Abstract
Background
Patient education on pharmacological therapy may increase medication adherence and decrease hospitalizations. Our aim is to evaluate the effectiveness of pharmaceutical care at emergency department discharge in patients with hypertension and/or diabetes.
Methods/design
This is a randomized controlled trial. Participants will be recruited from a public emergency department at Restinga district in Porto Alegre, southern Brazil. A total of 380 patients will be randomly assigned into 2 groups at the moment of emergency department discharge after receiving medical orientations: an intervention group, consisting of a structured individual counseling session by a pharmacist in addition to written orientations, or a control group, consisting only of written information about the disease. Outcomes will be assessed in an ambulatory visit 2 months after the randomization. The primary outcome is the proportion of patients with high medication adherence assessed using the Morisky-Green Test and the Brief Medication Questionnaire. The secondary outcomes are reduction of blood pressure, glycated hemoglobin, fasting plasma glucose, quality of life and number of visits to the emergency department.
Discussion
Pharmaceutical care interventions have shown to be feasible and effective in increasing medication adherence in both hospital outpatient and community pharmacy settings. However, there have been no previous assessments of the effectiveness of pharmacy care interventions initiated in patients discharged from emergency departments. Our hypothesis is that pharmaceutical counseling is also effective in this population.
Trial registration
ClinicalTrials.gov registration number: NCT01978925 (11 November 2013) and Brazilian Registry of Clinical Trials U1111-1149-8922 (5 November 2013).
Electronic supplementary material
The online version of this article (doi:10.1186/s13063-015-0579-3) contains supplementary material, which is available to authorized users.
Keywords: Pharmaceutical care, Medication adherence, Blood pressure, Diabetes, Emergency
Background
Cardiovascular disease (CVD) is the leading cause of mortality and burden of disease as measured in disability-adjusted life years (DALYs). Globally, high blood pressure and high blood glucose alone are responsible for 13% and 6% of deaths, respectively [1].
Treatment of chronic diseases commonly includes the long-term use of pharmacotherapy. Despite the efficacy of these treatments, inadequate medication adherence has been reported to approximately 50% of patients. Low adherence reduces the effectiveness of treatment, resulting in suboptimal illness control. As a consequence, an increased use of healthcare resources and reduction in patients’ quality of life has been observed [2-5]. About 20% of hospitalized patients experience adverse events after discharge. However, it is estimated that 60% of these events could be prevented with the proper use of medications [6-9].
Interventions focused on patient education related to pharmacological therapy may increase medication adherence and decrease morbidity [10]. Several interventions for improving adherence have been tested in clinical studies, including motivational and behavior strategies, simplification of dosing regimens, unit dose packaging, educational counseling, refill reminders and self-monitoring [11-16]. A systematic review of studies assessing interventions to improve medication adherence has shown an overall increase of 4 to 11%; however, no single strategy appeared to be superior [9].
Pharmaceutical care interventions for improving medication adherence seem to be feasible and effective, both in hospital and in community pharmacy settings [17,18]. Clinical pharmacists are able to identify and intervene to prevent potential problems with prescriptions written prior to discharge from the hospital, and studies have suggested that discharge counseling is able to improve medication adherence [19]. However, there is no evidence in the literature regarding the effectiveness of pharmaceutical care interventions initiated in patients discharged from emergency departaments (ED). In addition, few studies have assessed the impact of these interventions in developing countries, and the effect of these interventions in populations with lower income and educational level is uncertain.
The aim of this study is to compare pharmaceutical care at discharge from the ED with usual care in the adherence to medication prescriptions and in the control of hypertension and/or diabetes.
Methods/design
Study design
This is a randomized controlled, single-center study, with blinding of outcome assessors. A pilot study with ten patients was previously conducted in order to test study logistics and data collection instruments. Participants will be recruited from a public ED at Restinga district in Porto Alegre, southern Brazil. Porto Alegre is the 10th largest city in Brazil, with 1.5 million inhabitants. Restinga is a low-income district of Porto Alegre, with approximately 100,000 people and a Human Development Index (HDI) of 0.700 to 0.799, whereas Porto Alegre HDI ranges from 0.700 to 0.977 [20]. This ED is the single reference emergency service of the southern region of Porto Alegre, with 8,000 visits monthly. Eligible participants will be randomized to either the intervention or the control group, and will complete a follow-up interview after a period of 2 months (Figure 1).
Figure 1.
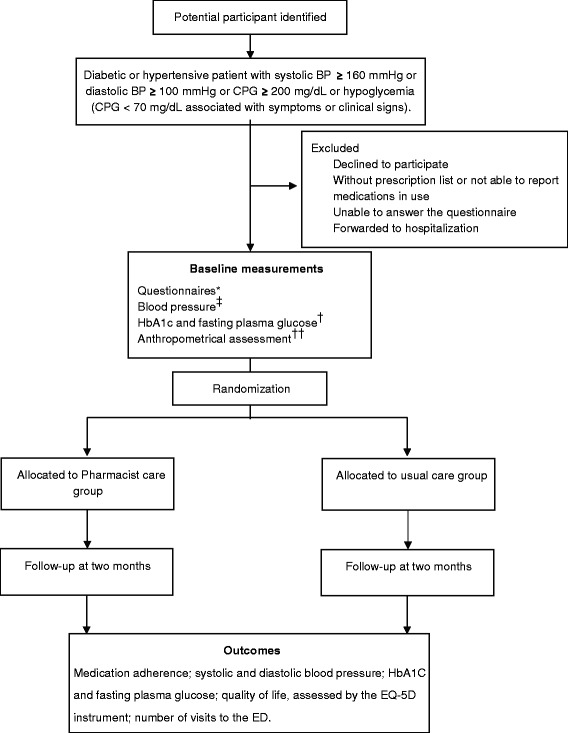
Flow diagram of the study design. *Questionnaires: sociodemographic variables; comorbidities; medication adherence; physical activity; alcohol consumption. ‡For patients with hypertension. †For patients with diabetes. ††Anthropometrical assessment: weight, height, waist and hip circumferences. BP = blood pressure; CPG = capillary plasma glucose; HbA1c = glycated hemoglobin.
Inclusion and exclusion criteria
All patients with ED discharge from 8 am to 6 pm, on weekdays, will be screened for eligibility.
Inclusion criteria
age ≥ 18 years;
previous diagnosis and drug treatment for hypertension and/or diabetes;
inadequate blood pressure (BP) control (systolic BP ≥ 160 mmHg or diastolic BP ≥ 100 mmHg), inadequate diabetes control (capillary plasma glucose ≥ 200 mg/dL) or moderate/severe hypoglycemia (capillary plasma glucose < 70 mg/dL associated with symptoms or clinical signs).
Exclusion criteria
patients without a prescription list or not able to report medications in use;
patients not residing in the city of Porto Alegre;
patients unable to answer the questionnaire or to sign the informed consent form;
patients forwarded to hospitalization.
Study conduct
Eligible participants will be identified through active screening by a research assistant, reviewing medical notes of admitted patients. The research assistant will describe the project to each potential participant, will provide a straightforward language statement and will invite them to participate in the study. If the participant accepts, written informed consent will be sought before baseline interview and physical examination. All participants will be referred to the study pharmacist for randomization after baseline data collection.
Baseline measures
Data collection at baseline includes measurement of BP (for patients with hypertension), glycated hemoglobin (HbA1c) and fasting plasma glucose (for patients with diabetes). In addition, medication in use, socio-demographic, comorbidity and anthropometrical variables (for example, gender, age, ethnicity, marital and smoking status, alcohol consumption, physical activity, circumferences and body mass index) will be recorded. The medical prescription at discharge will also be documented.
Randomization and participants allocation
Following baseline data collection, eligible participants will be randomized to either the intervention or the control group in a 1:1 ratio, using a telephonic central for allocation concealment. The randomization sequence will be generated by using a computer program in blocks of four, six and eight. Randomization will be stratified according to the number of different medications in use (<5 or ≥ 5).
Pharmaceutical care group
In the ED, immediately after discharge, participants randomized to the pharmaceutical care group will receive intervention coordinated by the study pharmacist. The clinical pharmacist will provide a structured 30-minute intervention for enhancing their medication adherence.
The recommendations include: discussion on hypertension and/or diabetes, risk of complications, prescribed drug therapy, correct use of medications and proper dosage, possible adverse effects, route of administration, schedule of administration and correct storage. The pharmacist will also emphasize the importance of lifestyle modifications. Printed educational material, with information on hypertension and/or diabetes medications, including suggested lifestyle interventions (for example, reduce salt and sugar intake, practice regular physical activity, smoking cessation, reducing alcohol consumption, monitor stress levels in day-to-day and reduce weight and keep it within the normal range) was prepared to assist in the intervention and will be handed to patients in the end of the session.
Control group
In addition to counseling provided by a physician and by the nursing staff during their stay in the ED (usual care), patients randomized to the control group will receive the same printed material information on hypertension and/or diabetes medications and lifestyle interventions in order to keep patients masked.
Follow-up visit
An ambulatory follow-up visit will occur 2 months after randomization for the assessment of the study’s outcomes. We will contact participants by phone prior to the follow-up visit in order to minimize losses; if necessary, home visits will be performed for those without conditions to attend the follow-up visit in the clinic. Data will be collected by a research assistant who is unaware of the randomization status.
Primary endpoint
The primary outcome is the proportion of patients with high medication adherence at 2 months. Medication adherence will be assessed subjectively using two validated questionnaires in the Portuguese language: the Morisky-Green Test (MGT) and Brief Medication Questionnaire (BMQ) [21,22]. The MGT assesses both intentional and unintentional nonadherence and comprises four items. Responses for each item are scored one for ‘yes’ and zero for ‘no’ and are added together. A total score of zero represents good adherence and a score of one or more represents suboptimal adherence. The BMQ is composed of eleven questions, divided into three domains (regime, beliefs and memory).
Secondary endpoints
The secondary outcomes are:
Sample size
The sample size was estimated assuming a percentage of 50% nonadherence to the medication treatment in the control group [25]. To detect a 15% absolute increase in the medication adherence in the intervention group, a sample size of 190 participants will be needed in each group, considering an alpha error of 5% (bilateral), statistical power of 80% and 10% of losses to follow-up.
One of the researchers of the study will be responsible for quality control of the collected information. The participants will be randomly selected to answer a simplified questionnaire containing key issues by telephone. This control will be performed in 10% of the sample.
Data analysis
The questionnaires will be entered into the database through processing by optical reader, using the Remark Office OMR® Software (version 8.0 Gravic, Inc., Malvern, PA) . All analyses will follow the intention-to-treat (ITT) principle. For the primary outcome, we will initially consider in the analysis patients with complete follow-up. A secondary analysis will be performed with data of all patients initially randomized, considering, as the worst plausible scenario, that 29% of the losses to follow-up, in both groups, are adherent. The worst plausible scenario was based on a previous observational study, conducted in 2012 in the same region, showing that 34.5% (95% confidence intervals (CI), 29% to 40%) of patients with hypertension assisted in basic health units are adherent according to the same instrument [26]. We will not use any imputation method for continuous outcomes, such as BP and blood glucose. For these outcomes, we will include only those participants who attended the follow-up visit.
Data will be presented as relative risk (RR) and mean difference (MD), with 95% CI. Categorical variables will be compared using the chi-square test; continuous variables will be compared using the Student t-test or the Mann–Whitney U-test. The analysis will be conducted in SPSS for Windows (version 19.0, Redmond, WA, USA) and Stata (version 10, College Station, TX, USA); the statistician will be blinded for the randomization status.
Ethics
This study has been approved by the Institutional Review Board of Hospital Moinhos de Vento of Porto Alegre (approval number 403.658). Written informed consent will be obtained from each participant at the time of enrolment. The trial has also been registered in the Clinical Trial Registry NCT01978925 and in the Brazilian Registry of Clinical Trials U1111-1149-8922, and according to the Brazilian Ethics in Human Research Regulations.
Discussion
We present a study protocol for a randomized controlled trial to investigate the impact of pharmaceutical care in patients with chronic conditions at discharge from an ED. The study will be conducted in a setting with social vulnerability in a developing country. We already knew that at least half of the people in treatment for hypertension and/or diabetes in the same community do not adhere adequately to medications. As consequence, many would develop undesirable outcomes, such as clinical complications and increase in the use of health services resources that could be avoided with adequate adherence to the medical prescription. The moment of the discharge from the ED may constitute an important opportunity for medication reconciliation, reviewing and providing information related to the medications of continuous use [27,28]. In this context, we are proposing an intervention focused on pharmaceutical care at emergency discharge in order to enhance adequate medication use. Our hypothesis is that patients receiving pharmaceutical orientation in the intervention group will present better adherence to pharmacological therapy, resulting in better control of BP and glycemic profile in our study population.
Several studies have shown that pharmaceutical care in the treatment of patients with hypertension and diabetes has been associated with significant reductions in hospitalizations, visits to the ED and increase in medication adherence [3,9,17-19]. These studies were conducted mainly in hospital and ambulatory settings in developed countries. To our knowledge, this study is the first designed to evaluate the effectiveness of pharmaceutical care interventions in patients discharged from a public ED. With this randomized controlled trial we expect to provide evidence regarding the effectiveness of pharmaceutical care for chronic conditions at ED discharge. As consequence, these results may be useful for policy -making related to the development and implementation of pharmaceutical care programs in emergency services.
Trial status
The trial is currently in the recruitment phase.
Acknowledgements
This study is supported by Institutional Development Program of the Brazilian National Health System (PROADI-SUS). Development Project Technical Operation and Management of Health Services in an intramunicipal Region of Porto Alegre, RS - Districts Restinga and extreme south, according signed by the Ministry of Health with the Hospital Moinhos de Vento, through the adjustment term number 05/2011, signed on 30 December 2011.
Abbreviations
- BMQ
Brief Medication Questionnaire
- BP
blood pressure
- CI
confidence interval
- CVD
cardiovascular disease
- DALY
disability-adjusted life year
- ED
emergency department
- EQ-5D
EuroQol five-dimension instrument
- HbA1c
glycated hemoglobin
- HDI
Human Development Index
- ITT
intention-to-treat
- MD
mean difference
- MGT
Morisky-Green Test
- RA
research assistant
- RR
relative risk
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
RAR and GANB conceived the study. RKN, KML, RAR, LSH, GANB, CAP, GAFSR, SC, CMG and MF participated in the study design. LEAL and TSD will include and collect clinical data of the patients. RKN, CMG, MF and SC developed the study intervention. CMG will deliver the proposed intervention. RKN, KML, RAR, MCSCS and MF will be responsible for data management and analysis. RKN and MF are the study coordinators and RAR is the principal investigator. RKN and MF wrote the manuscript. All authors reviewed and approved the final manuscript.
Contributor Information
Regina Kuhmmer, Email: regina.kuhmmer@hmv.org.br.
Karine Margarites Lima, Email: karine.lima@hmv.org.br.
Rodrigo Antonini Ribeiro, Email: rodrigo.ribeiro@hmv.org.br.
Luciano Serpa Hammes, Email: luciano.hammes@hmv.org.br.
Gisele Alsina Nader Bastos, Email: gisele.nader@hmv.org.br.
Maria Claudia Schardosim Cotta de Souza, Email: maria.souza@hmv.org.br.
Carisi Anne Polanczyk, Email: carisi.polanczyk@hmv.org.br.
Guilherme Alcides Flores Soares Rollin, Email: guilhermerollin@ibest.com.br.
Suhelen Caon, Email: suhelen.caon@hmv.org.br.
Cátia Moreira Guterres, Email: catiamoreiraguterres@gmail.com.
Leni Everson Araújo Leite, Email: leniaraujoleite@yahoo.com.br.
Tássia Scholante Delabary, Email: tatss.delabary@gmail.com.
Maicon Falavigna, Email: maicon.falavigna@hmv.org.br.
References
- 1.WHO . Global health risks: mortality and burden of disease attributable to selected major risks. Geneva: World Health Organization; 2009. pp. 1–170. [Google Scholar]
- 2.Melloni C, Alexander KP, Ou FS, LaPointe NM, Roe MT, Newby LK, et al. Predictors of early discontinuation of evidence-based medicine after acute coronary syndrome. Am J Cardiol. 2009;104(2):175–81. doi: 10.1016/j.amjcard.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 3.Sokol MC, McGuigan KA, Verbrugge RR, Epstein RS. Impact of medication adherence on hospitalization risk and healthcare cost. Med Care. 2005;43(6):521–30. doi: 10.1097/01.mlr.0000163641.86870.af. [DOI] [PubMed] [Google Scholar]
- 4.Bitton A, Choudhry NK, Matlin OS, Swanton K, Shrank WH. The impact of medication adherence on coronary artery disease costs and outcomes: a systematic review. Am J Med. 2013;126(4):357.e7–357.e27. doi: 10.1016/j.amjmed.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Iuga AO, McGuire MJ. Adherence and health care costs. Risk Manag Healthc Policy. 2014;7:35–44. doi: 10.2147/RMHP.S19801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Forster AJ, Clark HD, Menard A, Dupuis N, Chernish R, Chandok N, et al. Adverse events among medical patients after discharge from hospital. CMAJ. 2004;170(3):345–9. [PMC free article] [PubMed] [Google Scholar]
- 7.Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161–7. doi: 10.7326/0003-4819-138-3-200302040-00007. [DOI] [PubMed] [Google Scholar]
- 8.Pirmohamed M, James S, Meakin S, Green C, Scott AK, Walley TJ, et al. Adverse drug reactions as cause of admission to hospital: prospective analysis of 18 820 patients. BMJ. 2004;329(7456):15–9. doi: 10.1136/bmj.329.7456.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peterson AM, Takiya L, Finley R. Meta-analysis of trials of interventions to improve medication adherence. Am J Health Syst Pharm. 2003;60(7):657–65. doi: 10.1093/ajhp/60.7.657. [DOI] [PubMed] [Google Scholar]
- 10.Christensen M, Lundh A. Medication review in hospitalised patients to reduce morbidity and mortality. Cochrane Database Syst Rev. 2013;2 doi: 10.1002/14651858.CD008986.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schroeder K, Fahey T, Ebrahim S. Interventions for improving adherence to treatment in patients with high blood pressure in ambulatory settings. Cochrane Database Syst Rev. 2004;2 doi: 10.1002/14651858.CD004804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Skaer TL, Sclar DA, Markowski DJ, Won JK. Effect of value-added utilities on prescription refill compliance and health care expenditures for hypertension. J Hum Hypertens. 1993;7(5):515–8. [PubMed] [Google Scholar]
- 13.Márquez-Contreras E, Martell-Claros N, Gil-Guillén V, de la Figuera-Von WM, Casado-Martínez JJ, Martin-de Pablos JL, et al. Efficacy of a home blood pressure monitoring programme on therapeutic compliance in hypertension: the EAPACUM-HTA study. J Hypertens. 2006;24(1):169–75. doi: 10.1097/01.hjh.0000198023.53859.a2. [DOI] [PubMed] [Google Scholar]
- 14.Haynes RB, Yao X, Degani A, Kripalani S, Garg A, McDonald HP. Interventions to enhance medication adherence. Cochrane Database Syst Rev. 2005;4 doi: 10.1002/14651858.CD000011.pub2. [DOI] [PubMed] [Google Scholar]
- 15.Vervloet M, Linn AJ, van Weert JC, de Bakker DH, Bouvy ML, van Dijk L. The effectiveness of interventions using electronic reminders to improve adherence to chronic medication: a systematic review of the literature. J Am Med Inform Assoc. 2012;19(5):696–704. doi: 10.1136/amiajnl-2011-000748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gwadry-Sridhar FH, Manias E, Lal L, Salas M, Hughes DA, Ratzki-Leewing A, et al. Impact of interventions on medication adherence and blood pressure control in patients with essential hypertension: a systematic review by the ISPOR medication adherence and persistence special interest group. Value Health. 2013;16(5):863–71. doi: 10.1016/j.jval.2013.03.1631. [DOI] [PubMed] [Google Scholar]
- 17.McDonald HP, Garg AX, Haynes RB. Interventions to enhance patient adherence to medication prescriptions: scientific review. JAMA. 2002;288(22):2868–79. doi: 10.1001/jama.288.22.2868. [DOI] [PubMed] [Google Scholar]
- 18.Kaboli PJ, Hoth AB, McClimon BJ, Schnipper JL. Clinical pharmacists and inpatient medical care: a systematic review. Arch Intern Med. 2006;166(9):955–64. doi: 10.1001/archinte.166.9.955. [DOI] [PubMed] [Google Scholar]
- 19.Sarangarm P, London MS, Snowden SS, Dilworth TJ, Koselke LR, Sanchez CO, et al. Impact of pharmacist discharge medication therapy counseling and disease state education: Pharmacist Assisting at Routine Medical Discharge (project PhARMD) Am J Med Qual. 2013;28(4):292–300. doi: 10.1177/1062860612461169. [DOI] [PubMed] [Google Scholar]
- 20.Atlas of human development in the metropolitan region of Porto Alegre. Porto Alegre: City Hall /Secretariat for Political Coordination and Local Governance. Metroplan; PNUD; Foundation João Pinheiro, 2008, 32. Available in: http://www.pnud.org.br/publicacoes/atlas_portoalegre/LivroAtlasRMPA.pdf
- 21.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24(1):67–74. doi: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Ben AJ, Neumann CR, Mengue SS. The brief medication questionnaire and Morisky-Green test to evaluate medication adherence. Rev Saude Publica. 2012;46(2):279–89. doi: 10.1590/S0034-89102012005000013. [DOI] [PubMed] [Google Scholar]
- 23.Ferreira PL, Ferreira LN, Pereira LN. Contribution for the validation of the Portuguese version of EQ-5D. Acta Med Port. 2013;26(6):664–75. [PubMed] [Google Scholar]
- 24.Group E. EuroQol - a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 25.World Health Organization. Adherence to long-term therapies: evidence for action. World Health Organization; 2003: 1–194. Available in: http://whqlibdoc.who.int/publications/2003/9241545992.pdf
- 26.Notti RK. Effectiveness of multidisciplinary intervention on blood pressure control in primary health care [PhD thesis]. Federal University of Rio Grande do Sul, Faculty of Medicine; 2014.
- 27.Eisenhower C. Impact of pharmacist-conducted medication reconciliation at discharge on readmissions of elderly patients with COPD. Ann Pharmacother. 2014;48(2):203–8. doi: 10.1177/1060028013512277. [DOI] [PubMed] [Google Scholar]
- 28.VanSuch M, Naessens JM, Stroebel RJ, Huddleston JM, Williams AR. Effect of discharge instructions on readmission of hospitalised patients with heart failure: do all of the Joint Commission on Accreditation of Healthcare Organizations heart failure core measures reflect better care? Qual Saf Health Care. 2006;15(6):414–7. doi: 10.1136/qshc.2005.017640. [DOI] [PMC free article] [PubMed] [Google Scholar]


