reese TA, Liang HE, Tager AM, Luster AD, Van Rooijen N, Voehringer D, Locksley RM 2007. Chitin induces accumulation in tissue of innate immune cells associated with allergy. Nature 447: 92–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt RL, Liang HE, Locksley RM 2009. Cytokine-secreting follicular T cells shape the antibody repertoire. Nat Immunol 10: 385–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richet C 1967. Anaphylaxis. In Nobel lectures, physiology or medicine 1901–1921. Elsevier, Amsterdam. [Google Scholar]
- Rosenberg HF, Dyer KD, Foster PS 2013. Eosinophils: Changing perspectives in health and disease. Nat Rev Immunol 13: 9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudra D, deRoos P, Chaudhry A, Niec RE, Arvey A, Samstein RM, Leslie C, Shaffer SA, Goodlett DR, Rudensky AY 2012. Transcription factor Foxp3 and its protein partners form a complex regulatory network. Nat Immunol 13: 1010–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajic T, Hainard A, Scherl A, Wohlwend A, Negro F, Sanchez JC, Szanto I 2013. STAT6 promotes bi-directional modulation of PKM2 in liver and adipose inflammatory cells in Rosiglitazone-treated mice. Sci Rep 3: 2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz C, Gomez Perdiguero E, Chorro L, Szabo-Rogers H, Cagnard N, Kierdorf K, Prinz M, Wu B, Jacobsen SE, Pollard JW, et al. 2012. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science 336: 86–90. [DOI] [PubMed] [Google Scholar]
- Shelburne CP, Nakano H, St John AL, Chan C, McLachlan JB, Gunn MD, Staats HF, Abraham SN 2009. Mast cells augment adaptive immunity by orchestrating dendritic cell trafficking through infected tissues. Cell Host Microbe 6: 331–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H, Iwasaki A 2013. Tissue-resident memory T cells. Immunol Rev 255: 165–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soyer OU, Akdis M, Ring J, Behrendt H, Crameri R, Lauener R, Akdis CA 2013. Mechanisms of peripheral tolerance to allergens. Allergy 68: 161–170. [DOI] [PubMed] [Google Scholar]
- Spits H, Cupedo T 2012. Innate lymphoid cells: Emerging insights in development, lineage relationships, and function. Annu Rev Immunol 30: 647–675. [DOI] [PubMed] [Google Scholar]
- Stanya KJ, Jacobi D, Liu S, Bhargava P, Dai L, Gangl MR, Inouye K, Barlow JL, Ji Y, Mizgerd JP, et al. 2013. Direct control of hepatic glucose production by interleukin-13 in mice. J Clin Invest 123: 261–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan BM, Liang HE, Bando JK, Wu D, Cheng LE, McKerrow JK, Allen CDC, Locksley RM 2011. Genetic analysis of basophil function in vivo. Nat Immunol 12: 527–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talay O, Yan D, Brightbill HD, Straney EE, Zhou M, Ladi E, Lee WP, Egen JG, Austin CD, Xu M, et al. 2012. IgE memory B cells and plasma cells generated through a germinal-center pathway. Nat Immunol 13: 396–404. [DOI] [PubMed] [Google Scholar]
- Trasande L, Blustein J, Liu M, Corwin E, Cox LM, Blaser MJ 2013. Infant antibiotic exposures and early-life body mass. Int J Obesity 37: 16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tubo NJ, Pagan AJ, Taylor JJ, Nelson RW, Linehan JL, Ertelt JM, Huseby ES, Way SS, Jenkins MK 2013. Single naive CD4+ T cells from a diverse repertoire produce different effector cell types during infection. Cell 153: 785–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dyken SJ, Locksley RM 2013. Interleukin-4- and interleukin-13-mediated alternatively activated macrophages: Roles in homeostasis and disease. Annu Rev Immunol 31: 317–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voehringer D 2013. Protective and pathological roles of mast cells and basophils. Nat Rev Immunol 13: 362–375. [DOI] [PubMed] [Google Scholar]
- Wagner B 2009. IgE in horses: Occurrence in health and disease. Vet Immunol Immunopathol 132: 21–30. [DOI] [PubMed] [Google Scholar]
- Walker JA, McKenzie AN 2013. Development and function of group 2 innate lymphoid cells. Curr Opin Immunol 25: 148–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. 2007. Global surveillance, prevention and control of chronic respiratory diseases. WHO, Switzerland. [Google Scholar]
- Wilhelm C, Hirota K, Stieglitz B, Van Snick J, Tolaini M, Lahl K, Sparwasser T, Helmby H, Stockinger B 2011. An IL-9 fate reporter demonstrates the induction of an innate IL-9 response in lung inflammation. Nat Immunol 12: 1071–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Molofsky AB, Liang HE, Ricardo-Gonzalez RR, Jouihan HA, Bando JK, Chawla A, Locksley RM 2011. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science 332: 243–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Sullivan BM, Allen CD 2012. Fluorescent in vivo detection reveals that IgE+ B cells are restrained by an intrinsic cell fate predisposition. Immunity 36: 857–872. [DOI] [PubMed] [Google Scholar]
- Zhou T, Georgiev I, Wu X, Yang ZY, Dai K, Finzi A, Kwon YD, Scheid JF, Shi W, Xu L, et al. 2010. Structural basis for broad and potent neutralization of HIV-1 by antibody VRC01. Science 329: 811–817. [DOI] [PMC free article] [PubMed] [Google Scholar]