Abstract
Alzheimer's disease (AD) is a chronic neurodegenerative condition characterized by progressive memory. Mutations in genes involved in the production of amyloid-β (Aβ) are linked to the early-onset variant of AD. However, the most common form, sporadic AD, is considered the result of an interaction between environmental risk factors and various genes. Among them, recent work has highlighted the potential role that the 12/15Lipoxygenase (12/15LO) pathway may play in AD pathogenesis. 12/15LO is widely distributed in the central nervous system, and its levels are up-regulated in patients with AD or mild cognitive impairments. Studies using animal models implicated 12/15LO in the molecular pathology of AD, including the metabolism of Aβ and tau, synaptic integrity and cognitive functions. Here, we will provide an overview of this pathway and its relevance to AD pathogenesis, discuss the mechanism(s) involved, and provide an assessment of how targeting 12/15LO could lead to novel AD therapeutics.
Keywords: Alzheimer's disease, transgenic mouse models, 12/15-Lipoxygenase, amyloid beta, tau protein, synapse, behavior, therapeutics
Introduction
Lipoxygenases (LO) are key enzymes in the biosynthesis of a variety of biologically active lipids, but also by directly oxidizing lipid components in cell membranes inducers of structural changes that play a role in the maturation and differentiation of various cell types [1]. While the mouse has seven different lipoxygenase genes, only five genes have been found in humans. Nomenclature for the different LOs is based on the positional specificity of their substrate oxygenation. For example, the 12LO oxygenates the arachidonate substrate at carbon in position 12, whereas the 5LO at carbon 5. When more than one LO is present in the same species, they are named after the prototypical tissue of occurrence. The human LOs include 5LO, 12LO with platelet-type and leukocyte-type isoforms, and 15LO which is further separated into the reticulocyte or leukocyte-type, 15LO-1, and the epidermis-type, 15LO-2 [2, 3]. While some LOs exclusively form one compound from their substrate, others possess dual-specificity [4]. For example, leukocyte-type 12LO and reticulocyte-type 15LO-1 catalyze both carbon 12 and carbon 15 oxygenation to form two products 12- and 15-hydroxyeicosatetraenoic acid (12-HETE and 15-HETE), and for this reason they are also referred to as 12/15LO [5,6]. Among the dual specificity LOs is arachidonate 12LO, the brain isoform originally isolated from rat brain, which generates both 12-HETE and 15-HETE [7,8]. LOs are widely expressed throughout many tissues and have been involved in different diseases, including diabetes (both types 1 and 2), atherosclerosis, renal disease, and obesity [9,10]. Recently, LOs have been also implicated in some disorders of the central nervous system (CNS) including Alzheimer's disease (AD). In this article, we will provide an overview of this enzymatic pathway in the context of AD pathogenesis by exploring its contribution to the molecular and behavioral insults seen in the disease. In addition, we will present the rationale on why targeting 12/15LO could lead to viable therapeutics relevant not only for AD, but also for other diseases of the CNS.
Alzheimer's disease
Characterized by profound and irreversible memory impairment and cognitive deficits, AD is the most common neurodegenerative dementia. The disease is a global dilemma, with over 30 million patients worldwide and an economic burden exceeding half a trillion USD. Epidemiological studies suggest that 11% of those 65 and older and almost a third of those 85 and older have some form of the disease [11]. Since population demographics predict a worldwide increase in those aged 65 and older in the next 15 years, AD is a serious public health challenge. However, current therapeutic strategies are very limited for AD patients and do not modify disease course [12,13]. Therefore, investigation of new therapeutic targets that address multiple different facets of the AD phenotype and related pathophysiology must be actively sought to help address this problem.
Aβ and tau in Alzheimer's disease
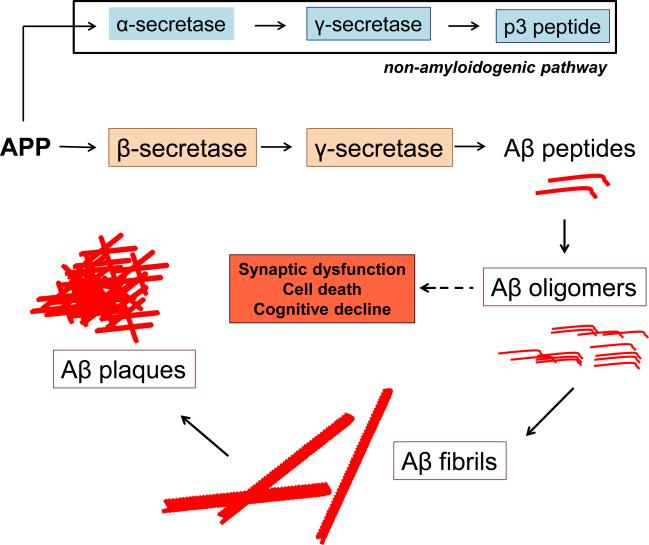
The two classical histopathological hallmarks of AD are extracellular insoluble deposits of amyloid β protein (Aβ) known as Aβ plaques, and intracellular accumulations of precipitated microtubule-associated tau protein known as neurofibrillary tangles [14]. Aβ peptides are produced as a result of the sequential cleavage of the Aβ precursor protein (APP) by the β-secretase (β APP cleavage enzyme, BACE-1) and the γ-secretase complex (composed of the nicastrin, presenilin-1 [PS1], anterior-pharynx defective-1 protein [APH-1], and presenilin enhancer protein-2 [Pen-2]) (Figure 1). APP may be also cleaved by α-secretase (ADAM [a disintegrin and metalloproteinase domain-containing protein]) family of proteins and then γ-secretase to produce nonamyloidogenic products but the Aβ producing pathway is thought to be advantaged in AD. As Aβ levels rise, soluble Aβ oligomers form, which are precursors to Aβ fibrils, eventually creating insoluble Aβ plaques. Although it was once assumed insoluble plaques cause cellular damage in AD, it is now thought that low-n Aβ oligomers cause neuronal damage and synaptic insult (Figure 1)[15].
Figure 1. Aβ metabolic pathway.
The Aβ precursor protein (APP) is processed in one of two main pathways that yield either Aβ peptides or non-amyloidogenic products. If APP is sequentially cleaved by the α-secretase, and then, the γ-secretase, then nonamyloidogenic products form. However, if APP is cleaved by β-secretase and then γ-secretase, then Aβ is produced. As Aβ peptides continue to be produced, they form low-n oligomers, fibrils and eventually plaques. It is believed that soluble low-n oligomers produce the neuronal and cytotoxic injury in Alzheimer's disease.
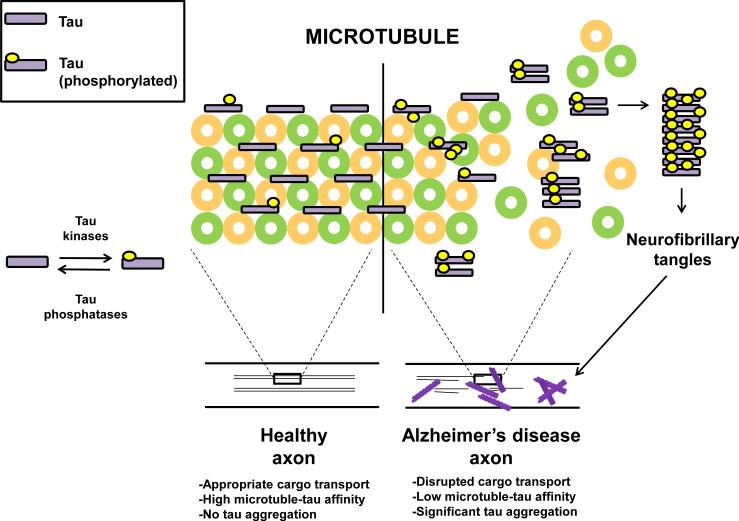
In addition to Aβ, the hyperphosphorylation of tau protein is also a critical event in AD pathogenesis. Tau is thought to serve as a physiological stabilizer of neuronal microtubules and contribute to axon stability and overall neuronal functioning [12]. In AD tau becomes hyperphosphorylated and by losing its affinity for microtubules tends to aggregate eventually forming neurofibrillary tangles (Figure 2). Although tau protein phosphorylation is typically regulated by the balanced action of both tau-associated kinases and phosphatases, in AD two tau-associated kinases are thought to be abnormally functional: cyclin-dependent kinase 5 and glycogen synthase kinase 3 beta [16-18].
Figure 2. Tau metabolic pathway.
The microtubule-associated tau protein maintains phosphorylation status through the combined actions of tau-associated kinases and tau-associated phosphatases. When appropriate physiological tau phosphorylation is maintained, tau affinity to microtubules is maintained and microtubule structure, axon integrity and cellular function are preserved. When tau is hyperphosphorylated as found in Alzheimer's disease, tau is thought to lose affinity from microtubules, form insoluble aggregates, eventually leading to impaired axonal transport, neuronal ultrastructure damage and cell death.
The 12/15Lipoxygenase
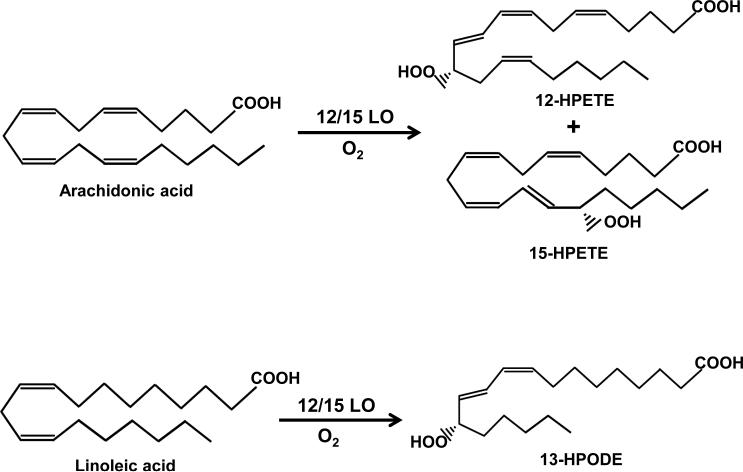
The 12/15LO catalyzes the oxidation of free and esterified fatty acids in phospholipids, generating bioactive lipid mediators such as 12-HETE and 15HETE from arachidonic acid, and 13-hydroxyoctadecadienoic acid (13-HODE) from linoleic acid, which have a multitude of functions in human tissue (Figure 3) [19]. 12/15LO lipid products are involved in protein kinase C mediated monocyte binding in vasculature, and in cell growth, acting through various mitogen-activated protein kinases [20,21]. In addition to cell signaling, 12/15LO can initiate oxidation of lipoproteins, with its genetic disruption significantly reducing systemic oxidative stress [22]. The 12/15LO-induced oxidative stress and direct cytotoxic effect of its metabolites have been implicated in mitochondrial dysfunction and altered tissue inflammatory responses [23-25]. Furthermore, pharmacological inhibition of this enzyme enhanced the survival of cells that were subjected to nitrosative-stress-induced cell death [26].
Figure 3. The 12/15Lipoxygnease pathway.
12/15Lipoxygenase catalyzes conversion of arachidonic acid to 12-hydroxyeicosatetranoic acid (12-HETE) and 15-hydroxyeicosatetranoic acid (15-HETE). 12/15-lipoxygenase can also catalyze conversion of other fatty acids such as linoleic acid, which it metabolizes to 13-hydroperoxyoctadecadienoic acid (13-HPODE).
Although these data provide evidence of the importance of 12/15LO in the periphery, the role of this enzyme in the CNS has only recently received much deserved attention. In the original report of 12/15LO in the brain, it was described to mainly localize in neurons and also some glial cells throughout the cerebrum, basal ganglia and hippocampus [27]. Later work showed that the metabolic products of 12/15LO activation were significantly increased in experimental model of brain ischemia-reperfusion injury, and suggested that this enzyme may be involved in neurodegeneration by oxidizing fatty acids in cell membranes [28]. Based on its pro-oxidant properties, this enzyme has been considered a potential source of brain oxidative stress since its genetic absence is sufficient to significantly reduce CNS oxidative stress in apoE-deficient mice, a model of hypercholesterolemia [29]. This role for 12/15LO in the CNS, hitherto underappreciated, has important implications for several neurodegenerative diseases including AD, in which brain oxidative stress reactions have been shown to be early events in their pathogenesis [30]. Studies using histopathologically-confirmed AD post-mortem brains demonstrated higher steady state levels and activity of 12/15LO than unaffected control brains, while no differences were detected in the cerebellum regions between the two groups [31]. Since elevated 12/15 LO expression and activity in AD brains occurs in areas known to be particularly vulnerable to AD insult (i.e., cortex, and subcortical structures such as hippocampus) and not in those regions found relatively spared from AD insult, such as cerebellum, these data suggested that 12/15LO is an AD-relevant molecular target.
This observation was confirmed by immunohistochemical studies in which the immunoreactivity of the enzyme was actually higher at the earlier rather than at the advanced stages of the disease. Importantly, a later study showed that the biochemical signature of 12/15LO enzymatic activation (i.e., 12-HETE and 15-HETE) was significantly increased also in cerebrospinal fluid of individuals with a clinical diagnosis of AD as well as mild cognitive impairments compared to aged-matched normal individuals [32]. Since mild cognitive impairment is believed to be a prodromal stage to AD in some patients, this finding has significant implications for AD biomarker development and early diagnostic screening. More recently, lipidomic analyses using brain tissues and plasma obtained from transgenic mice expressing mutated human APP and tau protein (Tg2576×JNPL3) (APP/tau mice) at 4 (pre-symptomatic phase), 10 (early symptomatic) and 15 months (late symptomatic) revealed increased levels of 12/15LO metabolic products during the early phase of their phenotype development [33].
12/15Lipoxygenase and Alzheimer's disease pathology
The observation that 12/5LO is not only up-regulated in brain regions particularly vulnerable in AD but also during the earliest stage of the disease pathology and in individuals with mild cognitive impairment, supported the original hypothesis that this protein may have a functional role in AD pathogenesis.
In a series of in vitro studies using neuronal cells stably expressing human APP with the Swedish mutant, a widely used cellular AD model, two structurally distinct and selective 12/15LO inhibitors, PD146176 and CDC, dose-dependently reduced Aβ formation without affecting total APP levels [34, 38]. Interestingly, these studies revealed for the first time that 12/15LO inhibition reduced BACE-1 levels and its enzymatic products, but not those of the α-secretase pathway [34]. Compared to wild-type mice, transgenic mice carrying the same APP Swedish mutation (known as Tg2576 mice, one of the most common models of AD-like amyloidosis) had significant elevation in brain levels and activity of 12/15LO but when Tg2576 mice were crossed with 12/15LO knockout mice, the newly generated animals had a significant reduction in levels and deposition of Aβpeptides. In the Tg2576 animals lacking 12/15LO, BACE-1 levels were reduced compared to Tg2576 animals with 12/15LO intact, mirroring the in vitro data [34]. By contrast, when Tg2576 mice were made to stably over-express 12/15LO they had a significant increase in Aβ peptides levels and deposition [36]. In vivo and in vitro studies showed that the effect of this enzymatic pathway on amyloidosis was indeed mediated by modulation of APP processing via the transcriptional regulation of BACE-1 mRNA levels, which involved the activation of the transcription factor Sp1 [36]. In total, these data showed that this enzymatic pathway directly influences Aβ formation via a BACE-1 dependent mechanism, and that its pharmacological inhibition could represent a novel therapeutic target for AD. These findings were further supported by pharmacological studies in which Tg2576 mice were administered a selective inhibitor of 12/15LO, PD 146176, for 6 weeks. At the end of the study, mice receiving the drug had a significant reduction (>70%) of 12/15LO enzyme activity, which was associated with a significant reduction in amyloid plaques, a decrease in brain Aβ levels via down-regulation of BACE-1, its cleavage products of APP, and the transcriptional factor Sp1 [36]. Interestingly, along with the effect on Aβ, endogenous tau phosphorylation also appeared to be modified by 12/15LO modulation, since Tg2576 mice over-expressing 12/15LO had higher phosphorylated tau but no changes in total tau levels [37]. Intriguingly both early- and advanced-stage tau epitopes were phosphorylated in the condition of 12/15LO overexpression suggesting its involvement in the entire range of AD-associated tauopathy.
However, while Tg2576 animals develop Aβ plaques, they do not develop neurofibrillary tangles. To see how advanced tau neuropathology was modified, in a recent paper we evaluated the effect of pharmacological inhibition of 12/15LO in the triple transgenic mice (3xTg) which develop both Aβ plaques and neurofibrillary tau tangles [38]. Compared with controls, 3xTg mice treated with PD146176, a specific 12/15LO inhibitor, had reduced Aβ peptides levels, burden of amyloid plaques, tau phosphorylation and insoluble tau deposition (i.e., neurofibrillary tangles). As with work in Tg2576 animals, changes in Aβ were associated with changes in BACE-1, whereas the phosphorylation of tau was linked to stress activated protein kinase/cJun N-terminal kinase (SAPK/JNK) [37]. In vitro data in that same paper revealed that 12/15LO modulation of tau occurs independently of Aβ, as pharmacological suppression of Aβ formation by selective γ-secretase inhibition was not sufficient in preventing 12/15LO-mediated increase in tau phosphorylation. This finding is particularly important as this suggest 12/15LO independently modulates Aβ production as well as tau phosphorylation, and suggests that 12/15LO inhibition can be used not only in amyloidotic diseases such as AD, but also other tauopathies.
12/15Lipoxygenase, synaptic integrity and memory
While manipulation of Aβ and tau pathology by 12/15LO is critical for pharmacologic effectiveness, arguably, its effects on synaptic integrity and, ultimately, cognitive functioning, are critical for symptom improvement in AD patients.
Interestingly, several groups have found 12/15LO to directly modulate synaptic function. Normandin and colleagues reported that pharmacological inhibition of 12/15LO modulates rat hippocampal long-term depression (LTD), the process by which neuronal synaptic activity is reduced in response to stimuli [38]. Other work has also detailed how 12/15LO metabolism is required for appropriate metabotropic glutamate receptor signaling, as well as long-term potentiation (LTP), the process by which stimulation-dependent enhancements between neurons occurs [39,40]
As we had already shown changes in the cardinal AD neuropathologies of Aβ and tau in vivo, we were interested to investigate whether, if at all, synaptic changes were present in our 12/15LO model systems. In Tg2576 mice, overexpressing 12/15LO reduced steady-state levels of two main synaptic proteins: post-synaptic density protein 95 (PSD-95) and synaptophysin. A similar result was obtained when the dendritic protein MAP2 was also assayed. These results were further confirmed in brain sections of the same mice when they were tested by immunohistochemical analyses [36]. By contrast, pharmacological inhibition of 12/15LO in 3xTg mice resulted in a significant increase in both PSD-95 and MAP2, suggesting an improvement of synaptic integrity [37]. As synaptic proteins were found to be modulated in the above studies, we next endeavored to assess whether 12/15LO modulated memory insults in these AD mouse models. Knockout of 12/15LO in Tg2576 mice significantly improved their learning in the fear conditioning paradigm, which reflects hippocampal functionality [32]. By contrast, overexpression of 12/15LO in Tg2576 mice lead to an exacerbation of contextual and cued recall memory impairments suggesting both hippocampal and amygdala involvement [35]. In 3xTg mice, pharmacological inhibition of 12/15LO improved performance on working memory, as assessed by the Y-maze, as well as fear-conditioned memory and spatial memory, as assessed by the Morris water maze paradigm [35]. Overall, these data suggest that 12/15LO plays a critical role in not only synaptic integrity but also in modulating the cognitive insult in AD (Figure 4).
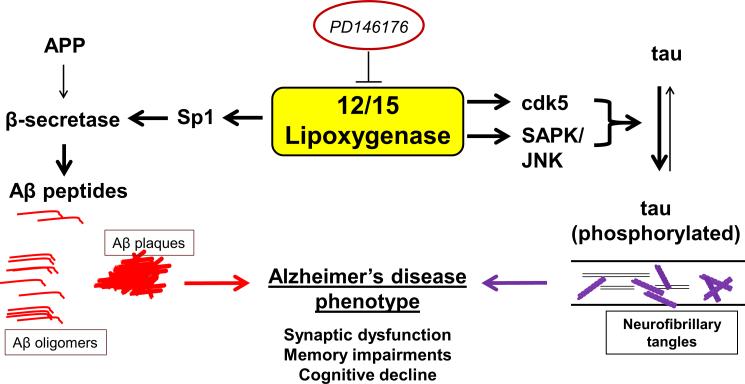
Figure 4. Synopsis of the 12/15Lipoxygenase pathway ion the context of Alzheimer's disease pathogenesis.
12/15Lipoxygenase (12/15LO) modulates Aβ production through the β-secretase by acting on the transcription factor Sp1. 12/15LO also modulates tau phosphorylation by acting on cdk5 and SAPK/JNK. As an end result, overexpression of 12/15 LO protein exacerbates the Alzheimer’ disease (AD) phenotype, while its knockout of pharmacological inhibition ameliorates synaptic dysfunction, memory impairments and cognitive decline in mouse models of the disease. Direct modulation of 12/15LO by PD146176 has demonstrated success in mitigating its effects on the AD phenotype.
12/15Lipoxygenase and neuroprotection
In addition to direct mitigation of the AD phenotype, work has also revealed protective benefits of 12/15LO modulation in other CNS injury contexts. As mentioned above, AD is the leading cause of neurodegenerative dementia, but vascular dementia (multi-infarcts) is the second leading cause of dementia, accounting for ~ 10% of all cases, and is related to chronic cerebral hypo-perfusion rather than direct toxic injury of Aβ or tau. A variety of vasculopathic insults over decades is thought to progressively contribute to vascular dementia, but a major risk factor is repeated ischemic injuries to the brain, as seen in stroke.
Stroke and ischemic damage share many common pathological mechanisms with AD pathogenesis such as oxidative stress, excitotoxicity, apoptosis, and inflammation [41-46]. In particular, data from various animals stroke models (i.e., multiple infarct embolic strokes, transient focal ischemia), as well as brain tissue from stroke patients, have shown consistently that caspase-mediated apoptosis plays a crucial role in ischemia, but also in AD, as APP and tau have been described to be caspase-cleaved substrates [47-49]. In animal models of ischemia, 12/15LO and its metabolites have been demonstrated to play a functional role in cell death, alteration of blood-brain-barrier permeability and edema, and pretreatment with 12/15LO inhibitors reduced infarct size and mitigated resultant apoptosis in these experimental animal models [50-53]. Most importantly, beyond reducing the molecular insults, pharmacological inhibition of 12/15LO in stroke has been linked to better function outcomes and improved recovery in animal models [54, 55].
Implications for Alzheimer's disease and beyond
In summary, 12/15LO presents a potentially viable molecular target for AD therapy. This protein not only regulates Aβ production and tau phosphorylation, but also mitigates AD-associated synaptic pathology and behavioral impairments. These pleotropic actions of 12/15LO, therefore, are a major boon for drug development since traditional drug development in AD has typically concentrated either on Aβ or tau, and a multifaceted target approach has the theoretical appeal of addressing multiple aspects of the AD phenotype at the same time. Moreover, pharmacological interventions aimed at modulating 12/15LO may also be useful in other diseases of the CNS which exclusively involve amyloid (i.e. amyloid angiopathy), or tau alone (i.e., frontotemporal dementia, traumatic encephalopathy).
Although many aspects of 12/15LO neurobiology remain elusive, further work on this protein and its metabolic effects would undoubtedly yield important knowledge, which could be very useful for understanding the pathogenesis and at the same time treating several neurological disorders.
Highlights.
-
1)
Alzheimer's disease (AD) is a progressive neurodegenerative disorder for which we do not have a cure.
-
2)
The 12/15Lipoxygenase (12/15LO) modulates the AD phenotype (Aβ production, tau phosphorylation, synaptic pathology, and cognitive impairment).
-
3)
Pharmacological blockade of 12/15LO represents a viable therapeutic opportunity for AD.
Acknowledgments
The work from the author's lab described in this article was supported in part by grants for the National Institute of Health, the Alzheimer's Association and the Alzheimer Art Quilt Initiative.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Ivanov I, Heydeck D, Hofheinz K, Roffeis J, O'Donnell VB, Kuhn H, Walther M. Molecular enzymology of lipoxygenases. Arch. Biochem. Biophys. 2010;503(2):161–174. doi: 10.1016/j.abb.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 2.Kuhn H, Banthiya S, van Leyen K. Mammalian lipoxygenases and their biological relevance. Biochim. Biophys. Acta. 2014 Oct 12;pii:S1388–1398. doi: 10.1016/j.bbalip.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Skrzypczak-Jankun E, Jankun J, Al-Senaidy A. Human lipoxygenase: developments in its structure, function, relevance to diseases and challenges in drug development. Curr. Med. Chem. 2012;19(30):5122–5127. doi: 10.2174/092986712803530520. [DOI] [PubMed] [Google Scholar]
- 4.Joo YC, Oh DK. Lipoxygenases: potential starting biocatalysts for the synthesis of signaling compounds. Biotechnol. Adv. 2012;30(6):1524–1532. doi: 10.1016/j.biotechadv.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 5.Kuhn H, Walther M, Kuban RJ. Mammalian arachidonate 15-lipoxygenases structure, function, and biological implications. Prostaglandins Other Lipid Mediat. 2002;68-69:263–290. doi: 10.1016/s0090-6980(02)00035-7. [DOI] [PubMed] [Google Scholar]
- 6.Berger M, et al. Simultaneous expression of leukocyte-type 12-lipoxygenase and reticulocyte-type 15-lipoxygenase in rabbits. J Mol Biol. 1998;278:935–948. doi: 10.1006/jmbi.1998.1737. [DOI] [PubMed] [Google Scholar]
- 7.Watanabe T, et al. Molecular cloning of a 12-lipoxygenase cDNA from rat brain. Eur. J. Biochem. 1993;212:605–612. doi: 10.1111/j.1432-1033.1993.tb17699.x. [DOI] [PubMed] [Google Scholar]
- 8.Nishiyama M, et al. Localization of arachidonate 12-lipoxygenase in canine brain tissue. J. Neurochem. 1992;58:1395–1400. doi: 10.1111/j.1471-4159.1992.tb11355.x. [DOI] [PubMed] [Google Scholar]
- 9.Natarajan R, Nadler JL. Lipid inflammatory mediators in diabetic vascular disease. Arterioscler Thromb Vasc Biol. 2004;24:1542–1548. doi: 10.1161/01.ATV.0000133606.69732.4c. [DOI] [PubMed] [Google Scholar]
- 10.Haeggström JZ, Funk CD. Lipoxygenase and leukotriene pathways: biochemistry, biology, and roles in disease. Chem. Rev. 2011;111(10):5866–98. doi: 10.1021/cr200246d. [DOI] [PubMed] [Google Scholar]
- 11.Thies W, Bleiler L. Alzheimer's disease facts and figures. Alzheimers Dement. 2013;2013;9:208–245. doi: 10.1016/j.jalz.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 12.Tan CC, et al. Efficacy and safety of donepezil, galantamine, rivastigmine, and memantine for the treatment of Alzheimer's disease: a systematic review and meta-analysis. J. Alzheim. Dis. 2014;41:615–631. doi: 10.3233/JAD-132690. [DOI] [PubMed] [Google Scholar]
- 13.Caraci F, et al. Clinical pharmacology of novel anti-Alzheimer disease modifying medications. Curr. Top Med. Chem. 2013;13:1853–1863. doi: 10.2174/15680266113139990141. [DOI] [PubMed] [Google Scholar]
- 14.Giannopoulos PF, Pratico D. In: Alzheimer's disease. Diet and Nutrition in Dementia and Cognitive Decline. Martin CR, Preddy VR, editors. Elsevier; 2014. pp. 13–21. [Google Scholar]
- 15.Ono K, Yamada M. Low-n oligomers as therapeutic targets of Alzheimer's disease. J. Neurochem. 2011;117:19–28. doi: 10.1111/j.1471-4159.2011.07187.x. [DOI] [PubMed] [Google Scholar]
- 16.Hanger DP, et al. Glycogen synthase kinase-3 induces Alzheimer's disease-like phosphorylation of tau: generation of paired helical filament epitopes and neuronal localization of the kinase. Neurosci. Lett. 1992;147:58–62. doi: 10.1016/0304-3940(92)90774-2. [DOI] [PubMed] [Google Scholar]
- 17.Baumann K, et al. Abnormal Alzheimer-like phosphorylation of tau-protein by cyclin-dependent kinases cdk2 and cdk5. FEBS Lett. 1993;336:417–424. doi: 10.1016/0014-5793(93)80849-p. [DOI] [PubMed] [Google Scholar]
- 18.Pei JJ, et al. Distribution of active glycogen cynthase kinase 3beta (GSK-3beta) in brains staged for Alzheimer disease neurofibrillary changes. J. Neuropathol. Exp. Neurol. 1999;58:1010–1019. doi: 10.1097/00005072-199909000-00011. [DOI] [PubMed] [Google Scholar]
- 19.Uderhardt S, Kronke G. 12/15-lipoxygenase during the regulation of inflammation, immunity, and self-tolerance. J. Mol. Med. 2012;90:1247–1256. doi: 10.1007/s00109-012-0954-4. [DOI] [PubMed] [Google Scholar]
- 20.Sultana C, et al. Lipoxygenase metabolites induced expression of adhesion molecules and transendothelial migration of monocyte-like HL-60 cells is linked to protein kinase C activation. J. Cell Physiol. 1996;167:477–487. doi: 10.1002/(SICI)1097-4652(199606)167:3<477::AID-JCP12>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 21.Reddy MA, et al. The oxidized lipid and lipoxygenase product 12(S)-hydroxyeicosatetraenoic acid induces hypertrophy and fibronectin transcription in vascular smooth muscle cells via p38 MAPK and cAMP response element-binding protein activation. Mediation of angiotensin II effects. J. Biol. Chem. 2002;277:9920–9928. doi: 10.1074/jbc.M111305200. [DOI] [PubMed] [Google Scholar]
- 22.Cyrus T, et al. Absence of 12/15-lipoxygenase expression decreases lipid peroxidation and atherogenesis in apolipoprotein e-deficient mice. Circulation. 2001;103:2277–2282. doi: 10.1161/01.cir.103.18.2277. [DOI] [PubMed] [Google Scholar]
- 23.McDuffie M, et al. Nonobese diabetic (NOD) mice congenic for a targeted deletion of 12/15-lipoxygenase are protected from autoimmune diabetes. Diabetes. 2008;57:199–208. doi: 10.2337/db07-0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sears DD, et al. 12/15-lipoxygenase is required for the early onset of high fat diet-induced adipose tissue inflammation and insulin resistance in mice. PLoS One. 2009;4:e7250. doi: 10.1371/journal.pone.0007250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reilly KB, et al. 12/15-Lipoxygenase activity mediates inflammatory monocyte/endothelial interactions and atherosclerosis in vivo. J. Biol. Chem. 2004;279:9440–9450. doi: 10.1074/jbc.M303857200. [DOI] [PubMed] [Google Scholar]
- 26.Strosznajder JB. Lipoxygenases and poly(ADP-ribose) polymerase in amyloid beta cytotoxicity. Neurochem. Res. 2011;36:839–848. doi: 10.1007/s11064-011-0412-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nishiyama M, et al. Arachidonate 12-lipoxygenase is localized in neurons, glial cells, and endothelial cells of the canine brain. J. Histochem. Cytochem. 1993;41:111–117. doi: 10.1177/41.1.8417106. [DOI] [PubMed] [Google Scholar]
- 28.Li Y, et al. A role for 12-lipoxygenase in nerve cell death casued by glutathione depletion. Neuron. 1997;19:453–463. doi: 10.1016/s0896-6273(00)80953-8. [DOI] [PubMed] [Google Scholar]
- 29.Chinnici CM, et al. Absence of 12/15 lipoxygenase reduces brain oxidative stress in apolipoprotein E-deficient mice. Am. J. Pathol. 2005;167:1371–1377. doi: 10.1016/S0002-9440(10)61224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Praticò D. Oxidative stress hypothesis in Alzheimer's disease: a reappraisal. Trends Pharm. Sci. 2008;29:609–615. doi: 10.1016/j.tips.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 31.Pratico D. 12/15-lipoxygenase is increased in Alzheimer's disease: possible involvement in brain oxidative stress. Am. J. Pathol. 2004;164:1655–1662. doi: 10.1016/S0002-9440(10)63724-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yao Y. Elevation of 12/15 lipoxygenase products in AD and mild cognitive impairment. Ann. Neurol. 2005;58:623–626. doi: 10.1002/ana.20558. [DOI] [PubMed] [Google Scholar]
- 33.Tajima Y, et al. Lipidomic analysis of brain tissues and plasma in a mouse model expressing mutated human amyloid precursor protein/tau for Alzheimer's disease. Lipids Health Dis. 2013;12:68. doi: 10.1186/1476-511X-12-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Succol F, Pratico D. A role for 12/15 lipoxygenase in the amyloid beta precursor protein metabolism. J. Neurochem. 2007;103:380–387. doi: 10.1111/j.1471-4159.2007.04742.x. [DOI] [PubMed] [Google Scholar]
- 35.Yang H, et al. Amelioration of the Alzheimer's disease phenotype by absence of 12/15-lipoxygenase. Biol. Psychiatry. 2010;68:922–929. doi: 10.1016/j.biopsych.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 36.Chu J, et al. Transcriptional regulation of beta-secretase-1 by 12/15-lipoxygenase results in enhanced amyloidogenesis and cognitive impairments. Ann. Neurol. 2012;71:57–67. doi: 10.1002/ana.22625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Giannopoulos PF, et al. The 12-15-lipoxygenase is a modulator of Alzheimer's-related tau pathology in vivo. Aging Cell. 2013;12:1082–1090. doi: 10.1111/acel.12136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chu J, et al. Pharmacologic blockade of 12/15-Lipoxygenase ameliorates memory deficits, Aβ and tau neuropathology in the triple transgenic mouse. Mol. Psychiatry. 2015 Jan 6; doi: 10.1038/mp.2014.170. doi: 10.1038/mp.2014.170. [Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 38.Oddo S, et al. Triple-transgenic model of Alzheimer's disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 39.Normandin M, et al. Involvement of the 12-lipoxygenase pathway of arachidonic acid metabolism in homosynaptic long-term depression of the rat hippocampus. Brain Res. 1996;730:40–46. doi: 10.1016/0006-8993(96)00428-3. [DOI] [PubMed] [Google Scholar]
- 40.Feinmark SJ. 12-lipoxygenase metabolites of arachidonic acid mediate metabotropic glutamate receptor-dependent long-term depression at hippocampal CA3-CA1 synapses. J. Neurosci. 2003;23:11427–11435. doi: 10.1523/JNEUROSCI.23-36-11427.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.DeCostanzo AJ, et al. 12-Lipoxygenase regulates hippocampal long-term potentiation by modulating L-type Ca2+ channels. J. Neurosci. 2010;30:1822–1831. doi: 10.1523/JNEUROSCI.2168-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lo EH, et al. Mechanisms, challenges and opportunities in stroke. Nat. Rev. Neurosci. 2003;4:399–415. doi: 10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- 43.Hardingham GE, Lipton SA. Regulation of neuronal oxidative and nitrosative stress by endogenous protective pathways and disease processes. Antioxid. Redox Signal. 2011;14:1421–1424. doi: 10.1089/ars.2010.3573. [DOI] [PubMed] [Google Scholar]
- 44.Moskowitz MA, et al. The science of stroke: mechanisms in search of treatments. Neuron. 2010;67:181–198. doi: 10.1016/j.neuron.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Niizuma K, et al. Oxidative stress and mitochondrial dysfunction as determinants of ischemic neuronal death and survival. J. Neurochem. 2009;109(Suppl 1):133–138. doi: 10.1111/j.1471-4159.2009.05897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saito A, et al. Oxidative stress and neuronal death/survival signaling in cerebral ischemia. Mol. Neurobiol. 2005;31:105–116. doi: 10.1385/MN:31:1-3:105. [DOI] [PubMed] [Google Scholar]
- 47.LeBlanc AC. The role of apoptotic pathways in Alzheimer's disease neurodegeneration and cell death. Curr. Alzheimer Res. 2005;2:389–402. doi: 10.2174/156720505774330573. [DOI] [PubMed] [Google Scholar]
- 48.Lebeau A, et al. Blockade of 12-lipoxygenase expression protects cortical neurons from apoptosis induced by beta-amyloid peptide. Cell Death Differ. 2004;11:875–884. doi: 10.1038/sj.cdd.4401395. [DOI] [PubMed] [Google Scholar]
- 49.Nakagomi T, et al. Effect of cyclooxygenase and lipoxygenase inhibitors on delayed neuronal death in the gerbil hippocampus. Stroke. 1989;20:925–929. doi: 10.1161/01.str.20.7.925. [DOI] [PubMed] [Google Scholar]
- 50.Gamblin TC, et al. Caspase cleavage of tau: linking amyloid and neurofibrillary tangles in Alzheimer's disease. Proc. Natl. Acad. Sci. US A. 2003;100:10032–10037. doi: 10.1073/pnas.1630428100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jin G, et al. Protecting against cerebrovascular injury: contributions of 12/15-lipoxygenase to edema formation after transient focal ischemia. Stroke. 2008;39:2538–2543. doi: 10.1161/STROKEAHA.108.514927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Leyen K, et al. Baicalein and 12/15-lipoxygenase in the ischemic brain. Stroke. 2006;37:3014–3018. doi: 10.1161/01.STR.0000249004.25444.a5. [DOI] [PubMed] [Google Scholar]
- 53.Yigitkanli K, et al. Inhibition of 12/15-lipoxygenase as therapeutic strategy to treat stroke. Ann. Neurol. 2013;73:129–135. doi: 10.1002/ana.23734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sun L, et al. Neuroprotection of hydroxysafflor yellow A in the transient focal ischemia: inhibition of protein oxidation/nitration, 12/15-lipoxygenase and blood-brain barrier disruption. Brain Res. 2012;1473:227–235. doi: 10.1016/j.brainres.2012.07.047. [DOI] [PubMed] [Google Scholar]
- 55.Pallast S, et al. Increased nuclear apoptosis-inducing factor after transient focal ischemia: a 12/15-lipoxygenase-dependent organelle damage pathway. J. Cereb. Blood Flow Metab. 2010;30:1157–1167. doi: 10.1038/jcbfm.2009.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lapchak PA, et al. Baicalein, an antioxidant 12/15-lipoxygenase inhibitor improves clinical rating scores following multiple infarct embolic strokes. Neuroscience. 2007;150:585–591. doi: 10.1016/j.neuroscience.2007.09.033. [DOI] [PubMed] [Google Scholar]