Abstract
Posttraumatic stress disorder (PTSD) often precedes comorbid substance use disorder and has been associated with aggression. Prior research has evidenced that alcohol use and other externalizing behaviors share genetic factors with PTSD; however, few studies have examined if specific genes are associated with externalizing behaviors in PTSD. The purpose of the current study was to investigate whether an α-synuclein gene polymorphism (SNCA rs356195) moderates the association of PTSD symptomatology with externalizing behaviors. We examined the separate and combined effects of PTSD symptomatology and SNCA rs356195 on alcohol- and aggression-related measures in nonclinical participants (N = 138 European Americans; 15 diagnosed with probable PTSD). Probable PTSD status and SNCA were both associated with externalizing measures. SNCA also moderated the association of PTSD symptomatology with hazardous alcohol use, but not with aggression-related measures. Current findings suggest that variations in SNCA may increase the likelihood that PTSD symptomatology results in excessive alcohol use.
Keywords: posttraumatic stress disorder, alpha-synuclein, alcohol use, impulsivity, aggression
1. Introduction
Posttraumatic stress disorder (PTSD) is a common psychiatric disorder that develops following a traumatic event and is estimated to affect approximately 9% of individuals in their lifetime.[1] The range of responses that can follow exposure to a traumatic event is broad and includes emotional numbing, hypervigilance, irritability, recklessness, and unwanted re-experiencing of the event through intrusive memories and flashbacks.[2] Notably, PTSD frequently co-occurs with substance use disorders.[3,4] For instance, estimated rates of lifetime alcohol use disorder range from about 22 to 52% in individuals with PTSD,[4–6] compared to 8 to 21% in individuals without PTSD.[5,6] Where temporal data are available, PTSD usually precedes comorbid substance use disorder.[3,4,7,8] PTSD has also been associated with interpersonal aggression,[9–12] and the robustness of this association has been supported by a meta-analysis of 39 studies.[13] Other studies have reported elevated rates of thrill-seeking behavior, risky sexual behavior, and unsafe driving associated with PTSD,[14,15] and these risky and impulsive behaviors frequently co-occur.[11,14,15]
Importantly, cluster analytic studies have revealed distinct patterns of personality and behavior among individuals who have been exposed to trauma.[16–19] One pattern is characterized by externalizing psychopathology, including high negative emotionality, low constraint, high levels of aggression, and elevated comorbidity with substance use disorders and antisocial personality disorder. This contrasts with an internalizing pattern characterized by low positive emotionality and high negative emotionality. The nature of the genetic and environmental influences on these outcomes is an area of ongoing research.
Twin studies using the Vietnam Era Twin Registry have revealed that PTSD and alcohol use disorder share common genetic influences.[20,21] However, examination of individual genes is better suited to determining which individuals with PTSD tend to display externalizing psychopathology following traumatic exposure.[22] The ANKK1/DRD2 A1 allele, which is associated with alcohol expectancies[23,24] and risk for alcohol dependence in general,[25] has been shown to be more frequent in combat veterans with PTSD who were harmful drinkers compared to those who were not harmful drinkers.[26] The ankyrin 3 gene (ANK3) has also been studied for its possible influence on externalizing behavior and PTSD: Logue and colleagues[27] reported that a single-nucleotide polymorphism (SNP; rs9801490 T allele) was associated with a reduced likelihood of PTSD and externalizing behavior, suggesting that it may reflect a common genetic factor underlying the development of both PTSD and externalizing behavior. Finally, variations in 5-HTTLPR have been found to interact with early life and family stress to predict alcohol use in maltreated children and adolescents.[28,29] Overall though, little is known about specific genes that are associated with externalizing behaviors in PTSD.
Alpha-synuclein is a presynaptic protein that attenuates dopamine (DA) biosynthesis and release,[30] and DA neurotransmission is known to play an important role in reward and addiction.[31,32] With regard to addictive behavior, the α-synuclein gene (SNCA) has been shown to be more highly expressed in the hippocampus and nucleus accumbens of alcohol-naïve rats bred to prefer alcohol compared to alcohol-naïve rats bred to not prefer alcohol.[33,34] In humans, blood α-synuclein levels have been positively correlated with alcohol and cocaine craving,[35,36] and several SNPs of SNCA have been associated with alcohol craving[37,38] and alcohol use disorder.[39] Recent studies have also linked SNCA to impulsivity in mice[40,41] and humans.[42] In regard to specific SNPs of SNCA, the C-allele of SNCA rs356195 has been associated with negative history of alcohol craving,[38] and SNCA rs356195 T-allele carriers have displayed greater impulsivity than individuals with the CC genotype.[42] Given the involvement of DA and impulsivity in aggressive behavior,[43,44] it is possible that SNCA is also associated with aggression. However, no study to date has examined this relationship.
Externalizing symptoms (e.g., aggression), characterized by behavioral disconstraint, have been prospectively associated with alcohol problems in individuals with PTSD.[45,46] The purpose of the current study was to investigate whether the SNCA rs356195 polymorphism, which has previously been associated with impulsivity, moderates the association of PTSD symptomatology with externalizing behaviors. To this end, we examined the separate and combined effects of PTSD symptomatology and SNCA rs356195 on alcohol- and aggression-related measures in a nonclinical sample. We hypothesized that SNCA T-allele carriers would display evidence of greater impairment in behavioral control in domains related to alcohol use and aggression relative to C homozygotes. Based on past research, we expected that probable PTSD status would be associated with more severe alcohol use and greater aggression and alcohol-related aggression expectancies. We also hypothesized that SNCA would moderate these relationships, such that PTSD symptomatology would be more strongly related to higher levels of hazardous alcohol use, aggression, and alcohol-related aggression expectancies in T-allele carriers.
2. Method
2.1. Participants and Procedures
Using the same methodology as a prior study,[47] a total of 222 participants were recruited from the university and community through university-based e-mail announcements, on- and off-campus fliers, and newspaper and online advertisements and were genotyped for SNCA rs356195. Blood samples for genotyping were obtained by puncturing the index finger with an automatic fingerstick lancet device and then storing three small blots of blood with 3MM chromatography paper (Whatman, Inc., Florham Park, NJ). Dried blood samples were analyzed at the Indiana Alcohol Research Center. DNA was isolated using the HotSHOT method,[48] after which TaqMan probes were used for allelic discrimination (Applied BioSystems, Inc., Foster City, CA). Thermocycling was carried out in MJ Research PTC-200 thermocyclers, and the PCR products were analyzed in an ABI PRISM® 7300 Sequence Detection System (SDS) instrument.
In order to limit the potentially confounding effects of population stratification, only participants who self-identified as “Caucasian” were retained. Of the remaining 145 participants, 7 were excluded because of incomplete self-report data, leaving a total sample of 138 European Americans (77 men and 61 women) between the ages of 21 and 55 (M = 25.96, SD = 7.48). The project was approved by The University of Southern Mississippi Human Subjects Protection Review Committee. Written informed consent was obtained prior to participation.
2.2. Measures
2.2.1. PTSD Checklist Civilian Version (PCL-C)
The PCL-C was used to diagnose probable PTSD.[49] The self-report instrument consists of 17 items corresponding to symptoms from PTSD Criterion B (trauma re-experiencing), Criterion C (trauma-related avoidance and general numbing), and Criterion D (increased arousal) of the 4th edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV).[50] Test-takers are asked to respond to each symptom consistent with how much they have been bothered by that problem during the past month, and items are answered on a 5-point severity scale scored from 1 (not at all) to 5 (extremely). Using the symptom cluster method, individuals are given a presumptive diagnosis of PTSD if they score 3 (moderately) or higher on at least one Criterion B item, three Criterion C items, and two Criterion D items (thus meeting Criterion B, C, and D, respectively). The symptom cluster method has yielded a sensitivity of 39–100% and a specificity of 79–94% in relation to interviewer-diagnosed PTSD.[49] In addition, PCL-C Criterion B, C, and D subscale scores and Total Scale scores were also computed.
2.2.2. Alcohol Use Disorders Identification Test (AUDIT)
The AUDIT is a self-report measure of hazardous and harmful alcohol use.[51] The first 3 items pertain to alcohol use frequency, typical drinking amount, and frequency of binge drinking, and the remaining 7 items inquire about alcohol-related difficulties and symptoms of alcohol dependence. Item responses are scored from 0 to 4, except for responses to the last two items, which are scored 0, 2, or 4, and higher scores indicate greater levels of hazardous drinking.
2.2.3. Alcohol Effects Questionnaire (AEQ) Aggression and Power Subscale
The AEQ Aggression and Power subscale is a 6-item self-report measure that assesses beliefs regarding the likelihood of engaging in aggressive behavior after consuming a few alcoholic drinks.[52] “True” and “False” item responses are scored 1 and 0, respectively, and higher scores are indicative of greater alcohol-related aggression expectancies.
2.2.4. Life History of Aggression (LHA) Aggression Subscale
The LHA Aggression subscale is a 5-item self-report measure that assesses history of aggressive behavior.[53] Items are answered on a 6-point frequency scale ranging from 0 (never happened) to 5 (happened so many times I couldn’t give a number), and higher scores are indicative of greater levels of past aggression. LHA Aggression scores have been shown to be highly correlated with other measures of aggression.[53]
2.3. Data Analysis
Alpha (two-tailed) was set at .05 for all analyses. We first obtained descriptive statistics and genotype frequencies and compared PTSD and SNCA groups on demographic variables. Hardy-Weinberg equilibrium was tested using an online Hardy-Weinberg equilibrium calculator.[54] Two-way between-subjects ANOVAs were used to evaluate the main and interactive effects of PTSD symptomatology and SNCA on continuous variables (i.e., AUDIT, AEQ Aggression and Power, and LHA Aggression scores). If there was a significant interaction between PTSD symptomatology and SNCA on a particular variable, then the interactive effects of PTSD Criterion (B, C, and D) and SNCA on that particular variable were also examined. For significant interactions, simple effects analyses were conducted with separate one-way between-subjects ANOVAs. Due to group size considerations (less than 5 TT participants per cell), SNCA group comparisons were limited to comparisons between C homozygotes (CC participants) and T-allele carriers (CT/TT participants), consistent with a prior SNCA study that used nearly the same (overlapping) sample.[42]
3. Results
3.1. Sample Characteristics
Using the PCL-C symptom cluster method, 47 participants met PTSD Criterion B; 35 participants met Criterion C; 25 participants met Criterion D; and 15 participants (3 men and 12 women) were diagnosed with probable PTSD. Fisher’s exact tests and one-way analyses of variance revealed that participants with and without a presumptive diagnosis of PTSD did not differ significantly in respect to age, years of education, or marital status (all ps > .18); however, women were significantly more likely to be diagnosed with probable PTSD (p = .005, RR = 5.0). Men and women did not differ significantly in regard to AUDIT or LHA Aggression scores (ps > .15), but women did score higher on AEQ Aggression and Power (t136 = 2.02, p = .046, d = .35. The genotypic frequency distribution for SNCA rs356195 in the total sample did not deviate significantly from expected Hardy-Weinberg equilibrium (78 CC, 49 CT, and 11 TT; χ2 = .69, p = .41). Fisher’s exact tests and one-way analyses of variance revealed that SNCA C homozygotes and T-allele carriers did not differ significantly in respect to sex, age, years of education, marital status, or probable PTSD diagnosis (all ps > .75). Descriptive statistics and Cronbach’s alphas for study measures are displayed in Table 1.
Table 1.
Descriptive Statistics and Cronbach’s Alphas for Self-Report Measures
| M (SD) | Cronbach’s α | |
|---|---|---|
| PCL-C Criterion B subscale | 8.3 (4.0) | .89 |
| PCL-C Criterion C subscale | 12.7 (5.0) | .81 |
| PCL-C Criterion D subscale | 7.7 (3.4) | .82 |
| PCL-C Total Scale | 28.7 (11.3) | .93 |
| AUDIT | 6.4 (3.5) | .67 |
| AEQ Aggression and Power subscale | 2.1 (1.5) | .58 |
| LHA Aggression subscale | 10.2 (4.7) | .77 |
Note. PCL-C = PTSD Checklist Civilian Version; AUDIT = Alcohol Use Disorders Identification Test; AEQ = Alcohol Effects Questionnaire; LHA = Life History of Aggression.
3.2. Hazardous Alcohol Use
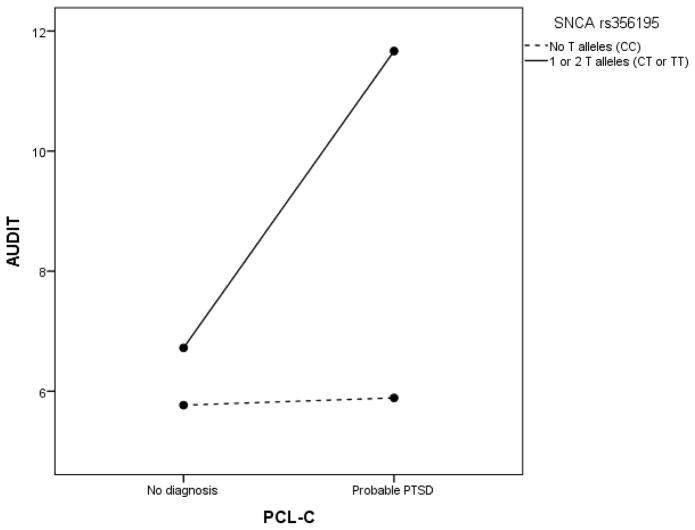
PTSD symptomatology (F(1, 134) = 7.52, p = .007, ηp2 = .053) and SNCA (F(1, 134) = 13.28, p = .0004, ηp2 = .090) were both related to AUDIT scores: Participants with probable PTSD (M = 8.2, SD = 5.2) displayed higher AUDIT scores than participants without probable PTSD (M = 6.2, SD = 3.2, d = .58), and T-allele carriers (M = 7.2, SD = 3.7) displayed higher AUDIT scores than C homozygotes (M = 5.8, SD = 3.2, d = .41). As displayed in Fig. 1, a significant interaction between PTSD symptomatology and SNCA (F(1, 134) = 6.82, p = .01, ηp2 = .048) was also found. Simple effects analyses showed that T-allele carriers with probable PTSD (M = 11.7, SD = 5.8) displayed higher AUDIT scores than T-allele carriers without probable PTSD (M = 6.7, SD = 3.1, p = .001, d = 1.46), whereas C homozygotes with probable PTSD (M = 5.9, SD = 3.3) scored nearly the same on the AUDIT as C homozygotes without probable PTSD (M = 5.8, SD = 3.2, p = .92).
Fig. 1.
PTSD and SNCA interaction effects on hazardous alcohol use levels. AUDIT = Alcohol Use Disorders Identification Test; PCL-C = PTSD Checklist Civilian Version.
Follow-up analyses were conducted to examine if meeting a specific PTSD criterion was primarily responsible for the significant interactive effect of PTSD symptomatology and SNCA on AUDIT scores. There was a significant interaction between SNCA and PTSD Criterion D (increased arousal), F(1, 134) = 7.82, p = .006, ηp2= .055. Simple effects analyses revealed that T-allele carriers who met Criterion D (M = 9.9, SD = 5.3) displayed higher AUDIT scores than T-allele carriers who did not meet Criterion D (M = 6.6, SD = 3.0, p = .007, d = .94), whereas C homozygotes who met Criterion D (M = 5.1, SD = 3.1) scored about the same on the AUDIT as C homozygotes who did not meet Criterion D (M = 5.9, SD = 3.2, p = .36). Neither Criterion B (p = .79) nor C (p = .58) significantly interacted with SNCA in relation to AUDIT scores.
3.3. Alcohol-Related Aggression Expectancies
PTSD symptomatology (F(1,134) = 10.38, p = .002, ηp2 = .072) and SNCA (F(1,134) = 13.95, p = .0003, ηp2 = .094) were both related to AEQ Aggression and Power scores: Participants with probable PTSD (M = 3.1, SD = 1.9) displayed higher AEQ Aggression and Power scores than participants without probable PTSD (M = 1.9, SD = 1.5, d = .78), and T-allele carriers (M = 2.6, SD = 1.7) displayed higher AEQ Aggression and Power scores than C homozygotes (M = 1.6, SD = 1.3, d = .67). However, the interaction of PTSD symptomatology and SNCA on AEQ Aggression and Power scores did not reach significance (p = .12). Therefore, no follow-up PTSD criterion analyses were conducted in regard to alcohol-related aggression expectancies.
3.4. History of Aggression
PTSD symptomatology (F(1,134) = 5.38, p = .022, ηp2 = .039) was related to LHA Aggression scores: Participants with probable PTSD (M = 13.0, SD = 4.9) displayed higher LHA Aggression scores than participants without probable PTSD (M = 9.9, SD = 4.6, d = .67). However, SNCA was not related to LHA Aggression scores (p = .96), and the interaction of PTSD symptomatology and SNCA on LHA Aggression scores did not reach significance (p = .63). Therefore, no follow-up PTSD criterion analyses were conducted in regard to aggression.
3.5 Supplemental Analyses
Although we chose to conduct primary analyses using ANOVA models with probable PTSD status as a predictor (for ease of interpretation and to be consistent with traditional categorical conceptualizations of PTSD[2,50]), taxometric studies have consistently revealed that the PTSD construct is optimally represented through the use of continuous (or dimensional) measures.[55–57] Therefore, we decided to conduct supplemental moderated regression analyses instead using PTSD severity (PCL-C Total Scale score) as a predictor. Because sex (female coded as 0; male coded as 1) was significantly correlated with PTSD severity (r = .21, p = .013) and AEQ Aggression and Power scores (p = .046, rpb = −.17), we also reran regression analyses controlling for gender. These moderated regression analyses were conducted with PROCESS for SPSS Version 2.13[58].
Results are reported as standardized regression coefficients (βs).
Moderated regression analyses revealed that the association between SNCA and AUDIT scores was significant (β = .21, p = .014), whereas the association between PTSD severity and AUDIT scores was not significant (p = .89). Moderated regression analyses also revealed that the interaction of PTSD severity and SNCA on AUDIT scores was significant (β = .17, p = .043). Follow-up analyses indicated that neither PTSD Criterion B scores (p = .10) nor C scores (p = .16) significantly interacted with SNCA in relation to AUDIT scores, but the interaction of SNCA and Criterion D scores (increased arousal) on AUDIT scores was significant (β = .24, p = .007), indicating that Criterion D scores were positively associated with AUDIT scores to a greater degree in T-allele carriers relative to C homozygotes. The significance of AUDIT-related regression results was not altered by controlling for gender.
Moderated regression analyses revealed that the association between PTSD severity and AEQ Aggression and Power scores (β = .22, p = .007) and the association between SNCA and AEQ Aggression and Power scores (β = .32, p = .0001) were both significant; however, the interaction of SNCA and PTSD severity on AEQ Aggression and Power scores was not significant (p = .20). Therefore, no follow-up PTSD criterion score analyses were conducted in regard to AEQ Aggression and Power scores. The significance of AEQ-related regression results was not altered by controlling for gender.
Moderated regression analyses revealed that the association between PTSD severity and LHA Aggression scores was significant (β = .27, p = .001); however, neither the association between SNCA and LHA Aggression scores (p = .49) nor the interaction of PTSD severity and SNCA on LHA Aggression scores was significant (p = .62). Therefore, no follow-up PTSD criterion score analyses were conducted in regard to LHA Aggression scores. The significance of LHA-related regression results was not altered by controlling for gender.
In summary, rerunning analyses with a dimensional PTSD measure altered the significance of results in only one instance: Whereas probable PTSD status was significantly related to AUDIT scores, PTSD severity was not.
4. Discussion
We examined whether an α-synuclein gene polymorphism (SNCA rs356195) moderates the association of PTSD symptomatology with externalizing behaviors (i.e., alcohol- and aggression related measures) in nonclinical participants. Probable PTSD status and SNCA were both associated with externalizing measures, although SNCA was more specific to alcohol. SNCA moderated the association of PTSD symptomatology with hazardous alcohol use, but not with aggression-related measures. Rerunning analyses with PTSD as a continuous measure did not substantially alter results. This is the first study to examine the potential influence of SNCA on the expression of externalizing behaviors in individuals with significant PTSD symptomatology.
As expected, individuals with probable PTSD reported higher levels of hazardous alcohol use than those without probable PTSD, and SNCA T-allele carriers reported higher levels of hazardous alcohol use than C homozygotes (small-to-medium effect sizes). There was also a significant interaction between PTSD symptomatology and SNCA: T-allele carriers with probable PTSD displayed higher AUDIT scores than T-allele carriers without probable PTSD (a large effect size), whereas C homozygotes with probable PTSD scored nearly the same on the AUDIT as C homozygotes without probable PTSD. Notably, the average AUDIT score for T-allele carriers with probable PTSD (which rounded to 12) was well above the cut-off score that has been recommended as indicating harmful or hazardous alcohol use (≥ 8),[51] suggesting that the moderating effect of SNCA on the relationship between PTSD symptomatology and hazardous alcohol use may be clinically significant. Further analysis indicated that this moderating effect was primarily due to the interaction of SNCA and DSM-IV PTSD Criterion D (analogous to DSM-5 PTSD Criterion E), which is related to increased arousal and reactivity.[2,50] Thus, it appears that SNCA T-allele carriers with PTSD may be particularly vulnerable to the development of harmful or hazardous alcohol use largely because of heightened arousal and reactivity. One potential explanation for this interaction is that variations in SNCA may confer risk for deficient impulse control,[42] thereby increasing the likelihood that PTSD-related arousal and reactivity result in alcohol use.
Also as expected, individuals with probable PTSD reported greater alcohol-related aggression expectancies than those without probable PTSD, and SNCA T-allele carriers reported greater alcohol-related aggression expectancies than C homozygotes (medium-to-large effect sizes). Hence, SNCA may influence the development of aggressive tendencies in the context of alcohol intoxication, which consequently may lead to greater expectations of aggressive behavior while under the influence of alcohol. In addition, individuals with probable PTSD reported a more severe history of aggression than those without probable PTSD (a medium effect size). Contrary to our hypotheses, however, SNCA was not related to history of aggression, and SNCA did not moderate the relationship between PTSD symptomatology and either of the aggression-related measures.
As stated previously, α-synuclein attenuates DA release,[30,40] and DA plays a key role in reward function.[31,32] Thus, one potential explanation for the presumed influence of SNCA on alcohol use and alcohol-related aggression in general is that α-synuclein may affect reward-related behaviors via regulation of DA neurotransmission.[34] Further backing this assertion, innately alcohol-preferring rats have displayed lower α-synuclein expression in the frontal cortex and caudate putamen compared to alcohol-nonpreferring rats;[59] mutant mice lacking α-synuclein have displayed increased reward-seeking behavior;[60] and human SNCA duplication carriers (who have enhanced levels of α-synuclein expression) have displayed impaired reward learning.[61] Although not tied to reward to the extent that addiction is, aggression in mice has been associated with DA activity in the nucleus accumbens, a region of the brain involved in the process of addiction,[62] and mice that were chronically victorious in aggressive encounters have displayed increased α-synuclein expression in the ventral tegmental area, a region of the brain involved in the process of reward.[63,64] As for SNCA being associated with expectations of aggression during alcohol intoxication but not aggression in general, it may be that the stimulant effects of alcohol tend to potentiate rewarding forms of aggression,[65] the susceptibility to which may be further increased by the effects of α-synuclein on DA and reward function.
In the current study, one unexpected finding was that women reported greater alcohol-related aggression expectancies than men. Notably, however, past studies that have used the AEQ Aggression and Power subscale to compare alcohol-related aggression expectancies between men and women have produced mixed results, with one study reporting that men scored higher,[66] another study reporting no significant difference in scores,[52] and another study reporting that women scored higher.[67] Although one study that differed from the current study used a sample that was 45% Black[66] (compared to 0% Black in the current study), the other two studies did not provide data regarding race, making it difficult to speculate if differences in sample composition might account for these discrepancies.
This study has several limitations worth noting. First, the current study is limited by its cross-sectional design. Although we conceived of PTSD symptomatology as likely preceding and contributing to hazardous alcohol use via symptoms of hyperarousal and hyperreactivity, it is instead possible that chronically excessive alcohol use led to hyperarousal as a result of alcohol-induced changes in central and peripheral stress pathways.[68] Another concern is that the majority of participants with probable PTSD were women, which may have confounded PTSD-related results. However, past research has indicated that PTSD is more prevalent in women than in men;[4] of the three criterion variables studied, gender was only correlated with alcohol-related aggression expectancies; and the significance of supplemental regression analyses was not altered by controlling for gender. The inclusion of only European Americans limits the ability to generalize results to other racial/ethnic groups (though this was done to minimize the potentially confounding effects of population stratification). Another limitation is that we only tested one SNP of SNCA (rs356195) using a single model of genetic inheritance (the dominant model) because of sample/group size considerations. We also administered self-report measures of PTSD symptoms and alcohol use to nonclinical participants and did not formally diagnose PTSD or alcohol use disorder and further did not include biological markers of alcohol use. Lastly, we speculated that α-synuclein may affect alcohol use and alcohol-related aggression through its effect on DA neurotransmission and reward function; however, the current study did not include measures related to α-synuclein expression, DA activity, or reward. Therefore, future studies would benefit from utilizing longitudinal designs, including individuals from various racial/ethnic backgrounds, genotyping multiple SNPs of SNCA, using samples large enough to test for multiple models of genetic inheritance, using clinical interviews to diagnose PTSD and alcohol use disorder (perhaps as part of a case-control design), measuring blood α-synuclein levels and biological markers of alcohol consumption, using functional brain imaging techniques, and including measures related to reward (e.g., anhedonia and reward responsiveness).
5. Conclusion
Past and present research indicates that PTSD and SNCA influence alcohol use. The current study extends past research by reporting a relationship between SNCA and alcohol-related aggression expectancies. It also extends past research by reporting that the association between PTSD symptomatology and hazardous alcohol use is moderated by genotypic variation in SNCA. Although preliminary, current findings suggest that variations in SNCA may increase the likelihood that PTSD symptomatology results in excessive alcohol use.
Highlights.
We genotyped the polymorphism SNCA rs356195 in 138 European Americans (EAs).
EAs reported their alcohol use, alcohol-aggression expectancies, and aggression.
We also diagnosed probable PTSD based on participant self-report.
SNCA moderated the association of PTSD symptomatology with hazardous alcohol use.
Variations in SNCA may increase risk of PTSD-related excessive drinking.
Acknowledgments
This study was supported by National Institute on Alcohol Abuse and Alcoholism grants P60-AA007611 and R21-AA14025 and a training grant from the National Cancer Institute (T32-CA009492). We also appreciate the technical support of Tamara Graves from the Indiana Alcohol Research Center, Genomics and Bioinformatics Core.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 2.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- 3.Jacobsen LK, Southwick SM, Kosten TR. Substance use disorders in patients with posttraumatic stress disorder: a review of the literature. Am J Psychiatry. 2001;158:1184–90. doi: 10.1176/appi.ajp.158.8.1184. [DOI] [PubMed] [Google Scholar]
- 4.Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52:1048–60. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- 5.Breslau N, Davis GC, Andreski P, Peterson E. Traumatic events and posttraumatic stress disorder in an urban population of young adults. Arch Gen Psychiatry. 1991;48:216–22. doi: 10.1001/archpsyc.1991.01810270028003. [DOI] [PubMed] [Google Scholar]
- 6.Breslau N, Davis GC, Peterson EL, Schultz L. Psychiatric sequelae of posttraumatic stress disorder in women. Arch Gen Psychiatry. 1997;54:81–7. doi: 10.1001/archpsyc.1997.01830130087016. [DOI] [PubMed] [Google Scholar]
- 7.Chilcoat HD, Breslau N. Investigations of causal pathways between PTSD and drug use disorders. Addict Behav. 1998;23:827–40. doi: 10.1016/s0306-4603(98)00069-0. [DOI] [PubMed] [Google Scholar]
- 8.Kline A, Weiner MD, Ciccone DS, Interian A, St Hill L, Losonczy M. Increased risk of alcohol dependency in a cohort of National Guard troops with PTSD: a longitudinal study. J Psychiatr Res. 2014;50:18–25. doi: 10.1016/j.jpsychires.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 9.Kulka RA, Schlenger WE, Fairbank JA, et al. Trauma and the Vietnam War generation: Report of findings from the National Vietnam Veterans Readjustment Study. New York: Brunner/Mazel; 1990. [Google Scholar]
- 10.Jordan BK, Marmar CR, Fairbank JA, et al. Problems in families of male Vietnam veterans with posttraumatic stress disorder. J Consult Clin Psychol. 1992;60:916–26. doi: 10.1037//0022-006x.60.6.916. [DOI] [PubMed] [Google Scholar]
- 11.Jakupcak M, Conybeare D, Phelps L, et al. Anger, hostility, and aggression among Iraq and Afghanistan War veterans reporting PTSD and subthreshold PTSD. J Trauma Stress. 2007;20:945–54. doi: 10.1002/jts.20258. [DOI] [PubMed] [Google Scholar]
- 12.Taft CT, Weatherill RP, Woodward HE, et al. Intimate partner and general aggression perpetration among combat veterans presenting to a posttraumatic stress disorder clinic. Am J Orthopsychiatry. 2009;79:461–8. doi: 10.1037/a0016657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Orth U, Wieland E. Anger, hostility, and posttraumatic stress disorder in trauma-exposed adults: a meta-analysis. J Consult Clin Psychol. 2006;74:698–706. doi: 10.1037/0022-006X.74.4.698. [DOI] [PubMed] [Google Scholar]
- 14.Fear NT, Iversen AC, Chatterjee A, et al. Risky driving among regular armed forces personnel from the United Kingdom. Am J Prev Med. 2008;35:230–6. doi: 10.1016/j.amepre.2008.05.027. [DOI] [PubMed] [Google Scholar]
- 15.Strom TQ, Leskela J, James LM, et al. An exploratory examination of risk-taking behavior and PTSD symptom severity in a veteran sample. Mil Med. 2012;177:390–6. doi: 10.7205/milmed-d-11-00133. [DOI] [PubMed] [Google Scholar]
- 16.Forbes D, Elhai JD, Miller MW, Creamer M. Internalizing and externalizing classes in posttraumatic stress disorder: a latent class analysis. J Trauma Stress. 2010;23:340–9. doi: 10.1002/jts.20526. [DOI] [PubMed] [Google Scholar]
- 17.Miller MW, Greif JL, Smith AA. Multidimensional Personality Questionnaire profiles of veterans with traumatic combat exposure: externalizing and internalizing subtypes. Psychol Assess. 2003;15:205–15. doi: 10.1037/1040-3590.15.2.205. [DOI] [PubMed] [Google Scholar]
- 18.Miller MW, Kaloupek DG, Dillon AL, Keane TM. Externalizing and internalizing subtypes of combat-related PTSD: a replication and extension using the PSY-5 scales. J Abnorm Psychol. 2004;113:636–45. doi: 10.1037/0021-843X.113.4.636. [DOI] [PubMed] [Google Scholar]
- 19.Miller MW, Resick PA. Internalizing and externalizing subtypes in female sexual assault survivors: implications for the understanding of complex PTSD. Behav Ther. 2007;38:58–71. doi: 10.1016/j.beth.2006.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McLeod DS, Koenen KC, Meyer JM, et al. Genetic and environmental influences on the relationship among combat exposure, posttraumatic stress disorder symptoms, and alcohol use. J Trauma Stress. 2001;14:259–75. doi: 10.1023/A:1011157800050. [DOI] [PubMed] [Google Scholar]
- 21.Xian H, Chantarujikapong SI, Scherrer JF, et al. Genetic and environmental influences on posttraumatic stress disorder, alcohol and drug dependence in twin pairs. Drug Alcohol Depend. 2000;61:95–102. doi: 10.1016/s0376-8716(00)00127-7. [DOI] [PubMed] [Google Scholar]
- 22.Norman SB, Myers US, Wilkins KC, et al. Review of biological mechanisms and pharmacological treatments of comorbid PTSD and substance use disorder. Neuropharmacology. 2012;62:542–51. doi: 10.1016/j.neuropharm.2011.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Young RM, Lawford BR, Feeney GF, Ritchie T, Noble EP. Alcohol-related expectancies are associated with the D2 dopamine receptor and GABAA receptor beta3 subunit genes. Psychiatry Res. 2004;127:171–83. doi: 10.1016/j.psychres.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 24.Connor JP, Young RM, Saunders JB, et al. The A1 allele of the D2 dopamine receptor gene region, alcohol expectancies and drinking refusal self-efficacy are associated with alcohol dependence severity. Psychiatry Res. 2008;160:94–105. doi: 10.1016/j.psychres.2007.06.030. [DOI] [PubMed] [Google Scholar]
- 25.Wang F, Simen A, Arias A, Lu QW, Zhang H. A large-scale meta-analysis of the association between the ANKK1/DRD2 Taq1A polymorphism and alcohol dependence. Hum Genet. 2013;132:347–58. doi: 10.1007/s00439-012-1251-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Young RM, Lawford BR, Noble EP, et al. Harmful drinking in military veterans with post-traumatic stress disorder: association with the D2 dopamine receptor A1 allele. Alcohol Alcohol. 2002;37:451–6. doi: 10.1093/alcalc/37.5.451. [DOI] [PubMed] [Google Scholar]
- 27.Logue MW, Solovieff N, Leussis MP, et al. The ankyrin-3 gene is associated with posttraumatic stress disorder and externalizing comorbidity. Psychoneuroendocrinology. 2013;38:2249–57. doi: 10.1016/j.psyneuen.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaufman J, Yang BZ, Douglas-Palumberi H, et al. Genetic and environmental predictors of early alcohol use. Biol Psychiatry. 2007;61:1228–34. doi: 10.1016/j.biopsych.2006.06.039. [DOI] [PubMed] [Google Scholar]
- 29.Nilsson KW, Sjoberg RL, Damberg M, et al. Role of the serotonin transporter gene and family function in adolescent alcohol consumption. Alcohol Clin Exp Res. 2005;29:564–70. doi: 10.1097/01.alc.0000159112.98941.b0. [DOI] [PubMed] [Google Scholar]
- 30.Venda LL, Cragg SJ, Buchman VL, Wade-Martins R. a-Synuclein and dopamine at the crossroads of Parkinson’s disease. Trends Neurosci. 2010;33:559–68. doi: 10.1016/j.tins.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berridge KC. The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology. 2007;191:391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- 32.Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–94. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- 33.Liang T, Spence J, Liu L, et al. alpha-Synuclein maps to a quantitative trait locus for alcohol preference and is differentially expressed in alcohol-preferring and -nonpreferring rats. Proc Natl Acad Sci U S A. 2003;100:4690–5. doi: 10.1073/pnas.0737182100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pelkonen A, Hiltunen M, Kiianmaa K, Yavich L. Stimulated dopamine overflow and alpha-synuclein expression in the nucleus accumbens core distinguish rats bred for differential ethanol preference. J Neurochem. 2010;114:1168–76. doi: 10.1111/j.1471-4159.2010.06844.x. [DOI] [PubMed] [Google Scholar]
- 35.Bonsch D, Reulbach U, Bayerlein K, Hillemacher T, Kornhuber J, Bleich S. Elevated alpha synuclein mRNA levels are associated with craving in patients with alcoholism. Biol Psychiatry. 2004;56:984–6. doi: 10.1016/j.biopsych.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 36.Mash DC, Adi N, Duque L, Pablo J, Kumar M, Ervin FR. Alpha synuclein protein levels are increased in serum from recently abstinent cocaine abusers. Drug Alcohol Depend. 2008;94:246–50. doi: 10.1016/j.drugalcdep.2007.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Agrawal A, Wetherill L, Bucholz KK, et al. Genetic influences on craving for alcohol. Addict Behav. 2013;38:1501–8. doi: 10.1016/j.addbeh.2012.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Foroud T, Wetherill LF, Liang T, et al. Association of alcohol craving with alpha-synuclein (SNCA) Alcohol Clin Exp Res. 2007;31:537–45. doi: 10.1111/j.1530-0277.2007.00337.x. [DOI] [PubMed] [Google Scholar]
- 39.Levey DF, Le-Niculescu H, Frank J, et al. Genetic risk prediction and neurobiological understanding of alcoholism. Transl Psychiatry. 2014;4:e391. doi: 10.1038/tp.2014.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pena-Oliver Y, Buchman VL, Dalley JW, et al. Deletion of alpha-synuclein decreases impulsivity in mice. Genes Brain Behav. 2012;11:137–46. doi: 10.1111/j.1601-183X.2011.00758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pena-Oliver Y, Sanchez-Roige S, Stephens DN, Ripley TL. Alpha-synuclein deletion decreases motor impulsivity but does not affect risky decision making in a mouse Gambling Task. Psychopharmacology. 2014;231:2493–506. doi: 10.1007/s00213-013-3416-y. [DOI] [PubMed] [Google Scholar]
- 42.Guillot CR, Fanning JR, Liang T, Berman ME. Evidence of a role for SNCA in impulse control in humans. Neurogenetics. 2014;15:77–8. doi: 10.1007/s10048-013-0379-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lesch KP, Merschdorf U. Impulsivity, aggression, and serotonin: a molecular psychobiological perspective. Behav Sci Law. 2000;18:581–604. doi: 10.1002/1099-0798(200010)18:5<581::aid-bsl411>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 44.Yanowitch R, Coccaro EF. The neurochemistry of human aggression. Adv Genet. 2011;75:151–69. doi: 10.1016/B978-0-12-380858-5.00005-8. [DOI] [PubMed] [Google Scholar]
- 45.Haller M, Chassin L. The influence of PTSD symptoms on alcohol and drug problems: internalizing and externalizing pathways. Psychol Trauma. 2013;5:484–93. [Google Scholar]
- 46.Miller MW, Vogt DS, Mozley SL, Kaloupek DG, Keane TM. PTSD and substance-related problems: the mediating roles of disconstraint and negative emotionality. J Abnorm Psychol. 2006;115:369–79. doi: 10.1037/0021-843X.115.2.369. [DOI] [PubMed] [Google Scholar]
- 47.Guillot CR, Fanning JR, Liang T, Berman ME. COMT associations with disordered gambling and drinking measures. J Gambl Stud. 2014 doi: 10.1007/s10899-013-9434-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Truett GE, Heeger P, Mynatt RL, Truett AA, Walker JA, Warman ML. Preparation of PCR-quality mouse genomic DNA with hot sodium hydroxide and tris (HotSHOT) Biotechniques. 2000;29:52, 4. doi: 10.2144/00291bm09. [DOI] [PubMed] [Google Scholar]
- 49.McDonald SD, Calhoun PS. The diagnostic accuracy of the PTSD checklist: a critical review. Clin Psychol Rev. 2010;30:976–87. doi: 10.1016/j.cpr.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 50.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Text Revision. 4. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 51.Babor TF, Higgins-Biddle JC, Saunders JB, Monteiro MG. The Alcohol Use Disorders Identification Test: Guidelines for Use in Primary Care. 2. Geneva, Switzerland: World Health Organization; 2001. [Google Scholar]
- 52.Rohsenow DJ. Drinking habits and expectancies about alcohol’s effects for self versus others. J Consult Clin Psychol. 1983;51:752–6. doi: 10.1037//0022-006x.51.5.752. [DOI] [PubMed] [Google Scholar]
- 53.Coccaro EF, Berman ME, Kavoussi RJ. Assessment of life history of aggression: development and psychometric characteristics. Psychiatry Res. 1997;73:147–57. doi: 10.1016/s0165-1781(97)00119-4. [DOI] [PubMed] [Google Scholar]
- 54.Rodriguez S, Gaunt TR, Day IN. Hardy-Weinberg equilibrium testing of biological ascertainment for Mendelian randomization studies. Am J Epidemiol. 2009;169:505–14. doi: 10.1093/aje/kwn359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Broman-Fulks JJ, Ruggiero KJ, Green BA, et al. Taxometric Investigation of PTSD: data from two nationally representative samples. Behav Ther. 2006;37:364–80. doi: 10.1016/j.beth.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 56.Forbes D, Haslam N, Williams BJ, Creamer M. Testing the latent structure of posttraumatic stress disorder: a taxometric study of combat veterans. J Trauma Stress. 2005;18:647–56. doi: 10.1002/jts.20073. [DOI] [PubMed] [Google Scholar]
- 57.Ruscio AM, Ruscio J, Keane TM. The latent structure of posttraumatic stress disorder: a taxometric investigation of reactions to extreme stress. J Abnorm Psychol. 2002;111:290–301. [PubMed] [Google Scholar]
- 58.Hayes AF. Introduction to mediation, moderation, and conditional process analysis: a regression-based approach. New York: Guilford Press; 2013. [Google Scholar]
- 59.Liang T, Kimpel MW, McClintick JN, et al. Candidate genes for alcohol preference identified by expression profiling in alcohol-preferring and -nonpreferring reciprocal congenic rats. Genome Biol. 2010;11:R11. doi: 10.1186/gb-2010-11-2-r11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Oksman M, Tanila H, Yavich L. Brain reward in the absence of alpha-synuclein. Neuroreport. 2006;17:1191–4. doi: 10.1097/01.wnr.0000230507.70843.51. [DOI] [PubMed] [Google Scholar]
- 61.Keri S, Moustafa AA, Myers CE, Benedek G, Gluck MA. {alpha}-Synuclein gene duplication impairs reward learning. Proc Natl Acad Sci U S A. 2010;107:15992–4. doi: 10.1073/pnas.1006068107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Couppis MH, Kennedy CH. The rewarding effect of aggression is reduced by nucleus accumbens dopamine receptor antagonism in mice. Psychopharmacology. 2008;197:449–56. doi: 10.1007/s00213-007-1054-y. [DOI] [PubMed] [Google Scholar]
- 63.Bondar NP, Boyarskikh UA, Kovalenko IL, Filipenko ML, Kudryavtseva NN. Molecular implications of repeated aggression: Th, Dat1, Snca and Bdnf gene expression in the VTA of victorious male mice. PLoS One. 2009;4:e4190. doi: 10.1371/journal.pone.0004190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kudryavtseva NN, Bondar NP, Boyarskikh UA, Filipenko ML. Snca and Bdnf gene expression in the VTA and raphe nuclei of midbrain in chronically victorious and defeated male mice. PLoS One. 2010;5:e14089. doi: 10.1371/journal.pone.0014089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pihl RO, Peterson JB, Lau MA. A biosocial model of the alcohol-aggression relationship. J Stud Alcohol Suppl. 1993;11:128–39. doi: 10.15288/jsas.1993.s11.128. [DOI] [PubMed] [Google Scholar]
- 66.Brown SA, Goldman MS, Inn A, Anderson LR. Expectations of reinforcement from alcohol: their domain and relation to drinking patterns. J Consult Clin Psychol. 1980;48:419–26. doi: 10.1037//0022-006x.48.4.419. [DOI] [PubMed] [Google Scholar]
- 67.Lundahl LH, Davis TM, Adesso VJ, Lukas SE. Alcohol expectancies: effects of gender, age, and family history of alcoholism. Addict Behav. 1997;22:115–25. doi: 10.1016/s0306-4603(96)00022-6. [DOI] [PubMed] [Google Scholar]
- 68.Sinha R. How does stress lead to risk of alcohol relapse? Alcohol Res. 2012;34:432–40. [PMC free article] [PubMed] [Google Scholar]