Abstract
The alpha carbonic anhydrases (α-CAs) are a group of structurally related zinc metalloenzymes that catalyze the reversible hydration of CO2 to HCO3 −. Humans have 15 different α-CAs with numerous physiological roles and expression patterns. Of these, 12 are catalytically active, and abnormal expression and activities are linked with various diseases, including glaucoma and cancer. Hence there is a need for CA isoform specific inhibitors to avoid off-target CA inhibition, but due to the high amino acid conservation of the active site and surrounding regions between each enzyme, this has proven difficult. However, residues towards the exit of the active site are variable and can be exploited to design isoform selective inhibitors. Here we discuss and characterize this region of “selective drug targetability” and how these observations can be utilized to develop isoform selective CA inhibitors.
1. Introduction
Carbonic anhydrases (CAs, EC 4.2.1.1) are a family of ubiquitous, mostly zinc metalloenzymes that catalyze the reversible hydration of carbon dioxide to bicarbonate and a proton [1, 2]. These enzymes are expressed in most living organisms and are encoded by five evolutionary distinct gene families: α-, β-, γ-, δ-, and ζ-CAs [3–5]. The α-CAs are expressed predominantly in vertebrates and are the only class observed in humans. β-CAs are found in prokaryotes, algae, and plants [6]; the γ-CAs are present in archaebacteria [7], while the δ- and ζ-CAs are found in diatoms [8]. The α-CAs have been extensively studied due to their role in human physiology and disease pathology [9]. Humans express 15 different isoforms, 12 of which are catalytically active and differ in their enzymatic efficiency. These isoforms also differ in cellular distribution and physiological function (Table 1). Specifically, there are eight cytosolic (CA I, II, III, VII, VIII, X, XI, and XIII), two mitochondrial (CA VA, and VB), one secreted (CA VI), three transmembrane (CA IX, XII and XIV), and one GPI-anchored (CA IV) isoforms of CA [10]. CA VIII, X and XI are noncatalytic due to the absence of one or more of the coordinating histidine residues and are termed CA related proteins (CA-RPs) [11].
Table 1.
Distribution, associated diseases, catalytic efficiency, and structural characterization of CAs.
| Isoform | Localization | K cat (s−1) |
K
cat/K
M
(M−1 s−1) |
pI | Oligomeric state | Number of PDB entries | References | |
|---|---|---|---|---|---|---|---|---|
| Organ/tissue | Subcellular | |||||||
| Associated disease | ||||||||
| I | RBCs, GI tract, and eye | Cytosol | 2.0 × 105 | 5.0 × 107 | 6.6 | Monomer | 19 | [2, 3, 9, 88, 89] |
| Hemolytic anemia | ||||||||
|
| ||||||||
| II | RBCs, kidney, osteoclasts, eye, GI tract, lung, brain, and testis | Cytosol | 1.4 × 106 | 1.5 × 108 | 6.9 | Monomer | 454 | [2, 3, 3, 9, 88–90] |
| Glaucoma, epilepsy, edema, altitude sickness | ||||||||
|
| ||||||||
| III | Adipocytes, skeletal muscle | Cytosol | 1.0 × 104 | 3.0 × 105 | 7.0 | Monomer | 6 | [2, 3, 9, 88, 89] |
| Oxidative stress | ||||||||
|
| ||||||||
| IV | Lung, kidney, brain, eye, RBCs, and colon | Membrane-bound | 1.1 × 106 | 5.1 × 107 | 6.4 | Monomer | 4 | [3, 9] |
| Retinitis pigmentosa, stroke, glaucoma | ||||||||
|
| ||||||||
| VA | Liver | Mitochondria | 2.9 × 106 | 2.9 × 107 | 7.2 | Monomer | 1* | [4, 91] |
| Obesity, insulin resistance | ||||||||
|
| ||||||||
| VB | Kidney, GI tract, spinal cord, heart and skeletal muscle, and pancreas | Mitochondria | 9.5 × 105 | 9.8 × 107 | 7.7 | Monomer | N/A | [4, 91] |
| Obesity, insulin resistance | ||||||||
|
| ||||||||
| VI | Salivary and mammary glands | Secreted | 3.4 × 105 | 4.9 × 107 | 6.5 | Dimer | 1 | [5, 9, 92, 93] |
| Dental caries | ||||||||
|
| ||||||||
| VII | Liver, colon, skeletal muscle, and brain | Cytosol | 9.5 × 105 | 8.3 × 107 | 6.9 | Monomer | 2 | [6, 9, 94] |
| Epilepsy | ||||||||
|
| ||||||||
| IX | GI mucosa, tumors | Transmembrane | 3.8 × 105 | 5.5 × 107 | 5.5 | Dimer | 2 | [3, 7, 9, 88, 89, 95] |
| Cancer | ||||||||
|
| ||||||||
| XII | Eye, tumors, reproductive epithelia, intestines, and kidney | Transmembrane | 4.2 × 105 | 3.5 × 107 | 5.8 | Dimer | 5 | [3, 8, 96] |
| Cancer, glaucoma | ||||||||
|
| ||||||||
| XIII | Kidney, thymus, submandibular glands, small intestine, and reproductive organs | Cytosol sterility |
1.5 × 105 | 1.1 × 107 | 6.5 | Monomer | 6 | [5, 44] |
|
| ||||||||
| XIV | Eye, brain, kidney, liver, bladder, and spinal cord | Transmembrane | 3.1 × 105 | 3.9 × 107 | 5.5 | Monomer | 1 | [9, 97] |
| Retinopathy, epilepsy | ||||||||
*murine.
The α-CA active site is located at the base of a large conical cavity spanning from the protein's surface to its center. This cavity is approximately 15 Å wide at its opening and 15 Å deep [4, 12, 13] based on observations in human CA II. At the core of the active site is a Zn(II) ion in a distorted tetrahedral coordination with His94, 96, and 119 (CA II numbering; used throughout) and a water/hydroxide molecule [14] (Figure 1). The active site of CA exhibits an amphiphilic nature and contains both a hydrophobic (Val121, Val143, Leu198, Val207, and Trp209 in purple, Figure 1(b)) and hydrophilic side (Tyr7, Asn62, His64, Asn67, Thr199, and Thr200 in green, Figure 1(b)) [15]. A high degree of residue conservation between the CA isoforms exists in each region.
Figure 1.
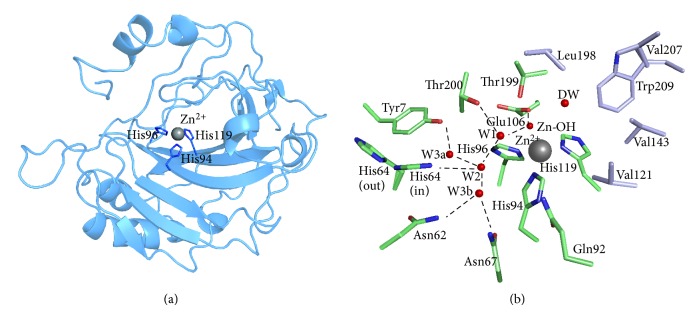
Structure of CA II. PDB ID: 3KS3. (a) Ribbon diagram depicting the overall structural fold. The active site zinc ion and coordinated histidines shown. (b) Active site and ordered waters (red spheres). Also shown are the hydrophilic (green) residues as well as the hydrophobic (purple) residues lining the active site.
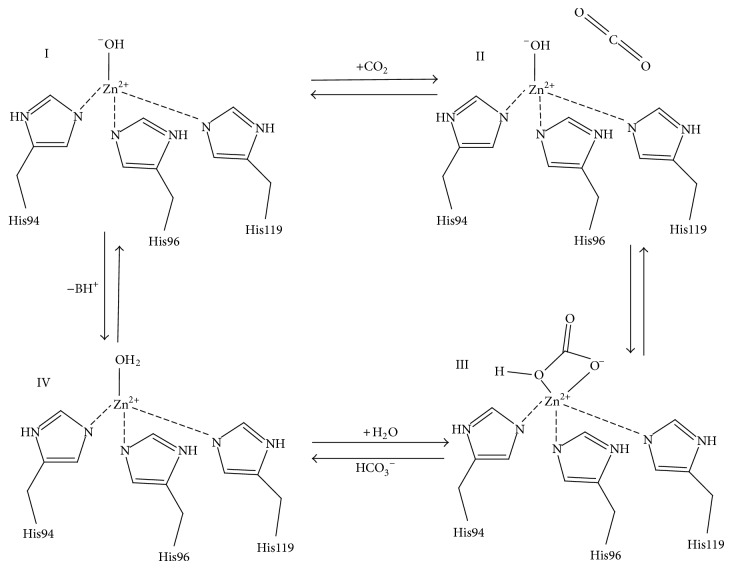
The first step of catalysis by CA is the nucleophilic attack of a Zn-bound OH− (active basic form) on a CO2 molecule, (Figure 2, I-II) to produce HCO3 − (III). The HCO3 − remains weakly bound to the Zn(II) ion (III) until it is displaced by a water molecule (III-IV) (inactive acidic form) and released into solution [16]. In the second step of CA catalysis (IV-I) the Zn-bound water regenerates to OH− through a proton transfer event mediated by a highly conserved (in most isoforms). Histidine residue in combination with a network of ordered water molecules that are stabilized by the adjacent hydrophilic region of the enzyme's active site [1, 2, 15] (Figure 1(b)). In crystal structures of CA II, His64 has been observed to occupy two distinct positions: inward (pointing towards the active site) and outward (pointing away from the active site) conformations (Figure 1(b)). The general consensus is that the inward conformation of His64 is poised to accept the proton that has been transferred from the catalytic zinc to the water network, while the outward conformation is in an orientation that favors proton shuttling to the bulk solvent [16–18].
Figure 2.
Schematic representation of CA catalytic mechanism.
CAs are among the most efficient catalysts known, however there is variation in catalytic efficiency between isoforms such that the members of the α-CAs with the exception of the CA-RPs can be divided into three generalized categories. As such, CA II, IV, VB, and VII are among the fastest of the human CAs with CA II exhibiting a k cat of 1.4 × 106 sec−1. CA VA, VI, IX, and XII exhibit relatively intermediate catalytic activity, and CA III, XIII and XIV are considered the least efficient CAs [3, 9] (Table 1). The efficiency of these enzymes depends on the speed of proton shuttling during the two-step catalytic mechanism [3, 18]. In most of the CAs, this proton shuttling residue is the aforementioned histidine at position 64 [19–21]. In CA III, which is considered the slowest among the CA isoforms (<1% of CA II activity), a lysine is at position 64 [16].
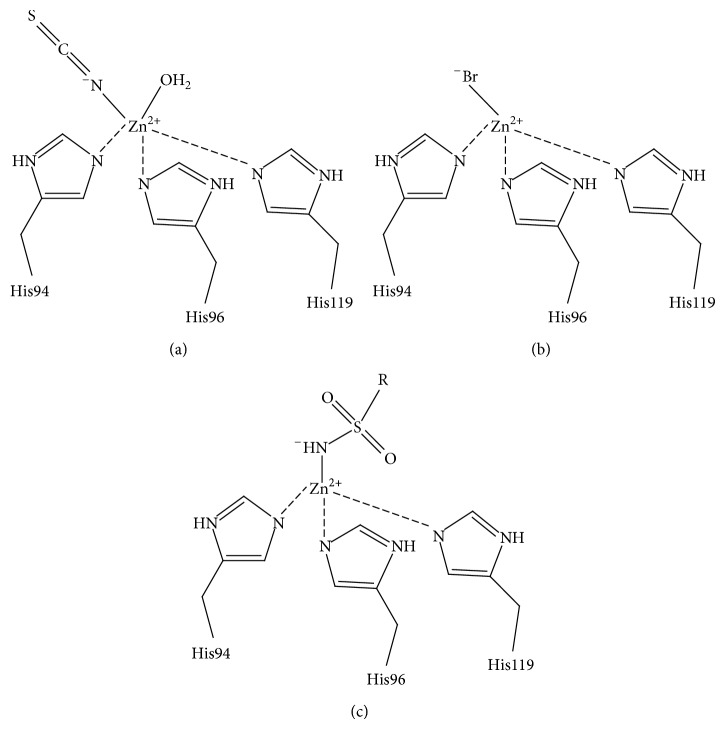
The human CAs are involved in various physiological functions, ranging from bone resorption to pH regulation, with abnormal levels or activities of these enzymes being commonly associated with various diseases (Table 1). Two main classes of CA inhibitors (CAIs) exist: the metal chelating anions and sulfonamide-based inhibitors. Both classes of CAIs are often referred to as “classical” inhibitors of CA and bind directly to the Zn(II) ion in the active site, displacing the Zn-bound solvent molecule. Metal chelating anions bind as either a trigonal-bipyramidal, distorted tetrahedral, or regular tetrahedral adduct [22] (Figures 3(a)–3(c)). Alternatively, sulfonamides generate a tetrahedral geometry upon binding to the catalytic zinc [9] (Figure 3(c)). This “classical” mode of binding of sulfonamide-based and anion CAI will be presented in more detail in later sections of this study.
Figure 3.
CA inhibition mechanism. (a) Anions such as thiocyanate form trigonal-bipyramidal adducts (b) Anions such as Br− form distorted tetrahedral adducts (c) sulfonamides as well as some anions form regular tetrahedral adducts.
As mentioned previously, the α-CAs display a remarkable diversity in regards to tissue distribution and overall physiological function. As such, a brief overview of each of these characteristics is presented and is summarized in Table 1.
Cytosolic CAs I and II are both expressed in red blood cells and are necessary for maintaining physiological pH of the blood through production of HCO3 − [23]. Abnormal levels of CA I in the blood are used as a marker for hemolytic anemia. CA II is ubiquitously expressed in other tissues including the kidney [24], bone, and also in ocular tissues [25]. Interestingly, CA II has also been shown to be associated with several transporters including the Cl−/HCO3 − exchanger, AEI [26], the Na+/HCO3 − cotransporter, NCB1 [27], and the Na+/H+ exchanger, NHE1 [28]. This suggests that CA II acts as a mediator of certain metabolic pathways by further providing the substrates for these various transporters [29]. As a result, CA II is often associated with several diseases such as glaucoma, renal tubular acidosis, and osteoporosis [3, 30]. In addition, CA II has also shown to be essential for the proper functioning of the water-transport channel, aquaporin-1 (AQP1) [31, 32]. Specifically, the relationship between CA II and AQP1 has been shown to be essential for maintaining proper CO2 transport in oocytes, regulation of AQP1 function, and also maintenance of a stable intracellular pH [31].
CA III expression is limited to skeletal muscle and adipose (both white and brown) tissue [33–35]. Unlike CA I and II, CA III displays (as mentioned previously) a remarkable 200-fold decrease in catalytic activity compared to CA II [36]. Furthermore, CA III contains two surface cysteine residues that can be glutathionylated thus acting as a vessel for reactive oxygen species (ROS) sequestration providing cell protection against oxidative damage [37]. These two attributes have caused speculation that CA III might serve a different physiological role unrelated to its primary catalytic function, although this notion is still unclear. It has been observed that CA III expression is directly correlated to adipogenesis and could potentially act as a regulator of peroxisome proliferator-activated receptor-γ2 (PPARγ2) expression [38]. As a result CA III has not currently been linked to any particular disease. CA VII is primarily expressed in colon, liver, skeletal muscle, and in the brain [39]. CA VII exists as two forms; one form displaying the complete amino acid sequence and the other containing a 56 residue N-terminal truncation [39]. Like CA III, CA VII has two surface cysteines that can be glutathionylated suggesting that it too can act in preventing cellular oxidative damage [40]. Though the physiological role of CA VII remains unclear, evidence suggests that this enzyme plays a role in neuronal excitement through HCO3 − production [41]. HCO3 − can mediate electric current through channels that are coupled to gamma-aminobutyric acid (GABAA) receptors, and upon inhibition of CA VII interruption of the current-gated channel is induced causing a suppression of neural excitement [42]. As a result CA VII has been a proposed target for treatment of seizures and neuropathic pain [43].
CA XIII is another active cytosolic CA. CA XIII expression has been shown to be localized to the thymus, kidney, submandibular gland, small intestine, and predominantly in both male and female reproductive organs [44]. It has been postulated that CA XIII plays a significant role in pH regulation of reproductive processes including sperm mobility [45]. To date, no significant physiological function regarding CA XIII has been observed. However, it should be noted that downregulation of CA XIII has been seen in cases of colorectal cancer; however the significance of this observation has not yet been concluded [45].
The CA-RPs: CA isoforms VIII, X, and XI are also located in the cytosol. It has been observed that the CA-RPs are expressed predominantly in the brain and as mentioned previously show no catalytic activity. To date, no known physiological roles, or relation to particular disease have been established [11]. As a result, we will not focus on these isoforms.
CA VA and VB are the only isoforms expressed in the mitochondrial matrix of hepatocytes and adipocytes, respectively [46]. CA VA has been shown to be directly associated with ureagenesis such that it provides HCO3 − to be utilized by carbamoyl phosphate synthetase I [47, 48]. Carbamoyl phosphate synthetase is responsible for synthesis of carbamoyl phosphate which is the rate-determining step of ureagenesis [47]. Furthermore, it has been shown that other necessary carboxylase reactions, including that of pyruvate carboxylase for gluconeogenesis, could be mediated by CA VA activity [48]. This indicates that CA VA can act as a key mediator in several metabolic pathways of the liver. In addition the same effect is seen in the mitochondria of adipocytes where CA VB facilitates carboxylase activity and thus causes induction of lipogenesis [49]. The relationship of CA VA and VB with certain metabolic pathways suggests that both enzymes could be considered as drug targets for modulating both gluconeogenesis and lipogenesis in cases of obesity and insulin resistance [50].
CA VI is the only CA that is secreted and has been found in tears, respiratory airways, epithelial lining of the alimentary canal, enamel organs, and most significantly in human saliva [51–55]. The physiological role of CA VI has not been established although it has been suggested that it is required for pH homeostasis of the mouth [56]. Maintenance of proper pH levels in saliva are necessary to protect against enamel erosions and acid neutralization in dental biofilms caused by bacteria [57, 58]. As a result it is suggested that CA VI plays a key role in these pathways. Interestingly, CA VI has also shown to be associated with taste and inhibition of CA VI has been shown to cause irregularities in taste perception or sometimes loss of taste completely [59]. This effect however is restored with exposure to high levels of zinc [60].
The membrane-associated CAs include the transmembrane isoforms: CA IX, XII, and XIV, and GPI-anchored isoform CA IV. CA IV is expressed both in the kidneys and lungs [61] and similarly to CA II, CA IV can interact with the same aforementioned transporters that span the renal cell surface [62]. It has therefore been established that the presence of CA IV in the kidney is necessary for bicarbonate resorption and normal kidney function [30]. Interestingly, mutant forms of CA IV have been shown to be associated with an autosomal dominant form of retinitis pigmentosa despite intrinsic levels of wild-type CA IV not being observed in ocular tissue [63].
Both CA IX and XII are often regarded as the tumor-associated CAs [64]. CA IX however has garnered the majority of the attention due to its intrinsically low level of expression in normal tissues [65], in combination with being a key modulator of tumor growth and survival. Specifically, CA IX acts as a mediator of tumorigenesis, pH control, tumor cell proliferation and migration, and cell adhesion [66–70]. CA IX has been shown to be regulated by tumor hypoxia and has not only been established as prognostic indicator for a variety of cancers but also as a generic anticancer target [71–73]. Similarly, CA XII expression has been observed to be upregulated in multiple tumor tissues but it has not been established as a prognostic marker [74–77]. Unlike CA IX, CA XII also shows a wider range of expression in normal tissue including the kidney, lung, prostate, ovaries, uterine endometrium, breast, and basolateral membrane of gut epithelium [64, 78–80]. Furthermore, it has been postulated that CA XII is important for normal kidney function [81].
CA XIV displays high sequence similarity with CA XII and has been shown to be expressed in most parts of the brain, colon, small intestine, urinary bladder, kidney, and retina [82, 83]. Interestingly, immunohistochemical analysis indicates that there is a strong correlation between CA XIV and CA IV expression suggesting there is functional overlap between the enzymes [84]. CA XIV has been shown to directly interact with membrane-transporters and has been observed to be important for pH balance in muscle and erythrocytes in response to chronic hypoxia. Furthermore, CA XIV activity is shown to be important in terms of hyperactivity of the heart and pH regulation in the retina [85–87].
2. Methods
A multiple sequence alignment of all the human α-CAs was performed in ClustalW2 [98, 99] and used to generate a cladogram that illustrated the evolutionary relationship between the isoforms. The primary sequence identity (%) and number of conserved residues (among the catalytically active isoforms) were calculated in ClustalW2 [98, 99] using the same sequence alignment information. The coordinate files for different CA II inhibitor-complexes were obtained from the Protein Data Bank (PDB) (http://www.wwpdb.org/) to compare the region in which these inhibitors bind in CA II's active site. One file was selected as a reference for the alignment to the other coordinate files in the molecular graphics program Coot [100]. A surface rendition of CA II in complex with each of the inhibitors was generated in Pymol [101]. The hydrophobicity scores for the residues constituting the hydrophobic cleft were calculated based on the Kyte-Doolittle hydropathy plot [102]. All figures were generated in Pymol [101].
3. Results and Discussion
3.1. Enzyme Inhibition
The α-CAs are very closely related (Figure 4) as per a >30% primary sequence identity amongst them (Table 2). It is this similarity that leads to complications when designing CAIs that are isoform selective as a majority of the sequence identity translates to residues located in the CA active site. Table 3 shows the number of conserved residues among the different isoforms for residues in the active site and surrounding areas. For example, the 60.5% primary sequence identity that exists between CA I and II (Table 2), in combination with both enzymes being expressed in RBCs, makes CA I a potential off-target isoform when targeting CA II for inhibition [103, 104]. Likewise, when designing selective inhibitors against CA IX, unwanted targeting of CA I and II (with 33.1 and 34.2% identity, resp.) can occur leading to an induced susceptibility to side-effects [9, 105]. The same is true when considering CA VI inhibition where CA II acts as an off-target isoform (33.5% identical) [9].
Figure 4.
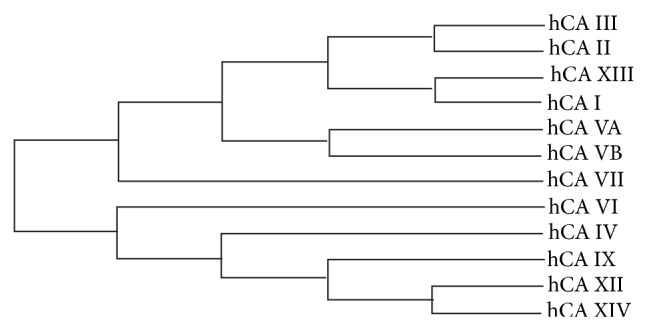
Cladogram of the human α-CAs.
Table 2.
Primary sequence identity (%) (lower left) and number of conserved residues (upper right) among catalytic CAs.
| I | II | III | IV | VA | VB | VI | VII | IX | XII | XIII | XIV | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | — | 158 | 141 | 78 | 126 | 128 | 82 | 132 | 83 | 91 | 154 | 85 |
| II | 60.5 | — | 152 | 88 | 133 | 138 | 90 | 147 | 85 | 89 | 157 | 96 |
| III | 54.2 | 58.5 | — | 82 | 120 | 117 | 87 | 130 | 80 | 86 | 151 | 90 |
| IV | 30.0 | 33.5 | 31.2 | — | 89 | 93 | 97 | 90 | 84 | 91 | 84 | 62 |
| VA | 48.1 | 50.8 | 45.4 | 23.6 | — | 184 | 93 | 131 | 83 | 84 | 124 | 88 |
| VB | 46.9 | 51.9 | 43.5 | 23.1 | 58.7 | — | 82 | 134 | 89 | 79 | 131 | 88 |
| VI | 31.9 | 33.5 | 32.3 | 27.0 | 27.9 | 24.4 | — | 93 | 107 | 104 | 90 | 106 |
| VII | 50.8 | 56.2 | 49.6 | 31.8 | 48.5 | 49.2 | 34.9 | — | 95 | 103 | 139 | 97 |
| IX | 33.1 | 34.2 | 31.1 | 27.2 | 31.9 | 32.7 | 38.9 | 37.0 | — | 101 | 90 | 113 |
| XII | 35.8 | 34.2 | 32.3 | 28.1 | 31.6 | 29.7 | 38.0 | 38.0 | 38.9 | — | 91 | 123 |
| XIII | 59.2 | 59.6 | 57.7 | 28.2 | 46.2 | 47.7 | 33.2 | 52.7 | 35.0 | 34.7 | — | 98 |
| XIV | 34.2 | 35.8 | 34.2 | 29.0 | 31.9 | 29.0 | 35.8 | 36.0 | 44.4 | 46.0 | 37.4 | — |
Table 3.
Active site residues of catalytic CAs (CA II numbering).
| Residues | Isozyme | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| I | III | IV | VA | VB | VI | VII | IX | XII | XIII | XIV | |
| Y7 | Y | Y | Y | W | Y | Y | Y | Y | Y | Y | Y |
| N62 | N | N | N | N | N | N | N | N | N | N | N |
| N67* | H | R | M | Q | L | Q | Q | Q | K | N | Q |
| I91* | F | R | K | K | K | Q | K | L | T | R | A |
| Q92 | Q | Q | Q | Q | Q | Q | Q | Q | Q | Q | Q |
| H94 | H | H | H | H | H | H | H | H | H | H | H |
| H96 | H | H | H | H | H | H | H | H | H | H | H |
| H119 | H | H | H | H | H | H | H | H | H | H | H |
| V121 | A | V | V | V | V | V | V | V | V | V | V |
| F131* | L | F | V | Y | F | Y | F | V | A | F | L |
| V135 | A | L | Q | V | A | Q | A | L | S | A | A |
| V143 | V | V | V | V | V | V | V | V | V | V | V |
| L198 | L | F | L | L | L | L | L | L | L | L | L |
| T199 | T | T | T | T | T | T | T | T | T | T | T |
| T200 | T | T | T | T | T | T | T | T | T | V | T |
| P202 | P | T | P | P | P | P | P | P | P | P | P |
| W209 | W | W | W | W | W | W | W | W | W | W | W |
*residues making up the selective pocket.
Therefore, to design highly selective CAIs requires the exploitation of subtle active site differences; predominantly residues found in the hydrophilic and hydrophobic pockets [22] (Figure 4). Comparative analysis of structures of ligand bound CA molecules shows that exploitable residues that contribute to ligand stabilization include residues N67, I91 and F131 (Figure 5), which are also highly variable between isoforms (Table 4). In addition, Q92, though conserved, has also shown to be important in inhibitor binding. Furthermore, structural interpretation of ligands bound to CA II show that inhibitors can extend out of the active site and form extensive and unique contacts with residues of either the hydrophilic or hydrophobic pocket.
Figure 5.
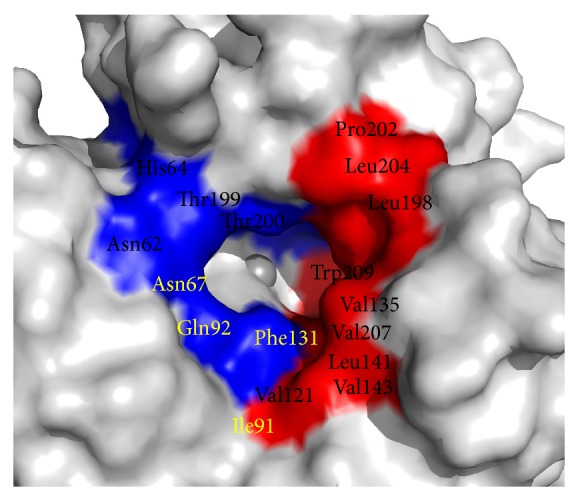
Solvent accessible residues in and around CA II active site. Hydrophilic cleft (blue) and hydrophobic cleft (red). Residues in yellow indicate residues of the “selective pocket.”
Table 4.
Hydrophobicity of CA active sites (CA II numbering).
| Residues | Isozyme | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| I1 | II2 | III3 | IV4 | V5 * | VI6 | VII7 | IX8 | XII9 | XIII10 | XIV11 | |
| I91 | F | I | R | K | K | Q | K | L | T | R | A |
| V121 | A | V | V | V | V | V | V | V | V | V | V |
| V135 | A | V | L | Q | S | Q | A | L | S | A | A |
| V141 | L | L | L | I | L | L | L | L | L | L | L |
| V143 | V | V | V | V | V | V | V | V | V | V | V |
| L198 | L | L | F | L | L | L | L | L | L | L | L |
| P202 | P | P | T | P | P | P | P | P | P | P | P |
| L204 | Y | L | E | D | A | T | S | A | N | L | Y |
| W209 | W | W | W | W | W | W | W | W | W | W | W |
|
| |||||||||||
| Total hydrophobicity | 14 | 26 | 8 | 4 | 11 | 7 | 11 | 23 | 9 | 15 | 16 |
12FOY; 23KS3; 33UYN; 41ZNC; 51DMX *murine; 63FE4; 73MDZ; 83IAI; 91JC2; 103DAZ; 114LU3.
3.2. Classical Inhibitors
Both the catalytic and inhibition mechanism of the α-CAs have been studied for several decades and have aided in designing potent isoform specific inhibitors that are important in a wide range of clinical applications (Table 1). This includes CAIs used such as antiglaucoma, antiepileptic, and antiobesity agents, as well as diagnostic tools [41]. A schematic of the basic components of a typical CAI is illustrated in Figure 6. It consists of a zinc-binding group (ZBG), a linker region (heterocyclic or benzene ring) and a variable “tail” region.
Figure 6.
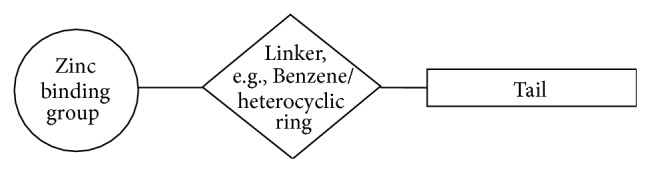
Schematic of the components of a classical CA inhibitor.
As discussed previously CAIs that bind directly to the Zn(II) ion can be divided into two groups based on how they coordinate to the metal center. Those that form trigonal-bipyramidal adducts through way of binding directly to the zinc-bound hydroxyl/water (e.g., cyanates and formates) [9, 16, 22], and those that form tetrahedral adducts and interact directly to the catalytic zinc (e.g., sulfonamides and bisulfites) (Figure 3) [9, 16, 22].
The classical CAIs: the metal-chelating anions and the sulfonamides and their isoesters (sulfamides/sulfamates) are the most studied of the CAIs [22]. However, “nonclassical” CAIs that do not bind directly to the Zn(II) ion also exist. This includes compounds such as coumarins and nitrates [106].
3.3. Metal-Chelating Anions
The inorganic anions (e.g., Br−) are weaker inhibitors than the sulfonamides and have inhibition constants (K i's) in the millimolar to submillimolar range [9]. However, for certain isoforms some anions show binding affinities in the low micromolar range (e.g., azide, cyanate, and trithiocarbonate) [88, 107–109]. Unlike the sulfonamides the anions may bind to the metal ion in three different coordination geometries: trigonal-bipyramidal geometry, tetrahedral geometry, or in a distorted tetrahedral geometry. The ability to bind in multigeometries is due primarily to the ligand's structural features. For example, hydrogen sulfide's (HS−) ability to act as an H-bond donor to Thr199 allows it to displace the hydroxyl bound zinc and maintain a tetrahedral coordination [9]. On the other hand, unprotonated ligands such as azide (N3 −) and bromide (Br−) adopt either the trigonal bipyramidal geometry or distorted tetrahedral geometry [9, 16, 22]. These inhibitors lack the ability to form H-bonds with the Oγ of Thr199 and so the geometry about the zinc sphere is distorted from the regular tetrahedral geometry [110, 111]. Formate and thiocyanate anions bind as a bipyramidal adduct shifting the zinc bound solvent [12, 112]. Other anions like the nitrates are not coordinated to the metal ion and instead are located in close proximity to it [9, 106].
3.4. Sulfonamide-Based CAIs
The sulfonamide-based compounds and their isoesters (sulfamides/sulfamates) are by far the most widely represented and clinically used CAIs. This class consists of several compounds, many of which have adapted long-term clinical applications [22]. Brinzolamide, dorzolamide, acetazolamide, methazolamide, and zonisamide have been used as antiglaucoma agents, diuretics, and antiepileptics [9]. Sulfonamides and their bioesters are potent inhibitors with K i's in the nanomolar range and bind in deprotonated forms to the Zn(II) ion displacing the zinc-bound hydroxyl/water while maintaining a tetrahedral coordination about the active site (Figure 3(c)) [113]. X-ray crystallographic structures of CA I, CA II, and CA IV in complex with these sulfonamide inhibitors are available in the PDB and in all complexes the deprotonated sulfonamide group is coordinated to the Zn(II) ion, while the Oγ atom of Thr199 makes a hydrogen bond with the sulfonamide's NH moiety. Thr199 also forms a second hydrogen bond to the carboxylate group of Glu106 [16]. Depending on the nature of the R-group, additional interactions with hydrophobic and/or hydrophilic residues in the region of the active site also influence inhibitor binding. However, it is the combination of the negative charge of the monoprotonated sulfonamide group with the positively charged zinc coupled with the ability of Thr199 to form two strong H-bonds that lends the sulfonamides their unique potency for CA inhibition [9].
3.5. Nonclassical CAIs
Aside from the classical metal chelating anion and sulfonamide-based inhibitors, which currently represent the majority of CAIs, other potent inhibitors exist. These include thiocarbonates, phenols [114, 115], coumarins [116, 117], polyamines [118], carbohydrate-based sulfonamide derivatives [119–121], and steroid sulfatases [122]. In addition peptidomimetic and monoclonal antibody CAIs have also been utilized [123–125].
The thiocarbamates are anion based chemotypes that exhibit monodentate coordination by way of one sulfur atom binding to the Zn(II) ion in the CA active site. This interaction is coupled with a hydrogen bond observed between an adjacent sulfur molecule reacting with Thr199 [126]. Several compounds currently exist of this chemotype that display nanomolar affinity for CA II and other isoforms. Structural data show that these compounds make unique contacts with several amino acids in the enzymes hydrophilic and hydrophobic binding pockets that can be exploited for design of isoform specific CAIs [127]. Other interesting “nonclassical” CAIs, the phenols, show an alternative mode of binding that is different from both classical sulfonamides and most anions (Figure 8(d)). These compounds anchor directly to the zinc-bound water molecule/hydroxyl rather than the Zn(II) ion itself [114]. However these compounds exhibit a reduction in potency typically in the millimolar range, but there is still a large interest to develop these compounds into potent isoform selective CAIs as they are derived from natural products [128].
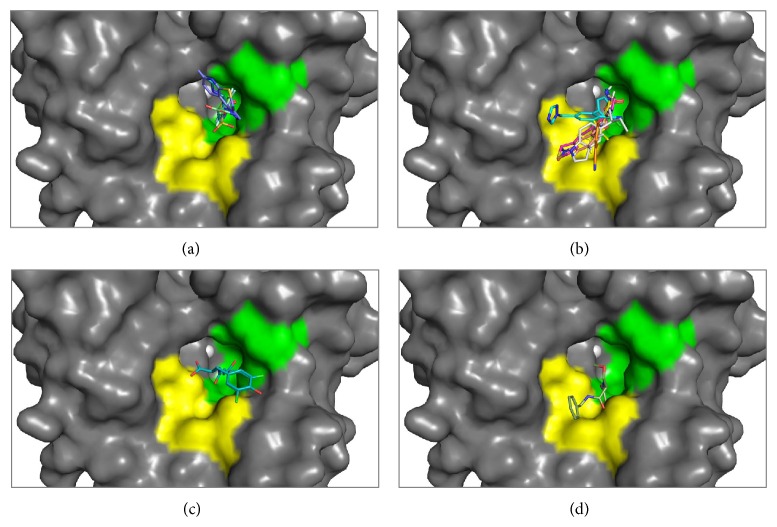
Figure 8.
CA inhibitor: (a) several inhibitors binding in the conserved region (green) of CA II's active site. These inhibitors are buried in the active site and are stabilized predominantly by hydrophobic residues (b). Several inhibitors occupying the “selective pocket” (yellow) of CA II. The tails of these inhibitors are extending out of the active site. (c) Coumarin binding on the perimeter of the active site. (d) Phenol binding in the proximity of the active site.
Other forms of nonclassical CAIs are the coumarins, which have been both engineered synthetically and isolated as natural products. These compounds vary in regards to isoform inhibition and selectivity [116, 117]. Coumarins, unlike classical CAIs, exhibit “prodrug” characteristics where, prior to binding to the active site, they are hydrolyzed by the esterase activity exhibited by CA that further induces binding at the entrance of the enzymes active site (Figure 8(c)) [116, 117]. This mechanism-based binding event of coumarins suggests that these compounds have potential use in CA isoform selectivity [129–134]. Based off of these observations, sulfur-based derivatives of this chemotype have been formulated and labeled as the “sulfocoumarins” [135]. These compounds also exhibit the same mechanism-based mode of CA binding but show increased affinity via the added sulfur moiety, which forms direct interactions with the catalytic zinc [135].
Polyamines, which belong to an alkaloid structural class, have also shown utility as CAIs [115, 118]. Several polyamine derivatives that have been isolated display high levels of CA isoform selectivity with potencies ranging from millimolar to low nanomolar levels [118]. Unlike the aforementioned CAIs, polyamines exhibit a mode of binding reliant on hydrogen bond formation throughout the active site cavity. Specifically, they anchor to the zinc-bound water/hydroxide (similar to phenols) with the terminal amine interacting with residues in positions 200 and 201 [118]. Most likely this attribute contributes to isoform selectivity of various polyamine CAIs and can thus be further developed to engineer more specific and potent CAIs of this class.
Several glycosyl primary sulfonamides and glycoconjugate sulfamates have been recognized as CAIs [120, 121]. These compounds are typically modifications of classical sulfonamide CAIs that usually have an aromatic-ring branched to the primary sulfonamide group (Figure 6). Instead these compounds replace the aromatic attachments of primary sulfonamides with mono- or disaccharide moieties [119–121]. Interestingly, the addition of a specific sugar moiety induces variable isoform selectivity ranging from micromolar to low nanomolar levels between CAs. More notably, these compounds have found use in inhibiting tumor associated isoforms IX and XII [119–121]. Not only do these compounds exhibit high affinity for CA IX/XII but the bulky sugar moieties cause a reduction in membrane permeability allowing for selective targeting of the extracellular facing catalytic domain of both tumor associated isoforms thus acting as location specific CAIs [119–121].
Similar to adding bulky-carbohydrate moieties to sulfonamides, steroid sulfatase inhibitors, which have been designed based on previously seen antimitotic inhibitors [136, 137] are able to take advantage of the variable residues in the hydrophobic pocket of specific CAs via van Der Waals contacts of the steroidal backbone [136–138]. The same trend was seen in energy calculations from molecular docking studies of such compounds with CA IX [137]. These particular compounds are also useful in locating specific targeting of extracellular CAs due to their reduced membrane permeability [136, 137].
In addition to the development of small-molecule inhibitors of CAs, there are several biologics used for CA inhibition. Utilization of monoclonal antibodies, such as M75 and G250, to recognize the proteoglycan-like (PG) domain (the N-terminal extension unique to this isoform) of CA IX have shown effectiveness in disrupting the ability of the enzymes function in regulating tumor cell adhesion and motility [139, 140]. More recently, the monoclonal antibody 6A10 has been developed to mediate CA XII activity also acting as a potential anticancer therapeutic [124, 125]. This becomes promising as such monoclonal antibodies exhibit high affinity to their target and can thus be used to distinguish between isoforms [124, 125]. More recently, peptide based inhibitors for CA IX have also been discovered utilizing a phage-display library [123]. However the benefits of these types of ligands are still unclear. Although there is postulation that the specific binding region of such peptides can be further exploited for the development of a biologic drug that is isoform selective [123].
3.6. Preferential Binding
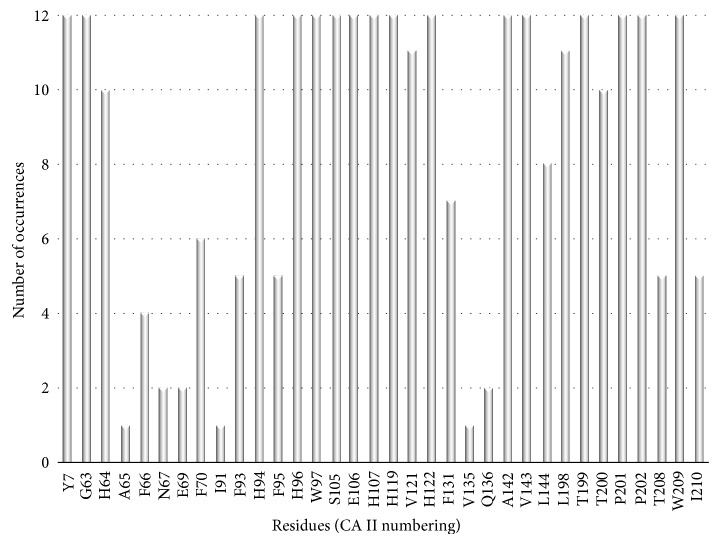
As we have seen the major hurdle in developing isoform selective CAIs is to design inhibitors that can distinguish between the similarities of the α-CA active site architecture. This would require the CAI to have limited interactions with conserved regions of the active site such as the three histidine residues coordinating the Zn(II) ion seen in all 12 catalytically active isoforms, residues that have shown to contribute to inhibitor binding such as Thr199 and Glu106 in CA II, and most of the residues that constitute both the hydrophobic and hydrophilic cleft as they are conserved (Figure 7).
Figure 7.
Bar graph of active site residues in the catalytic CA isozymes (CA II numbering).
Human CA II is the most well studied and characterized of the CA isoforms [141]. Over 400 X-ray crystallographic structures of CA II (both wild-type and variants) exist in the PDB with over 150 submissions containing CA II inhibitors [106]. Using the CA II active site as a reference it can be observed that the majority of inhibitors are buried deep in the enzymes active site (Figure 8(a)) and are restricted to the highly conserved region, which can be termed the “conserved pocket” (green shaded region, Figure 8(a)). Most of these inhibitors are sulfonamides (with short organic scaffolds) and so maintain the tetrahedral coordination about the zinc sphere while the variable “tails” of these inhibitors interact mainly with residues making up the hydrophobic and hydrophilic clefts. Furthermore, these variable “tail” regions are observed to be stabilized by H-bonds and hydrophobic interactions with Thr199, Thr200, Val121, Val143, and Leu198.
Despite the structural similarities observed between the CA isoforms, amino acid differences exist in specific regions of the active site. This region is defined as the “selective pocket” [106] (yellow shaded region, Figure 8(b)) and lies towards the edge of the active site relative to the catalytic zinc. Those inhibitors that are restricted to the conserved pocket are unable to form interactions with residues residing in the selective pocket due to the compact nature of their chemical scaffolds. Simply, the tails of these inhibitors are too short to interact with the residues that constitute the selective pocket and therefore cannot establish extensive contacts that can contribute to isoform selective inhibition. Residue positions 67, 91, and 131 establish this region termed the selective pocket (Table 3). Gln92, though conserved in all the isoforms, is also instrumental in contributing to inhibitor binding along with these select residues.
In addition to exploiting residues in the selective pocket between isoforms, selective CAIs can be designed based on overall hydrophobicity of the active site cleft. For example, CA II and CA IX display the most hydrophobic (hydrophobicity sores of ~26 and ~23, resp.) active site implying that designing CAIs with long flexible tails of a more hydrophobic nature may be beneficial to induce desired selective binding (Table 4). Notably, this attribute of the CA IX active-site coupled with its extracellular location provides an avenue to (1) design more hydrophobic CAIs that favor CA IX binding over other extracellular CAs and (2) engineer more bulky CAIs such that membrane permeability becomes poor thus eliminating the potential for CA II inhibition.
In order to design new isoform specific inhibitors that circumvent off-target CA inhibition, the structural dissimilarities that exist between the isoforms, particularly in the selective pocket, can be exploited. In addition, taking advantage of the global hydrophobic nature of the CA II or CA IX active site cleft provides a method to selective CAI design. It is already known that the sulfonamides are the most potent CAIs and this knowledge has been used to develop what is known as the “tail approach” to aid in the development of new inhibitors [142, 143]. This approach involves the appending of variable “tails” to the scaffolds of aromatic/heterocyclic sulfonamides to elongate the molecule. This allows the inhibitor to interact with amino acids from the middle to the edge of the active site relative to the catalytic zinc, which ultimately vary between different isoforms [106]. Small molecules such as phenols (Figure 8(c)) and coumarin (Figure 8(d)) also exhibit this same property by directly interacting with residues of the selective pocket.
4. Conclusions
A comparison of the conserved and nonconserved regions in the CA catalytic-site between isoforms revealed areas that can be exploited for rational design of selective CAIs. Specifically, highly variable areas amongst active site residues occur outwardly relative to the catalytic zinc in what has been defined as the selective pocket. Sequence alignments show that residues in positions 67, 91, and 131 vary between isoforms and structural analysis of CA II in complex with various inhibitors, show that “tails” of inhibitors make extensive contacts with these residues (Figures 5 and 8). Residues at position 91 seem to have the highest variability, in terms of specific residues type and between amino acid properties (i.e., hydrophilicity/hydrophobicity) between isoforms (Table 3). Interestingly, it is observed that CA II and IX exhibit the most hydrophobic catalytic domain and are the only isoforms (with exception to CA I and XIV) that contain hydrophobic residues at this position as well (Leu91 in CA IX). Position 91 can be termed a “hot-spot” for the design of isoform specific inhibitors, such that it contains both high variations between physical properties of amino acid, but (in the case of CA II and IX) there is also observable variation specific side-chain associated with the residues in this position. This attributes position 91 as being a key area that can be exploited by specific chemotypes and thus provides an alternative path for the design of selective CAIs. Overall, it is observed in Figure 8(b) that the residues farthest from the catalytic domain (relative to the zinc) remain the least conserved. This provides an exceptional advantage to the rational design of isoform specific inhibitors in that these variable regions can also be exploited by specific chemotypes. This notion is analogous to the idea of utilizing sulfonamide inhibitors with variable “tail” regions for isoform selective inhibitor development however in this study we have presented a more guided approach to this method of CAI design [106].
In summary our observations provide a template to exploit the variable regions of the catalytic domains of different CA isoforms. These guidelines can be utilized for the development of classical and nonclassical CAIs to overcome the potential of off-target CA inhibition and further lead to the development of more selective CAIs that can be employed in the clinic.
Supplementary Material
Surface rendition of carbonic anhydrase II showing how various inhibitors bind in and around the active site cleft. The “conserved region” (green) and “selective pocket” indicate regions of preferred binding by various carbonic anhydrase inhibitors.
Acknowledgments
The authors would like to acknowledge all the contributions of the carbonic anhydrase field that allowed for this study, especially Dr. David Silverman and Dr. Claudiu Supuran. This study was funded in part by the National Institutes of Health Grant GM25154.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Authors' Contribution
Melissa A. Pinard and Brian Mahon have contributed equally to this study.
References
- 1.Domsic J. F., Avvaru B. S., Chae U. K., et al. Entrapment of carbon dioxide in the active site of carbonic anhydrase II. Journal of Biological Chemistry. 2008;283(45):30766–30771. doi: 10.1074/jbc.M805353200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Avvaru B. S., Kim C. U., Sippel K. H., et al. A short, strong hydrogen bond in the active site of human carbonic anhydrase II. Biochemistry. 2010;49(2):249–251. doi: 10.1021/bi902007b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aggarwal M., Boone C. D., Kondeti B., McKenna R. Structural annotation of human carbonic anhydrases. Journal of Enzyme Inhibition and Medicinal Chemistry. 2013;28(2):267–277. doi: 10.3109/14756366.2012.737323. [DOI] [PubMed] [Google Scholar]
- 4.Krishnamurthy V. M., Kaufman G. K., Urbach A. R., et al. Carbonic anhydrase as a model for biophysical and physical-organic studies of proteins and protein-ligand binding. Chemical Reviews. 2008;108(3):946–1051. doi: 10.1021/cr050262p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Květoň J., Chegwidden W. R., Carter N. D., Edwards Y. H., editors. The Carbonic Anhydrases: New Horizons. Vol. 39. Photosynthetica; 2001. [Google Scholar]
- 6.Rowlett R. S. Structure and catalytic mechanism of the β-carbonic anhydrases. Biochimica et Biophysica Acta—Proteins and Proteomics. 2010;1804(2):362–373. doi: 10.1016/j.bbapap.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 7.Smith K. S., Ferry J. G. Prokaryotic carbonic anhydrases. FEMS Microbiology Reviews. 2000;24(4):335–366. doi: 10.1016/S0168-6445(00)00030-9. [DOI] [PubMed] [Google Scholar]
- 8.del Prete S., Vullo D., de Luca V., Supuran C. T., Capasso C. Biochemical characterization of the δ-carbonic anhydrase from the marine diatom Thalassiosira weissflogii, TweCA. Journal of Enzyme Inhibition and Medicinal Chemistry. 2014 doi: 10.3109/14756366.2013.868599. [DOI] [PubMed] [Google Scholar]
- 9.Alterio V., Di Fiore A., D'Ambrosio K., Supuran C. T., de Simone G. Multiple binding modes of inhibitors to carbonic anhydrases: how to design specific drugs targeting 15 different isoforms? Chemical Reviews. 2012;112(8):4421–4468. doi: 10.1021/cr200176r. [DOI] [PubMed] [Google Scholar]
- 10.Frost S. C. Physiological functions of the alpha class of carbonic anhydrases. Sub-Cellular Biochemistry. 2014;75:9–30. doi: 10.1007/978-94-007-7359-2_2. [DOI] [PubMed] [Google Scholar]
- 11.Aspatwar A., Tolvanen M. E. E., Ortutay C., Parkkila S. Carbonic anhydrase related protein VIII and its role in neurodegeneration and cancer. Current Pharmaceutical Design. 2010;16(29):3264–3276. doi: 10.2174/138161210793429823. [DOI] [PubMed] [Google Scholar]
- 12.Eriksson A. E., Jones T. A., Liljas A. Refined structure of human carbonic anhydrase II at 2.0 Å resolution. Proteins: Structure, Function and Genetics. 1988;4(4):274–282. doi: 10.1002/prot.340040406. [DOI] [PubMed] [Google Scholar]
- 13.Pocker Y., Sarkanen S. Carbonic anhydrase: structure catalytic versatility, and inhibition. Advances in Enzymology and Related Areas of Molecular Biology. 1978;47:149–274. doi: 10.1002/9780470122921.ch3. [DOI] [PubMed] [Google Scholar]
- 14.Fisher S. Z., Maupin C. M., Budayova-Spano M., et al. Atomic crystal and molecular dynamics simulation structures of human carbonic anhydrase II: insights into the proton transfer mechanism. Biochemistry. 2007;46(11):2930–2937. doi: 10.1021/bi062066y. [DOI] [PubMed] [Google Scholar]
- 15.Boone C. D., Pinard M., McKenna R., Silverman D. Catalytic mechanism of α-class carbonic anhydrases: CO2 hydration and proton transfer. Sub-cellular biochemistry. 2014;75:31–52. doi: 10.1007/978-94-007-7359-2_3. [DOI] [PubMed] [Google Scholar]
- 16.Supuran C. T., Scozzafava A., Casini A. Carbonic anhydrase inhibitors. Medicinal Research Reviews. 2003;23(2):146–189. doi: 10.1002/med.10025. [DOI] [PubMed] [Google Scholar]
- 17.Tu C., Silverman D. N., Forsman C., Jonsson B.-H., Lindskog S. Role of histidine 64 in the catalytic mechanism of human carbonic anhydrase II studied with a site-specific mutant. Biochemistry. 1989;28(19):7913–7918. doi: 10.1021/bi00445a054. [DOI] [PubMed] [Google Scholar]
- 18.Mikulski R., West D., Sippel K. H., et al. Water networks in fast proton transfer during catalysis by human carbonic anhydrase II. Biochemistry. 2013;52(1):125–131. doi: 10.1021/bi301099k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tu C., Sanyal G., Wynns G. C., Silverman D. N. The pH dependence of the hydration of CO2 catalyzed by carbonic anhydrase III from skeletal muscle of the cat. Steady state and equilibrium studies. The Journal of Biological Chemistry. 1983;258(14):8867–8871. [PubMed] [Google Scholar]
- 20.An H., Tu C., Duda D., et al. Chemical rescue in catalysis by human carbonic anhydrases II and III. Biochemistry. 2002;41(9):3235–3242. doi: 10.1021/bi0120695. [DOI] [PubMed] [Google Scholar]
- 21.Silverman D. N., Tu C. K., Wynns G. C. Role of buffer in catalysis of the hydration of CO2 by carbonic anhydrase. In: Bauer P. D. C., Gros P. D. G., Bartels P. D. H., editors. Biophysics and Physiology of Carbon Dioxide. Berlin, Germany: Springer; 1980. pp. 254–61. [Google Scholar]
- 22.McKenna R., Supuran C. T. Carbonic anhydrase inhibitors drug design. Sub-Cellular Biochemistry. 2014;75:291–323. doi: 10.1007/978-94-007-7359-2_15. [DOI] [PubMed] [Google Scholar]
- 23.Maren T. H., Swenson E. R. A comparative study of the kinetics of the Bohr effect in vertebrates. Journal of Physiology. 1980;303:535–547. doi: 10.1113/jphysiol.1980.sp013302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown D., Kumpulainen T., Roth J., Orci L. Immunohistochemical localization of carbonic anhydrase in postnatal and adult rat kidney. The American Journal of Physiology. 1983;245(1):F110–F118. doi: 10.1152/ajprenal.1983.245.1.F110. [DOI] [PubMed] [Google Scholar]
- 25.Gilmour K. M. Perspectives on carbonic anhydrase. Comparative Biochemistry and Physiology: A Molecular and Integrative Physiology. 2010;157(3):193–197. doi: 10.1016/j.cbpa.2010.06.161. [DOI] [PubMed] [Google Scholar]
- 26.Vince J. W., Reithmeier R. A. Carbonic anhydrase II binds to the carboxyl terminus of human band 3, the erythrocyte C1-/HCO3- exchanger. The Journal of Biological Chemistry. 1998;273(43):28430–28437. doi: 10.1074/jbc.273.43.28430. [DOI] [PubMed] [Google Scholar]
- 27.Pushkin A., Abuladze N., Gross E., et al. Molecular mechanism of kNBC1—carbonic anhydrase II interaction in proximal tubule cells. The Journal of Physiology. 2004;559(1):55–65. doi: 10.1113/jphysiol.2004.065110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li X., Alvarez B., Casey J. R., Reithmeier R. A. F., Fliegel L. Carbonic anhydrase II binds to and enhances activity of the Na+/H+ exchanger. The Journal of Biological Chemistry. 2002;277(39):36085–36091. doi: 10.1074/jbc.M111952200. [DOI] [PubMed] [Google Scholar]
- 29.McMurtrie H. L., Cleary H. J., Alvarez B. V., et al. The bicarbonate transport metabolon. Journal of Enzyme Inhibition and Medicinal Chemistry. 2004;19(3):231–236. doi: 10.1080/14756360410001704443. [DOI] [PubMed] [Google Scholar]
- 30.Sly W. S., Hewett-Emmett D., Whyte M. P., Yu Y. S., Tashian R. E. Carbonic anhydrase II deficiency identified as the primary defect in the autosomal recessive syndrome of osteopetrosis with renal tubular acidosis and cerebral calcification. Proceedings of the National Academy of Sciences of the United States of America. 1983;80(9):2752–2756. doi: 10.1073/pnas.80.9.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Henry R. P., Swenson E. R. The distribution and physiological significance of carbonic anhydrase in vertebrate gas exchange organs. Respiration Physiology. 2000;121(1):1–12. doi: 10.1016/S0034-5687(00)00110-9. [DOI] [PubMed] [Google Scholar]
- 32.Cooper G. J., Boron W. F. Effect of PCMBS on CO2 permeability of xenopus oocytes expressing aquaporin 1 or its C189S mutant. The American Journal of Physiology. 1998;275(6):C1481–C1486. doi: 10.1152/ajpcell.1998.275.6.C1481. [DOI] [PubMed] [Google Scholar]
- 33.Carter N. D. Hormonal and neuronal control of carbonic anhydrase III gene expression in skeletal muscle. In: Dodgson S. J., Tashian R. E., Gross G., Carter N. D., editors. The Carbonic Anhydrases: Cellular Physiology and Molecular Genetics. New York, NY, USA: Plenum Press; 1991. pp. 247–256. [Google Scholar]
- 34.Stanton L. W., Ponte P. A., Coleman R. T., Snyder M. A. Expression of CA III in rodent models of obesity. Molecular Endocrinology. 1991;5(6):860–866. doi: 10.1210/mend-5-6-860. [DOI] [PubMed] [Google Scholar]
- 35.Lyons G. E., Buckingham M. E., Tweedie S., Edwards Y. H. Carbonic anhydrase III, an early mesodermal marker, is expressed in embryonic mouse skeletal muscle and notochord. Development. 1991;111(1):233–244. doi: 10.1242/dev.111.1.233. [DOI] [PubMed] [Google Scholar]
- 36.Supuran C. T. Carbonic anhydrases—an overview. Current Pharmaceutical Design. 2008;14(7):603–614. doi: 10.2174/138161208783877884. [DOI] [PubMed] [Google Scholar]
- 37.Chai Y.-C., Jung C.-H., Lii C.-K., et al. Identification of an abundant S-thiolated rat liver protein as carbonic anhydrase III; characterization of S-thiolation and dethiolation reactions. Archives of Biochemistry and Biophysics. 1991;284(2):270–278. doi: 10.1016/0003-9861(91)90295-T. [DOI] [PubMed] [Google Scholar]
- 38.Mitterberger M. C., Kim G., Rostek U., Levine R. L., Zwerschke W. Carbonic anhydrase III regulates peroxisome proliferator-activated receptor-γ2. Experimental Cell Research. 2012;318(8):877–886. doi: 10.1016/j.yexcr.2012.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bootorabi F., Jänis J., Smith E., et al. Analysis of a shortened form of human carbonic anhydrase VII expressed in vitro compared to the full-length enzyme. Biochimie. 2010;92(8):1072–1080. doi: 10.1016/j.biochi.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 40.Truppo E., Supuran C. T., Sandomenico A., et al. Carbonic anhydrase VII is S-glutathionylated without loss of catalytic activity and affinity for sulfonamide inhibitors. Bioorganic and Medicinal Chemistry Letters. 2012;22(4):1560–1564. doi: 10.1016/j.bmcl.2011.12.134. [DOI] [PubMed] [Google Scholar]
- 41.Thiry A., Dogné J.-M., Supuran C. T., Masereel B. Carbonic anhydrase inhibitors as anticonvulsant agents. Current Topics in Medicinal Chemistry. 2007;7(9):855–864. doi: 10.2174/156802607780636726. [DOI] [PubMed] [Google Scholar]
- 42.Ruusuvuori E., Li H., Huttu K., et al. Carbonic anhydrase isoform VII acts as a molecular switch in the development of synchronous gamma-frequency firing of hippocampal CA1 pyramidal cells. The Journal of Neuroscience. 2004;24(11):2699–2707. doi: 10.1523/JNEUROSCI.5176-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Asiedu M., Ossipov M. H., Kaila K., Price T. J. Acetazolamide and midazolam act synergistically to inhibit neuropathic pain. Pain. 2010;148(2):302–308. doi: 10.1016/j.pain.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lehtonen J., Shen B., Vihinen M., et al. Characterization of CA XIII, a novel member of the carbonic anhydrase isozyme family. Journal of Biological Chemistry. 2004;279(4):2719–2727. doi: 10.1074/jbc.M308984200. [DOI] [PubMed] [Google Scholar]
- 45.Kummola L., Hämäläinen J. M., Kivelä J., et al. Expression of a novel carbonic anhydrase, CA XIII, in normal and neoplastic colorectal mucosa. BMC Cancer. 2005;5, article 41 doi: 10.1186/1471-2407-5-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shah G. N., Hewett-Emmett D., Grubb J. H., et al. Mitochondrial carbonic anhydrase CA VB: differences in tissue distribution and pattern of evolution from chose of CA VA suggest distinct physiological roles. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(4):1677–1682. doi: 10.1073/pnas.97.4.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lusty C. J. Carbamyl phosphate synthetase. Bicarbonate-dependent hydrolysis of ATP and potassium activation. The Journal of Biological Chemistry. 1978;253(12):4270–4278. [PubMed] [Google Scholar]
- 48.Dodgson S. J., Forster R. E., II Inhibition of CA V decreases glucose synthesis from pyruvate. Archives of Biochemistry and Biophysics. 1986;251(1):198–204. doi: 10.1016/0003-9861(86)90066-4. [DOI] [PubMed] [Google Scholar]
- 49.Lynch C. J., Fox H., Hazen S. A., Stanley B. A., Dodgson S., Lanoue K. F. Role of hepatic carbonic anhydrase in de novo lipogenesis. Biochemical Journal. 1995;310, part 1:197–202. doi: 10.1042/bj3100197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oommen K. J., Mathews S. Zonisamide: a new antiepileptic drug. Clinical Neuropharmacology. 1999;22(4):192–200. [PubMed] [Google Scholar]
- 51.Ogawa Y., Matsumoto K., Maeda T., et al. Characterization of lacrimal gland carbonic anhydrase VI. Journal of Histochemistry and Cytochemistry. 2002;50(6):821–827. doi: 10.1177/002215540205000608. [DOI] [PubMed] [Google Scholar]
- 52.Leinonen J. S., Saari K. A., Seppänen J. M., Myllylä H. M., Rajaniemi H. J. Immunohistochemical demonstration of carbonic anhydrase isoenzyme VI (CA VI) expression in rat lower airways and lung. Journal of Histochemistry and Cytochemistry. 2004;52(8):1107–1112. doi: 10.1369/jhc.4A6282.2004. [DOI] [PubMed] [Google Scholar]
- 53.Kaseda M., Ichihara N., Nishita T., Amasaki H., Asari M. Immunohistochemistry of the bovine secretory carbonic anhydrase isozyme (CA-VI) in bovine alimentary canal and major salivary glands. Journal of Veterinary Medical Science. 2006;68(2):131–135. doi: 10.1292/jvms.68.131. [DOI] [PubMed] [Google Scholar]
- 54.Smith C. E., Nanci A., Moffatt P. Evidence by signal peptide trap technology for the expression of carbonic anhydrase 6 in rat incisor enamel organs. European Journal of Oral Sciences. 2006;114, supplement 1:147–153. doi: 10.1111/j.1600-0722.2006.00273.x. [DOI] [PubMed] [Google Scholar]
- 55.Breiner Feldstein J., Silverman D. N. Purification and characterization of carbonic anhydrase from the saliva of the rat. Journal of Biological Chemistry. 1984;259(9):5447–5453. [PubMed] [Google Scholar]
- 56.Ship J. A. Diabetes and oral health: an overview. Journal of the American Dental Association (1939) 2003;134(Spec No:4S–10S) doi: 10.14219/jada.archive.2003.0367. [DOI] [PubMed] [Google Scholar]
- 57.Dowd F. J. Saliva and dental caries. Dental clinics of North America. 1999;43(4):579–597. [PubMed] [Google Scholar]
- 58.Kimoto M., Kishino M., Yura Y., Ogawa Y. A role of salivary carbonic anhydrase VI in dental plaque. Archives of Oral Biology. 2006;51(2):117–122. doi: 10.1016/j.archoralbio.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 59.Ortho-McNeil-Janssen Pharmaceuticals I. Topomax: drug summary, physicians’ desk reference, 2013.
- 60.Shatzman A. R., Henkin R. I. Gustin concentration changes relative to salivary zinc and taste in humans. Proceedings of the National Academy of Sciences of the United States of America. 1981;78(6 I):3867–3871. doi: 10.1073/pnas.78.6.3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhu X. L., Sly W. S. Carbonic anhydrase IV from human lung. Purification, characterization, and comparison with membrane carbonic anhydrase from human kidney. Journal of Biological Chemistry. 1990;265(15):8795–8801. [PubMed] [Google Scholar]
- 62.Yang Z., Alvarez B. V., Chakarova C., et al. Mutant carbonic anhydrase 4 impairs pH regulation and causes retinal photoreceptor degeneration. Human Molecular Genetics. 2005;14(2):255–265. doi: 10.1093/hmg/ddi023. [DOI] [PubMed] [Google Scholar]
- 63.Rebello G., Ramesar R., Vorster A., et al. Apoptosis-inducing signal sequence mutation in carbonic anhydrase IV identified in patients with the RP17 form of retinitis pigmentosa. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(17):6617–6622. doi: 10.1073/pnas.0401529101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ivanov S., Liao S.-Y., Ivanova A., et al. Expression of hypoxia-inducible cell-surface transmembrane carbonic anhydrases in human cancer. American Journal of Pathology. 2001;158(3):905–919. doi: 10.1016/S0002-9440(10)64038-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pastoreková S., Parkkila S., Parkkila A.-K., et al. Carbonic anhydrase IX, MN/CA IX: analysis of stomach complementary DNA sequence and expression in human and rat alimentary tracts. Gastroenterology. 1997;112(2):398–408. doi: 10.1053/gast.1997.v112.pm9024293. [DOI] [PubMed] [Google Scholar]
- 66.Lou Y., McDonald P. C., Oloumi A., et al. Targeting tumor hypoxia: suppression of breast tumor growth and metastasis by novel carbonic anhydrase IX inhibitors. Cancer Research. 2011;71:3364–3376. doi: 10.1158/0008-5472.CAN-10-4261. [DOI] [PubMed] [Google Scholar]
- 67.Swietach P., Hulikova A., Vaughan-Jones R. D., Harris A. L. New insights into the physiological role of carbonic anhydrase IX in tumour pH regulation. Oncogene. 2010;29(50):6509–6521. doi: 10.1038/onc.2010.455. [DOI] [PubMed] [Google Scholar]
- 68.Parkkila S., Rajaniemi H., Parkkila A.-K., et al. Carbonic anhydrase inhibitor suppresses invasion of renal cancer cells in vitro . Proceedings of the National Academy of Sciences of the United States of America. 2000;97(5):2220–2224. doi: 10.1073/pnas.040554897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Robertson N., Potter C., Harris A. L. Role of carbonic anhydrase IX in human tumor cell growth, survival, and invasion. Cancer Research. 2004;64(17):6160–6165. doi: 10.1158/0008-5472.CAN-03-2224. [DOI] [PubMed] [Google Scholar]
- 70.Švastová E., Žilka N., Zat'ovičová M., et al. Carbonic anhydrase IX reduces E-cadherin-mediated adhesion of MDCK cells via interaction with β-catenin. Experimental Cell Research. 2003;290(2):332–345. doi: 10.1016/S0014-4827(03)00351-3. [DOI] [PubMed] [Google Scholar]
- 71.Wykoff C. C., Beasley N. J. P., Watson P. H., et al. Hypoxia-inducible expression of tumor-associated carbonic anhydrases. Cancer Research. 2000;60(24):7075–7083. [PubMed] [Google Scholar]
- 72.Chia S. K., Wykoff C. C., Watson P. H., et al. Prognostic significance of a novel hypoxia-regulated marker, carbonic anhydrase IX, in invasive breast carcinoma. The American Society of Clinical Oncology: Journal of Clinical Oncology. 2001;19(16):3660–3668. doi: 10.1200/JCO.2001.19.16.3660. [DOI] [PubMed] [Google Scholar]
- 73.Generali D., Fox S. B., Berruti A., et al. Role of carbonic anhydrase IX expression in prediction of the efficacy and outcome of primary epirubicin/tamoxifen therapy for breast cancer. Endocrine-Related Cancer. 2006;13(3):921–930. doi: 10.1677/erc.1.01216. [DOI] [PubMed] [Google Scholar]
- 74.Creighton C. J., Cordero K. E., Larios J. M., et al. Genes regulated by estrogen in breast tumor cells in vitro are similarly regulated in vivo in tumor xenografts and human breast tumors. Genome Biology. 2006;7(4, article R28) doi: 10.1186/gb-2006-7-4-r28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nordfors K., Haapasalo J., Korja M., et al. The tumour-associated carbonic anhydrases CA II, CA IX and CA XII in a group of medulloblastomas and supratentorial primitive neuroectodermal tumours: an association of CA IX with poor prognosis. BMC Cancer. 2010;10, article 148 doi: 10.1186/1471-2407-10-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ilie M. I., Hofman V., Ortholan C., et al. Overexpression of carbonic anhydrase XII in tissues from resectable non-small cell lung cancers is a biomarker of good prognosis. International Journal of Cancer. 2011;128(7):1614–1623. doi: 10.1002/ijc.25491. [DOI] [PubMed] [Google Scholar]
- 77.Chien M.-H., Ying T.-H., Hsieh Y.-H., et al. Tumor-associated carbonic anhydrase XII is linked to the growth of primary oral squamous cell carcinoma and its poor prognosis. Oral Oncology. 2012;48(5):417–423. doi: 10.1016/j.oraloncology.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 78.Parkkila S., Rajaniemi H., Parkkila A.-K., et al. Carbonic anhydrase inhibitor suppresses invasion of renal cancer cells in vitro. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(5):2220–2224. doi: 10.1073/pnas.040554897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hynninen P., Parkkila S., Huhtala H., et al. Carbonic anhydrase isozymes II, IX, and XII in uterine tumors. APMIS. 2012;120(2):117–129. doi: 10.1111/j.1600-0463.2011.02820.x. [DOI] [PubMed] [Google Scholar]
- 80.Kivela A., Parkkila S., Saarnio J., et al. Expression of a novel transmembrane carbonic anhydrase isozyme XII in normal human gut and colorectal tumors. The American Journal of Pathology. 2000;156(2):577–584. doi: 10.1016/S0002-9440(10)64762-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Muhammad E., Leventhal N., Parvari G., et al. Autosomal recessive hyponatremia due to isolated salt wasting in sweat associated with a mutation in the active site of Carbonic Anhydrase 12. Human Genetics. 2011;129(4):397–405. doi: 10.1007/s00439-010-0930-4. [DOI] [PubMed] [Google Scholar]
- 82.Fujikawa-Adachi K., Nishimori I., Taguchi T., Onishi S. Human carbonic anhydrase XIV (CA14): cDNA cloning, mRNA expression, and mapping to chromosome 1. Genomics. 1999;61(1):74–81. doi: 10.1006/geno.1999.5938. [DOI] [PubMed] [Google Scholar]
- 83.Ochrietor J. D., Clamp M. F., Moroz T. P., et al. Carbonic anhydrase XIV identified as the membrane CA in mouse retina: strong expression in Müller cells and the RPE. Experimental Eye Research. 2005;81(4):492–500. doi: 10.1016/j.exer.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 84.Kaunisto K., Parkkila S., Rajaniemi H., Waheed A., Grubb J., Sly W. S. Carbonic anhydrase XIV: luminal expression suggests key role in renal acidification. Kidney International. 2002;61(6):2111–2118. doi: 10.1046/j.1523-1755.2002.00371.x. [DOI] [PubMed] [Google Scholar]
- 85.Juel C., Lundby C., Sander M., Calbet J. A. L., van Hall G. Human skeletal muscle and erythrocyte proteins involved in acid-base homeostasis: adaptations to chronic hypoxia. Journal of Physiology. 2003;548(2):639–648. doi: 10.1113/jphysiol.2002.035899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vargas L. A., Alvarez B. V. Carbonic anhydrase XIV in the normal and hypertrophic myocardium. Journal of Molecular and Cellular Cardiology. 2012;52(3):741–752. doi: 10.1016/j.yjmcc.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 87.Linser P., Moscona A. A. Variable CA II compartmentalization in vertebrate retina. Annals of the New York Academy of Sciences. 1984;429:430–446. doi: 10.1111/j.1749-6632.1984.tb12369.x. [DOI] [PubMed] [Google Scholar]
- 88.Supuran C. T. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nature Reviews Drug Discovery. 2008;7(2):168–181. doi: 10.1038/nrd2467. [DOI] [PubMed] [Google Scholar]
- 89.Supuran C. T. Carbonic anhydrase inhibitors: an editorial. Expert Opinion on Therapeutic Patents. 2013;23(6):677–679. doi: 10.1517/13543776.2013.778246. [DOI] [PubMed] [Google Scholar]
- 90.Eichhorn M. Mode of action, clinical profile and relevance of carbonic anhydrase inhibitors in glaucoma therapy. Klinische Monatsblatter fur Augenheilkunde. 2013;230(2):146–149. doi: 10.1055/s-0032-1328163. [DOI] [PubMed] [Google Scholar]
- 91.Nishimori I., Vullo D., Innocenti A., Scozzafava A., Mastrolorenzo A., Supuran C. T. Carbonic anhydrase inhibitors. The mitochondrial isozyme VB as a new target for sulfonamide and sulfamate inhibitors. Journal of Medicinal Chemistry. 2005;48(24):7860–7866. doi: 10.1021/jm050483n. [DOI] [PubMed] [Google Scholar]
- 92.Nishimori I., Minakuchi T., Onishi S., Vullo D., Scozzafava A., Supuran C. T. Carbonic anhydrase inhibitors. DNA cloning, characterization, and inhibition studies of the human secretory isoform VI, a new target for sulfonamide and sulfamate inhibitors. Journal of Medicinal Chemistry. 2007;50(2):381–388. doi: 10.1021/jm0612057. [DOI] [PubMed] [Google Scholar]
- 93.Pilka E. S., Kochan G., Oppermann U., Yue W. W. Crystal structure of the secretory isozyme of mammalian carbonic anhydrases CA VI: implications for biological assembly and inhibitor development. Biochemical and Biophysical Research Communications. 2012;419(3):485–489. doi: 10.1016/j.bbrc.2012.02.038. [DOI] [PubMed] [Google Scholar]
- 94.Vullo D., Voipio J., Innocenti A., et al. Carbonic anhydrase inhibitors. Inhibition of the human cytosolic isozyme VII with aromatic and heterocyclic sulfonamides. Bioorganic & Medicinal Chemistry Letters. 2005;15(4):971–976. doi: 10.1016/j.bmcl.2004.12.052. [DOI] [PubMed] [Google Scholar]
- 95.Gao R., Liao S., Zhang C., et al. Optimization of heterocyclic substituted benzenesulfonamides as novel carbonic anhydrase IX inhibitors and their structure activity relationship. European Journal of Medicinal Chemistry. 2013;62:597–604. doi: 10.1016/j.ejmech.2013.01.030. [DOI] [PubMed] [Google Scholar]
- 96.Whittington D. A., Waheed A., Ulmasov B., et al. Crystal structure of the dimeric extracellular domain of human carbonic anhydrase XII, a bitopic membrane protein overexpressed in certain cancer tumor cells. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(17):9545–9550. doi: 10.1073/pnas.161301298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Whittington D. A., Grubb J. H., Waheed A., Shah G. N., Sly W. S., Christianson D. W. Expression, assay, and structure of the extracellular domain of murine carbonic anhydrase XIV: implications for selective inhibition of membrane-associated isozymes. The Journal of Biological Chemistry. 2004;279(8):7223–7228. doi: 10.1074/jbc.M310809200. [DOI] [PubMed] [Google Scholar]
- 98.Thompson J. D., Higgins D. G., Gibson T. J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Research. 1994;22(22):4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Larkin M. A., Blackshields G., Brown N. P., et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23(21):2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 100.Emsley P., Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallographica Section D: Biological Crystallography. 2004;60(1):2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 101. The PyMOL Molecular Graphics System, Version 1.5.0.4 Schrödinger, LLC.n.d.
- 102.Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. Journal of Molecular Biology. 1982;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- 103.Esbaugh A. J., Tufts B. L. The structure and function of carbonic anhydrase isozymes in the respiratory system of vertebrates. Respiratory Physiology and Neurobiology. 2006;154(1-2):185–198. doi: 10.1016/j.resp.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 104.Swenson E. R. Respiratory and renal roles of carbonic anhydrase in gas exchange and acid-base regulation. EXS. 2000;(90):281–341. doi: 10.1007/978-3-0348-8446-4_15. [DOI] [PubMed] [Google Scholar]
- 105.Pinard M. A., Boone C. D., Rife B. D., Supuran C. T., McKenna R. Structural study of interaction between brinzolamide and dorzolamide inhibition of human carbonic anhydrases. Bioorganic and Medicinal Chemistry. 2013;21(22):7210–7215. doi: 10.1016/j.bmc.2013.08.033. [DOI] [PubMed] [Google Scholar]
- 106.Aggarwal M., Kondeti B., McKenna R. Insights towards sulfonamide drug specificity in α-carbonic anhydrases. Bioorganic and Medicinal Chemistry. 2013;21(6):1526–1533. doi: 10.1016/j.bmc.2012.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lindskog S. Structure and mechanism of Carbonic Anhydrase. Pharmacology and Therapeutics. 1997;74(1):1–20. doi: 10.1016/S0163-7258(96)00198-2. [DOI] [PubMed] [Google Scholar]
- 108.Supuran C. T., Winum J.-Y. Drug Design of Zinc-Enzyme Inhibitors: Functional, Structural, and Disease Applications. John Wiley & Sons; 2009. [Google Scholar]
- 109.Temperini C., Scozzafava A., Supuran C. T. Carbonic anhydrase inhibitors. X-ray crystal studies of the carbonic anhydrase II-trithiocarbonate adduct—an inhibitor mimicking the sulfonamide and urea binding to the enzyme. Bioorganic and Medicinal Chemistry Letters. 2010;20(2):474–478. doi: 10.1016/j.bmcl.2009.11.124. [DOI] [PubMed] [Google Scholar]
- 110.Jönsson B. M., Håkansson K., Liljas A. The structure of human carbonic anhydrase II in complex with bromide and azide. FEBS Letters. 1993;322(2):186–190. doi: 10.1016/0014-5793(93)81565-H. [DOI] [PubMed] [Google Scholar]
- 111.Mangani S., Håkansson K. Crystallographic studies of the binding of protonated and unprotonated inhibitors to carbonic anhydrase using hydrogen sulphide and nitrate anions. European Journal of Biochemistry. 1992;210(3):867–871. doi: 10.1111/j.1432-1033.1992.tb17490.x. [DOI] [PubMed] [Google Scholar]
- 112.Hakansson K., Carlsson M., Svensson L. A., Liljas A. Structure of native and apo carbonic anhydrase II and structure of some of its anion-ligand complexes. Journal of Molecular Biology. 1992;227(4):1192–1204. doi: 10.1016/0022-2836(92)90531-N. [DOI] [PubMed] [Google Scholar]
- 113.Supuran C. T., Scozzafava A. Carbonic-anhydrase inhibitors and their therapeutic potential. Expert Opinion on Therapeutic Patents. 2000;10(5):575–600. doi: 10.1517/13543776.10.5.575. [DOI] [Google Scholar]
- 114.Nair S. K., Ludwig P. A., Christianson D. W. Two-site binding of phenol in the active site of human carbonic anhydrase II: structural implications for substrate association. Journal of the American Chemical Society. 1994;116(8):3659–3660. doi: 10.1021/ja00087a086. [DOI] [Google Scholar]
- 115.Dictionary of Natural Products (DVD) version 21.1. CRC & Taylor & Francis; 2012. [Google Scholar]
- 116.Maresca A., Temperini C., Vu H., et al. Non-zinc mediated inhibition of carbonic anhydrases: coumarins are a new class of suicide inhibitors. Journal of the American Chemical Society. 2009;131(8):3057–3062. doi: 10.1021/ja809683v. [DOI] [PubMed] [Google Scholar]
- 117.Maresca A., Temperini C., Pochet L., Masereel B., Scozzafava A., Supuran C. T. Deciphering the mechanism of carbonic anhydrase inhibition with coumarins and thiocoumarins. Journal of Medicinal Chemistry. 2010;53(1):335–344. doi: 10.1021/jm901287j. [DOI] [PubMed] [Google Scholar]
- 118.Carta F., Temperini C., Innocenti A., Scozzafava A., Kaila K., Supuran C. T. Polyamines inhibit carbonic anhydrases by anchoring to the zinc-coordinated water molecule. Journal of Medicinal Chemistry. 2010;53(15):5511–5522. doi: 10.1021/jm1003667. [DOI] [PubMed] [Google Scholar]
- 119.Lopez M., Paul B., Hofmann A., et al. S-glycosyl primary sulfonamides—a new structural class for selective inhibition of cancer-associated carbonic anhydrases. Journal of Medicinal Chemistry. 2009;52(20):6421–6432. doi: 10.1021/jm900914e. [DOI] [PubMed] [Google Scholar]
- 120.Rodríguez O. M., Maresca A., Témpera C. A., Bravo R. D., Colinas P. A., Supuran C. T. N-β-glycosyl sulfamides are selective inhibitors of the cancer associated carbonic anhydrase isoforms IX and XII. Bioorganic and Medicinal Chemistry Letters. 2011;21(15):4447–4450. doi: 10.1016/j.bmcl.2011.06.031. [DOI] [PubMed] [Google Scholar]
- 121.Lopez M., Trajkovic J., Bornaghi L. F., et al. Design, synthesis, and biological evaluation of novel carbohydrate-based sulfamates as carbonic anhydrase inhibitors. Journal of Medicinal Chemistry. 2011;54(5):1481–1489. doi: 10.1021/jm101525j. [DOI] [PubMed] [Google Scholar]
- 122.Woo L. L., Purohit A., Malini B., Reed M. J., Potter B. V. Potent active site-directed inhibition of steroid sulphatase by tricyclic coumarin-based sulphamates. Chemistry & Biology. 2000;7(10):773–791. doi: 10.1016/S1074-5521(00)00023-5. [DOI] [PubMed] [Google Scholar]
- 123.Askoxylakis V., Garcia-Boy R., Rana S., et al. A new peptide ligand for targeting human carbonic anhydrase IX, identified through the phage display technology. PLoS ONE. 2010;5(12) doi: 10.1371/journal.pone.0015962.e15962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Li Y., Wang H., Oosterwijk E., et al. Antibody-specific detection of CAIX in breast and prostate cancers. Biochemical and Biophysical Research Communications. 2009;386(3):488–492. doi: 10.1016/j.bbrc.2009.06.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Dekaminaviciute D., Kairys V., Zilnyte M., et al. Monoclonal antibodies raised against 167-180 aa sequence of human carbonic anhydrase XII inhibit its enzymatic activity. Journal of Enzyme Inhibition and Medicinal Chemistry. 2014 doi: 10.3109/14756366.2013.856424. [DOI] [PubMed] [Google Scholar]
- 126.Carta F., Aggarwal M., Maresca A., Scozzafava A., McKenna R., Supuran C. T. Dithiocarbamates: a new class of carbonic anhydrase inhibitors. Crystallographic and kinetic investigations. Chemical Communications. 2012;48(13):1868–1870. doi: 10.1039/c2cc16395k. [DOI] [PubMed] [Google Scholar]
- 127.Carta F., Aggarwal M., Maresca A., et al. Dithiocarbamates strongly inhibit carbonic anhydrases and show antiglaucoma action in vivo. Journal of Medicinal Chemistry. 2012;55(4):1721–1730. doi: 10.1021/jm300031j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bayram E., Senturk M., Irfan Kufrevioglu O., Supuran C. T. In vitro inhibition of salicylic acid derivatives on human cytosolic carbonic anhydrase isozymes I and II. Bioorganic and Medicinal Chemistry. 2008;16(20):9101–9105. doi: 10.1016/j.bmc.2008.09.028. [DOI] [PubMed] [Google Scholar]
- 129.Carta F., Maresca A., Scozzafava A., Supuran C. T. Novel coumarins and 2-thioxo-coumarins as inhibitors of the tumor-associated carbonic anhydrases IX and XII. Bioorganic and Medicinal Chemistry. 2012;20(7):2266–2273. doi: 10.1016/j.bmc.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 130.Carta F., Vullo D., Maresca A., Scozzafava A., Supuran C. T. New chemotypes acting as isozyme-selective carbonic anhydrase inhibitors with low affinity for the offtarget cytosolic isoform II. Bioorganic and Medicinal Chemistry Letters. 2012;22(6):2182–2185. doi: 10.1016/j.bmcl.2012.01.129. [DOI] [PubMed] [Google Scholar]
- 131.Maresca A., Supuran C. T. Coumarins incorporating hydroxy- and chloro-moieties selectively inhibit the transmembrane, tumor-associated carbonic anhydrase isoforms IX and XII over the cytosolic ones i and II. Bioorganic & Medicinal Chemistry Letters. 2010;20(15):4511–4514. doi: 10.1016/j.bmcl.2010.06.040. [DOI] [PubMed] [Google Scholar]
- 132.Maresca A., Scozzafava A., Supuran C. T. 7,8-Disubstituted- but not 6,7-disubstituted coumarins selectively inhibit the transmembrane, tumor-associated carbonic anhydrase isoforms IX and XII over the cytosolic ones i and II in the low nanomolar/subnanomolar range. Bioorganic and Medicinal Chemistry Letters. 2010;20(24):7255–7258. doi: 10.1016/j.bmcl.2010.10.094. [DOI] [PubMed] [Google Scholar]
- 133.Davis R. A., Vullo D., Maresca A., Supuran C. T., Poulsen S.-A. Natural product coumarins that inhibit human carbonic anhydrases. Bioorganic and Medicinal Chemistry. 2013;21(6):1539–1543. doi: 10.1016/j.bmc.2012.07.021. [DOI] [PubMed] [Google Scholar]
- 134.Carta F., Maresca A., Scozzafava A., Supuran C. T. 5- and 6-Membered (thio)lactones are prodrug type carbonic anhydrase inhibitors. Bioorganic and Medicinal Chemistry Letters. 2012;22(1):267–270. doi: 10.1016/j.bmcl.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 135.Tars K., Vullo D., Kazaks A., et al. Sulfocoumarins (1,2-benzoxathiine-2,2-dioxides): a class of potent and isoform-selective inhibitors of tumor-associated carbonic anhydrases. Journal of Medicinal Chemistry. 2013;56(1):293–300. doi: 10.1021/jm301625s. [DOI] [PubMed] [Google Scholar]
- 136.Sippel K. H., Stander A., Tu C., et al. Characterization of carbonic anhydrase isozyme specific inhibition by sulfamated 2-ethylestra compounds. Letters in Drug Design and Discovery. 2011;8(8):678–684. doi: 10.2174/157018011796576105. [DOI] [Google Scholar]
- 137.Stander B. A., Joubert F., Tu C., Sippel K. H., McKenna R., Joubert A. M. In vitro evaluation of ESE-15-ol, an estradiol analogue with nanomolar antimitotic and carbonic anhydrase inhibitory activity. PLoS ONE. 2012;7(12) doi: 10.1371/journal.pone.0052205.e52205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.de Simone G., Supuran C. T. Carbonic anhydrase IX: biochemical and crystallographic characterization of a novel antitumor target. Biochimica et Biophysica Acta. 2010;1804(2):404–409. doi: 10.1016/j.bbapap.2009.07.027. [DOI] [PubMed] [Google Scholar]
- 139.Závada J., Závadová Z., Pastorek J., Biesovä Z., Ježek J., Velek J. Human tumour-associated cell adhesion protein MN/CA IX: identification of M75 epitope and of the region mediating cell adhesion. British Journal of Cancer. 2000;82(11):1808–1813. doi: 10.1054/bjoc.2000.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Buanne P., Renzone G., Monteleone F., et al. Characterization of carbonic anhydrase IX interactome reveals proteins assisting its nuclear localization in hypoxic cells. Journal of Proteome Research. 2013;12(1):282–292. doi: 10.1021/pr300565w. [DOI] [PubMed] [Google Scholar]
- 141.Duda D. M., Tu C., Fisher S. Z., et al. Human carbonic anhydrase III: structural and kinetic study of catalysis and proton transfer. Biochemistry. 2005;44(30):10046–10053. doi: 10.1021/bi050610h. [DOI] [PubMed] [Google Scholar]
- 142.Casini A., Scozzafava A., Mincione F., Menabuoni L., Ilies M. A., Supuran C. T. Carbonic anhydrase inhibitors: Water-soluble 4-sulfamoylphenylthioureas as topical intraocular pressure-lowering agents with long-lasting effects. Journal of Medicinal Chemistry. 2000;43(25):4884–4892. doi: 10.1021/jm001051+. [DOI] [PubMed] [Google Scholar]
- 143.Scozzafava A., Menabuoni L., Mincione F., Supuran C. T. Carbonic anhydrase inhibitors. A general approach for the preparation of water-soluble sulfonamides incorporating polyamino-polycarboxylate tails and of their metal complexes possessing long-lasting, topical intraocular pressure-lowering properties. Journal of Medicinal Chemistry. 2002;45(7):1466–1476. doi: 10.1021/jm0108202. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Surface rendition of carbonic anhydrase II showing how various inhibitors bind in and around the active site cleft. The “conserved region” (green) and “selective pocket” indicate regions of preferred binding by various carbonic anhydrase inhibitors.