Abstract
The secreted protein, YKL-40, has been proposed as a biomarker of a variety of human diseases characterized by ongoing inflammation, including chronic neurological pathologies such as multiple sclerosis (MS)2 and Alzheimer’s disease. However, inflammatory mediators and the molecular mechanism responsible for enhanced expression of YKL-40 remained elusive. Using several mouse models of inflammation, we now show that YKL-40 expression correlated with increased expression of both IL-1 and IL-6. Furthermore, IL-1 together with IL-6 or the IL-6 family cytokine, oncostatin M (OSM), synergistically upregulated YKL-40 expression in both primary human and mouse astrocytes in vitro. The robust cytokine-driven expression of YKL-40 in astrocytes required both STAT3 and NF-κB binding elements of the YKL-40 promoter. Additionally, YKL-40 expression was enhanced by constitutively active STAT3 and inhibited by dominant-negative IκBα. Surprisingly, cytokine-driven expression of YKL-40 in astrocytes was independent of the p65 subunit of NF-κB and instead required subunits RelB and p50. Mechanistically, we show that IL-1-induced RelB/p50 complex formation was further promoted by OSM and that these complexes directly bound to the YKL-40 promoter. Moreover, we found that expression of RelB was strongly upregulated during inflammation in vivo and by IL-1 in astrocytes in vitro. We propose that IL-1 and the IL-6 family of cytokines regulate YKL-40 expression during sterile inflammation via both STAT3 and RelB/p50 complexes. These results suggest that IL-1 may regulate the expression of specific anti-inflammatory genes in non-lymphoid tissues via the canonical activation of the RelB/p50 complexes.
Introduction
In the past several years YKL-40 (also known as chitinase 3-like protein 1, human cartilage glycoprotein 39, and breast regression protein 39) has attracted growing attention as a marker of ongoing inflammation and oncogenic transformation (1). This secreted glycoprotein, expressed in both invertebrates and vertebrates, belongs to the 18-glycosyl-hydrolase family of proteins but lacks glycolytic activity (2, 3). Although its biological functions are not fully understood, it is expressed by many cell types, including macrophages, neutrophils, synoviocytes, chondrocytes, smooth muscle cells, endothelial cells, microglia, and astrocytes, suggesting that its biological effects are not restricted to a particular cell type. Indeed, YKL-40 stimulates proliferation of synoviocytes and chondrocytes (4), promotes adhesion and migration of vascular smooth muscle and endothelial cells (5-7), and is a migration factor for astrocytes (8). Surprisingly, unchallenged YKL-40 knockout mice appear normal; however, when challenged with antigen they display impaired Th2-dependent immune responses, show diminished fibrosis and tissue inflammation, decreased accumulation of M2 macrophages, and increased apoptosis of CD4+ T cells and macrophages (9). In contrast, these mice display an exacerbated response to experimental autoimmune encephalomyelitis (EAE) (10) and enhanced inflammatory responses to hyperoxia (11), suggesting that YKL-40 differentially affects inflammatory responses depending on the type of immune activation and tissue involved. In agreement with the role of YKL-40 during inflammatory responses in mice, YKL-40 levels are elevated in patients with a wide array of human diseases characterized by ongoing inflammation, including rheumatoid arthritis, atherosclerosis, type 2 diabetes, pelvic inflammatory disease, chronic pancreatitis and secondary diabetes, severe pediatric asthma, cirrhosis, Crohn’s disease, and others (12-19). Elevated levels of YKL-40 are also present in the cerebrospinal fluids of patients with a variety of acute and chronic neurological pathologies, such as MS, Alzheimer disease, viral encephalitis, HIV-associated dementia, brain infarction, and traumatic brain injury (20-23), with activated microglia and reactive astrocytes both producing YKL-40 in the central nervous system. Thus, over the last decade it has become evident that elevated YKL-40 expression correlates with both infection-induced inflammation and sterile inflammation, a paradigm triggered by physical, chemical or metabolic noxia. Accordingly, YKL-40 is also expressed by several solid tumors, such as osteosarcoma, ovarian carcinoma and glioblastoma multiforme (GBM) (24, 25), and promotes angiogenesis and radioresistance of GBM tumors and angiogenesis of breast and colon cancer cells (26, 27). Since elevated expression of YKL-40 correlates with ongoing inflammation that is a component of many diseases, YKL-40 has been proposed as a biomarker for many pathologies, including cardiovascular disease, asthma, arthritis, MS, Alzheimer disease, and many cancers, including GBM, melanoma, hepatocellular carcinoma, breast and pancreatic cancers (20, 24).
Despite the numerous reports documenting elevated expression of YKL-40, relatively little is known about the inflammatory mediators and specific molecular mechanisms that control its expression. Proinflammatory cytokines, including TNF and IL-1, induce expression of YKL-40 in chondrocytes (28) and astrocytes (8); however, to a much lower extent than the conditioned media of macrophages (29). Both IL-1 and TNF are known to trigger a classical IκB kinase (IKK)γ-dependent activation of NF-κB, which involves IKKβ-mediated phosphorylation and subsequent degradation of IκBs, followed by the release and subsequent nuclear translocation of the p65/p50 NF-κB complexes (30). In contrast, these cytokines do not efficiently activate a noncanonical IKKγ-independent NF-κB pathway, which involves NF-κB-inducing kinase (NIK), IKKα-dependent processing of NF-κB2 p100, and generation of RelB/p52 complexes (31-33). Concordantly, the p65/p50 complex has been proposed to mediate TNF- and IL-1-induced YKL-40 expression in chondrocytes (28). In contrast however, p65/p50 has also been shown to recruit histone deacetylases-1 and −2 to the YKL-40 promoter and repress its expression in response to TNF in GBM cells (34). In addition, IL-6 and OSM moderately upregulate YKL-40 expression in human astrocytes, which requires STAT3 and formation of a complex between STAT3 and nuclear factor I-X3 on the YKL-40 promoter (8). However, profound activation of YKL-40 expression observed during ongoing inflammatory processes in vivo has not been adequately recapitulated in the in vitro experiments. Therefore in this study, we set up experiments to identify molecular mechanisms that govern YKL-40 expression during inflammation.
Materials and Methods
Mice
The doxycycline (DOX)-inducible, brain-specific HIV-Tat1-86 transgenic mice have been described previously (35). Tat expression was induced with chow containing 6 mg/g DOX (Harlan, Indianapolis, IN) for 1 week. C57BL/6 mice were obtained from Jackson Laboratory. Mice were housed at Virginia Commonwealth University according to guidelines of the Institutional Animal Care and Use Committee of Virginia Commonwealth University. The mouse protocols were approved by the Institutional Animal Care and Use Committee. Male and female mice 6–16 weeks of age were used.
Turpentine- and LPS-induced inflammation
Turpentine abscesses were initiated by subcutaneous injection of pure gum spirits of turpentine (50 μl) into anesthetized age-matched male and female mice (6–8 weeks of age). For LPS-induced inflammation, mice were injected subcutaneously with 50 μg LPS (in 50 μl PBS). Mice were killed after 8h and the skin and underlying muscles at the site of injection was collected for mRNA analysis.
Experimental autoimmune encephalomyelitis (EAE)
Each mouse received intradermally 300 μg of MOG35–55 peptide (AnaSpec, Fremont, CA) emulsified in CFA containing 500 μg of Mycobacterium tuberculosis H37Ra (Difco, Detroit, MI) and intraperitoneally 200 ng of Pertussis toxin (Enzo Life Sciences, Farmingdale, NY). An additional dose of 200 ng of Pertussis toxin was administered two days post immunization. A booster dose MOG35–55/CFA/Mycobacterium tuberculosis H37Ra was given seven days post immunization. Mice were examined daily (the first day following booster injection was assigned as day 1), weighed, and the severity of the disease was quantitated using a five point scale: 0 – no symptoms, 1 – limp tail, 2 – limp tail with loss of righting, 3 – paralysis of single hind limp, 4 – paralysis of both hind limps, and 5 – death. Tissues were collected at day 28 for RNA analysis.
Irradiation
Irradiations were carried out using a MDS Nordion Gamma-cell 40 research irradiator with a 137Cs source delivering a dose of 1.05 Gy/min to the head only. Mice received a dose of 20 Gy.
Clinical samples
Biopsy samples of oligodendroglioma tumors were obtained from VCU’s brain tumor bank (Department of Neurosurgery, Richmond, VA). Normal cortical tissue samples were obtained from healthy brain regions of patients with oligodendroglioma or epileptic patients. The biopsy samples represent primary grade II and III, and recurrent tumors.
Cell culture
Human glioblastoma U373-MG cells were obtained from American Type Culture Collection (Manassas, VA). Mouse astrocytes were prepared as described (36). Human cortical astrocytes were prepared from fetal tissue provided by Advanced Bioscience Resources and were cultured as previously described (37, 38). Primary human chondrocytes were purchased from PromoCell, Heidelberg, Germany. Cells were stimulated with 25 ng/ml IL-1, 25 ng/ml OSM, 25 ng/ml IL-6, 10 ng/ml TNF, or 25 ng/ml sIL-6R (all from R&D Systems, Minneapolis, MN).
Knock-down of target genes
Expression of RelB, cRel, p50, STAT3, p65 and p52 mRNAs was down-regulated using SmartPool siRNAs transfected into astrocytes using Dharmafect 1 (all from Dharmacon, Lafayette, CO).
Quantitative PCR
Total RNA was prepared utilizing TRIzol (Life Technologies, Carlsbad, CA). 1 μg of RNA was reverse-transcribed using the high capacity cDNA Archive kit (Life Technologies). Premixed primer-probe sets and TaqMan Universal PCR Master Mix (Life Technologies) were employed to examine mRNA levels. cDNAs were diluted 10-fold (for the target genes) or 100-fold (for GAPDH) and amplified using the ABI7900HT cycler. Gene expression levels were normalized to GAPDH and presented as a fold induction with the mean values ± SEM. A Power SYBRGreen PCR kit (Life Technologies) was used for chromatin immunoprecipitation qPCR assays as previously described (8).
Western Blotting
The cells were lysed in 10 mM Tris, pH 7.4, 150 mM sodium chloride, 1 mM EDTA, 0.5% Nonidet P-40, 1% Triton X-100, 1 mM sodium orthovanadate, 0.2 mM PMSF, and protease inhibitor mixture (Roche Applied Science, Indianapolis, IN). Samples were separated using SDS-PAGE and transferred onto nitrocellulose membranes (Schleicher & Schuell, Dassel, Germany). The anti-YKL-40, anti-RelB, anti-p65, anti-p50, anti-β-tubulin, and anti-GAPDH antibodies were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA) and anti-Stat3 antibody was obtained from Cell Signaling Technology (Danvers, MA). Antigen-antibody complexes were visualized by enhanced chemiluminescence using Immobilon Western blotting kit (Millipore, Temecula, CA).
Coimmunoprecipitation
200–300 μg of protein lysates, prepared as described above, were pre-cleared with 10 μl of the protein G-Sepharose beads (GE Healthcare, Pittsburgh, PA) for 1 h. The lysates were then incubated with 2 μg of anti-RelB or anti-p50 antibodies overnight at 4°C, 25 μl of protein G-Sepharose beads were added, and incubated for 1 h at 4°C. The beads were washed extensively with the lysis buffer, and immunoprecipitated proteins were eluted in sample buffer at 95 °C for 5 min.
Synthetic Oligonucleotides
The following oligonucleotides were synthesized to introduce mutations in the YKL-40 promoter: −669 NF-KB, 5’-CTGAATTCGATAGCTGTCTTTCCCTCTAA-3’ and 5’-ACAGCTATCGAA TTCAGAATGCTTTAAGC-3’; −717 NF-KB, 5’-ATCTCGAGAATAAAACAGAAGCAAAAT AG-3’ and 5’-TTATTCTCGAGATAAAGAGAGAGGATCTT-3’. Following oligonucleotides were used in EMSA: −669 NF-KB probe, 5’-GATCTTTCTTGGGAATTTCCC TGTCA-3’ and 5’-GATCTGACAGGGAAATTCCCAAGAAA-3’; −717 NF-KB probe, 5’-GATCTCTTTATGG GAATTTCAAAACAA-3’ and 5’-GATCTTGTTTTGAAATTCCCATAAAGA-3’.
Nuclear Extracts and EMSA
Nuclear extracts were prepared as previously described (39). Briefly, double-stranded DNA fragments were labeled by filling in the 5’-protruding ends with Klenow enzyme using [α-32P]dCTP (3000 Ci/mmol). 5 μg of nuclear extracts and ~10 fmol (10,000 cpm) of probe were utilized. Anti-RelB, anti-p50, anti-p65 (Santa Cruz Biotechnology, Santa Cruz, CA) antibodies were used for supershift studies.
Chromatin Immunoprecipitation (ChIP) Assay
ChIP assays were performed as described previously (8) with the following exceptions. The cells were cross-linked with 1% formaldehyde for 10 min at 37 °C and then washed with ice-cold PBS containing 125 mM glycine. RelB and p50 antibodies or non-immune rabbit serum were used to precipitate cross-linked proteins. The following primers to the YKL-40 promoter were used in the qPCR: 5’-GTGCAGCCGCCCCGTAG-3’ and 5’-GCCTGAAACTGAGCGCTCC-3’.
Plasmids and transfections
The pYKL(-1300)Luc reporter was provided by Dr. Michael Rehli (University of Regensberg, Regensberg, Germany) (40). The site-directed mutagenesis was performed using QuikChange II site-directed mutagenesis kit (Stratagene, La Jolla, CA) and the oligonucleotides described above and published previously (8). Plasmids expressing dominant negative IκBα and constitutively active STAT3 were described before (8). Human astrocytes were transiently transfected in 12-well clusters using the FuGENE6 transfection reagent (Roche Applied Science). Twenty four hours post-transfection, the cells were stimulated with IL-1 or OSM for 20h and cell extracts were prepared. Luciferase assays were performed using a dual luciferase reporter assay kit (Promega Corporation, Madison, WI). Luciferase activities were normalized to Renilla luciferase activities.
Statistical analysis
All experiments were repeated at least three times with consistent results. Data are presented as mean ± SEM. For mouse studies, four to six randomly chosen mice were used per experimental group. SPSS Statistics 21 software was used for statistical analyses. A Bonferroni post-hoc test was used for one-way ANOVA comparisons, with a P value of <0.05 being considered statistically significant. Independent sample Student's t-test was used for unpaired observations.
Results
Inflammation induces YKL-40, IL-1 and IL-6 expression
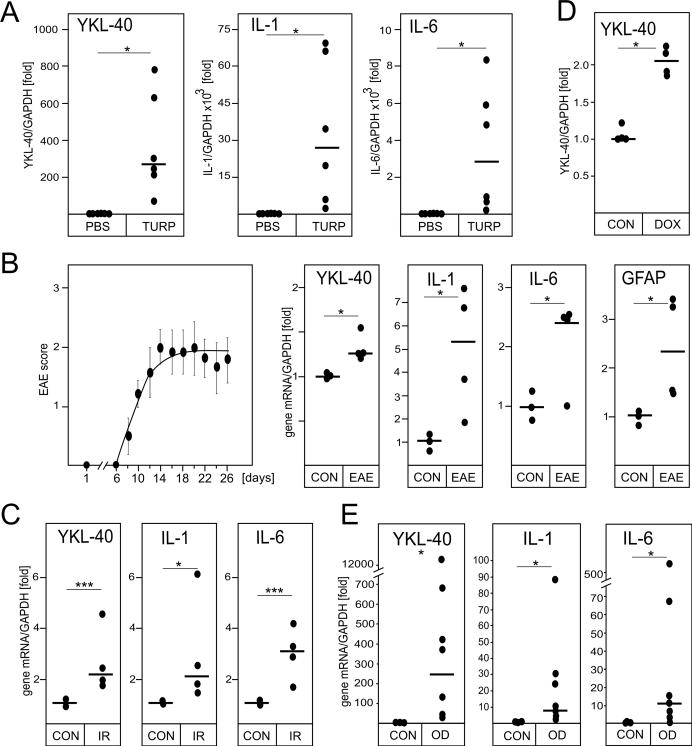
To model the induction of YKL-40 expression during inflammation, we first employed a turpentine mouse model of irritant-induced acute inflammation, which is IL-1R-dependent (41). Turpentine causes tissue destruction at the site of injection, local cytokine production, and infiltration of proinflammatory cells (38). The local reaction is subsequently followed by a systemic response, including the production of acute phase proteins in the liver (42). Indeed, YKL-40 mRNA expression was robustly activated in tissues surrounding the site of turpentine injection and its induction was accompanied by a very strong induction of both IL-1 and IL-6 mRNAs (Fig. 1A). To corroborate these findings in a central nervous system model of sterile inflammation, expression of YKL-40, IL-1, and IL-6 was subsequently analyzed using a mouse EAE model of MS. Immunization of mice with MOG35-50 peptide resulted in the induction of EAE, and modest upregulation of YKL-40 mRNA expression in the spinal cords of animals (Fig. 1B). The magnitude of YKL-40 induction was comparable to the results previously reported by others (10). In addition, expression of both IL-1 and IL-6 mRNAs was also enhanced. We also found an increase in the expression of glial fibrillary acidic protein mRNA, which indicated activation of astrocytes during EAE induction as reported previously (43). Subsequently, we analyzed expression of YKL-40, IL-1 and IL-6 in the brains of mice exposed to gamma irradiation, which is known to induce inflammation (44). In agreement with the turpentine and EAE data, levels of YKL-40, IL-1, and IL-6 mRNAs were significantly higher in the irradiated brains (Fig. 1C). We also used the HIV transactivator of transcription (TAT) transgenic mouse model in which induction of TAT protein expression in the brain induces local sterile inflammation and enhances expression of IL-1 and IL-6 (45). Using the TAT model, we found that YKL-40 mRNA expression was also enhanced upon activation of TAT expression (Fig. 1D). Lastly, we analyzed samples of human oligodendroglioma tumors and found that the expression of YKL-40, IL-1, and IL-6 mRNAs was strongly induced in comparison to normal brain (Fig. 1E). Similarly to sterile inflammation, YKL-40 mRNA expression also coincides with IL-1 and IL-6 mRNA expression during inflammation induced by bacterial LPS (Supplementary Fig. 1). We conclude that YKL-40 expression is enhanced during inflammation, which coincides with the increased expression of IL-1 and IL-6.
Fig. 1. Sterile inflammation induces YKL-40, IL-1 and IL-6 expression.
(A) Mice were injected s.c. with 50 μl turpentine or PBS (n=6). Tissue at the site of injection was collected after 8h and the expression of YKL-40, IL-1 and IL-6 mRNA was analyzed by qPCR. (B) EAE was induced in mice (n=6) and scored as described in methods section (left panel). Expression of YKL-40, IL-1, IL-6 and GFAP mRNA was analyzed in spinal cords at day 28 by qPCR (right panels). (C) Expression of YKL-40, IL-1 and IL-6 was analyzed in the hippocampus of control (CON) or irradiated (IR) mice after 24 h. (D) Tat expression was induced by DOX in Tat transgenic mice and the expression of YKL-40 mRNA was analyzed by qPCR. (E) Expression of YKL-40, IL-1 and IL-6 mRNA was analyzed by qPCR in biopsy samples of oligodendroglioma tumors (OD) and healthy brain tissue. Lines indicate median values. *P < 0.05 and ***P < 0.001 (t-test).
IL-1 and OSM synergistically induce YKL-40 expression in astrocytes
In the nervous system, both astrocytes and microglia express YKL-40 (8, 21) and these cell types also respond to a broad range of inflammatory stimuli (46). We used astrocytes as model cells to study activation of YKL-40 expression during sterile inflammation. Primary human astrocytes were stimulated with IL-1 and IL-6 together with soluble IL-6 receptor (sIL-6R) (due to the fact that human fetal astrocytes express limited amounts of IL-6R (37)). We also included OSM, a member of IL-6 cytokine family that signals via OSM receptors abundantly expressed by astrocytes. Although IL-1, IL-6/sIL-6R, or OSM alone only moderately activated YKL-40 mRNA expression, stimulation of astrocytes with IL-1 together with OSM resulted in synergistic activation (Fig. 2A). Since IL-1-treated astrocytes express IL-6, their costimulation with IL-1 and sIL-6R also caused synergistic induction of YKL-40 mRNA expression; however, to less extent than OSM alone. Similar effects of IL-1 and OSM on YKL-40 mRNA expression were also observed at the protein level (Fig. 2A, insert) and in mouse primary astrocytes (Fig. 2B). These data together with our in vivo studies suggest that YKL-40 expression is coordinately regulated by IL-1 and cytokines of the IL-6 cytokine family during sterile inflammation.
Fig. 2. Proinflammatory cytokines induce YKL-40 expression in astrocytes.
Primary human (A) and mouse (B) astrocytes were stimulated with IL-1, IL-6, OSM and sIL-6R for 18h and expression of YKL-40 mRNA was analyzed by qPCR. Secreted YKL-40 was analyzed by Western blotting in the culture medium (insert). ***P < 0.001 (one-way ANOVA).
STAT3 but not p65 (NF-κB) regulate cytokine-induced YKL-40 expression
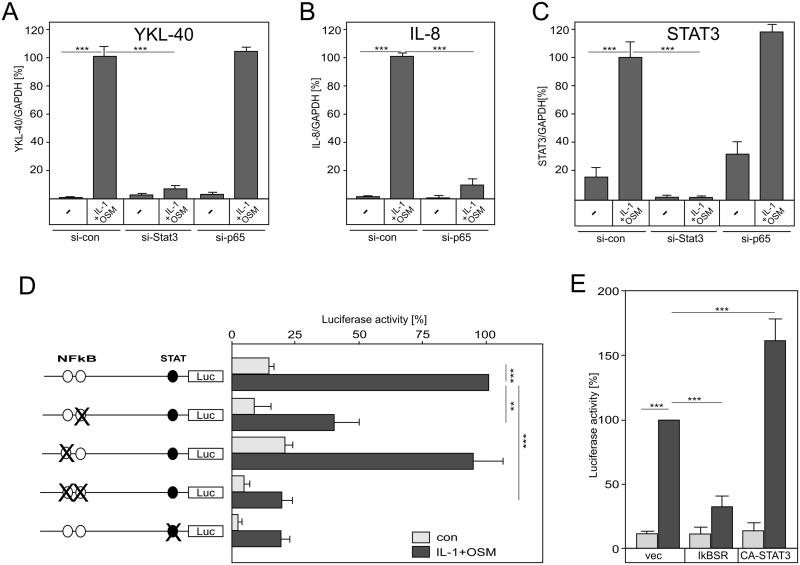
NF-κB and STAT3 are the major transcription factors activated by IL-1 and OSM, respectively (30, 47). Since p65 subunit of NF-κB and STAT3 were previously implicated in cytokine-induced expression of YKL-40 (8, 34), we knocked down their expression in human astrocytes. Downregulation of STAT3 (Fig. 3C) dramatically diminished IL-1/OSM-induced YKL-40 mRNA expression (Fig. 3A). Surprisingly, knockdown of p65 had no effect on YKL-40 mRNA expression (Fig. 3A), but did drastically diminish the expression of IL-8 mRNA, which is p65-dependent (Fig. 3B). To identify the mechanism of cytokine-induced YKL-40 expression, putative NF-κB and STAT3 binding sites of the YKL-40 promoter were mutated and the generated reporters were analyzed in astrocytes (Fig. 3D). Although cytokine-driven expression of YKL-40 is p65-independent (Fig. 3A), mutation of the proximal NF-κB site (−669 to −660 bp) alone substantially diminished activation of the reporter, whereas mutation of the distal NF-κB site (−717 to −708 bp) alone had no effect (Fig. 3D). In addition, mutation of the STAT3 site also drastically diminished reporter activity. In another approach, we overexpressed dominant-negative IκBα and constitutively active STAT3 and analyzed expression of the YKL-40 reporter (Fig. 3E). Constitutively active STAT3 enhanced, whereas dominant-negative IκBα diminished cytokine-responsiveness. Collectively, these results suggest that the proximal NF-κB and STAT3 elements of the YKL-40 promoter are required for full responsiveness to IL-1 and OSM; however, and in contrast to previous reports (34), p65 is dispensable for this response.
Fig. 3. Induction of YKL-40 expression by IL-1 and OSM is STAT3-dependent, but p65-independent.
Human astrocytes were transfected with the indicated si-RNAs. Forty hours post transfection, cells were stimulated with IL-1 and OSM for 18 hours and expression of YKL-40 (A), IL-8 (B), and STAT3 (C) was analyzed by qPCR. (D) Astrocytes were transfected with the indicated reporters, stimulated with IL-1 and OSM for 18 hours, and luciferase and renilla activities were determined. Data are presented in comparison to the induced wild-type reporter (set as 100%). (E) Astrocytes were transfected with pYKL(-1300)Luc and plasmids expressing either vector, dominant-negative IκB (IκBSR), or constitutively-active (CA-STAT3). Cells were stimulated with IL-1 and OSM for 18 hours and processed as described in D. **P < 0.01 and ***P < 0.001 (one-way ANOVA).
RelB and p50 regulate cytokine-induced YKL-40 expression
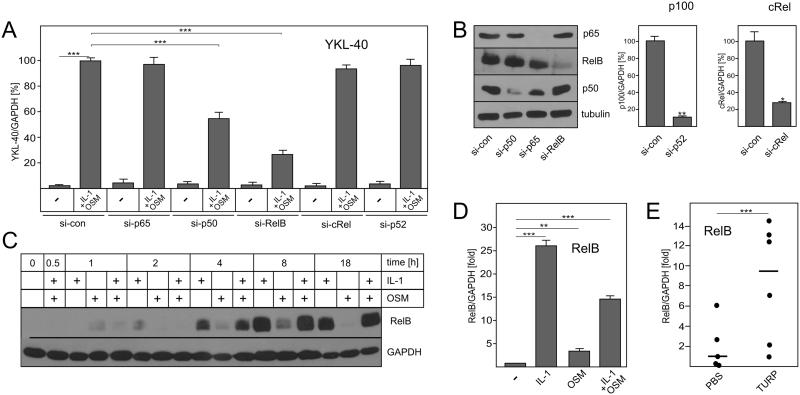
To determine which component of the NF-κB complex regulates cytokine-driven YKL-40 expression, expression of p65, cRel, RelB, p50 and p52 was effectively knocked-down in astrocytes (Fig. 4B). Knockdown of either RelB or p50 significantly diminished cytokine-induced YKL-40 mRNA expression, whereas knockdown of p65, cRel and p52 had no effect (Fig. 4A). This finding implicates both RelB and p50 in YKL-40 regulation. Interestingly, although human astrocytes constitutively express low levels of RelB, IL-1 induced dramatic RelB protein accumulation in these cells (Fig. 4C). Similarly, RelB mRNA expression was also up-regulated by IL-1 in mouse astrocytes (Fig. 4D). RelB mRNA expression was also strongly activated in vivo by turpentine in an IL-1-dependent model of irritant-induced sterile inflammation (Fig. 4E). Since TNF also efficiently activates NF-κB, we tested whether, similarly to IL-1, TNF regulates YKL-40 expression by a RelB-dependent mechanism. Although TNF and OSM could not induce YKL-40 mRNA expression in astrocytes, YKL40 mRNA expression was RelB-dependent in response to these cytokines in U373 glioma cells (Supplementary Fig. 2). These data suggests that RelB regulates expression of YKL-40 in response to proinflammatory cytokines, such as IL-1 and TNF, which induce canonical activation of RelB/p50 complexes. In contrast to astrocytes and U373 cells, we found that basal expression of YKL-40 and RelB mRNA was very high in primary human chondrocytes and not stimulated by IL-1 or OSM (Supplementary Fig 3). In these cells, basal expression of YKL-40 is RelB-, p65-, and STAT3-independent, implicating other unknown factors. Cumulatively, these data suggest that in cells expressing relatively low levels of YKL-40, such as astrocytes, cytokine-driven RelB promotes YKL-40 induction in vitro and in vivo.
Fig. 4. Cytokine-induced RelB/p50 complexes regulate YKL-40 expression.
Human astrocytes were transfected with the indicated si-RNAs. Forty hours post-transfection, cells were stimulated with IL-1 and OSM for 18 hours. Expression of YKL-40 (A), p100 and cRel (B, right panel) was analyzed by qPCR. Expression of p65, RelB, p50 and β-tubulin was analyzed by Western blotting (B, left panel). (C) Human astrocytes were stimulated with IL-1 and OSM for the indicated times and expression of RelB and GAPDH was analyzed by Western blotting. (D) Mouse astrocytes were stimulated with IL-1 and OSM for 18 hours and expression of RelB was analyzed by qPCR. (E) Mice were injected s.c. with 50 μl turpentine or PBS (n=6). Tissue at the site of injection was collected after 24h and the expression of RelB mRNA was analyzed by qPCR. Lines indicate median values. **P < 0.01 and ***P < 0.001 (one-way ANOVA).
RelB/p50 complexes bind to the YKL-40 promoter
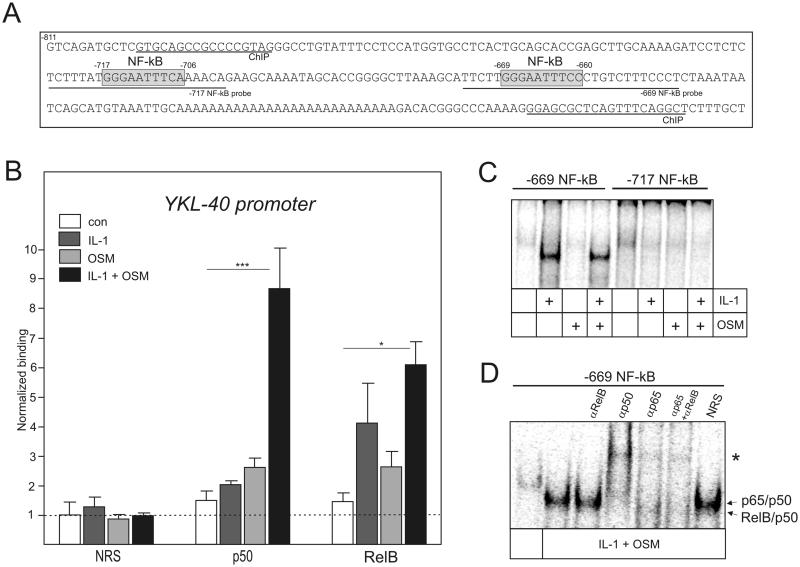
To determine whether RelB regulates YKL-40 expression directly or indirectly, binding of RelB and p50 to the YKL-40 promoter was analyzed by ChIP (Fig. 5A). These experiments were performed in U373 glioma cells, which similarly to human and mouse astrocytes, upregulate expression of both YKL-40 and RelB in response to IL-1 and OSM, and this cytokine-induced expression is diminished by the knockdown of p50 and RelB (Suppl. Fig. 4). Although RelB and p50 did not bind to the YKL-40 promoter in unstimulated U373 cells, binding of RelB and p50 was apparent in cytokine-treated cells (Fig. 5B), suggesting that RelB/p50 complexes directly regulate YKL-40 expression. To determine if IL-1/OSM-treatment induces RelB/p50 binding to the previously identified NF-κB sites, EMSAs were performed using oligonucleotides containing distal and proximal NF-κB sites. In agreement with the mutational analysis that showed the importance of the proximal NF-κB site (Fig. 3D), strong protein binding to the proximal, but not distal, NF-κB site of the YKL-40 promoter was induced by IL-1 alone or together with OSM (Fig. 5C). Cytokine stimulation facilitated the binding of protein complexes to the proximal NF-κB element in vitro, which super-shifted with anti-p65, anti-RelB and anti-p50 antibodies (Fig. 5D), indicating that the proximal NF-κB site can bind both p65/p50 and RelB/p50 complexes. We conclude that RelB/p50 complexes can directly bind to the proximal NF-κB site of the YKL-40 promoter and are essential for the cytokine-induced YKL-40 expression.
Fig. 5. RelB/p50 complexes bind at the distal NF-κB site of YKL-40 promoter.
(A) YKL-40 promoter. NF-κB sites are indicated by grey boxes. Positions of ChIP primers and probes used in EMSA (−717 and −669 probe) are indicated. (B) ChIP was performed using chromatin prepared from U373 cells treated with IL-1 and OSM for 2h. Binding of p50 and RelB to the YKL-40 promoter was analyzed using the antibodies described in the experimental procedures. NRS indicates normal rabbit serum used for immunoprecipitation. Results are shown as normalized binding (binding of NRS-immunoprecipitated untreated samples were set as 1). Experiments were performed three times. *P < 0.05 and ***P < 0.001 (two-way ANOVA). (C-D) Nuclear extracts were prepared from U373 and glioma cells stimulated with IL-1 and OSM for 8 hours. The binding was then analyzed by EMSA using the 32P-labeled oligonucleotide probes derived from the 5’ flanking region of the YKL-40 (−717 NF-kB and −669 NF-κB, as indicated). (C) Binding to the −717 NF-kB and −669 NF-κB elements in U373 glioma cells. (D) Binding to the −669 NF-kB probe in U373 cells. Specific antibodies or NRS were added to the binding reaction. Asterisk indicates super-shifted complexes.
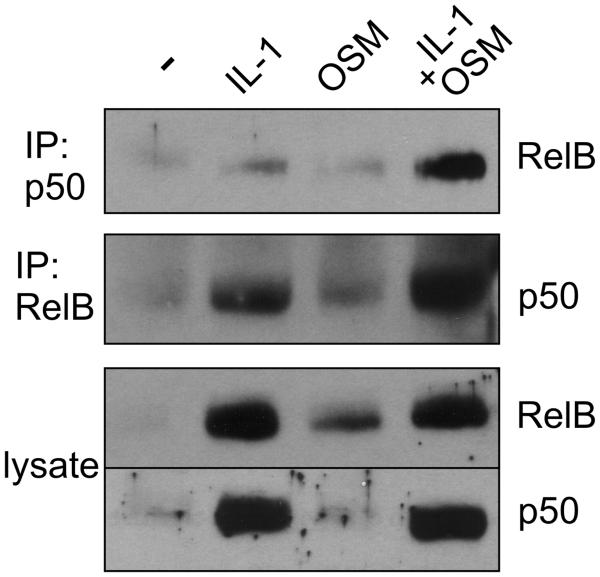
OSM enhances IL-1-induced RelB/p50 heterodimer formation
OSM and IL-1 efficiently regulated YKL-40 expression via STAT3 (Fig. 3A) and OSM promoted the recruitment of IL-1-induced p50 to the YKL-40 promoter (Fig. 5B). Since YKL-40 expression depends on RelB/p50 (Fig. 4A), we subsequently asked whether OSM promotes formation of IL-1-induced RelB/p50 heterodimers. RelB and p50 were immunoprecipitated from cytokine-treated cells and analyzed for the presence of coimmunoprecipitated RelB and p50 (Fig. 6). Indeed, p50/RelB complexes were formed in response to IL-1. More importantly, OSM significantly enhanced IL-1-induced formation of the p50/RelB complexes. These data suggest that in addition to STAT3 activation, OSM enhances YKL-40 expression by promoting formation of the RelB/p50 complexes, which bind to the YKL-40 promoter.
Fig. 6. OSM enhances IL-1-induced RelB/p50 heterodimer formation.
Human astrocytes were stimulated with IL-1 or OSM for 8 hours, p50 or RelB were immunoprecipitated, and co-immunoprecipitated p50 and RelB were detected by Western blotting, as indicated. Expression of RelB and p50 in the lysates is shown the lower panels (lysate).
Discussion
Although YKL-40 is upregulated in a broad range of human diseases associated with ongoing sterile inflammation, its biological functions still remain elusive (48-50). Nevertheless, exacerbated EAE (10) and enhanced responses to hyperoxia (11) in YKL-40 knock-out mice suggest that cytokine-induced YKL-40 is needed for the proper resolution of inflammation. Once inflammation is resolved, YKL-40 expression ceases. In contrast, chronic inflammation leads to continuous aberrant upregulation of YKL-40 expression, which is continuously driven by proinflammatory cytokines. Our data from the turpentine, TAT-overexpression, and EAE mouse models together with human oligodendroglioma data, demonstrate that YKL-40 expression correlates with the expression of both IL-1β and IL-6. Importantly, IL-1α and IL-1β are both critical initiators of sterile inflammation released in response to broad range of danger-associated molecular patterns. IL-1β is processed from its inactive precursor in response to inflammasome activation and subsequently secreted from activated cells, while active intracellular IL-1α, which does not require processing, is liberated from damaged cells (51). Since IL-1 efficiently activates expression of IL-6 in many cell types (52, 53), it is not surprising that these two cytokines are found at the sites of local inflammation. The synergistic upregulation of YKL-40 expression by IL-1 and IL-6 (or OSM) in in vitro experiments therefore likely recapitulates the regulation of YKL-40 in vivo. Similarly to the regulation of YKL-40, IL-1 and IL-6 (or OSM) are known to control expression of acute phase proteins in the liver, which are released to the blood to limit inflammation-associated damage and allow for the return to homeostasis (54). Thus, acute cytokine-driven expression of YKL-40 is likely highly beneficial and allows for proper resolution of inflammation. Nevertheless the mechanism by which YKL-40 limits inflammation remains elusive due to limited understanding of YKL-40 receptors and their signaling. In fact YKL-40 induces the interaction of αvβ3 integrins with syndecan-1 in endothelial cells (27), it activates ERK, AKT, and Wnt/β-catenin signaling in macrophages via IL-13 receptor alpha 2-dependent mechanism (55), and activates ERK, AKT, and p38 via protease activated receptor 2 in bronchial smooth muscle cells (56). It remains to be established whether these proposed receptors/mechanisms are important for the biological functions of YKL-40, which are associated with inflammation. However, in agreement with the proposed role of YKL-40 in limiting inflammation, it has recently been shown that YKL-40 also inhibits NF-κB activation and expression of IL-6, IL-8 and MCP-1 by skeletal muscle cells by a protease activated receptor 2-dependent mechanism (57).
It is generally accepted that IL-1 triggers a classical NF-κB pathway in many cell types, which leads to the induction of p65/p50 target genes, including those encoding proinflammatory cytokines such as IL-8 and IL-6 (58). It is also accepted that RelB is activated in lymphoid cells, such as dendritic cells, by a noncanonical NF-κB pathway and generation of RelB/p52 complexes that are important for proper dendritic cell functions (59). This non-canonical NF-κB activation pathway is activated by ligands such as CD40 and lymphotoxin-β (60), but cannot be activated by IL-1. However, it has recently been recognized that in dendritic cells, RelB can also be activated by a canonical pathway in response to TNF, LPS and CpG, and this activation results in RelB/p50 complex formation (60). This canonical RelB activation depends on TRAF6 (61), which is further supported by findings that the RelB/p50 complexes are bound to IκBα and IκBε in the cytoplasm (60). It has been also suggested that canonical activation of RelB in dendritic cells is possible because of a higher level of RelB expression in these cells and the constitutive activation of noncanonical pathway (60). The physiological importance of the RelB/p50 complexes is strongly supported by a more deleterious phenotype of RelB-deficient than the NIK-deficient mice (62), suggesting that RelB has NIK-independent functions that likely can be attributed to RelB/p50 complexes. Nevertheless, the mechanism of specific RelB/p50-dependent gene regulation is not clear since RelB/p50 and p65/p50 complexes bind the same regulatory sequences.
Although existence of RelB/p50 complexes in non-lymphoid tissues has been shown more than a decade ago (33), the function of these complexes remained elusive. Our data clearly demonstrate that RelB expression is strongly upregulated during sterile and LPS-induced inflammation in vivo, which is likely mediated by IL-1. Furthermore, IL-1 strongly activates expression of RelB in astrocytes in vitro. RelB and YKL-40 expression can also be upregulated strongly in cells responding to TNF. RelB subsequently forms complexes with p50 and activation of these complexes is controlled by IκBα. Once the RelB/p50 complexes are activated, they regulate expression of YKL-40 and likely other RelB/p50-responsive genes. Thus, it appears that RelB/p50 complexes may play critical role(s) during acute inflammation that normally leads to a return to homeostasis. However, chronic inflammation may also lead to pathological RelB/p50-dependent gene expression. Indeed, YKL-40 has recently been proposed as a marker of a particularly aggressive mesenchymal subtype of GBM (63). In agreement with our current findings indicating that RelB regulates YKL-40 expression, RelB is strongly expressed in mesenchymal subtype of GBM and promotes expression of mesenchymal genes, including YKL-40 (63). Furthermore, loss of RelB expression attenuates GBM cell survival, motility and invasion (64).
Our previous (8) and current data suggest that activation of YKL-40 expression requires both STAT3 and RelB. Although mechanistic details of YKL-40 promoter activation are not clear, robust cytokine-induced YKL-40 expression involves induction of RelB expression, binding of both STAT3 and RelB/p50 complexes to the YKL-40 promoter, and acetylation of histones. It remains to be established whether increased STAT3-dependent acetylation of histones (and opening of chromatin) allows for efficient binding of newly formed RelB/p50 complexes. Surprisingly, OSM also promotes formation of the RelB/p50 complexes by a mechanism, which currently remains unclear, but these complexes do not contain STAT3 (data not shown).
In summary, we propose that inflammation leads to the expression of YKL-40, which is induced by IL-1 and cytokines of the IL-6 family. Mechanistically, both STAT3 and the RelB/p50 complexes drive the expression of YKL-40 via the elements within the YKL-40 promoter.
Supplementary Material
Acknowledgments
We would like to thank Dr. Kristoffer Valerie (VCU) for irradiating mice.
Footnotes
This work was supported by a grant from the National Institutes of Health, NIAID (1R01AI093718 to T.K.) and from NIAMS (AR43510 to BFB).
- ChIP
- chromatin immunoprecipitation
- DOX
- doxycycline
- EAE
- experimental autoimmune encephalomyelitis
- GBM
- glioblastoma multiforme
- IKK
- IκB kinase
- MS
- multiple sclerosis
- NIK
- NF-κB-inducing kinase
- OSM
- oncostatin M
- sIL-6R
- soluble IL-6 receptor
- TAT
- transactivator of transcription
References
- 1.Roslind A, Johansen JS. YKL-40: a novel marker shared by chronic inflammation and oncogenic transformation. Methods Mol Biol. 2009;511:159–184. doi: 10.1007/978-1-59745-447-6_7. [DOI] [PubMed] [Google Scholar]
- 2.Kawamura K, Shibata T, Saget O, Peel D, Bryant PJ. A new family of growth factors produced by the fat body and active on Drosophila imaginal disc cells. Development. 1999;126:211–219. doi: 10.1242/dev.126.2.211. [DOI] [PubMed] [Google Scholar]
- 3.Hakala BE, White C, Recklies AD. Human cartilage gp-39, a major secretory product of articular chondrocytes and synovial cells, is a mammalian member of a chitinase protein family. The Journal of biological chemistry. 1993;268:25803–25810. [PubMed] [Google Scholar]
- 4.De Ceuninck F, Gaufillier S, Bonnaud A, Sabatini M, Lesur C, Pastoureau P. YKL-40 (cartilage gp-39) induces proliferative events in cultured chondrocytes and synoviocytes and increases glycosaminoglycan synthesis in chondrocytes. Biochemical and biophysical research communications. 2001;285:926–931. doi: 10.1006/bbrc.2001.5253. [DOI] [PubMed] [Google Scholar]
- 5.Nishikawa KC, Millis AJ. gp38k (CHI3L1) is a novel adhesion and migration factor for vascular cells. Experimental cell research. 2003;287:79–87. doi: 10.1016/s0014-4827(03)00069-7. [DOI] [PubMed] [Google Scholar]
- 6.Shackelton LM, Mann DM, Millis AJ. Identification of a 38-kDa heparin-binding glycoprotein (gp38k) in differentiating vascular smooth muscle cells as a member of a group of proteins associated with tissue remodeling. The Journal of biological chemistry. 1995;270:13076–13083. doi: 10.1074/jbc.270.22.13076. [DOI] [PubMed] [Google Scholar]
- 7.Malinda KM, Ponce L, Kleinman HK, Shackelton LM, Millis AJ. Gp38k, a protein synthesized by vascular smooth muscle cells, stimulates directional migration of human umbilical vein endothelial cells. Experimental cell research. 1999;250:168–173. doi: 10.1006/excr.1999.4511. [DOI] [PubMed] [Google Scholar]
- 8.Singh SK, Bhardwaj R, Wilczynska KM, Dumur CI, Kordula T. A complex of nuclear factor I-X3 and STAT3 regulates astrocyte and glioma migration through the secreted glycoprotein YKL-40. The Journal of biological chemistry. 2011;286:39893–39903. doi: 10.1074/jbc.M111.257451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee CG, Hartl D, Lee GR, Koller B, Matsuura H, Da Silva CA, Sohn MH, Cohn L, Homer RJ, Kozhich AA, Humbles A, Kearley J, Coyle A, Chupp G, Reed J, Flavell RA, Elias JA. Role of breast regression protein 39 (BRP-39)/chitinase 3-like-1 in Th2 and IL-13-induced tissue responses and apoptosis. The Journal of experimental medicine. 2009;206:1149–1166. doi: 10.1084/jem.20081271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonneh-Barkay D, Wang G, Laframboise WA, Wiley CA, Bissel SJ. Exacerbation of experimental autoimmune encephalomyelitis in the absence of breast regression protein 39/chitinase 3-like 1. Journal of neuropathology and experimental neurology. 2012;71:948–958. doi: 10.1097/NEN.0b013e31826eaee7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sohn MH, Kang MJ, Matsuura H, Bhandari V, Chen NY, Lee CG, Elias JA. The chitinase-like proteins breast regression protein-39 and YKL-40 regulate hyperoxia-induced acute lung injury. American journal of respiratory and critical care medicine. 2010;182:918–928. doi: 10.1164/rccm.200912-1793OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johansen JS, Stoltenberg M, Hansen M, Florescu A, Horslev-Petersen K, Lorenzen I, Price PA. Serum YKL-40 concentrations in patients with rheumatoid arthritis: relation to disease activity. Rheumatology. 1999;38:618–626. doi: 10.1093/rheumatology/38.7.618. [DOI] [PubMed] [Google Scholar]
- 13.Rathcke CN, Johansen JS, Vestergaard H. YKL-40, a biomarker of inflammation, is elevated in patients with type 2 diabetes and is related to insulin resistance. Inflammation research : official journal of the European Histamine Research Society … [et al.] 2006;55:53–59. doi: 10.1007/s00011-005-0010-8. [DOI] [PubMed] [Google Scholar]
- 14.Rathcke CN, Vestergaard H. YKL-40, a new inflammatory marker with relation to insulin resistance and with a role in endothelial dysfunction and atherosclerosis. Inflammation research : official journal of the European Histamine Research Society … [et al.] 2006;55:221–227. doi: 10.1007/s00011-006-0076-y. [DOI] [PubMed] [Google Scholar]
- 15.Hansen M, Nielsen AR, Vilsboll T, Lund A, Krarup T, Knop FK, Vestergaard H. Increased levels of YKL-40 and interleukin 6 in patients with chronic pancreatitis and secondary diabetes. Pancreas. 2012;41:1316–1318. doi: 10.1097/MPA.0b013e31824d9b93. [DOI] [PubMed] [Google Scholar]
- 16.Konradsen JR, James A, Nordlund B, Reinius LE, Soderhall C, Melen E, Wheelock AM, Lodrup Carlsen KC, Lidegran M, Verhoek M, Boot RG, Dahlen B, Dahlen SE, Hedlin G. The chitinase-like protein YKL-40: a possible biomarker of inflammation and airway remodeling in severe pediatric asthma. The Journal of allergy and clinical immunology. 2013;132:328–335. doi: 10.1016/j.jaci.2013.03.003. e325. [DOI] [PubMed] [Google Scholar]
- 17.Erzin Y, Uzun H, Karatas A, Celik AF. Serum YKL-40 as a marker of disease activity and stricture formation in patients with Crohn's disease. Journal of gastroenterology and hepatology. 2008;23:e357–362. doi: 10.1111/j.1440-1746.2007.05121.x. [DOI] [PubMed] [Google Scholar]
- 18.Lee SH, Lin CY, Wang PH, Han CP, Yang SF, Chang JT, Lee MC, Lin LY, Lee MS. Significant association of elevated concentration of plasma YKL-40 with disease severity in patients with pelvic inflammatory disease. Journal of clinical laboratory analysis. 2012;26:136–142. doi: 10.1002/jcla.21500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johansen JS, Moller S, Price PA, Bendtsen F, Junge J, Garbarsch C, Henriksen JH. Plasma YKL-40: a new potential marker of fibrosis in patients with alcoholic cirrhosis? Scandinavian journal of gastroenterology. 1997;32:582–590. doi: 10.3109/00365529709025104. [DOI] [PubMed] [Google Scholar]
- 20.Craig-Schapiro R, Perrin RJ, Roe CM, Xiong C, Carter D, Cairns NJ, Mintun MA, Peskind ER, Li G, Galasko DR, Clark CM, Quinn JF, D'Angelo G, Malone JP, Townsend RR, Morris JC, Fagan AM, Holtzman DM. YKL-40: a novel prognostic fluid biomarker for preclinical Alzheimer's disease. Biological psychiatry. 2010;68:903–912. doi: 10.1016/j.biopsych.2010.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonneh-Barkay D, Bissel SJ, Wang G, Fish KN, Nicholl GC, Darko SW, Medina-Flores R, Murphey-Corb M, Rajakumar PA, Nyaundi J, Mellors JW, Bowser R, Wiley CA. YKL-40, a marker of simian immunodeficiency virus encephalitis, modulates the biological activity of basic fibroblast growth factor. The American journal of pathology. 2008;173:130–143. doi: 10.2353/ajpath.2008.080045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Colton CA, Mott RT, Sharpe H, Xu Q, Van Nostrand WE, Vitek MP. Expression profiles for macrophage alternative activation genes in AD and in mouse models of AD. Journal of neuroinflammation. 2006;3:27. doi: 10.1186/1742-2094-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Comabella M, Fernandez M, Martin R, Rivera-Vallve S, Borras E, Chiva C, Julia E, Rovira A, Canto E, Alvarez-Cermeno JC, Villar LM, Tintore M, Montalban X. Cerebrospinal fluid chitinase 3-like 1 levels are associated with conversion to multiple sclerosis. Brain : a journal of neurology. 2010;133:1082–1093. doi: 10.1093/brain/awq035. [DOI] [PubMed] [Google Scholar]
- 24.Kzhyshkowska J, Gratchev A, Goerdt S. Human chitinases and chitinase-like proteins as indicators for inflammation and cancer. Biomarker insights. 2007;2:128–146. [PMC free article] [PubMed] [Google Scholar]
- 25.Ling H, Recklies AD. The chitinase 3-like protein human cartilage glycoprotein 39 inhibits cellular responses to the inflammatory cytokines interleukin-1 and tumour necrosis factor-alpha. The Biochemical journal. 2004;380:651–659. doi: 10.1042/BJ20040099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shao R, Hamel K, Petersen L, Cao QJ, Arenas RB, Bigelow C, Bentley B, Yan W. YKL-40, a secreted glycoprotein, promotes tumor angiogenesis. Oncogene. 2009;28:4456–4468. doi: 10.1038/onc.2009.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Francescone RA, Scully S, Faibish M, Taylor SL, Oh D, Moral L, Yan W, Bentley B, Shao R. Role of YKL-40 in the angiogenesis, radioresistance, and progression of glioblastoma. The Journal of biological chemistry. 2011;286:15332–15343. doi: 10.1074/jbc.M110.212514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Recklies AD, Ling H, White C, Bernier SM. Inflammatory cytokines induce production of CHI3L1 by articular chondrocytes. The Journal of biological chemistry. 2005;280:41213–41221. doi: 10.1074/jbc.M510146200. [DOI] [PubMed] [Google Scholar]
- 29.Bonneh-Barkay D, Bissel SJ, Kofler J, Starkey A, Wang G, Wiley CA. Astrocyte and macrophage regulation of YKL-40 expression and cellular response in neuroinflammation. Brain pathology. 2012;22:530–546. doi: 10.1111/j.1750-3639.2011.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 31.Pomerantz JL, Baltimore D. Two pathways to NF-kappaB. Molecular cell. 2002;10:693–695. doi: 10.1016/s1097-2765(02)00697-4. [DOI] [PubMed] [Google Scholar]
- 32.Oeckinghaus A, Hayden MS, Ghosh S. Crosstalk in NF-kappaB signaling pathways. Nature immunology. 2011;12:695–708. doi: 10.1038/ni.2065. [DOI] [PubMed] [Google Scholar]
- 33.Derudder E, Dejardin E, Pritchard LL, Green DR, Korner M, Baud V. RelB/p50 dimers are differentially regulated by tumor necrosis factor-alpha and lymphotoxin-beta receptor activation: critical roles for p100. The Journal of biological chemistry. 2003;278:23278–23284. doi: 10.1074/jbc.M300106200. [DOI] [PubMed] [Google Scholar]
- 34.Bhat KP, Pelloski CE, Zhang Y, Kim SH, deLaCruz C, Rehli M, Aldape KD. Selective repression of YKL-40 by NF-kappaB in glioma cell lines involves recruitment of histone deacetylase-1 and -2. FEBS letters. 2008;582:3193–3200. doi: 10.1016/j.febslet.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 35.Bruce-Keller AJ, Turchan-Cholewo J, Smart EJ, Geurin T, Chauhan A, Reid R, Xu R, Nath A, Knapp PE, Hauser KF. Morphine causes rapid increases in glial activation and neuronal injury in the striatum of inducible HIV-1 Tat transgenic mice. Glia. 2008;56:1414–1427. doi: 10.1002/glia.20708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stiene-Martin A, Zhou R, Hauser KF. Regional, developmental, and cell cycle-dependent differences in mu, delta, and kappa-opioid receptor expression among cultured mouse astrocytes. Glia. 1998;22:249–259. [PMC free article] [PubMed] [Google Scholar]
- 37.Kordula T, Rydel RE, Brigham EF, Horn F, Heinrich PC, Travis J. Oncostatin M and the interleukin-6 and soluble interleukin-6 receptor complex regulate alpha1-antichymotrypsin expression in human cortical astrocytes. The Journal of biological chemistry. 1998;273:4112–4118. doi: 10.1074/jbc.273.7.4112. [DOI] [PubMed] [Google Scholar]
- 38.Harikumar KB, Yester JW, Surace MJ, Oyeniran C, Price MM, Huang WC, Hait NC, Allegood JC, Yamada A, Kong X, Lazear HM, Bhardwaj R, Takabe K, Diamond MS, Luo C, Milstien S, Spiegel S, Kordula T. K63-linked polyubiquitination of transcription factor IRF1 is essential for IL-1-induced production of chemokines CXCL10 and CCL5. Nature immunology. 2014;15:231–238. doi: 10.1038/ni.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paugh BS, Bryan L, Paugh SW, Wilczynska KM, Alvarez SM, Singh SK, Kapitonov D, Rokita H, Wright S, Griswold-Prenner I, Milstien S, Spiegel S, Kordula T. Interleukin-1 regulates the expression of sphingosine kinase 1 in glioblastoma cells. The Journal of biological chemistry. 2009;284:3408–3417. doi: 10.1074/jbc.M807170200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rehli M, Niller HH, Ammon C, Langmann S, Schwarzfischer L, Andreesen R, Krause SW. Transcriptional regulation of CHI3L1, a marker gene for late stages of macrophage differentiation. The Journal of biological chemistry. 2003;278:44058–44067. doi: 10.1074/jbc.M306792200. [DOI] [PubMed] [Google Scholar]
- 41.Leon LR, Conn CA, Glaccum M, Kluger MJ. IL-1 type I receptor mediates acute phase response to turpentine, but not lipopolysaccharide, in mice. The American journal of physiology. 1996;271:R1668–1675. doi: 10.1152/ajpregu.1996.271.6.R1668. [DOI] [PubMed] [Google Scholar]
- 42.Glibetic MD, Baumann H. Influence of chronic inflammation on the level of mRNA for acute-phase reactants in the mouse liver. J Immunol. 1986;137:1616–1622. [PubMed] [Google Scholar]
- 43.Kothavale A, Di Gregorio D, Somera FP, Smith ME. GFAP mRNA fluctuates in synchrony with chronic relapsing EAE symptoms in SJL/J mice. Glia. 1995;14:216–224. doi: 10.1002/glia.440140307. [DOI] [PubMed] [Google Scholar]
- 44.Lee WH, Sonntag WE, Mitschelen M, Yan H, Lee YW. Irradiation induces regionally specific alterations in pro-inflammatory environments in rat brain. Int J Radiat Biol. 86:132–144. doi: 10.3109/09553000903419346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fitting S, Zou S, Chen W, Vo P, Hauser KF, Knapp PE. Regional heterogeneity and diversity in cytokine and chemokine production by astroglia: differential responses to HIV-1 Tat, gp120, and morphine revealed by multiplex analysis. Journal of proteome research. 2010;9:1795–1804. doi: 10.1021/pr900926n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liberto CM, Albrecht PJ, Herx LM, Yong VW, Levison SW. Pro-regenerative properties of cytokine-activated astrocytes. Journal of neurochemistry. 2004;89:1092–1100. doi: 10.1111/j.1471-4159.2004.02420.x. [DOI] [PubMed] [Google Scholar]
- 47.Heinrich PC, Behrmann I, Muller-Newen G, Schaper F, Graeve L. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. The Biochemical journal. 1998;334:297–314. doi: 10.1042/bj3340297. Pt 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Letuve S, Kozhich A, Arouche N, Grandsaigne M, Reed J, Dombret MC, Kiener PA, Aubier M, Coyle AJ, Pretolani M. YKL-40 is elevated in patients with chronic obstructive pulmonary disease and activates alveolar macrophages. J Immunol. 2008;181:5167–5173. doi: 10.4049/jimmunol.181.7.5167. [DOI] [PubMed] [Google Scholar]
- 49.Kuepper M, Bratke K, Virchow JC. Chitinase-like protein and asthma. N Engl J Med. 2008;358:1073–1075. doi: 10.1056/NEJMc073406. author reply 1075. [DOI] [PubMed] [Google Scholar]
- 50.Johansen JS. Studies on serum YKL-40 as a biomarker in diseases with inflammation, tissue remodelling, fibroses and cancer. Dan Med Bull. 2006;53:172–209. [PubMed] [Google Scholar]
- 51.Gross O, Thomas CJ, Guarda G, Tschopp J. The inflammasome: an integrated view. Immunological reviews. 2011;243:136–151. doi: 10.1111/j.1600-065X.2011.01046.x. [DOI] [PubMed] [Google Scholar]
- 52.Norris JG, Tang LP, Sparacio SM, Benveniste EN. Signal transduction pathways mediating astrocyte IL-6 induction by IL-1 beta and tumor necrosis factor-alpha. Journal of immunology. 1994;152:841–850. [PubMed] [Google Scholar]
- 53.Sparacio SM, Zhang Y, Vilcek J, Benveniste EN. Cytokine regulation of interleukin-6 gene expression in astrocytes involves activation of an NF-kappa B-like nuclear protein. Journal of neuroimmunology. 1992;39:231–242. doi: 10.1016/0165-5728(92)90257-l. [DOI] [PubMed] [Google Scholar]
- 54.Bode JG, Albrecht U, Haussinger D, Heinrich PC, Schaper F. Hepatic acute phase proteins--regulation by IL-6- and IL-1-type cytokines involving STAT3 and its crosstalk with NF-kappaB-dependent signaling. Eur J Cell Biol. 91:496–505. doi: 10.1016/j.ejcb.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 55.He CH, Lee CG, Dela Cruz CS, Lee CM, Zhou Y, Ahangari F, Ma B, Herzog EL, Rosenberg SA, Li Y, Nour AM, Parikh CR, Schmidt I, Modis Y, Cantley L, Elias JA. Chitinase 3-like 1 regulates cellular and tissue responses via IL-13 receptor alpha2. Cell reports. 2013;4:830–841. doi: 10.1016/j.celrep.2013.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bara I, Ozier A, Girodet PO, Carvalho G, Cattiaux J, Begueret H, Thumerel M, Ousova O, Kolbeck R, Coyle AJ, Woods J, Tunon de Lara JM, Marthan R, Berger P. Role of YKL-40 in bronchial smooth muscle remodeling in asthma. American journal of respiratory and critical care medicine. 2012;185:715–722. doi: 10.1164/rccm.201105-0915OC. [DOI] [PubMed] [Google Scholar]
- 57.Rallabhandi P, Nhu QM, Toshchakov VY, Piao W, Medvedev AE, Hollenberg MD, Fasano A, Vogel SN. Analysis of proteinase-activated receptor 2 and TLR4 signal transduction: a novel paradigm for receptor cooperativity. The Journal of biological chemistry. 2008;283:24314–24325. doi: 10.1074/jbc.M804800200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Matsusaka T, Fujikawa K, Nishio Y, Mukaida N, Matsushima K, Kishimoto T, Akira S. Transcription factors NF-IL6 and NF-kappa B synergistically activate transcription of the inflammatory cytokines, interleukin 6 and interleukin 8. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:10193–10197. doi: 10.1073/pnas.90.21.10193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bonizzi G, Karin M. The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends in immunology. 2004;25:280–288. doi: 10.1016/j.it.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 60.Shih VF, Davis-Turak J, Macal M, Huang JQ, Ponomarenko J, Kearns JD, Yu T, Fagerlund R, Asagiri M, Zuniga EI, Hoffmann A. Control of RelB during dendritic cell activation integrates canonical and noncanonical NF-kappaB pathways. Nature immunology. 2012;13:1162–1170. doi: 10.1038/ni.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kobayashi T, Walsh PT, Walsh MC, Speirs KM, Chiffoleau E, King CG, Hancock WW, Caamano JH, Hunter CA, Scott P, Turka LA, Choi Y. TRAF6 is a critical factor for dendritic cell maturation and development. Immunity. 2003;19:353–363. doi: 10.1016/s1074-7613(03)00230-9. [DOI] [PubMed] [Google Scholar]
- 62.Sun SC. The noncanonical NF-kappaB pathway. Immunological reviews. 2012;246:125–140. doi: 10.1111/j.1600-065X.2011.01088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bhat KP, Balasubramaniyan V, Vaillant B, Ezhilarasan R, Hummelink K, Hollingsworth F, Wani K, Heathcock L, James JD, Goodman LD, Conroy S, Long L, Lelic N, Wang S, Gumin J, Raj D, Kodama Y, Raghunathan A, Olar A, Joshi K, Pelloski CE, Heimberger A, Kim SH, Cahill DP, Rao G, Den Dunnen WF, Boddeke HW, Phillips HS, Nakano I, Lang FF, Colman H, Sulman EP, Aldape K. Mesenchymal differentiation mediated by NF-kappaB promotes radiation resistance in glioblastoma. Cancer cell. 2013;24:331–346. doi: 10.1016/j.ccr.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee DW, Ramakrishnan D, Valenta J, Parney IF, Bayless KJ, Sitcheran R. The NF-kappaB RelB protein is an oncogenic driver of mesenchymal glioma. PloS one. 2013;8:e57489. doi: 10.1371/journal.pone.0057489. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.