Abstract
The idea that memory is not a single mental faculty has a long and interesting history but became a topic of experimental and biologic inquiry only in the mid-20th century. It is now clear that there are different kinds of memory, which are supported by different brain systems. One major distinction can be drawn between working memory and long-term memory. Long-term memory can be separated into declarative (explicit) memory and a collection of nondeclarative (implicit) forms of memory that include habits, skills, priming, and simple forms of conditioning. These memory systems depend variously on the hippocampus and related structures in the parahippocampal gyrus, as well as on the amygdala, the striatum, cerebellum, and the neocortex. This work recounts the discovery of declarative and nondeclarative memory and then describes the nature of declarative memory, working memory, nondeclarative memory, and the relationship between memory systems.
There are different kinds of memory (e.g., declarative memory, nondeclarative memory, and working memory). Each has a distinct purpose and anatomy, and they operate independently and in parallel to support behavior.
The idea that memory is not a single faculty has a long history. In his Principles of Psychology, William James (1890) wrote separate chapters on memory and habit. Bergson (1910) similarly distinguished between a kind of memory that represents our past and memory that is not representational but nevertheless allows the effect of the past to persist into the present. One finds other antecedents as well. McDougall (1923) wrote about explicit and implicit recognition memory, and Tolman (1948) proposed that there is more than one kind of learning. These early proposals were often expressed as a dichotomy involving two forms of memory. The terminologies differed, but the ideas were similar. Thus, Ryle (1949) distinguished between knowing how and knowing that, and Bruner (1969) identified memory without record and memory with record. Later, the artificial intelligence literature introduced a distinction between declarative and procedural knowledge (Winograd 1975). Yet constructs founded in philosophy and psychology are often abstract and have an uncertain connection to biology, that is, to how the brain actually stores information. History shows that as biological information becomes available about structure and function, understanding becomes more concrete and less dependent on terminology.
THE DISCOVERY OF DECLARATIVE AND NONDECLARATIVE MEMORY SYSTEMS
Biological and experimental inquiry into these matters began with studies of the noted patient H.M. (Scoville and Milner 1957; Squire 2009). H.M. developed profound memory impairment following a bilateral resection of the medial temporal lobe, which had been performed to relieve severe epilepsy. The resection included much of the hippocampus and the adjacent parahippocampal gyrus (Annese et al. 2014; Augustinack et al. 2014). H.M.’s memory impairment was disabling and affected all manner of material (scenes, words, faces, etc.), so it was quite unexpected when he proved capable of learning a hand–eye coordination skill (mirror drawing) over a period of 3 days (Milner 1962). He learned rapidly and efficiently but on each test day had no memory of having practiced the task before. This finding showed that memory is not a single entity. Yet, at the time, discussion tended to set aside motor skills as an exception, a less cognitive form of memory. The view was that all of the rest of memory was impaired in H.M. and that the rest of memory is of one piece.
There were early suggestions in the animal literature that more than just motor skills were intact after lesions of hippocampus or related structures (Gaffan 1974; Hirsh 1974; O’Keefe and Nadel 1978). However, these proposals differed from each other, and they came at a time when the findings in experimental animals did not conform well to the findings for human memory and amnesia. In particular, animals with hippocampal lesions often succeeded at tasks that were failed by patients with similar lesions. It gradually became clear that animals and humans can approach the same task with different strategies (and using different brain systems), and also that patients with medial temporal lobe lesions, like experimental animals with similar lesions, can in fact succeed at a wide range of learning and memory abilities.
First came the finding that memory-impaired patients could acquire, at a normal rate, the perceptual skill of reading mirror-reversed words, despite poor memory for the task and for the words that were read (Cohen and Squire 1980). Thus, perceptual skills, not just motor skills, were intact. This finding was presented in the framework of a brain-based distinction between two major forms of memory that afford either declarative or procedural knowledge. Declarative knowledge is knowledge available as conscious recollection, and it can be brought to mind as remembered verbal or nonverbal material, such as an idea, sound, image, sensation, odor, or word. Procedural knowledge refers to skill-based information. What is learned is embedded in acquired procedures and is expressed through performance.
Subsequently, other forms of experience-dependent behaviors were found to be distinct from declarative memory. One important phenomenon was priming—the improved ability to detect, produce, or classify an item based on a recent encounter with the same or related item (Tulving and Schacter 1990; Schacter and Buckner 1998). For example, individuals will name objects faster on their second presentation, and independent of whether they recognize the objects as having been presented before.
Another important discovery was that the neostriatum (not the medial temporal lobe) is important for the sort of gradual feedback-guided learning that results in habit memory (Mishkin et al. 1984; Packard et al. 1989; Knowlton et al. 1996). Tasks that assess habit learning are often structured so that explicit memorization is not useful (e.g., because the outcome of each trial is determined probabilistically), and individuals must depend more on a gut feeling. After learning, it is more accurate to say that individuals have acquired a disposition to perform in a particular way than to say that they have acquired a fact (i.e., declarative knowledge) about the world.
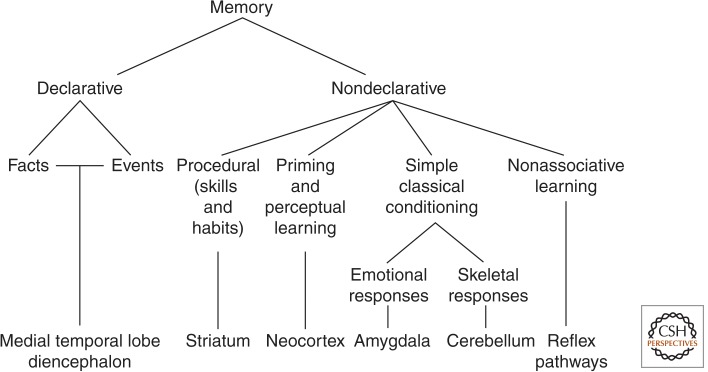
Given the wide variety of learning and memory phenomena that could eventually be shown in patients (e.g., priming and habit learning as well as simple forms of classical conditioning), the perspective eventually shifted to a framework involving multiple memory systems rather than just two kinds of memory (Fig. 1). Accordingly, the term “nondeclarative” was introduced with the idea that nondeclarative memory is an umbrella term referring to multiple forms of memory that are not declarative (Squire and Zola-Morgan 1988). Nondeclarative memory includes skills and habits, simple forms of conditioning, priming, and perceptual learning, as well as phylogenetically early forms of behavioral plasticity like habituation and sensitization that are well developed in invertebrates. The various memory systems can be distinguished in terms of the different kinds of information they process and the principles by which they operate.
Figure 1.
Organization of mammalian long-term memory systems. The figure lists the brain structures thought to be especially important for each form of memory. In addition to its central role in emotional learning, the amygdala is able to modulate the strength of both declarative and nondeclarative memory.
Declarative memory provides a way to represent the external world. It is the kind of memory we typically have in mind when we use the term memory in everyday language. Declarative memory has two major components, semantic memory (facts about the world) and episodic memory (the ability to re-experience a time-and-place-specific event in its original context) (Tulving 1983). The acquisition of episodic memory requires the involvement of brain systems in addition to medial temporal lobe structures, especially the frontal lobes (Tulving 1989; Shimamura et al. 1991). There is some uncertainty around the issue of whether nonhuman animals have the capacity for episodic memory (i.e., the capacity for mental time travel that can return an animal to the scene of an earlier event), and the idea has been difficult to put to the test (Tulving 2005). For elegant demonstrations of episodic-like memory in nonhuman animals, see Clayton and Dickinson (1998).
Nondeclarative memory is dispositional and is expressed through performance rather than recollection. An important principle is the ability to gradually extract the common elements from a series of separate events. Nondeclarative memory provides for myriad unconscious ways of responding to the world. The unconscious status of nondeclarative memory creates some of the mystery of human experience. Here arise the habits and preferences that are inaccessible to conscious recollection, but they nevertheless are shaped by past events, they influence our current behavior and mental life, and they are a fundamental part of who we are.
Sherry and Schacter (1987) suggested that multiple memory systems evolved because they serve distinct and fundamentally different purposes. For example, the gradual changes that occur in birdsong learning are different from, and have a different function than, the rapid learning that occurs when a bird caches food for later recovery. These memory systems operate in parallel to support and guide behavior. For example, imagine an unpleasant event from early childhood, such as being knocked down by a large dog. Two independent consequences of the event could potentially persist into adulthood as declarative and nondeclarative memories. On the one hand, the individual might have a conscious, declarative memory of the event itself. On the other hand, the individual might have a fear of large dogs, quite independently of whether the event itself is remembered. Note that a fear of dogs would not be experienced as a memory but rather as a part of personality, a preference, or an attitude about the world.
THE NATURE OF DECLARATIVE MEMORY
Studies of patients and experimental animals with medial temporal lobe damage have identified four task requirements that reliably reveal impaired memory: (1) tasks where learning occurs in a single trial or single study episode (Mishkin 1978); (2) tasks where associations between stimuli are learned across space and time (e.g., Higuchi and Miyashita 1996; Fortin et al. 2002); (3) tasks where the acquired information can be used flexibly (e.g., Bunsey and Eichenbaum 1996; Smith and Squire 2005); and (4) tasks where learning depends on awareness of what is being learned (Clark and Squire 1998; Smith and Squire 2008).
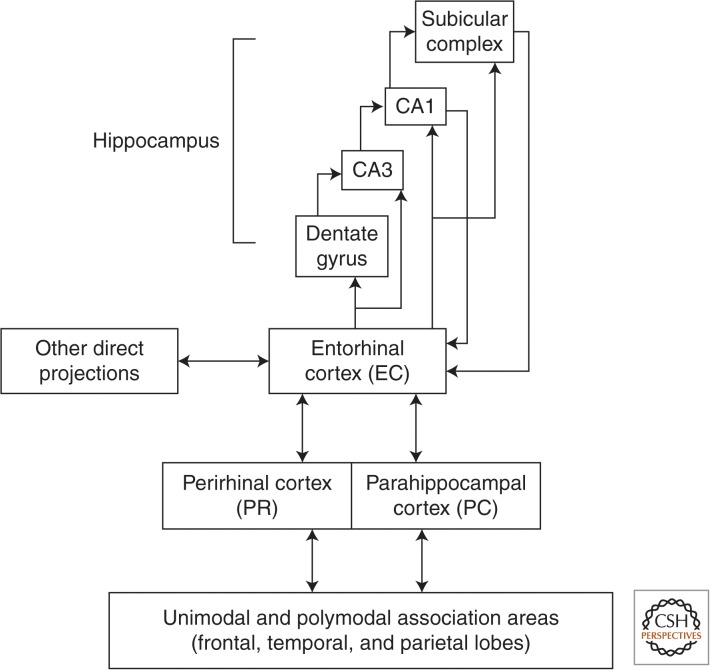
Declarative memory (sometimes termed explicit memory) is well adapted for the rapid learning of specific events. Declarative memory allows remembered material to be compared and contrasted. The stored representations are flexible, accessible to awareness, and can guide performance in multiple different contexts. The key structures that support declarative memory are the hippocampus and the adjacent entorhinal, perirhinal, and parahippocampal cortices, which make up much of the parahippocampal gyrus (Fig. 2) (Squire and Zola-Morgan 1991). These structures are organized hierarchically, and their anatomy suggests how the structures might contribute differently to the formation of declarative memory, for example, in the encoding of objects (perirhinal cortex) or scenes (parahippocampal cortex) and in the forming of associations between them (hippocampus) (Squire et al. 2004; Davachi 2006; Staresina et al. 2011).
Figure 2.
Schematic view of the medial temporal lobe memory system for declarative memory, which is composed of the hippocampus and the perirhinal, entorhinal, and parahippocampal cortices. In addition to the connections shown here, there are also weak projections from the perirhinal and parahippocampal cortices to the CA1-subiculum border.
Structures in the diencephalic midline (mammillary nuclei, medial dorsal nucleus, anterior thalamic nuclei, together with the internal medullary lamina and the mammillothalamic tract) are also important for declarative memory. Damage to these structures causes the same core deficit as damage to the medial temporal lobe, probably because these nuclei and tracts are anatomically related to the medial temporal lobe (see Markowitsch 1988; Victor et al. 1989; Harding et al. 2000; Squire and Wixted 2011).
Discussion continues about the nature of declarative memory and about when exactly the hippocampus (and related structures) is involved in learning and memory. One proposal is that, whereas conscious recollection depends on the hippocampus as described above, the hippocampus is also important for unconscious memory under some circumstances (Henke 2010; Hannula and Greene 2012; Shohamy and Turk-Browne 2013). One way to explore this issue has been to record eye movements while volunteers are making behavioral memory judgments. In some situations (e.g., when a change has occurred in the layout of a scene), the eyes do not reveal signs of memory (by moving to the changed location) unless participants are aware of where the change occurred (Smith et al. 2008). Furthermore, these eye movement effects require the integrity of the hippocampus. Nevertheless, in other situations, eye movements can signal which item is correct, and correlate with hippocampal activity, even when behavioral memory judgments are incorrect and participants are therefore thought to be unaware (Hannula and Ranganath 2009). Such a finding could mean that eye movements (and hippocampal activity) can index unaware memory. Yet, it is also true that awareness is (presumably) continuous, and a low amount of awareness is not the same as a complete lack of awareness (Kumaran and Wagner 2009). Just as recognition memory can succeed when free recall fails, eye movements might reveal signs of aware memory when recognition fails. It would be instructive in this circumstance to obtain confidence ratings in association with memory judgments and ask whether there is any detectable awareness of which items are correct.
Other work has implicated medial temporal lobe structures in the unaware learning of sequences and other tasks with complex contingencies (Chun and Phelps 1999; Rose et al. 2002; Schendan et al. 2003). This idea is often based on functional magnetic resonance imaging (fMRI) evidence of medial temporal lobe activity during unaware learning. Strategic factors (e.g., explicit attempts to memorize) may explain some of these effects (Westerberg et al. 2011), together with the possibility that awareness may not be entirely absent (Poldrack and Rodriguez 2003). In addition, considering that fMRI data cannot establish a necessary role for a particular structure, it will be useful to supplement fMRI data with evidence that patients with hippocampal lesions are impaired at the same tasks that afford unaware learning. Interestingly, for some of these tasks, hippocampal patients were not impaired (Reber and Squire 1994; Manns and Squire 2001), but impairment has been reported in patients when the damage was undescribed or extended beyond the hippocampus (Chun and Phelps 1999). Studies that combine fMRI, patient data, and rigorous measures of awareness will be useful in pursuing this interesting issue.
WORKING MEMORY
Working memory refers to the capacity to maintain a limited amount of information in mind, which can then support various cognitive abilities, including learning and reasoning (Baddeley 2003). Within cognitive neuroscience, the term “working memory” has largely replaced the less precise term “short-term memory.” (Note that the term “short-term memory” remains useful in cellular neuroscience where it has a different and distinct meaning [Kandel et al. 2014].) Historically, working memory has been considered to be distinct from long-term memory and independent of the medial temporal lobe structures that support the formation of long-term memory (Drachman and Arbit 1966; Atkinson and Shiffrin 1968; Baddeley and Warrington 1970; Milner 1972). Long-term memory is needed when the capacity of working memory is exceeded or when working memory is disrupted by diverting attention to different material.
Uncertainty can arise when determining in any particular case, whether performance depends on working memory or whether so much information needs to be kept in mind that working memory capacity is exceeded and performance must rely on long-term memory. What is the situation when patients fail at tasks with short retention intervals, or no retention interval (Hannula et al. 2006; Olson et al. 2006; Warren et al. 2011, 2012; Yee et al. 2014)? Are these long-term memory tasks or, as has been suggested, do such findings show that working memory sometimes depends on the medial temporal lobe (Ranganath and Blumenfeld 2005; Graham et al. 2010). It is important to note that working memory cannot be defined in terms of any particular retention interval (Jeneson and Squire 2012). Even when the retention interval is measured in seconds, working memory capacity can be exceeded such that performance must depend, in part, on long-term memory (e.g., immediately after presentation of a list of 10 words).
Findings from a scene-location task illustrate the problem. The task involved scenes containing a number of different objects. On each trial, a scene was presented together with a question (e.g., is the plant on the table?). A few seconds later, the same scene was presented again but now the object queried about might or might not appear in a different location. In this condition, patients with hippocampal lesions were accurate at detecting whether or not the object had moved (Jeneson et al. 2011). However, when attention was drawn to four different objects, any one of which might move, patients were impaired (Yee et al. 2014). It is likely that the impairment in the second condition occurred because working memory capacity was exceeded. Visual working memory capacity is quite limited and, typically, even healthy young adults can maintain only three to four simple visual objects in working memory (Cowan 2001; Fukuda et al. 2010).
In any case, there are ways to distinguish working memory and long-term memory (Shrager et al. 2008; Jeneson et al. 2010). For example, one can vary the number of items or associations to be remembered and ask whether patients show a sharp discontinuity in performance as the number of items increases and working memory capacity is exceeded. In one study (Jeneson et al. 2010), patients with hippocampal lesions or large medial temporal lobe lesions saw different numbers of objects (1 to 7) on a tabletop and then immediately tried to reproduce the array on an adjacent table. Performance was intact when only a few object locations needed to be remembered. However, there was an abrupt discontinuity in performance with larger numbers of object locations. One patient (who had large medial temporal lobe lesions similar to H.M.) learned one-, two-, or three-object locations as quickly as did controls, never needing more than one or two trials to succeed. Yet when four-object locations needed to be remembered, he did not succeed even after 10 trials with the same array. These findings indicate that the ability to maintain small numbers of object–place associations in memory is intact after medial temporal lobe lesions. An impairment was evident only when a capacity limit was reached, at which point performance needed to depend on long-term memory. A similar conclusion was reached in studies of a single patient with restricted hippocampal lesions. Performance was fully intact on an extensive working memory battery, including tasks of relational (associative) memory (Baddeley et al. 2010, 2011).
Spatial tasks like path integration can also be performed normally by patients with medial temporal lobe lesions, as long as the task can be managed within working memory (Shrager et al. 2008; Kim et al. 2013). The findings are different for rats with hippocampal lesions (Kim et al. 2013), either because for the rat the task relies on long-term memory or because the rat hippocampus is needed for some online spatial computations, as suggested previously (Whitlock et al. 2008).
fMRI findings are relevant to these issues, because medial temporal lobe activity is sometimes found in association with short-delay recognition memory tasks (Ranganath and D’Esposito 2001; Piekema et al. 2006; Toepper et al. 2010). Yet, it is noteworthy that the extent of medial temporal lobe activity in short-delay tasks can be modulated by memory demands. For example, in some studies, the medial temporal lobe activity that occurred while maintaining information in memory was correlated with subsequent retention of the material being learned (Schon et al. 2004; Ranganath et al. 2005; Nichols et al. 2006). In addition, in a study that required maintaining faces in memory, the connectivity between the hippocampus and the fusiform face area increased with higher mnemonic load (one face vs. four faces) (Rissman et al. 2008). Concurrently, with higher load, the connectivity decreased between frontal regions traditionally linked to working memory (Goldman-Rakic 1995; Postle 2006) and the fusiform face area. These findings suggest that fMRI activity in the medial temporal lobe reflects processes related to the formation of long-term memory rather than processes related to working memory itself (for review, see Jeneson and Squire 2012).
NONDECLARATIVE MEMORY
Nondeclarative memory (sometimes termed implicit memory) refers to a collection of abilities that are expressed through performance without requiring conscious memory content. Study of nondeclarative memory began with motor skills and perceptual skills, as described above, but soon included additional abilities as well. The next of these to come under study was the phenomenon of priming. Priming is evident as improved access to items that have been recently presented or improved access to associates of those items. This improvement is unconscious and is experienced as part of perception, as perceptual fluency, not as an expression of memory. A key finding was that priming effects were intact in memory-impaired patients. For example, patients can perform normally on tests that use word stems as cues for recently presented words (e.g., study BRICK, CRATE; test with BRI___, CRA___). Importantly, performance was intact in patients only when they were instructed to complete each cue to form the first word that comes to mind. With conventional memory instructions (use the cue to help recall a recently presented word), healthy volunteers outperformed the patients (Graf et al. 1984). Priming can occur for material that has no preexisting memory representation (e.g., nonsense letter strings) (Hamann and Squire 1997a) and for material that is related by meaning to recently studied items. Thus, when asked to free associate to a word (e.g., strap), volunteers produced a related word (e.g., belt) more than twice as often when that word (belt) was presented recently (Levy et al. 2004). Importantly, severely amnesic patients showed fully intact word priming, even while performing at chance levels in parallel memory tests (Hamann and Squire 1997b; Levy et al. 2004). Thus, priming occurs but it does not benefit conscious memory decisions. Indeed, direct measurements showed that priming provides only a weak and unreliable cue for conscious judgments of familiarity (Conroy et al. 2005).
Priming is presumably advantageous because animals evolved in a world where things that are encountered once are likely to be encountered again. Priming improves the speed and efficiency with which organisms interact with a familiar environment and may influence feature-based attentional processes (Hutchinson and Turk-Browne 2012; Theeuwes 2013). Evoked-potential studies indicate that the electrophysiological signature of priming occurs early and well before the activity that signals conscious recognition of a past event (Paller et al. 2003). In neuroimaging studies, priming is often associated with reduced activity in regions of neocortex relevant to the task (Squire et al. 1992; Schacter et al. 2007). A similar finding (repetition suppression) (Desimone 1996) has been described in nonhuman primates (a stimulus-specific attenuation in firing rate with repeated presentation of a stimulus), and may underlie the phenomenon of priming (Wiggs and Martin 1998). Models have been proposed to explain how a net reduction in cortical activity could allow for faster perceptual processing (i.e., priming) (Grill-Spector et al. 2006). Some studies have found a correlation between behavioral measures of priming and reduced activity in the prefrontal cortex (Maccotta and Buckner 2004). This result has not been found in ventral temporal cortex for either humans or nonhuman primates (Maccotta and Buckner 2004; McMahon and Olson 2007).
Changes in cortex also underlie the related phenomenon of perceptual learning (Gilbert et al. 2009). Perceptual learning refers to gradual improvement in the detection or discrimination of visual stimuli with repeated practice. Changes in cortical circuitry during perceptual learning are detectable as early as primary visual cortex (V1) and may depend in part on structural changes in the long-range horizontal connections formed by V1 pyramidal cells (Gilbert and Li 2012). This circuitry is under the control of bottom-up processes as well as top-down influences related to attention and behavioral context (Gilbert and Li 2013).
Another early example of nondeclarative memory was simple classical conditioning, best illustrated in the literature of delay eyeblink conditioning. In delay conditioning, a neutral conditioned stimulus (CS), such as a tone, is presented just before an unconditioned stimulus (US), such as an airpuff to the eye. The two stimuli then overlap and coterminate. Critically, delay eyeblink conditioning is intact in amnesia and is acquired independently of awareness (Gabrieli et al. 1995; Clark and Squire 1998). Participants who did not become aware of the relationship between the CS and US (i.e., that the CS predicts the US) learned just as well as volunteers who did become aware (Manns et al. 2001). Indeed, when CS–US association strength was varied (by changing the number of consecutive CS alone or CS–US presentations), the probability of a conditioned response increased with association strength but was inversely related to how much the US was expected (Clark et al. 2001). Largely on the basis of work with rabbits, delay eyeblink conditioning proved to depend on the cerebellum and associated brain stem circuitry (Thompson and Krupa 1994; Thompson and Steinmetz 2009). Forebrain structures are not necessary for acquisition or retention of classically conditioned eyeblink responses.
Evaluative information, that is, whether a stimulus has positive or negative value, is acquired largely as nondeclarative memory. Biological study of this kind of memory has focused especially on the associative learning of fear (Davis 2006; Adolphs 2013; LeDoux 2014). Its nondeclarative status is illustrated by the fact that, in humans, associative fear learning proceeded normally after hippocampal lesions, even though the CS–US pairings could not be reported (Bechara et al. 1995). The amygdala has a critical role in fear learning, and its function (as well as its connectivity) appears to be conserved widely across species. In human neuroimaging studies, the amygdala was activated not only by fear but by strongly positive emotions as well (Hamann et al. 2002). Thus, the amygdala appears to be critical for associating sensory stimuli with stimulus valence. Ordinarily, animals express fear learning by freezing behavior (immobility). However, in a task where learned fear must instead be expressed by executing an avoidance response (an escape), freezing is maladaptive. In this case, prefrontal cortex inhibits defense behaviors (such as freezing) that are mediated by the amygdala, thereby allowing the animal to escape (Moscarello and LeDoux 2013). Inhibitory action of the prefrontal cortex on the amygdala (from infralimbic prefrontal cortex in rat or from ventromedial prefrontal cortex in humans) has also been found to occur during the reversal of fear learning (i.e., extinction) (Milad and Quirk 2012). This work has relevance for clinical disorders, such as phobias and posttraumatic stress disorder (Davis 2011).
In addition to these functions, it is important to note that the amygdala also exerts a modulatory influence on both declarative and nondeclarative memory. This role of the amygdala is the basis for the fact that emotionally arousing events are typically remembered better than emotionally neutral events. The mechanism for this effect is understood and depends on the release of stress hormones from the adrenal gland, which affects the forebrain via the vagus nerve, the nucleus of the solitary tract, and the locus coeruleus. Ultimately, the effect is mediated by the amygdala through its basolateral nucleus (McGaugh and Roozendaal 2009).
The gradual trial-and-error learning that leads to the formation of habits was proposed in the 1980s to be supported by the striatum (Mishkin et al. 1984), and habit memory subsequently became an important focus of study (Yin and Knowlton 2006; Graybiel 2008; Liljeholm and O’Doherty 2012). Habit memory is characterized by automatized, repetitive behavior and, unlike declarative memory, is insensitive to changes in reward value (Dickinson 1985). An early demonstration of the distinction between declarative memory and habit memory came from rats with fornix lesions or caudate lesions tested on two ostensibly similar tasks. Rats with fornix lesions, which disrupt hippocampal function, failed when they needed to acquire a flexible behavior but succeeded when they needed to respond repetitively. Rats with caudate lesions showed the opposite pattern (Packard et al. 1989). A similar contrast between declarative memory and habit memory was shown for memory-impaired patients with hippocampal lesions and patients with nigrostriatal damage caused by Parkinson’s disease (Knowlton et al. 1996). In the task, probabilistic classification, participants gradually learned which of two outcomes (sun or rain) would occur on each trial, given the particular combination of four cues that appeared. One, two, or three cues could appear on any trial, and each cue was independently and probabilistically related to the outcome. Patients with hippocampal lesions learned the task at a normal rate but could not report facts about the task. Parkinson patients remembered the facts but could not learn the task.
Tasks that can be learned quickly by memorization can also be learned by a trial-and-error, habit-based strategy, albeit much more slowly. In one study, healthy volunteers were able to learn eight separate pairs of “junk objects” within a single session of 40 trials (i.e., choose the correct object in each pair). Two severely amnesic patients with large medial temporal lobe lesions also learned but only gradually, requiring more than 25 test sessions and 1000 trials (Bayley et al. 2005). Unlike declarative memory, which is flexible and can guide behavior in different contexts, the acquired knowledge in this case was rigidly organized. Patient performance collapsed when the task format was altered by asking participants to sort the 16 objects into correct and incorrect groups (a trivial task for controls). In addition, although by the end of training the patients were consistently performing at a high level, at the start of each test day they were never able to describe the task, the instructions, or the objects. Indeed, during testing they expressed surprise that they were performing so well. These findings provide particularly strong evidence for the distinction between declarative (conscious) and nondeclarative (unconscious) memory systems.
Reward-based learning of this kind depends on dopamine neurons in the midbrain (substantia nigra and ventral tegmental area), which project to the striatum and signal the information value of the reward (Schultz 2013). The dorsolateral striatum is crucial for the development of habits in coordination with other brain regions. Neurophysiological studies in mice during skill learning documented that the dorsolateral striatum was increasingly engaged as performance became more automatic and habit-like (Yin et al. 2009). In contrast, the dorsomedial striatum was engaged only early in training. Similarly, in rats learning a conditioned T-maze task, activity gradually increased in the dorsolateral striatum as training progressed, and this activity correlated with performance (Thorn et al. 2010). In the dorsomedial striatum, activity first increased but then decreased as training progressed. Increased activity in the dorsolateral striatum during the later stages of habit formation occurred together with late-developing activity in infralimbic cortex (Smith and Graybiel 2013). Moreover, disruption of infralimbic cortex during late training prevented habit formation. Thus, these two regions (dorsolateral striatum and infralimbic cortex) appear to work together to support a fully formed habit.
THE RELATIONSHIP BETWEEN MEMORY SYSTEMS
The memory systems of the mammalian brain operate independently and in parallel to support behavior, and how one system or another gains control is a topic of considerable interest (Poldrack and Packard 2003; McDonald and Hong 2013; Packard and Goodman 2013). In some circumstances, memory systems are described as working cooperatively to optimize behavior and in other circumstances are described as working competitively. However, it is not easy to pin down what should count for or against cooperativity, competition, or independence in any particular case. For example, the fact that the manipulation of one memory system can affect the operation of another has been taken as evidence for competition between systems (Schwabe 2013). Yet, even for systems that are strictly independent, the loss of one system would be expected to affect the operation of another system by affording it more opportunity to control behavior.
Much of the experimental work on the relationship between memory systems has focused on hippocampus-dependent declarative memory and dorsolateral striatum-dependent habit memory. In an illustrative study (Packard and McGaugh 1996), rats were trained in a four-arm, plus-shaped maze to go left, always beginning in the south arm (and with the north arm blocked). In this situation, rats could learn either a place (the left arm) or a response (turn left). To discriminate between these two possibilities, rats were occasionally started in the north arm (with the south arm now blocked). When these north-arm starts were given early in training, rats tended to enter the same arm that had been rewarded. They had learned a place. However, with extended training, rats tended to repeat the learned left-turn response and enter the unrewarded arm. Place learning was abolished early in training by lidocaine infusions into the hippocampus. In this case, rats showed no preference for either arm. Correspondingly, later in training, response learning was abolished by lidocaine infusions into the caudate nucleus. In this case, however, rats showed place responding. In other words, even though behavior later in training was guided by caudate-dependent response learning, information remained available about place. When the caudate nucleus was inactive, the parallel memory system supported by the hippocampus was unmasked.
A similar circumstance has been described in humans performing a virtual navigation task that could be solved by either a spatial or nonspatial (habit-like) strategy (Iaria et al. 2003). At the outset, spatial and nonspatial strategies were adopted equally often, but as training progressed participants tended to shift to a nonspatial strategy. Participants who used the spatial strategy (navigating in relation to landmarks) showed increased activity in the right hippocampus early in training. Participants using the nonspatial strategy (counting maze arms) showed increased activity in the caudate nucleus, which emerged as training progressed.
Several factors increase the tendency to adopt a striatal strategy, including stress (Kim et al. 2001; Schwabe 2013), psychopathology (Wilkins et al. 2013), aging (Konishi et al. 2013), and a history of alcohol and drug use (Bohbot et al. 2013). Prefrontal cortex may also be important in determining which memory system gains control over behavior (McDonald and Hong 2013).
Although many tasks can be acquired by more than one memory system, other tasks strongly favor one system over another. In this circumstance, engaging the less optimal system can interfere with performance. Thus, fornix lesions in rats facilitated acquisition of a caudate-dependent maze habit that required repeated visits to designated arms (Packard et al. 1989). The fornix lesion presumably disrupted the tendency to use a nonoptimal declarative memory strategy. Similarly, a familiar feature of skill learning in humans is that trying to memorize, and use declarative memory, can disrupt performance.
Neuroimaging studies show that feedback-guided learning typically engages the striatum. A task described earlier, probabilistic classification, requires participants to make a guess on each trial based on cues that are only partially reliable. Participants typically begin by trying to memorize the task structure but then turn to the habit-like strategy of accumulating response strength in association with the cues. Correspondingly, fMRI revealed activity in the medial temporal lobe early during learning (Poldrack and Gabrieli 2001). As learning progressed, activity decreased in the medial temporal lobe, and activity increased in the striatum. Moreover, when the task was modified so as to encourage the use of declarative memory, less activity was observed in the striatum and more activity was observed in the medial temporal lobe.
CONCLUSION
The memory system framework is fundamental to the contemporary study of learning and memory. Within this framework, the various memory systems have distinct purposes and distinct anatomy, and different species can solve the same task using different systems. Interestingly, efforts have been made to account for some findings (e.g., priming or classification learning) with models based on a single system (Zaki et al. 2003; Zaki 2004; Berry et al. 2012). Yet, these accounts have difficulty explaining double dissociations (e.g., Packard et al. 1989; Knowlton et al. 1996), chance performance on tests of declarative memory when priming is intact (Hamann and Squire 1997b), and successful habit learning in the face of expressed ignorance about the task (Bayley et al. 2005).
One implication of these facts is that the therapeutic targets for various kinds of memory disorders are quite different. For example, for extreme fear-based memories like phobias, one must target the amygdala, for strong habit-based memories like obsessive–compulsive disorders, one must target the striatum, and for severe forgetfulness, as in Alzheimer’s disease, one must target the hippocampus and adjacent structures. The notion of multiple memory systems is now widely accepted and establishes an important organizing principle across species for investigations of the biology of memory.
Footnotes
Editors: Eric R. Kandel, Yadin Dudai, and Mark R. Mayford
Additional Perspectives on Learning and Memory available at www.cshperspectives.org
REFERENCES
- Adolphs R 2013. The biology of fear. Curr Biol 23: R79–R93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annese J, Schenker-Ahmed NM, Bartsch H, Maechler P, Sheh C, Thomas N, Kayano J, Ghatan A, Bresler N, Frosch MP, et al. 2014. Postmortem examination of patient H.M.’s brain based on histological sectioning and digital 3D reconstruction. Nat Commun 5: 3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson RC, Shiffrin RM 1968. Human memory: A proposed system and its control processes. In Psychology of learning and motivation: Advances in research and theory (ed. Spence KW, Spence JT), pp. 89–195 Academic, New York. [Google Scholar]
- Augustinack JC, van der Kouwe AJW, Salat DH, Benner T, Stevens AA, Annese J, Fischl B, Frosch MP, Corkin S 2014. H.M.’s contributions to neuroscience: A review and autopsy studies. Hippocampus 24: 1267–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley A 2003. Double dissociations: Not magic, but still useful. Cortex 39: 129–131. [DOI] [PubMed] [Google Scholar]
- Baddeley AD, Warrington EK 1970. Amnesia and the distinction between long- and short-term memory. J Verb Learn Verb Behav 9: 176–189. [Google Scholar]
- Baddeley A, Allen R, Vargha-Khadem F 2010. Is the hippocampus necessary for visual and verbal binding in working memory? Neuropsychologia 48: 1089–1095. [DOI] [PubMed] [Google Scholar]
- Baddeley A, Jarrold C, Vargha-Khadem F 2011. Working memory and the hippocampus. J Cog Neurosci 23: 3855–3861. [DOI] [PubMed] [Google Scholar]
- Bayley PJ, Frascino JC, Squire LR 2005. Robust habit learning in the absence of awareness and independent of the medial temporal lobe. Nature 436: 550–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Tranel D, Damasio H, Adolphs R, Rockland C, Damasio AR 1995. Double dissociation of conditioning and declarative knowledge relative to the amygdala and hippocampus in humans. Science 269: 1115–1118. [DOI] [PubMed] [Google Scholar]
- Bergson HL 1910. Matter and memory (trans. Paul NM, Palmer WS). George Allen, London. [Google Scholar]
- Berry CJ, Shanks DR, Speekenbrink M, Henson RNA 2012. Models of recognition, repetition priming, and fluency: Exploring a new framework. Psych Rev 119: 40–79. [DOI] [PubMed] [Google Scholar]
- Bohbot VD, Del Balso D, Conrad K, Konishi K, Leyton M 2013. Caudate nucleus-dependent navigational strategies are associated with increased use of addictive drugs. Hippocampus 23: 973–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruner JS 1969. Modalities of memory. In The pathology of memory (ed. Talland GA, et al. ). pp. 253–259 Academic, New York. [Google Scholar]
- Bunsey M, Eichenbaum H 1996. Conservation of hippocampal memory function in rats and humans. Nature 379: 255–257. [DOI] [PubMed] [Google Scholar]
- Chun MM, Phelps EA 1999. Memory deficits for implicit contextual information in amnesic subjects with hippocampal damage. Nat Neurosci 2: 844–847. [DOI] [PubMed] [Google Scholar]
- Clark RE, Squire LR 1998. Classical conditioning and brain systems: A key role for awareness. Science 280: 77–81. [DOI] [PubMed] [Google Scholar]
- Clark RE, Manns JR, Squire LR 2001. Trace and delay eyeblink conditioning: Contrasting phenomena of declarative and nondeclarative memory. Psychol Sci 12: 304–308. [DOI] [PubMed] [Google Scholar]
- Clayton RS, Dickinson A 1998. Episodic-like memory during cache recovery by scrub jays. Nature 395: 272–274. [DOI] [PubMed] [Google Scholar]
- Cohen NJ, Squire LR 1980. Preserved learning and retention of pattern analyzing skill in amnesia: Dissociation of knowing how and knowing that. Science 210: 207–209. [DOI] [PubMed] [Google Scholar]
- Conroy MA, Hopkins RO, Squire LR 2005. On the contribution of perceptual fluency and priming to recognition memory. Cogn Affect Behav Neurosci 5: 14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan N 2001. The magical number 4 in short-term memory: A reconsideration of mental storage capacity. Behav Brain Sci 24: 87–185. [DOI] [PubMed] [Google Scholar]
- Davachi L 2006. Item, context and relational episodic encoding in humans. Curr Opin Neurobiol 16: 693–700. [DOI] [PubMed] [Google Scholar]
- Davis M 2006. Neural systems involved in fear and anxiety measured with fear-potentiated startle. Am Psychol 61: 741–756. [DOI] [PubMed] [Google Scholar]
- Davis M 2011. NMDA receptors and fear extinction: Implications for cognitive behavioral therapy. Dialogues Clin Neurosci 13: 463–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desimone R 1996. Neural mechanisms for visual memory and their role in attention. Proc Natl Acad Sci 93: 13494–13499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson A 1985. Actions and habits: the development of behavioural autonomy. Phil Trans R Soc Lond B Biol Sci 308: 67–78. [Google Scholar]
- Drachman DA, Arbit J 1966. Memory and the hippocampal complex. II. Is memory a multiple process? Arch Neurol 15: 52–61. [DOI] [PubMed] [Google Scholar]
- Fortin NJ, Agster KL, Eichenbaum HB 2002. Critical role of the hippocampus in memory for sequences of events. Nat Neurosci 5: 458–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda K, Awh E, Vogel EK 2010. Discrete capacity limits in visual working memory. Curr Opin Neurobiol 20: 177–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrieli JDE, McGlinchey-Berroth R, Carrillo MC, Gluck MA, Cermak LS, Disterhoft JF 1995. Intact delay-eyeblink classical conditioning in amnesia. Behav Neurosci 109: 819–827. [DOI] [PubMed] [Google Scholar]
- Gaffan D 1974. Recognition impaired and association intact in the memory of monkeys after transaction of the fornix. J Comp Physiol Psychol 86: 1100–1109. [DOI] [PubMed] [Google Scholar]
- Gilbert CD, Li W 2012. Adult visual cortical plasticity. Neuron 75: 250–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert CD, Li W 2013. Top-down influences on visual processing. Nat Rev Neurosci 14: 350–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert CD, Li W, Piech V 2009. Perceptual learning and adult cortical plasticity. J Physiol 587: 2743–2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS 1995. Architecture of the prefrontal cortex and the central executive. Ann NY Acad Sci 769: 71–83. [DOI] [PubMed] [Google Scholar]
- Graf P, Squire LR, Mandler G 1984. The information that amnesic patients do not forget. J Exp Psychol Learn Mem Cog 10: 164–178. [DOI] [PubMed] [Google Scholar]
- Graham KS, Barense MD, Lee AC 2010. Going beyond LTM in the MTL: A synthesis of neuropsychological and neuroimaging findings on the role of the medial temporal lobe in memory and perception. Neuropsychologia 48: 831–853. [DOI] [PubMed] [Google Scholar]
- Graybiel AM 2008. Habits, rituals, and the evaluative brain. Ann Rev Neurosci 31: 359–387. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Henson R, Martin A 2006. Repetition and the brain: Neural models of stimulus-specific effects. Trends Cognit Sci 10: 14–23. [DOI] [PubMed] [Google Scholar]
- Hamann SB, Squire LR 1997a. Intact priming for novel perceptual representations in amnesia. J Cog Neurosci 9: 699–713. [DOI] [PubMed] [Google Scholar]
- Hamann SB, Squire LR 1997b. Intact perceptual memory in the absence of conscious memory. Behav Neurosci 111: 850–854. [DOI] [PubMed] [Google Scholar]
- Hamann SB, Ely TD, Hoffman JM, Kilts CD 2002. Ecstasy and agony: Activation of the human amygdala in positive and negative emotion. Psychol Sci 13: 135–141. [DOI] [PubMed] [Google Scholar]
- Hannula DE, Greene AJ 2012. The hippocampus reevaluated in unconscious learning and memory: At a tipping point? Front Human Neurosci 6: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannula DE, Ranganath C 2009. The eyes have it: Hippocampal activity predicts expression of memory in eye movements. Neuron 63: 592–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannula DE, Tranel D, Cohen NJ 2006. The long and the short of it: Relational memory impairments in amnesia, even at short lags. J Neurosci 26: 8352–8359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding A, Halliday G, Caine D, Krill J 2000. Degeneraton of anterior thalamic nuclei differentiates alcoholics with amnesia. Brain 123: 141–154. [DOI] [PubMed] [Google Scholar]
- Henke K 2010. A model for memory systems based on processing modes rather than consciousness. Nat Rev Neurosci 11: 523–532. [DOI] [PubMed] [Google Scholar]
- Higuchi S, Miyashita Y 1996. Formation of mnemonic neuronal responses to visual paired associates in inferotemporal cortex is impaired by perirhinal and entorhinal lesions. Proc Natl Acad Sci 93: 739–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsh R 1974. The hippocampus and contextual retrieval of information from memory: A theory. Behav Biol 12: 421–444. [DOI] [PubMed] [Google Scholar]
- Hutchinson JB, Turk-Browne NB 2012. Memory-guided attention: control from multiple memory systems. Trends Cogn Sci 16: 576–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iaria G, Petrides M, Dagher A, Pike B, Bohbot VD 2003. Cognitive strategies dependent on the hippocampus and caudate nucleus in human navigation: Variability and change with practice. J Neurosci 13: 5945–5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James W 1890. Principles of psychology. Holt, New York. [Google Scholar]
- Jeneson A, Squire LR 2012. Working memory, long-term memory, and medial temporal lobe function. Learn Mem 19: 15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeneson A, Mauldin KN, Squire LR 2010. Intact working memory for relational information after medial temporal lobe damage. J Neurosci 30: 13624–13629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeneson A, Mauldin KN, Hopkins RO 2011. The role of the hippocampus in retaining relational information across short delays: The importance of memory load. Learn Mem 18: 301–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel ER, Dudai Y, Mayford MR 2014. The molecular and systems biology of memory. Cell 157: 163–186. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Lee HJ, Han JS, Packard MG 2001. Amygdala is critical for stress-induced modulation of hippocampal long-term potentiation and learning. J Neurosci 21: 5222–5228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Sapiurka M, Clark R, Squire LR 2013. Contrasting effects on path integration after hippocampal damage in humans and rats. Proc Natl Acad Sci 110: 4732–4737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowlton BJ, Mangels JA, Squire LR 1996. A neostriatal habit learning system in humans. Science 273: 1399–1402. [DOI] [PubMed] [Google Scholar]
- Konishi K, Etchamendy N, Roy S, Marighetto A, Rajah N, Bohbot VD 2013. Decreased functional magnetic resonance imaging activity in the hippocampus in favor of the caudate nucleus in older adults tested in a virtual navigation task. Hippocampus 23: 1005–1014. [DOI] [PubMed] [Google Scholar]
- Kumaran D, Wagner AD 2009. It’s in my eyes, but it doesn’t look that way to me. Neuron 63: 561–563. [DOI] [PubMed] [Google Scholar]
- LeDoux J 2014. Coming to terms with fear. Proc Natl Acad Sci 111: 2871–2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy DA, Stark CEL, Squire LR 2004. Intact conceptual priming in the absence of declarative memory Psychol Sci 15: 680–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljeholm M, O’Doherty JP 2012. Contributions of the striatum to learning, motivation, and performance: An associative account. Trends Cogn Sci 16: 467–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccotta L, Buckner RL 2004. Evidence for neural effects of repetition that directly correlate with behavioral priming. J Cog Neurosci 16: 1625–1632. [DOI] [PubMed] [Google Scholar]
- Manns JR, Squire LR 2001. Perceptual learning, awareness, and the hippocampus. Hippocampus 11: 776–782. [DOI] [PubMed] [Google Scholar]
- Manns JM, Clark RE, Squire LR 2001. Single-cue delay eyeblink classical conditioning is unrelated to awareness. Cogn Affect Behav Neurosci 1: 192–198. [DOI] [PubMed] [Google Scholar]
- Markowitsch H 1988. Diencephalic amnesia: A reorientation towards tracts? Brain Res Rev 13: 351–370. [DOI] [PubMed] [Google Scholar]
- McDonald RJ, Hong NS 2013. How does a specific learning and memory system in the mammalian brain gain control of behavior? Hippocampus, 23: 1084–1102. [DOI] [PubMed] [Google Scholar]
- McDougall W 1923. Outline of psychology. Scribners, New York. [Google Scholar]
- McGaugh JL, Roozendaal B 2009. Drug enhancement of memory consolidation: Historical perspective and neurobiological implications. Psychopharmacology 1: 3–14. [DOI] [PubMed] [Google Scholar]
- McMahon DB, Olson CR 2007. Repetition suppression in monkey inferotemporal cortex: Relation to behavioral priming. J Neurophysiol 97: 3532–3543. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ 2012. Fear extinction as a model for translational neuroscience: Ten years of progress. Ann Rev Psychol 63: 129–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner B 1962. Les troubles de la memoire accompagnant des lésions hippocampiques bilaterales. In Physiologie de l’hippocampe, pp. 257–272 Centre National de la Recherche Scientifique, Paris. [Google Scholar]
- Milner B 1972. Disorders of learning and memory after temporal lobe lesions in man. Clin Neurosurg 19: 421–466. [DOI] [PubMed] [Google Scholar]
- Mishkin M 1978. Memory in monkeys severely impaired by combined but not by separate removal of amygdala and hippocampus. Nature 273: 297–298. [DOI] [PubMed] [Google Scholar]
- Mishkin M, Malamut B, Bachevalier J 1984. Memories and habits: Two neural systems. In Neurobiology of learning and memory (ed. Lynch G, et al. ), pp. 65–77 Guilford, New York. [Google Scholar]
- Moscarello JM, LeDoux JE 2013. Active avoidance learning requires prefrontal suppression of amygdala-mediated defensive reactions. J Neurosci 33: 3815–3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols EA, Kao YC, Verfaellie M, Gabrieli JD 2006. Working memory and long-term memory for faces: Evidence from fMRI and global amnesia for involvement of the medial temporal lobes. Hippocampus 16: 604–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keefe J, Nadel L 1978. The hippocampus as a cognitive map. Oxford University Press, London. [Google Scholar]
- Olson IR, Page K, Moore KS, Chatterjee A, Verfaellie M 2006. Working memory for conjunctions relies on the medial temporal lobe. J Neurosci 26: 4596–4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard MG, Goodman J 2013. Factors that influence the relative use of multiple memory systems. Hippocampus 23: 1044–1052. [DOI] [PubMed] [Google Scholar]
- Packard MG, McGaugh JL 1996. Inactivation of hippocampus or caudate nucleus with lidocaine differentially affects expression of place and response learning. Neurobiol Learn Mem 65: 65–72. [DOI] [PubMed] [Google Scholar]
- Packard MG, Hirsh R, White NM 1989. Differential effects of fornix and caudate nucleus lesions on two radial maze tasks: Evidence for multiple memory systems. J Neurosci 9: 1465–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paller KA, Hutson CA, Miller BB, Boehm SG 2003. Neural manifestations of memory with and without awareness. Neuron 38: 507–516. [DOI] [PubMed] [Google Scholar]
- Piekema C, Kessels RP, Mars RB, et al. 2006. The right hippocampus participates in short-term memory maintenance of object–location associations. NeuroImage 33: 374–382. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Gabrieli JD 2001. Characterizing the neural mechanisms of skill learning and repetition priming: Evidence from mirror reading. Brain 124: 67–82. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Packard MG 2003. Competition among multiple memory systems: Converging evidence from animal and human brain studies. Neuropsychologia 41: 245–251. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Rodriguez P 2003. Sequence learning: What’s the hippocampus to do? Neuron 37: 891–893. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Clark J, Pare-Blagoev EJ, Shohamy D, Creso Moyano J, Myers C, Gluck MA 2001. Interactive memory systems in the human brain. Nature 414: 546–550. [DOI] [PubMed] [Google Scholar]
- Postle BR 2006. Working memory as an emergent property of the mind and brain. Neurosci 139: 23–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C, Blumenfeld RS 2005. Doubts about double dissociations between short- and long-term memory. Trends Cogn Sci 9: 374–380. [DOI] [PubMed] [Google Scholar]
- Ranganath C, D'Esposito M 2001. Medial temporal lobe activity associated with active maintenance of novel information. Neuron 31: 865–873. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Cohen MX, Brozinsky CJ 2005. Working memory maintenance contributes to long-term memory formation: Neural and behavioral evidence. J Cogn Neurosci 17: 994–1010. [DOI] [PubMed] [Google Scholar]
- Reber PJ, Squire LR 1994. Parallel brain systems for learning with and without awareness. Learn Mem 1: 217–229. [PubMed] [Google Scholar]
- Rissman J, Gazzaley A, D’Esposito M 2008. Dynamic adjustments in prefrontal, hippocampal, and inferior temporal interactions with increasing visual working memory load. Cerebr Cortex 18: 1618–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose M, Haider H, Weiller C, Buchel C 2002. The role of medial temporal lobe structures in implicit learning: An event-related fMRI study. Neuron 36: 1221–1231. [DOI] [PubMed] [Google Scholar]
- Ryle G 1949. The concept of mind. Hutchinson, San Francisco. [Google Scholar]
- Schacter DL, Buckner RL 1998. Priming and the brain. Neuron 20: 185–195. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Wig GS, Stevens WD 2007. Reductions in cortical activity during priming. Curr Opin Neurobiol 17: 171–176. [DOI] [PubMed] [Google Scholar]
- Schendan HE, Searl MM, Melrose RJ, Stern CE 2003. An fMRI study of the role of the medial temporal lobe in implicit and explicit sequence learning. Neuron 37: 1013–1025. [DOI] [PubMed] [Google Scholar]
- Schon D, Lorber B, Spacal M, Semenza C 2004. A selective deficit in the production of exact musical intervals following right-hemisphere damage. Cogn Neuropsychol 21: 773–784. [DOI] [PubMed] [Google Scholar]
- Schultz W 2013. Updating dopamine reward signals. Curr Opin Neurobiol 23: 229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe L 2013. Stress and the engagement of multiple memory systems: Integration of animal and human studies. Hippocampus 23: 1035–1043. [DOI] [PubMed] [Google Scholar]
- Scoville WB, Milner B 1957. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry 20: 11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherry DF, Schacter DL 1987. The evolution of multiple memory systems. Psychol Rev 94: 439–454. [Google Scholar]
- Shimamura AP, Janowsky JS, Squire LR 1991. What is the role of frontal lobe damage in memory disorders? In Frontal lobe functioning and dysfunction (ed. Levin HD, et al. ), pp. 173–195 Oxford University Press, New York. [Google Scholar]
- Shohamy D, Turk-Browne NB 2013. Mechanisms for widespread hippocampal involvement in cognition. J Exp Psychol Gen 142: 1159–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrager Y, Levy DA, Hopkins RO, Squire LR 2008. Working memory and the organization of brain systems. J Neurosci 28: 4818–4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KS, Graybiel AM 2013. A dual operator view of habitual behavior reflecting cortical and striatal dynamics. Neuron 79: 361–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C, Squire LR 2005. Declarative memory, awareness, and transitive inference. J Neurosci 25: 10138–10146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CN, Squire LR 2008. Experience-dependent eye movements reflect hippocampus-dependent (aware) memory. J Neurosci 28: 12825–12833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR 2009. Memory and brain systems: 1969–2009. J Neurosci 29: 12711–12716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Wixted JT 2011. The cognitive neuroscience of human memory since H.M. Ann Rev Neurosci 34: 259–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Zola-Morgan S 1988. Memory: Brain systems and behavior. Trends Neurosci 11: 170–175. [DOI] [PubMed] [Google Scholar]
- Squire LR, Zola-Morgan S 1991. The medial temporal lobe memory system. Science 253: 1380–1386. [DOI] [PubMed] [Google Scholar]
- Squire LR, Ojemann JG, Miezin FM, Petersen SE, Videen TO, Raichle ME 1992. Activation of the hippocampus in normal humans: A functional anatomical study of memory. Proc Natl Acad Sci 89: 1837–1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Stark CEL, Clark RE 2004. The medial temporal lobe. Ann Rev Neurosci 27: 279–306. [DOI] [PubMed] [Google Scholar]
- Staresina BP, Duncan KD, Davachi L 2011. Perirhinal and parahippocampal cortices differentially contribute to later recollection of object- and scene-related event details. J Neurosci 31: 8739–8747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theeuwes J 2013. Feature-based attention: It is all bottom-up priming. Phil Trans R Soc B Biol Sci 368: 20130055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RF, Krupa DJ 1994. Organization of memory traces in the mammalian brain. Ann Rev Neurosci 17: 519–550. [DOI] [PubMed] [Google Scholar]
- Thompson RF, Steinmetz JE 2009. The role of the cerebellum in classical conditioning of discrete behavioral responses. Neurosci 162: 732–755. [DOI] [PubMed] [Google Scholar]
- Thorn CA, Atallah H, Howe H, Graybiel AM 2010. Differential dynamics of activity changes in dorsolateral and dorsomedial striatal loops during learning. Neuron 66: 781–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toepper M, Markowitsch HJ, Gebhardt H, Beblo T, Thomas C, Gallhofer B, Driessen M, Sammer G 2010. Hippocampal involvement in working memory encoding of changing locations: An fMRI study. Brain Res 1354: 91–99. [DOI] [PubMed] [Google Scholar]
- Tolman RC 1948. Cognitive maps in rats and man. Psychol Rev 55: 189–208. [DOI] [PubMed] [Google Scholar]
- Tulving E 1983. Elements of episodic memory. Oxford University Press, Cambridge, MA. [Google Scholar]
- Tulving E 1989. Remembering and knowing the past. American Scientist 77: 361–367. [Google Scholar]
- Tulving E 2005. Episodic memory and autonoesis: Uniquely human? In The missing link in cognition: Evolution of self-knowing consciousness (ed. Terrace H, et al. ), pp. 3–56 Oxford University Press, New York. [Google Scholar]
- Tulving E, Schacter DL 1990. Priming and human memory systems. Science 247: 301–306. [DOI] [PubMed] [Google Scholar]
- Victor M, Adams RD, Collins GH 1989. The Wernicke–Korsakoff syndrome and related neurological disorders due to alcoholism and malnutrition. Davis, Philadelphia. [Google Scholar]
- Warren DE, Duff MC, Tranel D, Cohen NJ 2011. Observing degradation of visual representations over short intervals when medial temporal lobe is damaged. J Cogn Neurosci 23: 3862–3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren DE, Duff MC, Jensen U, Tranel D, Cohen NJ 2012. Hiding in plain view: Lesions of the medial temporal lobe impair online representation. Hippocampus 22: 1577–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerberg CE, Miller BB, Reber PJ, Cohen NJ, Paller KA 2011. Neural correlates of contextual cueing are modulated by explicit learning. Neuropsychologia 49: 3439–3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock JR, Sutherland RJ, Witter MP, Moser MB, Moser EI 2008. Navigating from hippocampus to parietal cortex. Proc Natl Acad Sci 105: 14755–14762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggs CL, Martin A 1998. Properties and mechanisms of perceptual priming. Curr Opin Neurobiol 8: 227–233. [DOI] [PubMed] [Google Scholar]
- Wilkins LK, Girard TA, Konishi K, King M, Herdman KA, King J, Christensen B, Bohbot VD 2013. Selective deficit in spatial memory strategies contrast to intact response strategies in patients with schizophrenia spectrum disorders tested in a virtual navigation task. Hippocampus 23: 1015–1024. [DOI] [PubMed] [Google Scholar]
- Winograd T 1975. Frame representations and the declarative-procedural controversy. In Representation and understanding: Studies in cognitive science (ed. Bobrow D, et al. ), pp. 185–210 Academic, New York. [Google Scholar]
- Yee LT, Hannula DE, Tranel D, Cohen NJ 2014. Short-term retention of relational memory in amnesia revisited: Accurate performance depends on hippocampal integrity. Front Hum Neurosci 8: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ 2006. The role of the basal ganglia in habit formation. Nat Rev Neurosci 7: 464–476. [DOI] [PubMed] [Google Scholar]
- Yin HH, Mulcare SP, Hilário MRF, Clouse E, Holloway T, Davis MI, Hansson AC, Lovinger DM, Costa RM, et al. 2009. Dynamic reorganization of striatal circuits during the acquisition and consolidation of a skill. Nat Neurosci 12: 333–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki SR 2004. Is categorization performance really intact in amnesia? A meta-analysis. Pysch Bull Rev 11: 1048–1054. [DOI] [PubMed] [Google Scholar]
- Zaki SR, Nosofsky RM, Jessup NM, Unversagt FR 2003. Categorization and recognition performance of a memory-impaired group: Evidence for single system models. J Int Neuropsychol Soc 9: 94–406. [DOI] [PubMed] [Google Scholar]