Abstract
There still is an unmet need for a safe and sustained intravitreal drug delivery system. In this study we are proposing and characterizing a micelle based, clear-media intravitreal drug delivery system using the lipid derivatized nucleoside analog, hexadecyloxypropyl-cidofovir (HDP-CDV, CMX 001). HDP-CDV forms micelles in water and in vitreous supernatant with the critical micelle concentration of 19 μg/mL and 9 μg/mL, respectively at 37°C. The formed micelles had the average size of 274.7 nm and the Zeta potential of −47.1 mV. Drug release study in the excised rabbit vitreous showed a sustained release profile with a half-life of 2.7 days. The micelle formulation of HDP-CDV demonstrated a good safety profile in two animal species (rabbit and guinea pig) following intravitreal injection. The sustained efficacy was tested in a pretreatment study design and the drug potency was tested in an ongoing herpes simplex virus (HSV-1) retinitis model. The pretreatment studies using single intravitreal injection and later HSV-1 infection revealed at least 9 weeks of vitreous presence and therapeutic level of HDP-CDV, with 71% eyes protection from infection. The treatment study demonstrated that intravitreal administration halted active HSV-1 retinitis in 80% of the infected eyes while cidofovir (CDV) treatment failed to suppress active HSV-1 retinitis. In summary, lipid derivatized nucleoside analogs can be formulated as a micelle intravitreal injection and provides a sustained drug release in vitreous for chronic retinal diseases.
Keywords: Micelles formulation, intravtreal drug delivery, Cidofovir, Lipid prodrug, Herpes Simplex Virus-1 retinitis, Rabbit eye, Guinea pig eye, Micelle
1. INTRODUCTION
Many eye diseases affecting the posterior portion of the globe are refractory and lingering. For example, acute retinal necrosis or cytomegalovirus retinitis are vision threatening and hard to treat. Often times frequent intravitreal injections of antiviral drugs are required to maintain the drug level at the target tissue because systemic treatment could have severe side effects outside of the eye [1, 2]. In recent years, long-lasting ocular drug delivery systems such as vectorial porous silicon based intravitreal delivery [3, 4], particulate intravitreal drug delivery [5, 6], or lipid prodrug based delivery systems are being developed [7, 8]. Cidofovir is a FDA approved small molecule anti-viral drug and has been used as a local intravitreal injection for viral retinitis [9] to avoid systemic complications. However, intravitreal cidofovir has ocular complications of iritis and hypotony even with a lowered intravitreal dose [9, 10]. There is an unmet need to develop safe and sustainable ocular formulation of cidofovir. Previously we have tried to develop a crystalline drug depository formulation using lipid prodrug strategy, which allowed formation of a drug depot in vitreous and offer a slow release, similar to triamcinolone acetonide intravitreal application [11]. However, this type of delivery can cause unpredictable localized retinal toxicity if the drug depot touches the retina [7, 12]. This type of drug delivery also has the disadvantage of creating vitreous floaters [13, 14] and may cause visual discomfort in some patients as seen after intravitreal triamcinolone [15]. We hypothesize that micelles may form in vitreous after intravitreal injection of appropriate lipid prodrugs such as Hexadecyloxypropyl-Cidofovir (HDP-CDV, CMX 001) and may provide sustained release while offering clear vitreous without drug aggregates. HDP-CDV is a lipid prodrug of cidofovir (CDV) and has much more potent antiviral and anti-proliferation effects than the unmodified parent drug, cidofovir [16, 17]. In a recent study, we tested our hypothesis by intravitreal injection of HDP-CDV and examined its ocular pharmacokinetics [18]. That study demonstrated a longer vitreous half-life than most small molecules (including CDV) and suggested the formation of vitreous micelles. We believe that vitreous micelle drug delivery may be a superior intravitreal drug delivery system compared to the crystalline intravitreal drug delivery we featured previously [7, 8].
The current study was designed to characterize the in vitro physicochemical properties and ex vivo release properties of the micelle delivery system of HDP-CDV, and to test its in vivo efficacy in prophylactic and real time treatment settings using the rabbit HSV-1 retinitis model.
2. MATERIALS AND METHODS
2.1 HDP-CDV formulation preparation
HDP-CDV (molecular weight as sodium salt = 583.67) was synthesized as described previously [16]. HDP-CDV was prepared in various media for different testing purposes; however, the drug concentration was kept constant at 42 μg/mL, which is roughly the resultant drug concentration in rabbit vitreous [18].
2.2 Size distribution and Zeta potential of the micelles
The size distribution and zeta potential of HDP-CDV micelles were measured using a Malvern Zetasizer Nanoseries-ZS zeta potential analyzer (Malvern Instruments, Malvern, Worcestershire, UK). Samples with the concentration of 1.49×10−3 M were measured in 1 mL disposable plastic cuvettes (Malvern Instruments). Each experiment was conducted at 25 °C by dispersing the samples in deionized water and analyzed in triplicate. The electrophoretic mobility (μm/s) was converted to zeta potential by built-in software using Helmholtz-Smoluchowski equation.
2.3 Transmission electron microscopy (TEM) of the micelles
Transmission Electron Microscopy was performed using a FEI Tecnai transmission electron microscope (FEI, Hillsboro, OR USA). HDP-CDV micelles were dispersed in deionized water and one drop was applied to a carbon coated copper grid. This copper grid was fixed onto the sample holder and placed in the vacuum chamber of the transmission electron microscope. Deionized water only served as a control.
2.4 Critical Micelle Concentration (CMC) determinations
HDP-CDV was dissolved into deionized water with the final concentration of 1.49×10−3 M as the stock solution. The stock solution was diluted to a set of ten solutions with various concentrations of HDP-CDV from 0 to 1.49×10−3 M. 10 mL of each solution was transferred into small jars and 10 drops of a saturated 1-(2-pyridylazo)-2-naphthol solution in pentane were added. The jars were then gently swirled for 20 min to allow the pentane to evaporate and the color to develop [19]. The absorbance of each solution was measured at 470 nm using a spectrofluorometer (SpectraMax M5, Molecular Devices Corp. USA.). The results were analyzed with the software OriginPro 8.0 (OriginLab Corporation. Northampton, USA)
For CMC in vitreous, the rabbit vitreous was first homogenized with a tissue grinder, and then centrifuged at 13,000 rpm for 10min and the precipitate was discarded. HDP-CDV was dissolved into the supernatant with the final concentration of 1.49×10−3 M as the stock solution. The subsequent steps were the same as above described for CMC in deionized water.
2.5 Ex Vivo drug Release
Dynamic drug release was performed in the excised vitreous using a custom-made diffuse/flow chamber connected to a syringe pump [4, 20]. The apparatus includes three flow chambers so that each experiment can be performed in triplicate. Briefly, 42μg HDP-CDV dissolved in 50μl PBS was injected into the excised rabbit vitreous in the flow chamber using the same syringe and needle as for an intravitreal injection for rabbit eye. Each flow chamber volume was 1.5 mL to mimic innate rabbit vitreous and was pumped by a NE-1000 syringe pump (New Era Pump Systems, Farmingdale, NY) with a constant flow rate of 1 μL/min using PBS. The entire setup was maintained at 37 °C. The operation was performed in a sterile fashion and the chamber was airtight and infused with dynamic fluid. All these elements kept the vitreous free of infection. During the 8-day release, the infusate was collected once daily. The samples were stored at −80 °C. Before HPLC analysis, each sample was centrifuged at 13,000 rpm for 10min and 200μL of the supernatant was transferred to a 1.5ml EP tube and 100ng octyl-CDV was added as an internal standard. The tube was then vortexed for 30s, 200μL 2-propanol (HPLC grade) was added and the tube vortexed for another 2 min. After centrifugation at 13,000rpm for 10min, the supernatant was transferred to an EP tube then frozen and lyophilized for HPLC analysis [18]. The chromatographic separation was performed on a Shiseido Capcell Pak MG III C-18 column (2.0 mm ID × 50 mm length, 3μm) with a guard column. Mobile phase A was 2.5% by volume methanol (CH3OH, Sigma Aldrich, HPLC-grade) in water with 0.1% formic acid (HCOOH, Sigma-Aldrich). Pure methanol with 0.1% by volume formic acid was used as mobile phase B. The mobile phase was delivered at a rate of 200 μL/min under gradient conditions as follows: 30% phase B to 95% phase B in 10 min, followed by 6 min with 95% phase B and then back to 30% phase B in 1 min. An additional time of 8 min at 30% phase B was used to equilibrate the column and return the system to the initial conditions for subsequent analysis. The HPLC was operated under selected reaction monitoring (SRM) scan mode to detect HDP-CDV and the spiked internal standard, octyl-cidofovir. A HPLC run of pure BSS was used as a blank control. The sample was re-dissolved with 100 μL of the mobile phase and 20 μL was injected into HPLC. Each sample was analyzed three times. HDP-CDV linear range was 0.6–6000 ng; limit of quantitation (LOQ) and limit of detection (LOD) were 1.27 ng and 0.42 ng, respectively.
2.6 Animal studies
2.6.1 Drug formulations and dosage
Dosing was based on our previous pharmacokinetic/safety study [18] in which the highest non-toxic HDP-CDV intravitreal dose was 42 μg/eye. The equimolar dose of CDV (20 μg/eye, molecular weight = 279.18) was used for comparison, and both drugs were dispersed in 50 μL of 5% dextrose/dose. For the confirmatory safety study in guinea pig eyes, the equivalent HDP-CDV dose (7 μg/eye) was based on rabbit vitreous volume of 1.5 mL vs. 250 μL vitreous volume for guinea pig. For guinea pig eye injection, 8.3 μL of the drug solution was injected with a 30-gauge needle connected to an extension tubing which again was connected to a 50 μL Hamilton syringe.
All procedures adhered to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Only the right eyes of the animals were tested.
2.6.2 Confirmatory safety study using a second species (Guinea pig)
The right eyes of eight albino guinea pigs were intravitreally injected with either 7μg HDP-CDV or 3.3 μg CDV dispersed in 8.3 μL of 5% dextrose. Injection of 5% dextrose (8.3μL) was used as a control. Slit-lamp examination, fundus examination, and intraocular pressure (IOP) measurements were performed on day 3, day 7, day 14 and day 28 after drug injection. Before euthanasia, full field scotopic and photopic electroretinography (ERG) were obtained from all eyes using published methods [21]. After euthanasia, eyes were enucleated, fixed in 1.25% glutaraldehyde and 2% paraformaldehyde for 48 hours, embedded in paraffin and sectioned, then stained with hematoxylin and eosin (HE) and examined under light microscope. Transmission electron microscopy (TEM) was also performed to confirm the light microscopic findings.
2.6.3 Efficacy studies in rabbit HSV-1 retinitis model
HSV-1 (PH strain) was provided by Jang Oh (Proctor Foundation, University of California, San Francisco) and cultured as previously described [22]. The stock was titered by Virapur, Inc. (San Diego, CA) prior to use.
Prophylaxis study
Twenty-seven New Zealand Pigmented rabbits were divided into two groups: 5 week and 9 week. In the first (5 week pretreatment) experiment, ten rabbits were divided equally into the HDP-CDV group and the CDV group.
In the second (9 week pretreatment) experiment, rabbits were divided into: HDP-CDV group (seven rabbits), CDV group (five rabbits), and 5% dextrose control group (five rabbits).
For all the treatment groups, baseline examinations were documented, including intraocular pressure (IOP) with handheld Tonopen (Medtronic, Jacksonville, FL), anterior segment examination with slit-lamp biomicroscopy, and indirect ophthalmoscopy. The right eyes of the animals were intravitreally injected with either HDP-CDV (42μg in 50 μL of 5% dextrose), or CDV (28 μg in 50μL of 5% dextrose), or vehicle (50 μL 5% dextrose alone). Five weeks or 9 weeks after the treatments, 5×10−5 dilution of a 108 TCID/mL herpes simplex virus-1 (HSV-1) was inoculated into the rabbit vitreous to induce HSV-1 retinitis. Retinitis grading was evaluated on day 3, day 6, day 9 and day 14 after virus injection using our published grading system (Figure 1) [22]. Histology was performed after euthanasia on day 14 for pathologic evaluation.
Figure 1.

Left panel shows scattered micelles most of which are within the size range of 50 to 200 nm. Right panel serves as control, which is from deionized water without addition of HDP-CDV
Treatment study
Fifteen New Zealand pigmented rabbits were used for a study in which HSV-1 retinitis was induced as above, and then, 4 days after virus inoculation, the treatments (HDP-CDV, CDV, vehicle) were administered using the same dosing as the prophylaxis study, and five rabbit eyes for each treatment.
Histologic evaluation
Histology examination was performed to confirm the presence, extent and the severity of retinitis and the accompanying vitritis and choroiditis. Four vitreous areas, including the superior and inferior vitreous at pars plana, the vitreous next to posterior lens capsule, as well as vitreous next to optic nerve, were carefully inspected and the inflammatory cells counted. The total inflammatory cells from all four locations were used as an inflammation severity score for statistical analysis. Retinal and choroidal thicknesses were measured at eight locations as we described in our previous study [7], with five locations in the inferior retina and three locations in the superior retina. The thickness of the retina was measured from internal limiting membrane (ILM) to external limiting membrane (ELM) because retinal detachment is common in this type of retinitis and outer segments of photoreceptors shrink and shorten when retinal detachment is present. If measured from internal limiting membrane to retinal-pigmented epithelium (RPE), significant variations from outer segments shrinkage would factor in. Canon EOS6D photographed the measurement areas with the magnification of 33X. Vitreous inflammation cell counts and retinal or choroidal thickness measurement were conducted with ImageJ (developed by National Institutes of Health) from the histology images.
2.7 Data analysis
The continuous variables, such as ERG, IOP, vitreous cell counts, thickness of the retina, thickness of the choroid were compared within, between or among groups by t-test or the Tukey’s Honestly Significant Difference (HSD) test. The ordinal variables, such as retinitis scores were analyzed across groups by the Kruskal-Wallis test followed by a nonparametric Tukey-type test to locate the difference if a null hypothesis was rejected by the Kruskal-Wallis test. Differences of P<0.05 were considered to be statistically significant.
3. RESULTS
3.1 Physicochemical properties of the HDP-CDV micelles
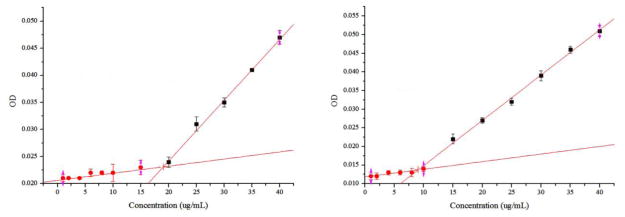
The HDP-CDV molecule consists of a hydrophobic hexadecyl (C16) alkyl chain and a hydrophilic polar cidofovir group attached to a propanediol backbone, which mimics the glycerol portion of lysophospholipids. The amphipathic nature of HDP-CDV results in surfactant and detergent effects in aqueous solution, including micelle formation when the HDP-CDV concentration exceeds the critical micelle concentration (CMC). The HDP-CDV micellar formulations are stable for at least 2 weeks at room temperature. The micelle solution is visually thicker than water but transparent, which is desirable for intravitreal use. SEM imaging as shown in Figure 1 confirmed micelle formation in the injecting solution. No filtration was performed either for in vitro, ex vivo, or in vivo studies. The micelles in Figure 1 were heterogeneous in size and some aggregation was observed. The presence of some large aggregates can bias dynamic laser scattering (DLS) measurement and result in a larger average size of test micelles. In the current study, the mean size of the test micelles was 274.7 nm with PDI (polydispersity index) of 0.478; and the Zeta potential was −47.1 mV. In addition to DLS bias from the presence of small portion of large micelles aggregates, SEM image of micelles tend to be smaller than the ones in micellar solution due to evaporation of water and condensation. The critical micelles concentration at 37°C for HDP-CDV in PBS was 19 μg/mL and 9 μg/mL in vitreous supernatant (Figure 2).
Figure 2.
Plots of the absorbance at 470 nm versus the HDP-CDV concentration; right panel is for HDP-CDV in PBS (X=19.03, Y=0.02), left panel is for HDP-CDV in vitreous supernatant (X=9.01, Y=0.01).
3.2 Ex Vivo drug Release
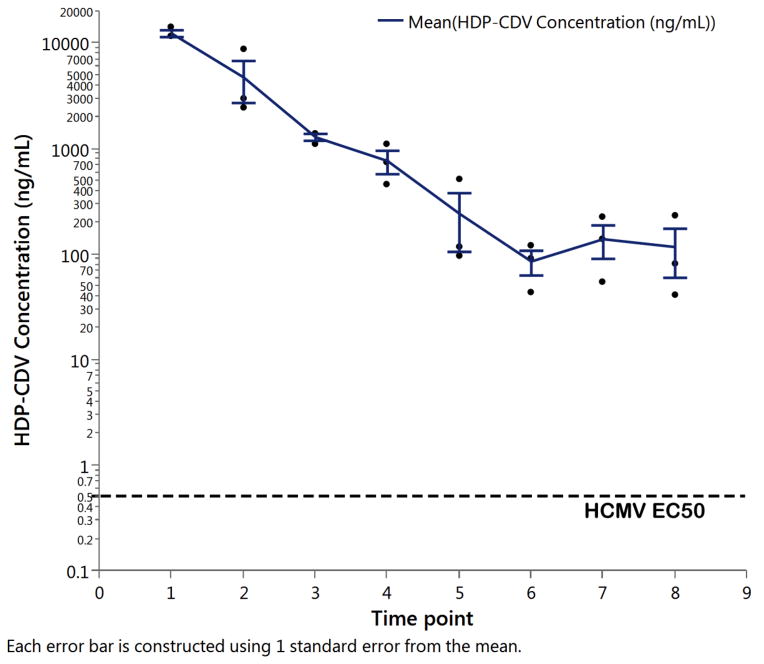
A non-compartmental analysis of the HDP-CDV concentration of the infusate over time using software Phoenix WinNonlin version 6.3 revealed the estimated half-life as 2.7 days and mean resident time of 1.8 days. The observed maximum concentration (Cmax) was 12.4 μg/mL 24 hours after the injection and the last concentration at 8 days after the injection was 0.12 μg/mL or 120 ng/mL (Figure 3). The molecular weight of HDP-CDV is 561.7 and 120 ng/mL is converted into a molar concentration of 214 nM which is 238 times higher than the EC50 (0.9 nM) for this drug against wild-type strains of CMV (Figure 3) [16].
Figure 3.
HDP-CDV concentration versus time in excised rabbit vitreous at 37°C in the irrigated dissolution chambers. HDP-CDV= Hexadecyloxypropyl-Cidofovir; HCMV=Human Cytomegalovirus; EC50=Effective concentration of HDP-CDV reduced viral replication by 50% (DNA reduction assay). Each error bar is constructed using 1 standard error from the mean.
3.3 Confirmatory Safety in Guinea Pig Eyes
After injection, HDP-CDV dispersed in the vitreous of the guinea pig eyes with the vitreous clear at all examination time points. No anterior chamber cells or retina abnormalities were observed by slit lamp or indirect ophthalmoscopy during the 8-week study period. The IOP difference between the treated eyes and their fellow eyes were compared across the three groups and no significant difference was noted (HDP-CDV 0.08 ± 1.13 mmHg vs. CDV 0.97 ± 2.59 mmHg vs. dextrose 0.082 ± 2.68 mmHg; p >0.05 Tukey’s HSD).
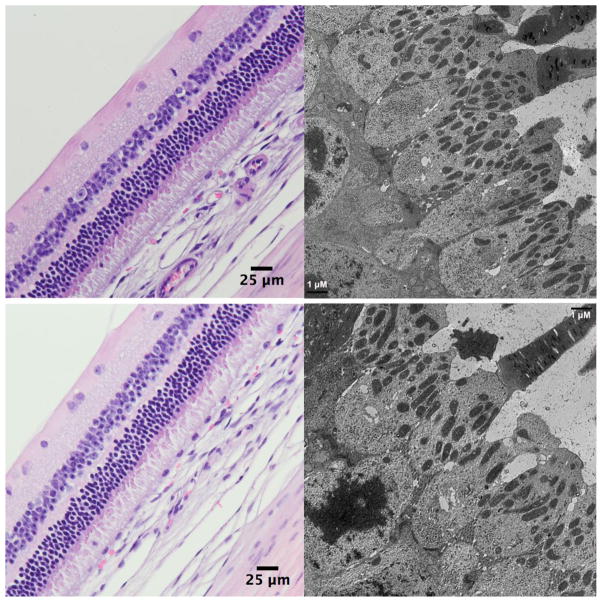
ERG examination before euthanasia did not show significant difference between the treated eyes and the contralateral eyes (paired t test, b-wave implicit and amplitude difference of the fellow eyes for Dextrose group 1.45 ± 13.03 and 2.25 ± 16.14; for CDV group 3.21 ± 14.9 and 2.71 ± 13.3; for HDP-CDV group 0.68 ± 9.38 and 2.28 ± 20.32) or among the three study groups (Dunnett’s test with control of dextrose). Histology of light and electron microscopy revealed normal retinal structures (Figure 4).
Figure 4.
Light microscopic images (left) and electron microscopic images (right) of the injected eye (upper panels) and its contralateral non-injected eyes (lower panels), showing comparable normal retinal layers under light microscope (62.5X) and normal ultrastructures of the photoreceptors under transmission electron microscope (3000X) at 4 weeks after the injection.
3.4 Efficacy Studies in Rabbit HSV-1 Retinitis Model
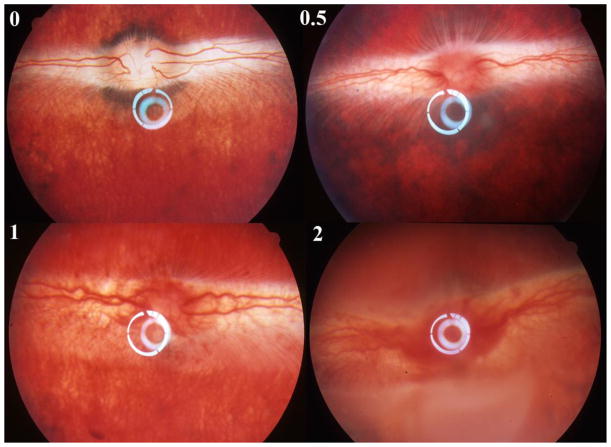
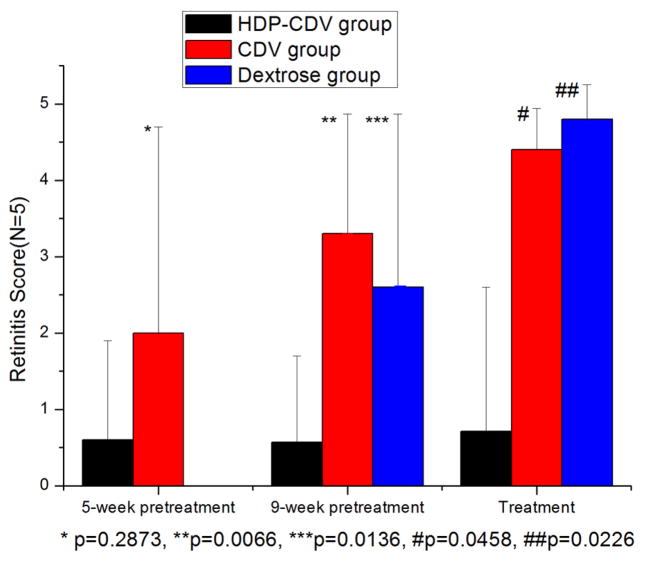
HSV-1 retinitis in rabbit is a rapidly spreading retinitis model. It appears on day 3 or day 4 after the virus inoculation and spreads quickly to the whole retina within 2 weeks. The retinitis can be better clinically scored (Figure 5) using indirect ophthalmoscope before day 9 beyond which the vitreous becomes cloudy and retinal detachment develops (Grade 3 with these changes confined within inferior retina, and Grade 4 with the changes spread to superior retina) [12]. As shown on Treatment in Figure 6, HDP-CDV treated rabbits had significantly lower retinitis scores than that from the rabbits treated with CDV or Dextrose.
Figure 5.
Natural course of HSV-1 rabbit retinitis. Upper-left panel, normal rabbit fundus; Upper-right panel, retinitis grade 0.5 that is characterized by the hyperemia of optic nerve and no obvious signs of hemorrhage yet; Bottom-left panel, retinitis grade 1 that is characterized by the hemorrhages of the optic nerve and the medullary ray but no obvious retina infection (whitening foci) yet. The vitreous is still clear at this stage. Bottom-right, retinitis grade 2 is characterized by increased vitritis with decreased vitreous clarity as well as presence of discrete white patches of necrosis involving the inferior retina.
Figure 6.
Retinitis scores distribution within each treatment strategy. In each treatment category, the retinitis scores of the other two groups were statistically compared with HDP-CDV intervention group. Except for 5-week pretreatment in which retinitis scores was not significantly different between HDP-CDV and CDV interventions, all other intervention paradigms showed significantly lower retinitis scores from HDP-CDV groups.
3.5 Prophylaxis Study
The prophylaxis study was designed to test therapeutic duration of this drug delivery system, in which test drug and system is intravitreally-administered weeks before the disease model induction.
For the 5-week prophylaxis study, a non-inferiority test was conducted. Out of five eyes treated with HDP-CDV, four did not develop retinitis and one developed a mild and delayed retinitis. In contrast, of five eyes with CDV injection, two developed retinitis, consistent with earlier studies, which indicated that the therapeutic duration of a single intravitreal CDV injection is about 5 weeks [9]. Retinitis score between HDP-CDV group (0.6 ± 1.3) and CDV group (2 ± 2.7) was not significantly different (p = 0.2873) Pathologic finding was consistent with clinical grading. Since HDP-CDV was at least as good as CDV, we proceeded with a 9-week study. For the 9-week pretreatment study, seventeen rabbits were used. Out of seven rabbit eyes treated with HDP-CDV, two developed retinitis, while all five CDV-treated rabbits developed severe retinitis, as did rabbits treated with vehicle. The retinitis score of the HDP-CDV group (0.57 ± 1.13) was significantly lower than both the CDV group (3.3 ± 1.57, p=0.0066) and the 5% dextrose treated group (2.6 ± 2.27, p=0.0136) as shown in Figure 6. Pathologic findings were in agreement with clinical grading. The pre-treatment strategy was used to test sustainability of this delivery system and is clinically relevant to estimate the intervals between subsequent dosing.
3.6 Real-time Treatment Study
This study was designed to test the strength of therapeutic effect of the drug therefore the drug administration was after the model disease manifests. Fifteen rabbits were used in the treatment study. On day 4 after virus inoculation, nine of the rabbits had developed grade 0.5 retinitis, three had developed grade 1 retinitis, and three did not show retinitis. The rabbits were grouped into HDP-CDV, CDV and vehicle control groups based on even distribution of retinitis severity and the corresponding intravitreal injection (HDP-CDV, CDV, or Dextrose) was performed. By day 6 following initial virus inoculations, out of the five rabbits that were injected with HDP-CDV, four showed halted retinitis, and one showed slower progressive retinitis. In contrast, all the CDV and dextrose control treated rabbits had similar fast progressing HSV-1 retinitis. The mean retinitis score of the HDP-CDV group (0.71 ± 1.89) was significantly lower than the CDV group (4.4 ± 0.54, p=0.0458) and control group (4.8 ± 0.45, p = 0.0226) at the end of the 2-week study. There was no significant difference between the CDV and control groups (Figure 6, p= 0.2455).
Around 2 weeks following virus inoculation, this retinitis model usually has completed its malicious retina infection process and vitritis subsides substantially with better vitreous clarity. The damage left by the infection was better appreciated in histopathology. Pathology evaluation revealed that vitreous inflammatory cells were significantly higher in the control group (355 ± 275) than CDV-treated group (193 ± 127); and HDP-CDV had the fewest inflammatory cells (29 ± 72, p<0.0001 each pair using Wilcoxon method).
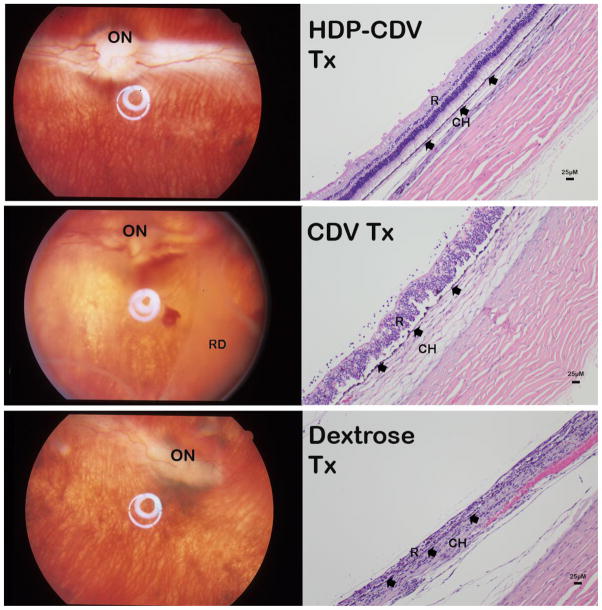
The means of retina thickness of the eight measurement locations [7] in HDP-CDV, CDV, and 5% dextrose control groups were 87.31 ± 25.04 μm, 71.84 ± 39.45 μm, and 59.60 ± 44.02 μm: significantly different from each other (HSD all pairs, P<0.0001). Mean choroid thicknesses were 31.83 ± 11.92 μm, 56.37 ± 26.74 μm, and 92.87 ± 40.94 μm, which also showed significant difference among the groups; the thicker choroid indicates severe chorioretinitis from HSV infection (P<0.0001) (Figure 7). HSV-1 infection causes retina necrosis and retina becomes thinner while HSV-1 choroiditis causes severe swelling of choroid and the choroid becomes thicker.
Figure 7.
Top-panels: HDP-CDV treated eye (HDP-CDV Tx) presented relatively normal fundus (left image); histology revealed only mild vitritis with inflammatory cells seen along the retina surface (right image). Mid-panels: CDV treated eye (CDV Tx) showed hazy vitreous with extensive retinal detachment (RD) (left image); histology revealed disorganized retina (R) layers with much more inflammatory cells along the retina surface (right image). Both RPE (arrowheads) and choroid (CH) are swelling. Bottom-panels: Dextrose injected eye revealed distorted optic nerve (ON) and shortened right side medullary ray (left image); histology showed no recognizable retinal layers and RPE was barely recognizable (arrowheads) with inflammatory cells packed in both retina area (R) and in Choroid (CH).
4. DISCUSSION
To bypass the ocular blood barriers and to avoid systemic side effects, ocular local drug delivery has become a mainstay for chorioretinal diseases. Intravitreal injection of therapeutics is the most effective route of all ocular drug delivery modalities. It has become a standard care for many retinal diseases [23]. However, frequent intravitreal injection is also becoming a burden for both patient and healthcare provider. A sustained drug delivery system has become an unmet need for chronic retinal disease care. The current study has demonstrated a micelle based intravitreal drug delivery system. Unlike other delivery systems, these micelles are formed by the drug molecules themselves without addition of non-therapeutic components such as cholesterol for liposomes [24] or polymers for nanoparticles and microspheres [25, 26]. The non-therapeutic addition imposes stress to ocular metabolic system and fluid circulation pathways. For example, intravitreal liposomes or polymer particles can trigger vitreous inflammation and at the same time impair vitreous clarity [27].
The current study demonstrated a clear vitreous without drug precipitates and we demonstrated micelle formation and presence in both the injected formulation and in the rabbit vitreous after injection. The size of the micelles was about 275 nm and they are negatively charged (−47.1 mV). Vitreous is also negatively charged due to the presence of negatively charged collagens, therefore these micelles disperse well in vitreous and good vitreous clarity is maintained. Kim et al [28] also reported superior intravitreal dispersion with the use of negatively charged particles. It is expected that micelle formation comes easier in a more ionized environment such as vitreous humor than in water. Our measured critical micelle concentration (CMC) also demonstrated lower CMC (9 μg/mL) in vitreous than in PBS (19 μg/mL). Although how the micelles form in gel vitreous is not clear, it is possible that micelles reside in micro fluid pockets between collagen bundles. Clearance of macromolecular DDS (drug delivery system) from vitreal liquid depends on the specific delivery system and drug being delivered. In the current study, HDP-CDV forms micelles in many micro vitreous fluid pockets among vitreous collagens. We believe that there is a dynamic balance between micelles and HDP-CDV molecules which is constantly being eliminated along with the vitreous water turnover reported to be 1 to 2 μL/min [29]. Once the HDP-CDV drop below the CMC level in that particular micro fluid pocket, micelles collapse into many individual HDP-CDV molecules and exit the vitreous through retina-choroid pathway [30]. Micelles formation in vitreous may have greatly enhanced HDP-CDV vitreous residence time.
In this study, ex vivo drug release showed a half-life of 2.7 days which is quite a long vitreous half-life for a small molecule drug and supports formation of micelles in excised rabbit vitreous because similar small molecule drugs such as ganciclovir or cidofovir have vitreous half-lives of only hours [31, 32]. The current study did not perform an ex vivo release for CDV because its vitreous half-life was well characterized [32–34]. The half-life of HDP-CDV in excised rabbit vitreous (2.7 days) was shorter than that in living rabbit eyes (6.2 days) [18]. This may be due to the excised vitreous liquefying at 37°C over time, a change that may lead to quicker elimination of test drug as well as alter the CMC of HDP-CDV. With the current ex vivo infusate, the sample was only subjected to HDP-CDV detection. Based on our previous publication, CDV and various metabolites are possibly present in the infusate and some of metabolites may not be qualified [18, 35]. Therefore, instead of characterizing metabolites in vitro we conducted in vivo efficacy studies to evaluate this drug delivery system.
In the current study, we used HDP-CDV as a model drug to demonstrate the concept and possibility of using micelles as a formulation to achieve sustained intravitreal drug delivery. Similarly, many small molecules or nucleoside analogs such as 5-fluorouridine and cytarabine can be lipid derivatized [8, 20] and may be delivered in micelle formulation for treatment of unwanted retinal proliferation. Currently, CMV retinitis has become less threatening due to induction of highly active antiretroviral therapy (HAART). However, challenges remain for HIV patients who are poorly responsive to HARRT, as well as for hematopoietic stem cell (HSCT) or solid organ (SOT) transplant recipients [36, 37]. In the current efficacy studies, HDP-CDV demonstrated much superior protection and treatment efficacy than CDV in an equimolar dose which is 20 μg per eye as for clinical human eye injection [38]. It is even more encouraging that 9-week pretreatment study provided complete protection for 71% of the eyes while CDV and dextrose did not provide any protection at all. From our in vivo study, vitreous half-life of HDP-CDV was 6.2 days, which would provide about 40 days of vitreous residence if 6 half-lives would clear the 95% of drug from the vitreous. HDP-CDV is metabolized into CDV upon entering the cells and CDV along with its metabolites persist for a sustained time inside the cell (half-life 4 days) [39]. Long vitreous half-life of HDP-CDV and long intracellular half-life of CDV and its metabolites warranty a lasting therapeutic effect. Indeed, the 9-week prophylaxis success supported this pharmacokinetics and the contribution from the formation of micelles. The current retinitis model is much more rapid progressing than retinitis seen in human eye, this delivery system may provide better than 71% protection for human retinitis and translate into a longer than 9 weeks of therapeutic effect. In the current treatment study, we chose drug administration 4 days after virus inoculation based on the natural history of this HSV-1 retinitis model [7, 21], which allows us to allocate the animals to achieve a balanced baseline retinitis among the comparison groups. HDP-CDV showed obviously better treatment efficacy than the concurrent CDV intravitreal injection. The retina was significantly thicker and choroid was significantly thinner in the HDP-CDV group than in the other two groups. The good treatment efficacy is likely attributed to longer sustained release and better retina penetration for HDP-CDV than for CDV. Such a local drug therapy will provide long protection while eliminating any systemic complication from intravenous drug infusion, which is considered a standard care.
In summary, HDP-CDV forms micelles in PBS and vitreous supernatant in which micelles form easier than in PBS. The long ex vivo and in vivo vitreous half-life may stem from micelle formation and uniform distribution due to negative charges of the micelles. These physicochemical properties of the micelles offered a long vitreous half-life and clear vitreous, which transformed to a potent intravitreal sustained antiviral prophylaxis and treatment.
Highlights for review.
Lipid prodrug of cidofovir, HDP-CDV, forms micelles in vitreous
No change of Vitreous clarity or retinal function after intravitreal 42 μg
Single dose of 42 μg can provide 9 weeks of protection from HSV-1 infection
HDP-CDV is much more potent than cidofovir on active HSV-1 retinitis
Vitreal micellar formulation of lipid prodrug is a long-lasting delivery system
Acknowledgments
Funding Support:
This work was supported by National Institutes of Health grant #: EY020617 (Cheng L); EY 018589 (Freeman, WR); and P30EY022589.
The work was also partially supported by Research to Prevent Blindness, UCSD.
We want to thank Miss Anna Nilsson for proofreading of this manuscript.
Footnotes
Chemical compounds studied in this article: Hexadecyloxypropyl-cidofovir (PubChem CID: 483477); pentane (PubChem CID: 8003); Cidofovir (PubChem CID: 60613); 2-Propanol (PubChem CID: 3776); Methanol (PubChem CID: 887); Formic acid (PubChem CID: 284); Dextrose (PubChem CID: 5793); Glutaraldehyde (PubChem CID: 3485); Formaldehyde (PubChem CID: 712); Disodium hydrogen phosphate (PubChem CID: 24203)
Potential conflicts of interest: NONE
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Izzedine H, Launay-Vacher V, Deray G. Antiviral drug-induced nephrotoxicity. American journal of kidney diseases: the official journal of the National Kidney Foundation. 2005;45:804–17. doi: 10.1053/j.ajkd.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 2.Jacobsen T, Sifontis N. Drug interactions and toxicities associated with the antiviral management of cytomegalovirus infection. Am J Health Syst Pharm. 2010;67:1417–25. doi: 10.2146/ajhp090424. [DOI] [PubMed] [Google Scholar]
- 3.Chhablani J, Nieto A, Hou HY, et al. Oxidized Porous Silicon Particles Covalently Grafted with Daunorubicin as a Sustained Intraocular Drug Delivery System. Invest Ophth Vis Sci. 2013;54:1268–79. doi: 10.1167/iovs.12-11172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hou HY, Nieto A, Ma FY, Freeman WR, Sailor MJ, Cheng LY. Tunable sustained intravitreal drug delivery system for daunorubicin using oxidized porous silicon. J Control Release. 2014;178:46–54. doi: 10.1016/j.jconrel.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Merodio M, Irache JM, Valamanesh F, Mirshahi M. Ocular disposition and tolerance of ganciclovir-loaded albumin nanoparticles after intravitreal injection in rats. Biomaterials. 2002;23:1587–94. doi: 10.1016/s0142-9612(01)00284-8. [DOI] [PubMed] [Google Scholar]
- 6.Koo H, Moon H, Han H, et al. The movement of self-assembled amphiphilic polymeric nanoparticles in the vitreous and retina after intravitreal injection. Biomaterials. 2012;33:3485–93. doi: 10.1016/j.biomaterials.2012.01.030. [DOI] [PubMed] [Google Scholar]
- 7.Cheng L, Hostetler KY, Lee J, et al. Characterization of a novel intraocular drug-delivery system using crystalline lipid antiviral prodrugs of ganciclovir and cyclic cidofovir. Invest Ophthalmol Vis Sci. 2004;45:4138–44. doi: 10.1167/iovs.04-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng L, Hostetler KY, Valiaeva N, et al. Intravitreal Crystalline Drug Delivery for Intraocular Proliferation Diseases. Invest Ophthalmol Vis Sci. 2010;51:474–81. doi: 10.1167/iovs.09-3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rahhal FM, Arevalo JF, Chavez de la Paz E, Munguia D, Azen SP, Freeman WR. Treatment of cytomegalovirus retinitis with intravitreous cidofovir in patients with AIDS. A preliminary report. Ann Intern Med. 1996;125:98–103. doi: 10.7326/0003-4819-125-2-199607150-00003. [DOI] [PubMed] [Google Scholar]
- 10.Taskintuna I, Rahhal FM, Arevalo JF, et al. Low-dose intravitreal cidofovir (HPMPC) therapy of cytomegalovirus retinitis in patients with acquired immune deficiency syndrome. Ophthalmology. 1997;104:1049–57. doi: 10.1016/s0161-6420(97)30188-2. [DOI] [PubMed] [Google Scholar]
- 11.Nicolo M, Nasciuti F, Lai S, Ghiglione D, Borgia L, Calabria G. Intravitreal triamcinolone acetonide as primary treatment for diffuse diabetic macular edema: a prospective noncomparative interventional case series. European journal of ophthalmology. 2006;16:129–33. [PubMed] [Google Scholar]
- 12.Cheng L, Hostetler KY, Chaidhawangul S, et al. Intravitreal toxicology and duration of efficacy of a novel antiviral lipid prodrug of ganciclovir in liposome formulation. Invest Ophthalmol Vis Sci. 2000;41:1523–32. [PubMed] [Google Scholar]
- 13.Cheng LY, Rivero ME, Garcia CR, et al. Evaluation of intraocular pharmacokinetics and toxicity of prinomastat (AG3340) in the rabbit. J Ocul Pharmacol Th. 2001;17:295–304. doi: 10.1089/108076801750295326. [DOI] [PubMed] [Google Scholar]
- 14.Falkenstein IA, Cheng L, Jones TR, et al. Intraocular properties of a repository urokinase receptor antagonist a36 Peptide in rabbits. Current eye research. 2010;35:742–50. doi: 10.3109/02713683.2010.486519. [DOI] [PubMed] [Google Scholar]
- 15.Sutter FKP, Gillies MC. Pseudo-endophthalmitis after intravitreal injection of triamcinolone. British Journal of Ophthalmology. 2003;87:972–4. doi: 10.1136/bjo.87.8.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beadle JR, Hartline C, Aldern KA, et al. Alkoxyalkyl esters of cidofovir and cyclic cidofovir exhibit multiple-log enhancement of antiviral activity against cytomegalovirus and herpesvirus replication in vitro. Antimicrob Agents Ch. 2002;46:2381–6. doi: 10.1128/AAC.46.8.2381-2386.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hostetler KY, Rought S, Aldern KA, Trahan J, Beadle JR, Corbeil J. Enhanced antiproliferative effects of alkoxyalkyl esters of cidofovir in human cervical cancer cells in vitro. Mol Cancer Ther. 2006;5:156–9. doi: 10.1158/1535-7163.MCT-05-0200. [DOI] [PubMed] [Google Scholar]
- 18.Wang H, Chhablani J, Freeman WR, et al. Intraocular safety and pharmacokinetics of hexadecyloxypropyl-cidofovir (HDP-CDV) as a long-lasting intravitreal antiviral drug. Invest Ophthalmol Vis Sci. 2011;52:9391–6. doi: 10.1167/iovs.11-8293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Furton KG, Norelus A. Determining the Critical Micelle Concentration of Aqueous Surfactant Solutions - Using a Novel Colorimetric Method. J Chem Educ. 1993;70:254–7. [Google Scholar]
- 20.Kim JS, Beadle JR, Freeman WR, et al. A novel cytarabine crystalline lipid prodrug: hexadecyloxypropyl cytarabine 3′,5′-cyclic monophosphate for proliferative vitreoretinopathy. Molecular vision. 2012;18:1907–17. [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng L, Hostetler KY, Chaidhawangul S, et al. Treatment or prevention of herpes simplex virus retinitis with intravitreally injectable crystalline 1-O-hexadecylpropanediol-3-phospho-ganciclovir. Invest Ophthalmol Vis Sci. 2002;43:515–21. [PubMed] [Google Scholar]
- 22.Cheng L, Hostetler KY, Chaidhawangul S, et al. Treatment of herpes retinitis in an animal model with a sustained delivery antiviral drug, liposomal 1-O-octadecyl-SN-glycerol-3-phosphonoformate. Retina. 1999;19:325–31. doi: 10.1097/00006982-199907000-00010. [DOI] [PubMed] [Google Scholar]
- 23.Group CR, Martin DF, Maguire MG, et al. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. The New England journal of medicine. 2011;364:1897–908. doi: 10.1056/NEJMoa1102673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li N, Zhuang C, Wang M, Sun X, Nie S, Pan W. Liposome coated with low molecular weight chitosan and its potential use in ocular drug delivery. International Journal of Pharmaceutics. 2009;379:131–8. doi: 10.1016/j.ijpharm.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 25.Nagarwal RC, Kant S, Singh PN, Maiti P, Pandit JK. Polymeric nanoparticulate system: A potential approach for ocular drug delivery. J Control Release. 2009;136:2–13. doi: 10.1016/j.jconrel.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 26.Gao Y, Sun Y, Ren F, Gao S. PLGA–PEG–PLGA hydrogel for ocular drug delivery of dexamethasone acetate. Drug Development and Industrial Pharmacy. 2010;36:1131–8. doi: 10.3109/03639041003680826. [DOI] [PubMed] [Google Scholar]
- 27.Short BG. Safety Evaluation of Ocular Drug Delivery Formulations: Techniques and Practical Considerations. Toxicol Pathol. 2008;36:49–62. doi: 10.1177/0192623307310955. [DOI] [PubMed] [Google Scholar]
- 28.Kim H, Robinson SB, Csaky KG. Investigating the Movement of Intravitreal Human Serum Albumin Nanoparticles in the Vitreous and Retina. Pharm Res-Dord. 2009;26:329–37. doi: 10.1007/s11095-008-9745-6. [DOI] [PubMed] [Google Scholar]
- 29.Davson H, Luck CP. Chemistry and Rate of Turnover of the Ocular Fluids of the Bush Baby (Galago-Crassicaudatus-Agisymbanus) J Physiol-London. 1959;145:433. doi: 10.1113/jphysiol.1959.sp006151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moseley H, Foulds WS, Allan D, Kyle PM. Routes of Clearance of Radioactive Water from the Rabbit Vitreous. British Journal of Ophthalmology. 1984;68:145–51. doi: 10.1136/bjo.68.3.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Henry K, Cantrill H, Fletcher C, Chinnock BJ, Balfour HH. Use of intravitreal ganciclovir (dihydroxy propoxymethyl guanine) for cytomegalovirus retinitis in a patient with AIDS. Am J Ophthalmol. 1987;103:17–23. doi: 10.1016/s0002-9394(14)74163-7. [DOI] [PubMed] [Google Scholar]
- 32.Cundy K, Lynch G, Shaw J, Hitchcock M, Lee W. Distribution and metabolism of intravitreal cidofovir and cyclic HPMPC in rabbits. Current eye research. 1996;15:569–76. doi: 10.3109/02713689609000768. [DOI] [PubMed] [Google Scholar]
- 33.Dolnak DR, Munguia D, Wiley CA, et al. Lack of Retinal Toxicity of the Anticytomegalovirus Drug (S)-1-(3-Hydroxy-2-Phosphonylmethoxypropyl) Cytosine. Invest Ophth Vis Sci. 1992;33:1557–63. [PubMed] [Google Scholar]
- 34.Taskintuna I, Rahhal FM, Capparelli EV, Cundy KC, Freeman WR. Intravitreal and plasma cidofovir concentrations after intravitreal and intravenous administration in AIDS patients with cytomegalovirus retinitis. J Ocul Pharmacol Th. 1998;14:147–51. doi: 10.1089/jop.1998.14.147. [DOI] [PubMed] [Google Scholar]
- 35.Cheng LY, Hostetler KY, Toyoguchi M, et al. Ganciclovir release rates in vitreous from different formulations of 1-O-hexadecylpropanediol-3-phospho-ganciclovir. J Ocul Pharmacol Th. 2003;19:161–9. doi: 10.1089/108076803321637690. [DOI] [PubMed] [Google Scholar]
- 36.Jabs DA, Ahuja A, Van Natta M, Lyon A, Srivastava S, Gangaputra S. Course of cytomegalovirus retinitis in the era of highly active antiretroviral therapy: five-year outcomes. Ophthalmology. 2010;117:2152–61. e1–2. doi: 10.1016/j.ophtha.2010.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. The New England journal of medicine. 1998;338:853–60. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 38.Freeman WR. New developments in the treatment of CMV retinitis. Ophthalmology. 1996;103:999–1000. doi: 10.1016/s0161-6420(96)30548-4. [DOI] [PubMed] [Google Scholar]
- 39.De Cian W. Cidofovir, a new addition to the available therapies against cytomegalovirus. Mg Virology. 1998;21:215–28. [Google Scholar]