Abstract
ES-62 is an anti-inflammatory phosphorylcholine-containing glycoprotein secreted by the filarial nematode Acanthocheilonema viteae. Accelerated atherosclerosis frequently occurs in systemic lupus erythematosus (SLE), resulting in substantial cardiovascular morbidity and mortality. We examined the effects of ES-62 in the gld.apoE−/− mouse model of this condition. Treatment with ES-62 did not substantially modulate renal pathology but caused decreased anti-nuclear autoantibody levels. Moreover, a striking 60% reduction in aortic atherosclerotic lesions was observed, with an associated decrease in macrophages and fibrosis. We believe that these latter findings constitute the first example of a defined parasitic worm product with therapeutic potential in atherosclerosis: ES-62-based drugs may represent a novel approach to control accelerated atherosclerosis in SLE.
Keywords: Atherosclerosis, ES-62, Helminth, Systemic lupus erythematosus
In human populations, parasitic worm infection is linked to a decreased incidence of diseases associated with aberrant inflammation such as autoimmune conditions. For example, an inverse relationship has recently been observed between filarial nematode infection and both type I diabetes (Aravindhan et al., 2010) and rheumatoid arthritis (Panda et al., 2013). ES-62 is a protein secreted by the filarial nematode Acanthocheilonema viteae during parasitism of rodents (Harnett et al., 2003). Structural analysis of the molecule revealed the attachment of phosphorylcholine (PC) to an N-type glycan, a post-translational modification not previously described (Houston and Harnett, 2004). The PC moiety confers a wide range of immunomodulatory properties on ES-62, which are broadly anti-inflammatory in nature (Harnett et al., 2003; Pineda et al., 2014). As a consequence, ES-62 is able to inhibit inflammatory responses and thus protect against the development of disease in mouse models of arthritis and asthma (McInnes et al., 2003; Melendez et al., 2007).
Patients with systemic lupus erythematosus (SLE) and other autoimmune conditions such as rheumatoid arthritis are at higher risk of developing accelerated atherosclerosis with a consequent increase in morbidity and mortality from cardiovascular disease (Nikpour et al., 2005; Skaggs et al., 2012; Choy et al., 2014). Atherosclerosis is the underlying cause of most cardiovascular disease, accounting for the majority of deaths in the Western world. It is a disorder in which intimal thickening and lipid deposition occur in the elastic arteries such as the aorta and places of turbid flow, as well as in the larger arteries such as the coronary arteries (Ross, 1993). The apoE−/− mouse is a well-established mouse model that has spontaneous hypercholesterolemia and is susceptible to atherosclerotic lesion formation (Plump et al., 1992).
The traditional Framingham risk factors for atherosclerosis in the general population include hypertension, hypercholesterolemia, diabetes mellitus and smoking. However, after controlling for differences in traditional Framingham risk factors, the contribution of lupus-specific risk factors still demonstrate increased relative risk for fatal myocardial infarction and stroke in SLE patients (Manzi et al., 1997; Esdaile et al., 2001). Increased proteinuria and serum creatinine, elevated triglycerides, anti-phospholipid antibodies and pro-inflammatory high density lipoprotein (HDL) are a few lupus-associated factors that may contribute to cardiovascular disease in lupus patients (Bessant et al., 2006; Skaggs et al., 2012). However, larger cohort studies are needed to determine the actual risk factors. The exact causative mechanism of these lupus-specific risk factors remains poorly understood although dysregulated inflammation is thought to play a contributory role. Given the well-documented range of anti-inflammatory properties of ES-62, we therefore investigated whether it had any protective activity in the gld.apoE−/− mouse model of lupus and lupus-associated atherosclerosis.
Gld.apoE−/− mice were generated as described previously (Aprahamian et al., 2004) and male animals maintained on a high cholesterol Western diet (Harlan-Teklad Research Diet, TD.88137, Harlan Laboratories, Madison, Wisconsin, USA), starting at 7 weeks of age, for 12 weeks. Highly purified, endotoxin-free ES-62 was prepared as described previously (McInnes et al., 2003) and administered using Alzet osmotic pumps at a rate of 0.2 μg/h. We previously showed that this release rate provides a serum concentration equivalent to that found for PC-containing molecules during filarial nematode infection of humans (Wilson et al., 2003). Control animals received PBS (termed vehicle). Pumps were removed and replaced every 3 weeks to supply treatment for the 12 week duration of the study. The experiments were approved by the Institutional Animal Care and Use Committee of Boston University, Massachusetts, USA.
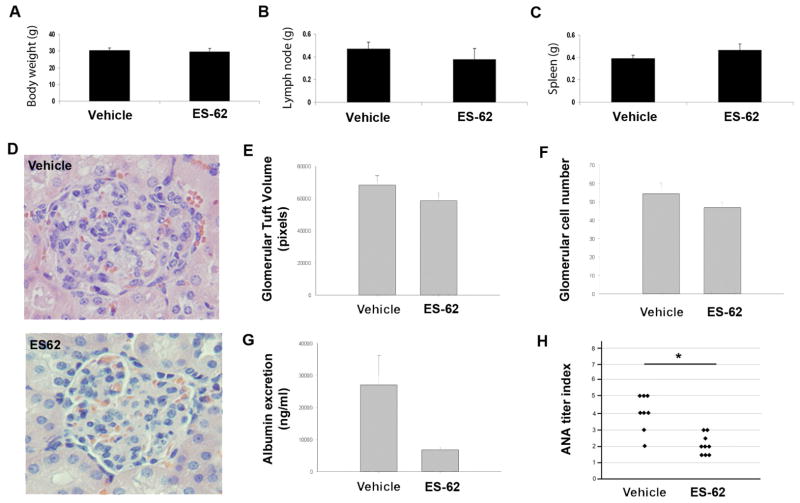
Hallmarks of the gld.apoE−/− phenotype are pronounced enlargement of lymph nodes and spleen, proteinuria, enlarged glomeruli and renal tubular vacuolization (Aprahamian et al., 2004). After 12 weeks of ES-62 treatment, the mice were euthanized and the final body and organ weights were recorded, with no significant change in body weight (Fig. 1A), lymphadenopathy of submandibular lymph nodes (Fig. 1B) or splenomegaly (Fig. 1C) being observed. However, we found that ES-62 treatment tended to reduce renal disease as measured by glomerular tuft volume and glomerular cell count (Fig. 1D – F), and urine albumin levels (Fig. 1G), while significantly decreasing circulating levels of anti-nuclear antibodies (ANA) (Fig. 1H), suggesting overall that the nematode product may exhibit some protection against lupus pathology in this mouse model.
Fig. 1.
Effect of Acanthocheilonema viteae ES-62 on body weight and autoimmune characteristics in gld.apoE−/− mice. Twelve weeks after the start of treatment (A) body weight was measured and (B) submandibular lymph nodes and (C) spleens were harvested and weighed. (D) Representative H&E-stained sections of kidney. (E) Glomerular tuft size and (F) cell numbers were measured by computer-assisted pixel counting and presented as the mean values ± S.E.M. of individual mice. At least 25 glomeruli were measured from H&E-stained kidney sections from each animal as previously described (Aprahamian et al., 2004). (G) Forty-eight hours prior to euthanasia, urine (albumin) samples were obtained using metabolic cages and measured using a protein assay (Bio-Rad Laboratories, USA) according to the manufacturer’s instructions. (H) Circulating anti-nuclear antibodies (ANA) were measured by immunofluorescence using HEp-2 coated slides (The Binding Site Inc., San Diego, California, USA). Slides were incubated for 1 h with serial log-scale dilutions (1:100 to 1:90,000) of mouse serum, washed in PBS, and then incubated with FITC-labeled goat anti-mouse IgG (whole molecule; Sigma-Aldrich, St. Louis, Missouri, USA). Slides were viewed using fluorescent microscopy and scored using the value of the last positive dilution (*, P <0.05) where the data represent the titers for individual mice (vehicle, n = 8; ES-62, n = 9).
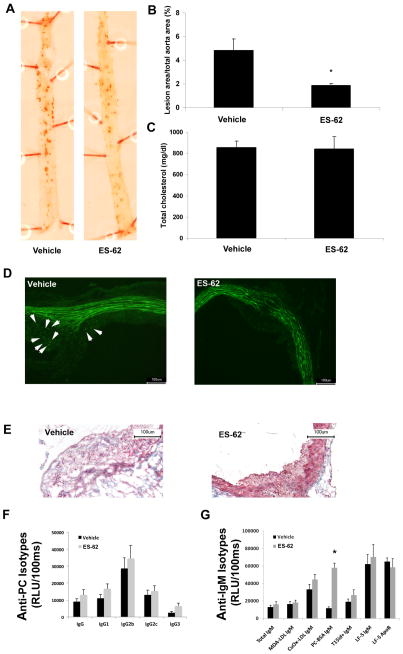
Aortic atherosclerosis was analyzed with the use of Oil Red O staining as previously described (Aprahamian et al., 2004). Examination of dissected aortae revealed a significant reduction in percentage of atherosclerotic lesion area by nearly 60% in the ES-62-treated mice compared with vehicle: 1.79 ± 0.38 and 4.77 ± 2.55, respectively (Fig. 2A, B). The levels of total serum cholesterol were similar to those previously reported in the gld.apoE−/− mouse (Aprahamian et al., 2004) and did not change with ES-62 treatment (Fig. 2C). The extent of inflammation in the atherosclerotic lesion was subsequently evaluated by performing immunohistochemical analysis to determine macrophage presence whereupon it was found that lesions from vehicle-treated mice displayed increased amounts of macrophage staining compared with those of ES-62 treated animals (Fig. 2D). In addition, further analysis of lesion composition demonstrated decreased levels of collagen in lesions of mice that received ES-62 compared with PBS (Fig. 2E).
Fig. 2.
Acanthocheilonema viteae ES-62 treatment decreases atherosclerosis in gld.apoE−/− mice and not through induction of protective cross-reactive anti-phosphorylcholine (PC) antibodies. (A) Representative photographs and (B) quantification of Oil Red O-stained aortae from mice maintained on high cholesterol Harlan-Teklad Research Western diet for 12 weeks and treated with ES-62 or vehicle (*, P <0.01). (C) Quantification of total serum cholesterol, determined by a microtiter procedure according to the manufacturer’s instructions (Wako Diagnostics, Japan). Representative images of immunohistochemical analysis of atherosclerotic lesion by (D) F4/80 staining for macrophages (Caltag Laboratories, United Kingdom) and (E) trichrome staining for collagen shown within the lesion in blue. (F,G) Microtiter wells were coated with either antigens (malondialdehyde oxidized low density lipoprotein (MDA-LDL), copper oxidized LDL (CuOx-LDL), PC-BSA) or antibodies (AB1-2) at 2–5 μg/mL and serum antibodies to respective antigens were determined at different dilutions as described previously (Chang et al., 1999) (F) Serum levels of IgG isotypes binding to PC-BSA were determined by chemiluminescent ELISA. (G) IgM levels to indicated antigens as well as T15id+ IgM immune complexes were measured. Plasma levels of T15id+ natural IgM antibodies (EO6) were determined using the anti-T15-idiotypic monoclonal antibody AB1-2 (a mouse IgG1, which is absolutely specific for both the canonical T15 VH and the T15 VL regions) for capture, followed by detection steps using an anti-mouse IgM antibody. LF5 is a monoclonal antibody against mouse Apolipoprotein B (ApoB) and was used to capture ApoB containing particles on an ELISA plate. It was a kind gift from Stephen Young (University of California-Los Angeles, USA). IgM associated with LF5-ApoB particles was measured using anti-mouse IgM to detect IgM-ApoB immune complexes. In parallel wells, the amount of captured ApoB particles was measured using a commercial goat-anti ApoB to determine whether equal amounts of ApoB were captured in the assay. Values are given as relative light units (RLU) per 100 ms and represent the mean of triplicate determinations. Data are shown as mean ± S.E.M. values of all mice in each group (vehicle, n = 8; ES-62-treated, n = 9). The significant differences were assessed by a Mann Whitney test. P < 0.05 was considered significant.
Although the striking reduction in atherosclerotic lesion area was consistent with the well-characterized anti-inflammatory activity of ES-62, as the nematode product contains PC we decided to examine whether an anti-PC antibody response had been elicited, as recent data indicate that certain anti-PC antibodies can protect against atherosclerosis. For example, immunization of mice with Streptococcus pneumoniae, which contains PC components, induces IgM anti-PC antibodies of the T15 idiotype (Chang et al., 1999), which are atheroprotective (Binder et al., 2003). The observed cross-protection probably reflects the fact that PC is a major component of low density lipoprotein (LDL), and there is increasing evidence that antibodies against oxidized LDL (OxLDL) appear to be protective in atherosclerosis (Su et al., 2006). While not fully understood, it has been suggested that by binding to OxLDL, the antibodies could prevent lipoprotein uptake by scavenger receptors on macrophages, thereby preventing foam cell formation (Binder et al., 2007).
No significant increase in anti-PC reactivity was seen in ES-62-treated mice with respect to any IgG isotype (Fig. 2F), although an increase was observed with respect to IgM anti-PC antibodies (Fig. 2G). However, no significant increase was seen with respect to anti-malondialdehyde oxidized (MDA)-LDL IgM, anti-copper oxidized (CuOx)-LDL IgM, or T15/E06 idiotypic IgM antibodies in sera derived from ES-62-treated mice (Fig. 2G). This is consistent with a report demonstrating that different classes of anti-PC antibodies exhibit varying degrees of binding to OxLDL (Shaw et al. 2003). Our data therefore suggest that the protective effects of ES-62 are not due to T15/OxLDL cross-reactive antibodies.
Here, we demonstrate that ES-62 protects against atherosclerosis, as observed by a significant 60% decrease in lesion area with no effect on cholesterol levels. We have previously noted protection in this model with simvastatin, due to its immunomodulatory properties, correlating with a shift from a Th1 (pro-inflammatory) to a Th2 (anti-inflammatory) immunological phenotype (Aprahamian et al., 2006). Similarly, ES-62 has previously been shown to inhibit Th1 cytokine production (IFNγ) in the mouse collagen-induced arthritis model (McInnes et al., 2003). Moreover, atherogenic lesions contain many immune cells including macrophages and dendritic cells (Binder et al., 2007) and while we have previously established that ES-62 directly targets these cell types, modulating the immune response towards an anti-inflammatory phenotype (Harnett et al., 2003; Harnett and Harnett, 2006), we also demonstrate here that ES-62 reduces the number of macrophages within the atherosclerotic lesion. Taken together, this suggests that ES-62 is protective in this mouse model of atherosclerosis, by generation of an anti-inflammatory environment that includes targeting of macrophages rather than reducing cholesterol levels or generating cross-protective anti-PC antibodies.
To a specialized patient population, such as those with SLE or other autoimmune diseases susceptible to accelerated atherosclerosis, the immunomodulatory anti-inflammatory effects of a helminth-derived molecule could be beneficial in limiting disease severity or progression. The potentially protective effects of parasitic helminths have been suspected for years and indeed a decrease in plaque size has been observed when using live worms (Doenhoff et al., 2002) or undefined, multi-component soluble egg antigen extract from the parasitic platyhelminth Schistosoma mansoni (Stanley et al., 2009). Here, for the first known time, we report decreased atherosclerotic lesion area when focusing on a member of the nematode phylum and, unlike the other studies referred to, a single, purified, highly characterized molecule has been employed. We thus believe that ES-62 now merits additional exploration in terms of further elucidating the mechanism by which it protects in this model (as mentioned earlier, ES-62 has a wide range of anti-inflammatory activities) as the next step in a novel approach to drug development for atherosclerosis.
Highlights.
ES-62 is the first known defined parasitic worm product to protect against atherosclerosis
ES-62 may exhibit protection against lupus pathology
Drugs based on ES-62 could be a novel approach to control atherosclerosis in systemic lupus erythematosus
Acknowledgments
The authors thank Maria Ozsvar Kozma for excellent technical assistance. This work was supported by grants from the Wellcome Trust, UK (086852 to W and MM Harnett) the National Institutes of Health (NIH), USA (PO1 R050256 to I.R.R.), by K01 AR055965-02 from US National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS)/NIH to T.A., and R21 AR063387-01 from NIAMS/NIH to X.Z.
Footnotes
Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIAMS or NIH.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aprahamian T, Bonegio R, Rizzo J, Perlman H, Lefer DJ, Rifkin IR, Walsh K. Simvastatin treatment ameliorates autoimmune disease associated with accelerated atherosclerosis in a murine lupus model. J Immunol. 2006;177:3028–3034. doi: 10.4049/jimmunol.177.5.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aprahamian T, Rifkin I, Bonegio R, Hugel B, Freyssinet JM, Sato K, Castellot JJ, Jr, Walsh K. Impaired clearance of apoptotic cells promotes synergy between atherogenesis and autoimmune disease. J Exp Med. 2004;199:1121–1131. doi: 10.1084/jem.20031557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aravindhan V, Mohan V, Surendar J, Rao MM, Ranjani H, Kumaraswami V, Nutman TB, Babu S. Decreased prevalence of lymphatic filariasis among subjects with type-1 diabetes. Am J Trop Med Hyg. 2010;83:1336–1339. doi: 10.4269/ajtmh.2010.10-0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessant R, Duncan R, Ambler G, Swanton J, Isenberg DA, Gordon C, Rahman A. Prevalence of conventional and lupus-specific risk factors for cardiovascular disease in patients with systemic lupus erythematosus: A case-control study. Arthritis Rheum. 2006;55:892–899. doi: 10.1002/art.22343. [DOI] [PubMed] [Google Scholar]
- Binder CJ, Hartvigsen K, Witztum JL. Promise of immune modulation to inhibit atherogenesis. J Am Coll Cardiol. 2007;50:547–550. doi: 10.1016/j.jacc.2007.04.054. [DOI] [PubMed] [Google Scholar]
- Binder CJ, Horkko S, Dewan A, Chang MK, Kieu EP, Goodyear CS, Shaw PX, Palinski W, Witztum JL, Silverman GJ. Pneumococcal vaccination decreases atherosclerotic lesion formation: molecular mimicry between Streptococcus pneumoniae and oxidized LDL. Nat Med. 2003;9:736–743. doi: 10.1038/nm876. [DOI] [PubMed] [Google Scholar]
- Chang MK, Bergmark C, Laurila A, Horkko S, Han KH, Friedman P, Dennis EA, Witztum JL. Monoclonal antibodies against oxidized low-density lipoprotein bind to apoptotic cells and inhibit their phagocytosis by elicited macrophages: evidence that oxidation-specific epitopes mediate macrophage recognition. Proc Natl Acad Sci U S A. 1999;96:6353–6358. doi: 10.1073/pnas.96.11.6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy E, Ganeshalingam K, Semb AG, Szekanecz Z, Nurmohamed M. Cardiovascular risk in rheumatoid arthritis: recent advance in the understanding of the pivotal role of inflammation, risk predictors, and the impact of treatment. Rheumatology. 2014;53:2143–54. doi: 10.1093/rheumatology/keu224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doenhoff MJ, Stanley RG, Griffiths K, Jackson CL. An anti-atherogenic effect of Schistosoma mansoni infections in mice associated with a parasite-induced lowering of blood total cholesterol. Parasitology. 2002;125:415–421. doi: 10.1017/s0031182002002275. [DOI] [PubMed] [Google Scholar]
- Esdaile JM, Abrahamowicz M, Grodzicky T, Li Y, Panaritis C, du Berger R, Cote R, Grover SA, Fortin PR, Clarke AE, Senecal JL. Traditional Framingham risk factors fail to fully account for accelerated atherosclerosis in systemic lupus erythematosus. Arthritis Rheum. 2001;44:2331–2337. doi: 10.1002/1529-0131(200110)44:10<2331::aid-art395>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Harnett W, Harnett MM, Byron O. Structural/functional aspects of ES-62--a secreted immunomodulatory phosphorylcholine-containing filarial nematode glycoprotein. Curr Protein Pept Sci. 2003;4:59–71. doi: 10.2174/1389203033380368. [DOI] [PubMed] [Google Scholar]
- Houston KM, Harnett W. Structure and synthesis of nematode phosphorylcholine-containing glycoconjugates. Parasitology. 2004;129:655–661. doi: 10.1017/s0031182004006171. [DOI] [PubMed] [Google Scholar]
- Manzi S, Meilahn EN, Rairie JE, Conte CG, Medsger TA, Jr, Jansen-McWilliams L, D’Agostino RB, Kuller LH. Age-specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: comparison with the Framingham Study. Am J Epidemiol. 1997;145:408–415. doi: 10.1093/oxfordjournals.aje.a009122. [DOI] [PubMed] [Google Scholar]
- McInnes IB, Leung BP, Harnett M, Gracie JA, Liew FY, Harnett W. A novel therapeutic approach targeting articular inflammation using the filarial nematode-derived phosphorylcholine-containing glycoprotein ES-62. J Immunol. 2003;171:2127–2133. doi: 10.4049/jimmunol.171.4.2127. [DOI] [PubMed] [Google Scholar]
- Melendez AJ, Harnett MM, Pushparaj PN, Wong WS, Tay HK, McSharry CP, Harnett W. Inhibition of Fc epsilon RI-mediated mast cell responses by ES-62, a product of parasitic filarial nematodes. Nat Med. 2007;13:1375–1381. doi: 10.1038/nm1654. [DOI] [PubMed] [Google Scholar]
- Nikpour M, Urowitz MB, Gladman DD. Premature atherosclerosis in systemic lupus erythematosus. Rheum Dis Clin North Am. 2005;31:329–354. vii–viii. doi: 10.1016/j.rdc.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Panda AK, Ravindran B, Das BK. Rheumatoid arthritis patients are free of filarial infection in an area where filariasis is endemic: comment on the article by Pineda et al. Arthritis Rheum. 2013;65:1402–1403. doi: 10.1002/art.37883. [DOI] [PubMed] [Google Scholar]
- Pineda MA, Lumb F, Harnett MM, Harnett W. ES-62, a therapeutic anti-inflammatory agent evolved by the filarial nematode Acanthocheilonema viteae. Mol Biochem Parasitol. 2014;194:1–8. doi: 10.1016/j.molbiopara.2014.03.003. [DOI] [PubMed] [Google Scholar]
- Plump AS, Smith JD, Hayek T, Aalto-Setälä K, Walsh A, Verstuyft JG, Rubin EM, Breslow JL. Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell. 1992;71:343. doi: 10.1016/0092-8674(92)90362-g. [DOI] [PubMed] [Google Scholar]
- Shaw PX, Goodyear CS, Chang MK, Witztum JL, Silverman GJ. The autoreactivity of anti-phosphorylcholine antibodies for atherosclerosis-associated neo-antigens and apoptotic cells. J Immunol. 2003;170:6151–6157. doi: 10.4049/jimmunol.170.12.6151. [DOI] [PubMed] [Google Scholar]
- Skaggs BJ, Hahn BH, McMahon M. Accelerated atherosclerosis in patients with SLE-mechanisms and management. Nat Rev Rheumatol. 2012;8:214–223. doi: 10.1038/nrrheum.2012.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley RG, Jackson CL, Griffiths K, Doenhoff MJ. Effects of Schistosoma mansoni worms and eggs on circulating cholesterol and liver lipids in mice. Atherosclerosis. 2009;207:131–138. doi: 10.1016/j.atherosclerosis.2009.04.037. [DOI] [PubMed] [Google Scholar]
- Su J, Georgiades A, Wu R, Thulin T, de Faire U, Frostegard J. Antibodies of IgM subclass to phosphorylcholine and oxidized LDL are protective factors for atherosclerosis in patients with hypertension. Atherosclerosis. 2006;188:160–166. doi: 10.1016/j.atherosclerosis.2005.10.017. [DOI] [PubMed] [Google Scholar]
- Van Doornum S, McColl G, Wicks IP. Accelerated atherosclerosis: an extraarticular feature of rheumatoid arthritis? Arthritis Rheum. 2002;46:862–873. doi: 10.1002/art.10089. [DOI] [PubMed] [Google Scholar]
- Wilson EH, Deehan MR, Katz E, Brown KS, Houston KM, O’Grady J, Harnett MM, Harnett W. Hyporesponsiveness of murine B lymphocyts exposed to the filarial nematode secreted product ES-62 in vivo. Immunology. 2003;109:238–245. doi: 10.1046/j.1365-2567.2003.01661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]