Abstract
Several potent and broadly neutralizing antibodies to HIV-1 have been isolated recently from peripheral blood B cells of infected individuals, based on pre-screening of antibody activity in the serum. However, little is known regarding the cells that make the antibodies that circulate in the blood. Accordingly, we investigated the most likely source, the bone marrow, of chronically HIV-1-infected individuals who were not receiving antiretroviral therapy. Increased frequencies of plasma cells, as well as B cell precursors, namely preB-I, preB-II, and decreased frequencies of mature B cells were observed in bone marrow aspirates of these individuals compared to HIV-negative counterparts. Increased frequencies of bone marrow plasma cells are consistent with known hallmarks of HIV-1 infection, namely hypergammaglobulinemia and increased frequencies of peripheral blood plasmablasts. Levels of HIV-1 envelope (Env)-binding and HIV-1-neutralizing antibodies were measured in serum and corresponding frequencies of antibody-secreting or Env-binding cells were measured in the blood (plasmablasts and memory B cells) and in the bone marrow (plasma cells). A strong correlation was observed between serum HIV-1-specific antibodies and Env-specific bone marrow-derived plasma cells, but not circulating plasmablasts or memory B cells. These findings demonstrate that despite HIV-1-induced phenotypic and functional B-cell dysregulation in the peripheral blood and secondary lymphoid tissues, bone marrow plasma cells remain a primary source for circulating HIV-1-specific antibodies in HIV-1-infected individuals.
Introduction
Despite the effectiveness and scale-up of antiretroviral therapy in the treatment of HIV-1 infection, development of an antibody-based HIV-1 vaccine is a critical element in strategies to end this pandemic (1). Such an endeavor has remained an elusive goal for over two decades, largely due to the inadequacy of the natural immune response to HIV-1 infection and difficulty in establishing a correlate of immunity upon which to model a vaccine. However, over the past five years, there has been a rapid succession of advances in the isolation of broadly neutralizing antibodies (bnAbs) from memory B cells in the peripheral blood of HIV-1-infected individuals (2-6). These bnAbs target a variety of different epitopes within HIV-1 envelope proteins gp120 and gp41, described as sites of vulnerability of the virus, and have been derived by a number of different methods (7, 8). However, most methods begin with the same approach, that of screening serum for the presence of HIV-1-specific bnAbs, which arise in approximately 10-25% of individuals after several months to years of infection (8). These approaches are premised on an assumption that has not been widely validated, with only two known examples (3), that HIV-1-specific circulating memory B cells from which bnAbs are cloned are closely related to the antibodies in the serum from which neutralization screens are performed. There is also evidence for recapitulation of serum neutralization breadth by a small number of antibodies derived from memory B cells (4, 9), although the individuals in these studies were selected on the basis of potent HIV-1-neutralizing activity in their serum. It remains unclear whether this phenomenon applies to the vast majority of individuals whose serum does not show potent HIV neutralizing capacity. Other studies have described large numbers of specificities, either from B-cell clones or in serum of each individual (10, 11), although in these cases, the link between cellular and serologic sources of antibodies was not investigated. However, another study reported discordance between HIV-1 envelope-specific memory B-cell responses and circulating antibodies in infected individuals who naturally control viremia (12).
Antibodies are produced by B cells that have undergone partial differentiation, referred to as plasmablasts (PBs), or have completed the differentiation process, and are referred to as plasma cells (PCs). Several other features distinguish these two populations of antibody-secreting cells. Both populations in humans express high levels of CD27 and CD38 while having lost expression of CD20; PBs have recently cycled (Ki-67+) and maintain expression of CD19 more than do PCs whereas PCs express CD138, a marker of differentiation rarely observed on PBs (13, 14). PBs arise during the early stages of an immune response in secondary lymphoid tissues and can circulate between tissues and into the peripheral blood (14-16). PBs may arise directly from naïve B cells in extrafollicular sites following antigenic stimulation; however, they can accumulate relatively high levels of somatic hypermutation, as has been shown in acute HIV-1 infection (17), a process more consistent with having undergone affinity maturation in germinal centers. Furthermore, those PBs, which were directed against gp41 of the HIV-1 envelope, likely arose from pre-existing memory B cells (17), suggesting there may exist multiple routes of B-cell differentiation, and not necessarily linear relationships between naïve and memory B cells, as well as PBs and PCs.
In contrast to the high turnover/short-lived properties of PBs, PCs are by definition long-lived and sessile, residing primarily in the bone marrow, and to a lesser and possibly more short-lived extent, in secondary lymphoid tissues such as spleen, lymph nodes, tonsils and mucosal associated lymphoid tissues (15, 16). The processes that dictate the survival of PCs and their homing to the bone marrow are not completely understood, although CXCR4 and its ligands are essential for homing and survival is maintained by a “bone marrow niche” comprised of reticular cells, stromal cells, fibroblasts, eosinophils and macrophages, and the factors they secrete, including IL-6, IL-5, APRIL, BAFF, and TNF (16, 18).
Relatively little is known regarding the cellular/tissue origin of HIV-specific antibodies present in serum of infected or even vaccinated individuals, although as with other pathogens and immunogens, the bone marrow PCs are thought to be a major source (3, 15, 16). In addition, there are several unique factors to consider in HIV-1 infection, and in particular, in the setting of chronic viremia where hypergammaglobulinemia and increased frequencies of circulating PBs are observed, although only a relatively small fraction of PBs are HIV-1-specific (19). Given the uncertainty of the origin of antibodies that circulate in the blood, together with their importance in the screening process in the generation of HIV-neutralizing antibodies from B cells, we investigated HIV-1-specific B cells in the peripheral blood and bone marrow of chronically HIV-1-infected individuals. We found an increased frequency of plasma cells in the bone marrow from HIV-1-infected individuals compared to HIV-negative counterparts, and more importantly, a strong correlation between HIV-1-specific circulating antibodies and HIV-1 envelope-specific PCs in the bone marrow but no correlation with circulating PBs or memory B cells. Our results demonstrate that despite the strong evidence of B-cell hyperactivity in the blood and secondary lymphoid tissues in HIV-1-infected individuals, the bone marrow remains the major source of HIV-1-specific antibodies in the blood.
Materials and Methods
Study Participants
Bone marrow aspirates and core biopsies were obtained from HIV-1-infected individuals after providing informed consent and in accordance with the Institutional Review Board of the National Institute of Allergy and Infectious Diseases, National Institutes of Health and the Declaration of Helsinki. Eight individuals were recruited as detailed in Table I. HIV-1-infected participants were not receiving antiretroviral therapy at time of study and all were classified as chronically infected. Specimens from HIV-negative individuals were obtained from the Department of Laboratory Medicine, Clinical Research Center, National Institutes of Health. Bone marrow biopsies and aspirates were obtained from posterior iliac crests under conscious sedation and local anesthesia. In addition, cryopreserved bone marrow mononuclear cells (BMMCs) were obtained from commercially available sources (Allcells or Stemcell Technologies).
Table I. Demographic and immunologic profile of HIV-1-infected individuals.
| Patient number | Age (y) | Gender | Ethnicity | Estimateda time of infection at biopsy (months) | Plasma HIV-1 RNA at time of biopsyb (copies/ml) | CD4 count at time of biopsy (cells/μl) | Serum gp140 binding ELISA (OD) |
|---|---|---|---|---|---|---|---|
| 1 | 26 | Male | Hispanic | 14 | 1118 | 777 | 0.428 |
| 2 | 34 | Male | Hispanic | 16-28 | 14200 | 460 | 0.816 |
| 3 | 31 | Male | Black | >120 | 164 | 714 | 2.461 |
| 4 | 26 | Female | Black | 19 | 2549 | 479 | 0.95 |
| 5 | 44 | Male | White | >96 | 3739 | 403 | 2.593 |
| 6 | 29 | Female | Hispanic | >101 | 1562 | 434 | 2.091 |
| 7 | 28 | Male | Hispanic | 27 | 20964 | 397 | 3.189 |
| 8 | 23 | Male | Hispanic | 23 | 19156 | 875 | 2.989 |
Estimated from clinical history taken at screening visit.
All were ART-naïve, except #5 and #6 discontinued ART 35 and 75 months prior to study, respectively.
Morphologic analyses
Sections from decalcified formalin-fixed paraffin embedded core bone marrow biopsies were stained with CD138 antibody using an autostaining system (Ventana). Immunohistochemistry stains were digitally scanned and analyzed to determine the percentage of CD138+ cells in the cellular bone marrow.
Processing of bone marrow aspirates and peripheral blood
Three serial bone marrow aspirate pulls were obtained from each participant. Quality assessment of each pull was performed by establishing their hemodilution, based on the presence of mature neutrophils (CD13+/CD16+/CD45+), which should be less than 30% within the myeloid cells gate, as previously described (20). BMMCs from each bone marrow aspirate pull and PBMCs from blood draws or leukapheresis were obtained by Ficoll-Hypaque density gradient centrifugation. BMMCs corresponding to the pull with the lowest hemodilution were used for further processing and analyses. CD38hi/CD138+ PCs were sorted from BMMCs using a FACSAria cell sorter (BD Biosciences), with a median purity of 95%. For functional and certain phenotyping analyses, mature (CD10-) B cells were enriched to ∼95% purity from PBMCs by negative selection (StemCell Technologies), as described previously (21).
Phenotypic analyses
Neutrophil staining for analysis of hemodilution was performed with the following mAbs: CD13-PE, CD16-brilliant violet (BV) 510, and CD45-FITC from Biolegend. Freshly processed BMMCs were used for phenotypic analyses. Cryopreserved BMMCs were also included in the HIV-negative group after verifying that there were minimal differences between fresh and thawed samples. Cryopreserved PBMCs were used to measure the frequency of PBs among HIV-1-infected individuals and the frequency of gp140-specific memory B cells. The following mAbs were used for cell surface staining: CD3-BV510, CD19- PE-Cy7, CD27-PerCP-Cy5.5, and CD38-BV421 from Biolegend; CD10-APC, CD20-APC-H7, and IgG-PE-Cy7 from BD Biosciences; and CD138-PE from eBioscience. For Ig isotype staining of PBs and PCs, cells were permeabilized for intracellular staining (Permeabilizing solution 2, BD Biosciences), and the following mAbs were used: IgM-BV510 and IgG-APC from BD Biosciences, and IgA-FITC from Dako. Frequencies of HIV-1 envelope-specific memory B cells were measured with a trimeric HIV-1 envelope gp140 probe (YU2-gp140-F), as previously described (22). In brief, the probe containing an Avitag biotinylation motif at the C termini was generated as previously described (23), and fluorescently labeled with streptavidin-APC (Invitrogen). Flow cytometry was performed on a FACSCanto flow cytometer (BD Biosciences), with data analyses on FlowJo Version 9.6 software (TreeStar).
Functional analyses
Frequencies of PBs and PCs secreting IgG, IgA and IgM, as well as those specific for gp140, were measured by ELISPOT, as previously described (24). Briefly, Immobilon-P polyvinylidene difluoride membrane plates (MAIPSWU10; Millipore) were coated with 5 μg/ml anti-Ig light-chain antibodies (Rockland Immunochemicals), followed by addition of sorted plasma cells, incubation overnight, and detection with biotinylated antibodies against each of the Ig classes or biotinylated YU2-gp140-F. Unlabeled or biotinyated Keyhole limpet hemocyanin served as negative control antigen.
ELISA
Maxisorp plates (Nunc) were coated with 2.5μg/ml goat anti-human IgG (H+L) (Jackson Immuno-Research) in PBS overnight at 4°C. Serum samples from study participants and an HIV-negative control individual were diluted 5-fold, starting from a 1:50 dilution in PBS-0.02% azide, mixed 1:1 with blocking buffer (PBS containing 2% skim milk and 5% FBS), and added to the plate. Consecutive incubations of 1 h interspaced with vigorous washing (PBS-0.02% Tween 20) were as follows: serum sample mix at room temperature; biotinylated YU2-gp140-F (2.5μg/ml) at 37°C; and streptavidin-horseradish peroxidase (Sigma; 1/1000) at room temperature. Plates were developed 5 min with TMB peroxidase substrate (Biorad), stopped with an equal volume of 3% HCl, and the optical density (OD) was measured at 450nm. An OD of two standard deviations above that of HIV-negative serum was considered positive and OD values at 1/50 dilution of end-point dilution curves (Supplemental Fig. 1) were used in data analyses.
Serum neutralizing activity
Serum samples were tested for HIV-1-neutralizing activity using a pseudovirus assay with a tier 2 panel developed and performed by Monogram Biosciences (25). The panel consisted of viruses pseudotyped with HIV-1 envelopes from strains 92BR020, MGRM-C-026, 92TH021, 93IN905, 94UG103 and JRCSF. A tier 1 pseudovirus, NL4/3 was also included, as well as a negative control, virus pseudotyped with the murine leukemia virus envelope aMLV. Neutralization titers were reported as the reciprocal serum dilution that inhibited virus infection by 50% (IC50).
Statistical analyses
Statistical analyses were performed using Prism software (version 6.0 for Mac). Two-group comparisons were performed using the Mann-Whitney U test (Figures 2,3,4 and 5). The Spearman rank method was used to test for correlation (Figure 6). A P value of ≤0.05 was considered significant.
Figure 2.
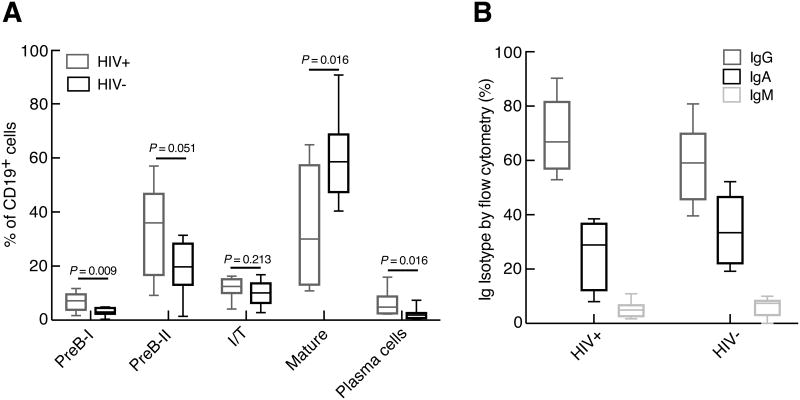
Comparison of B-cell subsets in the bone marrow of HIV-1-infected and HIV-negative individuals. (A) Frequencies of B-cell subsets were measured, as defined by markers shown in Figure 1. (B) Distribution of Ig isotypes among bone marrow PCs was determined following permeabilization of cells. Median (horizontal bar) and scatter boxes shown represent analyses of 8 HIV-1-infected and 8 HIV-negative individuals.
Figure 3.
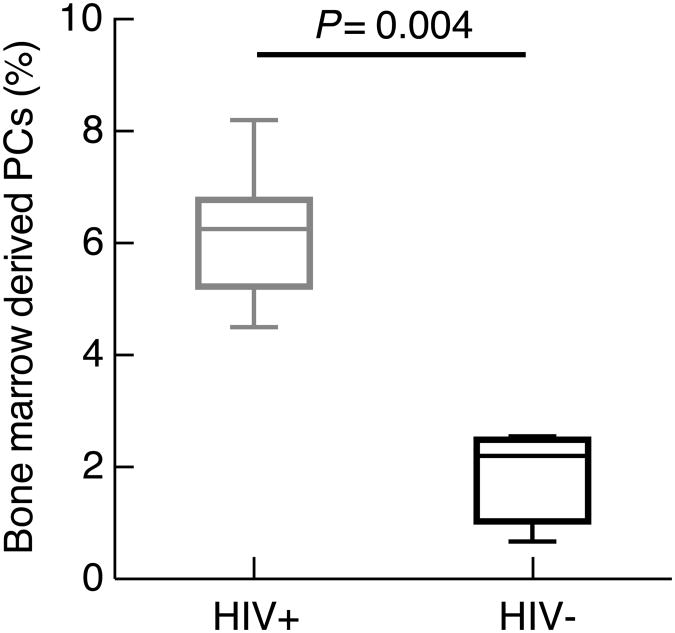
Frequencies of bone marrow core biopsy-derived PCs calculated from immunohistochemistry. Frequencies were determined by immunohistochemistry following staining with CD138 and quantification by digital analysis. Median (horizontal bar) and scatter boxes shown represent analyses of 8 HIV-1-infected and 4 HIV-negative individuals.
Figure 4.
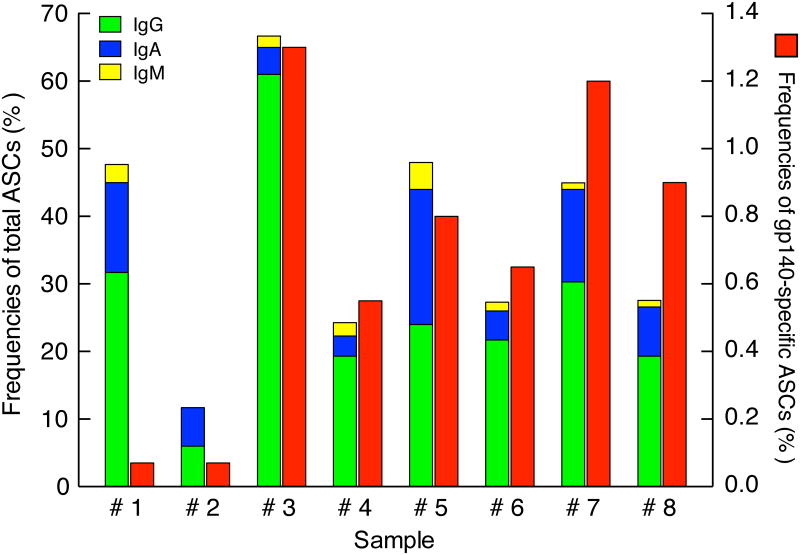
Frequencies of total and Env-specific PCs in bone marrow of HIV-1-infected individuals. CD38++/CD138+ PCs were sorted from bone marrow aspirates and evaluated for total Ig (left y-axis) and HIV-1 gp140-specific (right y-axis) ASC frequencies by ELISPOT. Sample numbers refer to individuals described in Table I.
Figure 5.
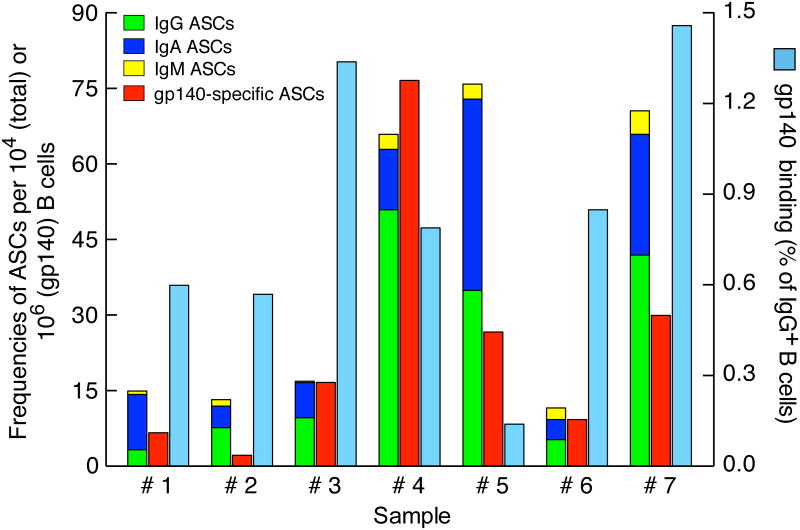
Frequencies of total Ig and Env-specific ASCs and memory B cells in the peripheral blood of HIV-1-infected individuals. Mature B cells were enriched from PBMCs and evaluated for total Ig (left y-axis) and HIV-1 gp140-specific ASC frequencies by ELISPOT (left y-axis), as well as for binding to HIV-1 gp140 probe by flow cytometry (right y-axis). Sample numbers refer to individuals described in Table I; analyses were performed on 7 individuals.
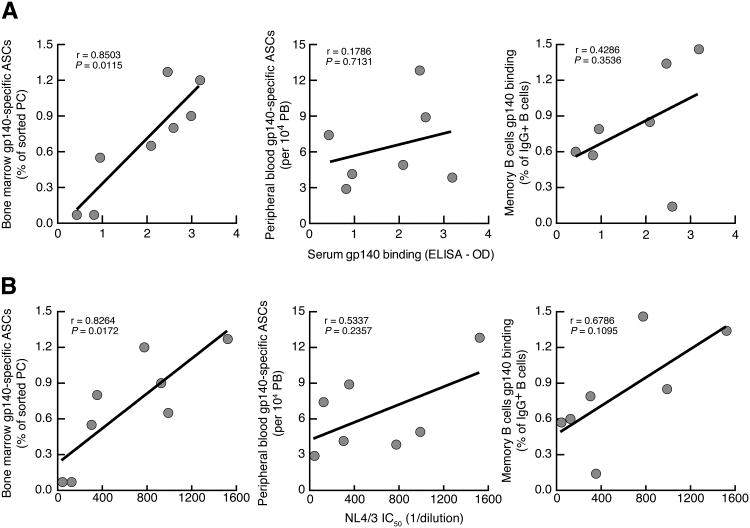
Figure 6.
Relationship between HIV-1-specific antibodies in the serum of HIV-1-infected individuals and corresponding frequencies of Env-specific B cells in bone marrow and peripheral blood. Graphs showing correlation of serum (A) Env-binding antibodies and (B) HIV-neutralizing activity (reported as IC50) against NL4/3 pseudovirus with the frequency of Env-specific cells among bone marrow PCs (left), PBMC PBs (center), and IgG+ PBMC (memory) B cells (right). For PCs and PBs, the assay was ELISPOT and for IgG+ B cells the assay was flow cytometry and data are those shown in Figure 4 and 5, except Env-specific peripheral blood ASCs is reported relative to total frequencies of PBs among B cells, as measured by flow cytometry.
Results
B-cell subsets in the bone marrow
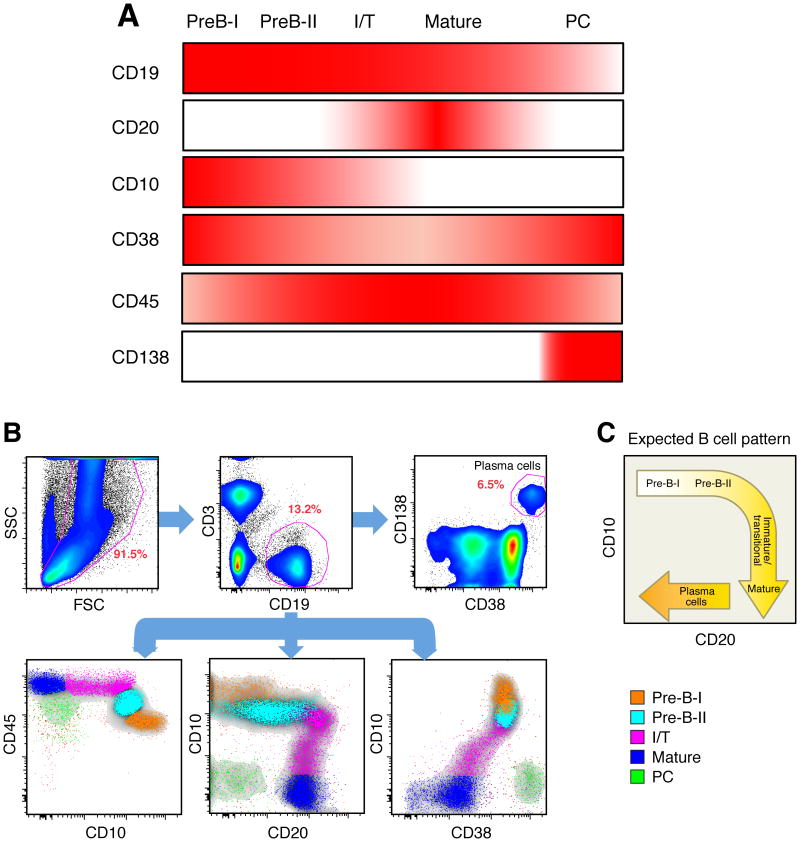
In humans, the bone marrow is the primary site of B-cell development as well as the major repository of long-lived PCs. Given the paucity of current studies on bone marrow B cells in HIV-1 infection, we first sought to analyze all B-cell subsets among aspirate BMMCs of study participants (Table I). All participants had detectable HIV-1 viremia and were not receiving ART at time of study. Of note, six of the participants were therapy-naïve whereas two had discontinued ART several years prior to participation (Table I). A panel of markers was chosen, based on our preliminary analyses (data not shown) and published studies (26-29), to highlight the different stages of B-cell development in the bone marrow (Fig. 1A). The earliest B cells to express CD19 are PreB-I, followed by PreB-II, immature/transitional (I/T) and mature B cells. With CD19 as the pan B cell marker, subsets were defined as follows: PreB-I cells, CD10hi/CD45lo/CD20-/CD38hi; preB-II, CD10int/CD45int/CD20-/CD38int; I/T B cells, CD10lo/CD45hi/CD20+/CD38lo; mature B cells, CD10-/CD45hi/CD20+/CD38lo; and plasma cells, CD10-/CD45lo/CD20-/CD138+/CD38hi. As shown for the representative plots in Fig. 1B, all five B-cell subsets were clearly delineated with distinct patterns of expression for CD10, CD20, CD38, and CD45. Of note, the profile for CD10 by CD20 was similar to the one that is commonly observed for B-cell subsets in the human bone marrow (Fig. 1C) (27). The PCs in the bone marrow were also distinguished by the expression of CD38 and CD138 (Fig. 1B). In group analyses, the frequencies of PCs, PreB-I and PreB-II cells, as a percentage of total B cells, were significantly increased in the bone marrow of HIV-1-infected compared to HIV-negative individuals (Fig. 2A). The frequency of mature B cells was significantly decreased in HIV-1-infected compared to HIV-negative individuals (Fig. 2A), and based on available sub-analyses, this difference was due to lower frequencies of CD27+ memory B cells (data not shown). There was no significant difference in the frequency of I/T B cells between the two groups (Fig. 2A). Finally, we assessed the Ig isotype distribution among the PCs in the bone marrow with a staining strategy that included a cell permeabilization, a necessary step because Igs produced by PCs are predominantly intracellular with little or no expression on the cell surface. As shown in Fig. 2B, the majority of bone marrow PCs of both HIV-1-infected and HIV-negative individuals expressed IgG, fewer expressed IgA and only a fraction expressed IgM. There was no significant difference in the Ig isotype distribution in bone marrow PCs between the two groups. Collectively, these findings reveal that there exist differences in the frequencies of B cells at early and later stages of development and differentiation in the bone marrow of HIV-1-infected individuals compared to uninfected individuals.
Figure 1.
Phenotypic characterization of B cells in the bone marrow. (A) Combination of cell-surface markers used to identify B-cell developmental stages and PCs in the bone marrow. Intensity of color indicates level of expression. I/T refers to immature/transitional. (B) Phenotypic and subset analyses of B cells in the bone marrow of an HIV-1-infected individual, identified based on their expression of CD45 and CD10, CD20 and CD10 and CD38. In the upper panels, CD19+CD3- cells were gated from all live cells and PCs were identified from CD19+ cells based on the expression profile CD38hi/CD138+. (C) Cartoon of B-cell subsets in bone marrow reflecting the expected B cell pattern delineated by their expression of CD10 and CD20.
Morphologic analyses of bone marrow core biopsies
Bone marrow core biopsies from eight HIV-1-infected and four HIV-negative individuals were available for hematopathologic review and analysis. Evidence of progressive trilineage (erythroid, myeloid, and lymphoid) hematopoiesis indicative of adequate maturation was observed in all specimens. Enumeration of PCs, based on morphology and expression of CD138, was performed on core biopsies by immunohistochemistry combined with a digital quantification method. As shown in Fig. 3, the frequency of PCs was significantly increased in the core biopsies of HIV-1-infected (median of 6.2%) compared to HIV-negative (median of 2.2%) individuals. These findings confirmed the increased frequency of plasma cells observed by flow cytometry in the bone marrow aspirates of HIV-1-infected individuals.
Frequencies of HIV-1-specific PCs in the bone marrow of infected individuals
Analyses relating to HIV-1-specific PC responses in the bone marrow of HIV-1-infected individuals have been conducted in a limited number of studies and study subjects (3, 17, 30). To gain a better understanding of the contribution of the bone marrow to the antibody response against HIV-1, we measured frequencies ofHIV-1 Env-specific (hereafter referred to as Env-specific) bone marrow PCs using an ELISPOT assay that was previously described for similar measurements among PBs in the peripheral blood (19). PCs were sorted from BMMCs based on the expression of CD19, CD138, and CD38 (Fig. 1B), and both total and Env-specific frequencies of antibody-secreting cells (ASCs) were measured. The distribution of Ig isotype among total ASCs was similar to that determined by flow cytometry on unfractionated BMMCs (Fig. 2B), in that IgG represented the major isotype, followed by IgA and lastly, IgM (Fig.4). One caveat to the ASC analysis was that we expected our isotype analysis to account for 100% of sorted cells given that they were all PCs. We do not have an explanation for the variations observed among individuals relative to total ASCs given that purity of sorted cells were similar, although the lower frequencies were observed in individuals with the lowest percentage of PCs in the aspirate and loss of viability associated with a longer sorting period may have been a factor. Nonetheless, the sorting step was deemed essential to accurately measure the relatively low frequencies of Env-specific ASCs. In this regard, the frequencies of Env-specific BMMC-derived PCs ranged from 0.1% to 1.3% (Fig. 4). While this may be considered a low frequency, the median of 0.7% for this group is nonetheless approximately 10-fold higher than frequencies of tetanus toxoid-specific PCs reported following immunization with tetanus toxoid (31). Of note, the approach taken to measure the Env-specific ASC frequencies does not provide information on Env-specificity per Ig isotype and obtaining such information would be technically difficult and require twice or more the number of cells used in the current assay. Collectively, these data demonstrate that ELISPOT is an assay that can provide information on the Ig isotype distribution of BMMC-sorted PCs that is comparable to that observed by flow cytometry and furthermore, this approach provides a means for measuring HIV-1-specific responses among these PCs.
Frequencies of HIV-1-specific PBs and memory B cells in the peripheral blood
We previously evaluated Env-specific PBs and memory B cells in the peripheral blood of large cohorts of HIV-1-infected individuals (19, 22). For the purpose of comparisons with the bone marrow compartment, we extended these analyses to the present study. Mature (CD10-) B cells were isolated from PBMCs seven of the eight individuals listed in Table I on whom there were stored cells available within 6 months of the bone marrow biopsy. Frequencies of total Ig and Env-specific PB ASCs were measured among mature B cells by ELISPOT (Fig. 5), as performed on the BMMCs PCs, although without prior enrichment by cell sorting. Similar to bone marrow PCs, IgG was the main isotype contributor to the total ASCs, followed by IgA and then IgM (Fig. 5). When calculated as a percentage of total Ig ASCs, the frequencies of Env-specific PBs ranged from 0.2% to 1.2% (data not shown), with a median of 0.6% that was similar to that previously reported (19).
Whereas ELISPOT remains the assay of choice for evaluating antigen-specific responses among PBs and PCs, HIV-1 specificity among memory B cells can be assessed by flow cytometry with HIV-1 envelope probes since these cells express high levels of surface Ig (22, 32, 33). Of note, the same probe was used for both ELISPOT and flow cytometry, namely an Avitag-based biotinylated form of trimerized YU2 gp140 that was used directly for detection of Env-specific ASCs, as well as a fluorochrome-conjugated form that was used for evaluation of Env-specific memory B cells. As shown in Fig. 5, the frequencies of Env-specific memory B cells among PBMCs ranged from 0.1% to 1.46% of total IgG-expressing B cells, with a median of 0.8 % that was similar to that described previously for a similar group of HIV-1-infected individuals (22). Collectively, the frequencies of Env-specific memory B cells and PBs in the peripheral blood of individuals who underwent a bone marrow biopsy were comparable to previous studies and thus representative of larger similar cohorts.
Contribution of PCs, PBs and memory B cells to the HIV-1-specific B cell immunity
Finally, we wished to evaluate the association between the different sources of Env-specific B cells and titers of Env-binding antibodies in the serum. Accordingly, we measured reactivities by ELISA of serum antibodies to HIV-1 gp140 from the eight individuals on whom we had BMMC B-cell data at a time point that was within 6 months of the bone marrow biopsy. Of note, the same biotinylated gp140, used to measure cellular specificities by flow cytometry and ELISPOT, was used to measure serologic specificities by ELISA, with the latter two assays also set up in the same orientation, namely gp140 was added in the detection phase of both assays. The individual OD values are shown in Table I and were used to assess correlations with various Env-specific cellular measurements made in this study. As shown in Fig. 6A, levels of Env-specific serum antibodies were strongly and significantly correlated with frequencies of Env-specific bone marrow-derived PCs whereas there was no significant correlation between serum Env-binding antibodies and frequencies of peripheral blood Env-specific PBs or memory B cells. Of note, eight individuals were included in the correlation between serum and bone marrow while only seven were included in the correlations involving peripheral blood cells. Nonetheless, the bone marrow correlation remained significant when only those seven individuals were included in the analysis (r = 0.8829; P = 0.015). With regard to the bone marrow ASCs, the correlation with serum antibodies was strongest when Env-specific PCs were reported as a percentage of sorted cells whereas for the peripheral blood ASCs, there was no significant correlation whether the values were reported as an absolute count or as a fraction of total ASCs or as a percentage of PBs among B cells (data not shown). However, in extended analyses of values shown in Figs. 4 and 5, we found one additional significant correlation between frequencies of Env-specific and total ASCs in the peripheral blood (r = 0.9643, P = 0.003; data not shown). This was consistent with our previous findings that plasmablasts in the blood are associated with HIV-1 viremia, which in turn drives HIV-1-specific responses (19, 22). Finally, we also evaluated the HIV-1-neutralizing activity of the serum of our participants using a panel of five tier 2 and one tier 1 pseudoviruses described in the Materials and Methods. As shown in Supplemental Table I, neutralization against tier 2 viruses was weak, rarely two-fold above negative control aMLV, whereas neutralization against the tier 1 NL4/3 strain was above this threshold in seven of the eight individuals. When IC50 values were correlated with the Env-specific cellular responses shown in Fig. 6A, we found significant correlations between serum neutralizing activity against NL4/3 and bone marrow Env-specific ASCs but not peripheral blood Env-specific ASCs or memory B cells (Fig. 6B). Furthermore, when the contribution of the negative control, aMLV, was subtracted from test values in Supplemental Table I, significant correlations between bone marrow Env-specific ASCs and serum neutralizing activity were observed for four of the seven strains (Supplemental Fig. 2), whereas none of the correlations were significant between serum activity and peripheral blood Env-specific ASCs or memory B cells (data not shown). Collectively, these data suggest that PCs in the bone marrow are the main contributors to the antibodies, including those with neutralizing activity, that circulate in the blood, at least with regard to Env-specific responses in infected individuals.
Discussion
In the present study, we demonstrate that bone marrow-derived PCs are the main source of circulating HIV-1-specific antibodies in HIV-1-infected individuals. The past few years has seen extraordinary progress in understanding and harnessing antibody responses to HIV-1 in infected individuals (8, 34-36). However, circulating antibodies remain poorly understood in terms of cellular origin and relationships among the various B-cell populations from which bnAbs can potentially arise. It has been assumed, largely based on murine models (37, 38), that long-lived PCs in the human bone marrow are the source of serum antibodies, providing long lasting immunity against infectious agents. However, antigen-specific PCs in the bone marrow compartment and their contribution to the pool of antigen-specific circulating antibodies in serum has not been previously ascertained in humans. In the setting of HIV-1 infection, where hypergammaglobulinemia is common and increased frequencies of PBs and/or PCs have been observed in the peripheral blood and secondary lymphoid tissues (19, 39), the contribution of bone marrow to circulating antibodies could come from many different cellular sources. Despite the fact that there are multiple potential cellular sources of HIV-1-specific serum antibody in the blood, secondary lymphoid tissues, and bone marrow, we demonstrate a strong correlation between HIV-1-specific antibodies circulating in the serum of HIV-1-infected individuals and the frequencies of Env-specific PCs in the bone marrow. These findings suggest that HIV-1-specific bone marrow-derived PCs are the main source of HIV-1-specific serum antibodies.
The HIV-1-infected individuals we studied were chronically infected and all were viremic to varying degrees. However, they were also relatively healthy, with a median CD4+ T-cell count of 470 cells/μl. These demographics likely explain the absence of gross hematologic abnormalities in this group, contrasting with studies performed on individuals with more advanced HIV-1 disease where bone marrow pathology was reported (40-42). Nonetheless, we did observe several abnormalities within B-cell subsets of the bone marrow in our cohort that are perhaps indicative of systemic effects of chronic HIV-1 infection on lymphoid cells, including homeostatic dysregualtion. On the one hand, we found increased frequencies of plasma cells in the aspirates and core biopsy, consistent with chronic antigenic stimulation in general, and in this case HIV-1-induced B-cell terminal differentiation (43, 44). On the other hand, we also found evidence of perturbations of B cell development in the bone marrow. Frequencies of B-cell precursors were elevated whereas those of mature B cells were reduced. The increased precursor frequency could be a response to HIV-1-induced B-cell depletion, similar to although not as profound as CD4+ T-cell depletion (45). A lower frequency of mature B cells could either reflect a block in maturation due to limiting factors needed for this process, or a premature exit from the bone marrow of I/T B cells. In this regard, HIV-1 infection is associated with an increased frequency of I/T B cells in the periphery (46). Whether these changes in the bone marrow also contribute to the paucity of memory B cells in the periphery in HIV-1 infection is a possibility that warrants further investigation, especially given the importance of the memory B-cell compartment in responding to HIV and other pathogens (47, 48).
Finally, the important contribution of HIV-1-specific bone marrow PCs to the circulating HIV-1-specific antibodies raises several points for discussion and future consideration relative to bnAbs. One study has shown a link between bone marrow PCs and HIV-1-specific bnAbs present in the serum in two patients (3). In addition, in that study, the serum antibody was shown to be linked to the antibody reconstituted from memory B cells in the peripheral blood and antibody gene sequences in the bone marrow PCs. A link between HIV-1-neutralizing serum antibodies and memory B cells has also been established in studies of individuals with potent HIV-1-neutralizing serum activity (4, 9), and our data indicate a similar trend in individuals who do not have strong neutralizing responses (Fig. 6). However, these potentially important relationships need to be further explored in larger, more diverse cohorts. Nonetheless, given that bone marrow PCs are likely the durable repository of antibodies responsible for maintaining long-term immunity, based on our study of the relationship between bone marrow PCs and HIV-1-specific antibodies, this compartment should be included as part of the evaluation of responses to immunogens being considered in HIV vaccine strategies aimed at inducing broadly reactive HIV-specific antibodies.
Supplementary Material
Acknowledgments
We thank the patients for their willingness to participate in this study. We thank Shyam Kottilil for patient recruitment and care and Catherine Rehm and Sara Jones for specimen processing.
This work was supported by the Intramural Research Program of the NIAID, NIH. Y.L. was supported by National Institutes of Health/NIAID Grant R01AI102766 and Development Grant (a sub-award from NIAID P30AI36214) from the Center for AIDS Research, University of California, San Diego.
Abbreviations used in this article
- ASC
antibody-secreting cells
- BMMCs
bone marrow mononuclear cells
- bnAbs
broadly neutralizing antibodies
- BV
brilliant violet
- Env
HIV-1 envelope
- IC50
50% inhibitory concentration
- I/T
immature/transitional
- PBs
plasmablasts
- PCs
plasma cells
References
- 1.Fauci AS, Marston HD. Ending AIDS--is an HIV vaccine necessary? N Engl J Med. 2014;370:495–498. doi: 10.1056/NEJMp1313771. [DOI] [PubMed] [Google Scholar]
- 2.Walker LM, Phogat SK, Chan-Hui PY, Wagner D, Phung P, Goss JL, Wrin T, Simek MD, Fling S, Mitcham JL, Lehrman JK, Priddy FH, Olsen OA, Frey SM, Hammond PW, Protocol GPI, Kaminsky S, Zamb T, Moyle M, Koff WC, Poignard P, Burton DR. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science. 2009;326:285–289. doi: 10.1126/science.1178746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scheid JF, Mouquet H, Ueberheide B, Diskin R, Klein F, Oliveira TY, Pietzsch J, Fenyo D, Abadir A, Velinzon K, Hurley A, Myung S, Boulad F, Poignard P, Burton DR, Pereyra F, Ho DD, Walker BD, Seaman MS, Bjorkman PJ, Chait BT, Nussenzweig MC. Sequence and structural convergence of broad and potent HIV antibodies that mimic CD4 binding. Science. 2011;333:1633–1637. doi: 10.1126/science.1207227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walker LM, Huber M, Doores KJ, Falkowska E, Pejchal R, Julien JP, Wang SK, Ramos A, Chan-Hui PY, Moyle M, Mitcham JL, Hammond PW, Olsen OA, Phung P, Fling S, Wong CH, Phogat S, Wrin T, Simek MD, Protocol GPI, Koff WC, Wilson IA, Burton DR, Poignard P. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature. 2011;477:466–470. doi: 10.1038/nature10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu X, Yang ZY, Li Y, Hogerkorp CM, Schief WR, Seaman MS, Zhou T, Schmidt SD, Wu L, Xu L, Longo NS, McKee K, O'Dell S, Louder MK, Wycuff DL, Feng Y, Nason M, Doria-Rose N, Connors M, Kwong PD, Roederer M, Wyatt RT, Nabel GJ, Mascola JR. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science. 2010;329:856–861. doi: 10.1126/science.1187659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang J, Ofek G, Laub L, Louder MK, Doria-Rose NA, Longo NS, Imamichi H, Bailer RT, Chakrabarti B, Sharma SK, Alam SM, Wang T, Yang Y, Zhang B, Migueles SA, Wyatt R, Haynes BF, Kwong PD, Mascola JR, Connors M. Broad and potent neutralization of HIV-1 by a gp41-specific human antibody. Nature. 2012;491:406–412. doi: 10.1038/nature11544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moir S, Malaspina A, Fauci AS. Prospects for an HIV vaccine: leading B cells down the right path. Nat Struct Mol Biol. 2011;18:1317–1321. doi: 10.1038/nsmb.2194. [DOI] [PubMed] [Google Scholar]
- 8.Mascola JR, Haynes BF. HIV-1 neutralizing antibodies: understanding nature's pathways. Immunol Rev. 2013;254:225–244. doi: 10.1111/imr.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonsignori M, Montefiori DC, Wu X, Chen X, Hwang KK, Tsao CY, Kozink DM, Parks RJ, Tomaras GD, Crump JA, Kapiga SH, Sam NE, Kwong PD, Kepler TB, Liao HX, Mascola JR, Haynes BF. Two distinct broadly neutralizing antibody specificities of different clonal lineages in a single HIV-1-infected donor: implications for vaccine design. J Virol. 2012;86:4688–4692. doi: 10.1128/JVI.07163-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Georgiev IS, Doria-Rose NA, Zhou T, Kwon YD, Staupe RP, Moquin S, Chuang GY, Louder MK, Schmidt SD, Altae-Tran HR, Bailer RT, McKee K, Nason M, O'Dell S, Ofek G, Pancera M, Srivatsan S, Shapiro L, Connors M, Migueles SA, Morris L, Nishimura Y, Martin MA, Mascola JR, Kwong PD. Delineating antibody recognition in polyclonal sera from patterns of HIV-1 isolate neutralization. Science. 2013;340:751–756. doi: 10.1126/science.1233989. [DOI] [PubMed] [Google Scholar]
- 11.Scheid JF, Mouquet H, Feldhahn N, Seaman MS, Velinzon K, Pietzsch J, Ott RG, Anthony RM, Zebroski H, Hurley A, Phogat A, Chakrabarti B, Li Y, Connors M, Pereyra F, Walker BD, Wardemann H, Ho D, Wyatt RT, Mascola JR, Ravetch JV, Nussenzweig MC. Broad diversity of neutralizing antibodies isolated from memory B cells in HIV-infected individuals. Nature. 2009;458:636–640. doi: 10.1038/nature07930. [DOI] [PubMed] [Google Scholar]
- 12.Guan Y, Sajadi MM, Kamin-Lewis R, Fouts TR, Dimitrov A, Zhang Z, Redfield RR, DeVico AL, Gallo RC, Lewis GK. Discordant memory B cell and circulating anti-Env antibody responses in HIV-1 infection. Proc Natl Acad Sci U S A. 2009;106:3952–3957. doi: 10.1073/pnas.0813392106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jackson SM, Wilson PC, James JA, Capra JD. Human B cell subsets. Adv Immunol. 2008;98:151–224. doi: 10.1016/S0065-2776(08)00405-7. [DOI] [PubMed] [Google Scholar]
- 14.Fink K. Origin and Function of Circulating Plasmablasts during Acute Viral Infections. Front Immunol. 2012;3:78. doi: 10.3389/fimmu.2012.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tangye SG. Staying alive: regulation of plasma cell survival. Trends Immunol. 2011;32:595–602. doi: 10.1016/j.it.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Chu VT, Berek C. The establishment of the plasma cell survival niche in the bone marrow. Immunol Rev. 2013;251:177–188. doi: 10.1111/imr.12011. [DOI] [PubMed] [Google Scholar]
- 17.Liao HX, Chen X, Munshaw S, Zhang R, Marshall DJ, Vandergrift N, Whitesides JF, Lu X, Yu JS, Hwang KK, Gao F, Markowitz M, Heath SL, Bar KJ, Goepfert PA, Montefiori DC, Shaw GC, Alam SM, Margolis DM, Denny TN, Boyd SD, Marshal E, Egholm M, Simen BB, Hanczaruk B, Fire AZ, Voss G, Kelsoe G, Tomaras GD, Moody MA, Kepler TB, Haynes BF. Initial antibodies binding to HIV-1 gp41 in acutely infected subjects are polyreactive and highly mutated. J Exp Med. 2011;208:2237–2249. doi: 10.1084/jem.20110363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Belnoue E, Tougne C, Rochat AF, Lambert PH, Pinschewer DD, Siegrist CA. Homing and adhesion patterns determine the cellular composition of the bone marrow plasma cell niche. J Immunol. 2012;188:1283–1291. doi: 10.4049/jimmunol.1103169. [DOI] [PubMed] [Google Scholar]
- 19.Buckner CM, Moir S, Ho J, Wang W, Posada JG, Kardava L, Funk EK, Nelson AK, Li Y, Chun TW, Fauci AS. Characterization of plasmablasts in the blood of HIV-infected viremic individuals: evidence for nonspecific immune activation. J Virol. 2013;87:5800–5811. doi: 10.1128/JVI.00094-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loken MR, Chu SC, Fritschle W, Kalnoski M, Wells DA. Normalization of bone marrow aspirates for hemodilution in flow cytometric analyses. Cytometry B Clin Cytom. 2009;76:27–36. doi: 10.1002/cyto.b.20429. [DOI] [PubMed] [Google Scholar]
- 21.Moir S, Ho J, Malaspina A, Wang W, DiPoto AC, O'Shea MA, Roby G, Kottilil S, Arthos J, Proschan MA, Chun TW, Fauci AS. Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals. J Exp Med. 2008;205:1797–1805. doi: 10.1084/jem.20072683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kardava L, Moir S, Shah N, Wang W, Wilson R, Buckner CM, Santich BH, Kim LJ, Spurlin EE, Nelson AK, Wheatley AK, Harvey CJ, McDermott AB, Wucherpfennig KW, Chun TW, Tsang JS, Li Y, Fauci AS. Abnormal B cell memory subsets dominate HIV-specific responses in infected individuals. J Clin Invest. 2014;124:3252–3262. doi: 10.1172/JCI74351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Y, Svehla K, Louder MK, Wycuff D, Phogat S, Tang M, Migueles SA, Wu X, Phogat A, Shaw GM, Connors M, Hoxie J, Mascola JR, Wyatt R. Analysis of neutralization specificities in polyclonal sera derived from human immunodeficiency virus type 1-infected individuals. J Virol. 2009;83:1045–1059. doi: 10.1128/JVI.01992-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buckner CM, Kardava L, Moir S. Evaluation of B cell function in patients with HIV. Curr Protoc Immunol. 2013;Chapter 12 doi: 10.1002/0471142735.im1213s100. Unit 12 13. [DOI] [PubMed] [Google Scholar]
- 25.Binley JM, Wrin T, Korber B, Zwick MB, Wang M, Chappey C, Stiegler G, Kunert R, Zolla-Pazner S, Katinger H, Petropoulos CJ, Burton DR. Comprehensive cross-clade neutralization analysis of a panel of anti-human immunodeficiency virus type 1 monoclonal antibodies. J Virol. 2004;78:13232–13252. doi: 10.1128/JVI.78.23.13232-13252.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perez-Andres M, Paiva B, Nieto WG, Caraux A, Schmitz A, Almeida J, Vogt RF, Jr, Marti GE, Rawstron AC, Van Zelm MC, Van Dongen JJ, Johnsen HE, Klein B, Orfao A MBL Primary Health Care Group of Salamanca for the Study of MBL. Human peripheral blood B-cell compartments: a crossroad in B-cell traffic. Cytometry B Clin Cytom. 2010;78(Suppl 1):S47–60. doi: 10.1002/cyto.b.20547. [DOI] [PubMed] [Google Scholar]
- 27.van Lochem EG, van der Velden VH, Wind Hk, te Marvelde JG, Westerdaal Na, van Dongen JJ. Immunophenotypic differentiation patterns of normal hematopoiesis in human bone marrow: reference patterns for age-related changes and disease-induced shifts. Cytometry B Clin Cytom. 2004;60:1–13. doi: 10.1002/cyto.b.20008. [DOI] [PubMed] [Google Scholar]
- 28.Sedek L, Bulsa J, Sonsala A, Twardoch M, Wieczorek M, Malinowska I, Derwich K, Niedzwiecki M, Sobol-Milejska G, Kowalczyk JR, Mazur B, Szczepanski T. The immunophenotypes of blast cells in B-cell precursor acute lymphoblastic leukemia: How different are they from their normal counterparts? Cytometry B Clin Cytom. 2014 doi: 10.1002/cytob.21176. [DOI] [PubMed] [Google Scholar]
- 29.Agrawal S, Smith SA, Tangye SG, Sewell WA. Transitional B cell subsets in human bone marrow. Clin Exp Immunol. 2013;174:53–59. doi: 10.1111/cei.12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burton DR, Barbas CF, 3rd, Persson MA, Koenig S, Chanock RM, Lerner RA. A large array of human monoclonal antibodies to type 1 human immunodeficiency virus from combinatorial libraries of asymptomatic seropositive individuals. Proc Natl Acad Sci U S A. 1991;88:10134–10137. doi: 10.1073/pnas.88.22.10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giesecke C, Frolich D, Reiter K, Mei HE, Wirries I, Kuhly R, Killig M, Glatzer T, Stolzel K, Perka C, Lipsky PE, Dorner T. Tissue distribution and dependence of responsiveness of human antigen-specific memory B cells. J Immunol. 2014;192:3091–3100. doi: 10.4049/jimmunol.1302783. [DOI] [PubMed] [Google Scholar]
- 32.Scheid JF, Mouquet H, Feldhahn N, Walker BD, Pereyra F, Cutrell E, Seaman MS, Mascola JR, Wyatt RT, Wardemann H, Nussenzweig MC. A method for identification of HIV gp140 binding memory B cells in human blood. J Immunol Methods. 2009;343:65–67. doi: 10.1016/j.jim.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sundling C, Li Y, Huynh N, Poulsen C, Wilson R, O'Dell S, Feng Y, Mascola JR, Wyatt RT, Karlsson Hedestam GB. High-resolution definition of vaccine-elicited B cell responses against the HIV primary receptor binding site. Sci Transl Med. 2012;4:142ra196. doi: 10.1126/scitranslmed.3003752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.West AP, Jr, Scharf L, Scheid JF, Klein F, Bjorkman PJ, Nussenzweig MC. Structural insights on the role of antibodies in HIV-1 vaccine and therapy. Cell. 2014;156:633–648. doi: 10.1016/j.cell.2014.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kwong PD, Mascola JR. Human antibodies that neutralize HIV-1: identification, structures and B cell ontogenies. Immunity. 2012;37:412–425. doi: 10.1016/j.immuni.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burton DR, Poignard P, Stanfield RL, Wilson IA. Broadly neutralizing antibodies present new prospects to counter highly antigenically diverse viruses. Science. 2012;337:183–186. doi: 10.1126/science.1225416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Slifka MK, Antia R, Whitmire JK, Ahmed R. Humoral immunity due to long-lived plasma cells. Immunity. 1998;8:363–372. doi: 10.1016/s1074-7613(00)80541-5. [DOI] [PubMed] [Google Scholar]
- 38.Manz RA, Thiel A, Radbruch A. Lifetime of plasma cells in the bone marrow. Nature. 1997;388:133–134. doi: 10.1038/40540. [DOI] [PubMed] [Google Scholar]
- 39.Lindqvist M, van Lunzen J, Soghoian DZ, Kuhl BD, Ranasinghe S, Kranias G, Flanders MD, Cutler S, Yudanin N, Muller MI, Davis I, Farber D, Hartjen P, Haag F, Alter G, Schulze zur Wiesch J, Streeck H. Expansion of HIV-specific T follicular helper cells in chronic HIV infection. J Clin Invest. 2012;122:3271–3280. doi: 10.1172/JCI64314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dhurve SA, Dhurve AS. Bone Marrow Abnormalities in HIV Disease. Mediterr J Hematol Infect Dis. 2013;5:e2013033. doi: 10.4084/MJHID.2013.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meggetto F, Cesarman E, Mourey L, Massip P, Delsol G, Brousset P. Detection and characterization of human herpesvirus-8-infected cells in bone marrow biopsies of human immunodeficiency virus-positive patients. Hum Pathol. 2001;32:288–291. doi: 10.1053/hupa.2001.22749. [DOI] [PubMed] [Google Scholar]
- 42.Amara S, Dezube BJ, Cooley TP, Pantanowitz L, Aboulafia DM. HIV-associated monoclonal gammopathy: a retrospective analysis of 25 patients. Clin Infect Dis. 2006;43:1198–1205. doi: 10.1086/508351. [DOI] [PubMed] [Google Scholar]
- 43.Moir S, Malaspina A, Pickeral OK, Donoghue ET, Vasquez J, Miller NJ, Krishnan SR, Planta MA, Turney JF, Justement JS, Kottilil S, Dybul M, Mican JM, Kovacs C, Chun TW, Birse CE, Fauci AS. Decreased survival of B cells of HIV-viremic patients mediated by altered expression of receptors of the TNF superfamily. J Exp Med. 2004;200:587–599. [PubMed] [Google Scholar]
- 44.Morris L, Binley JM, Clas BA, Bonhoeffer S, Astill TP, Kost R, Hurley A, Cao Y, Markowitz M, Ho DD, Moore JP. HIV-1 antigen-specific and -nonspecific B cell responses are sensitive to combination antiretroviral therapy. J Exp Med. 1998;188:233–245. doi: 10.1084/jem.188.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moir S, Malaspina A, Ho J, Wang W, Dipoto AC, O'Shea MA, Roby G, Mican JM, Kottilil S, Chun TW, Proschan MA, Fauci AS. Normalization of B cell counts and subpopulations after antiretroviral therapy in chronic HIV disease. J Infect Dis. 2008;197:572–579. doi: 10.1086/526789. [DOI] [PubMed] [Google Scholar]
- 46.Malaspina A, Moir S, Ho J, Wang W, Howell ML, O'Shea MA, Roby GA, Rehm CA, Mican JM, Chun TW, Fauci AS. Appearance of immature/transitional B cells in HIV-infected individuals with advanced disease: correlation with increased IL-7. Proc Natl Acad Sci U S A. 2006;103:2262–2267. doi: 10.1073/pnas.0511094103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Amu S, Ruffin N, Rethi B, Chiodi F. Impairment of B-cell functions during HIV-1 infection. AIDS. 2013;27:2323–2334. doi: 10.1097/QAD.0b013e328361a427. [DOI] [PubMed] [Google Scholar]
- 48.Moir S, Fauci AS. Insights into B cells and HIV-specific B-cell responses in HIV-infected individuals. Immunol Rev. 2013;254:207–224. doi: 10.1111/imr.12067. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.