Abstract
Blood and lymphatic vessels deliver oxygen and nutrients, remove waste and CO2, and regulate interstitial pressure in tissues and organs. These vessels begin life early in embryogenesis using transcription factors and signaling pathways that regulate differentiation, morphogenesis, and proliferation. Here we describe how these vessels develop in the mouse embryo, and the signals that are important to their development.
There are 60,000 miles of blood vessels in a typical adult human. These vessels are an integral part of virtually every organ, so their developmental programs must adapt to numerous organ-specific signals and switches.
1. INTRODUCTION
This article describes the development of blood and lymphatic vessels, two organ systems that form extensive networks throughout the body. Blood vessels deliver oxygen and nutrients to virtually all cells in developing mammalian embryos and adults, whereas lymphatic vessels drain the interstitial fluid that collects in tissues, and serve as a conduit for immune cell trafficking and fat absorption. The mammal has been at the forefront of studies of blood and lymphatic vessel development, starting with the seminal studies of Florence Sabin on pig embryos in 1902 (Sabin 1902, 1917). Blood vessels of a typical adult human compose 8% of body mass, and end to end would go around the earth 2.5 times, a distance of 60,000 miles.
Blood and lymphatic vessels develop similarly to other organs and tissues in several ways. Signals produced by embryonic cells activate transcription factor programs to initiate vascular development. Endothelial cells form the inner lining of all blood vessels, and they differentiate from mesoderm under the influence of specific transcription factors, such as those of the ETS and Fox families, that are expressed in response to signaling pathways commonly deployed in development, such as BMP (bone morphogenetic protein), Wnt, and Notch (Lammerts van Bueren and Black 2012). ETS/Fox transcription factors regulate expression of other transcription factors such as Scl and Fli1, and signaling receptors such as VEGFR-2 and Tie-2. In fact, 27% of endothelial genes, including VEGFR-2 and Tie-2, have a hybrid enhancer motif that binds both FoxC2 and Etv2 (De Val et al. 2008). Signaling pathways regulate cellular behaviors such as division and migration. Lymphatic endothelial cells differentiate from venous endothelial cells through a stepwise process of transcription factor expression, including COUP-TFII, Sox18, and Prox1, and growth-factor-mediated chemotaxis and proliferation (Francois et al. 2011). Endothelial cells also recruit other cell types to developing blood vessels, including pericytes and smooth muscle cells that stabilize vessels. In large vessels, these mural cells form complex layers that provide contractility and tone. Both vascular and lymphatic endothelial cells form cell–cell contacts with neighboring endothelial cells, and undergo migration and morphogenetic changes as a unit to expand vessel networks as the embryo grows and develops. Finally, stem and progenitor cells are associated with several aspects of blood and lymphatic vessel development and diseases (Bautch 2011).
However, several aspects of blood and lymphatic vessel development are quite special. Blood and lymphatic vessels are an integral part of virtually every organ and tissue in the animal, so their developmental and morphogenetic programs must adapt to the signals and switches of numerous organ-specific developmental programs. For example, blood vessels respond to brain-derived signals to sprout into the central nervous system and pattern in concert with the patterning of the developing brain and neural tube (Bautch and James 2009; Quaegebeur et al. 2011). In contrast to the brain or the lungs, blood vessels, and to some extent lymphatic vessels, must function as soon as they form; blood vessels provide oxygen and nutrients to embryonic organs and tissues even as the vessels are developing, expanding, and remodeling in response to ongoing developmental programs, and lymphatic vessels facilitate fluid exchange within tissues as they expand. Ultimately, both blood and lymphatic vessels must develop as a barrier to unregulated exchange of materials between the inside and outside of the vessel, yet they must also provide for regulated movement of fluids, solids, and even cells into and out of the vessels. For example, lymphocytes egress from blood vessels at sites of inflammation in the adult, and then they are cleared through lymphatic vessels. Finally, blood and lymphatic vessels are exquisitely responsive to mechanical cues that allow them to develop in response to the needs of diverse environments. Blood vessels initially form a primitive network or plexus, which is then remodeled by the shear stress produced by blood flow and further stabilized via recruitment of pericytes and sometimes smooth muscle cells (Culver and Dickinson 2010). In adults, the lymphatic vasculature responds to a different cue—interstitial pressure, which changes the permeability of lymphatic endothelial cells to increase fluid reabsorption.
This article will examine the development of blood and lymphatic vessels during mouse embryogenesis, with brief references to seminal discoveries in other model organisms. Several excellent reviews have provided more depth and detail on this subject (Risau and Flamme 1995; Risau 1997; Coultas et al. 2005; Eichmann et al. 2005). We cover the sources and specification of blood and lymphatic vascular cells, then examine how vessel networks are assembled and expanded during development, and bring forth urgent major questions and future directions of the field.
2. DEVELOPMENT OF BLOOD VESSELS
2.1. Vasculogenesis—Specification and Migration of Angioblasts
Endothelial cells arise from mesoderm that forms at gastrulation and migrates extensively in the early embryo. Initial differentiation produces a precursor called the angioblast. Historically, it was debated whether blood vessels first arise in the yolk sac and then “seed” the embryo, or whether they arise independently in both the extraembryonic yolk sac and the embryo proper. However, it is now clear that blood vessels arise more or less simultaneously in both the embryo and the yolk sac. Within the mouse embryo, at approximately embryonic day 7.5 (E7.5), mesodermal cells expressing angioblast markers begin to coalesce in the paraxial region and form two cords that subsequently lumenize (Drake and Fleming 2000; Chong et al. 2011). A recent study identifies a repulsive signal, Sema 3E, as important in the initial placement of the dorsal aortas (Meadows et al. 2012). At about the same time endothelial cells are specified in the nascent yolk sac. Extraembryonic mesoderm that emerged from the posterior streak region condenses in discrete areas called “blood islands” to form a ring around the visceral yolk sac, immediately above the embryonic–extraembryonic border (Risau and Flamme 1995; Ferkowicz and Yoder 2005). The blood islands contain both vascular and hematopoietic precursor cells that do not appear to be clonal in origin (Ueno and Weissman 2006). VEGF-A, which is secreted by both the yolk sac endoderm and a mesothelial layer that subsequently forms over the hematovascular layer, is important for the proper formation of yolk sac vessels (Miquerol et al. 1999; Damert et al. 2002).
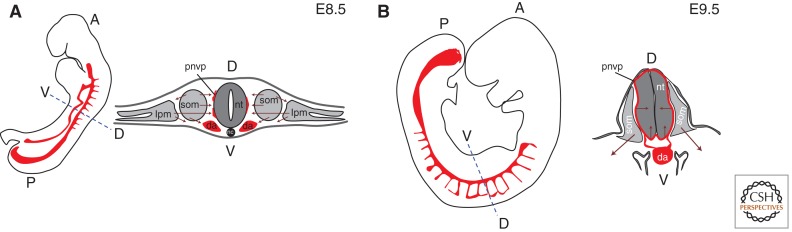
There are multiple mesodermal sources that provide endothelial precursor cells as embryonic development proceeds. Lateral plate mesoderm-derived angioblasts migrate medially and contribute to the perineural vascular plexus that surrounds the neural tube and intersomitic vessels (Fig. 1A). Somites also produce angioblasts that migrate medially to contribute to the perineural vascular plexus, laterally to contribute to the limb vasculature, and ventrally to contribute to the dorsal aorta and the developing kidney vessels (Pardanaud et al. 1996; Ambler et al. 2001). A recent avian study showed that cells from the posterior of each somite contribute to both endothelial cells and smooth muscle cells of the dorsal aorta subsequent to its initial formation (Sato et al. 2008). Lineage tracing studies reveal that somites contain cells that are bipotential for skeletal muscle and endothelium, or smooth muscle and endothelium (Esner et al. 2006; Lagha et al. 2009), suggesting that somitic angioblasts arise as part of the dermomyotome. Subsequently, most organs have a mesodermal component or a nearby source of mesenchyme that provides angioblasts for local vessel networks, and angiogenic sprouting produces conduit vessels and contributes to network expansion (see next section). One exception is the central nervous system (CNS), which does not have an internal source of angioblasts. During development, angioblasts form the perineural vascular plexus on the outer surface of the brain and neural tube. In an orchestrated program, CNS signals induce vascular sprouting into the neural tissue (Fig. 1B), and subsequently pattern the vessel network within the CNS (Bautch and James 2009; Quaegebeur et al. 2011).
Figure 1.
Blood vessel development in early postimplantation mouse embryos. (A) At E8.5, angioblasts and endothelial cells migrate medially and ventrally from lateral plate mesoderm and somites, and laterally from somites. (B) At E9.5, angiogenic sprouting from the perineural vascular plexus (pnvp) vascularizes the neural tube. Embryo silhouettes show pattern of trunk and intersomitic vessels in red (heart and head vessels are excluded); maroon arrows indicate migratory streams or direction of sprouting; black line denotes pnvp; da, dorsal aorta; lpm, lateral plate mesoderm; nc, notochord; nt, neural tube; som, somite; A, anterior; P, posterior; D, dorsal; V, ventral.
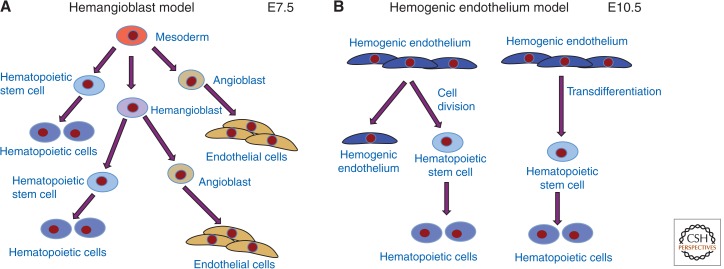
Potential lineage relationship(s) between the vascular and hematopoietic systems have been intensely investigated, starting with Florence Sabin, who observed that blood cells and blood vessels formed in close proximity and thus hypothesized a common precursor cell called the hemangioblast (Fig. 2A) (Sabin 1920). Embryonic stem cells undergo a programmed differentiation in culture to produce numerous embryonic cell types, including mesoderm that differentiates into several lineages such as primitive hematopoietic cells and vascular endothelial cells (Kearney and Bautch 2003; Keller 2005; Jakobsson et al. 2007). Keller and colleagues showed that mouse and human embryonic stem (ES) cells produce progenitor cells that give rise to both hematopoietic and endothelial cells in culture and in E7.5 mouse embryos, and Vogeli et al. lineage traced single cells in the zebrafish embryo to provide rigorous proof for a link between the lineages (Choi et al. 1998; Huber et al. 2004; Vogeli et al. 2006; Yang et al. 2008). Other investigators provided evidence that endothelial cells at specific sites in mid-gestation embryos, such as the ventral floor of the dorsal aorta, are associated with clusters of hematopoietic cells, suggesting a lineage relationship (de Bruijn et al. 2002). Endothelial cells that give rise to hematopoietic cells are called hemogenic endothelium, and recently this concept has been verified with elegant live imaging in both zebrafish and E10.5 mouse embryos (Fig. 2B) (Zovein et al. 2008; Eilken et al. 2009; Bertrand et al. 2010; Boisset et al. 2010; Kissa and Herbomel 2010). These studies show that differentiated endothelial cells can produce hematopoietic cells that appear to contribute to the hematopoietic stem cell pool, and they suggest that this process involves transdifferentiation of single endothelial cells into hematopoietic cells (Zape and Zovein 2011). The data supporting the hemangioblast model is also consistent with hemogenic endothelium, because the hemangioblast studies identified the progeny of single cells rather than the mechanisms leading to these relationships. Indeed, it is possible that both models are correct, and one recent study using mouse ES cells provided evidence that hemangioblast-derived endothelial cells have hemogenic capacity (Lancrin et al. 2009).
Figure 2.
Relationships of vascular and hematopoietic lineages. (A) The hemangioblast is the hypothesized bipotential precursor of some endothelial and hematopoietic cells. (B) An alternative model has differentiated endothelial cells dividing or transdifferentiating to produce hematopoietic stem cells.
2.2. Sprouting Angiogenesis and Blood Vessel Network Formation
Once the major blood vessels form via vasculogenesis, sprouting angiogenesis leads to the expansion of vessel networks and the formation of new connections (Risau 1997). Although vasculogenesis temporally precedes angiogenesis in development, the two processes often occur simultaneously once angiogenesis commences, and many vessel networks expand via both de novo differentiation of endothelial progenitor cells and sprouting angiogenesis.
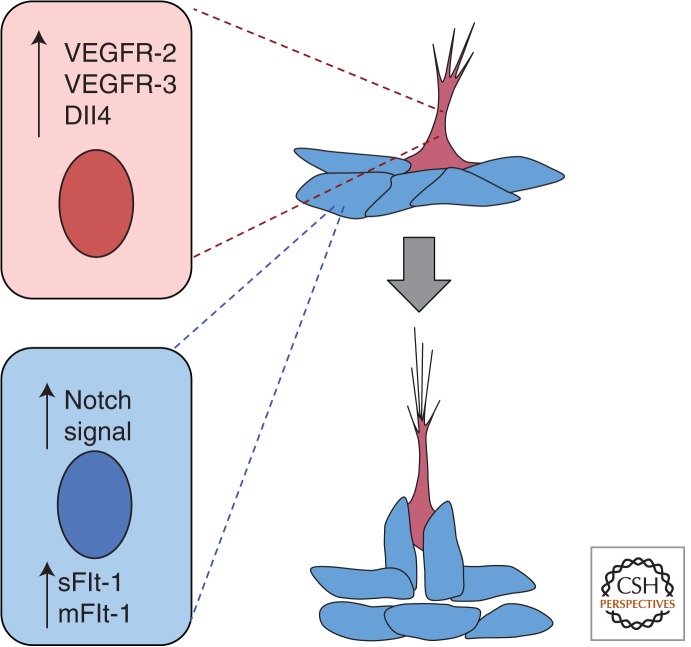
Angiogenesis involves the division and sprouting migration of differentiated endothelial cells. In recent years much effort has gone into a cellular and molecular description of angiogenesis, starting with the seminal observations by Gerhardt and colleagues (Gerhardt et al. 2003) in the postnatal mouse retina that led to the concept of tip cells and stalk cells in developing blood vessels. The retinal vasculature develops in the early postnatal period in the mouse, starting with expansion from the optic nerve at the center of the retina over the retinal tissue during the first week; the stereotypical nature of this vessel network allows for identification of the vascular front, which is the part of the network that is actively expanding at any given time (Fruttiger 2002). Proangiogenic signals, such as VEGF-A, cause some endothelial cells in a nascent vessel to migrate toward the cue, whereas other endothelial cells respond by cell division but do not overtly migrate. This heterogeneity in the responsiveness of endothelial cells was explained by the finding that endothelial cells in developing blood vessels show different phenotypes, and tip cells respond to VEGF-A signal by initiating migration, whereas cells behind the tip cell assume a stalk cell phenotype and divide in response to VEGF-A but do not migrate independently of the tip cell. Notch signaling, which is involved in many binary developmental decisions, is also critical for tip cell versus stalk cell phenotype (Hellstrom et al. 2007; Leslie et al. 2007; Siekmann and Lawson 2007). Tip cells up-regulate Notch ligands such as Dll4 and signal to neighboring cells to induce the acquisition of the stalk cell phenotype, in part through up-regulation of VEGFR-1 (Flt-1) (Fig. 3). The tip cell phenotype is plastic, as eventually the tip fuses to form a connection, with a new cell assuming a tip cell phenotype if branching continues. A recent study suggested that there is competition for the tip cell position mediated by VEGF and Notch; and in an in vitro model, tip cells were replaced in a time frame of hours (Jakobsson et al. 2010).
Figure 3.
Blood vessel sprouting and lumen formation. As vessels initiate sprouting, a cell (pink) adopts a tip cell phenotype, and through signaling of the Notch ligand Dll4 to neighboring cells sets up the stalk cell phenotype (blue), which is accompanied by up-regulation of Flt-1. As the sprout extends, the lumen forms behind the tip cell. sFlt-1, soluble Flt-1; mFlt-1, membrane Flt-1.
2.3. Lumen Formation
Before blood flow commences, blood vessels must form a lumen, and several recent studies have examined this process in detail (Iruela-Arispe and Davis 2009). Studies in zebrafish, a model organism amenable to live image analysis, suggested that vessel lumens form by a cord-hollowing mechanism (Kamei et al. 2006; Blum et al. 2008). Similar cord hollowing is also found during formation of the lumen in the dorsal aorta of the mouse embryo (Strilic et al. 2009). A model has been proposed whereby negatively charged glycoproteins on the future luminal surface of the endothelial cells provide a repulsive signal, and ensuing contraction and cell-shape changes expand the lumen. This hollowing process requires signaling by the small GTPase Rho, as genetic loss of a Rho regulator prevents lumen formation (Xu et al. 2011). Once a lumen forms, it extends as the vessel network expands (Fig. 3). Tip cells do not have a lumen, but the stalk cells immediately behind the tip cell begin to lumenize in the retina. To form a lumen, endothelial cells in vessels must be polarized in the apical (luminal)–basal (abluminal) axis. The regulation of polarity is critical to proper blood vessel function, and it is an area of intense research (Lee and Bautch 2011).
2.4. Artery–Vein Differentiation and Blood Vessel Remodeling
Another developmental program of import is the process whereby arteries and veins are distinguished, and blood vessels are remodeled in response to blood flow. The two aspects of blood vessel development are linked in that stabilization of at least arterial identity involves flow-mediated input, and arteries have higher shear flow and higher circumferential strain than veins. However, the initial differentiation of arteries versus veins initiates before and independent of blood flow (Wang et al. 1998). In arteries, a pathway that includes VEGF-A and Notch regulates arterial identity, and the transcription factors FoxC1 and FoxC2 regulate expression of Notch and Dll4 in arteries (Seo et al. 2006; Swift and Weinstein 2009). In veins, the orphan nuclear receptor COUP-TFII regulates venous identity, and acts to repress Notch (You et al. 2005). A recent in-depth analysis of arterial and venous marker expression in the developing mouse embryo revealed that arterial specification precedes venous specification, and provided evidence that blood flow is necessary for expression of some, although not all, arterial markers (Chong et al. 2011). In the mouse embryo, the heart starts to beat at approximately E8.0, but initially the heartbeats are irregular and weak, and blood does not begin to circulate until E8.5. It is at this time that flow-mediated remodeling begins, and it is operative throughout life. For example, small vessels called collaterals do not normally experience directed blood flow, but remodel in response to directed flow on blockade of normal conduits, such as occurs with a heart attack or stroke (Heil et al. 2006). Several molecular complexes are reputed to sense flow and transduce signals in endothelial cells, including a complex of PECAM–VEGFR-2–VEcadherin (Tzima et al. 2005). Many mutations in the mouse are embryonic lethal at mid-gestation with a cardiovascular phenotype. Some of these mutations affect flow-mediated remodeling, often downstream from heart defects (Culver and Dickinson 2010). In general, remodeling defects result in loss of the hierarchical organization of vessels in the network from larger conduits to smaller vessels and capillaries, lack of recruitment of support cells such as pericytes and smooth muscle cells, and often loss of arterial identity. Interestingly, a recent report suggests that flow-mediated signals can switch lymphatic vessels (see next section) to venous blood vessels, perhaps via flow-mediated regulation of the lymphatic transcription factor Prox-1 (Chen et al. 2012).
3. DEVELOPMENT OF LYMPHATIC VESSELS
Compared to blood vessel development, much less is known about the process of embryonic lymphangiogenesis (Fig. 4). Until recently, our knowledge of the genetic and developmental paradigms that drive lymphangiogenesis was limited because of lack of lymphatic-specific markers. However, the discovery of a handful of genes that can distinguish early lymphatic endothelial cells from blood endothelial cells has provided scientists with new tools that have helped to drive the field forward over the past 15 years.
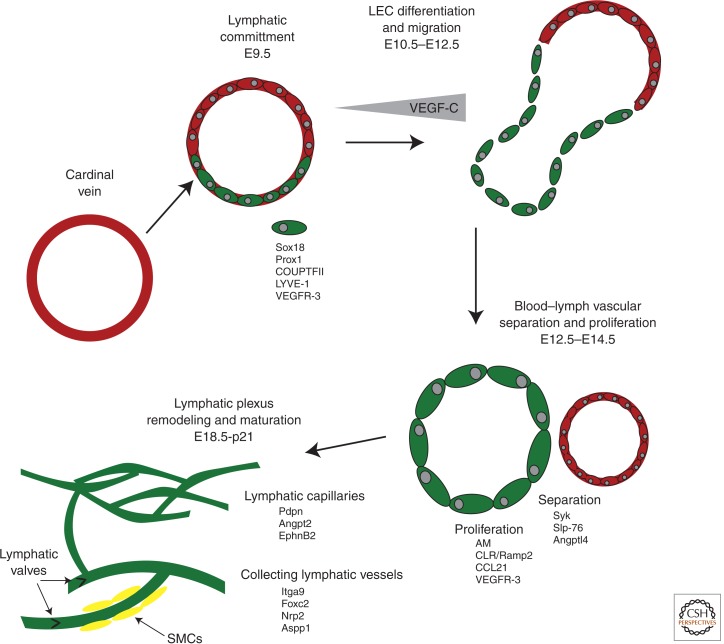
Figure 4.
Stages of lymphangiogenesis. Lymphatic endothelial cells (LECs) are derived from venous endothelial precursors during development. The expression of lymphatic-specific receptors, like VEGFR-3, allows for LEC to migrate and separate from veins in response to chemotactic guidance cues. Following the establishment of lymphatic sacs, a cohort of growth factors drives the proliferation and remodeling of the early lymphatic plexus from mid-gestation through the early postnatal period. The maturation of large collecting lymphatic vessels, which include valves and lymphatic smooth muscle cells, also occurs during the late gestation and postnatal periods. AM, adrenomedulin; CLR/Ramp2, calcitonin-like receptor/receptor activity modifying protein-2.
3.1. Transcriptional Regulation of Lymphatic Competence and Specification
Venous endothelial cells of the cardinal vein serve as the precursors of lymphatic endothelial cells (LECs). At E9.5 of mouse development, venous endothelial cells of the cardinal vein express the classical adult lymphatic marker, lymphatic vascular endothelial hyaluronan receptor-1 (LYVE-1). However, the specification and commitment of precursor venous endothelial cells to the lymphatic lineage occurs through the stepwise expression of a series of transcription factors, including Sox18, COUPTFII, and Prox1. Although the polarized induction cues have yet to be identified, LYVE-1+ dorsolateral cells of the cardinal vein begin to express SRY-related HMG-box 18 (Sox18), which in turn elicits the expression of prospero-related homeobox domain 1 (Prox1) (Francois et al. 2008). Although the functions of Sox18 can be redundant with other SoxF family members (Sox7 and Sox17) in certain strains of mice (Hosking et al. 2009), the importance of Sox18 in the lymphatic vasculature is underscored by human mutations in SOX18 causing hypotriochosis–lymphdedema–telangiectasia, which is a congenital condition characterized by hair loss and lymphedema (Irrthum et al. 2003).
The orphan nuclear receptor chicken ovalbumin upstream transcription factor II (CoupTFII) also plays an important and synergistic role in establishing LEC specification. CoupTFII is coexpressed with Prox1 in developing LECs at E11.5 and in vitro studies have shown that CoupTFII directly binds to Prox1 and participates in the activation of several lymphatic-specific gene promoters, including Prox1 itself (Lee et al. 2009). Although the expression of CoupTFII is not restricted to lymphatic progenitors or LECs, its expression in venous progenitors of the cardinal vein is required for the initiation of Prox1 expression and establishing LEC specification (Srinivasan et al. 2010).
The expression of prospero-related homeobox domain 1 (Prox1) defines LEC identity, because loss of Prox1 expression in mice results in arrested lymphangiogenesis (Wigle and Oliver 1999). Therefore, Prox1 is necessary for lymphangiogenesis and helps to drive the expression of numerous lymphatic-specific genes that transform venous progenitor cells into functional lymphatic endothelial cells. Indeed, the expression of many lymphatic-specific or lymphatic-enriched genes can be directly attributed to their transcriptional induction by Prox1. Moreover, the continued expression of Prox1 in lymphatic endothelial cells of the adult animal is required for the maintenance of these vessels, because conditional deletion of Prox1 in adult animals causes the reversion of lymphatic endothelium to venous endothelium (Johnson et al. 2008). For these reasons, Prox1 is often referred to as the “global regulator” of lymphatic fate because it plays an essential role in the establishment and maintenance of lymphatic endothelial cells. However, it is interesting to note that the functions of Prox1 are not exclusive to lymphatics. Recent studies have shown that Prox1 is required for the development and maintenance of venous valves (Bazigou et al. 2011). In addition, Prox1 also has important roles in the development of the retina and heart (Dyer et al. 2003; Risebro et al. 2009). Therefore, although Prox1 clearly plays central functions in lymphatics, it is most likely that the specialized fate and maintenance of lymphatic endothelial cells is a process that is ultimately governed by several interrelated transcriptional pathways and downstream target genes.
3.2. Lymphatic Endothelial Cell Differentiation and Migration
Once the fate of LECs has been specified by transcriptional regulators, they become capable of responding to external guidance cues and growth factors. The most well-characterized lymphatic growth factor is VEGF-C, which binds to and signals through the lymphatic tyrosine kinase receptor, VEGFR-3 (Flt-4 in mouse)—a downstream target gene of Prox1. It is widely believed that LECs respond to peripheral gradients of VEGF-C to migrate and separate from venous precursor vessels; however, the source and spatial distribution of this VEGF-C gradient has yet to be determined. Nevertheless, LECs in VEGF-C knockout mice are unable to migrate away from veins, despite retaining their lymphatic identity (Karkkainen et al. 2004), and overexpression of VEGF-C in mouse skin causes lymphatic vessel hyperplasia (Jeltsch et al. 1997). Thus, the expression of VEGF-C plays a critical role in enabling nascent LECs to migrate away from their originating vein.
The dorsolateral migration of LECs away from veins is accompanied by their coalescence into primitive lymph sacs—the earliest form of lymphatic vessel, consisting of overlapping endothelial cell–cell junctions, a lumen, and fibrillin-rich anchoring filaments. Because lymphatic vessels lack a basement membrane, these anchoring filaments serve the important functions of tethering lymphatic vessels to the interstitial matrix and of sensing and responding to increased interstitial pressure through an integrin-mediated change in cell–cell junctions.
For many years, the predominating model for early lymph sac formation was that individual LECs migrate away from their precursor vein and then reunite and coalesce into an early lymphatic sac. However, this simple model does not account for the heterogeneous, and yet simultaneous, development of large lymphatic trunks versus superficial dermal lymphatics. More recent studies using ultra-high-resolution techniques have revealed a more complex mechanism of LEC migration from the veins (Francois et al. 2012). Some LECs—those expressing relatively high levels of neuropilin-2 (a VEGF-C coreceptor) and low levels of LYVE-1—migrate away from the precursor vein as a stream of individual cells and ultimately contribute to the formation of superficial lymphatic vessels. In contrast, LECs that express low levels of neuropilin-2 and high LYVE-1 tend to form a sheet of cells that resemble a balloonlike structure adjacent to the vein, and these structures give rise to the larger lymphatic trunks.
In a similar fashion to the requirement of blood flow for normal blood vascular development (described in Section 2), the complete separation of lymphatic sacs from veins occurs between E12.5 and E14.5 in the mouse and is dependent on the formation and signaling of platelet microthrombi. Platelet aggregation and activation at the site of lymph sac separation is mediated by the interaction of podoplanin (expressed on LECs) with its receptor CLEC-2 (expressed on platelets) (Schacht et al. 2003; Bertozzi et al. 2010; Finney et al. 2012). As a consequence of this ligand–receptor interaction, the activation of the tyrosine kinase Syk and the adaptor protein SLP-76 in platelets fulfills the separation of lymphatic sacs from the progenitor vein (Abtahian et al. 2003). Failure of appropriate lymphatic–venous separation during embryogenesis results in blood-filled lymphatic vessels.
Interestingly, the preservation of distinct and separate blood and lymphatic vascular systems is a process that must be maintained postnatally. As discussed above, the persistent expression of Prox1 in adult lymphatics is required for their maintenance as lymphatic endothelial cells (Johnson et al. 2008). Likewise, the expression of Prox1 in a small subset of venous progenitors allows for an intercalation of venous and lymphatic endothelial cells that form the functional connection between the lymphatic and venous circulatory systems at the juncture of the jugular and subclavian veins (Srinivasan and Oliver 2011). As another example, loss of the lymphangiogenic factor angiopoietin-like protein 4 (Angptl4) results in lymphatic–venous shunts in the small intestine of postnatal mice (Backhed et al. 2007). Therefore, it is likely that there exist additional signaling factors that stimulate and maintain the separation of lymphatic vessels from blood vessels, and likewise allow for their connection at the junction of the thoracic duct and subclavian vein.
Additional development and remodeling of the lymphatic vascular system occurs from E14.5 throughout the early postnatal period. Similar to the processes that govern the transition from early blood vasculogenesis to angiogenesis, early lymphatic vessels undergo a remodeling and maturation process that includes robust endothelial cell proliferation and lymphatic sprouting. Some lymphatic vessels must also develop one-way valves and acquire lymphatic smooth muscle cells, distinguishing features of larger collecting lymphatics that are required for their function as drainage conduits. There are numerous signaling molecules that regulate the later stages of lymphangiogenesis. Although most fall within the category of cytokine-like growth factors that signal through receptor tyrosine kinases (like VEGFs, angiopoietins, ephrins, neuropilins, and growth factors) there are also important G protein-coupled receptor signaling molecules that play essential functions in the later stages of lymphangiogenesis (like adrenomedullin, CCL21, and lysophospholipids) (Dunworth and Caron 2009).
4. GENETIC NETWORKS IN BLOOD AND LYMPHATIC VESSEL FORMATION
Most of the signals and pathways that regulate vascular development are also used in multiple developmental programs: FGF, Wnt, Notch, and BMP. In addition, VEGF-A signaling is critical for multiple aspects of vascular development. Lymphangiogenesis also heavily relies on the VEGF pathway, although VEGF-C and VEGFR-3 are the predominant players. Although much is known about the individual functions of these signaling pathways, the challenges for the future are elucidating how these signaling pathways interact with one another within an endothelial cell, and the mechanisms by which different types of endothelial cells respond differently to the same signals.
VEGF-A is a ligand produced in a dynamic expression pattern throughout development by numerous tissues just before and during vascularization, and it is a major regulator of vascular development in all vertebrates. Genetic ablation of a single allele of the ligand, and homozygous deletion of either of two receptor tyrosine kinases (VEGFR-1 and Flt-1 in the mouse; VEGFR-2 and flk-1 in the mouse) is embryonic lethal with vascular defects (Fong et al. 1995; Shalaby et al. 1995; Carmeliet et al. 1996; Ferrara et al. 1996). Alternative splicing produces three major VEGF-A isoforms with differing affinity for extracellular matrix, and the secretion of these from target tissues is thought to help set up a gradient of signal that instructs new vessel growth (Ruhrberg et al. 2002; Stalmans et al. 2002); indeed, expression of a single VEGF-A isoform is embryonic or early postnatal lethal in the mouse for two of the three isoforms. VEGF-A binding activates Flk-1, which activates numerous downstream signaling pathways that contribute to proliferation, migration, and survival (Koch et al. 2011). In contrast, Flt-1 acts as a negative regulator of the pathway during development, and alternative splicing of the Flt-1 RNA leads to both a membrane-localized form and a secreted form that binds VEGF-A and inhibits binding to Flk-1 (Kendall and Thomas 1993; Roberts et al. 2004). Interestingly, spatial localization of Flt-1 expression is also important and contributes to network heterogeneity and local sprout guidance (Chappell et al. 2009).
Our understanding of how Wnt and BMP signaling affect vascular development is less complete. Wnt affects both endothelial cell specification and vessel morphogenesis via its canonical signaling pathway (Dejana 2010). A novel connection between several chromatin remodeling complexes and regulation of Wnt signaling in vascular development has recently been established (Griffin et al. 2011; Curtis and Griffin 2012). The noncanonical Wnt pathway has also been implicated in vessel morphogenesis in the developing retina, via regulation of the VEGF receptor Flt-1 (Stefater et al. 2011). BMP is clearly important for vascular development, although exactly how and where it affects blood vessel formation is obscure, and different ligands appear to mediate either positive (BMP2, BMP6) or negative (BMP9) responses (David et al. 2009). A recent study found that BMP2 was necessary and sufficient for development of the venous plexus in zebrafish, but not required in sprouting of the intersegmental vessels from the dorsal aorta (Wiley et al. 2011). BMP2 signaling also positively regulates sprouting and branching of mammalian vessels in vivo and in vitro (DM Wiley, SM Meadows, DC Chong, et al., in prep.). Other studies suggest that Smad signaling that is presumably downstream from BMP can also affect vessel development in complex ways, and the input of different BMP ligands and/or the use of different coreceptors may affect the overall phenotypes (Larrivee et al. 2012; Moya et al. 2012).
Notch is critical to vascular development at several different stages, as it intersects with and integrates numerous signaling inputs. Notch is involved in early arterial identity in both zebrafish and mouse. Several Notch receptors and ligands are specifically expressed by arterial endothelial cells (Mailhos et al. 2001; Chong et al. 2011), suggesting the importance of the Notch pathway in establishing artery versus vein specification within the developing vasculature, and Notch signaling up-regulates artery-specific markers such as ephrinB2 (Lawson et al. 2001; Grego-Bessa et al. 2007; Siekmann and Lawson 2007). Notch is involved in migration of somitic angioblasts to the dorsal aorta in avians, as only Notch-active somitic cells are competent for this migration (Sato et al. 2008). Notch intersects with the VEGF pathway to regulate arterial endothelial cell identity during development and after the onset of blood flow (Lawson et al. 2002; Masumura et al. 2009). Notch signaling is also required in endothelial cells to determine the relative level of VEGF signaling among neighbors, which allows for adoption of a tip cell or stalk cell phenotype—loss of Notch leads to excess tip cells and elevated Notch signaling promotes the stalk cell phenotype (Hellstrom et al. 2007; Jakobsson et al. 2010). In contrast, little is known about Notch–BMP interactions. Both Notch and BMP stimulate expression of ER71, an Ets family transcription factor that stimulates formation of Flk-1-positive mesoderm, giving rise to both blood and vessel progenitors (Lee et al. 2008). In endothelial cells, cell–cell contact activated Notch signaling, and this synergized with BMP signals to activate Herp2 (Hey1), another Notch target, and block endothelial migration (Itoh et al. 2004). Smad 1/5 and the ALK1 BMP/TGFβ receptor also intersect with Notch in complex ways (Larrivee et al. 2012; Moya et al. 2012).
The most well-characterized lymphatic signaling pathway is that of VEGF-C/VEGFR-3 (Flt4). However, VEGF-A and VEGF-D also have established and potent effects in promoting tumor lymphangiogenesis. Because VEGF proteins are expressed broadly, lymphatic endothelial cells must develop ways to distinguish their responsiveness to VEGF ligands from those of veins and arteries. This level of endothelial cell heterogeneity is achieved through selective expression of VEGF receptors VEGFR-2 (Flk1) and VEGFR-3 (Flt4), as well as their coreceptors, the neuropilins (Bahram and Claesson-Welsh 2010). As a consequence, the VEGF ligands can sense, respond, and activate different downstream signaling pathways in lymphatic endothelial cells compared to blood endothelial cells, and this heterogeneity has already been exploited for the therapeutic targeting of the lymphatic vascular system in conditions of lymphedema or tumor metastasis (Wissmann and Detmar 2006; Tervala et al. 2008).
Similarly, the function of the broadly expressed peptide adrenomedullin is preferentially targeted to lymphatic endothelial cells through expression of its receptors. Adrenomedullin peptide binds to a G-protein-coupled receptor, calcitonin-like receptor (CLR), when the receptor is heterodimerized with a chaperone protein called RAMP2. The selectively enhanced expression of CLR and RAMP2 by Prox1 in lymphatic endothelial cells serves to sensitize the lymphatic vasculature to adrenomedullin signaling, compared to the blood vasculature (Fritz-Six et al. 2008). Therefore, like the VEGF signaling pathway, the heterogeneous expression of receptors underlies much of the responsiveness of lymphatic endothelial cells to external growth and guidance cues.
5. COMPELLING QUESTIONS IN BLOOD AND LYMPHATIC VESSEL DEVELOPMENT
5.1. Do Endothelial Cells from Distinct Sources Have Similar or Different Developmental Programs?
We know that, given the right signaling inputs, most mesoderm can give rise to vascular endothelium. However, it is not clear whether endothelial cells differentiate using “common” pathways or tissue-specific pathways. It is likely that both pathways will be important. For example, VEGF-A signaling is important for vascular differentiation at most embryonic sites, and thus it may be a “common” differentiation pathway, whereas BMP signaling seems important in some but not all blood vessel development. This question also relates to the next question.
5.2. Do Endothelial Cells from Different Sources Have Different Potential and/or Functions?
The simple answer to this question may be “no,” because endothelial cells from most tissues/organs, when transplanted, can adopt the functional attributes of the vessel bed at the transplantation site, suggesting that environmental cues are dominant over “source” for organ-specific attributes such as fenestration. However, there are likely to be exceptions, especially with regard to the potential of endothelial cells, because that attribute is difficult to assess rigorously. For example, there is evidence that only the endothelial cells on the floor of the dorsal aorta have hemogenic potential, and one idea is that these endothelial cells derive from a hemangioblast intermediate, and as a result of this lineage have a potential distinct from their neighbors that derives from a different mesodermal layer. This concept, however, remains to be rigorously tested. Any differences in potential and/or function attributed to lineage may be influenced by epigenetic changes in the genome, and we have barely begun to understand how this regulatory system impacts vascular development.
5.3. What Are the Factors that Control Lymphatic Vessel Heterogeneity?
Continuing on the concept discussed above, we have developed a fairly sophisticated understanding of the genetic program and environmental cues that govern heterogeneity of blood endothelial cells throughout different vascular beds. However, we have barely begun to broach this question with regard to lymphatic vessels. A confounding issue to tackling this question for lymphatic endothelial cells is that lymphatic vessels participate in a wide variety of biological functions that range from lipid absorption in the gut to immune cell trafficking and maturation in lymph nodes, in addition to their “classical” role of mediating interstitial fluid absorption and homeostasis. Therefore, in many ways, the lymphatic vascular system has adopted additional important physiological functions that are likely derived from their anatomical position and environmental cues. However, the factors, both genetic and environmental, that program lymphatic endothelial cells to regulate these diverse functions remain unknown.
5.4. What Is the Role of Stem/Progenitor Cells in Vascular Development?
This is a controversial concept in the adult, and the existence and definition of endothelial progenitor cells has been debated vigorously. In the developmental context, there is no strong evidence that stem/progenitor cells play a major role, other than the hemangioblast. However, congenital vascular tumors called infantile hemangiomas are thought to arise clonally from a stem or progenitor cell, suggesting that these entities exist normally during development and are coopted in diseases (Boscolo and Bischoff 2009). However, it is also possible that non-progenitor cells acquire the ability to expand clonally. A congenital malformation results from a specific genetic mutation in Tie-2, and this mutation was found in several distinct lesions in one patient, suggesting that a circulating progenitor cell was the mutational target (Limaye et al. 2009). Finally, the intricate cross talk between endothelial cells and mural cells such as pericytes that stabilizes vessels has been hypothesized to hold the progenitor potential of pericytes in “suspended animation,” and disease and/or injury may release this block and allow pericytes to show progenitor cell potential (Bianco 2011). It will be exciting to see how these and other questions will be answered in the future, and how their answers help us both better understand vascular development and how to combat diseases linked to development of blood and lymphatic vessels.
ACKNOWLEDGMENTS
We thank Diana Chong and Scott Espenschied for help with artwork, and we apologize to colleagues whose work was not cited owing to space constraints. This work was supported by NIH-NHLBI grants (43174 and 86564 to V.L.B. and 91973 to K.M.C.), a UNC Lineberger Comprehensive Cancer Center Innovation grant (V.L.B.), and a Career Award in Biomedical Sciences from the Burroughs Wellcome Fund (K.M.C.).
Footnotes
Editors: Patrick P.L. Tam, W. James Nelson, and Janet Rossant
Additional Perspectives on Mammalian Development available at www.cshperspectives.org
REFERENCES
- Abtahian R, Guerriero A, Sebzda E, Lu M, Zhou R, Mocsai A, Myers E, Huang B, Jackson D, Ferrari V, et al. 2003. Regulation of blood and lymphatic vascular separation by signaling proteins SLP-76 and Syk. Science 299: 247–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambler CA, Nowicki JL, Burke AC, Bautch VL 2001. Assembly of trunk and limb blood vessels involves extensive migration and vasculogenesis of somite-derived angioblasts. Dev Biol 234: 352–364. [DOI] [PubMed] [Google Scholar]
- Backhed F, Crawford PA, O’Donnell D, Gordon JI 2007. Postnatal lymphatic partitioning from the blood vasculature in the small intestine requires fasting-induced adipose factor. Proc Natl Acad Sci 104: 606–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahram F, Claesson-Welsh L 2010. VEGF-mediated signal transduction in lymphatic endothelial cells. Pathophysiology 17: 253–261. [DOI] [PubMed] [Google Scholar]
- Bautch VL 2011. Stem cells and the vasculature. Nat Med 17: 1437–1443. [DOI] [PubMed] [Google Scholar]
- Bautch VL, James JM 2009. Neurovascular development: The beginning of a beautiful friendship. Cell Adh Migr 3: 199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazigou E, Lyons OTA, Smith A, Venn GE, Cope C, Brown NA, Makinen T 2011. Genes regulating lymphangiogenesis control venous valve formation and maintenance in mice. J Clin Invest 121: 2984–2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertozzi CC, Schmaier AA, Mericko P, Hess PR, Zou Z, Chen M, Chen C-Y, Xu B, Lu M-m, Zhou D, et al. 2010. Platelets regulate lymphatic vascular development through CLEC-2-SLP-76 signaling. Blood 116: 661–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand JY, Chi NC, Santoso B, Teng S, Stainier DYR, Traver D 2010. Haematopoietic stem cells derive directly from aortic endothelium during development. Nature 464: 108–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco P 2011. Back to the future: Moving beyond “mesenchymal stem cells.” J Cell Biochem 112: 1713–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum Y, Belting H-G, Ellertsdottir E, Herwig L, Loders F, Affolter M 2008. Complex cell rearrangements during intersegmental vessel sprouting and vessel fusion in the zebrafish embryo. Dev Biol 316: 312–322. [DOI] [PubMed] [Google Scholar]
- Boisset J-C, van Cappellen W, Andrieu-Soler C, Galjart N, Dzierzak E, Robin C 2010. In vivo imaging of haematopoietic cells emerging from the mouse aortic endothelium. Nature 464: 116–120. [DOI] [PubMed] [Google Scholar]
- Boscolo E, Bischoff J 2009. Vasculogenesis in infantile hemangioma. Angiogenesis 12: 197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, et al. 1996. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature 380: 435–439. [DOI] [PubMed] [Google Scholar]
- Chappell JC, Taylor SM, Ferrara N, Bautch VL 2009. Local guidance of emerging vessel sprouts requires soluble Flt-1. Dev Cell 17: 377–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C-Y, Bertozzi C, Zou Z, Yuan L, Lee JS, Lu M, Stachelek SJ, Srinivasan S, Guo L, Vincente A, et al. 2012. Blood flow reprograms lymphatic vessels to blood vessels. J Clin Invest 122: 2006–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi K, Kennedy M, Kazarov A, Papadimitriou J, Keller G 1998. A common precursor for hematopoietic and endothelial cells. Development 125: 725–732. [DOI] [PubMed] [Google Scholar]
- Chong DC, Koo Y, Xu K, Fu S, Cleaver O 2011. Stepwise arteriovenous fate acquisition during mammalian vasculogenesis. Dev Dyn 240: 2153–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coultas L, Chawengsaksophak K, Rossant J 2005. Endothelial cells and VEGF in vascular development. Nature 438: 937–945. [DOI] [PubMed] [Google Scholar]
- Culver JC, Dickinson ME 2010. The effects of hemodynamic force on embryonic development. Microcirculation 17: 164–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis CD, Griffin CT 2012. The chromatin-remodeling enzymes BRG1 and CHD4 antagonistically regulate vascular Wnt signaling. Mol Cell Biol 32: 1312–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damert A, Miquerol L, Gertsenstein M, Risau W, Nagy A 2002. Insufficient VEGFA activity in yolk sac endoderm compromises haematopoietic and endothelial differentiation. Development 129: 1881–1892. [DOI] [PubMed] [Google Scholar]
- David L, Feige J-J, Bailly S 2009. Emerging role of bone morphogenetic proteins in angiogenesis. Cytokine Growth Factor Rev 20: 203–212. [DOI] [PubMed] [Google Scholar]
- de Bruijn MFTR, Ma X, Robin C, Ottersbach K, Sanchez M-J, Dzierzak E 2002. Hematopoietic stem cells localize to the endothelial cell layer in the midgestation mouse aorta. Immunity 16: 673–683. [DOI] [PubMed] [Google Scholar]
- Dejana E 2010. The role of Wnt signaling in physiological and pathological angiogenesis. Circ Res 107: 943–952. [DOI] [PubMed] [Google Scholar]
- De Val S, Chi NC, Meadows SM, Minovitsky S, Anderson JP, Harris IS, Ehlers ML, Agarwal P, Visel A, Xu S-M, et al. 2008. Combinatorial regulation of endothelial gene expression by Ets and Forkhead transcription factors. Cell 135: 1053–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake CJ, Fleming PA 2000. Vasculogenesis in the day 6.5 to 9.5 mouse embryo. Blood 95: 1671–1679. [PubMed] [Google Scholar]
- Dunworth WP, Caron KM 2009. G protein-coupled receptors as potential drug targets for lymphangiogenesis and lymphatic vascular diseases. Arterioscler Thromb Vasc Biol 29: 650–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer MA, Livesey FJ, Cepko CL, Oliver G 2003. Prox1 function controls progenitor cell proliferation and horizontal cell genesis in the mammalian retina. Nat Genet 34: 53–58. [DOI] [PubMed] [Google Scholar]
- Eichmann A, Yuan L, Moyon D, leNoble F, Pardanaud L, Breant C 2005. Vascular development: From precursor cells to branched arterial and venous networks. Int J Dev Biol 49: 259–267. [DOI] [PubMed] [Google Scholar]
- Eilken HM, Nishikawa S-I, Schroeder T 2009. Continuous single-cell imaging of blood generation from haemogenic endothelium. Nature 457: 896–900. [DOI] [PubMed] [Google Scholar]
- Esner M, Meilhac SM, Relaix F, Nicolas J-F, Cossu G, Buckingham ME 2006. Smooth muscle of the dorsal aorta shares a common clonal origin with skeletal muscle of the myotome. Develpment 133: 737–749. [DOI] [PubMed] [Google Scholar]
- Ferkowicz MJ, Yoder MC 2005. Blood island formation: Longstanding observations and modern interpretations. Exp Hematol 33: 1041–1047. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O’Shea KS, Powell- Braxton L, Hillan KJ, Moore MW 1996. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature 380: 439–442. [DOI] [PubMed] [Google Scholar]
- Finney BA, Schweighoffer E, Navarro-Nunez L, Benezech C, Barone F, Hughes CE, Langan SA, Lowe KL, Pollitt AY, Mourao-Sa D, et al. 2012. CLEC-2 and Syk in the megakaryocytic/platelet lineage are essential for development. Blood 119: 1747–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong GH, Rossant J, Gertsenstein M, Breitman ML 1995. Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature 376: 66–70. [DOI] [PubMed] [Google Scholar]
- Francois M, Caprini A, Hosking B, Orsenigo F, Wilhelm D, Browne C, Paavonen K, Karnezis T, Shayan R, Downes M, et al. 2008. Sox18 induces development of the lymphatic vasculature in mice. Nature 456: 643–647. [DOI] [PubMed] [Google Scholar]
- Francois M, Harvey NL, Hogan BM 2011. The transcriptional control of lymphatic vascular development. Physiology 26: 146–155. [DOI] [PubMed] [Google Scholar]
- Francois M, Short K, Secker GA, Combes A, Schwarz Q, Davidson T-L, Smyth I, Hong Y-K, Harvey NL, Koopman P 2012. Segmental territories along the cardinal veins generate lymph sacs via a ballooning mechanism during embryonic lymphangiogenesis in mice. Dev Biol 364: 89–98. [DOI] [PubMed] [Google Scholar]
- Fritz-Six KL, Dunworth WP, Li M, Caron KM 2008. Adrenomedullin signaling is necessary for murine lymphatic vascular development. J Clin Invest 118: 40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruttiger M 2002. Development of the mouse retinal vasculature: Angiogenesis versus vasculogenesis. Invest Ophthalmol Vis Sci 43: 522–527. [PubMed] [Google Scholar]
- Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D, et al. 2003. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol 161: 1163–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grego-Bessa J, Luna-Zurita L, del Monte G, Bolus V, Melgar P, Arandilla A, Garratt AN, Zang H, Mukouyama Y, Chen H, et al. 2007. Notch signaling is essential for ventricular chamber development. Dev Cell 12: 415–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin CT, Curtis CD, Davis RB, Muthukumar V, Magnuson T 2011. The chromatin-remodeling enzyme BRG1 modulates vascular Wnt signaling at two levels. Proc Natl Acad Sci 108: 2282–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil M, Eitenmüller I, Schmitz-Rixen T, Schaper W 2006. Arteriogenesis versus angiogenesis: Similarities and differences. J Cell Mol Med 10: 45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellström M, Phng LK, Hofmann JJ, Wallgard E, Coultas L, Lindblom P, Alva J, Nilsson AK, Karlsson L, Gaiano N, et al. 2007. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature 445: 776–780. [DOI] [PubMed] [Google Scholar]
- Hosking B, Francois M, Wilhelm D, Orsenigo F, Caprini A, Svingen T, Tutt D, Davidson T, Browne C, Dejana E, et al. 2009. Sox7 and Sox17 are strain-specific modifiers of the lymphangiogenic defects caused by Sox18 dysfunction in mice. Development 136: 2385–2391. [DOI] [PubMed] [Google Scholar]
- Huber TL, Kouskoff V, Joerg Fehling H, Palis J, Keller G 2004. Haemangioblast commitment is initiated in the primitive streak of the mouse embryo. Nature 432: 625–630. [DOI] [PubMed] [Google Scholar]
- Irrthum A, Devriendt K, Chitayat D, Matthijs G, Glade C, Steijlen PM, Fryns J-P, Van Steensel MAM, Vikkula M 2003. Mutations in the transcription factor gene SOX18 underlie recessive and dominant forms of hypotrichosis-lymphedema-telangiectasia. Am J Human Genet 72: 1470–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iruela-Arispe ML, Davis GE 2009. Cellular and molecular mechanisms of vascular lumen formation. Dev Cell 16: 222–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh F, Itoh S, Goumans M-J, Valdimarsdottir G, Iso T, Dotto GP, Hamamori Y, Kedes L, Kato M, Dijke Pt 2004. Synergy and antagonism between Notch and BMP receptor signaling pathways in endothelial cells. EMBO J 23: 541–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsson L, Kreuger J, Claesson-Welsh L 2007. Building blood vessels: Stem cell models in vascular biology. J Cell Biol 177: 751–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsson L, Franco CA, Bentley K, Collins RT, Ponsioen B, Aspalter IM, Rosewell I, Busse M, Thurston G, Medvinsky A, et al. 2010. Endothelial cells dynamically compete for the tip cell position during angiogenic sprouting. Nat Cell Biol 12: 943–953. [DOI] [PubMed] [Google Scholar]
- Jeltsch M, Joukov V, Meng X, Lakso M, Rauvala H, Swartz M, Fukumura D, Jain R, Alitalo K 1997. Hyperplasia of lymphatic vessels in VEGF-C transgenic mice. Science 276: 1423–1425. [DOI] [PubMed] [Google Scholar]
- Johnson NC, Dillard ME, Baluk P, McDonald DM, Harvey NL, Frase SL, Oliver G 2008. Lymphatic endothelial cell identity is reversible and its maintenance requires Prox1 activity. Genes Dev 22: 3282–3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamei M, Saunders WB, Bayless KJ, Dye L, Davis GE, Weinstein BM 2006. Endothelial tubes assemble from intracellular vacuoles in vivo. Nature 442: 453–456. [DOI] [PubMed] [Google Scholar]
- Karkkainen MJ, Haiko P, Sainio K, Partanen J, Taipale J, Petrova TV, Jeltsch M, Jackson DG, Talikka M, Rauvala H, et al. 2004. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat Immunol 5: 74–80. [DOI] [PubMed] [Google Scholar]
- Kearney JB, Bautch VL 2003. In vitro differentiation of mouse ES cells: Hematopoietic and vascular development. Methods Enzymol 365: 83–98. [DOI] [PubMed] [Google Scholar]
- Keller G 2005. Embryonic stem cell differentiation: Emergence of a new era in biology and medicine. Genes Dev 19: 1129–1155. [DOI] [PubMed] [Google Scholar]
- Kendall RL, Thomas KA 1993. Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc Natl Acad Sci 90: 10705–10709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissa K, Herbomel P 2010. Blood stem cells emerge from aortic endothelium by a novel type of cell transition. Nature 464: 112–115. [DOI] [PubMed] [Google Scholar]
- Koch S, Tugues S, Li X, Gualandi L, Claesson-Welsh L 2011. Signal transduction by vascular endothelial growth factor receptors. Biochem J 437: 169–183. [DOI] [PubMed] [Google Scholar]
- Lagha M, Brunelli S, Messina G, Cumano A, Kume T, Relaix F, Buckingham ME 2009. Pax3:Foxc2 reciprocal repression in the somite modulates muscular versus vascular cell fate choice in multipotent progenitors. Dev Cell 17: 892–899. [DOI] [PubMed] [Google Scholar]
- Lammerts van Bueren K, Black B 2012. Regulation of endothelial and hematopoietic development by the ETS transcription factor Etv2. Curr Opin Hematol 19: 199–205. [DOI] [PubMed] [Google Scholar]
- Lancrin C, Sroczynska P, Stephenson C, Allen T, Kouskoff V, Lacaud G 2009. The haemangioblast generates haematopoietic cells through a haemogenic endothelium stage. Nature 457: 892–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrivee B, Prahst C, Gordon E, del Toro R, Mathivet T, Duarte A, Simons M, Eichmann A 2012. ALK1 signaling inhibits angiogenesis by cooperating with the Notch pathway. Dev Cell 22: 489–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson ND, Scheer N, Pham VN, Kim C-H, Chitnis AB, Campos-Ortega JA, Weinstein BM 2001. Notch signaling is required for arterial-venous differentiation during embryonic vascular development. Development 128: 3675–3683. [DOI] [PubMed] [Google Scholar]
- Lawson ND, Vogel AM, Weinstein BM 2002. Sonic hedgehog and vascular endothelial growth factor Act upstream of the Notch pathway during arterial endothelial differentiation. Dev Cell 3: 127–136. [DOI] [PubMed] [Google Scholar]
- Lee CY, Bautch VL 2011. Ups and downs of guided vessel sprouting: The role of polarity. Physiology 26: 326–333. [DOI] [PubMed] [Google Scholar]
- Lee D, Park C, Lee H, Lugus JJ, Kim SH, Arentson E, Chung YS, Gomez G, Kyba M, Lin S, et al. 2008. ER71 acts downstream of BMP, Notch, and Wnt signaling in blood and vessel progenitor specification. Cell Stem Cell 2: 497–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Kang J, Yoo J, Ganesan SK, Cook SC, Aguilar B, Ramu S, Lee J, Hong Y-K 2009. Prox1 physically and functionally interacts with COUP-TFII to specify lymphatic endothelial cell fate. Blood 113: 1856–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie JD, Ariza-McNaughton L, Bermange AL, McAdow R, Johnson SL, Lewis J 2007. Endothelial signalling by the Notch ligand delta-like 4 restricts angiogenesis. Development 134: 839–844. [DOI] [PubMed] [Google Scholar]
- Limaye N, Wouters V, Uebelhoer M, Tuominen M, Wirkkala R, Mulliken JB, Eklund L, Boon LM, Vikkula M 2009. Somatic mutations in angiopoietin receptor gene TEK cause solitary and multiple sporadic venous malformations. Nat Genet 41: 118–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailhos C, Modlich U, Lewis J, Harris A, Bicknell R, Ish-Horowicz D 2001. Delta4, an endothelial specific Notch ligand expressed at sites of physiological and tumor angiogenesis. Differentiation 69: 135–144. [DOI] [PubMed] [Google Scholar]
- Masumura T, Yamamoto K, Shimizu N, Obi S, Ando J 2009. Shear stress increases expression of the arterial endothelial marker ephrinB2 in murine ES cells via the VEGF-Notch signaling pathways. Arterioscler Thromb Vasc Biol 29: 2125–2131. [DOI] [PubMed] [Google Scholar]
- Meadows SM, Fletcher PJ, Moran C, Xu K, Neufeld G, Chauvet S, Mann F, Krieg PA, Cleaver O 2012. Integration of repulsive guidance cues generates avascular zones that shape mammalian blood vessels. Circ Res 110: 34–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miquerol L, Gertsenstein M, Harpal K, Rossant J, Nagy A 1999. Multiple developmental roles of VEGF suggested by a LacZ-tagged allele. Dev Biol 212: 307–322. [DOI] [PubMed] [Google Scholar]
- Moya IM, Umans L, Maas E, Pereira PNG, Beets K, Francis A, Sents W, Robertson EJ, Mummery CL, Huylebroeck D, et al. 2012. Stalk cell phenotype depends on integration of Notch and Smad1/5 signaling cascades. Dev Cell 22: 501–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardanaud L, Luton D, Prigent M, Bourcheix L-M, Catala M, Dieterlen-Lievre F 1996. Two distinct endothelial lineages in ontogeny, one of them related to hemopoiesis. Development 122: 1363–1371. [DOI] [PubMed] [Google Scholar]
- Quaegebeur A, Lange C, Carmeliet P 2011. The neurovascular link in health and disease: Molecular mechanisms and therapeutic implications. Neuron 71: 406–424. [DOI] [PubMed] [Google Scholar]
- Risau W 1997. Mechanisms of angiogenesis. Nature 386: 671–674. [DOI] [PubMed] [Google Scholar]
- Risau W, Flamme I 1995. Vasculogenesis. Ann Rev Cell Dev Biol 11: 73–91. [DOI] [PubMed] [Google Scholar]
- Risebro CA, Searles RG, Melville AAD, Ehler E, Jina N, Shah S, Pallas J, Hubank M, Dillard M, Harvey NL, et al. 2009. Prox1 maintains muscle structure and growth in the developing heart. Development 136: 495–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DM, Kearney JB, Johnson JH, Rosenberg MP, Kumar R, Bautch VL 2004. The vascular endothelial growth factor (VEGF) receptor Flt-1 (VEGFR-1) modulates Flk-1 (VEGFR-2) signaling during blood vessel formation. Am J Pathol 164: 1531–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhrberg C, Gerhardt H, Golding M, Watson R, Ioannidou S, Fujisawa H, Betsholtz C, Shima DT 2002. Spatially restricted patterning cues provided by heparin-binding VEGF-A control blood vessel branching morphogenesis. Genes Dev 16: 2684–2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabin F 1902. On the origin of the lymphatic system from the veins and the development of the lymph hearts and thoracic duct in the pig. Am J Anat 1: 367–389. [Google Scholar]
- Sabin FR 1917. Origin and development of the primitive vessels of the chick and of the pig. Contrib Embryol Carnegie Inst 6: 61–124. [Google Scholar]
- Sabin FR 1920. Studies on the origin of the blood vessels and of red blood corpuscles as seen in the living blastoderm of chick during the second day of incubation. Contrib Embryol Carnegie Inst 9: 215–262. [Google Scholar]
- Sato Y, Watanabe T, Saito D, Takahashi T, Yoshida S, Kohyama J, Ohata E, Okano H, Takahashi Y 2008. Notch mediates the segmental specification of angioblasts in somites and their directed migration toward the dorsal aorta in avian embryos. Dev Cell 14: 890–901. [DOI] [PubMed] [Google Scholar]
- Schacht V, Ramirez MI, Hong Y-K, Hirakawa S, Feng D, Harvey N, Williams M, Dvorak AM, Dvorak HF, Oliver G, et al. 2003. T1α/podoplanin deficiency disrupts normal lymphatic vasculature formation and causes lymphedema. EMBO J 22: 3546–3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo S, Fujita H, Nakano A, Kang M, Duarte A, Kume T 2006. The forkhead transcription factors, Foxc1 and Foxc2, are required for arterial specification and lymphatic sprouting during vascular development. Dev Biol 294: 458–470. [DOI] [PubMed] [Google Scholar]
- Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman ML, Schuh AC 1995. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature 376: 62–66. [DOI] [PubMed] [Google Scholar]
- Siekmann AF, Lawson ND 2007. Notch signalling limits angiogenic cell behaviour in developing zebrafish arteries. Nature 445: 781–784. [DOI] [PubMed] [Google Scholar]
- Srinivasan RS, Oliver G 2011. Prox1 dosage controls the number of lymphatic endothelial cell progenitors and the formation of the lymphovenous valves. Genes Dev 25: 2187–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan RS, Geng X, Yang Y, Wang Y, Mukatira S, Studer Ml, Porto MPR, Lagutin O, Oliver G 2010. The nuclear hormone receptor Coup-TFII is required for the initiation and early maintenance of Prox1 expression in lymphatic endothelial cells. Genes Dev 24: 696–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalmans I, Ng Y-S, Rohan R, Fruttiger M, Bouche A, Yuce A, Fujisawa H, Hermans B, Shani M, Jansen S, et al. 2002. Arteriolar and venular patterning in retinas of mice selectively expressing VEGF isoforms. J Clin Invest 109: 327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefater JA III, Lewkowich I, Rao S, Mariggi G, Carpenter AC, Burr AR, Fan J, Ajima R, Molkentin JD, Williams BO, et al. 2011. Regulation of angiogenesis by a non-canonical Wnt-Flt1 pathway in myeloid cells. Nature 474: 511–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strilic B, Kucera T, Eglinger J, Hughes MR, McNagny KM, Tsukita S, Dejana E, Ferrara N, Lammert E 2009. The molecular basis of vascular lumen formation in the developing mouse aorta. Dev Cell 17: 505–515. [DOI] [PubMed] [Google Scholar]
- Swift MR, Weinstein BM 2009. Arterial-venous specification during development. Circ Res 104: 576–588. [DOI] [PubMed] [Google Scholar]
- Tervala T, Suominen E, Saaristo A 2008. Targeted treatment for lymphedema and lymphatic metastasis. Ann NY Acad Sci 1131: 215–224. [DOI] [PubMed] [Google Scholar]
- Tzima E, Irani-Tehrani M, Kiosses WB, Dejana E, Schultz DA, Engelhardt B, Cao G, DeLisser H, Schwartz MA 2005. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature 437: 426–431. [DOI] [PubMed] [Google Scholar]
- Ueno H, Weissman IL 2006. Clonal analysis of mouse development reveals a polyclonal origin for yolk sac blood islands. Dev Cell 11: 519–533. [DOI] [PubMed] [Google Scholar]
- Vogeli KM, Jin S-W, Martin GR, Stainier DYR 2006. A common progenitor for haematopoietic and endothelial lineages in the zebrafish gastrula. Nature 443: 337–339. [DOI] [PubMed] [Google Scholar]
- Wang HU, Chen Z-F, Anderson DJ 1998. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell 93: 741–753. [DOI] [PubMed] [Google Scholar]
- Wigle JT, Oliver G 1999. Prox1 function is required for the development of the murine lymphatic system. Cell 98: 769–778. [DOI] [PubMed] [Google Scholar]
- Wiley DM, Kim JD, Hao JJ, Hong CC, Bautch VL, Jin SW 2011. Distinct signalling pathways regulate sprouting angiogenesis from the dorsal aorta and the axial vein. Nat Cell Biol 13: 686–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wissmann C, Detmar M 2006. Pathways targeting tumor lymphangiogenesis. Clin Cancer Res 12: 6865–6868. [DOI] [PubMed] [Google Scholar]
- Xu K, Sacharidou A, Fu S, Chong DC, Skaug B, Chen ZJ, Davis GE, Cleaver O 2011. Blood vessel tubulogenesis requires Rasip1 regulation of GTPase signaling. Dev Cell 20: 526–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Soonpaa MH, Adler ED, Roepke TK, Kattman SJ, Kennedy M, Henckaerts E, Bonham K, Abbott GW, Linden RM, et al. 2008. Human cardiovascular progenitor cells develop from a KDR+ embryonic- stem-cell-derived population. Nature 453: 524–528. [DOI] [PubMed] [Google Scholar]
- You L-R, Lin F-J, Lee CT, DeMayo FJ, Tsai M-J, Tsai SY 2005. Suppression of Notch signalling by the COUP-TFII transcription factor regulates vein identity. Nature 435: 98–104. [DOI] [PubMed] [Google Scholar]
- Zape JP, Zovein AC 2011. Hemogenic endothelium: Origins, regulation, and implications for vascular biology. Semin Cell Dev 22: 1036–1047. [DOI] [PubMed] [Google Scholar]
- Zovein AC, Hofmann JJ, Lynch M, French WJ, Turlo KA, Yang Y, Becker MS, Zanetta L, Dejana E, Gasson JC, et al. 2008. Fate tracing reveals the endothelial origin of hematopoietic stem cells. Cell Stem Cell 3: 625–636. [DOI] [PMC free article] [PubMed] [Google Scholar]