Abstract
Introduction
Deficits in attentional abilities can significantly impact rehabilitation and recovery from traumatic brain injury (TBI). This study investigated the nature and recovery of pre-attentive (parallel) and attentive (serial) visual search abilities after TBI.
Methods
Participants were 40 individuals with moderate to severe TBI who were tested following emergence from post-traumatic amnesia and approximately 8-months post-injury, as well as 40 age and education matched controls. Pre-attentive (automatic) and attentive (controlled) visual search situations were created by manipulating the saliency of the target item amongst distractor items in visual displays. The relationship between pre-attentive and attentive visual search rates and follow-up community integration were also explored.
Results
The results revealed intact parallel (automatic) processing skills in the TBI group both post-acutely and at follow-up. In contrast, when attentional demands on visual search were increased by reducing the saliency of the target, the TBI group demonstrated poorer performances compared to the control group both post-acutely and 8-months post-injury. Neither pre-attentive nor attentive visual search slope values correlated with follow-up community integration.
Conclusions
These results suggest that utilizing intact pre-attentive visual search skills during rehabilitation may help to reduce high mental workload situations, thereby improving the rehabilitation process. For example, making commonly used objects more salient in the environment should increase reliance or more automatic visual search processes and reduce visual search time for individuals with TBI.
Keywords: attention, closed head injury, outcome, pre-attentive processes, parallel and serial search
In the everyday environment, visual search is a common activity. Sometimes visual search abilities are so efficient that we may not notice we have searched for an item because the item is so salient in its surroundings. At other times, however, effort and time may need to be spent to locate an item in its environment. For example, imagine searching for a child in a school cafeteria where all the children are wearing the same uniform. This effortful search task can be made much easier if the child you are searching for is the only child wearing a bright orange hat. Although the mechanisms that underlie visual search are not fully understood (Chan & Hayward, 2009; Muller et al., 2010; Tsotsos, Rodrigues-Sanchez, Rothenstein, A. L., & Simine, 2008; Wolfe, Palmer, & Horowitz, 2010), a dominant view that has emerged from laboratory studies is that visual search efficiency depends on two interrelated yet distinct processes: pre-attentive and attentive processes (Palmer, Fencsik, Flusberg, Horowitz, & Wolfe, 2011; Smilek, Frischen, Reynold, Gerritsen, & Eastwood, 2007). In this study, we used a visual search paradigm and created visual search situations that relied heavily on either pre-attentive or attentive processing. We examined visual search abilities in individuals who suffered moderate to severe traumatic brain injuries (TBI) both post-acutely (i.e., following emergence from post-traumatic amnesia; PTA) and after approximately eight months of recovery.
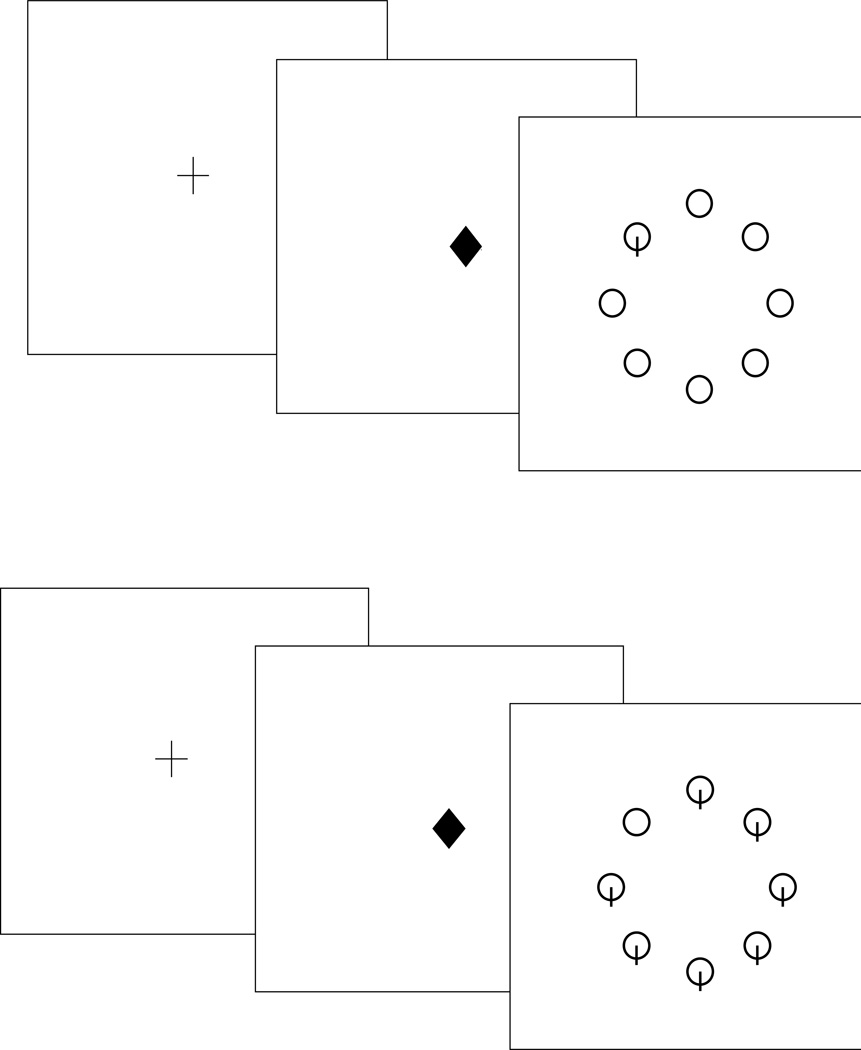
In the typical visual search paradigm, participants must search for a target item among a varying number of distractor items in a visual display and indicate whether the target item is present or absent. Reaction times (RT) are measured as a function of the total number of items presented in the visual display (i.e., display or set size). By using a target that is salient or uniquely defined from distractor items by a basic feature (single-feature search; see Figure 1a), search situations can be created where the target “pops out” of its surrounding involuntarily capturing attention, with RT showing little to no change with display size (e.g., Plude & Doussard-Roosevelt, 1989, Treisman & Gelade, 1980; Wolfe et al., 1990). In these situations, visual search has been described as relying primarily on pre-attentive processes, which are considered to be largely automatic (bottom-up) as they allow the search process to occur quickly, in parallel and with little attentional effort (Palmer et al., 1993; Muller-Oerhing, 2013). In contrast, by using a non-salient target item that overlaps in features with distractor items (conjunction search; see Figure 1b), search situations can be created where RT increases significantly as the number of items in the display size increases. In these situations, visual search has been described as relying heavily on attentive processes, which require an effortful, and typically serial search of items (perceptual grouping in conjunction search can also involve pre-attentive mechanisms) to identify the presence or absence of the target item in the visual display (Bundesen, 1990, Sung, 2008; Treisman & Gelade, 1980; Wolfe et al., 1989; Wilkinson, Halligan, Henson, & Dolan, 2002).
Figure 1.
Example of pre-attentive stimuli with target-present (top). Example of attentive stimuli with target-present (bottom).
Within the TBI literature, prior visual search studies have shown that individuals with TBI require more time to search for target items when the target and distracter items have a high degree of feature overlap (Bate, Mathias, & Crawford, 2001a, Hatta, Yoshizaki, Ito, Mase, & Kabasawa, 2012; Heinze, Munte, Gobiet, Niemann & Ruff, 1992; Rasmussen et al., 2008). For example, Bates et al. (2001a) found that TBI participants required more time than controls to search for designated target symbols on a colored map and in a simulated classified telephone directory, both within one year post-injury and at more than two years post-injury. Furthermore, Schmitter-Edgecombe and colleagues (Schmitter-Edgecombe & Beglinger, 2001; Schmitter-Edgecombe & Kibby, 1998) found that when searching for non salient target items, RTs of individuals with moderate to severe TBI (> 1 year post-injury) slowed more than those of controls as visual display size increased. These finding indicate that in attention-demanding visual search situations, individuals with TBI require more time to process task-irrelevant information and search the visual displays.
The visual search study by Schmitter-Edgecombe and Kibby (1998) also showed that when attention was directed to the target location ahead of time with a visual cue, under conditions of low (but not high) target-distractor similarity, TBI participants (> 1 year post-injury) successfully ignored irrelevant task information. Furthermore, a study by Schmitter and Beglinger (2001) revealed that, following extended practice on an effortful visual search task, individuals with severe TBI (> 1 year post-injury) were able to develop an automatic attention response similar to controls in a consistent mapping condition. A study by Bates and colleagues (Bates, Mathias, & Crawford, 2001b), which used Posner’s Covert Orienting of Attention Task to examine orienting of visual attention, revealed no difference between TBI and control groups in their ability to disengage, move and engage attention even under dual-task conditions. The authors suggested that performance on the visual orienting task may have become automated with practice. Taken together, these studies indicate that by one-year post-injury TBI participants perform similarly to controls in visual search situations that minimize the amount of attentional effort involved in the search process.
This dissociation between TBI participants’ performances on visual search tasks that require varying degrees of attentional effort is consistent with other prior literature which generally suggests that unlike more explicit, attention demanding (controlled) processes, tasks that rely heavily on automatic processes are intact at one year post moderate to severe TBI. Examples of such tasks include the automatic activation of words (Perri, Carlesimo, Loasses, & Caltagirone, 2000; Schmitter-Edgecombe, Marks, & Fahy, 1993), perceptually-based implicit learning (Nissley & Schmitter-Edgecombe, 2002), automatic retrieval processes (Ries & Marks, 1995) and perceptual implicit memory processes (Schmitter-Edgecombe, 1996; Shum, Sweeper & Murray, 1996; Vakil, Biederman, Liran, Groswasser, & Aberbuch, 1994). To date, little research has investigated how relatively automatic processes are affected in the initial stages of recovery from TBI. An early study by Vakil and colleagues (Vakil, Blachstein, & Hoofien, 1991) showed that by 3 to 6 months post-injury, TBI patients can implicitly show knowledge for information that they cannot access explicitly. Understanding the recovery of automatic processes has important implications for rehabilitation. For example, if relatively automatic cognitive processes recover early, or are relatively intact following emergence from PTA, then early cognitive interventions that utilize and build on these intact skills could help expedite recovery and thereby improve the rehabilitation process and cognitive outcome.
In study 1, we utilized visual search tasks that relied heavily on pre-attentive (single-feature search) and attentive processing to investigate more automatic and controlled aspects of visual search, respectively, in the post-acute stage of recovery from moderate to severe TBI (i.e., following emergence from PTA). In study 2, we investigated the recovery of pre-attentive and attentive visual search processes by retesting a subsample of the TBI participants approximately eight months post-injury. Based on prior research, we expected that post acutely the TBI participants would search the visual displays at a slower rate than controls when cognitively-demanding, controlled processes were required in the attentive search task. We were especially interested in whether more automatic, pre-attentive visual search processes involved in the extraction of simple featural information would be impaired in the early stage of recovery following TBI. We expected that the TBI group would exhibit improvements in their visual search rates on both the heavily attentive and pre-attentive search tasks following six months or more of recovery. We further hypothesized that visual search rates would remain disproportionately slowed in the attentive search condition for the TBI group, while pre-attentive search would not differ from controls at follow-up.
As a secondary goal of this study, we also explored the relationship between pre-attentive and attentive visual search rates, community integration, and neuropsychological measures assessing attention and speeded processing, verbal learning and memory, and executive functioning. A continuing challenge for TBI research is identification of the best early predictors of long-term psychosocial outcome following TBI. A study by Millis and colleagues (Millis, Rosenthal & Lourie, 1994) suggested that cognitive speed and flexibility, complex attention, and memory during the acute phase of recovery may be the best predictors of later community integration. We were especially interested in whether individuals with TBI who post-acutely exhibited the greatest difficulties with more foundational automatic processes, as characterized by pre-attentive search, would have poorer long-term psychosocial outcomes.
Experiment 1: Cross-sectional analysis
The purpose of Experiment 1 was to determine whether individuals with TBI would exhibit intact visual search rates in the post-acute phase of recovery when the visual search task relied more heavily on automatic, pre-attentive processes as compared to effortful, attentive processes.
Methods
Participants
Participants included 40 individuals with TBI (10 female, 30 male) and 40 matched controls (17 female, 23 male) between the ages of 16 and 55. The participants with TBI were recruited from consecutive admissions to a regional inpatient rehabilitation program in the Pacific Northwest and received information about their cognitive testing performances in return for their involvement. Participants were excluded from the study if they had a history of multiple head injuries, preexisting neurological, psychiatric, or developmental disorder, recent (i.e., past year) history of treatment for substance abuse, a visual field deficit that would disrupt viewing of a computer screen, poor visual acuity at a distance of 16 inches (i.e., 20/60 vision using both eyes), or severe motor deficits in both upper limbs that would preclude accurate measurement of RT. Control participants were recruited from the community through the use of advertisements and were given monetary compensation in return for their time.
All TBI participants suffered a moderate or severe TBI. Severe TBI was defined by a Glasgow Coma Scale (GCS, Teasdale & Jennett, 1974) score of 8 or less (n = 25), documented at the scene of the accident or in the emergency room. Moderate TBI was defined by a GCS between 9 and 12 (n = 4), or by a GCS > 12 if accompanied by positive neuroimaging findings and/or neurosurgery (n = 11) (Dennis et al., 2001; Fletcher et al., 1990; Taylor et al., 2002; Williams, Levin, & Eisenberg, 1990). All participants exhibited a period of extended PTA (M = 20.18 days; SD = 13.88 days; range = 1–56 days). Emergence from PTA was measured either prospectively (n = 27) by repeated administration of the Galveston Orientation and Amnesia Test (GOAT, Levin, O’Donnell, & Grossman, 1979), or retrospectively (n = 13) when PTA had resolved prior to arrival at the rehabilitation facility, by carefully assessing recall of post-injury memories until the evaluator was persuaded that the participant displayed normal continuous memory (King et al, 1997; McMillan, Jongen, & Greenwood, 1996). The majority of head injuries resulted from a motor vehicle or motorcycle accident (n = 28), eight were a result of a fall, two were a pedestrian in a motor vehicle accident, one resulted from an assault, and one from a sports related injury.
For time 1 testing, participants took part in this study an average of 21 days after emergence from PTA (M = 21.03 days; SD = 16.10 days; range = 1–68 days). Time since injury (TSI) ranged from 12 – 89 days (M = 41.20 days; SD= 19.85 days). Comparisons between the TBI and control groups revealed the groups were well matched on the demographic variables of age, education and gender, X2 (1, n=80) = 2.74 (see Table 1). In addition, TBI and control groups were well matched on an estimate of premorbid intelligence derived from the Barona Index equation (Barona, Reynolds & Chastain, 1984), which takes into account 6 demographic variables (i.e., age, sex, race, education, occupation and region). Consistent with sustaining a recent moderate to severe brain injury, Table 1 shows that participants with TBI performed more poorly than controls on measures assessing general mental status (Telephone Interview of Cognitive Status [TICS], Brandt & Folstein, 2003), attention/speeded processing (Symbol Digit Modalities Test [SDMT], Smith, 1991; Trail Making Test [TMT], Part A, Reitan, 1958), verbal learning and memory (Rey Auditory Verbal Learning Test [RAVLT], Majdan, Sziklas, & Jones-Gotman, 1996), and executive functioning (Controlled Oral Word Association test [COWA, PRW], Benton, Hamsher, & Sivan, 1994; Letter-Number sequencing sub-test from the Wechsler Adult Intelligence Scale-Third Edition [WAIS-III]; Wechsler, 1997; TMT, Part B, Reitan, 1958).
Table 1.
Demographic Data and Mean Neuropsychological Summary Data for Traumatic Brain Injury (TBI) and Control Groups
| Group | ||||||
|---|---|---|---|---|---|---|
| TBI (n=40) |
Control (n=40) |
|||||
| Variable or Test | M | SD | M | SD | t-test | Cohen’s d |
| Demographics | ||||||
| Age | 31.43 | 13.34 | 29.83 | 13.38 | −.20 | .05 |
| Education (years) | 12.73 | 1.92 | 13.60 | 2.25 | 1.87 | .42 |
| Gender (% male) | 75% | 58% | ||||
| eFSIQ | 102.22 | 6.55 | 105.13 | 6.97 | 1.93 | .43 |
| Global Cognitive Status | ||||||
| TICS total score | 33.14† | 3.86 | 38.19‡ | 3.97 | 5.35** | 1.29 |
| Attention/Processing Speed | ||||||
| SDMT Oral total | 37.13§ | 12.50 | 58.23 | 8.91 | 8.65** | 1.96 |
| SDMT Written total | 44.85§ | 11.68 | 68.95 | 11.34 | 9.31** | 2.11 |
| Trails A | 39.10§ | 13.68 | 23.33 | 7.84 | −6.31** | 1.43 |
| Verbal Memory | ||||||
| RAVLT List Learning | 43.70† | 9.35 | 56.35 | 7.45 | 6.59** | 1.50 |
| RAVLT Delayed Recall† | 7.49† | 3.31 | 11.28 | 3.08 | 5.20** | 1.19 |
| Executive Skills | ||||||
| WAIS-III LN Sequencing | 9.67§ | 2.59 | 12.35 | 3.00 | 4.25** | .96 |
| COWAT | 25.21§ | 9.33 | 40.78 | 11.08 | 6.75** | 1.53 |
| Trails B | 106.87§ | 47.68 | 53.70 | 19.94 | 6.50** | 1.47 |
Note. Unless otherwise indicated, mean scores are raw scores. TICS = Telephone Interview for Cognitive Status; SDMT = Symbol Digit Modalities Test; RAVLT = Rey Auditory Verbal Learning Test; LN Sequencing= Letter Number Sequencing subtest of the Weschler Adult Intelligence Scale- Third Edition; COWAT= Controlled Oral Word Association Test (PRW).
p < .001.
n = 37;
n = 32;
n = 39
Apparatus and Stimuli
The stimuli were displayed on an IBM-compatible personal computer programmed with SuperLab Pro Beta Version Experimental Lab Software (Cedrus Corporation, 1999). Stimuli consisted of circles and circles with intersecting vertical lines (see Figure 1). Each stimulus item subtended a visual angle of two degrees. The stimulus items were situated at the vertices of an imaginary octagon centered at fixation with radius of five degrees. A circular configuration was chosen as it allows all locations to have the same properties (Yantis & Johnson, 1990). The stimuli were black against a white background. The number of stimuli present in one display (set size) was either two or eight. Target and distractor stimuli were defined by the nature of the search task (i.e., pre-attentive or attentive).
Procedure
All participants completed a battery of standardized and experimental neuropsychological tests, which included the neuropsychological tests listed in Table 1 and the visual search tasks. Administration of the tasks typically occurred across two sessions post-acutely for the TBI participants and took approximately 3–4 hours in total. The pre-attentive and attentive visual search tasks were always administered in the same testing session.
Each participant completed the visual search task in both a pre-attentive and attentive search condition. Task order was counterbalanced across participants. Both tasks consisted of 128 test trials preceded by 16 practice trials. Participants received a self-paced rest break half way through each task. Four different conditions were created by manipulating the number of items in the visual display (2 or 8) and the response required (target-present or target-absent). In addition, each location of the visual display served as the target location an equal number of times. For both tasks, each trial was signaled by a computer-generated auditory warning tone (medium pitch) presented simultaneously with a 400 ms fixation cross displayed in the center of the screen. Participants were instructed to focus their eyes on the fixation cross and told that this would help make their responses faster. Just prior to the presentation of the search display, the fixation cross changed to a central diamond for 200ms. Prior research has shown that a state of distributed attentional resources can be induced by presentation of an informationless, neutral cue (e.g., central diamond) prior to the visual display presentation (Eriksen & Yeh, 1985). The search display then appeared on the computer screen until the participant made a response. Participants were told to press the button labeled “yes” if the target was present in the display and to press the button labeled “no” if the target was absent (see Figure 1). Responses were made with the index and middle fingers of the participant’s dominant hand. To minimize RTs, participants kept their fingers resting on the response keys throughout the duration of the tasks. Half of the visual displays required target-present responses and half target-absent responses.
In the pre-attentive search condition, the target was a circle with an intersecting vertical line, and the distractors were circles with no intersecting lines (see Figure 1a). For the pre-attentive condition, the target contained a simple feature (vertical line) that differentiated it from the distractors. In this situation, the target should automatically “pop out” resulting in a highly efficient, parallel search, and a flat RT function (Plude & Doussard-Roosevelt, 1989, Treisman & Gelade, 1980). For the attentive search condition, targets and distractors were reversed, such that the target was a circle and the distractors were circles with intersecting lines (see Figure 1b). In this condition, the target lacked a distinct feature that the distractors possessed. Thus, search should be less efficient and cognitively-demanding, as evidenced by a positively sloping RT function (Plude & Doussard-Roosevelt, 1989, Treisman & Gelade, 1980). For both the pre-attentive and attentive search tasks, error trials were signaled by a low pitch tone presented simultaneously with the word “WRONG”. Participants were told to respond as quickly and accurately as possible. If their accuracy dropped below 93%, they were instructed to respond more carefully. Conversely, if their accuracy went above 97% they were instructed to respond more quickly. We wanted participants to operate at the same level of accuracy across groups and search tasks. The inter-trial interval was 1500 ms.
Analysis
SPSS statistical software was used for data analysis. Reaction times for error trials were removed from analysis. The data were trimmed by removing trials that were 2.5 standard deviations away from the individual means. This resulted in removal of less than 3% of the data for both the TBI (2.65%) and control (2.60%) groups. To examine the robustness of the interactions (Wagenmakers & Krypotos, 2012), normalize the data, and take effects of differences in processing speed into account (Faust, Balota, Spieler & Frerraro, 1999; Hoelfsloot, Westerhuis, Smilde, & van der Werf, 2006), transformed RTs [i.e., log(RT) and 1/RT] were analyzed along with the raw data. Because the logarithmic transformation is easier to conceptually translate than the reciprocal transformation, this data is presented in the text and Tables. The raw data is also presented in the Tables. The findings did not change materially based on method (i.e., raw data and two transformations) and instances where the data did not yield identical findings are indicated in the results sections.
Reaction time and accuracy rates for the pre-attentive and attentive visual search tasks were subjected to separate mixed-model analyses of variance (ANOVA) with group (TBI, control) as the between subjects factor and display size (two, eight) and response type (target-present, target-absent) as the within-subjects factors. Correlation analyses were performed for the TBI group to examine for relationships between target-present and target-absent search slope values and injury characteristics, demographic variables, and standardized neuropsychological tests (see Table 1). The visual search slope values for the functions relating RT to the number of items in the visual display represented the increase in log normalized reaction time with additional distracters (i.e., log(RT)/additional distracters). Because of the large number of correlations conducted, a more conservative value of p < .005 was used to establish significance.
Pre-attentive Data
As seen in Table 2, analysis of the pre-attentive search RTs revealed that RTs were faster for the control group, F(1, 78) = 41.427, MSE = .045, p < .001, ηp2= .347, and for display size 2, F(1, 78) = 9.076, MSE = .001, p < .005, ηp2= .104. The main effect of response, F(1, 78) = 4.284, MSE = .002, p = .042, ηp2= .052, was modified by a significant response type by group interaction, F(1, 78) = 8.863, MSE = .002, p = .004, ηp2= .102. Breakdown of this interaction revealed that response type did not influence the RTs of the control participants in the pre-attentive search condition, t = −.720, p = .476. In contrast, the RTs of the TBI participants were significantly slower for target-absent responses compared to target-present responses, t(1, 39) = 3.251, p = .002. No additional two-way interactions reached significance, Fs < 1.366, nor was the 3-way interaction significant, F = 1.030. Both the raw and reciprocal transformation data revealed a similar pattern of findings to the log transformed data. Lack of a group by display size interaction suggests that the groups did not differ in the rate at which they searched the visual displays in the pre-attentive condition. In addition, the percent slowing between display sizes 2 and 8 was minimal (i.e., < 1%) for both the TBI (0.46% slowing) and control (0.30% slowing) groups.
Table 2.
Mean Log Transformed Reaction Times, Standard Deviations and Accuracy Rates as a Function of Group, Display Size, Response Type and Visual Search Task (raw RTs in parentheses)
| Pre-attentive Visual Search Task | ||||
|---|---|---|---|---|
| TBI (n = 40) | Control (n = 40) | |||
| Display Size | Display Size | |||
| Condition | 2 | 8 | 2 | 8 |
| Target-present | ||||
| M | 2.86 (752.79) |
2.87 (770.55) |
2.72 (533.35) |
2.73 (541.26) |
| SD | 0.13 (242.57) |
0.13 (269.76) |
0.07 (82.16) |
0.06 (76.75) |
| % Correct | 96.25 | 98.12 | 96.09 | 97.97 |
| Target-absent | ||||
| M | 2.88 (790.10) |
2.90 (854.92) |
2.72 (528.61) |
2.73 (540.40) |
| SD | 0.13 (261.36) |
0.16 (406.07) |
0.07 (90.47) |
0.08 (104.44) |
| % Correct | 97.73 | 98.44 | 96.41 | 97.42 |
| Attentive Visual Search Task | ||||
| TBI (n = 40) | Control (n = 40) | |||
| Display Size | Display Size | |||
| Condition | 2 | 8 | 2 | 8 |
| Target-present | ||||
| M | 2.91 (832.10) |
3.04 (1123.32) |
2.78 (603.72) |
2.90 (803.25) |
| SD | 0.10 (205.89) |
0.11 (300.03) |
0.06 (85.70) |
0.08 (152.99) |
| % Correct | 97.77 | 98.05 | 97.19 | 97.42 |
| Target-absent | ||||
| M | 2.95 (926.18) |
3.25 (1892.60) |
2.82 (661.79) |
3.04 (1144.18) |
| SD | 0.11 (262.29) |
0.16 (710.67) |
0.07 (98.31) |
0.13 (362.40) |
| % Correct | 95.31 | 97.19 | 93.67 | 96.64 |
The mixed model ANOVA analysis on accuracy rates revealed higher accuracy rates for target-absent as compared to target-present responses, F(1, 78) = 15.896, MSE = .001, p < .001, ηp2= .169. There was no main effect of group, F = 1.237, and no other main effects or interactions reached significance, Fs < 2.248. As seen in Table 2, accuracy rates generally fell within the overall expected range of 93% – 97% for both the TBI and control groups, and showed a similar pattern across groups. Because accuracy rates were higher for target absent responses, and TBI participants showed disproportionately slower RTs to target-absent as compared to target-present responses, we assessed for a speed-accuracy trade off. Correlation analyses revealed no significant association between RTs and accuracy rates for either the target-present (r = −.18) or target absent (r = −.15) responses of the TBI group, indicating that a speed-accuracy trade-off was unlikely to explain the group by response type interaction found for the RT data.
Attentive Data
As seen in Table 2, analysis of the attentive search RTs revealed that RTs were faster for the control group, F(1, 78) = 48.101, MSE = .039, p < .001, ηp2= .381, for display size 2, F(1, 78) = 626.672, MSE = .005, p < .001, ηp2= .889, and for target-present responses, F(1, 78) = 346.146, MSE = .003, p < .001, ηp2= .816. The significant interaction between display size and response type, F(1, 78) = 227.526, MSE = .002, p < .001, ηp2= .750, indicated that the display size effect was greater for target-absent (8.94% slowing) compared to target-present responses (4.37% slowing). Three significant group interactions were also observed: Group X Display Size, F(1, 78) = 6.518, p = .013, ηp2= .077, Group X Response Type, F(1, 78) = 9.782, p = .002, ηp2= .111, and Group X Display Size X Response Type, F(1, 78) = 12.803, p < .001, ηp2= .141. The group by display size effect indicated that the addition of distracters increased the visual search time more for the TBI participants than controls. The significant group by response type and three-way interaction reflected the fact that the display size effect was larger in magnitude for the target-absent compared to the target-present trials and this pattern was more pronounced for the TBI group compared to controls (see Table 2). The raw data revealed a similar pattern of findings, while the reciprocal transformation data showed only the display size by response type and group by display size interactions. Although there were some differences across methods, there was consistency in the finding that the display size effect slowed the visual search rate of the TBI participants (7.33% slowing) more than that of the controls (6.07% slowing) in the attentive search condition.
Analysis of task accuracy revealed that accuracy rates were higher for display size 2, F(1, 78) = 25.393, MSE = .001, p < .001, ηp2= .246. The significant main effect of response type, F(1, 78) = 11.508, p = .001, ηp2= .129, was modified by an interaction between display size and response type, F(1, 78) = 7.367, p = .008, ηp2= .086. Breakdown of the interaction revealed no difference in accuracy rate at display size 2 for the target-present and target-absent responses, t = −.571, p = .570. In contrast, target-absent responses showed higher accuracy rates for display size 8 compared to target-present responses, t(79) = −3.851, p < .001. There were no significant main effect of group, F = 1.441, and no interactions involving group, Fs < .549, suggesting that the RT findings are unlikely to be confounded by speed-accuracy trade-off differences across groups.
Correlations
For the TBI group, we conducted correlational analyses to determine whether the pre-attentive and attentive visual search rates for target-absent and target-present log normalized slope measures exhibited a relationship with injury characteristics (i.e., GCS, PTA, TSI), demographic variables, and the standardized neuropsychological tasks administered in Table 1. At a more conservative significance level of p < .005, no significant correlations emerged between the slope estimates (preattentive: target present = .001, target absent = .003; attentive: target present = .021, target absent = .049) and the standardized neuropsychological measures, rs between −.26 and .16, or the demographic variables of age, education, gender and eFSIQ, rs between −.14 and .29. For injury characteristics, there were no significant correlations with GCS or TSI, rs between −.22 and .35, while duration of PTA showed a positive correlation with the pre-attentive target absent slope, r = .46, p = .003. For the raw RT and reciprocal transformation slope estimates, no significant correlations reached the .005 level of significance.
Discussion
Consistent with prior research conducted with TBI individuals in the chronic phase of recovery (> one year post-injury; e.g., Hatta et al., 2012; Schmitter-Edgecombe & Beglinger, 2001; Rasmussen et al., 2008), TBI participants in the post-acute phase of recovery exhibited slower visual search rates than controls in the attentive search condition. The finding that target absent trials (8.94% slowing) were more influenced by the display size manipulation than target present trials (4.37% slowing) supports the supposition that more cognitively-demanding, serial processing was required (Bundesen, 1990; Sung, 2008) in the attentive search condition. In contrast, the display size manipulation resulted in a minimal amount of slowing (< .05 percent) for both the TBI and control groups in the pre-attentive search condition. This suggests that the search process in the pre-attentive condition was largely automatic, efficient, and occurred in parallel for both groups. This study extends to the post-acute phase of recovery the findings of prior studies which showed that relatively automatic processes are intact by one year post moderate to severe TBI (e.g., Nissley & Schmitter-Edgecombe, 2001; Perri, et al., 2000). Of interest, while the feature extraction process involved in pre-attentive search appeared intact, the TBI group exhibited slower RTs for target absent compared to target present responses found compared to the control group. This suggests that the TBI participants may be experiencing difficulties with other processes involved, such as detecting or selecting the absence of a target (Potter, 2012). In addition, the slower overall RTs of the TBI participants in both the pre-attentive and attentive search conditions indicates that processes involved in the stimulus identification and/or response selection aspects of the search tasks are slowed relative to those of controls in the post-acute phase of recovery.
Experiment 2: Longitudinal Analysis
The purpose of Experiment 2 was to evaluate the recovery of visual search processes in individuals who had sustained moderate to severe TBIs. We were also interested in whether visual search measures could be useful as early predictors of later community integration.
Methods
Participants
Twenty-one (6 female, 15 male) of the 38 participants completed follow-up testing. The follow-up session occurred an average of 8.5 months after the baseline testing session (M= 8.49 months; SD= 2.78 months; range= 6–14 months), with TSI ranging from 208 – 475 days (M = 305 days; SD = 93 days). The participants with TBI who were retested did not differ from the non-returning TBI participants in age, education or eFSIQ (see Table 3). There was also no difference in the gender distribution of the returning and non-returning TBI participants, X2 (1, n=40) = .30. In addition, returning and non-returning TBI participants did not differ in terms of severity of injury, as measured by both the GCS, t = .221, (returners: M = 8.05, SD = 4.50; non-returners: M = 7.74, SD = 4.37) and duration of PTA, t = −.649, (returners: M = 18.81, SD = 11.65; non-returners: M = 21.68, SD = 16.19). Furthermore, as seen in Table 3, there were no significant differences between the TBI returners and non-returners on the administered tests of attention and speeded processing, verbal learning and memory, and executive functioning skills. This suggests that the characteristics of the TBI returners were generally similar to those of the TBI non-returners.
Table 3.
Demographic Data and Mean Neuropsychological Summary Data for Returning and Non-returning Traumatic Brain Injury (TBI) Participants
| Group | ||||||
|---|---|---|---|---|---|---|
| TBI Returners (n=21) |
TBI Non-returners (n=19) |
|||||
| Variable or Test | M | SD | M | SD | t-test | Cohen’s d |
| Demographics | ||||||
| Age | 33.57 | 14.55 | 26.95 | 11.20 | 1.60 | .51 |
| Education (years) | 12.90 | 1.99 | 12.53 | 1.87 | .62 | .20 |
| Gender (% male) | 71% | 79% | ||||
| eFSIQ | 103.14 | 6.72 | 101.20 | 6.37 | .93 | .30 |
| Global Cognitive Status | ||||||
| TICS total score | 33.86 | 3.77 | 32.22† | 3.89 | 1.32 | .45 |
| Attention/Processing Speed | ||||||
| SDMT Oral total | 38.76 | 10.94 | 35.22‡ | 14.19 | .87 | .29 |
| SDMT Written total | 46.14 | 10.15 | 43.33‡ | 13.39 | .74 | .24 |
| Trails A | 40.57 | 11.78 | 37.39‡ | 15.79 | .72 | .24 |
| Verbal Memory | ||||||
| RAVLT List Learning | 46.25 | 8.88 | 40.32§ | 8.81 | 2.07 | .67 |
| RAVLT Delayed Recall† | 8.25 | 2.61 | 6.21§ | 3.50 | 2.03 | .66 |
| Executive Skills | ||||||
| WAIS-III LN Sequencing | 9.81 | 2.96 | 9.50‡ | 2.15 | .37 | .12 |
| COWAT | 26.00 | 10.21 | 24.28‡ | 8.37 | .57 | .19 |
| Trails B | 104.47 | 45.41 | 109.67‡ | 51.38 | −.34 | .11 |
Note. Unless otherwise indicated, mean scores are raw scores. TICS = Telephone Interview for Cognitive Status; SDMT = Symbol Digit Modalities Test; RAVLT = Rey Auditory Verbal Learning Test; LN Sequencing= Letter Number Sequencing subtest of the Weschler Adult Intelligence Scale- Third Edition; COWAT= Controlled Oral Word Association Test (PRW).
n = 16;
n = 18;
n = 17
Follow-up testing for the controls occurred an average of 7.20 months after the baseline testing session (SD= 2.05 months; range= 6–12 months) and did not differ from that of the TBI group, t = 1.720. Retested controls (8 female, 13 male) and participants with TBI were well matched on the demographic variables of age, t = .001, (control: M = 33.57, SD = 13.95; TBI: M =33.52, SD = 14.61), education, t = −1.328, (control: M = 13.81, SD = 2.40; TBI: M = 12.90, SD = 2.00), eFSIQ, t = −1.296, (control: M = 105.90, SD = 7.08; TBI: M = 103.14, SD = 6.72) and gender, X2 (1, N = 42) = .43. At follow-up, with the exception of the WAIS-III letter-number sequencing subtest (TBI: M = 9.81, SD = 2.96; control: M = 12.10, SD = 2.88; t(40) = .2.537, p = .015), the TBI returners continued to differ from the retested controls on all other administered neuropsychological tests that assessed global cognitive status, t = 3.811, p < .001, attention and speeded processing, ts > 5.226, ps < .001, verbal learning and memory, ts > 2.689, ps < .015, and executive skills, ts > 4.355, ps < .001.
Procedure
The standardized neuropsychological tests administered and the materials and procedures used for the pre-attentive and attentive visual search tasks were identical to those used in Experiment 1. The order of presentation of the pre-attentive and attentive tasks was identical to that given at time 1 for each participant. Most participants completed the testing battery in one session that lasted between 3–4 hours with breaks.
At follow-up, the TBI participants were also administered the Community Integration Questionnaire (CIQ, Willer, Rosenthal, Kreutzer, Gordon & Rempel, 1993). The CIQ is a 15-item questionnaire that was administered to the TBI participant as a structured interview. The CIQ assesses for degree of participation in a variety of everyday activities that evaluate home integration, social integration and productivity (e.g., shopping, meal preparation, housework, finances, leisure activities, travel). Coefficient alpha for the CIQ was .76, and test-retest reliability with TBI participants as informants was .91 (Willer et al., 1993).
Analysis
Reaction time and accuracy rates for the pre-attentive and attentive visual search tasks were subjected to separate mixed-model ANOVAs with group (TBI, control) as the between subjects factor and time (post-acute, follow-up), display size (two, eight) and response type (target-present, target-absent) as the within-subjects factors. Error trial RTs were removed from the analyses and data were trimmed by removing trials that were 2.5 standard deviations away from the individual means. Transformed RTs [i.e., log(RT) and 1/RT] were analyzed along with the raw data, and data from the logarithmic transformation is presented in the text and Tables (raw data also in Tables). Again, the primary findings did not change materially based on method. Correlation analyses were performed for the TBI group to examine for relationships between post-acute and follow-up visual search slope values and the measure of community integration obtained at follow-up. Correlation analyses were also conducted between the CIQ and injury characteristics, demographic variables, and the standardized neuropsychological testing data collected post-acutely and at follow-up. Because of the large number of correlations conducted, a more conservative value of p < .005 was used to establish significance.
Pre-attentive search
Table 4 displays RTs for correct responses as a function of time (baseline, follow-up), group (CHI, control), display size (two, eight) and response type (target-present, target-absent). Analysis of the pre-attentive search RTs revealed that RTs were faster at follow-up, F(1, 40) = 4.95, MSE = .019, p = .032, ηp2= .110, for the control group, F(1, 40) = 10.359, MSE = .064, p = .003, ηp2= .206, and for display size 2, F(1, 40) = 7.395, MSE = .001, p = .010, ηp2= .156. There was no main effect of response type, F = 2.033. The significant response type by group interaction, F(1, 40) = 9.621, MSE = .002, p = .004, ηp2= .194, reflected the finding that RTs were slower for target-absent compared to target-present responses for the TBI group while the control group showed the reverse effect. No additional two-way interactions, Fs < 3.812, or three-way interactions reached significance, Fs < 2.122, nor was the 4-way interaction significant, F = .381. With the exception of a lack of a main effect for time with the raw data, the pattern of the data was identical across methods of analysis. Similar to the post-acute data, the consistent finding of no significant group by display size interaction indicates that the TBI and control groups did not differ in their visual search rates in the pre-attentive condition. In addition, percent slowing between display sizes 2 and 8 was minimal (i.e., < .05) at both time 1 and follow-up for the TBI and the control groups.
Table 4.
Mean Log Transformed Reaction Times, Standard Deviations and Accuracy Rates as a Function of Group, Display Size, Response Type, Visual Search Task and Time (raw data in parentheses)
| Pre-attentive Visual Search Task | ||||||||
|---|---|---|---|---|---|---|---|---|
| TBI (n = 21) | Control (n = 21) | |||||||
| Display Size | Display Size | |||||||
| Base | Follow-up | Base | Follow-up | |||||
| Condition | 2 | 8 | 2 | 8 | 2 | 8 | 2 | 8 |
| Target-present | ||||||||
| M | 2.83 (695.69) |
2.83 (700.03) |
2.78 (629.68) |
2.78 (635.49) |
2.74 (558.77) |
2.75 (564.55) |
2.72 (545.94) |
2.73 (548.25) |
| SD | 0.10 (164.59) |
0.10 (179.64) |
0.12 (195.44) |
0.12 (222.13) |
0.06 (80.95) |
0.06 (77.14) |
0.11 (154.90) |
0.10 (138.72) |
| % Correct | 97.17 | 98.36 | 97.32 | 97.47 | 96.73 | 96.88 | 95.24 | 97.47 |
| Target-absent | ||||||||
| M | 2.84 (714.46) |
2.85 (742.24) |
2.80 (671.70) |
2.82 (716.25) |
2.74 (551.43) |
2.75 (569.09) |
2.71 (522.94) |
2.72 (535.08) |
| SD | 0.10 (162.26) |
0.12 (217.48) |
0.13 (267.84) |
0.16 (371.80) |
0.07 (94.47) |
0.08 (111.74) |
0.09 (119.94) |
0.10 (144.27) |
| % Correct | 98.21 | 98.36 | 98.07 | 98.81 | 97.77 | 97.62 | 96.58 | 97.77 |
| Attentive Visual Search Task | ||||||||
| TBI (n = 21) | Control (n = 21) | |||||||
| Display Size | Display Size | |||||||
| Base | Follow-up | Base | Follow-up | |||||
| Condition | 2 | 8 | 2 | 8 | 2 | 8 | 2 | 8 |
| Target-present | ||||||||
| M | 2.88 (780.58) |
3.02 (1072.78) |
2.85 (727.58) |
2.99 (1090) |
2.79 (627.54) |
2.92 (849.27) |
2.79 (623.34) |
2.90 (802.48) |
| SD | 0.09 (182.92) |
0.11 (270.83) |
0.12 (235.10) |
0.17 (727) |
0.05 (75.41) |
0.07 (124.06) |
0.09 (147.06) |
0.09 (181.43) |
| % Correct | 98.21 | 95.98 | 97.92 | 94.64 | 97.77 | 94.64 | 91.67 | 91.07 |
| Target-absent | ||||||||
| M | 2.94 (897.17) |
3.23 (1827.62) |
2.88 (801.94) |
3.16 (1622.78) |
2.83 (687.61) |
3.09 (1267.69) |
2.82 (668.15) |
3.01 (1052.83) |
| SD | 0.11 (262.20) |
0.16 (751.19) |
0.13 (313.37) |
0.19 (1049.83) |
0.06 (89.06) |
0.12 (354.50) |
0.09 (160.61) |
0.11 (258.77) |
| % Correct | 97.62 | 98.07 | 97.02 | 97.77 | 97.47 | 96.58 | 96.88 | 94.35 |
Analysis of the accuracy data revealed higher accuracy rates for set size 8, F(1, 40) = 4.296, MSE = .001, p = .045, ηp2= .097, and for target-absent responses, F(1, 40) = 5.802, MSE = .001, p = .021, ηp2= .127. Neither the main effect of group or time were significant, Fs < 1.760. There were also no significant interactions, Fs < 1.904, indicating a similar pattern of accuracy across groups and time.
Attentive search
As seen in Table 4, analysis of the attentive search RTs revealed that RTs were faster for the control group, F(1, 40) = 11.736, MSE = .072, p = .001, ηp2= .2279, at follow-up, F(1, 40) = 7.385, MSE = .019, p = .010, ηp2= .156, for display size 2, F(1, 40) = 387.270, MSE = .008, p < .001, ηp2= .906, and for target-present responses, F(1, 40) = 212.203, MSE = −.004, p < .001, ηp2= ..841. Similar to the baseline data, there were also significant interactions between display size and response type, F(1, 40) = 186.457, MSE = .002, p < .001, ηp2= .823, group and display size, F(1, 40) = 4.447, MSE = .008, p = .041, ηp2= .100, group and response type, F(1, 40) = 5.202, MSE = .004, p = .028, ηp2= .115, and group, display size and response type, F(1, 40) = 4.179, MSE = .002, p = .048, ηp2= .095. The significant time by display size, F(1, 40) = 5.786, MSE = .002, p = .021, ηp2= .126, and three-way interaction between time, display size and response type, F(1, 40) = 5.972, MSE = .001, p = .019, ηp2= .130, reflected the fact that the display size effect was larger in magnitude for the target-absent compared to the target-present trials and this pattern was more pronounced at time 1 compared to follow-up (see Table 4). There was also a significant three-way interaction between group, time, and display size, F(1, 40) = 4.179, MSE = .002, p = .048, ηp2= .095. Breakdown of the interaction revealed no change in the display size effect across time for the TBI group (baseline = 7.39% slowing, follow-up = 7.33% slowing), F = .050, whereas the control group exhibited a reduced display size effect at follow-up (5.34% slowing) compared to baseline (6.93% slowing), F(1, 20) = 14.084, p = .001. No other interactions were significant, Fs < 238. For the reciprocal transformation data the only interactions to remain significant were the display size by response, and the group by time by display size. Across methods, the data consistently indicated that the search rates of the TBI remained slowed relative to controls at follow-up in the attentive search condition.
Analysis of the attentive search task accuracy rates revealed higher accuracy rates at time 1, F(1, 40) = 4.262, MSE = .007, p = .045, ηp2= .096, for display size 2, F(1, 40) = 4.036, MSE = .004, p = .05, ηp2= .092, and for target-absent responses, F(1, 40) = 6.460, MSE = .004, p = .015, ηp2= .139. Neither the group main effect, F = 3.478, or any of the interactions reached significance, Fs < 1.877, indicating a similar pattern of accuracy across groups and time.
Correlations
We conducted correlation analyses to determine whether pre-attentive or attentive visual search rates (defined by target-absent and target-present log normalized slope measures), obtained post-acutely and at follow-up, would predict community integration as measured by the CIQ. Because of the large number of correlations, we used a p-value of less than .005 for significance. The correlation analyses revealed no significant relationships between follow-up CIQ score and the post-acute slope estimates (preattentive: target present = .000, target absent = .002; attentive: target present = .023, target absent = .049) for either the pre-attentive (target-present: r = .32, target-absent: r = .35) or attentive, (target-present: r = .29, target-absent: r = .48, p = .034) visual search tasks. Similarly no significant correlations emerged between the community integration measure and follow-up slope estimates (preattentive: target present = .000, target absent = .003; attentive: target present = .024, target absent = .046) for the pre-attentive (target-present: r = −.07, target-absent: r = .02) and attentive (target-present: r = .04, target-absent: r = .23) conditions. The raw and reciprocal transformation slope estimates similarly revealed no significant relationships with the follow-up CIQ score.
Injury characteristics (i.e., GCS, PTA, TSI), rs between −.23 and .20, and demographic variables, rs between −.29 and .30, also showed no significant correlations with follow-up community integration. With the exception of the letter fluency subtest, no other standardized neuropsychological tests (see Table 1) administered post-acutely, rs between −.23 and .20, or at follow-up, rs between −.30 and .37, were significantly correlated with the community integration measure. At follow-up, the letter fluency test was positively correlated with community integration, r = .63, p = .003, such that better scores on the fluency tests were associated with better community integration; this relationship did not reach the .005 level of significance for the post-acute letter fluency score, r = .51, p = .022.
Discussion
Replicating findings from Experiment 1, the TBI participants exhibited intact visual search performance in the pre-attentive condition and slowed visual search performance in the attentive condition at follow-up. In the pre-attentive condition, the continued slower RTs for target absent compared to target present responses for the TBI group compared to controls suggests continued difficulties with processes outside of feature extraction. In the attentive condition, unlike control participants who showed faster visual search rates at follow-up (6.93% slowing) compared to baseline (7.33% slowing), the TBI participants exhibited no improvement in their visual search rate across time in the attentive condition (7.39% and 7.33% slowing, respectively). This contrasts with the overall faster follow-up RTs for both the attentive and pre-attentive search conditions, which suggest improvement for the TBI group in other processes necessary for completion of visual search, such as perceptual encoding and/or the response-related components of visual search. The overall faster response rates at follow-up by the TBI participants could reflect recovery, practice effects, or a combination of the two.
General Discussion
The findings from this work have several important implications. The results illustrate the importance of understanding the specific type of visual search situation being investigated. The analyses revealed that the visual search abilities of individuals with moderate to severe TBI did not differ from those of controls when the visual search process was largely automatic, being performed quickly and in parallel. In contrast, in the attentive search condition where more cognitively-demanding, controlled processes were required for the search process, the TBI group required a significantly longer time to search the visual displays compared to controls. Furthermore, the TBI group experienced difficulties directing their visual search and ignoring irrelevant information in the attentive condition both post-acutely and at follow-up, with no significant recovery evident. The findings also showed that in the post-acute phase of recovery from a moderate to severe TBI, a visual search process that required mainly automatic processes and involved the extraction of simple featural information appeared to be relatively intact and could be utilized in rehabilitation.
While both the TBI and control participants utilized an efficient, parallel visual search process in the pre-attentive condition, both post-acutely and at follow-up the TBI participants took longer responding to target-absent relative to target-present trials. This indicates that although the TBI participants did not experience difficulty with the feature extraction processes characteristic of feature searches, they did experience difficulty with other processes involved with pre-attentive feature search. For example, the TBI participants may have experienced difficulty detecting or selecting the absence of a target, or they may have set a higher criterion than controls for saying that the target was not there. Similar difficulties with target-absent responses have also been noted in the aging population (Hommell, Li, & Li, 2004; Potter et al., 2012), and the underlying nature of this finding requires further investigation. This finding, combined with the consistent finding of a slower visual search process in the attentive search condition that did not improve with time in contrast to overall RT, suggests that the TBI participants were experiencing difficulties with specific aspects of the visual search process rather than experiencing a general reduction in processing speed.
Early theories of visual search hypothesized a dichotomous distinction between parallel and serial search (e.g., Treisman & Gelade, 1980). The more recently accepted view is that bottom-up (pre-attentive) and top-down (attentive) processes are both involved in feature and conjunction searches (e.g., Madden, 1997; Palmer et al., 2011), with the role of attention being more significant when participants must search for target items that overlap in visual features with distractor items. In addition to the attentional processes involved in spatially directing attention to items in the visual display, other processes that could be compromised in TBI include the time involved in processing each item, as well as the ability to inhibit additional processing of task-irrelevant information, and moving or shifting to a new item. Even in “pop out” visual search situations, visual search performance can be degraded due to other factors such as lateral masking or crowding, which might occur if the visual display was densely populated (Egeth, 2012). Therefore, it will be important to understand the boundary conditions wherein individuals with moderate to severe TBI can search visual displays with the same degree of proficiency as controls.
The brain areas involved in supporting bottom-up and top-down visual search have been investigated in a number of functional imaging and neurophysiological studies. Functional imaging research has shown that frontoparietal attention networks play an important role in visual search (Corbetta, Shulman, Miezin, & Petersen, 1995; Donner et al., 2002). Data from this body of research appears mixed with regard to whether the dorsal and ventral attention networks are differentially engaged when the search target differs from distracters in single (bottom-up) versus multiple features (Anderson et al., 2007; Mantini, Corbetta, Perucci, Romani, & del Gratta, 2009; Parks & Madden, 2013). Electrophysiological research suggests that the parietal cortex may be more involved in bottom-up search processes and the prefrontal cortex when top-down visual search processes are involved (Li, Gratton, Yao, & Ruthruff, 2011; Li, Gratton, Fabiani, & Knight, 2013). If top-down visual search processes are linked to the prefrontal cortex, this may be related to the poorer controlled search abilities of the TBI participants as the typical neuropathology of TBIs is usually greater in the prefrontal cortex compared to the parietal area (Stuss, 2011). Future work is needed to better understand the brain areas that support visual search abilities and how they are affected by TBI.
No relationships were found between a measure of community integration (i.e., activities at home, in the community, and on the job) and the slope values for either the pre-attentive or the attentive search conditions. However, the general intactness of visual search in the pre-attentive condition made it difficult to assess our hypothesis that psychosocial outcome would be more compromised in individuals who initially exhibited the greatest difficulties with more foundational automatic processes. It is also important to point out that the CIQ was administered as a structured self-report interview. If some of the participants lacked awareness for the extent of their difficulties, this may have affected the validity of this measure. Future research may want to additionally gather this data from a knowledgeable informant. In a prior study (Millis et al., 1994), two of 12 neuropsychological measures that the authors administered acutely (i.e., Trails B and Rey Auditory Verbal Learning Test list learning) following moderate to severe TBI, were found to correlate with self-reported CIQ obtained at about 1 year. In the current study, the only standardized neuropsychological measure that correlated significantly with follow-up CIQ was performance on the letter fluency test. This could represent a spurious finding and should be interpreted with caution. However, similar to the study by Millis et al (1994), this measure does rely to some extent on executive abilities and requires flexibility and cognitive speed. Understanding early predictors of long-term psychosocial outcome following TBI remains an important area of study.
Prior research suggests that deficits in attention are common in individuals who suffer severe TBIs (e.g., Catroppa et al., 2011; Mathias & Wheaton, 2007; Zocolotti et al., 2000), and can have a major impact on recovery and be an impediment to successful rehabilitation (Bate, Mathias, & Crawford, 2001a). The results of this study have several important implications for rehabilitation strategies. First, the findings suggest that individuals with TBI will have less difficulty locating items in their environment when the items are salient and distinct from other items in the environment. For example, a pink hairbrush would be easier to locate than a brown hairbrush on a brown nightstand with other brown hairstyling tools. Therefore, making commonly used objects more salient should increase reliance on automatic visual search processes and reduce visual search time for individuals with TBI. Of note, the findings also suggest that in cases where the salient pink hairbrush is not on the nightstand, it may take individuals with TBI longer to realize that the target is absent than to find it when it is present. At a broader level, gaining a better understanding of the types of more automatic processes that remain relatively intact in the post-acute stage following TBI is important, as these intact skills could be incorporated into training and rehabilitation techniques to help reduce the high mental workload situations that are often required of more attention-demanding, controlled tasks (Schmitter-Edgecombe, 1996). For example, if schemata for overlearned daily rituals remain intact following TBI, such routine could be capitalized upon to reduce mental workload, frustration and distress. In some cases, however, it may be important to teach methods for overriding more automatic responses so that individuals can successfully adjust to changing situations.
The individuals with TBI assessed in this study were primarily Caucasian and suffered moderate to severe injuries mainly as a result of a motor vehicle accident. Therefore, the findings may not generalize to other ethnic groups or to individuals with less severe injuries or who experience TBIs as a result of a different mechanism (e.g., blast). All TBI participants were also actively participating in inpatient rehabilitation at the time of post acute testing, therefore the findings may not generalize to TBI participants who do not receive inpatient services. Although the TBI participants exhibited intact visual search performance when compared to controls on the pre-attentive task and impaired performance on the attentive task, the exact mechanism that underlies this differential visual search performance remains unclear. Furthermore, the sensitivity of our ability to detect a relationship between the CIQ and the neuropsychological measures was also limited by the specific test administered.
In conclusions, whether differences are identified between individuals who suffered moderate to severe TBI and control participants in visual search is dependent on the type of visual search required. Both post-acutely and following more than six months of recovery, the TBI participants were at a clear disadvantage compared to controls when visual search required predominantly attentive, serial search processes. Reducing the attentional demands on visual search by using salient stimuli that appeared to “pop out” from the surroundings and required more pre-attentive processes eliminated the group differences in target detection performance both post-acutely and at follow-up, suggesting that these processes could be capitalized on in rehabilitation. The TBI participants did, however, appear to experience difficulty with other aspects of the pre-attentive search process, namely identifying the absence of a target, and this finding requires further exploration.
Acknowledgments
This study was supported by the National Institute of Neurological Disorders and Stroke under grant #R01 NS47690. We would like to thank Randi McDonald, Shital Pavawalla, Jonathan Anderson, Jennifer McWilliams, Michelle Nuegen, Matthew Wright, and Ellen Woo for their support in coordinating data collection. We would also like to thank the TBI participants and the members of the Head Injury Research Team for their help in collecting and scoring the data.
Footnotes
We have no financial interests or benefits to disclose.
References
- Anderson EJ, Mannan SK, Husain M, Rees G, Sumner P, Mort DJ. Involvement of prefrontal cortex in visual search. Experimental Brain Research. 2007;180:289–302. doi: 10.1007/s00221-007-0860-0. [DOI] [PubMed] [Google Scholar]
- Barona A, Reynolds CR, Chastain R. A demographically based index of prem orbid intelligence for the WAIS-R. Journal of Consulting and Clinical Psychology. 1984;52:885–887. [Google Scholar]
- Bate AJ, Mathias JL, Crawford JR. Performance on the test of everyday attention and standard tests of attention following severe traumatic brain injury. The Clinical Neuropsychologist. 2001a;15(3):405–422. doi: 10.1076/clin.15.3.405.10279. [DOI] [PubMed] [Google Scholar]
- Bate AJ, Mathias JL, Crawford JR. The covert orienting of visual attention following severe traumatic brain injury. Journal of Clinical and Experimental Neuropsychology. 2001b;23(3):386–398. doi: 10.1076/jcen.23.3.386.1190. [DOI] [PubMed] [Google Scholar]
- Benton AL, Hamsher K, Sivan AB. Multilingual Aphasia Examination. 3rd ed. Iowa City, IA: AJA Associates; 1994. [Google Scholar]
- Brandt J, Folstein MF. Telephone Interview for Cognitive Status. Lutz, FL: Psychological Assessment Resources; 2003. [Google Scholar]
- Bundesen C. A theory of visual attention. Psychological Review. 1990;97(4):523–547. doi: 10.1037/0033-295x.97.4.523. [DOI] [PubMed] [Google Scholar]
- Catroppa C, Anderson V, Godfrey C, Rosenfeld JV. Attentional skills 10 years post-paediatric traumatic brain injury (TBI) Brain Injury. 2011;25:858–869. doi: 10.3109/02699052.2011.589794. [DOI] [PubMed] [Google Scholar]
- Cedrus Corporation. SuperLab Pro 1.75 [Computer program] San Pedro, CA: 1999. [Google Scholar]; Dennis M, Sinopoli K, Flectcher JM, Schachar R. Puppets, robots, critics, and actors within a taxonomy of attention for developmental disorders. Journal of the International Neuropsychological Society. 2008;14:673–690. doi: 10.1017/S1355617708080983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan LKH, Hayward WG. Feature integration theory revisited: Dissociating feature detection and attentional guidance in visual search. Journal of Experimental Psychology: Human Perception & Performance. 2009;35:119–132. doi: 10.1037/0096-1523.35.1.119. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL, Miezin FM, Petersen SE. Superior parietal cortex activation during spatial attention shifts and visual feature conjunction. Science. 1995;270:802–805. doi: 10.1126/science.270.5237.802. [DOI] [PubMed] [Google Scholar]
- Donner TH, Kettermann A, Diesch E, Ostendorf F, Villringer A, Brandt SA. Visual feature and conjunction searches of equal difficulty engage only partially overlapping frontoparietal networks. Neuroimage. 2002;15:16–25. doi: 10.1006/nimg.2001.0951. [DOI] [PubMed] [Google Scholar]
- Egeth HE. From Perception to Consciousness: Searching with Anne Treisman. New York, NY: Oxford University Press; 2012. Some reflections on the processing of perceptual features; pp. 164–171. [Google Scholar]
- Eriksen CW, Yeh Y-Y. Allocation of attention in the visual field. Journal of Experimental Psychology: Human Perception and Performance. 1985;11(5):583–597. doi: 10.1037//0096-1523.11.5.583. [DOI] [PubMed] [Google Scholar]
- Fletcher JM, Ewing-Cobbs L, Miner ME, Levin HS, Eisenberg H. Behavioral changes after closed head injury in children. Journal of Consulting and Clinical Psychology. 1990;58:93–98. doi: 10.1037//0022-006x.58.1.93. [DOI] [PubMed] [Google Scholar]
- Hatta T, Yoshizaki K, Ito Y, Mase M, Kabasawa H. Reliability and validity of the Digit Cancellation Test, a brief screen of attention. Psychologia: An International Journal of Psychological Sciences. 2012;55(4):246–256. [Google Scholar]
- Heinze H-J, Munte TF, Gobiet W, Niemann H, Ruff RM. Parallel and serial visual search after closed head injury: Electrophysiological evidence for perceptual dysfunctions. Neuropsychologia. 1992;30(6):495–514. doi: 10.1016/0028-3932(92)90054-p. [DOI] [PubMed] [Google Scholar]
- Hommel B, Li KZ, Li SC. Visual search across the lifespan. Developmental Psychology. 2004;40:545–558. doi: 10.1037/0012-1649.40.4.545. [DOI] [PubMed] [Google Scholar]
- King NS, Crawford S, Wenden FJ, Moss N, Wade DT. Interventions and service need following mild and moderate head injury: The Oxford Head Injury Service. Clinical Rehabilitation. 1997;11:13–27. doi: 10.1177/026921559701100104. [DOI] [PubMed] [Google Scholar]
- Levin HS, O’Donnell VM, Grossman RG. The Galveston Orientation and Amnesia Test: A practical scale to assess cognition after head injury. Journal of Nervous and Mental Disease. 1979;167(11):675–684. doi: 10.1097/00005053-197911000-00004. [DOI] [PubMed] [Google Scholar]
- Li L, Gratton C, Fabiani M, Knight RT. Age-related frontotemporal changes during the control of botton-up and top-down attention: an ERP study. Neurobiology of Aging. 2013;34:477–488. doi: 10.1016/j.neurobiolaging.2012.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Gratton, Tao D, Knight RT. Role of the frontal and parietal cortices in the control of bottom-up and top-down attention in humans. Brain Research. 2010;1344:173–184. doi: 10.1016/j.brainres.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majdan A, Sziklas V, Jones-Gotman M. Performance of healthy subjects and patients with resection from the anterior temporal lobe on matched tests of verbal and visuoperceptual learning. Journal of Clinical and Experimental Neuropsychology. 1996;18(3):416–430. doi: 10.1080/01688639608408998. [DOI] [PubMed] [Google Scholar]
- Mantini D, Corbetta M, Perrucci MG, Romani GL, Del Gratta C. Large-scale brain networks account for sustained and transient activity during target detection. NeuroImage. 2009;44:265–274. doi: 10.1016/j.neuroimage.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathias JL, Wheaton P. Changes in attention and information-processing speed following severe traumatic brain injury: A meta-analytic review. Neuropsychology. 2007;21(2):212–223. doi: 10.1037/0894-4105.21.2.212. [DOI] [PubMed] [Google Scholar]
- McMillan TM, Jongen ELMM, Greenwood RJ. Assessment of post-traumatic amnesia after severe closed head injury: Retrospective or prospective? Journal of Neurology, Neurosurgery, & Psychiatry. 1996;60(4):422–427. doi: 10.1136/jnnp.60.4.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millis SR, Rosenthal M, Lourie IF. Predicting community integration after traumatic brain injury with neuropsychological measures. International Journal of Neuroscience. 1994;79(3–4):165–167. doi: 10.3109/00207459408986077. [DOI] [PubMed] [Google Scholar]
- Muller-Oehring EM, Schulte T, Rohlfing T, Pfefferbaum A, Sullivan EV. Visual search and the aging brain: discerning the effects of age-related volume shrinkage on alertness, feature binding, and attentional control. Neuropsychology. 2013;27:48–59. doi: 10.1037/a0030921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller HJ, T¨ollner T, Zehetleitner M, Geyer T, Rangelov D, Krummenacher J. Dimension-based attention modulates feed-forward visual processing. Acta Psychologica. 2010;135:117–122. doi: 10.1016/j.actpsy.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Nissley HM, Schmitter-Edgecombe M. Perceptually based implicit learning in severe closed-head injury patients. Neuropsychology. 2002;16(1):111–122. doi: 10.1037//0894-4105.16.1.111. [DOI] [PubMed] [Google Scholar]
- Parks EL, Madden DJ. Brain connectivity and visual attention. Brain Connectivity. 2013;3:317–338. doi: 10.1089/brain.2012.0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer J, Ames CT, Lindsey DT. Measuring the effect of attention on simple visual search. Journal of Experimental Psychology: Human Perception and Performance. 1993;19(1):108–130. doi: 10.1037//0096-1523.19.1.108. [DOI] [PubMed] [Google Scholar]
- Palmer EM, Fencsik DE, Flusberg SJ, Horowitz TS, Wolfe JM. Signal detection evidence for limited capacity in visual search. Attention, Perception, & Psychophysics. 2011;73(8):2413–2424. doi: 10.3758/s13414-011-0199-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perri R, Calesimo GA, Loasses A, Caltagirone C. Deficient intentional access to semantic knowledge in patients with severe closed-head injury. Cortex: A Journal Devoted to the Study of the Nervous System and Behavior. 2000;36(2):213–225. doi: 10.1016/s0010-9452(08)70525-2. [DOI] [PubMed] [Google Scholar]
- Plude DJ, Doussard-Roosevelt JA. Aging, selective attention, and feature integration. Psychology and Aging. 1989;4(1):98–105. doi: 10.1037/0882-7974.4.1.98. [DOI] [PubMed] [Google Scholar]
- Potter LM, Grealy MA, Elliott MA, Andres P. Aging and performance on an everyday-based visual search task. Acta Psychologica. 2012;140:208–217. doi: 10.1016/j.actpsy.2012.05.001. [DOI] [PubMed] [Google Scholar]
- Rasmussen I, Xu J, Antonsen IK, Brunner J, Skandsen T, Axelson DE, Haberg A. Simple dual tasking recruits prefrontal cortices in chronic severe traumatic brain injury patients, but not in controls. Journal of Neurotrauma. 2008;25:1057–1070. doi: 10.1089/neu.2008.0520. [DOI] [PubMed] [Google Scholar]
- Ries M, Mark W. Selective attention deficits following severe closed head injury: The role of inhibitory processes. Neuropsychology. 2005;19(4):476–483. doi: 10.1037/0894-4105.19.4.476. [DOI] [PubMed] [Google Scholar]
- Reitan RM. Validity of the Trail Making Test as an indicator of organic brain damage. Perceptual and Motor Skills. 1958;8:271–276. [Google Scholar]
- Schmitter-Edgecombe M. Effects of traumatic brain injury on cognitive performance: An attentional resource hypothesis in search of data. The Journal of Head Trauma Rehabilitation. 1996;11(2):17–30. [Google Scholar]
- Schmitter-Edgecombe M, Beglinger L. Acquisition of skilled visual search performance following severe closed-head injury. Journal of the International Neuropsychological Society. 2001;7(5):615–630. doi: 10.1017/s1355617701755099. [DOI] [PubMed] [Google Scholar]
- Schmitter-Edgecombe M, Kibby M. Visual selective attention after severe closed head injury. Journal of the International Neuropsychological Society. 1998;4(2):144–159. doi: 10.1017/s1355617798001441. [DOI] [PubMed] [Google Scholar]
- Schmitter-Edgecombe M, Marks W, Fahy JF. Semantic priming after severe closed head trauma: Automatic and attentional processes. Neuropsychology. 1993;7(2):136–148. [Google Scholar]
- Schmitter-Edgecombe M, Nissley HM. Effects of aging on implicit covariation learning. Aging, Neuropsychology, and Cognition. 2002;9(1):61–75. [Google Scholar]
- Shum D, Sweeper S, Murray R. Performance on verbal implicit and explicit memory tasks following traumatic brain injury. The Journal of Head Trauma Rehabilitation. 1996;11(2):43–53. [Google Scholar]
- Smith A. Symbol Digit Modalities Test. Los Angeles, CA: Western Psychological Services; 1991. [Google Scholar]
- Smilek D, Frischen A, Reynolds MG, Gerritsen C, Eastwood JD. What infeluences visual search efficiency? Disentangling contributions of preattentive and postattentive processes. Perception & Psychophysics. 2007;69(7):1105–1116. doi: 10.3758/bf03193948. [DOI] [PubMed] [Google Scholar]
- Sung K. Serial and parallel attentive visual searches: Evidence from cumulative distribution functions of response times. Journal of Experimental Psychology: Human Perception and Performance. 2008;34(6):1372–1388. doi: 10.1037/a0011852. [DOI] [PubMed] [Google Scholar]
- Stuss DT. Traumatic brain injury: relation to executive dysfunction and the frontal lobes. Current Opinion in Neurology. 2011;24:584–589. doi: 10.1097/WCO.0b013e32834c7eb9. [DOI] [PubMed] [Google Scholar]
- Taylor HG, Yeates KO, Wade SL, Drotar D, Stancin T, Minich N. A prospective study of short- and long-term outcome after traumatic brain injury in children: Behavior and Achievement. Neuropsychology. 2002;16:15–27. doi: 10.1037//0894-4105.16.1.15. [DOI] [PubMed] [Google Scholar]
- Wade SL, Drotar D, Stancin T, Minich N. A prospective study of short- and long-term outcomes after traumatic brain injury in children: Behavior and achievement. Neuropsychology. 2002;15:15–27. doi: 10.1037//0894-4105.16.1.15. [DOI] [PubMed] [Google Scholar]
- Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2(7872):81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- Treisman A, Gelade G. A feature integration theory of attention. Cognitive Psychology. 1980;12:97–136. doi: 10.1016/0010-0285(80)90005-5. [DOI] [PubMed] [Google Scholar]
- Tsotsos JK, Rodriguez-Sanchez AJ, Rothenstein AL, Simine E. The different stages of visual recognition need different attentional binding strategies. Brain Research. 2008;1225:119–132. doi: 10.1016/j.brainres.2008.05.038. [DOI] [PubMed] [Google Scholar]
- Vakil E, Biederman Y, Liran G, Groswasser Z, Aberbuch S. Head-injured patients and control group: Implicit versus explicit measures of frequency of occurrence. Journal of Clinical and Experimental Neuropsychology. 1994;16(4):539–546. doi: 10.1080/01688639408402665. [DOI] [PubMed] [Google Scholar]
- Vakil E, Blachstein H, Hoofien D. Automatic temporal order judgment: The effect of intentionality of retrieval on closed-head-injured patients. Journal of Clinical and Experimental Neuropsychology. 1991;13(2):291–298. doi: 10.1080/01688639108401044. [DOI] [PubMed] [Google Scholar]
- Verghese P. Visual search and attention: A signal detection theory approach. Neuron. 2001;31:523–535. doi: 10.1016/s0896-6273(01)00392-0. [DOI] [PubMed] [Google Scholar]
- Wechsler D. The WAIS-III administration and scoring manual. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- Wilkinson DT, Halligan PW, Henson RN, Dolan RJ. The effects of interdistracter similarity on search processes in superior parietal cortex. NeuroImage. 2002;15:611–619. doi: 10.1006/nimg.2001.0993. [DOI] [PubMed] [Google Scholar]
- Williams DH, Levin HS, Eisenber HM. Mild head injury classification. Neurosurgery. 1990;27(3):422–428. doi: 10.1097/00006123-199009000-00014. [DOI] [PubMed] [Google Scholar]
- Willer B, Rosenthal M, Kreutzer JS, Gordon WA, Rempel R. Assessment of community integration following rehabilitation for traumatic brain injury. The Journal of Head Trauma Rehabilitation. 1993;8(2):75–87. [Google Scholar]
- Wolfe JM, Cave KR, Franzel SL. Guided search: An alternative to the feature integration model for visual search. Journal of Experimental Psychology: Human Perception and Performance. 1989;15(3):419–433. doi: 10.1037//0096-1523.15.3.419. [DOI] [PubMed] [Google Scholar]
- Wolfe JM, Yu KP, Stewart MI, Shorter AD, Friedman.-Hill SR, Cave KR. Limitations on the parallel guidance of visual search: Color x colors and orientation x orientation conjunctions. Journal of Experimental Psychology: Human Perception and Performance. 1990;16(4):879–892. doi: 10.1037//0096-1523.16.4.879. [DOI] [PubMed] [Google Scholar]
- Wolfe JM, Palmer EM, Horowitz Reaction time distributions constrain models of visual search. Vision Research. 2010;50:1304–1311. doi: 10.1016/j.visres.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yantis S, Johnston JC. On the locus of visual selection: Evidence from focused attention tasks. Journal of Experimental Psychology: Human Perception and Performance. 1990;16:135–149. doi: 10.1037//0096-1523.16.1.135. [DOI] [PubMed] [Google Scholar]