Abstract
Humans use anticipatory and compensatory postural strategies to maintain and restore balance when perturbed. Inefficient generation and utilization of anticipatory postural adjustments (APAs) is one of the reasons for postural instability. The aim of the study was to investigate the role of training in improvement of APAs and its effect on subsequent control of posture. Thirteen healthy young adults were exposed to predictable external perturbations before and after a single training session consisting of catches of a medicine ball thrown at the shoulder level. 3-D body kinematics, EMG activity of thirteen trunk and leg muscles, and ground reaction forces were recorded before and immediately after a single training session. Muscle onsets, EMG integrals, center of pressure (COP), and center of mass (COM) displacements were analyzed during the anticipatory and compensatory phases of postural control. The effect of a single training session was seen as significantly early muscle onsets and larger anticipatory COP displacements. As a result, significantly smaller peak COM displacements were observed after the perturbation indicating greater postural stability. The outcome of this study provides a background for examining the role of training in improvement of APAs and its effect on postural stability in individuals in need.
Keywords: balance control, anticipatory postural adjustments, training, ball catching, postural stability, rehabilitation
Introduction
Anticipatory postural control strategies are mostly acquired through learning and based on previous experience of the postural disturbance (Massion, 1992, Schmitz et al., 2002, Witherington et al., 2002). Historically, it was considered that APAs are acquired only in preparation of a self-generated perturbation, for example, resulting from a focal movement. Anticipatory postural adjustments were considered to differ from compensatory postural adjustments (CPAs) because their organization was thought to be based on experience in performing intentional actions (Massion, 1992). However, it has long been shown that while APAs are certainly generated prior to an intentional motor action, they are also produced in preparation of an external predictable perturbation. Thus, several studies involving bimanual loading and unloading tasks, have demonstrated the presence of anticipatory adjustments in the postural forearm and the trunk and leg muscles, even in the absence of an explicit voluntary movement (Aruin et al., 2001, Massion, 1992, Shiratori and Latash, 2001). Moreover, the outcome of the experiments where the kinetic energy of the impact could be estimated through visual or proprioceptive cues, confirmed that anticipatory adjustments can be produced based on available visual information about the forthcoming body perturbation (Aruin, Shiratori, 2001, Massion, 1992, Shiratori and Latash, 2001). Likewise, it was also shown that APAs are generated in trunk and leg muscles based on an accurate prediction of the timing of the whole body perturbation (induced with pendulum-impact paradigm) using visual inputs (Santos and Aruin, 2008, 2009). Thus, APAs are generated prior to a “predictable” perturbation, irrespective of its internal or external origin.
While APAs are acquired based on previous experiences and learning, they are also capable of short-term adaptation in response to immediate environmental changes. Thus, APAs are scaled according to the actual or perceived level of body stability. For example, in experiments where a standard action initiated a standard perturbation, APA amplitude was reduced in conditions of low initial stability (such as standing on a narrow beam or see-saw) and the magnitude of APAs was scaled according to the magnitude of postural instability (Aruin et al., 1998, Gantchev and Dimitrova, 1996). In some muscles the onset of activation was also delayed as the instability levels increased. Also, the effect of instability was stronger when the direction of perturbation coincided with the direction of instability (Aruin et al, 1998). In other experiments, where leg flexions were performed from initial bipedal and unipedal stance conditions, APA amplitudes were reduced when the initial conditions were unstable (unipedal stance) (Nouillot et al., 1992). This suppression of APAs was thought to be protective in nature, since APAs by definition are based on an approximation of the perturbation and they may act as a source of instability if inappropriately executed. Besides the presence of instability, it has been found that the nature of the instability is also important to the generation of APAs. If the instability is due to reduced base of support, APA amplitudes are reduced, however if the instability is due to diminished friction between the feet or shoes and the surface (such as standing on roller skates) or due to other causes that are not mechanical in nature (such as under conditions of muscle vibration that induce postural illusion), APAs are increased in magnitude (Shiratori and Latash, 2000, Slijper and Latash, 2004). At the other extreme are highly stable conditions, wherein APA amplitudes are also reduced, for example in sitting condition (Aruin and Shiratori, 2003), or with an additional finger touch support during arm flexion in standing (Slijper and Latash, 2000) or hand support during rocking on heels movement (Noe et al., 2003). In highly stable conditions, APAs would not be required to maintain equilibrium and they are therefore scaled down. These changes in APAs that occur immediately following modifications in the environmental factors are considered to be short-term adaptations based on sensory cues about the new environmental conditions (Massion, 1992).
On the other hand, changes in feedforward postural strategies that are observed after several trials or after several days of exposure to new environmental conditions signify short-term learning related changes. For example, it was reported that patients with low back pain frequently show delayed anticipatory activation of the deep trunk muscle, transversus abdominis (TrA); such a delay is considered as a consistent marker of dysfunction in trunk motor control (Hodges and Richardson, 1996). Thus, in experiments involving patients with low back pain, a single training session involving isolated voluntary contraction of the TrA muscle in supine resulted in early APA onset in this muscle prior to arm flexion movements (Tsao and Hodges, 2007). Moreover, with four weeks of such training early onsets were seen along with consistent activation of this muscle during walking as opposed to phasic activity prior to training (Tsao and Hodges, 2008). These effects were retained at six month follow up. Additionally, with training, the patterns of anticipatory activation of the deep trunk muscle seen in individuals with low back pain became closer to the patterns of activity observed in healthy individuals and may have led to possible improvements in symptoms (Tsao and Hodges, 2008).
While motor adaptive changes in APAs have been seen with training, it is not known how training-related modifications in anticipatory postural activity can affect the functional role of APAs, mainly control of equilibrium. Particularly, previous studies on training based changes in APAs have focused on the role of APAs with respect to perturbations that were self-initiated, either directly or indirectly (Massion et al., 1999, Tsao and Hodges, 2007). It is not known how a short training session that involves a functional activity such as catching a ball may affect the generation of APAs prior to a predictable external perturbation and the effect of these motor learning induced changes in APAs on subsequent balance control. Thus, the objective of the study was to investigate the immediate effects of a single training session in enhancing the utilization of anticipatory postural adjustments in balance control of healthy young adults. We hypothesized that early onset of anticipatory muscle activity will be observed following training. This enhanced postural preparation will result in reduced COM and COP peak displacements (indicating greater postural stability) following a predictable external perturbation.
Methods
Subjects
Thirteen healthy young adults (7 males and 6 females) without any neurological or musculoskeletal disorders participated in the study. The mean age of the group was 26.69 ± 3.72 years; mean body mass 68.10 ± 13.61 kg, and mean height 1.74 ± 0.09 m. All the subjects signed a written informed consent approved by the university’s Institutional Review Board.
Experimental Set-up and Procedure
The subjects participated in a single training session and they were tested twice, before and immediately after the end of training. During the tests the subjects stood on the force platform and were exposed to external perturbations induced by a pendulum (Santos and Aruin, 2008, 2009). A load (mass, m = 3% of the subjects’ body weight) was attached to the pendulum next to its distal end (Fig. 1). The subjects were required to receive each pendulum impact with their hands, while their arms, wrists, and fingers were extended at the shoulder level, and to maintain their balance after the perturbation. The impact was induced in a sagittal plane and posterior direction; the amplitude of the pendulum impact when it hit the subject’s hands was about 90 Nm. Two to three practice trials were given prior to initial testing. The subjects received a series of predictable perturbations (eyes were open) before (pre-training) and immediately after (post-training) a short training session. Ten trials, each five seconds in duration, were performed in each experimental condition involving assessment with pendulum perturbations.
Fig. 1.
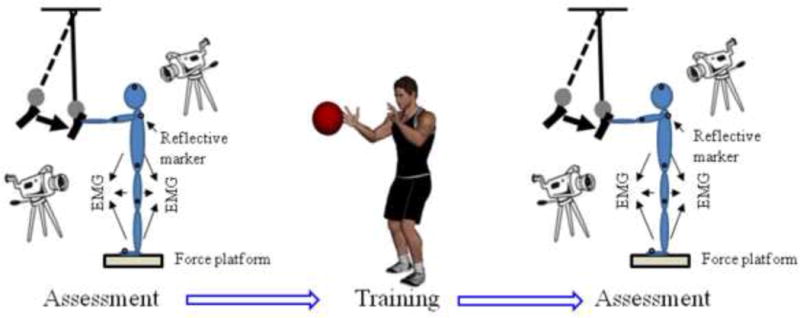
Schematic representation of the experimental set up. The pendulum impact paradigm was used for the assessment. EMG activity, displacements of the reflective markers, and ground reaction forces were recorded. Training involved catches of a medicine ball.
The training session consisted of 130 catches of a medicine ball thrown at the shoulder level from a distance of 3 m and lasted for about 20–25 minutes. The catches included the ball being thrown either directly towards the subjects’ midline or slightly to the right or left of midline. The subjects caught the ball while standing with feet shoulder width apart. Thus, the training session involved perturbations that were predictable and as such would generate both, APAs and CPAs. A 0.9 or 1.8 kg medicine ball was used for subjects weighing below or above 55 kg, respectively. The weight of the ball and the number of catches were decided based on pilot experiments in which subjects were surveyed about their tolerance to catching balls of different weights. For safety purposes in all the experiments, the subjects wore a harness (NeuroCom, USA) with two straps loosely attached to the ceiling. All participants were allowed to have rest periods as needed.
Instrumentation
Electromyographic (EMG) activity of thirteen right trunk and lower limb muscles was recorded with disposable surface electrodes (Red Dot 3M). After the skin area was cleaned with alcohol preps, electrodes were attached to the muscle belly of erector spinae longus (ESL, 3 cm lateral to the first lumbar vertebra), rectus abdominis (RA, 3 cm lateral to the umbilicus), external oblique (EO, midpoint on the axial line between the 10th rib and the anterior superior iliac spine (ASIS)), gluteus medius (GMED, midpoint on the line from the iliac crest to the greater trochanter), semitendinosus (ST, midpoint on the line from the ischial tuberosity to the medial epicondyle of the tibia), biceps femoris (BF, midpoint on the line from the ischial tuberosity to the lateral epicondyle of the tibia), vastus lateralis (VL, at 2/3rd on the line between ASIS and the lateral side of the patella), vastus medialis (VM, at lower 25% on the line between ASIS and medial knee joint space), rectus femoris (RF, midpoint on the line from the ASIS to the superior part of the patella), medial gastrocnemius (GASM, on the most prominent bulge of the muscle), lateral gastrocnemius (GASL, at 1/3rd of the line from the head of the fibula to the lateral side of the Achilles tendon insertion), soleus (SOL, at 2/3rd of the line between the medial femoral condyle and the medial malleolus), and tibialis anterior (TA, at 1/3rd on the line between the tip of the fibula and the tip of the medial malleolus). The placement of electrodes for recording EMG activity was based on recommendations reported in the literature (Basmajian, 1980, Hermens et al., 2000). The distance between two electrodes in a pair was 25 mm. A ground electrode was attached to the anterior aspect of the leg over the tibial bone. EMG signals were collected, filtered, and amplified (10–500 Hz, gain 2000) with differential amplifiers (Myopac, RUN Technologies, USA). Ground reaction forces and moments of force were recorded using a force platform (model OR-5, AMTI, USA). The moment of the perturbation (pendulum impact) was identified by using an accelerometer (Model 208CO3, PCB Piezotronics, Inc, USA) attached to the pendulum.
Three-dimensional kinematic data was collected using a six-camera VICON 612 system (Oxford Metrics, UK). Retroreflective markers were placed over anatomical landmarks bilaterally according to the Plug-In-Gait (PIG) model (Vicon®, 2002), which includes: second metatarsal head, calcaneus, lateral malleolus, lateral epicondyle of the femur, a marker on the lateral border of the leg (between the lateral malleolus and femoral epicondyle markers), anterior/posterior superior iliac spines, a marker on the lateral border of the thigh (between the femoral epicondyle and anterior superior iliac spines), second metacarpal, lateral epicondyle of the humerus, acromio-clavicular joint, and a marker on the lateral border of the arm (between the humeral epicondyle and the acromio-clavicular joint markers). Also, subjects wore head and wrists bands with 4 and 2 markers attached on them, respectively. Finally, 5 additional markers were attached over the following landmarks: 7th cervical vertebra, 10th thoracic vertebra, inferior angle of the right scapula, between the 2 sternoclavicular joints, and xiphoid process of the sternum bone.
EMG, forces, moments of force, and accelerometer signals were digitized with a 16-bit resolution at 1000 Hz and kinematic data were acquired at 100 Hz by means of the VICON 612 data station that controlled data collection of all signals.
Data Processing
The data were analyzed off-line using MATLAB (MathWorks, Natick, MA) programs. Five to seven trials were used for further analysis since we had to discard trials with incomplete kinematic data even when the trial contained good EMG and force platform data. EMG signals were full-wave rectified and filtered with a 100 Hz low-pass, 2nd order, zero-lag Butterworth filter, while the ground reaction forces, moments, and COM data were filtered with a 40 Hz low-pass, 2nd order, zero-lag Butterworth filter. The „time-zero’ (T0=0, moment of pendulum impact) was calculated from the accelerometer signal. First the maximum magnitude was obtained and then the point of time at which the acceleration magnitude reached 5% of the maximum was considered as T0. This value was confirmed by visual inspection by an experienced researcher. Data in the range from −600 ms (before T0) to +1000 ms (after T0) were selected for further analysis. Individual trials were aligned according to T0 and this was used as a common reference point for all the signals.
Muscle onset/latency for each trial was detected in a time window from −250 ms to +250 ms in relation to T0 by a combination of computer algorithm and visual inspection of the trials. The latency for a specific muscle was defined as the instant lasting for at least 50 ms when its EMG amplitude was greater (activation) or smaller (inhibition) than the mean ± 2 SD of its baseline value, measured from −500 to −400 ms. The onset latencies for each muscle were then averaged across the trials within each condition for each subject.
Integrals of anticipatory and compensatory EMG activity were derived using average trials for each subject. Integrals of the EMG activities (IntEMGi) were calculated for 4 different epochs, each of 150 ms duration in relation to T0. The time windows for the 4 epochs were: 1) from −250 ms to −100 ms (anticipatory activity, APA1); 2) −100 ms to +50 ms (anticipatory activity, APA2); 3) +50 ms to 200 ms (compensatory reactions, CPA1); and 4) + 200 ms to 350 ms (late compensatory reactions, CPA2). The IntEMGi for each of the 4 epochs was further corrected by the EMG integral of the baseline activity from −600 ms to −450 ms in relation to T0 (the same 150 ms duration as the duration of each of the four epochs). The integrals of EMG activity were then normalized by the peak muscle activity across all conditions within an experiment for each muscle for each subject. For a given subject’s specific muscle, the normalization steps were: (1) to find a maximum absolute value for each muscle (∫EMG max) among all conditions, and (2) to divide each integral by the maximum (∫EMG max) (Krishnan and Aruin, 2011, Li and Aruin, 2009). All the normalized integrals (IEMGNORM) are thus within the range from +1 to −1, with positive values indicating an increase in the activation of the muscle and negative values indicating a decrease in the background activity (inhibition).
Displacements of the center of pressure (COP) in the anterior-posterior direction were calculated based on previous literature (Santos et al., 2010, Winter et al., 1996). The baseline activity used for calculation was from −500 to −400 ms in relation to T0. Initial processing of the kinematic data was done using the Vicon and BodyBuilder 3D modeling software. The PIG model consisted of fifteen body segments, including pelvis, femur (2), tibia (2), feet (2), humerus (2), radius (2), hands (2), thorax, and head. Body mass and height, 7 anthropometrical measures such as leg length, knee, ankle, elbow, and wrist width and shoulder offset and hand thickness for each subject were entered in the PIG model. These measures together with the kinematic data were used to calculate body’s COM position. Further analysis was performed using customized MATLAB programs. Aligned trials were averaged for each subject for each condition. The anterior-posterior displacements of COP and COM at T0 which is anticipatory in nature and the peak displacement (maximum displacement after T0) that is compensatory in nature were calculated. It is important to note that larger peak displacements following the perturbation indicate greater postural instability.
Statistical Analysis
Statistical analysis was performed in SPSS 17 for Windows XP (SPSS Inc., Chicago, USA). Means with standard errors are reported. A paired t-test was used for comparison of latencies of individual muscles between the two experimental conditions (pre-training and post-training). For IEMGNORM, separate 2 × 4 repeated measures ANOVAs were performed for each muscle. There were two within-subject factors: experimental condition (2 levels: pre-training and post-training) and epoch (4 levels: APA1, APA2, CPA1, and CPA2). When condition x epoch interactions were significant, paired t-tests with Bonferroni’s correction were used for post hoc comparisons. Paired t-tests were also used for comparing displacements of COP and COM at T0 and at peak between the two experimental conditions (pre-training and post-training). Statistical significance was set at p < 0.05 for all tests except for the post-hoc comparisons performed for IEMGNORM. When post-hoc comparisons were performed for investigating the differences in IEMGNORM between the two conditions across each epoch, Bonferroni’s correction was applied such that p < 0.012 was considered statistically significant. When post-hoc comparisons were performed for investigating the differences in IEMGNORM between the four epochs within a given condition, Bonferroni’s correction was applied such that p < 0.008 was considered statistically significant.
Results
Onset of Muscle Activity (Muscle Latency)
Overall, after a single training session, postural activity in anticipation of the perturbation occurred earlier as compared to the pre-training condition. The difference between the two conditions was statistically significant for six out of thirteen muscles, namely: SOL, RF, VM, VL, BF, and ST (Fig. 2). The onset of APA activity before and after training was as follows: SOL (pre-training: −122.65 ± 11.43 ms, post-training: −157.26 ± 12.01 ms, t = 2.726, p = 0.020); RF (pre-training: −113.04 ± 7.22 ms, post-training: −157.40 ± 14.68 ms, t = 2.627, p = 0.030); VM (pre-training: −92.91 ± 7.98 ms, post-training: −138.95 ± 17.72 ms, t = 2.961, p = 0.025); VL (pre-training: −93.42 ± 8.35 ms, post-training: −136.17 ± 13.56 ms, t = 2.410, p = 0.047); BF (pre-training: −132.60 ± 8.89 ms, post-training: −171.02 ± 14.18 ms, t = 3.643, p = 0.004); ST (pre-training: −144.91 ± 12.80 ms, post-training: −178.95 ± 15.58 ms, t = 2.855, p = 0.016). There was a trend toward earlier APA onsets following training as seen in GASM (pre-training: −144.17 ± 18.58 ms, post-training: −199.68 ± 19.88 ms, t= 2.077, p = 0.071) and TA (pre-training: −86.69 ± 14.72 ms, post-training: −118.65 ± 19.83 ms, t = 2.008, p = 0.076) muscles, but the differences were not statistically significant.
Fig. 2.
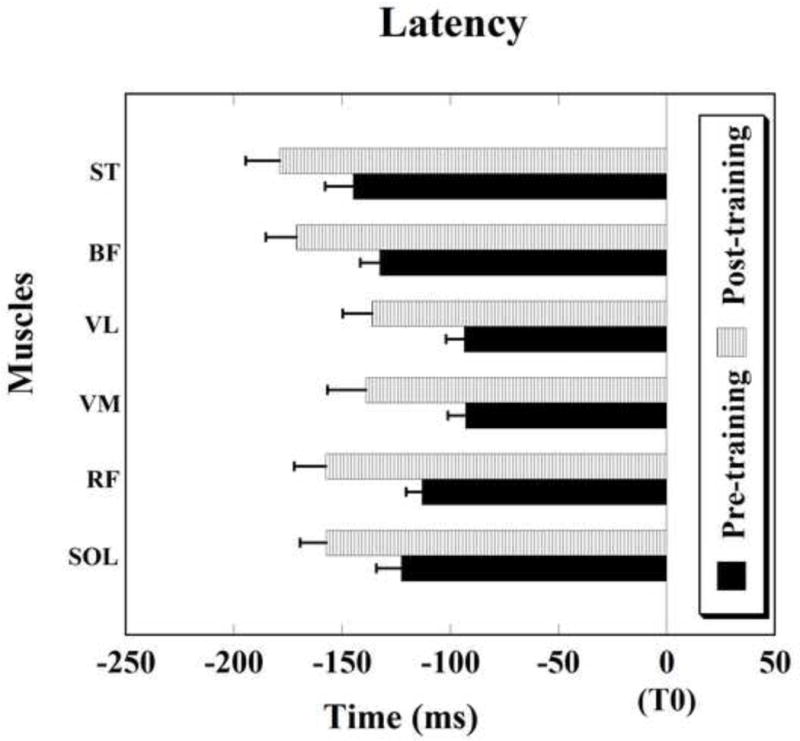
Muscle activity onsets. Note the early onsets of anticipatory postural activity (prior to the moment of perturbation (T0)) after a single training session in young adults. Muscle abbreviations: SOL-soleus, RF – rectus femoris, VM – vastus medialis, VL – vastus lateralis, BF- biceps femoris, and ST-semitendinosus. Differences in latencies are significant for all muscles shown, p < 0.05. Mean±SE are shown.
Integrated Electromyographic Activity
A significant interaction between condition and epoch was seen for the TA muscle (F3,36= 3.681, p = 0.021). The condition and epoch interaction was close to significance for the VL muscle (F3,36 = 2.781, p = 0.055). The post-hoc effects were, however, not statistically significant for both the muscles.
Displacements of Center of Pressure and Center of Mass
Figures 3 and 4 depict the displacements of COP and COM respectively, at T0 and the peak displacements after T0 for the two conditions. It can be seen that the anticipatory COP displacement (at T0) following training (24.53 ± 1.57 mm) was significantly larger than before training (20.99 ± 1.25 mm) (t = −2.834, p = 0.020). At the same time, the peak displacements were similar between the two conditions (before and after the training).
Fig. 3.
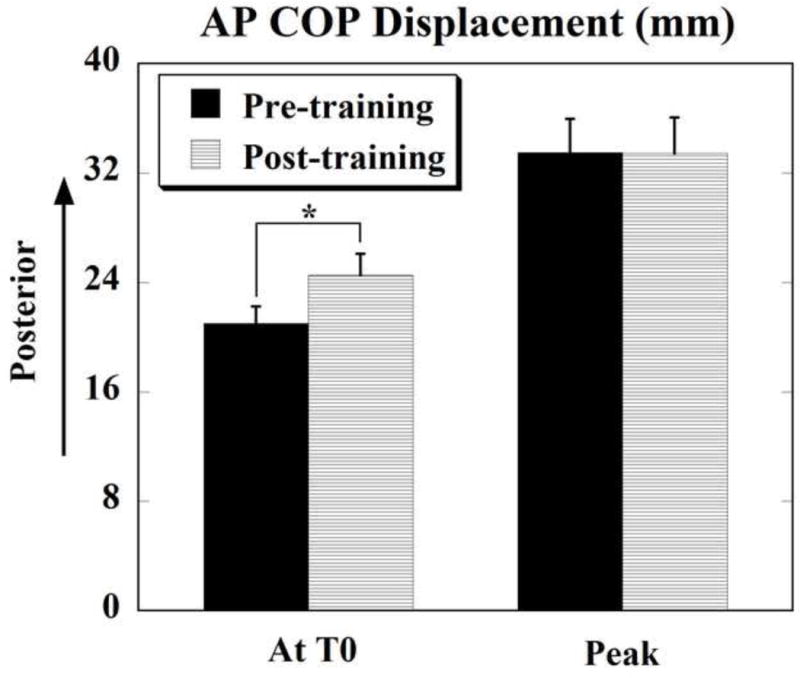
Anterior-posterior (AP) displacement of COP at T0 (anticipatory) and peak displacement (after T0, compensatory) before (pre-training) and after (post-training) training. Positive values indicate displacement in the posterior/backward direction. * denotes p < 0.05. Mean±SE are shown.
Fig. 4.
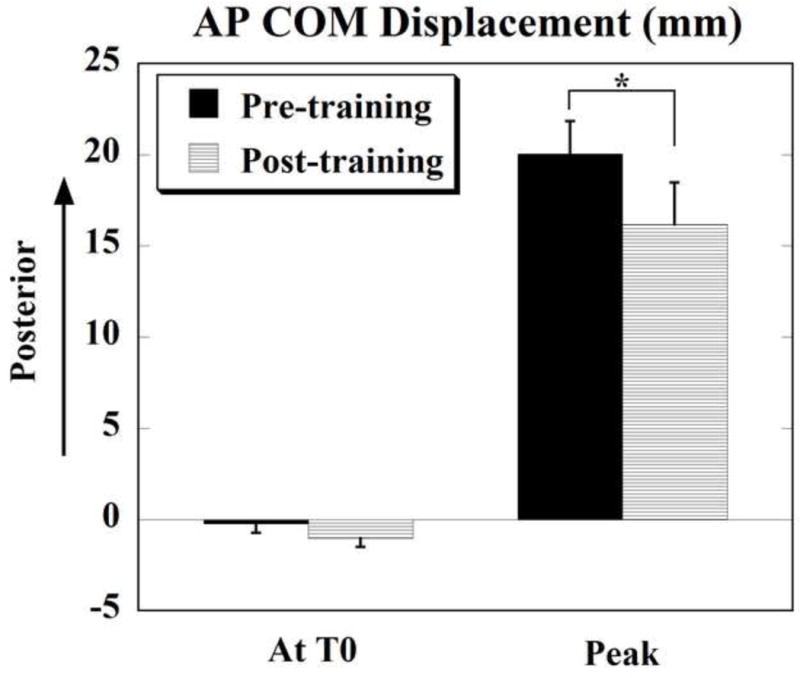
Anterior-posterior (AP) displacement of COM at T0 (anticipatory) and peak displacement (after T0, compensatory) before (pre-training) and after (post-training) training. Positive values indicate displacement in the posterior/backward direction and negative values indicate displacement in the anterior/forward direction. * denotes p < 0.01. Mean±SE are shown.
The anticipatory COM displacement in the forward direction (i.e. opposite to the direction of perturbation) following training was −1.04 ± 0.48 mm as compared to the pre-training condition (−0.22 ± 0.52 mm), the difference was, however, not statistically significant (p=0.149). The peak COM displacement following training (16.18 ± 2.30 mm) was significantly smaller than the peak displacement before training (20.04 ± 1.79 mm) (t = 3.873, p = 0.003).
Discussion
The present study was focused on examining the immediate effects of training on the utilization of anticipatory postural adjustments in balance control of healthy young adults. The findings of the study demonstrated the effectiveness of a single training session involving a functional activity (ball catching) in enhancing anticipatory postural adjustments. The greater postural preparation due to improvement of APAs resulted in greater postural stability following a perturbation.
After the training session, the anticipatory activity was earlier in the leg and thigh muscles; however, no changes were seen in the trunk muscles. The lack of changes in the trunk muscles is in contrast to previously reported findings (Tsao and Hodges, 2007, 2008). One of the possible reasons for the difference in findings observed between the studies is that we studied healthy young adults while these previous investigations included patients with chronic low back pain. As such not all muscles of young subjects could be expected to show improvement with training. Another reason relates to the differences in the experimental tasks used to induce perturbation: external symmetrical perturbations vs. unilateral rapid arm flexion. Moreover, this study was focused on the immediate effects of a single session of training. It needs to be studied whether longer training would result in an improvement of APA activity in the trunk muscles of healthy young adults. Early muscle onsets observed after training indicate greater postural preparation in anticipation of the expected disturbance. As a result of this preparation, smaller compensatory postural adjustments were required to deal with the destabilizing effects of the perturbation. Changes in EMG activity (early activation of muscles) were also associated with changes in COP and COM displacements. Thus, following a single training session larger displacements of the COP in the posterior direction that could help in body stabilization were observed in preparation to the forthcoming perturbation. As a result of this enhanced preparation, the eventual peak displacement of the body’s COM after the impact was significantly smaller, indicating improved postural stability.
In the past, some studies have shown training related improvements in anticipatory postural activity in healthy individuals or in people with low back pain. Thus, one of the early studies showed that healthy individuals could acquire APAs in their postural forearm during a load lifting task initiated by the opposite hand without a direct movement of the triggering hand after about 40 to 60 repetitions (Paulignan et al., 1989). However when the arms were switched, the acquisition was not transferred to the new postural forearm (Massion, Ioffe, 1999). In experiments involving patients with low back pain, isolated voluntary contraction of the transverses abdominis muscle was found to cause early APA onset in this muscle during arm flexion movements immediately after a single training session (Tsao and Hodges, 2007) as opposed to training that involved sit-ups where all abdominal muscles were exercised as a group. Thus, these studies provide preliminarily evidence that motor adaptive changes in feedforward postural adjustments or APAs are possible with training and changes in APAs depend on the type of motor training (Tsao and Hodges, 2008). However, these previous studies have shown improvements in APAs with respect to their role in stabilization of a postural segment or with respect to movement performance. Since APAs are known to play a significant role in whole body balance control and are also known to be impaired with aging (Inglin and Woollacott, 1988, Rogers et al., 1992, Woollacott and Manchester, 1993) or neurological disorders such as stroke (Garland et al., 1997), Parkinson’s disease (Latash et al., 1995), and multiple sclerosis (Krishnan et al., 2012a, b), it is important to understand how training-related modifications in anticipatory postural activity can affect the functional role of APAs in control of equilibrium following a perturbation. This study for the first time demonstrates that training involving a functional activity such as catching a ball, that causes whole body predictable perturbation, is capable of enhancing anticipatory postural activity in several lower limb muscles. Moreover, when exposed to a predictable but external perturbation, the improved anticipatory postural control is used more efficiently such that the compensatory postural responses are scaled down and greater postural stability is achieved.
Conclusion
This study has demonstrated that anticipatory postural activity in lower limb muscles prior to a predictable external perturbation can be augmented in healthy young adults after a single training session. Training-related changes are reflected as early onsets of APA activity as well as larger anticipatory COP displacements. The ability to utilize increased anticipatory activity is improved following training and is reflected in smaller displacements of COM after the perturbation, indicating greater postural stability. The outcome of the study suggests that training could be used in enhancement of APAs in individuals in need.
Acknowledgments
This work was supported in part by the NIH grant # HD064838. We thank Vennila Krishnan and Xiaoyan Li for assistance in data processing.
Biographies

Neeta Kanekar received her PhD in Kinesiology and Rehabilitation Sciences from the University of Illinois at Chicago (UIC) and prior to that her MS in Physical Therapy from UIC. After receiving her Bachelor’s degree in Physiotherapy from Maharashtra University of Health Sciences, Seth G.S. Medical College & K.E.M Hospital, India, she practiced as a PT for a few years before pursuing active research. She is currently doing her postdoctoral fellowship with her research focus on brain plasticity and stroke rehabilitation using non-invasive brain stimulation methods. Her primary area of research is related to motor control and learning, brain plasticity, biomechanics, and rehabilitation. She is actively involved in developing new rehabilitation approaches for improving mobility and function in older adults and individuals with neurological disorders such as stroke and multiple sclerosis.

Alexander S. Aruin received his PhD in Medical Cybernetics from the Moscow Institute of Artificial Organs and Transplantation in 1978 and a D.Sc. (PhD) in Biomechanics from the Latvian Institute of Traumatic Injuries and Orthopedics in 1990. He was affiliated with the Moscow Institute of Electronic Engineering as Full Professor and Director of the Laboratory of Ergonomics and Biomechanics. After moving to the United States in 1992, he became a faculty at Rush-Presbyterian St. Luke’s Medical Center in Chicago and later a faculty member at Pennsylvania State University. He is currently a Professor of Physical Therapy, Bioengineering, and the Director of the Knecht Movement Science Laboratory at the University of Illinois at Chicago, a Professor of Physical Medicine & Rehabilitation at Rush University, Chicago, and a Senior Clinical Researcher at Marianjoy Rehabilitation Hospital in Wheaton, Illinois. He has co-authored more than 140 referred papers, two monographs, and 44 patents in the fields of biomechanics, motor control, neuromuscular disorders, and rehabilitation. Dr. Aruin develops new technologies for training healthy people and for providing physical therapy and rehabilitation to injured and disabled individuals. He is a recipient of federally supported grants and is a member of the American Society for Biomechanics, Society for Neuroscience, and International Society of Motor Control.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aruin A, Shiratori T. Anticipatory postural adjustments while sitting: the effects of different leg supports. Exp Brain Res. 2003;151:46–53. doi: 10.1007/s00221-003-1456-y. [DOI] [PubMed] [Google Scholar]
- Aruin AS, Forrest WR, Latash ML. Anticipatory postural adjustments in conditions of postural instability. Electroencephalogr Clin Neurophysiol. 1998;109:350–9. doi: 10.1016/s0924-980x(98)00029-0. [DOI] [PubMed] [Google Scholar]
- Aruin AS, Shiratori T, Latash ML. The role of action in postural preparation for loading and unloading in standing subjects. Exp Brain Res. 2001;138:458–66. doi: 10.1007/s002210100729. [DOI] [PubMed] [Google Scholar]
- Basmajian JV. Electromyography–dynamic gross anatomy: a review. Am J Anat. 1980;159:245–60. doi: 10.1002/aja.1001590302. [DOI] [PubMed] [Google Scholar]
- Gantchev GN, Dimitrova DM. Anticipatory postural adjustments associated with arm movements during balancing on unstable support surface. Int J Psychophysiol. 1996;22:117–22. doi: 10.1016/0167-8760(96)00016-5. [DOI] [PubMed] [Google Scholar]
- Garland SJ, Stevenson TJ, Ivanova T. Postural responses to unilateral arm perturbation in young, elderly, and hemiplegic subjects. Arch Phys Med Rehabil. 1997;78:1072–7. doi: 10.1016/s0003-9993(97)90130-1. [DOI] [PubMed] [Google Scholar]
- Hermens HJ, Freriks B, Disselhorst-Klug C, Rau G. Development of recommendations for SEMG sensors and sensor placement procedures. J Electromyogr Kinesiol. 2000;10:361–74. doi: 10.1016/s1050-6411(00)00027-4. [DOI] [PubMed] [Google Scholar]
- Hodges PW, Richardson CA. Inefficient muscular stabilization of the lumbar spine associated with low back pain. A motor control evaluation of transversus abdominis. Spine (Phila Pa 1976) 1996;21:2640–50. doi: 10.1097/00007632-199611150-00014. [DOI] [PubMed] [Google Scholar]
- Inglin B, Woollacott M. Age-related changes in anticipatory postural adjustments associated with arm movements. J Gerontol. 1988;43:M105–13. doi: 10.1093/geronj/43.4.m105. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Aruin AS. Postural control in response to a perturbation: role of vision and additional support. Exp Brain Res. 2011;212:385–97. doi: 10.1007/s00221-011-2738-4. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Kanekar N, Aruin AS. Anticipatory postural adjustments in individuals with multiple sclerosis. Neurosci Lett. 2012a;506:256–60. doi: 10.1016/j.neulet.2011.11.018. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Kanekar N, Aruin AS. Feedforward postural control in individuals with multiple sclerosis during load release. Gait Posture. 2012b;36:225–30. doi: 10.1016/j.gaitpost.2012.02.022. [DOI] [PubMed] [Google Scholar]
- Latash ML, Aruin AS, Neyman I, Nicholas JJ. Anticipatory postural adjustments during self inflicted and predictable perturbations in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1995;58:326–34. doi: 10.1136/jnnp.58.3.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Aruin AS. The effect of short-term changes in body mass distribution on feed-forward postural control. J Electromyogr Kinesiol. 2009;19:931–41. doi: 10.1016/j.jelekin.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Massion J. Movement, posture and equilibrium: interaction and coordination. Prog Neurobiol. 1992;38:35–56. doi: 10.1016/0301-0082(92)90034-c. [DOI] [PubMed] [Google Scholar]
- Massion J, Ioffe M, Schmitz C, Viallet F, Gantcheva R. Acquisition of anticipatory postural adjustments in a bimanual load-lifting task: normal and pathological aspects. Exp Brain Res. 1999;128:229–35. doi: 10.1007/s002210050842. [DOI] [PubMed] [Google Scholar]
- Noe F, Quaine F, Martin L. Mechanical effect of additional supports in a rocking on heels movement. Gait Posture. 2003;18:78–84. doi: 10.1016/s0966-6362(02)00163-7. [DOI] [PubMed] [Google Scholar]
- Nouillot P, Bouisset S, Do MC. Do fast voluntary movements necessitate anticipatory postural adjustments even if equilibrium is unstable? Neurosci Lett. 1992;147:1–4. doi: 10.1016/0304-3940(92)90760-5. [DOI] [PubMed] [Google Scholar]
- Paulignan Y, Dufosse M, Hugon M, Massion J. Acquisition of co-ordination between posture and movement in a bimanual task. Exp Brain Res. 1989;77:337–48. doi: 10.1007/BF00274991. [DOI] [PubMed] [Google Scholar]
- Rogers MW, Kukulka CG, Soderberg GL. Age-related changes in postural responses preceding rapid self-paced and reaction time arm movements. J Gerontol. 1992;47:M159–65. doi: 10.1093/geronj/47.5.m159. [DOI] [PubMed] [Google Scholar]
- Santos MJ, Aruin AS. Role of lateral muscles and body orientation in feedforward postural control. Exp Brain Res. 2008;184:547–59. doi: 10.1007/s00221-007-1123-9. [DOI] [PubMed] [Google Scholar]
- Santos MJ, Aruin AS. Effects of lateral perturbations and changing stance conditions on anticipatory postural adjustment. J Electromyogr Kinesiol. 2009;19:532–41. doi: 10.1016/j.jelekin.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Santos MJ, Kanekar N, Aruin AS. The role of anticipatory postural adjustments in compensatory control of posture: 1. Electromyographic analysis. J Electromyogr Kinesiol. 2010;20:388–97. doi: 10.1016/j.jelekin.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Schmitz C, Martin N, Assaiante C. Building anticipatory postural adjustment during childhood: a kinematic and electromyographic analysis of unloading in children from 4 to 8 years of age. Exp Brain Res. 2002;142:354–64. doi: 10.1007/s00221-001-0910-y. [DOI] [PubMed] [Google Scholar]
- Shiratori T, Latash M. The roles of proximal and distal muscles in anticipatory postural adjustments under asymmetrical perturbations and during standing on rollerskates. Clin Neurophysiol. 2000;111:613–23. doi: 10.1016/s1388-2457(99)00300-4. [DOI] [PubMed] [Google Scholar]
- Shiratori T, Latash ML. Anticipatory postural adjustments during load catching by standing subjects. Clin Neurophysiol. 2001;112:1250–65. doi: 10.1016/s1388-2457(01)00553-3. [DOI] [PubMed] [Google Scholar]
- Slijper H, Latash M. The effects of instability and additional hand support on anticipatory postural adjustments in leg, trunk, and arm muscles during standing. Exp Brain Res. 2000;135:81–93. doi: 10.1007/s002210000492. [DOI] [PubMed] [Google Scholar]
- Slijper H, Latash ML. The effects of muscle vibration on anticipatory postural adjustments. Brain Res. 2004;1015:57–72. doi: 10.1016/j.brainres.2004.04.054. [DOI] [PubMed] [Google Scholar]
- Tsao H, Hodges PW. Immediate changes in feedforward postural adjustments following voluntary motor training. Exp Brain Res. 2007;181:537–46. doi: 10.1007/s00221-007-0950-z. [DOI] [PubMed] [Google Scholar]
- Tsao H, Hodges PW. Persistence of improvements in postural strategies following motor control training in people with recurrent low back pain. J Electromyogr Kinesiol. 2008;18:559–67. doi: 10.1016/j.jelekin.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Vicon®. Plug-in-Gait modelling instructions. Oxford, UK: Oxford Metrics Ltd; 2002. [Google Scholar]
- Winter DA, Prince F, Frank JS, Powell C, Zabjek KF. Unified theory regarding A/P and M/L balance in quiet stance. Journal of neurophysiology. 1996;75:2334–43. doi: 10.1152/jn.1996.75.6.2334. [DOI] [PubMed] [Google Scholar]
- Witherington D, von Hofsten C, Rosander K, Robinette A, Woollacott M, Bertenthal B. The Development of Anticipatory Postural Adjustments in Infancy. INFANCY. 2002;3:495–517. [Google Scholar]
- Woollacott MH, Manchester DL. Anticipatory postural adjustments in older adults: are changes in response characteristics due to changes in strategy? J Gerontol. 1993;48:M64–70. doi: 10.1093/geronj/48.2.m64. [DOI] [PubMed] [Google Scholar]


