Abstract
Background
Clinical evidence shows that bipolar disorder (BD) is characterized by white matter (WM) microstructural abnormalities. However, little is known about the biological mechanisms associated with these abnormalities and their relationship with cognitive functioning.
Methods
49 adult BD patients ((M±SD): 29.27 ± 7.92 years; 17 males, 32 females; 34 BD-I, 10 BD-II, and 5 BD-NOS) and 28 age-matched normal subjects ((M±SD): 29.19 ± 7.35 years; 10 males and 18 females) underwent diffusion tensor imaging (DTI) imaging. DTI metrics were computed using whole-brain tract-based spatial statistics (TBSS) as part of the FMRIB Software Library. Measures of WM coherence (fractional anisotropy - FA) and axonal structure (mean, axial and radial diffusivity - MD, AD and RD) were employed to characterize the microstructural alterations in the limbic, commissural, association and projection fiber tracts. All participants performed the Brief Assessment of Cognition for Affective disorders (BAC-A).
Results
BD patients performed poorly on verbal fluency tasks and exhibited large clusters of altered FA, RD and MD values within the retrolenticular part of the internal capsule, the superior and anterior corona radiata, and the corpus callosum. Increased FA values in the left IFOF and the forceps minor correlated positively with verbal fluency scores. Altered RD parameters in the corticospinal tract and the forceps minor were associated with reduced visuomotor abilities.
Conclusions
The reported verbal fluency deficits and FA, RD and MD alterations in WM structures are potential cognitive and neural markers of BD. Abnormal RD values may be associated with progressive demyelination.
Keywords: bipolar disorder, diffusion tensor imaging, cognitive, demyelination, TBSS
Introduction
Bipolar disorder (BD) is a devastating illness with significant functional and social consequences for both affected individuals and their relatives [1, 2]. In BD the alternation of periods of euphoria and periods of depression is accompanied by significant cognitive impairment that persists during the euthymic and acute phases [3-6]. In particular, BD patients have been shown to present with deficits in visual motion perception [7], visuomotor speed, visual and verbal memory, sustained attention [5, 6, 8-11] and conceptual reasoning [12].
Microstructural abnormalities in white matter (WM) fiber tracts connecting to the limbic-striatal, cingulate, thalamus, corpus callosum and prefrontal regions have been observed in children, adolescents and adults with BD [13-20]. The corpus callosum and thalamic radiation of middle-aged and drug-naïve BD populations showed abnormal axial (AD) and radial diffusivity (RD) values [21-23]. Increased RD values have been observed in the cingulum, the superior and inferior uncinated fasciculus, the corpus callosum and the internal capsule of BD I patients when compared to their unaffected relatives and healthy controls [24]. Another study found similar abnormalities in the hippocampus, thalamus and caudate nucleus [25]. The reduction in WM integrity observed in BD has been linked to processes of demyelination, cerebral hypoperfusion, neuroinflammation and reduced mitochondrial metabolism [25, 26] but there is little empirical evidence supporting either of these biological hypotheses.
Diffusion tensor imaging (DTI) is a non-invasive neuroimaging technique that enables visualization of the WM fibers, and characterization of microstructural changes based on the degree of water diffusion at a voxel and regional level. Traditional metrics derived from DTI are fractional anisotropy (FA) and mean, axial and radial diffusivity (MD, AD and RD). FA represents the proportion of water diffusion parallel to the axons compared to that perpendicular to the axons. AD and RD estimate water diffusivity along and across the axons, and MD is a linear combination of AD and RD where MD=(AD+2*RD)/3 = 1/3*AD + 2/3*RD. While a decrease in FA in projection fibers such as the thalamic radiation and the posterior corona radiata has been associated with disruptions in the overall WM organization, FA abnormalities in association tracts (e.g. longitudinal fasciculus) and commissural fibers (corpus callosum) have been linked to poor intra- or interhemispheric connectivity [27]. Further, findings from animal studies indicate that increased AD values may reflect axonal injury, and that RD values increase as a result of demyelination processes [28-30].
To date only a limited number of studies has investigated the relationship between DTI metrics and cognitive functioning in BD. A recent publication found WM alterations in the corpus callosum and the anterior thalamic radiation bilaterally in middle-aged BD I patients when compared to healthy controls [21]. Notably, the authors found a positive correlation between problem solving abilities and FA, MD and RD values in the thalamic radiation and fornix in BD but not in the HC group. In another study middle-aged BD I patients showed decreased FA values in the internal capsule, the right uncinate fasciculus and the corpus callosum along with decreased accuracy in set shifting and risk taking tasks [31]. In adolescents with BD I reduced mean FA values in fiber tracts connecting to fronto-temporal and cingulate regions were associated with psychomotor retardation on the Trail Making Test [32]. These findings show a link between WM abnormalities and reduced visuomotor processing speed and executive functions. It is, however, unclear whether this relationship is specific to BD I or represents a trait marker of the bipolar illness. Further, considering that the brain morphology changes considerably over the lifespan [33-35], it is difficult to compare the results of these studies as they included adolescents and middle-aged adults. Another potential confounding factor is related to the type of cognitive tests employed in previous studies as they may not be suitable for assessing cognition in BD.
Thus, the purpose of this study is to elucidate the relationship between WM integrity and cognitive functioning in young adults by using multiple parameters of WM integrity and the Brief Assessment of Cognition in Affective Disorder (BAC-A) – a well-validated cognitive battery designed specifically for BD [36]. Based on previous findings we expected to find WM abnormalities in the BD group when compared to HC. We also predicted to find positive correlations between indices of WM integrity and memory, visuomotor processing and executive scores, as these cognitive domains have been found to be impaired in BD.
Materials and methods
Participants
The sample included 49 adult BD patients (M±SD: 29.10±7.86 years; 19 males, 30 females; 34 BD-I, 10 BD-II, and 5 BD-NOS) and 28 age-matched healthy controls (HC) (M±SD: 29.03±7.34 years; 9 males and 19 females). Patients were recruited from inpatient and outpatient clinics of the University of North Carolina at Chapel Hill (UNC). HC were recruited through local media advertisements and flyers posted in public areas. All patients met the DSM-IV-R criteria for BD. The diagnosis of BD among patients and the absence of mental disorders among controls were ascertained by the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders Axis I (SCID I) [37], which was administered to all participants by an independent psychiatrist or trained research assistant. The interview also included the Montgomery–Åsberg Depression Rating Scale (MADRS) [38] and the Young mania Rating Scale (YMRS) [39]. Functional impairment was evaluated using the Global Assessment of Functioning (GAF) which is Axis V of the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition Text Revision (DSM-IV-TR) [40]. Participating BD patients and HC were aged between 18 and 48 years, had no history of substance abuse in the previous 6 months and no current medical problems. 41 of the 49 BD participants took psychotropic medication at the time of enrollment. HC with a history of any Axis I disorder in first-degree relatives and use of psychoactive medication less than 2 weeks prior to the start of the study were excluded. All female participants underwent a urine pregnancy test and urine drug screen to exclude pregnancy and illegal drug use. Subjects suffering from chronic medical issues including cardiovascular and neurological disorders were excluded. The study protocol was approved by the local Institutional Review board and informed consent was obtained from all the participants.
Imaging data acquisition and image processing
All imaging was performed on a 3T Siemens Allegra scanner at the UNC imaging facility. Whole-brain diffusion-weighted images were acquired using a spin echo-planar imaging protocol. Image acquisition parameters included: repetition time=9200 ms, echo time=79 ms, slice thickness=2mm, imaging matrix=128×104, voxel size= 2mm, b-value =1000 sec/mm2. Two images without gradient loading (b-value =0 s/mm2) were acquired prior to the acquisition of 30 directions (each containing 80 slices) with uniform gradient loading (b0 = 1000 s/mm2). To correct for eddy currents we used the first b0 image as a template. In addition to diffusion-weighted images we also acquired T1-weighted structural images for the purpose of anatomical localization.
DTI processing
The FMRIB's Diffusion Toolbox (FDT) within FSL (http://www.fmrib.ox.ac.uk/fsl) was used to preprocess diffusion weighted images and correct for eddy current distortions. FA maps of both BD and control subjects were affine and then non-linearly registered to an MNI template. FA images were created by fitting a tensor model to the raw diffusion data using the DTIFIT Reconstruct Diffusion Tensor tool. Brain Extraction Tool (BET) was used to remove non-brain tissue from images of the brain with a fractional intensity threshold of 0.3. DTIfit was then used to fit the diffusion tensor to each voxel thus creating voxel-wise maps of FA, AD, RD and MD.
Voxelwise statistical analysis of data was carried out using Tract-Based Spatial Statistics (TBSS) within FSL. All subject's FA data were affine-registered to MNI152 space using the FSL nonlinear registration tool FNIRT. The mean FA image was created and optimized to create a mean FA skeleton to represent common tracts among all individuals. Individual subjects' FA data was projected onto this skeleton. Voxelwise statistics across subjects (adjusted for gender and age) were carried out on each point of the FA skeleton using permutation-based non-parametric testing (RANDOMISE - as implemented in FSL), using 5000 permutations [40] to compare differences between BD patients and HC. A false discovery rate (FDR) correction was used to correct the threshold for multiple comparisons and the minimum threshold of statistical significance was set at p≤0.01. Based on the results of the voxel-wise analyses, we superimposed significant clusters containing ≥ 5 voxels on the Johns Hopkins University (JHU)-ICBM-DTI-81 WM labels atlas and extracted FA, RD, MD and AD values for each participant.
Cognitive performance
All participants were administered the Wechsler Abbreviated Scale of Intelligence (WASI) a screener of verbal, non-verbal, and general cognitive ability [41] and the reading test of the Wide Range Achievement Test-4 (WRAT-4), which is a measure of premorbid intellectual quotient (IQ) [42]. Cognitive functioning was assessed by the Brief Assessment of Cognition in Affective Disorders (BAC-A) [36]. The BAC-A has been found to have strong psychometric properties in patients with BD Type I compared to HC and takes approximately 35 minutes to administer [36]. It assesses psychomotor speed, memory, attention and executive functions and comprises the following tests: Token Motor, Symbol Coding, List Learning, Digit Sequencing, the Category Instances/Controlled Oral Word Association Test and the Tower of London [43]. In addition to these 6 subtests the BAC-A includes the Emotional Stroop task [44] and the Affective interference test - a n affective auditory verbal learning test similar in structure to the Auditory Verbal Learning Test (AAVLT) [45].
Emotional Stroop test
Participants are presented with sheets of papers with 4 columns of words of either neutral or affective polarity in colored (red, blue, green and yellow) or blank ink. They are then instructed to either read the words (Word naming) or the color of the words (Color naming) going down the columns. Participants are given 30 seconds to read as many words as they can on each page. The goal of this task is to determine the individual's ability to suppress irrelevant stimuli and read a word whose meaning identifies a different color from the color of presentation of the word (Interference). The Interference index is calculated by subtracting the number of correct responses to emotional words during the Color naming condition from the number of correct responses to emotional words during the Word naming condition. This index was adjusted for the number of correctly identified neutral words during the “Word” and “Color naming” conditions. The following formula was used: (Emotional Word Naming divided by Word Naming) minus (Emotional Color Naming divided by Color naming).
Affective interference
In this task participants are given three trials to learn 10 non-affective words (fruits and vegetables) and 10 affective words (.e.g. “cancer,” “triumphant,” “enraged”). After a 20-minute delay, recognition memory is tested by presenting the initial 20 words (10 emotional and 10 fruits and vegetables) along with 20 words that had not been presented earlier. This task evaluates components of short-term and delayed affective and non-affective memory.
The scores on the BAC-A were summarized in eight cognitive domains: visuomotor (number of correct responses on the Symbol coding and Token Motor tasks), short-term affective memory (number of correctly recalled words during the affective learning trials of the Affective Interference test), short-term non-affective memory (number of correct words during the non-affective learning trials of the List Learning and Affective Interference tests and number of correct answers on the Digit Sequencing task), delayed affective memory (number of correct words during the delayed free recall of affective words of the Affective Interference test), delayed non-affective memory (number of correct words during the delayed free recall of non-affective words of the Affective Interference test), fluency (number of correct responses on the Category and Controlled Oral Word Association tests), inhibition (Interference index of the Stroop task) and problem solving (number of correct responses on the Tower of London test).
Statistical analyses
Statistical analyses were performed using IBM SPSS statistics (Version 21.0). Normality of each variable was investigated. Where appropriate, outliers were winsorised. Demographic, clinical and cognitive differences between groups were assessed with χ2-square and multivariate analyses of variance (MANOVA). Age and the WRAT-4 Reading score were entered as covariates in analyses on the BAC-A cognitive summary scores. Pearson's correlation coefficients were measured to explore the relationship between cognitive functioning and DTI metrics in the BD sample. Individuals with a MADRS score ≥20 (marker of moderate to severe depression) were excluded from the correlational analyses. This is based on previous evidence suggesting that measures of cognitive functioning collected during the severe phase of depression are not reliable indicators of individuals' mental abilities [46]. Demographic and cognitive results were considered to be statistically significant at a Bonferroni-adjusted p-value <.05.
Results
Group characteristics
Demographics and clinical features for BD and HC are reported in Table 1. There was no significant difference in age and gender between the two groups. Pre-morbid IQ (estimated by the reading score of the WRAT-4) was significantly reduced in BD patients compared to HC. The mean MADRS score of our BD sample was 16.82 (SD: 12.33) which is an indicator of mild to moderate depression. However, 15 subjects had a score ≥ 20, a marker of moderate to severe depression.
Table 1. Demographic and clinical characteristics of the sample.
| Group | N (BD/HC) | Bipolar disorder | Healthy Controls | p/χ2 |
|---|---|---|---|---|
| Mean (SD) | ||||
| Gender Male/female, N | 49/28 | 19/30 | 9/19 | .37 |
| Age (years) | 49/28 | 29.10(7.86) | 29.03(7.34) | .97 |
| WRAT Reading | 42/18 | 104.57(15.18) | 118.94(16.89) | .002 |
| WASI Vocabulary | 45/27 | 57.15(12.84) | 59.59(11.5) | .42 |
| WASI Matrix | 45/26 | 54.13(7.50) | 55.73(6.56) | .37 |
| WASI Full Scale IQ | 45/26 | 110.44(14.093) | 114.46(13.27) | .24 |
| YMRS | 45/27 | 6 (5.68) | .40(.63) | .00 |
| MADRS | 35/16 | 16.82(12.33) | 0 | .00 |
| GAF | 37/15 | 60.32(12.59) | 92.27(4.301) | .00 |
| Age of onset of mood disorders (years) | 41/0 | 20.85(6.87) | - | - |
| Lifetime number of manic episodes | 46/28 | 7.108 (11.83) | - | - |
| Lifetime number of depressive episodes | 43/28 | 20 (34.02) | - | - |
Abbreviations: GAF: Global Assessment of Functioning, MADRS: Montgomery–Åsberg Depression Rating Scale, YMRS: Young Mania Rating Scale, WASI: Wechsler Abbreviated Scale of Intelligence, WRAT: Wide Range Achievement Test.
DTI metrics
As illustrated in Figures 1 and 2, BD patients exhibited reduced FA and increased RD and MD values in all major WM tracts. The largest clusters (≥ 10 voxels) with abnormal FA values were located within the right corticospinal tract, the left superior longitudinal fasciculus (L-SLF), the left inferior fronto-occipital fasciculus (IFOF) and the forceps minor. Altered RD values were found within the right corticospinal tract and the IFOF bilaterally.
Figure 1.
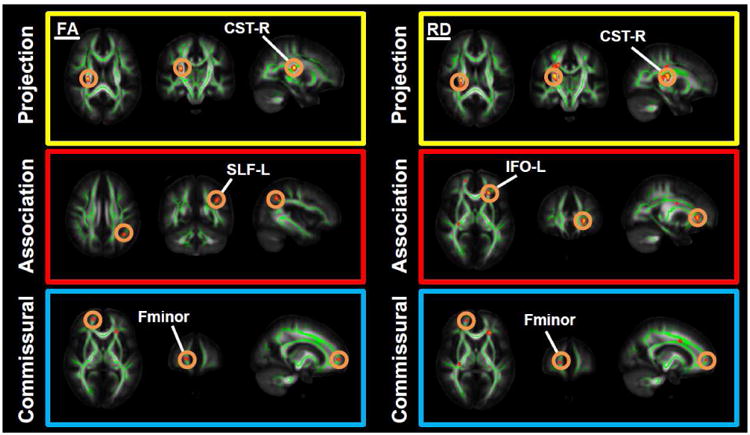
Results from TBSS analyses showing differences in fractional anisotropy (FA) and radial diffusivity (RD) in bipolar patients (BD) versus healthy controls (HC). Voxels are superimposed on the white matter skeleton (green). The background images are MNI152 template (MNI - Montreal Neurological Institute). Yellow-red clusters represent abnormalities in FA and RD in commissural, association and projection fibers (FDR-corrected p<0.01). Abbreviations: CST-R: right corticospinal tract, SLF-L: left superior longitudinal fasciculus, IFO-L: left inferior fronto-occipital fasciculus, Fminor: forceps minor.
Figure 2.
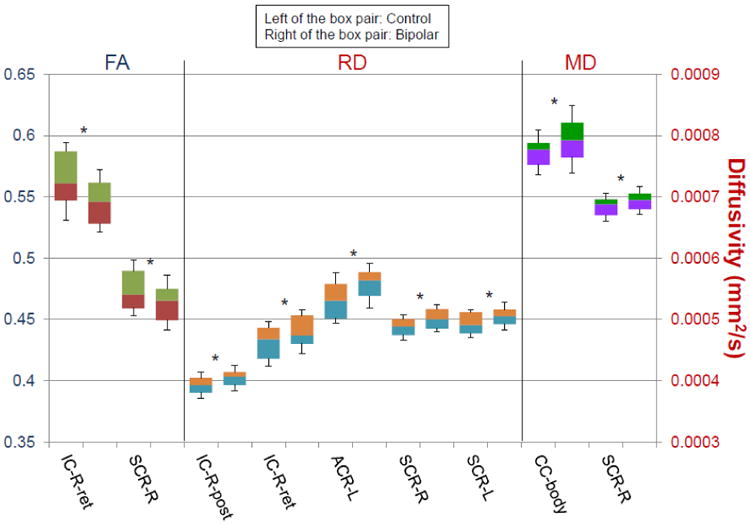
Mean fractional anisotropy (FA), radial diffusivity (RD), and mean diffusivity (MD) values in the retrolenticular part of the internal capsule (IC-ret), the posterior limb of internal capsule (IC-post), the superior corona radiata (SCR), the anterior corona radiata (ACR), and the body of the corpus callosum (CC-body) of BD patients (right of the box pair) and HC (left of the box pair). Tracts are labeled with reference to the JHU ICBM-DTI-81 white-matter labels atlas. L and R denote the left and right hemispheres.*FDR-corrected p<.05.
By superimposing these results on the JHU-ICBM-DTI-81 atlas clusters with reduced FA were found to be located in the right retrolenticular portion of the internal capsule and the right superior corona (see Table 1S). Clusters with increased RD were situated in the right posterior limb and the right retrolenticular portion of the internal capsule, the left anterior corona radiata, and the superior corona radiata bilaterally (Table 2S). Increased MD values were found in the body of the corpus callosum and the right superior corona radiata (Table 3S).
Cognitive performance
Multivariate analyses showed a significantly reduced verbal fluency performance in BD patients [F(1,53)=7.19, p=.01, η2=.12]. Secondary analyses showed that BD patients generated a lower number of S-words [F(1,56)=6.09, p=.017, η2=.09] and names of animals [F(1,56)=6.61, p=.013, η2=.106] compared to HC (Figure 3).
Figure 3.
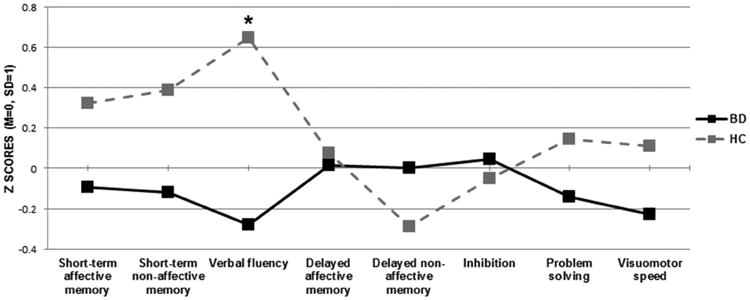
Mean standardized scores of the eight cognitive summary scores of the BAC-A in BD patients and HC; Inhibition refers to the interference index of the Emotional Stroop test; *Bonferroni-corrected p<.05.
Cognitive performance and DTI metrics in BD
In the BD sample affective and non-affective short-term memory scores correlated negatively with the RD values in the left superior corona radiata (r=-55, p=.017 STM; r=-49, p=.04 STNM). The MD values in the right corticospinal tract were inversely related to short-term affective and non-affective memory (r=-.52, p=.02; r=-.52, p=.026), and delayed affective memory (r=.49, p=.04) (Figure 4S).
Discussion
The purpose of the current study was to examine WM integrity and its relationship with cognitive functioning in a group of young adults with BD. We performed unbiased whole-brain TBSS analyses and found that clusters with reduced FA and altered (increased) RD and MD values were predominantly located in the retrolenticular part of the internal capsule, the superior and anterior corona radiata, and the corpus callosum. These DTI abnormalities are consistent with previous results in BD [47] and provide valuable insight into the relationship between the integrity of WM pathways and the deficits in cognitive processing observed in BD. Indeed the internal capsula and the corona radiata are important WM nodes that facilitate the transfer of sensorimotor and cognitive information between the brain stem, the thalamus, and the fronto-striatal circuit [20, 48]. Further, the corpus callosum mediates the transfer of interhemispheric information, which is crucial for higher order cognitive functions, and exhibits morphological abnormalities in mood disorders [49, 50].
Increased FA values in WM fibers connecting to the frontolimbic and cingulate regions have consistently been reported in previous DTI studies in BD [47, 51, 52]. By contrast, only a small number of studies has examined markers of water diffusivity such as RD and AD. These parameters are important as they provide information on the nature of the microstructural changes observed in BD [22, 51]. Animal models of WM injury show that elevated RD and MD values reflect a surge in brain water mobility and possibly reduced integrity of the axonal wall [53]. Other studies indicate that increased RD values are associated with myelin dysfunction [28-30]. Further, findings from postmortem brain studies in BD patients are consistent with the hypothesis that glial dysfunction and a reduced number of myelinating cells (e.g. oligodendrocytes) contribute to the pathophysiology of BD [54]. In addition, myelin genes have been found to be underexpressed in BD patients [55, 56]. Thus, the reported alterations in RD and MD values may be due to processes of myelin degeneration [57].
In this study cognitive functioning was assessed using the BAC-A, a comprehensive and well-validated cognitive battery for BD [36, 58]. Compared to HC, BD patients performed poorly on tests of verbal fluency and generated a reduced number of S- and animal words. Furthermore, in BD patients with a MADRS score < 20 reduced performance in affective and non-affective short-term memory tasks was associated with altered RD and MD values within the right superior corona radiata and corticospinal tract. Verbal fluency impairment has been previously reported in the literature on BD [5, 6, 10, 59-65] and has been linked to executive dysfunction [66], inefficient information retrieval techniques [67] and poor verbal skills [59, 60]. Given the participants' preserved vocabulary scores on the WASI, the present results suggest that poor planning and/or word retrieval skills may be potential explanations for the impaired verbal fluency skills observed in our BD sample. Further, the significant correlations between short-term memory and DTI metrics provide preliminary evidence for a potential association between white matter integrity and cognitive functioning in BD. In particular, the corticospinal tract is involved in semantic processing [68], an important mechanism underlying high order cognitive functions typically impaired in BD such as memory [6].
One of the strengths of the current study is the heterogeneity of the sample in terms of BD subtype as previous studies restricted their analyses to type I BD. Thus the reported changes in WM integrity and related cognitive deficits could be considered as being markers for BD. Furthermore, in this study cognitive functioning was assessed using the BAC-A, a comprehensive cognitive battery that has been recently validated in a sample of middle-aged depressed BD patients [36]. A potential confounder is that the majority of BD participants took one or more psychotropic medications. Psychotropic medications have been associated with volumetric changes in the fronto-limbic and hippocampal regions [69-71]. However their effects on the WM structure remain unexplored. It is noteworthy that we found significant differences in WRAT-4 scores, but not in WASI, between BD and HC. A possible explanation for the divergent results could be related to the smaller number of data points available for the WRAT-4 (42 BD /18 HC) compared to the WASI (45 BD/26 HC). In other words, it is possible that a larger number of WRAT scores in the HC group would have led to a less pronounced mean difference between HC and BD. Although our BD sample did not include rapid cyclers, at least 15 participants reported experiencing more than 30 manic or depressive episodes (up to a maximum of 100). This is the primary reason for the high mean lifetime number of mood episodes observed in Table 1 (20±34.02 for depressive episodes and 7.108±11.83 for manic episodes). However, given that these figures are based on self-report these findings should be interpreted with great caution and rather be viewed as “rough” estimates of illness chronicity in our sample.
It is noteworthy to mention that we chose the FDR correction over the more traditional threshold-free cluster enhancement (TFCE) because the TFCE correction would have required two optimization parameters to give proper weights to clusters depending on their height and spatial extent [70]. This cannot be known prior to analysis, thus different data may potentially require different parameters. To address this issue we used the FDR correction – a conventional correction method used in previous DTI studies [72, 73]. FDR clustering method may result in false positives located away from the cluster's “true” center. Despite this potential spatial error we were able to associate tracts to their cluster locations.
In summary, our findings provide evidence of the association between aberrant WM fiber integrity and cognitive deficits in young adults with BD. The reported impairment in verbal fluency and changes in WM integrity in fiber tracts connecting to the internal capsula, the corona radiata and the corpus callosum may serve as cognitive and neural markers of BD. The abnormalities in FA and RD observed in these regions could be related to mechanisms of progressive demyelination.
Supplementary Material
Table 1S. Fractional anisotropy (FA) differences in healthy controls (HC) versus bipolar (BD) patients - HC > BD. Data are presented as means and standard deviations; p-values are FDR-corrected. Labels are based on Johns Hopkins University (JHU)-ICBM-DTI-81 WM atlas.
Table 2S. Radial Diffusivity (RD) differences in bipolar (BD) patients vs healthy controls (HC), BD>HC. Data are presented as means and standard deviations in 1×10-3 mm2/s; p-values are FDR-corrected. Labels are based on Johns Hopkins University (JHU)-ICBM-DTI-81 WM atlas.
Table 3S. Fractional anisotropy (FA), radial diffusivity (RD) and mean diffusivity (MD) values in healthy controls (HC) vs bipolar (BD) patients. Tracts are labeled with reference to the JHU ICBM-DTI-81 white-matter labels atlas in the right (R) and left (L) hemisphere (FA: HC > BD; MD,RD: BD>HC). Data are presented as means and standard deviations. Unit of measure for RD and MD is 1×10-3 mm2/s. p-values are FWE-corrected. Labels are based on Johns Hopkins University (JHU)-ICBM-DTI-81 WM atlas.
Table 4S. Pearson's correlation coefficients among DTI metrics and cognitive summary scores. Abbreviations: DTI metrics: CST-R = right corticospinal tract, FM = forceps minor, IFOF-L = left inferior fronto-occipital fasciculus, Inhibition = Interference index of the Emotional Stroop test, PS = problem solving, SLF-L = left superior longitudinal fasciculus; SCR-R = right superior corona radiata. Cognitive summary scores: STAM = short-term affective memory, STNM = short-term non-affective memory, DAM = delayed affective memory, DNM = delayed non-affective memory, PS = problem solving, VM = visuomotor skills in bipolar disorder (BD) and healthy controls (HC). Significant correlations are denoted with asterisks at significance level *p< 0.05, **p< 0.01. N=67.
Highlights.
Tract-based spatial statistics for the whole-brain estimation of DTI parameters.
BD patients displayed abnormal FA, RD and MD values in major white matter tracts.
Cognitive functioning was assessed with the BAC-A.
BD patients exhibited verbal fluency deficits.
Abnormal DTI values were associated with reduced cognitive performance.
Acknowledgments
Funding: Dr. Sanches has served on the speakers' bureau for Astra Zeneca and has received research support from Janssen.
Dr. Richard Keefe currently or in the past 3 years has received investigator-initiated research funding support from the Brain Plasticity, Inc., Department of Veteran's Affair, Feinstein Institute for Medical Research, GlaxoSmithKline, National Institute of Mental Health, Novartis, PsychoGenics, Research Foundation for Mental Hygiene, Inc., and the Singapore National Medical Research Council. He currently or in the past 3 years has received honoraria, served as a consultant, or advisory board member for Abbvie, Akebia, Amgen, Astellas, Asubio, AviNeuro/ChemRar, BiolineRx, Biogen Idec, Biomarin, Boehringer-Ingelheim, Eli Lilly, EnVivo, GW Pharmaceuticals, Helicon, Lundbeck, Merck, Minerva Neurosciences, Inc., Mitsubishi, Novartis, Otsuka, Pfizer, Roche, Shire, Sunovion, Takeda, Targacept. Dr. Keefe receives royalties from the BACS and BAC-A testing batteries and the MATRICS Battery (Symbol Coding). He is also a shareholder in NeuroCog Trials, Inc. and Sengenix.
Dr J. C. Soares has received grants/research support from Forrest, BMS, Merck, Elan, Stanley Medical Research Institute, NIH and has been a speaker for Pfizer and Abbott. This work was supported by NIH grant 1R01MH085667 and Pat Rutherford, Jr Chair in Psychiatry at UTHealth.
Footnotes
Disclosure: Drs Bauer, Ouyang, Mwangi, Zunta-Soares, and Huang have no conflicts of interest
Contributors: IB wrote the first draft of the manuscript; RK, MS, GZS and JCS designed the study and managed data collection; IB, BM, AO, HH analysed the data; all the authors revised and approved the final version of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Isabelle E. Bauer, University of Texas Health Science Center at Houston, Department of Psychiatry and Behavioral Sciences, 77054 Houston, TX, United States
Austin Ouyang, Advanced Imaging Research Center, University of Texas Southwestern Medical Center, Dallas, TX, United States.
Benson Mwangi, University of Texas Health Science Center at Houston, Department of Psychiatry and Behavioral Sciences, 77054 Houston, TX, United States.
Marsal Sanches, University of Texas Health Science Center at Houston, Department of Psychiatry and Behavioral Sciences, 77054 Houston, TX, United States.
Giovana B. Zunta-Soares, University of Texas Health Science Center at Houston, Department of Psychiatry and Behavioral Sciences, 77054 Houston, TX, United States
Richard S. E. Keefe, Division of Medical Psychology, Duke University, Medical Centre, 27710 Durham, NC, United States
Hao Huang, Department of Radiology, Children's Hospital of Philadelphia, University of Pennsylvania PA, United States.
Jair C. Soares, University of Texas Health Science Center at Houston, Department of Psychiatry and Behavioral Sciences, 77054 Houston, TX, United States
References
- 1.Geddes JR, Miklowitz DJ. Treatment of bipolar disorder. The Lancet. 2013;381(9878):1672–1682. doi: 10.1016/S0140-6736(13)60857-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mathers CD, Fat DM, Boerma J. The global burden of disease: 2004 update. World Health Organization; Geneva: 2008. [Google Scholar]
- 3.APA. Practice guideline for the treatment of patients with bipolar disorder (revision) Am J Psychiatry. 2002;159:4. [PubMed] [Google Scholar]
- 4.MacQueen GM, Young LT, Joffe RT. A review of psychosocial outcome in patients with bipolar disorder. Acta Psychiatrica Scandinavica. 2001;103(3):163–170. doi: 10.1034/j.1600-0447.2001.00059.x. [DOI] [PubMed] [Google Scholar]
- 5.Bora E, Yucel M, Pantelis C. Cognitive endophenotypes of bipolar disorder: a meta-analysis of neuropsychological deficits in euthymic patients and their first-degree relatives. Journal of affective disorders. 2009;113(1):1–20. doi: 10.1016/j.jad.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 6.Quraishi S, Frangou S. Neuropsychology of bipolar disorder: a review. Journal of affective disorders. 2002;72(3):209. doi: 10.1016/s0165-0327(02)00091-5. [DOI] [PubMed] [Google Scholar]
- 7.O'Bryan RA, et al. Disturbances of visual motion perception in bipolar disorder. Bipolar disorders. 2013 doi: 10.1111/bdi.12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldberg TE, et al. Contrasts between patients with affective disorders and patients with schizophrenia on a neuropsychological test battery. Am J Psychiatry. 1993;150(9):1355–62. doi: 10.1176/ajp.150.9.1355. [DOI] [PubMed] [Google Scholar]
- 9.Albus M, et al. Contrasts in neuropsychological test profile between patients with first -episode schizophienia and first -episode affective disorders. Acta Psychiatrica Scandinavica. 1996;94(2):87–93. doi: 10.1111/j.1600-0447.1996.tb09830.x. [DOI] [PubMed] [Google Scholar]
- 10.Martínez - Arán A, et al. Cognitive impairment in euthymic bipolar patients: implications for clinical and functional outcome. Bipolar disorders. 2004;6(3):224–232. doi: 10.1111/j.1399-5618.2004.00111.x. [DOI] [PubMed] [Google Scholar]
- 11.Martínez-Arán A, et al. Cognitive function across manic or hypomanic, depressed, and euthymic states in bipolar disorder. American Journal of Psychiatry. 2004;161(2):262–270. doi: 10.1176/appi.ajp.161.2.262. [DOI] [PubMed] [Google Scholar]
- 12.Ryan KA, et al. Differential executive functioning performance by phase of bipolar disorder. Bipolar disorders. 2012;14(5):527–536. doi: 10.1111/j.1399-5618.2012.01032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adler CM, et al. Evidence of white matter pathology in bipolar disorder adolescents experiencing their first episode of mania: a diffusion tensor imaging study. Am J Psychiatry. 2006;163(2):322–4. doi: 10.1176/appi.ajp.163.2.322. [DOI] [PubMed] [Google Scholar]
- 14.Adler CM, et al. Abnormal frontal white matter tracts in bipolar disorder: a diffusion tensor imaging study. Bipolar Disord. 2004;6(3):197–203. doi: 10.1111/j.1399-5618.2004.00108.x. [DOI] [PubMed] [Google Scholar]
- 15.Benedetti F, et al. Tract -specific white matter structural disruption in patients with bipolar disorder. Bipolar Disorders. 2011;13(4):414–424. doi: 10.1111/j.1399-5618.2011.00938.x. [DOI] [PubMed] [Google Scholar]
- 16.Frazier JA, et al. White matter abnormalities in children with and at risk for bipolar disorder. Bipolar Disord. 2007;9(8):799–809. doi: 10.1111/j.1399-5618.2007.00482.x. [DOI] [PubMed] [Google Scholar]
- 17.Beyer JL, et al. Cortical white matter microstructural abnormalities in bipolar disorder. Neuropsychopharmacology. 2005;30(12):2225–9. doi: 10.1038/sj.npp.1300802. [DOI] [PubMed] [Google Scholar]
- 18.Liu JX, et al. Differences in white matter abnormalities between bipolar I and II disorders. J Affect Disord. 2010;127(1-3):309–15. doi: 10.1016/j.jad.2010.05.026. [DOI] [PubMed] [Google Scholar]
- 19.Nortje G, et al. Systematic review and voxel-based meta-analysis of diffusion tensor imaging studies in bipolar disorder. Journal of affective disorders. 2013;150(2):192–200. doi: 10.1016/j.jad.2013.05.034. [DOI] [PubMed] [Google Scholar]
- 20.Mahon K, et al. A voxel-based diffusion tensor imaging study of white matter in bipolar disorder. Neuropsychopharmacology. 2009;34(6):1590–600. doi: 10.1038/npp.2008.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oertel-Knöchel V, et al. Frontal white matter alterations are associated with executive cognitive function in euthymic bipolar patients. Journal of affective disorders. 2014;155:223–233. doi: 10.1016/j.jad.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 22.Barysheva M, et al. White matter microstructural abnormalities in bipolar disorder: A whole brain diffusion tensor imaging study. Neurolmage: Clinical. 2013;2:558–68. doi: 10.1016/j.nicl.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lagopoulos J, et al. Microstructural White Matter Changes in the Corpus Callosum of Young People with Bipolar Disorder: A Diffusion Tensor Imaging Study. PloS one. 2013;8(3):e59108. doi: 10.1371/journal.pone.0059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Emsell L, et al. White matter differences in euthymic bipolar I disorder: a combined magnetic resonance imaging and diffusion tensor imaging voxel - based study. Bipolar disorders. 2013;15(4):365–76. doi: 10.1111/bdi.12073. [DOI] [PubMed] [Google Scholar]
- 25.Canales-Rodríguez EJ, et al. Structural Abnormalities in Bipolar Euthymia: A Multicontrast Molecular Diffusion Imaging Study. Biological psychiatry. 2013;76(3):239–48. doi: 10.1016/j.biopsych.2013.09.027. [DOI] [PubMed] [Google Scholar]
- 26.Mahon K, Burdick KE, Szeszko PR. A role for white matter abnormalities in the pathophysiology of bipolar disorder. Neuroscience & Biobehavioral Reviews. 2010;34(4):533–554. doi: 10.1016/j.neubiorev.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heng S, Song AW, Sim K. White matter abnormalities in bipolar disorder: insights from diffusion tensor imaging studies. Journal of neural transmission. 2010;117(5):639–654. doi: 10.1007/s00702-010-0368-9. [DOI] [PubMed] [Google Scholar]
- 28.Song SK, et al. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage. 2003;20(3):1714–1722. doi: 10.1016/j.neuroimage.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 29.Song SK, et al. Dysmyelination Revealed through MRI as Increased Radial (but Unchanged Axial) Diffusion of Water. Neurolmage. 2002;17(3):1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- 30.Song SK, et al. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage. 2005;26(1):132–140. doi: 10.1016/j.neuroimage.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 31.Linke J, et al. Impaired Anatomical Connectivity and Related Executive Functions: Differentiating Vulnerability and Disease Marker in Bipolar Disorder. Biological psychiatry. 2013;74(12):908–16. doi: 10.1016/j.biopsych.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 32.Kafantaris V, et al. Lower Orbital Frontal White Matter Integrity in Adolescents With Bipolar I Disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 2009;48(1):79–86. doi: 10.1097/CHI.0b013e3181900421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yap QJ, et al. Tracking cerebral white matter changes across the lifespan: insights from diffusion tensor imaging studies. Journal of Neural Transmission. 2013;120(9):1369–1395. doi: 10.1007/s00702-013-0971-7. [DOI] [PubMed] [Google Scholar]
- 34.Wu M, et al. Development of superficial white matter and its structural interplay with cortical gray matter in children and adolescents. Human brain mapping. 2014;35(6):2806–2816. doi: 10.1002/hbm.22368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brenhouse HC, Andersen SL. Developmental trajectories during adolescence in males and females: a cross-species understanding of underlying brain changes. Neuroscience & Biobehavioral Reviews. 2011;35(8):1687–1703. doi: 10.1016/j.neubiorev.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keefe RSE, et al. The brief assessment of cognition in affective disorders (BAC-A):performance of patients with bipolar depression and healthy controls. Journal of Affective Disorders. 2014;166:86–92. doi: 10.1016/j.jad.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 37.First MB, et al. Structured Clinical Interview for DSM-IVo Axis I Disorders (SCID-I), Clinician Version, Administration Booklet. American Psychiatric Pub; 2012. [Google Scholar]
- 38.Williams JB, Kobak KA. Development and reliability of a structured interview guide for the Montgomery–Åsberg Depression Rating Scale (SIGMA) The British Journal of Psychiatry. 2008;192(1):52–58. doi: 10.1192/bjp.bp.106.032532. [DOI] [PubMed] [Google Scholar]
- 39.Young R, et al. A rating scale for mania: reliability, validity and sensitivity. The British Journal of Psychiatry. 1978;133(5):429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 40.American Psychiatric Association. DSM-IV-TR: Diagnostic and statistical manual of mental disorders, text revision 2000: (Text Revision (DSM-IV TR) fourth. American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- 41.Wechsler D. Wechsler abbreviated scale of intelligence. San Antonio: The Psychological Corporation; 1999. [Google Scholar]
- 42.Wilkinson GS, Robertson G. Wide Range Achievement Test (WRAT4) Psychological Assessment Resources; Lutz: 2006. [Google Scholar]
- 43.Keefe R, et al. The Brief Assessment of Cognition in Schizophrenia: reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophrenia Research. 2004;68(2–3):283–297. doi: 10.1016/j.schres.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 44.LaMonica HM, et al. Differential effects of emotional information on interference task performance across the life span. Frontiers in aging neuroscience. 2010;2 doi: 10.3389/fnagi.2010.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Snyder KA, Harrison DW. The affective auditory verbal learning test. Archives of Clinical Neuropsychology. 1997;12(5):477–482. [PubMed] [Google Scholar]
- 46.van der Werf-Eldering MJ, et al. Cognitive Functioning in Patients with Bipolar Disorder: Association with Depressive Symptoms and Alcohol Use. PLoS ONE. 2010;5(9):e13032. doi: 10.1371/journal.pone.0013032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marlinge E, Bellivier F, Houenou J. White matter alterations in bipolar disorder: potential for drug discovery and development. Bipolar disorders. 2014;16(2):97–112. doi: 10.1111/bdi.12135. [DOI] [PubMed] [Google Scholar]
- 48.Schmahmann JD, et al. Cerebral white matter. Annals of the New York Academy of Sciences. 2008;1142(1):266–309. doi: 10.1196/annals.1444.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brambilla P, et al. Corpus callosum signal intensity in patients with bipolar and unipolar disorder. Journal of Neurology, Neurosurgery & Psychiatry. 2004;75(2):221–225. [PMC free article] [PubMed] [Google Scholar]
- 50.Schneider MR, et al. Neuroprogression in bipolar disorder. Bipolar disorders. 2012;14(4):356–374. doi: 10.1111/j.1399-5618.2012.01024.x. [DOI] [PubMed] [Google Scholar]
- 51.Emsell L, et al. Limbic and callosal white matter changes in euthymic bipolar I disorder: an advanced diffusion magnetic resonance imaging tractography study. Biol Psychiatry. 2013;73(2):194–201. doi: 10.1016/j.biopsych.2012.09.023. [DOI] [PubMed] [Google Scholar]
- 52.Leow A, et al. Impaired inter-hemispheric integration in bipolar disorder revealed with brain network analyses. Biol Psychiatry. 2013;73(2):183–93. doi: 10.1016/j.biopsych.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Budde MD, et al. Axial diffusivity is the primary correlate of axonal injury in the experimental autoimmune encephalomyelitis spinal cord: a quantitative pixelwise analysis. The Journal of neuroscience. 2009;29(9):2805–2813. doi: 10.1523/JNEUROSCI.4605-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. Journal of Magnetic Resonance, Series B. 1996;111(3):209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- 55.Tkachev D, et al. Oligodendrocyte dysfunction in schizophrenia and bipolar disorder. The Lancet. 2003;362(9386):798–805. doi: 10.1016/S0140-6736(03)14289-4. [DOI] [PubMed] [Google Scholar]
- 56.Hakak Y, et al. Genome-wide expression analysis reveals dysregulation of myelination-related genes in chronic schizophrenia. Proceedings of the National Academy of Sciences. 2001;98(8):4746–4751. doi: 10.1073/pnas.081071198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chambers JS, Perrone-Bizzozero NI. Altered myelination of the hippocampal formation in subjects with schizophrenia and bipolar disorder. Neurochemical research. 2004;29(12):2293–2302. doi: 10.1007/s11064-004-7039-x. [DOI] [PubMed] [Google Scholar]
- 58.Bauer IE, et al. Evaluation of cognitive function in bipolar disorder using the Brief Assessment of Cognition in Affective Disorders (BAC-A) Journal of psychiatric research. 2015;60:81–86. doi: 10.1016/j.jpsychires.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rossell SL. Category fluency performance in patients with schizophrenia and bipolar disorder: The influence of affective categories. Schizophrenia Research. 2006;82(2–3):135–138. doi: 10.1016/j.schres.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 60.Martinez - Aran A, et al. Functional outcome in bipolar disorder: the role of clinical and cognitive factors. Bipolar disorders. 2007;9(1 - 2):103–113. doi: 10.1111/j.1399-5618.2007.00327.x. [DOI] [PubMed] [Google Scholar]
- 61.Lopes R, Fernandes L. Bipolar disorder: clinical perspectives and implications with cognitive dysfunction and dementia. Depression research and treatment. 2012;2012 doi: 10.1155/2012/275957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Glahn DC, et al. The neurocognitive signature of psychotic bipolar disorder. Biological psychiatry. 2007;62(8):910–916. doi: 10.1016/j.biopsych.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 63.Cavanagh J, et al. Case—control study of neurocognitive function in euthymic patients with bipolar disorder: an association with mania. The British Journal of Psychiatry. 2002;180(4):320–326. doi: 10.1192/bjp.180.4.320. [DOI] [PubMed] [Google Scholar]
- 64.Sapin LR, et al. Mediational factors underlying cognitive changes and laterality in affective illness. Biological Psychiatry. 1987;22(8):979–986. doi: 10.1016/0006-3223(87)90007-2. [DOI] [PubMed] [Google Scholar]
- 65.Zubieta JK, et al. Cognitive function in euthymic bipolar I disorder. Psychiatry research. 2001;102(1):9–20. doi: 10.1016/s0165-1781(01)00242-6. [DOI] [PubMed] [Google Scholar]
- 66.Juselius S, et al. Executive functioning in twins with bipolar I disorder and healthy co-twins. Archives of clinical neuropsychology. 2009;24(6):599–606. doi: 10.1093/arclin/acp047. [DOI] [PubMed] [Google Scholar]
- 67.Calev A, Nigal D, Chazan S. Retrieval from semantic memory using meaningful and meaningless constructs by depressed, stable bipolar and manic patients. British Journal of Clinical Psychology. 1989;28(1):67–73. doi: 10.1111/j.2044-8260.1989.tb00813.x. [DOI] [PubMed] [Google Scholar]
- 68.Martino J, et al. Anatomic dissection of the inferior fronto-occipital fasciculus revisited in the lights of brain stimulation data. Cortex. 2010;46(5):691–699. doi: 10.1016/j.cortex.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 69.Savitz J, et al. Amygdala volume in depressed patients with bipolar disorder assessed using high resolution 3TMRI: the impact of medication. Neuroimage. 2010;49(4):2966–2976. doi: 10.1016/j.neuroimage.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Foland LC, et al. Increased volume of the amygdala and hippocampus in bipolar patients treated with lithium. Neuroreport. 2008;19(2):221–4. doi: 10.1097/WNR.0b013e3282f48108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hajek T, et al. Neuroprotective effect of lithium on hippocampal volumes in bipolar disorder independent of long-term treatment response. Psychological medicine. 2013:1–11. doi: 10.1017/S0033291713001165. [DOI] [PubMed] [Google Scholar]
- 72.Skranes J, et al. Clinical findings and white matter abnormalities seen on diffusion tensor imaging in adolescents with very low birth weight. Brain. 2007;130(3):654–666. doi: 10.1093/brain/awm001. [DOI] [PubMed] [Google Scholar]
- 73.Blumenfeld-Katzir T, et al. Diffusion MRI of structural brain plasticity induced by a learning and memory task. PloS one. 2011;6(6):e20678. doi: 10.1371/journal.pone.0020678. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table 1S. Fractional anisotropy (FA) differences in healthy controls (HC) versus bipolar (BD) patients - HC > BD. Data are presented as means and standard deviations; p-values are FDR-corrected. Labels are based on Johns Hopkins University (JHU)-ICBM-DTI-81 WM atlas.
Table 2S. Radial Diffusivity (RD) differences in bipolar (BD) patients vs healthy controls (HC), BD>HC. Data are presented as means and standard deviations in 1×10-3 mm2/s; p-values are FDR-corrected. Labels are based on Johns Hopkins University (JHU)-ICBM-DTI-81 WM atlas.
Table 3S. Fractional anisotropy (FA), radial diffusivity (RD) and mean diffusivity (MD) values in healthy controls (HC) vs bipolar (BD) patients. Tracts are labeled with reference to the JHU ICBM-DTI-81 white-matter labels atlas in the right (R) and left (L) hemisphere (FA: HC > BD; MD,RD: BD>HC). Data are presented as means and standard deviations. Unit of measure for RD and MD is 1×10-3 mm2/s. p-values are FWE-corrected. Labels are based on Johns Hopkins University (JHU)-ICBM-DTI-81 WM atlas.
Table 4S. Pearson's correlation coefficients among DTI metrics and cognitive summary scores. Abbreviations: DTI metrics: CST-R = right corticospinal tract, FM = forceps minor, IFOF-L = left inferior fronto-occipital fasciculus, Inhibition = Interference index of the Emotional Stroop test, PS = problem solving, SLF-L = left superior longitudinal fasciculus; SCR-R = right superior corona radiata. Cognitive summary scores: STAM = short-term affective memory, STNM = short-term non-affective memory, DAM = delayed affective memory, DNM = delayed non-affective memory, PS = problem solving, VM = visuomotor skills in bipolar disorder (BD) and healthy controls (HC). Significant correlations are denoted with asterisks at significance level *p< 0.05, **p< 0.01. N=67.


