Abstract
Rejection remains a major clinical challenge limiting allograft survival after solid organ transplantation. Both cellular and humoral immunity contribute to this complication, with increased recognition of antibody-mediated damage during acute and chronic rejection. Using a mouse model of MHC-mismatched heart transplantation, we report markedly protective effects of Notch inhibition, dampening both T cell and antibody-driven rejection. T cell-specific pan-Notch blockade prolonged heart allograft survival and decreased IFNγ and IL-4 production by alloreactive T cells, especially when combined with depletion of recipient CD8+ T cells. These effects were associated with decreased infiltration by conventional T cells and an increased proportion of regulatory T cells in the graft. Transient administration of neutralizing antibodies specific for Delta-like1/4 (Dll1/4) Notch ligands in the peri-transplant period led to prolonged acceptance of allogeneic hearts, with superior outcome over Notch inhibition only in T cells. Systemic Dll1/4 inhibition decreased T cell cytokines and graft infiltration, but also germinal center B cell and plasmablast numbers as well as production of donor-specific alloantibodies and complement deposition in the transplanted hearts. Dll1 or Dll4 inhibition alone provided partial protection. Thus, pathogenic signals delivered by Dll1/4 Notch ligands early after transplantation promote organ rejection through several complementary mechanisms. Transient interruption of theses signals represents a new attractive therapeutic strategy to enhance long-term allograft survival.
Introduction
Immune-mediated rejection limits the success of organ transplantation in patients. Acute rejection causes morbidity and mortality, as well as a need for urgent retransplantation in selected patients. Despite current immunosuppressive strategies, chronic allograft rejection occurs in a majority of recipients, limiting the life span of transplanted organs. Alloreactive conventional T cells play a central role in the rejection process and represent the main target of existing interventions, while regulatory T cells (Tregs) have protective effects (1). Alternative pathogenic mechanisms are increasingly recognized in both acute and chronic rejection, including a central role for donor-specific antibodies and complement deposition (2-6). New therapeutic interventions are needed to better preserve allografts from these different forms of immune-mediated damage.
Notch signaling was first recognized for its requirement at early stages of T cell development in the thymus (7, 8). Subsequently, other effects of Notch signaling were discovered in the regulation of T cell differentiation and function as well as in selected B cell subsets and innate lymphoid cells (9-11). Notch signals are mediated by the interaction of cell-surface Notch receptors (Notch1-4) with agonistic Delta-like (Dll1/4) or Jagged (Jagged1/2) ligands (12). Notch ligand-receptor binding triggers regulated proteolysis of the receptor, leading to the release of intracellular Notch (ICN) (13). ICN migrates into the nucleus where it interacts with the DNA-binding transcription factor CSL/RBP-Jk and a member of the Mastermind-like (MAML) family of transcriptional coactivators (14-16). Truncated N-terminal MAML fragments with potent and specific dominant negative activity (DNMAML) block transcriptional activation downstream of all Notch receptors (17, 18). DNMAML expression represents a powerful approach to capture the overall effects of canonical Notch signaling in specific cell types (17, 19-23). In addition, targeted inhibition of specific Notch ligands and receptors can identify the unique effects of individual family members in vivo and provide new therapeutic opportunities (21, 24, 25).
Major regulatory effects of Notch signaling in alloreactive T cell immunity were recently discovered in mouse models of allogeneic bone marrow transplantation (21, 23, 26). Inhibition of all Notch signals in donor T cells led to potent protection from acute graft-versus-host disease (GVHD) (21, 23). Notch1/2 receptors and Dll1/4 Notch ligands accounted for all the effects of Notch signaling in GVHD, with dominant roles for Notch1 and Dll4 (21). Transient inhibition of Dll1/4 in the peri-transplant period led to prolonged GVHD control. Notch blockade markedly reduced the production of inflammatory cytokines, while increasing Treg expansion. Notch-deprived alloreactive T cells showed features of acquired hyporesponsiveness, suggesting that Notch should be considered as a new major regulator of alloreactivity and tolerance (21, 26, 27). In organ rejection, initial work using exposure of T cells to overexpressed Notch ligands showed a potential role of Notch in tolerance induction (27-30). However, due to the artificial nature of this experimental system, no definitive information could be gathered about the role of endogenous Notch signals in transplant rejection. Riella and collaborators targeted Dll1 Notch ligands with monoclonal antibodies in a mouse model of heart transplantation (31). In combination with B7/CD28 blockade, they observed a significant although modest protective effect of Dll1 blockade associated with STAT6-dependent Th2 polarization. In contrast, Jagged2-mediated agonism mediated increased rejection (32). These observations are consistent with a tolerogenic effect of Notch inhibition during graft rejection. However, they may markedly underestimate the full impact of the Notch pathway as the study focused only on blocking a single Notch ligand and only partial inhibition of Notch signaling was achieved, as evidenced by the persistence of Dll1-dependent marginal zone B cells in this model (31, 33). In addition, the mechanisms of protection may differ from those seen with more efficient methods of Notch inhibition across multiple ligands or receptors.
To comprehensively evaluate the role of Notch signaling in transplant rejection, we combined a genetic approach to block all canonical Notch signals in host T cells and a biochemical strategy to achieve potent systemic inhibition of Delta-like1/4 Notch ligands. This approach allowed us to capture the overall effects of Notch signaling in alloreactive T cells, while investigating both T cell-intrinsic and extrinsic consequences of systemic Dll1/4 inhibition. Notch blockade in T cells increased the survival of heart allografts, especially when combined with transient CD8+ T cell depletion in the recipients, suggesting that alloreactive CD4+ T cells are particularly sensitive to the effects of Notch inhibition. Dll1/4 blockade induced superior protection from rejection as compared to inhibition of Notch signaling only in T cells. Importantly, transient Dll1/4 inhibition was sufficient to induce long-term graft acceptance and inhibited pathogenic T cells, while decreasing the numbers of germinal center B cells and plasmablasts as well as the production of donor-specific alloantibodies. This represents a different, broader and more efficient immune intervention than previously reported only with partial Dll1 inhibition. Our data identify potent immunobiological effects of Notch signaling in multiple pathogenic aspects of alloreactivity. These observations suggest new Notch-based strategies to control organ rejection that could be considered for human interventions.
Material and Methods
Mice
BALB/c (H-2d) and C57BL/6 (H-2b) mice were obtained from Charles River Laboratories (Raleigh, NC). Cd4-Cre+ x ROSADNMAMLf mice on a C57BL/6 background (abbreviated DNMAML) were described previously to express the DNMAML-GFP pan-Notch inhibitor in all mature CD4+ and CD8+ T cells (21-23, 26). This strategy allowed for efficient blockade of Notch transcriptional activation downstream of all Notch receptors in T cells, without interference with Notch signaling at early stages of T cell development. All mice were housed under specific pathogen-free conditions in the Unit for Laboratory Animal Medicine at the University of Michigan. Experiments were performed according to NIH guidelines and approved by the University of Michigan’s Committee on the Use and Care of Animals.
Vascularized cardiac transplantation
Heterotopic transplantation of intact allogeneic BALB/c hearts into C57BL/6 (B6) or B6-DNMAML (DNMAML) recipients was performed as described (34, 35). Briefly, the aorta and pulmonary artery of donor hearts were anastomosed end-to-side to the recipient’s abdominal aorta and inferior vena cava, respectively. Upon perfusion with the recipient’s blood, the transplanted heart resumes contraction. Graft function was monitored by abdominal palpation. Rejection was scored based on the cessation of heart contraction.
In vivo depletion of CD8+ T cells
The hybridoma secreting anti-CD8 monoclonal antibody (clone 2.43) was obtained from American Type Culture Collection (Manassas, VA). Anti-CD8 antibodies were purified and resuspended in PBS by Bio X Cell (West Lebanon, NH). Where indicated, allograft recipients received 1 mg of anti-CD8 mAb i.p. on days −1, 0, and 7 relative to the day of transplantation. The efficiency of CD8 depletion was verified in pilot experiments (typically near complete depletion for about 2 weeks, followed by gradual return to approximately 50% of normal levels by day 50) (36, 37).
In vivo antibody-mediated inhibition of Notch ligands
Humanized IgG1 neutralizing monoclonal antibodies specific for the Dll1 or Dll4 extracellular domain were described previously (21, 24). An irrelevant human IgG1 antibody specific for herpes simplex virus gD protein (anti-GD) was used as isotype control in selected experiments. Antibodies were administered i.p. (5 mg/kg). The potency and specificity of each batch of antibody was verified by in vivo administration and assessment of Dll1-dependent marginal zone B cells and Dll4-dependent thymocytes, as described (21). Antibodies were administered on day 0 after surgery and repeated on days 3, 7 and 10 after transplantation.
Histology and assessment of graft-infiltrating cells
Allografts were recovered either at the time of rejection or at prespecified time points, fixed in formalin, and embedded in paraffin. When prespecified time points were used, graft survival data from these particular recipients were censored. Sections were stained with H&E to assess myocyte viability (presence of cross striation and myocyte nuclei) and the nature, intensity, and localization of graft-infiltrating cells (GICs). For isolation and quantification of GICs, portions of the transplanted hearts were weighed, pooled, minced, and digested with 1 mg/ml collagenase A (Roche Diagnostics, Indianapolis, IN) for 30 min at 37°C. After tissue debris settled, suspended GICs were harvested. RBCs were lysed by hypotonic shock, and GICs were passed through a 30 μm nylon mesh. Viable cells were enumerated by Trypan blue exclusion and/or assessed by flow cytometry.
Flow cytometry
The following antibodies were used: anti-CD4, CD8α, CD19, CD45.2, CD95, CD138, B220, I-A/I-E, TCRβ, Thy1.2, GL7 (Biolegend, San Diego, CA); and anti-FoxP3 (eBioscience, San Diego, CA). For intracellular FoxP3 staining, we used a fixation/permeabilization kit according to the manufacturer’s instructions (eBioscience). Dead cell exclusion was performed though the addition of DAPI or Ghost Violet (Tonbo Biosciences, San Diego, CA). Samples were evaluated on a BD Fortessa analyzer. In selected cases, sorting was performed on BD Aria III (Becton Dickinson, San Jose, CA). Flow cytometry files were analyzed using FlowJo (TreeStar, Ashland, OR).
Immunohistochemistry for C4d deposition in allografts
Paraffin embedded allografts were sectioned, then de-paraffinized and processed for antigen retrieval in Trilogy (Cell Marque, Rocklin, CA). As previously described (38), sections were incubated with rabbit anti-mouse C4d (kindly provided by Dr. William Baldwin, Cleveland Clinic) at a 1:500 dilution followed by detection and DAB development using the SuperPicture™ Polymer Detection Kit (Invitrogen, Grand Island, NY), then counterstained with hematoxylin.
ELISPOT assays
For enumeration of alloreactive cytokine-producing cells, ELISPOT assays were performed as previously described (39, 40). Splenocytes were cultured in RPMI 1640 supplemented with 2% FCS, 1 mM sodium pyruvate, 100 U/ml penicillin, 100 μg/ml streptomycin, 1.6 mM L-glutamine, 10 mM HEPES buffer (Gibco Life Technologies, Grand Island, NY), 0.27 mM L-asparagine, 0.55 mM L-arginine HCl, 14 μM folic acid, and 50 μM 2-ME (Sigma-Aldrich, St. Louis, MO). Capture and detection antibodies (BD Biosciences, San Jose, CA) were as follows: IFN-γ (R4-6A2, XMG1.2), IL-4 (11B11, BVD6-24G2), and IL-17 (TC11-18H10, TC11-8H4.1). Polyvinylidene difluoride-backed microtiter plates (Millipore, Billerica, MA) were coated with unlabeled mAb and blocked with 1% BSA in PBS. Irradiated (1000 rad) BALB/c splenocytes (4×105) and 1×106 recipient splenocytes were added to the plates for 24 hours. After washing, a 1/1000 dilution of anti-biotin alkaline phosphatase conjugate (Vector Laboratories, Burlingame, CA) was added to IFN-γ and IL-17 plates, and a 1/2000 dilution of HRP conjugated streptavidin (Dako, Carpinteria, CA) was added to IL-4 plates. Plates were washed and spots were visualized by addition of NBT (Bio-Rad, Hercules, CA)/3-bromo-4-chloro indolyl phosphate (Sigma-Aldrich, St. Louis, MO) to IFN-γ and IL-17 plates, or 3-amino-9-ethylcarbazole (Pierce) to IL-4 plates. Color development continued until spots were visible and stopped by adding H2O. Plates were dried and spots were quantified with an Immunospot Series 1 ELISPOT analyzer (Cellular Technology Ltd., Shaker Heights, OH).
Quantitative reverse transcription-PCR
For RNA extraction, cardiac allografts were homogenized in Trizol (Life Technologies) followed by extraction according to the manufacturer’s protocol. cDNA was prepared with Superscript II (Invitrogen). Real-time PCR was performed in triplicate for each sample with SYBR Green PCR Master Mix and analyzed on a Mastercycler ep realplex (Eppendorf). Relative transcript abundance was calculated using the ΔΔCt method after normalization with Cd3 to account for the abundance of T cells in the graft. Primer sequences were obtained from the PrimerBank (http://pga.mgh.harvard.edu/primerbank). For analysis of Dtx1 Notch target gene expression, RNA was extracted from sorted lymphocyte populations and processed as above, but using Taqman primers with normalization to Hprt (Applied Biosystems).
Quantification of donor-reactive antibodies
As described (38, 41), P815 (H-2d) cells (American Type Culture Collection, Manassas, VA) were stained for flow cytometric analysis using a 1/50 dilution of sera obtained from cardiac allograft recipients as the primary antibody, followed by FITC-conjugated rabbit anti-mouse IgG antibody (Invitrogen Life Technologies, Grand Island, NY) used at a 1/50 dilution. Data are reported as the mean channel fluorescence determined on a Becton Dickinson FACScan (San Jose, CA).
Statistical analysis
Allograft survival curves were analyzed using a log-rank test. Significance of ELISPOT and alloantibody results was determined by an unpaired t test with Welch’s correction. All data were analyzed using GraphPad Prism v. 6.0. p values ≤0.05 were considered statistically different.
Results
Delayed rejection of allogeneic hearts upon T cell-specific pan-Notch inhibition
To evaluate the role of Notch signaling in host T cells during allograft rejection, we first used transplantation of MHC-mismatched BALB/c hearts into C57BL/6 (B6) recipient mice. This model triggers an acute form of cellular rejection dominated by a Th1 pattern of cytokine release as well as production of donor-reactive alloantibodies (37, 42-44). To block Notch signaling in T cells, we studied mice expressing the pan-Notch inhibitor DNMAML in all mature CD4+ and CD8+ T cells (Cd4-Cre+ x ROSADNMAMLf or DNMAML mice) (21-23, 26, 45). DNMAML expression blocks transcriptional activation downstream of all Notch receptors, an efficient strategy to capture all the effects of canonical Notch signaling in T cells (17, 21-23, 26). In wild-type B6 recipients, hearts were rejected after a median observation of only 7 days, consistent with past observations (Fig. 1A) (42-44). In DNMAML recipients, allograft survival was doubled with a median rejection occurring at day 14. DNMAML expression in T cells led to decreased numbers of graft-infiltrating cells when assessed at the time of rejection (Fig. 1B-C). DNMAML mice had lower numbers of alloreactive IFNγ-producing cells in the spleen, without increase in IL-4-producing cells (Fig. 1D). Despite this effect on cytokine production, Notch inhibition in T cells did not prevent the appearance of donor-reactive IgG antibodies (Fig. 1E). Thus, Notch blockade in T cells increased allograft survival in this acute rejection model, although without providing long-term protection.
Figure 1. T cell-specific Notch inhibition delays the rejection of heart allografts.
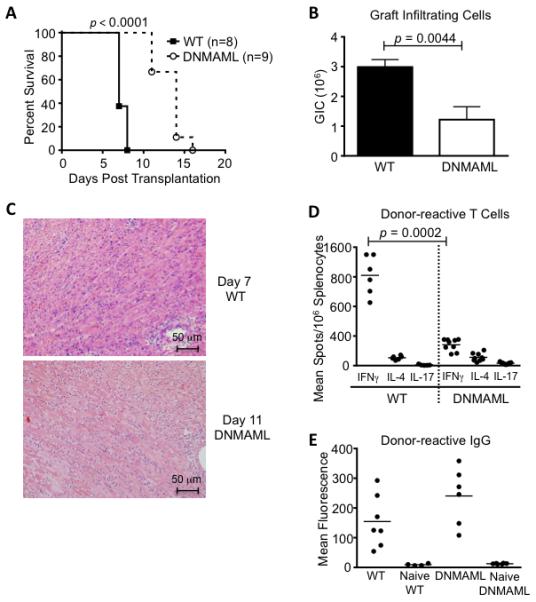
(A) Vascularized cardiac BALB/c allografts were established in wild-type C57BL/6 recipients (WT, n=8) or in C57BL/6 mice expressing the pan-Notch inhibitor DNMAML in mature T cells (DNMAML, n=9). Arrows indicate hearts still functioning at the time of sacrifice. Survival of the heart allografts was prolonged in DNMAML recipients (p<0.0001, % survival scored based on abdominal palpation); (B) Decreased numbers of graft-infiltrating cells (GICs) in DNMAML as compared to WT recipients at the time of rejection (n=8-9/group); (C) Histology showing cellular infiltrates in the rejected hearts (WT: day 7; DNMAML: day 11); (D) Enumeration of donor-reactive cells producing IFNγ, IL-4 and IL-17 in the spleen of transplant recipients, showing decreased IFNγ-producing cells in DNMAML vs. WT recipients (p=0.0002); (E) Quantification of donor-reactive IgG in the serum of WT and DNMAML recipients. DNMAML did not prevent the emergence of these antibodies (p=0.1226 for WT vs. DNMAML IgG). Naïve WT and DNMAML sera served as negative controls.
Markedly delayed organ rejection when combining transient CD8 depletion and pan-Notch inhibition in T cells
To evaluate the impact of Notch inhibition specifically in CD4+ T cells, we depleted CD8+ T cells in wild-type and DNMAML mice (Fig. 2). This inductive strategy eliminated CD8+ T cells from the periphery for at least 2 weeks, with a gradual return to ca. 50% of levels observed in non-depleted mice by day 50 after treatment ((36, 37) and data not shown). CD8+ T cell depletion is accompanied by a decrease in IFNγ-producing cells and an increase in IL-4-producing cells in this model (43). In wild-type recipients, transient antibody-mediated depletion of CD8+ T cells in the peri-transplant period provided modest prolongation of allograft survival, as previously reported (Fig. 2A compared to Fig. 1A) (43). In contrast, rejection was markedly delayed to a median of 45 days in CD8-depleted B6 DNMAML recipients. DNMAML expression decreased the number of graft-infiltrating cells at day 14 and day 30 (Fig. 2B-C). ELISPOT assays in the spleen showed a trend for decreased IFNγ-producing cells and a significant decrease in IL-4-producing cells in DNMAML as compared to B6 recipients (Fig. 2D). DNMAML expression also decreased the titers of donor-specific alloantibodies at day 14, although not at later time points (Fig. 2E). Altogether, alloreactive CD4+ T cells appeared particularly sensitive to Notch inhibition in the setting of CD8 depletion. Pan-Notch inhibition in CD4+ T cells decreased both Th1 and Th2 cytokines as well as T cell help to alloantibody-secreting cells.
Figure 2. Combined Notch inhibition in T cells and CD8 depletion markedly delays heart allograft rejection and decreases the number of cytokine-producing donor-reactive T cells.
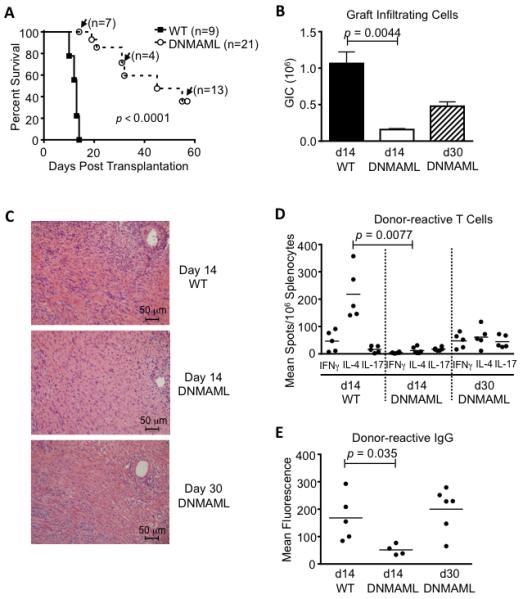
(A) After antibody-mediated CD8 depletion, vascularized cardiac BALB/c allografts were established in wild-type C57BL/6 recipients (WT, n=9) or in C57BL/6 mice expressing DNMAML in mature T cells (DNMAML, n=21). Survival of the heart allografts was markedly prolonged in DNMAML recipients (p<0.0001). Arrows indicate functioning grafts that were harvested at defined time points before rejection for preplanned immunological analysis (with subsequent graft survival data censored); (B) Decreased numbers of graft-infiltrating cells at day 14 in DNMAML recipients. Slightly higher numbers were recovered subsequently at day 30 (d14 WT: n=9; d14 DNMAML n=7; d30 DNMAML n=6); (C) Histology showing cellular infiltrates in the rejected hearts at day 14 or day 30; (D) Enumeration of donor-reactive cells producing IFNγ, IL-4 and IL-17 in the spleens of transplant recipients. IL-4 production dominates in the CD8-depleted model (43) and was markedly decreased in DNMAML recipients (p=0.0077); (E) Donor-reactive serum IgG, showing decreased levels at day 14 in DNMAML recipients (p=0.0351), but subsequent rise at day 30.
Decreased cytokine production and T cell graft infiltration upon transient CD8 depletion and inhibition of Dll1/Dll4 Notch ligands
We previously showed dominant collective effects of Dll1 and Dll4 Notch ligands in the pathogenesis of acute GVHD after allogeneic bone marrow transplantation (21). Thus, we compared the effects of DNMAML-mediated pan-Notch inhibition in T cells to those of systemic inhibition of Dll1/4 Notch ligands during allograft rejection. To achieve potent and specific Dll1/4 blockade, we injected humanized neutralizing monoclonal antibodies, starting immediately after heart transplantation and on days 3, 7 and 10 post-transplant (21, 24, 25). DNMAML was expressed in the vast majority of CD4+ T cells as shown by detection of the DNMAML-GFP fusion protein in these cells at day 14 after transplantation (Fig. 3A), as well as in residual CD8+ T cells (data not shown). Both DNMAML expression and systemic Dll1/4 blockade profoundly decreased the abundance of Dtx1 Notch target gene transcripts in T cells, indicating efficient Notch inhibition (Fig. 3A). Transient Dll1/4 blockade had superior activity in reducing the number of graft-infiltrating cells at early and at late time points after transplantation (Fig. 3B). T cell-specific DNMAML expression and systemic Dll1/4 blockade both decreased the numbers of IFNγ and IL-4-producing cells at day 14 (Fig. 3C). Interestingly, the effects of Dll1/4 blockade were more persistent than those of DNMAML expression at day 30 and 50, even though anti-Dll1/4 antibodies were only administered transiently for 10 days after transplantation.
Figure 3. Transient CD8 depletion and inhibition of Dll1/Dll4 Notch ligands decrease T cell graft infiltration and production of multiple inflammatory cytokines.
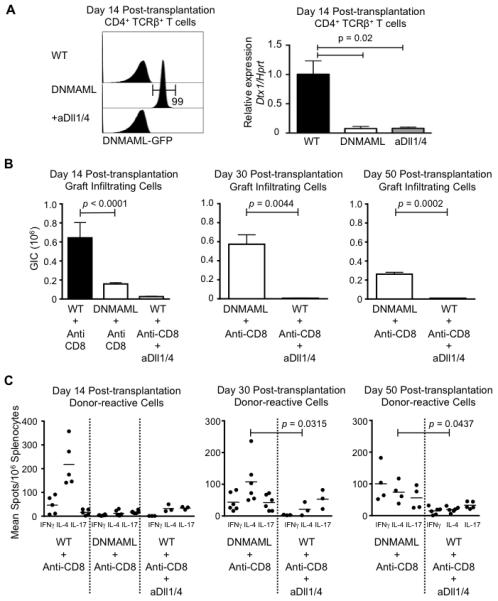
(A) Analysis of Notch inhibition in T cells via DNMAML expression or systemic anti-Dll1/4 treatment. Left: flow cytometric analysis of DNMAML-GFP expression in CD4+ T cells at day 14 after transplantation, showing DNMAML expression in nearly all T cells. Right: abundance of Dtx1 Notch target gene transcripts in sort-purified CD4+ T cells at day 14 after transplantation (qRT-PCR), showing an equivalent decrease in DNMAML and anti-Dll1/4-treated WT T cells (aDll1/4); (B) After CD8 depletion, BALB/c heart allografts were established in wild-type (WT) B6 recipients with intact Notch signaling (WT + anti-CD8), T cell-specific Notch inhibition (DNMAML + anti-CD8) and peri-transplant systemic inhibition of Dll1/4 Notch ligands (days 0, 3, 5, 7) (WT + anti-CD8 + anti-Dll1/4). Recovery of graft-infiltrating cells was markedly decreased in anti-Dll1/Dll4-treated wild-type mice at day 14, 30 and 50 after transplantation (n=3-7/group). The effects of anti-Dll1/4 therapy were more pronounced and persistent than with DNMAML-mediated Notch inhibition in T cells; (C) Cytokine-producing cells were enumerated in the spleen at day 14, day 30 and day 50 post-transplantation. Both DNMAML and Dll1/4 blockade decreased IFNγ and IL-4 production, although the effects of Dll1/4 blockade were more persistent. Day 14 WT or DNMAML and day 30 DNMAML data are also shown in Fig. 2D but repeated here for the sake of comparison.
To further understand the effects of Notch inhibition, we assessed expression of Ifng and key mediators T cell cytotoxicity (Fig. 4A). Ifng, Prf1 and Gzmb transcripts were decreased in the heart allografts of DNMAML and anti-Dll1/Dll4-treated wild-type recipients. In contrast, Foxp3 expression was relatively increased upon Notch inhibition. Consistent with these findings, flow cytometric analysis showed a relative increase in the frequency of FoxP3-expressing in graft-infiltrating CD4+ T cells of DNMAML and anti-Dll1/4-treated transplant recipients, with no change among CD4+ splenocytes (Fig. 4B). In terms of absolute numbers, graft-infiltrating FoxP3− conventional T cells were decreased in both DNMAML and anti-Dll4-treated mice, while absolute numbers of FoxP3+ Tregs were not significantly changed (Fig. 4C). The Tconv/Treg ratio was decreased in the graft but not in the spleen of DNMAML or anti-Dll1/4-treated mice (Fig. 4D). Thus, both methods of Notch inhibition decreased the numbers of conventional effector T cells in the graft while increasing the relative frequency of Tregs.
Figure 4. Both DNMAML expression and systemic Dll1/Dll4 inhibition decrease conventional effector T cells while increasing the relative numbers of FoxP3+ regulatory T cells in the allograft.
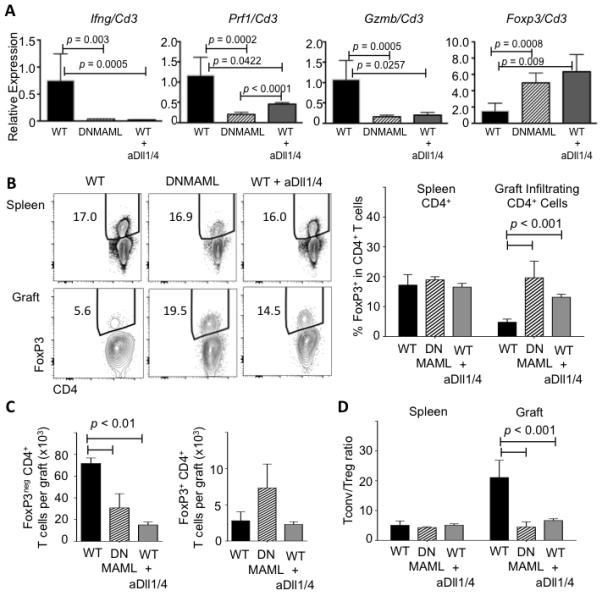
(A) qRT-PCR analysis of Ifng, Prf1, Gzmb and Foxp3 transcripts in RNA extracted from heart allografts on day 14 post-transplantation (n=3-7/group). Normalization was with Cd3 transcripts to account for variable numbers of infiltrating T cells; (B) Flow cytometric detection of intracellular FoxP3 in splenic (top) and graft-infiltrating CD4+ T cells (bottom) at day 14 post-transplantation in WT, DNMAML and anti-Dll1/4-treated WT recipients (n=4/group); (C) Absolute number of FoxP3− conventional (Tconv) and FoxP3+ regulatory T cells (Treg); (D) Tconv/Treg CD4+ T cell ratio. Left: splenocytes. Right: graft-infiltrating T cells.
Decreased production and deposition of donor-reactive antibodies after systemic Dll1 and Dll4 blockade in CD8-depleted wild-type mice
To evaluate if systemic Dll1/4 inhibition had effects outside of the T cell lineage that could contribute to decreased rejection, we assessed the impact of Dll1/Dll4 blockade on alloantibody production (Fig. 5). At early time points after transplantation (day 14), both DNMAML expression in T cells and systemic Dll1/4 inhibition decreased serum titers of donor-reactive IgG antibodies, but with more potent inhibition upon Dll1/4 blockade (Fig. 5A). At later time points (day 30 and 50), wild-type recipients were not available for comparison due to prior allograft rejection, while DNMAML recipients had rising titers of donor-reactive alloantibodies (Fig. 2A, Fig. 5A). The emergence of donor-reactive antibodies was associated with eventual graft loss in DNMAML mice (Fig. 2A). In contrast, alloantibody titers remained very low at day 30-50 after systemic Dll1/4 inhibition (Fig. 5A). Next, we compared the effects of DNMAML expression and systemic Dll1/4 inhibition on CD138+ plasmablasts in the spleen (Fig. 5B). DNMAML-mediated Notch inhibition in T cells induced a significant but modest decrease in plasmablasts, presumably through an indirect effect on T cell help. Anti-Dll1/4 antibodies markedly reduced plasmablast numbers, to the same extent as their effects on alloantibody titers (Fig. 5A, 5B). Numbers of splenic B220+CD95+GL7+ germinal center B cells were also profoundly decreased in anti-Dll1/4-treated but not in DNMAML recipients (Fig. 5C). Finally, systemic Dll1/4 inhibition but not DNMAML expression in T cells eliminated marginal zone B cells (data not shown), consistent with the Dll1-dependence of this population (33) and further revealing the direct impact of Dll1/4 Notch ligands on B cell populations. At the termination of the experiment, immunohistochemistry showed complement deposition within the heart allografts in DNMAML recipients, but not in anti-Dll1/4-treated mice (Fig. 5D). These findings suggest that systemic blockade of Dll1/4 Notch ligands decreased the number of B lineage cells differentiating into antibody-producing cells, controlling the accumulation of pathogenic alloantibodies and complement deposition in this rejection model.
Figure 5. Systemic Dll1/Dll4 blockade affects B cell populations and leads to decreased production of allograft-reactive antibodies in CD8-depleted mice.
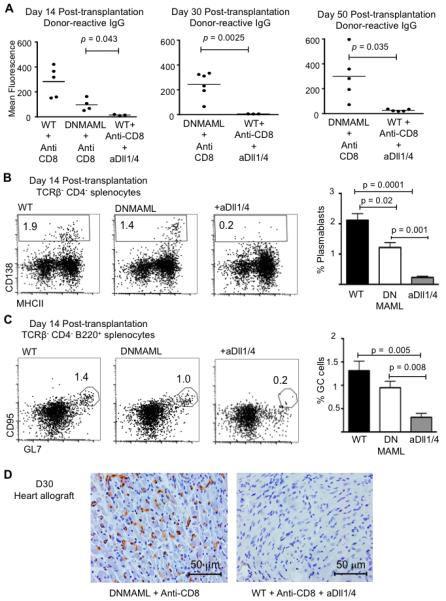
(A) Quantification of donor-reactive IgG antibodies in the serum of transplant recipients at day 14, day 30 and day 50 after transplantation. IgG levels were decreased at day 14 in DNMAML as compared to WT recipients, but rose subsequently. Systemic Dll1 and Dll4 blockade profoundly decreased the accumulation of alloantibodies over the entire observation period; (B) Flow cytometric quantification of CD138+ plasmablasts in the spleen at day 14 post-transplantation in WT, DNMAML and anti-Dll1/4-treated WT recipients (n=4/group). Representative plots are shown after gating on live TCRβ−CD4− cells. Bar graphs show mean ± SEM; (C) Flow cytometric detection of splenic B220+CD95+GL7+ germinal center (GC) B cells at day 14 post-transplantation in WT, DNMAML and WT anti-Dll1/4-treated recipients (n=4/group). Bar graphs show mean ± SEM; (D) Immunohistochemical analysis of C4d deposition in the transplanted hearts at day 30 reveals decreased deposition in WT recipients treated with anti-Dll1/4 as compared to DNMAML recipients. Data are representative of n=5 (DNMAML + anti-CD8) and n=3 allografts analyzed (WT + anti-CD8 + anti-Dll1/4).
Additive effects of Dll1 and Dll4 blockade prevent heart rejection in CD8-depleted mice
Based on the superior effect of transient Dll1/4 blockade on surrogate endpoints of alloreactivity, we tested the overall impact of this strategy on allograft survival and the individual contribution of Dll1 and Dll4 ligands in CD8-depleted recipients (Fig. 6). Either Dll1 or Dll4 inhibition alone markedly prolonged allograft survival and decreased the number of graft-infiltrating cells, indicating that both Notch ligands contributed to promote rejection (Fig. 6A-B). Remarkably, peri-transplant inhibition of both Dll1 and Dll4 allowed 100% long-term graft survival during the entire observation period (Fig. 6A). This is superior to graft survival in CD8-depleted DNMAML recipients (Fig. 2A). Combined Dll1/4 blockade decreased graft infiltration more profoundly than Dll1 or Dll4 inhibition alone (Fig. 6B), while also blocking the emergence of donor-specific alloantibodies (Fig. 6C). Thus, when combined with CD8 depletion, transient systemic Dll1/4 blockade induced long-term acceptance of MHC-mismatched heart allografts.
Figure 6. Additive effects of Dll1 and Dll4 blockade prevent heart rejection in CD8-depleted mice.
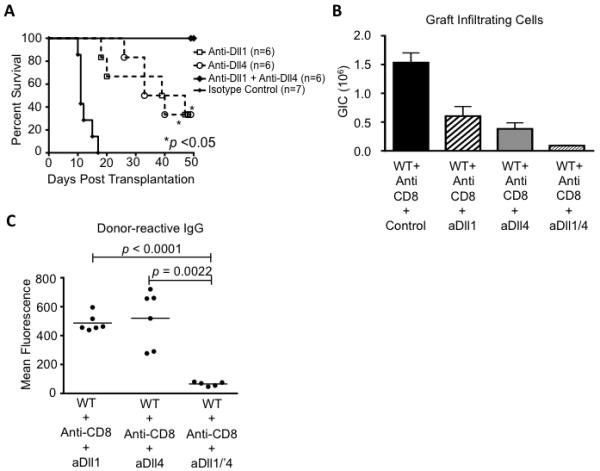
(A) After CD8 depletion, BALB/c heart allografts were established in B6 recipients treated with isotype control (anti-GD, n=7), anti-Dll1 (n=6), anti-Dll4 (n=6) or anti-Dll1+anti-Dll4 antibodies (n=6) (i.p. day 0, 3, 7, 10). Allograft survival was prolonged upon either Dll1 or Dll4 blockade alone (p<0.05). Upon combined Dll1/4 inhibition, no rejection was observed over the entire observation period (50 days); (B) Dll1, Dll4 and Dll1/4 blockade all decrease the number of graft-infiltrating cells at day of rejection or at termination of the experiment, but with more profound effects for combined blockade (p=0.028 vs. anti-Dll1 alone; p=0.038 vs. anti-Dll4 alone) (n=6-7/group); (C) Dll1/4 blockade but not isolated Dll1 or Dll4 inhibition prevents the accumulation of donor-reactive serum IgG antibodies (day 50 after transplantation).
Prolonged protective effects of Dll1/4 blockade even without CD8 depletion
Given its profound effects in the CD8-depleted mice, we assessed the impact of combined Dll1/4 inhibition on rejection of MHC-mismatched allografts in the absence of T cell depletion. In contrast to the modest protective effects of T cell-specific DNMAML expression, systemic Dll1/4 blockade in wild-type recipients markedly prolonged allograft survival to a median duration of 37 days (Fig. 1A, 7A). Rejection in these animals correlated with the presence of serum alloantibodies. When combined with DNMAML expression, peri-transplant Dll1/4 blockade led to long-term graft acceptance for at least 50 days after transplantation, even in the absence of CD8 depletion (Fig. 7A). Anti-Dll1/4 antibodies decreased graft infiltration and production of inflammatory cytokines more profoundly than DNMAML expression alone (Fig. 7B-C). The combination of DNMAML expression and anti-Dll1/4 antibodies was most effective at decreasing the number of cytokine-producing cells and donor-reactive alloantibodies, correlating with absence of rejection in non-CD8-depleted mice (Fig. 7C-D). In this model with an intact CD8+ T cell compartment, our findings suggest that long-term T cell-specific inhibition was important to promote the maximum degree of prolonged graft acceptance, indicating the temporal effects of Notch signaling in CD4+ and CD8+ alloreactive T cells may differ. However, systemic peri-transplant Dll1/4 blockade was required for maximal protection (Fig. 1A, Fig. 7A), suggesting that non-T cell effects of Dll1/4 inhibition play an important role.
Figure 7. Transient Dll1/4 blockade provides prolonged protection from rejection even without CD8 depletion.
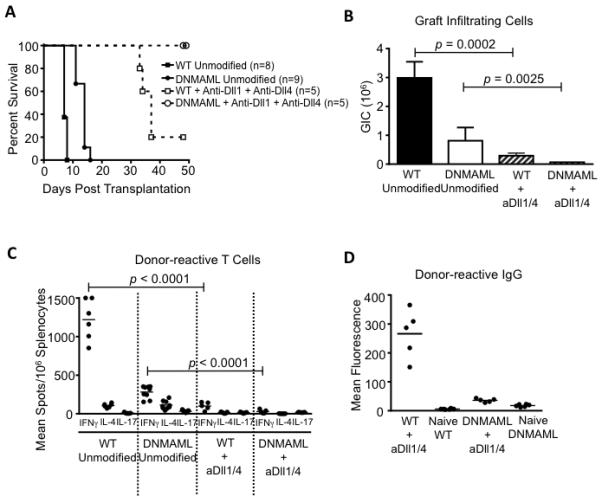
(A) BALB/c heart allografts were established in B6 recipients in the absence of T cell depletion, with intact Notch signaling (WT unmodified, n=8), T cell-specific DNMAML expression (DNMAML unmodified, n=9), Dll1/4 blockade (WT+anti-Dll1+anti-Dll4, n=5) or both DNMAML expression and Dll1/4 blockade (DNMAML+anti-Dll1+anti-Dll4, n=5). DNMAML only modestly prolonged allograft survival over WT recipients. Dll1/4 blockade (day 0, 3, 7, 10) in WT recipients markedly prolonged graft survival (median rejection >35 days). Dll1/4 blockade in DNMAML recipients led to 100% graft survival over the entire observation period; (B) Number of graft-infiltrating cells at the time of rejection or at termination of the experiment (n=5-9/group); (C) Enumeration of cytokine-producing cells in the spleen at the time of rejection or at termination of the experiment; (D) Quantification of serum donor-reactive IgG antibodies. Unlike Dll1/4 inhibition alone, DNMAML expression and anti-Dll1/4 treatment blocked the accumulation of alloantibodies. Selected data for WT and DNMAML recipients presented in Fig. 1 are repeated in Fig. 7A-C for comparison with anti-Dll1/4 treatment.
Discussion
Notch signaling is emerging as a powerful context-specific regulator of antigen-driven immune responses (10, 46, 47). Understanding these effects is essential to characterize the versatile immunobiological effects of Notch signaling, as well as to discover new Notch-based therapeutic interventions with translational potential. Our findings identify a major pathogenic role for Notch signaling driven by two Delta-like Notch ligands (Dll1/4) in cellular and humoral rejection of mouse heart allografts. These effects are consistent with the protective effects of Dll1 inhibition that were previously reported, although those were modest and only identified upon concomitant inhibition of B7/CD28 signaling (31). Past observations were obtained using systemic antibodies that only partially inhibited Dll1-mediated signals, as evidenced by persistence of Dll1-dependent marginal zone B cells (31, 33). In contrast, we used a genetic approach to fully inhibit Notch signaling downstream of all Notch receptors in T cells, as well as systemic administration of potent anti-Dll1 and anti-Dll4 monoclonal antibodies to achieve near complete inhibition of both Delta-like ligands in vivo. Using this strategy, we observed a high degree of protection even without interfering with CD28-mediated signals. Thus, our findings suggest a much broader impact of Notch inhibition than previously observed with systemic Dll1 blockade only (31).
Transient inhibition of Dll1 and Dll4 ligands in the peri-transplant period was sufficient to confer long-term protection, suggesting a tolerogenic effect. Dll1/4 inhibition blocked the production of multiple T cell inflammatory cytokines and decreased the accumulation of conventional effector T cells in the graft, while leading to a relative increase in the frequency of FoxP3+ T cells. These effects of Notch inhibition on the Tconv/Treg balance during graft rejection are reminiscent of our observations in GVHD, suggesting the existence of conserved effects of Notch signaling in alloreactivity (21, 23, 26). In GVHD, we previously showed that the increased relative accumulation of Notch-deficient Tregs correlated with enhanced in vivo expansion of preexisting Tregs (26). In addition to its effects on T cells, systemic administration of anti-Dll1/4 antibodies also blocked the production and deposition of donor-reactive alloantibodies, a major mediator of acute and chronic rejection (2-6). Altogether, these findings suggest a central pathogenic role for signals delivered by Dll1 and Dll4 Notch ligands during early stages after organ transplantation, with long-term immunobiological effects observed even upon transient interruption of these signals. Thus, Dll1 and Dll4 Notch ligands are attractive therapeutic targets to prevent multiple aspects of organ allograft rejection.
In the acute rejection model that we studied, the protective effects of Notch inhibition were most pronounced in CD8-depleted recipients, suggesting that CD4+ alloreactive T cells were particularly sensitive to Notch inhibition. Notch inhibition markedly decreased the number of IL-4-producing alloreactive T cells that were previously shown to dominate in the CD8-depleted model (43). This effect of complete Notch blockade in T cells differed from the Th2 bias observed upon concomitant partial Dll1 inhibition and B7/CD28 blockade (31). Moreover, the effects of Notch blockade were not limited to Th2 differentiation, as IFNγ-producing cells were also affected in this model as well as in the absence of CD8+ T cell depletion. These observations differ from past reports linking Notch signaling preferentially to Th2 differentiation (22, 31, 48), but are consistent with recent observations that Notch can regulate the differentiation and function of multiple T helper lineages (21-23, 26, 48-51). In particular, our observations in transplant rejection have multiple features in common with our findings in GVHD (21, 23, 26). In both cases, transient Notch blockade was sufficient to provide long-term protection and was associated with decreased production of multiple inflammatory cytokines, while preserving or enhancing the expansion of regulatory T cells. After bone marrow transplantation, we showed that Notch blockade induces a hyporesponsive state in alloreactive T cells including features previously reported in models of T cell anergy (26). Thus, Notch signaling may exert a conserved set of effects in T cell alloimmunity, with tolerogenic effects of Notch inhibition in both graft-versus-host and host-versus-graft reactivity.
An interesting consequence of Notch inhibition was to blunt the accumulation of donor-specific alloantibodies, as well as complement deposition in the allograft. Dll1/4 inhibition had particularly profound effects on B lineage cells differentiating into antibody-producing cells and blocked the alloantibody response that correlated with long-term allograft rejection in the CD8-depleted rejection model. Since T cell-specific Notch inhibition with DNMAML also delayed alloantibody production, decreased T cell cytokine production may be involved in this effect. Alternatively, Notch inhibition may block the differentiation and function of T follicular helper cells that support the germinal center reaction, as shown recently with model antigens (52). However, systemic Dll1/4 inhibition had more profound and durable effects on alloantibody production than T cell-specific Notch inhibition in the CD8-depleted model, suggesting that direct effects of Dll1/4 blockade on B lineage cells could play a major role. Possible targets include germinal center B cells and plasma cells, consistent with the markedly decreased numbers of these cells in anti-Dll1/4-treated mice (33, 53-55). Alternatively, it is possible that anti-Dll1/4 antibodies but not DNMAML expression inhibit putative non-canonical effects of Notch signaling in T cells that are independent of transcriptional activation by CSL/RBP-Jk and MAML proteins (47, 56-58). Such non-canonical pathways could control T cell help beyond the effects of canonical Notch signaling. Regardless of the pathways involved, our findings are highly significant, as antibody-mediated mechanisms are increasingly recognized to play an important role in acute and chronic organ rejection (2-6).
We found that Dll1 and Dll4 Notch ligands both contributed to the rejection process, with additive benefits of Dll1 and Dll4 blockade. Thus, the effects of Notch inhibition were underestimated by work using Dll1 blockade alone, especially since only partial Dll1 inhibition was achieved (31). In mouse models of acute graft-versus-host disease, we also observed individual effects of Delta-like family ligands (21), although the relative importance of Dll1 appeared higher in transplant rejection. It remains to be determined if Dll1 and Dll4 exert their effects on the same immune cells or on distinct aspects of the alloimmune response. As anti-Dll1/4 antibodies were administered systemically, both T cell and non-T cell effects could contribute to the protection. These include inhibition of B cell or plasma cell function, effects on dendritic cells and thymic effects (7, 33, 53-55, 59, 60). Dll4 inhibition was reported to block early T cell development, but with rebound production of natural Tregs after recovery from Dll4 blockade (60). These effects were shown to dampen autoimmune diabetes and could thus be involved as well in mitigating transplant rejection, although it cannot explain protection during administration of the antibodies during the initial two weeks after transplantation.
Altogether, our findings have fundamental immunobiological as well as translational implications. Notch appears to play a central role in the regulation of alloreactivity. Its full impact is best revealed by efficient in vivo loss-of-function strategies that completely block all Notch effects in T cells or all systemic Delta-like-mediated signals. In view of these profound effects, it will be essential to understand how this pathway is controlled and how it interacts with other critical regulators of T cell differentiation and function. In terms of translational applications, humanized anti-Dll1/4 used in this study crossreact with mouse and human Notch ligands and could thus be considered in principle for human interventions, especially since short-term blockade in the peri-transplant period has the potential to confer long-term allograft survival.
Acknowledgments
This work was supported by the National Institutes of Health (HL070613 to DKB; AI091627 to IM), the Damon Runyon Cancer Research Foundation and the Leukemia and Lymphoma Society (IM). Individual support included T32 training grants from the University of Michigan’s Medical Scientist Training Program (GM07863 to JC), Graduate Program of Cell and Molecular Biology (GM007315 to JC) and Graduate Program of Immunology (AI007413 to AS), a Miller Fund Award for innovative immunology research (AS) and a Research Training Award for Fellows from the American Society of Hematology (VR). Flow cytometry was partially supported by a University of Michigan Cancer Center Grant (P30-CA46592).
References
- 1.Li XC, Turka LA. An update on regulatory T cells in transplant tolerance and rejection. Nature Reviews Nephrology. 2010;6:577–583. doi: 10.1038/nrneph.2010.101. [DOI] [PubMed] [Google Scholar]
- 2.Baldwin WM, 3rd, Valujskikh A, Fairchild RL. Antibody-mediated rejection: emergence of animal models to answer clinical questions. American Journal of Transplantation. 2010;10:1135–1142. doi: 10.1111/j.1600-6143.2010.03065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kwun J, Knechtle SJ. Overcoming Chronic Rejection-Can it B? Transplantation. 2009;88:955–961. doi: 10.1097/TP.0b013e3181b96646. [DOI] [PubMed] [Google Scholar]
- 4.Vongwiwatana A, Tasanarong A, Hidalgo LG, Halloran PF. The role of B cells and alloantibody in the host response to human organ allografts. Immunol Rev. 2003;196:197–218. doi: 10.1046/j.1600-065x.2003.00093.x. [DOI] [PubMed] [Google Scholar]
- 5.Stegall MD, Raghavaiah S, Gloor JM. The (re)emergence of B cells in organ transplantation. Current Opinion in Organ Transplantation. 2010;15:451–455. doi: 10.1097/MOT.0b013e32833b9c11. [DOI] [PubMed] [Google Scholar]
- 6.Salvadori M, Bertoni E. Impact of donor-specific antibodies on the outcomes of kidney graft: Pathophysiology, clinical, therapy. World Journal of Transplantation. 2014;4:1–17. doi: 10.5500/wjt.v4.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Radtke F, Wilson A, Stark G, Bauer M, van Meerwijk J, MacDonald HR, Aguet M. Deficient T cell fate specification in mice with an induced inactivation of Notch1. Immunity. 1999;10:547–558. doi: 10.1016/s1074-7613(00)80054-0. [DOI] [PubMed] [Google Scholar]
- 8.Pui JC, Allman D, Xu L, DeRocco S, Karnell FG, Bakkour S, Lee JY, Kadesch T, Hardy RR, Aster JC, Pear WS. Notch1 expression in early lymphopoiesis influences B versus T lineage determination. Immunity. 1999;11:299–308. doi: 10.1016/s1074-7613(00)80105-3. [DOI] [PubMed] [Google Scholar]
- 9.Maillard I, Fang T, Pear WS. Regulation of lymphoid development, differentiation and function by the Notch pathway. Annu Rev Immunol. 2005;23:945–974. doi: 10.1146/annurev.immunol.23.021704.115747. [DOI] [PubMed] [Google Scholar]
- 10.Radtke F, Macdonald HR, Tacchini-Cottier F. Regulation of innate and adaptive immunity by Notch. Nature Reviews Immunology. 2013 doi: 10.1038/nri3445. [DOI] [PubMed] [Google Scholar]
- 11.Sandy AR, Maillard I. Notch signaling in the hematopoietic system. Expert Opin Biol Ther. 2009;9:1383–1398. doi: 10.1517/14712590903260777. [DOI] [PubMed] [Google Scholar]
- 12.Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137:216–233. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Strooper B, Annaert W, Cupers P, Saftig P, Craessaerts K, Mumm JS, Schroeter EH, Schruvers V, Wolfe MS, Ray WJ, Goate A, Kopan R. A presenilin-1-dependent -secretase-like protease mediates release of Notch intracellular domain. Nature. 1999;398:518–522. doi: 10.1038/19083. [DOI] [PubMed] [Google Scholar]
- 14.Tamura K, Taniguchi Y, Minoguchi S, Sakai T, Tun T, Furukawa T, Honjo T. Physical interaction between a novel domain of the receptor Notch and the transcription factor RBP-J kappa/Su(H) Current Biology. 1995;5:1416–1423. doi: 10.1016/s0960-9822(95)00279-x. [DOI] [PubMed] [Google Scholar]
- 15.Wu L, Aster JC, Blacklow SC, Lake R, Artavanis-Tsakonas S, Griffin JD. MAML1, a human homologue of drosophila mastermind, is a transcriptional co-activator for NOTCH receptors. Nat Genet. 2000;26:484–489. doi: 10.1038/82644. [DOI] [PubMed] [Google Scholar]
- 16.Nam Y, Sliz P, Song L, Aster JC, Blacklow SC. Structural basis for cooperativity in recruitment of MAML coactivators to Notch transcription complexes. Cell. 2006;124:973–983. doi: 10.1016/j.cell.2005.12.037. [DOI] [PubMed] [Google Scholar]
- 17.Maillard I, Weng AP, Carpenter AC, Rodriguez CG, Sai H, Xu L, Allman D, Aster JC, Pear WS. Mastermind critically regulates Notch-mediated lymphoid cell fate decisions. Blood. 2004;104:1696–1702. doi: 10.1182/blood-2004-02-0514. [DOI] [PubMed] [Google Scholar]
- 18.Weng AP, Nam Y, Wolfe MS, Pear WS, Griffin JD, Blacklow SC, Aster JC. Growth suppression of pre-T acute lymphoblastic leukemia cells by inhibition of notch signaling. Mol Cell Biol. 2003;23:655–664. doi: 10.1128/MCB.23.2.655-664.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maillard I, Koch U, Dumortier A, Shestova O, Xu L, Sai H, Pross SE, Aster JC, Bhandoola A, Radtke F, Pear WS. Canonical Notch signaling is dispensable for the maintenance of adult hematopoietic stem cells. Cell Stem Cell. 2008;2:356–366. doi: 10.1016/j.stem.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maillard I, Schwarz BA, Sambandam A, Fang T, Shestova O, Xu L, Bhandoola A, Pear WS. Notch-dependent T-lineage commitment occurs at extrathymic sites following bone marrow transplantation. Blood. 2006;107:3511–3519. doi: 10.1182/blood-2005-08-3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tran IT, Sandy AR, Carulli AJ, Ebens C, Chung J, Shan GT, Radojcic V, Friedman A, Gridley T, Shelton A, Reddy P, Samuelson LC, Yan M, Siebel CW, Maillard I. Blockade of individual Notch ligands and receptors controls graft-versus-host disease. J Clin Invest. 2013;123:1590–1604. doi: 10.1172/JCI65477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tu L, Fang TC, Artis D, Shestova O, Pross SE, Maillard I, Pear WS. Notch signaling is an important regulator of type 2 immunity. J Exp Med. 2005;202:1037–1042. doi: 10.1084/jem.20050923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Y, Sandy AR, Wang J, Radojcic V, Shan GT, Tran IT, Friedman A, Kato K, He S, Cui S, Hexner E, Frank DM, Emerson SG, Pear WS, Maillard I. Notch signaling is a critical regulator of allogeneic CD4+ T-cell responses mediating graft-versus-host disease. Blood. 2011;117:299–308. doi: 10.1182/blood-2010-03-271940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ridgway J, Zhang G, Wu Y, Stawicki S, Liang WC, Chanthery Y, Kowalski J, Watts RJ, Callahan C, Kasman I, Singh M, Chien M, Tan C, Hongo JA, de Sauvage F, Plowman G, Yan M. Inhibition of Dll4 signalling inhibits tumour growth by deregulating angiogenesis. Nature. 2006;444:1083–1087. doi: 10.1038/nature05313. [DOI] [PubMed] [Google Scholar]
- 25.Wu Y, Cain-Hom C, Choy L, Hagenbeek TJ, de Leon GP, Chen Y, Finkle D, Venook R, Wu X, Ridgway J, Schahin-Reed D, Dow GJ, Shelton A, Stawicki S, Watts RJ, Zhang J, Choy R, Howard P, Kadyk L, Yan M, Zha J, Callahan CA, Hymowitz SG, Siebel CW. Therapeutic antibody targeting of individual Notch receptors. Nature. 2010;464:1052–1057. doi: 10.1038/nature08878. [DOI] [PubMed] [Google Scholar]
- 26.Sandy A, Chung J, Toubai T, Shan G, Tran I, Friedman A, Blackwell T, Reddy P, King P, Maillard I. T cell-specific Notch inhibition blocks graft-versus-host disease by inducing a hyporesponsive program in alloreactive CD4+ and CD8+ T cells. J Immunol. 2013;190:5818–5828. doi: 10.4049/jimmunol.1203452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chung J, Maillard I. Notch Signaling in Alloreactive T Cell Immunity. Curr Top Microbiol Immunol. 2012;360:135–150. doi: 10.1007/82_2012_226. [DOI] [PubMed] [Google Scholar]
- 28.Vigouroux S, Yvon E, Wagner HJ, Biagi E, Dotti G, Sili U, Lira C, Rooney CM, Brenner MK. Induction of antigen-specific regulatory T cells following overexpression of a Notch ligand by human B lymphocytes. J Virol. 2003;77:10872–10880. doi: 10.1128/JVI.77.20.10872-10880.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yvon ES, Vigouroux S, Rousseau RF, Biagi E, Amrolia P, Dotti G, Wagner HJ, Brenner MK. Over expression of the Notch ligand, Jagged-1 induces alloantigen-specific human regulatory T cells. Blood. 2003;102:3815–3821. doi: 10.1182/blood-2002-12-3826. [DOI] [PubMed] [Google Scholar]
- 30.Wong KK, Carpenter MJ, Young LL, Walker SJ, McKenzie G, Rust AJ, Ward G, Packwood L, Wahl K, Delriviere L, Hoyne G, Gibbs P, Champion BR, Lamb JR, Dallman MJ. Notch ligation by Delta1 inhibits peripheral immune responses to transplantation antigens by a CD8+ cell-dependent mechanism. J Clin Invest. 2003;112:1741–1750. doi: 10.1172/JCI18020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Riella LV, Ueno T, Batal I, De Serres SA, Bassil R, Elyaman W, Yagita H, Medina-Pestana JO, Chandraker A, Najafian N. Blockade of Notch ligand delta1 promotes allograft survival by inhibiting alloreactive Th1 cells and cytotoxic T cell generation. J Immunol. 2011;187:4629–4638. doi: 10.4049/jimmunol.1004076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Riella LV, Yang J, Chock S, Safa K, Magee CN, Vanguri V, Elyaman W, Lahoud Y, Yagita H, Abdi R, Najafian N, Medina-Pestana JO, Chandraker A. Jagged2-signaling promotes IL-6-dependent transplant rejection. Eur J Immunol. 2013;43:1449–1458. doi: 10.1002/eji.201243151. [DOI] [PubMed] [Google Scholar]
- 33.Hozumi K, Negishi N, Suzuki D, Abe N, Sotomaru Y, Tamaoki N, Mailhos C, Ish-Horowicz D, Habu S, Owen MJ. Delta-like 1 is necessary for the generation of marginal zone B cells but not T cells in vivo. Nat Immunol. 2004;5:638–644. doi: 10.1038/ni1075. [DOI] [PubMed] [Google Scholar]
- 34.Corry RJ, Winn HJ, Russell PS. Primarily vascularized allografts of hearts in mice. The role of H-2D, H-2K, and non-H-2 antigens in rejection. Transplantation. 1973;16:343–350. doi: 10.1097/00007890-197310000-00010. [DOI] [PubMed] [Google Scholar]
- 35.Corry RJ, Winn HJ, Russell PS. Heart transplantation in congenic strains of mice. Transplantation Proceedings. 1973;5:733–735. [PubMed] [Google Scholar]
- 36.Bishop DK, Chan Wood S, Eichwald EJ, Orosz CG. Immunobiology of allograft rejection in the absence of IFN-gamma: CD8+ effector cells develop independently of CD4+ cells and CD40-CD40 ligand interactions. J Immunol. 2001;166:3248–3255. doi: 10.4049/jimmunol.166.5.3248. [DOI] [PubMed] [Google Scholar]
- 37.Burrell BE, Csencsits K, Lu G, Grabauskiene S, Bishop DK. CD8+ Th17 mediate costimulation blockade-resistant allograft rejection in T-bet-deficient mice. J Immunol. 2008;181:3906–3914. doi: 10.4049/jimmunol.181.6.3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Csencsits K, Burrell BE, Lu G, Eichwald EJ, Stahl GL, Bishop DK. The classical complement pathway in transplantation: unanticipated protective effects of C1q and role in inductive antibody therapy. American Journal of Transplantation. 2008;8:1622–1630. doi: 10.1111/j.1600-6143.2008.02295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matesic D, Lehmann PV, Heeger PS. High-resolution characterization of cytokine-producing alloreactivity in naive and allograft-primed mice. Transplantation. 1998;65:906–914. doi: 10.1097/00007890-199804150-00008. [DOI] [PubMed] [Google Scholar]
- 40.Faust SM, Lu G, Marini BL, Zou W, Gordon D, Iwakura Y, Laouar Y, Bishop DK. Role of T cell TGFbeta signaling and IL-17 in allograft acceptance and fibrosis associated with chronic rejection. J Immunol. 2009;183:7297–7306. doi: 10.4049/jimmunol.0902446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burrell BE, Lu G, Li XC, Bishop DK. OX40 costimulation prevents allograft acceptance induced by CD40-CD40L blockade. J Immunol. 2009;182:379–390. doi: 10.4049/jimmunol.182.1.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bishop DK, Li W, Chan SY, Ensley RD, Shelby J, Eichwald EJ. Helper T lymphocyte unresponsiveness to cardiac allografts following transient depletion of CD4-positive cells. Implications for cellular and humoral responses. Transplantation. 1994;58:576–584. doi: 10.1097/00007890-199409150-00009. [DOI] [PubMed] [Google Scholar]
- 43.Chan SY, DeBruyne LA, Goodman RE, Eichwald EJ, Bishop DK. In vivo depletion of CD8+ T cells results in Th2 cytokine production and alternate mechanisms of allograft rejection. Transplantation. 1995;59:1155–1161. [PubMed] [Google Scholar]
- 44.Piccotti JR, Chan SY, Goodman RE, Magram J, Eichwald EJ, Bishop DK. IL-12 antagonism induces T helper 2 responses, yet exacerbates cardiac allograft rejection. Evidence against a dominant protective role for T helper 2 cytokines in alloimmunity. J Immunol. 1996;157:1951–1957. [PubMed] [Google Scholar]
- 45.Sandy AR, Stoolman J, Malott K, Pongtornpipat P, Segal BM, Maillard I. Notch Signaling Regulates T Cell Accumulation and Function in the Central Nervous System during Experimental Autoimmune Encephalomyelitis. J Immunol. 2013;191:1606–1613. doi: 10.4049/jimmunol.1301116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Amsen D, Antov A, Flavell RA. The different faces of Notch in T-helper-cell differentiation. Nat Rev Immunol. 2009;9:116–124. doi: 10.1038/nri2488. [DOI] [PubMed] [Google Scholar]
- 47.Osborne BA, Minter LM. Notch signalling during peripheral T-cell activation and differentiation. Nat Rev Immunol. 2007;7:64–75. doi: 10.1038/nri1998. [DOI] [PubMed] [Google Scholar]
- 48.Amsen D, Antov A, Jankovic D, Sher A, Radtke F, Souabni A, Busslinger M, McCright B, Gridley T, Flavell RA. Direct regulation of Gata3 expression determines the T helper differentiation potential of Notch. Immunity. 2007;27:89–99. doi: 10.1016/j.immuni.2007.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bailis W, Yashiro-Ohtani Y, Fang TC, Hatton RD, Weaver CT, Artis D, Pear WS. Notch simultaneously orchestrates multiple helper T cell programs independently of cytokine signals. Immunity. 2013;39:148–159. doi: 10.1016/j.immuni.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Amsen D, Blander JM, Lee GR, Tanigaki K, Honjo T, Flavell RA. Instruction of distinct CD4 T helper cell fates by different notch ligands on antigen-presenting cells. Cell. 2004;117:515–526. doi: 10.1016/s0092-8674(04)00451-9. [DOI] [PubMed] [Google Scholar]
- 51.Fang TC, Yashiro-Ohtani Y, Del Bianco C, Knoblock DM, Blacklow SC, Pear WS. Notch directly regulates Gata3 expression during T helper 2 cell differentiation. Immunity. 2007;27:100–110. doi: 10.1016/j.immuni.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Auderset F, Schuster S, Fasnacht N, Coutaz M, Charmoy M, Koch U, Favre S, Wilson A, Trottein F, Alexander J, Luther SA, MacDonald HR, Radtke F, Tacchini-Cottier F. Notch signaling regulates follicular helper T cell differentiation. J Immunol. 2013;191:2344–2350. doi: 10.4049/jimmunol.1300643. [DOI] [PubMed] [Google Scholar]
- 53.Tanigaki K, Han H, Yamamoto N, Tashiro K, Ikegawa M, Kuroda K, Suzuki A, Nakano T, Honjo T. Notch-RBP-J signaling is involved in cell fate determination of marginal zone B cells. Nat Immunol. 2002;3:443–450. doi: 10.1038/ni793. [DOI] [PubMed] [Google Scholar]
- 54.Santos MA, Sarmento LM, Rebelo M, Doce AA, Maillard I, Dumortier A, Neves H, Radtke F, Pear WS, Parreira L, Demengeot J. Notch1 engagement by Delta-like-1 promotes differentiation of B lymphocytes to antibody-secreting cells. Proc Natl Acad Sci U S A. 2007;104:15454–15459. doi: 10.1073/pnas.0702891104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thomas M, Calamito M, Srivastava B, Maillard I, Pear WS, Allman D. Notch activity synergizes with B-cell-receptor and CD40 signaling to enhance B-cell activation. Blood. 2007;109:3342–3350. doi: 10.1182/blood-2006-09-046698. [DOI] [PubMed] [Google Scholar]
- 56.Shin HM, Minter LM, Cho OH, Gottipati S, Fauq AH, Golde TE, Sonenshein GE, Osborne BA. Notch1 augments NF-kappaB activity by facilitating its nuclear retention. Embo J. 2006;25:129–138. doi: 10.1038/sj.emboj.7600902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dongre A, Surampudi L, Lawlor RG, Fauq AH, Miele L, Golde TE, Minter LM, Osborne BA. Non-Canonical Notch Signaling Drives Activation and Differentiation of Peripheral CD4(+) T Cells. Front Immunol. 2014;5:54. doi: 10.3389/fimmu.2014.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shin HM, Tilahun ME, Cho OH, Chandiran K, Kuksin CA, Keerthivasan S, Fauq AH, Golde TE, Miele L, Thome M, Osborne BA, Minter LM. NOTCH1 Can Initiate NF-kappaB Activation via Cytosolic Interactions with Components of the T Cell Signalosome. Front Immunol. 2014;5:249. doi: 10.3389/fimmu.2014.00249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lewis KL, Caton ML, Bogunovic M, Greter M, Grajkowska LT, Ng D, Klinakis A, Charo IF, Jung S, Gommerman JL, Ivanov II, Liu K, Merad M, Reizis B. Notch2 receptor signaling controls functional differentiation of dendritic cells in the spleen and intestine. Immunity. 2011;35:780–791. doi: 10.1016/j.immuni.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Billiard F, Lobry C, Darrasse-Jeze G, Waite J, Liu X, Mouquet H, DaNave A, Tait M, Idoyaga J, Leboeuf M, Kyratsous CA, Burton J, Kalter J, Klinakis A, Zhang W, Thurston G, Merad M, Steinman RM, Murphy AJ, Yancopoulos GD, Aifantis I, Skokos D. Dll4-Notch signaling in Flt3-independent dendritic cell development and autoimmunity in mice. J Exp Med. 2012;209:1011–1028. doi: 10.1084/jem.20111615. [DOI] [PMC free article] [PubMed] [Google Scholar]


