Introduction
Ibuprofen is a traditional nonsteroidal anti-inflammatory drug (NSAID) widely used for its analgesic, anti-inflammatory, and antipyretic properties [1,2]. At low over-the-counter doses (800–1200 mg/day), ibuprofen is indicated to relieve minor pain and inflammation, including headache, muscular aches, toothache, fever, backache, and dysmenorrhea. At prescription doses (1800–2400 mg/day), it is used for the long-term treatment of rheumatoid arthritis, osteoarthritis, ankylosing spondylitis, and other chronic conditions [2]. Ibuprofen has also been used off-label to promote closure of patent ductus arteriosus (PDA) in preterm neonates [3]. It is commonly used in pediatric patients for the treatment of acute pain and fever (5–10 mg/kg every 6–8 h) due to its relative safety compared with aspirin and its high efficacy compared with acetaminophen [2]. Prescription doses of ibuprofen (adult: 200–800 mg every 6–8 h; pediatric: 5–10 mg/kg every 6–8 h) have greater antipyretic and analgesic effects in both children and adults compared with commonly used doses of acetaminophen (adult: 500–1000 mg every 6–8 h; pediatric: 10–15 mg/kg every 4–6 h) [4].
Pharmacokinetics
Ibuprofen is most commonly administered orally, but an intravenous formulation is also approved for use in the USA. Other formulations, most notably topical and rectal, can be prepared by compounding pharmacies in the USA and may be commercially available in other countries. Ibuprofen is rapidly and completely absorbed following oral administration (tmax, ~ 1–2 h depending on the specific oral formulation), and unbound concentrations show linear pharmacokinetics at commonly used doses [1,5]. It is extensively (>98%) bound to plasma proteins at therapeutic concentrations [1]. Although ibuprofen may displace other highly protein-bound drugs, this is unlikely to result in clinically relevant drug–drug interactions for agents with a low extraction ratio, such as warfarin [6] and phenytoin [7]. Consistent with the high degree of plasma protein binding, ibuprofen exhibits a low apparent volume of distribution that approximates plasma volume (~0.1–0.2 l/kg), but it is able to penetrate into the central nervous system (CNS) and accumulate at peripheral sites where its analgesic and anti-inflammatory effects are required. Ibuprofen is present in a free, unbound form in cerebrospinal fluid and is retained in the synovial fluid in the inflamed joints of arthritic patients [2]. Ibuprofen has a wide therapeutic concentration range for its analgesic, antipyretic, and anti-inflammatory effects (~10–50 mg/l) and a relatively short plasma half-life (t1/2, ~ 1–3 h), necessitating frequent administration to maintain therapeutic plasma concentrations [1,2].
The pharmacokinetic profile of ibuprofen in the pediatric population (age > 0.5 years) appears to be similar to that observed in adults in general, although some studies have indicated that young children (0.5–5 years) have higher rates of ibuprofen clearance [2]. In contrast, the half-life of ibuprofen in premature neonates is in the order of 30–45 h following intravenous administration, which may be due to several factors including developmental effects on cytochrome P450 (CYP) enzyme activity and lower glomerular filtration rates in neonates compared with adults [2,3].
Metabolism
Like most NSAIDs, ibuprofen is administered as a racemic mixture of R and S enantiomers, with S-ibuprofen being largely responsible for its pharmacologic activity [1]. Following administration, an estimated 50–65% of R-ibuprofen undergoes inversion to the S enantiomer through an acyl-CoA thioester by the enzyme α-methylacyl-coenzyme A racemase (encoded by gene AMACR) [1,8,9]. This appears to occur predominantly systemically in the liver [1,10], but may occur pre-systemically in the gut as well [11].
Ibuprofen is almost completely metabolized, with little to no unchanged drug found in the urine [1,9,12]. The primary route of elimination is oxidative metabolism by CYP enzymes to inactive metabolites (Fig. 1). Urinary excretion of the two major metabolites, carboxy-ibuprofen and 2-hydroxy-ibuprofen (and their corresponding acyl glucuronides), accounts for ~37 and 25% of an administered dose, respectively [1,9]. Small amounts of other hydroxylated metabolites (3-hydroxy-ibuprofen and 1-hydroxy-ibuprofen) have also been detected in urine [12].
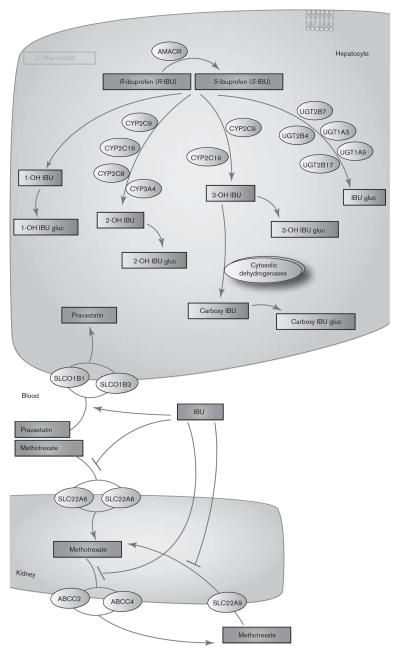
Fig. 1.
Metabolism and transport of ibuprofen in the liver and kidney. IBU, ibuprofen; IBU gluc, ibuprofen glucuronide. A fully interactive version is available online at http://www.pharmgkb.org/pathway/PA166041114.
CYP2C9 is the primary CYP isoform responsible for ibuprofen clearance, catalyzing the formation of 3-hydroxy-ibuprofen (most of which is subsequently converted to carboxy-ibuprofen by cytosolic dehydrogenases [12,13]) and 2-hydroxy-ibuprofen [13,14]. Consequently, coadministration of ibuprofen with CYP2C9 inhibitors (i.e. selective serotonin-reuptake inhibitors) or other CYP2C9 substrates (i.e. warfarin) may precipitate a pharmacokinetic drug–drug interaction, thereby increasing the risk for an adverse drug event (for a broader discussion, refer to the Drug–drug interactions section) [6,15,16]. Whereas CYP2C9 can readily metabolize both enantiomers of ibuprofen in vitro, CYP2C8, which plays a minor role in ibuprofen clearance, exhibits stereoselectivity, preferentially catalyzing the 2-hydroxylation of R-ibuprofen [13,14,17]. CYP3A4 also contributes to ibuprofen clearance at high concentrations through 2-hydroxylation, whereas CYP2C19 appears to play a minor role [14].
Approximately 10–15% of an ibuprofen dose is directly glucuronidated to ibuprofen-acyl glucuronide [1,9]. In-vitro experiments indicate that multiple uridine 5′diphosphoglucuronosyltransferases (UGTs) are capable of metabolizing ibuprofen, including UGT1A3, UGT1A9, UGT2B4, UGT2B7, and UGT2B17 [18–20]. UGT1A10, which is predominantly expressed in the gut, can also generate ibuprofen-acyl glucuronide [21]. CYP-derived hydroxy and carboxy metabolites are metabolized to the corresponding acyl glucuronides, but the UGTs that catalyze this reaction have not been investigated. Further studies are necessary to characterize the relative contributions of individual UGTs to ibuprofen metabolism in vivo.
Although glucuronidation is generally considered a detoxification pathway, acyl glucuronides are potentially reactive metabolites. They can undergo intramolecular rearrangement and are capable of binding covalently to macromolecules and contributing to toxicity [22]. Consistent with this, covalent binding of ibuprofen-acyl glucuronide to plasma proteins has been detected in vitro and in elderly individuals chronically treated with ibuprofen in vivo [23]. However, ibuprofen-acyl glucuronide was relatively less reactive than other compounds investigated, and the degree of covalent binding to plasma proteins was low, suggesting that ibuprofen-acyl glucuronide is not a key contributor to toxicity in most individuals [23]. Conjugation to thiols has also been reported, although these conjugates account for less than 1% of urinary metabolites [24]. Like acyl glucuronides, these metabolites are considered reactive and may contribute to adverse drug events; however, evidence demonstrating the toxicity of these metabolites in humans in vivo is lacking [25].
Transport
NSAIDs interact with various classes of transporters. It is still unclear which, if any, transporters facilitate the uptake or efflux of ibuprofen in vivo or whether this influences the distribution or clearance. Ibuprofen is a weak acid and is lipid soluble; hence, it is feasible that it may be able to cross membranes without the need for specific transporters [1]. However, the interaction of ibuprofen with various transporters may result in clinically relevant drug–drug interactions.
In-vitro studies have demonstrated that ibuprofen is a substrate for SLC22A6 and SLC22A8 [26] and can inhibit various transporters, including SLC22A6 (hOAT1), SLC22A7 (hOAT2), SLC22A8 (hOAT3), SLC22A9 (hOAT4), SLC22A1 (OCT1), SLC15A1 (hPEPT1), SLC5A8 (hSMCT1), and SLC16A1 (MCT1) [26–31]. Stereoselectivity in transporter inhibition has been observed in some cases, with S-ibuprofen being a more potent inhibitor of SLC22A6 than R-ibuprofen, whereas both enantiomers inhibited SLC22A8 equipotently [32]. Although ibuprofen is not a substrate for the organic anion-transporting polypeptides, it does interact with SLCO1B1 (hOATP1B1) and SLCO1B3 (hOATP1B3) to increase the uptake of pravastatin and inhibit the uptake of bromosulfophthalein [33]. Additional studies are necessary to determine whether these transporter interactions observed in vitro lead to clinically relevant drug–drug interactions in vivo.
One drug–drug interaction in which transporters may play a role is the well-recognized interaction between methotrexate and ibuprofen. Coadministration of NSAIDs with methotrexate reduces methotrexate clearance, resulting in elevated systemic concentrations [1,6]. Ibuprofen inhibited methotrexate uptake by SLC22A6, SLC22A8, and SLC22A9 in vitro [32,34,35], suggesting that inhibition of these transporters in the kidney may contribute to the reduction in renal clearance of methotrexate upon coadministration with ibuprofen. Another possible mechanism is through the inhibition of ABCC2 (MRP2)-mediated and ABCC4 (MRP4)-mediated transport of methotrexate, which would also be hypothesized to decrease the renal clearance of methotrexate in vivo [36].
Although the interaction between methotrexate and ibuprofen is potentially fatal, some transporter-mediated interactions with ibuprofen may enhance the efficacy or limit the toxicity of the interacting drug. For example, ibuprofen was shown to modulate the activity of ABCB1 (P-glycoprotein) such that treatment of human sarcoma cells with ibuprofen reversed ABCB1-mediated efflux of doxorubicin and led to increased drug accumulation, cytotoxicity, and apoptosis [37]. Ibuprofen may increase intracellular concentrations and potentiate the antiviral efficacy of nucleoside reverse transcriptase inhibitors, including zidovudine, lamivudine, tenofovir, and abacavir, through the inhibition of ABCC4, which mediates the export of these drugs out of T cells [38]. Through the inhibition of SLC22A6, ibuprofen may limit the nephrotoxicity of the antiviral drug adefovir, known for its cytotoxicity in the renal proximal tubules [39].
It is important to note that studies to date have been performed in vitro, largely with cells transfected with the transporter of interest. Although the majority of these studies used concentrations of ibuprofen in the range of the total drug concentrations observed in plasma, it is unclear how well these conditions approximate the unbound concentrations that would be available to inhibit transport in vivo. Thus, additional studies are necessary to clarify the clinical relevance of these transporter-mediated drug–drug interactions in vivo.
Pharmacodynamics
The main mechanism of action of ibuprofen is the non-selective, reversible inhibition of the cyclooxygenase enzymes COX-1 and COX-2 (coded for by PTGS1 and PTGS2, respectively; Fig. 2) [1]. In-vitro studies have indicated that, of the two enantiomers, S-ibuprofen is a more potent inhibitor of COX enzymes compared with R-ibuprofen [40,41]. In an in-vitro human whole-blood assay, S-ibuprofen was seen to have comparable inhibitory activities toward COX-1 and COX-2 (IC50 2.1 and 1.6 μmol/l, respectively). In contrast, R-ibuprofen was ~ 15-fold less potent than S-ibuprofen as a COX-1 inhibitor (IC50 34.9 μmol/l) and did not inhibit COX-2 at concentrations of up to 250 μmol/l [42]. COX-1 and COX-2 catalyze the first committed step in the synthesis of prostanoids – prostaglandin (PG) E2, PGD2, PGF2α, PGI2 (also known as prostacyclin), and thromboxane (Tx) A2 – from arachidonic acid. Prostanoids produce a diverse array of biologic effects through the activation of prostanoid receptors, and play important roles in a variety of homeostatic and pathologic processes [43].
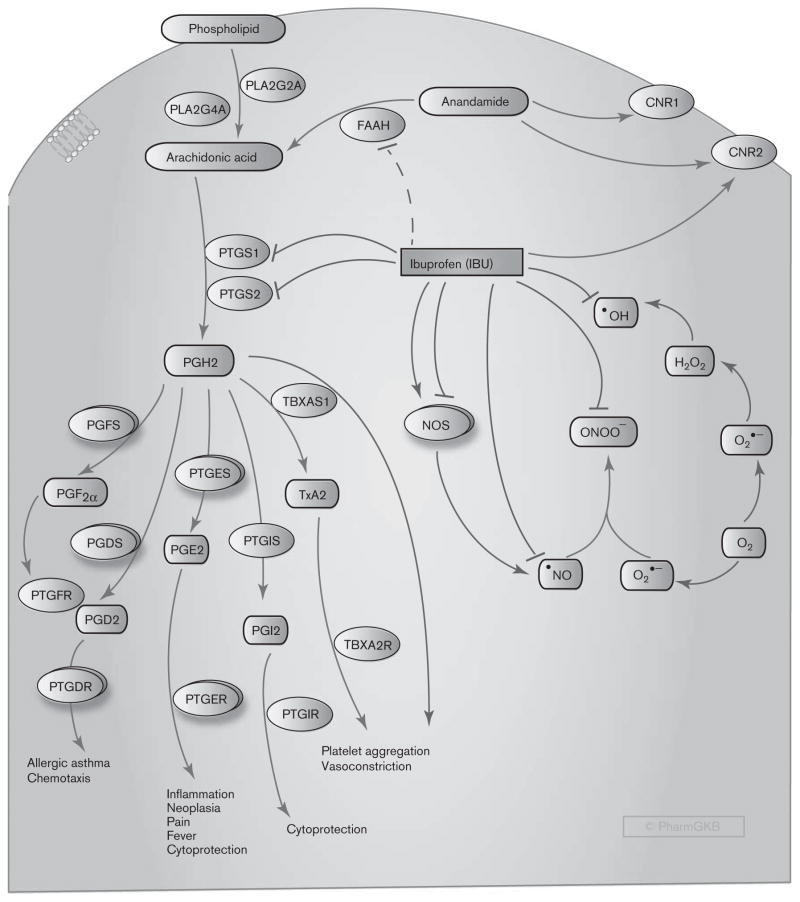
Fig. 2.
Stylized cell depicting the mechanism of action of ibuprofen (IBU). Arachidonic acid is released from the cell membrane phospholipids by phospholipase A2 (PLA2), encoded by PLA2G4A (cytosolic, calcium-dependent) and PLA2G2A (in platelets and synovial fluid). Arachidonic acid is converted to the unstable intermediate prostaglandin (PG) H2 by cytosolic prostaglandin G/H synthases, termed cyclooxygenases (COX), that exist in two forms, COX-1 and COX-2, and are encoded by PTGS1 and PTGS2, respectively. PGH2 is converted by tissue-specific synthases to various prostanoids – that is, PGE2, PGD2, PGF2α, PGI2, and TxA2. These bioactive lipids act through their corresponding receptors to trigger a series of biological effects. Ibuprofen exerts its anti-inflammatory and analgesic effects through inhibition of both COX isoforms. In addition, ibuprofen scavenges HO•, •NO, and ONOO− radicals and can potentiate or inhibit nitric oxide formation through its effects on nitric oxide synthase (NOS) isoforms. Ibuprofen may activate the antinociceptive axis through binding to the cannabinoid receptors and through inhibition of fatty acid amide hydrolase (FAAH), which metabolizes the endocannabinoid anandamide. CNR1 and CNR2, cannabinoid receptors 1 and 2; H2O2, hydrogen peroxide; FAAH, fatty acid amide hydrolase; •NO, nitric oxide; NOS, nitric oxide synthase; ONOO−, peroxynitrite anion; O2•−, superoxide anion; PGD2, prostaglandin D2; PGDS, prostaglandin D synthase; PGE2, prostaglandin E2; PGF2α, prostaglandin F2α; PGFS, prostaglandin F synthase; PGH2, prostaglandin H2; PGI2, prostacyclin; PTGDR, prostaglandin D receptors; PTGER, prostaglandin E receptors; PTGES, prostaglandin E synthase; PTGFR, prostaglandin F receptors; PTGIR, prostacyclin receptor; PTGIS, prostacyclin synthase; TBXA2R, TxA2 receptor; TBXAS1, thromboxane A synthase 1; TxA2, thromboxane A2. A fully interactive version is available online at http://www.pharmgkb.org/pathway/PA166121942.
Many of the pharmacodynamic effects of ibuprofen can be directly linked to the inhibition of prostanoid synthesis. Single and repeated oral doses of ibuprofen inhibited the production of COX-1-derived TxB2 (a stable metabolite of TxA2) ex vivo by ~ 96 and ~ 90%, respectively, whereas COX-2-derived PGE2 production ex vivo was inhibited by ~ 84 and ~ 76%, respectively [44]. PGE2 and PGI2 are proinflammatory prostanoids that enhance edema formation, increase vascular permeability, and promote leukocyte infiltration. They also reduce the threshold of nociceptor sensory neurons to stimulation [43]. Ibuprofen exerts its anti-inflammatory and analgesic effects largely by inhibiting the formation of these prostanoids. PGE2 is also a primary mediator of pyresis, and its synthesis is triggered in the hypothalamus by pyrogens such as cytokines, endotoxin, and products from activated leukocytes [45]. Thus, the antipyretic effects of ibuprofen can be attributed to inhibition of PGE2 synthesis. Inhibition of both PGF2α and PGE2, which trigger spasm of the uterine smooth muscles and inflammatory pain, is responsible for the therapeutic efficacy of ibuprofen in primary dysmenorrhea [46]. TxA2, a major product of COX-1 in platelets, causes vasoconstriction and promotes platelet activation and aggregation, thereby leading to thrombus formation [43,47]. Consequently, ibuprofen exhibits a mild, transient antiplatelet effect through reversible inhibition of platelet COX-1, as evidenced by its ability to inhibit stimulus-triggered platelet aggregation in vitro [48].
In addition to the direct inhibition of prostanoid synthesis, ibuprofen exerts other biologic effects that may contribute to its anti-inflammatory action and might be consequent to the suppression of prostaglandin synthesis. Several studies have suggested that ibuprofen can inhibit neutrophil aggregation and degranulation as well as proinflammatory cytokine production by immune cells in vitro and in vivo [49–53]. During inflammation, immune cells, such as macrophages, mast cells, eosinophils, and neutrophils, robustly produce reactive oxygen species (e.g. superoxide anion, O2•−, hydroxyl radical, HO•, and other unstable molecules) and reactive nitrogen species (e.g. nitric oxide, •NO, and peroxynitrite anion, ONOO−) that contribute to the pathophysiology of the inflammatory processes [54]. Using noncellular in-vitro screening systems, ibuprofen was reported to scavenge HO•, •NO, and ONOO− radicals at concentrations comparable to the high doses prescribed for chronic inflammatory conditions [55]. Cell-based in-vitro studies have indicated that ibuprofen can activate or inhibit nitric oxide production through constitutive nitric oxide synthases (cNOS) (neuronal NOS, encoded by NOS1, and endothelial NOS, encoded by NOS3) or inflammation-induced nitric oxide synthase (iNOS, encoded by NOS2) depending on the type of the enzyme and the cellular system used [48,56]. In healthy human individuals, therapeutic doses of ibuprofen triggered a reduction in exhaled NO and urinary excretion of nitrite and nitrate, consistent with an inhibitory effect of the drug on nitric oxide production [57]. Taken together, these findings suggest that ibuprofen exhibits pleiotropic anti-inflammatory effects by inhibiting prostanoid synthesis, interfering with immune cell function, scavenging reactive oxygen and nitrogen species, and altering nitric oxide synthesis. However, further studies are required to determine whether the effects of ibuprofen on immune cells and reactive oxygen and nitrogen species result from the inhibition of prostaglandin production.
Additional analgesic effects of ibuprofen may be attributable to elevated levels of the endocannabinoid anandamide (also known as arachidonoylethanolamide), which activates the antinociceptive axis through the cannabinoid receptors (CB1 and CB2) in the CNS. Animal studies have suggested that, at therapeutic concentrations, ibuprofen inhibits anandamide metabolism [58,59] and, together with anandamide, exerts a synergistic antinociceptive effect in a model of inflammatory pain [60]. In in-vitro studies, ibuprofen was shown to inhibit the binding of a potent synthetic agonist to the human CB2 cannabinoid receptor, indicating that it may compete with endogenous ligands for receptor binding and activation of the analgesic pathway [61]. However, the clinical relevance of these findings is still to be investigated in human participants.
Adverse events
The short plasma half-life, a wide therapeutic window, and the lack of prolonged retention in specific body compartments make ibuprofen a relatively safe drug. There is no evidence of ibuprofen accumulation in the elderly and relatively little impact of chronic disease states (arthritis) or mild renal/hepatic impairments on the pharmacokinetics of ibuprofen [1,2]. Although serious skin diseases, such as the Stevens–Johnson syndrome and toxic epidermal necrolysis, have been reported in patients with ibuprofen use, these are exceedingly rare, at a rate of less than 1 per 1 million users per week for most NSAIDs [62].
Like other NSAIDs, ibuprofen can cause serious gastrointestinal and possibly cardiovascular adverse events, especially at high doses [1,2,63–66]. Most observational studies with ibuprofen have reported no increased risk for cardiovascular events, such as myocardial infarction and sudden cardiac death [67–69]. However, the risk for cardiovascular events might increase with prolonged exposure to ibuprofen (i.e. greater than 1 year) [70]. It still remains to be determined how ibuprofen compares with COX-2-selective inhibitors, known to pose a cardiovascular risk [66]. In the Therapeutic Arthritis Research and Gastrointestinal Event Trial, gastrointestinal safety and cardiovascular safety were compared between a COX-2 inhibitor lumiracoxib and traditional NSAIDs ibuprofen and naproxen [64,65]. Despite a higher incidence of cardiovascular events in the lumiracoxib group, the Therapeutic Arthritis Research and Gastrointestinal Event Trial involved patients at low risk, was under-powered, and used an intention-to-treat analysis [65]. A recent meta-analysis of 280 randomized trials of NSAIDs versus placebo and 474 trials of one NSAID versus another NSAID focused on the cardiovascular and gastrointestinal risks of this class of drugs among different patient populations, especially those at increased risk for vascular disease [63]. Compared with placebo, high-dose ibuprofen significantly increased the risk for major coronary events (nonfatal myocardial infarction or coronary death), although the number of events was low and, similarly to other NSAIDs, was associated with increased upper gastrointestinal complications. All NSAIDs, including ibuprofen, doubled the risk for heart failure causing hospital admission, and none of the NSAIDs studied was associated with an increased risk for stroke. Although high-dose ibuprofen significantly increased the risk for major coronary events, further studies are required to verify whether the cardiovascular risks associated with ibuprofen are comparable to those associated with COX-2-selective inhibitors [63]. Moreover, the cardiovascular risk associated with short-term, low-dose ibuprofen use is a topic of some debate, as prospective studies defining this risk are lacking. Overall, relative to other NSAIDs, especially COX-2-selective inhibitors, ibuprofen might have lower gastrointestinal and cardiovascular risks, especially when used over a short term at over-the-counter doses [1,2,63,66]. However, resolving the issue of cardiovascular risk from ibuprofen alone or relative to COX-2 inhibitors would require a large-scale, long-term, adequately powered, randomized, controlled outcome trial.
Drug–drug interactions
Ibuprofen exhibits pharmacodynamic interactions with a variety of drugs. Ibuprofen antagonizes the cardioprotective effect of low-dose aspirin (acetylsalicylic acid) through competition for the NSAID binding site of COX-1 in platelets [71]. Low-dose aspirin is recommended as an effective antiplatelet therapy for secondary prevention of myocardial infarction and stroke [72,73]. Consumption of low-dose aspirin results in maximum inhibition of TxA2 synthesis by platelets, with subsequent inhibition of platelet aggregation. Under chronic dosing conditions, when ibuprofen is administered three times a day, this interaction undermines aspirin-induced inhibition of platelet aggregation irrespective of which of the drugs precedes the other in the morning [71]. The follow-up studies on ibuprofen–aspirin interactions range from confirming ibuprofen antagonistic effect on aspirin antiplatelet action [64,74,75] to reporting no change after the concurrent administration of the two drugs [69,76], although a well-powered, clinical end-point study has never been conducted.
Because it reversibly inhibits COX-1 in platelets, ibuprofen has a transient antiplatelet effect for 1 h during the 8 h dosing interval, which may increase bleeding risk when administered with other anticoagulant or antiplatelet agents. Concomitant administration of warfarin with ibuprofen was reported to prolong the bleeding time [77] and increase the international normalized ratio, a measure of the clotting tendency of blood [78]. An increased risk for gastrointestinal bleeding has been reported after coadministration of NSAIDs with selective serotonin-reuptake inhibitors (SSRIs). SSRIs block serotonin reuptake by platelets and downregulate serotonin receptors, leading to the inhibition of platelet function and increased bleeding risk [15]. SSRI use alone increases the risk for bleeding by 30% as compared with non-NSAID/non-SSRI use, and the risk for gastrointestinal events increases to 50–60% when SSRIs are coadministered with NSAIDs [6]. These effects may be compounded by a concomitant pharmacokinetic drug–drug interaction through CYP2C9 (see the Pharmacokinetics section). Individuals with CYP2C9*2 and CYP2C9*3 variants, who comprise about 20% of the white population, may be especially susceptible to the bleeding events [6].
In patients with a bipolar affective disorder, the concomitant use of lithium with NSAIDs has been reported to increase the serum lithium level and reduce lithium clearance, thus causing acute lithium intoxication [79]. Mechanistically this might be due to inhibition of prostaglandin-mediated excretion of lithium in the distal tubule. However, the degree of elevation in serum lithium concentrations with concomitant ibuprofen treatment has been inconsistent across studies [80–83]. In a geropsychiatric population, coadministration of lithium with ibuprofen for 6 days was found to increase the serum lithium level and decreased lithium clearance, with pronounced interindividual variability [82]. The magnitude of increase in the serum lithium level ranged from 12 to 66.5%, with an average increase of 34%, suggesting that there is substantial interindividual variability in the clinical significance of this drug–drug interaction. Thus, frequent monitoring of serum lithium levels upon initiation of concomitant therapy with ibuprofen is recommended to identify those individuals in whom a reduction in lithium dosage is necessary. The effect of long-term ibuprofen therapy in lithium-treated patients needs to be further investigated [82].
Finally, NSAIDs, including ibuprofen, interfere with the efficacy of many antihypertensive agents, including β-adrenergic blockers, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and diuretics [84–87]. This is mediated through the inhibition of the production of vasodilatory prostanoids in the kidneys, thereby inducing vasoconstriction of afferent renal arterioles, fluid retention, and reduction in renal blood flow, promoting activation of the rennin–angiotensin system [2]. Although a well-designed trial, adjusting for drug exposure and comparing the hypertensive effects of NSAIDs, has not been conducted to date, a retrospective study reported that ibuprofen appears to be less likely than diclofenac and piroxicam to necessitate an intensification of antihypertensive treatment [86].
Pharmacogenomics
Pharmacogenomic studies on ibuprofen have examined the effects of genetic polymorphisms on pharmacokinetics (clearance, half-life, area under the curve) [88–92], pharmacodynamics (inhibition of COX-1 and COX-2) [88,90], the safety profile (gastrointestinal adverse events) [88,93–97], and therapeutic efficacy (analgesia, PDA closure, cancer chemoprevention) [3,98–107].
Several studies have investigated the effect of genetic variations in CYP2C8 and CYP2C9 on ibuprofen pharmacokinetics because of the key role of these enzymes in ibuprofen clearance. Ibuprofen clearance is significantly reduced in carriers of the CYP2C9*3 variant allele compared with individuals with the CYP2C9*1/*1 genotype, whereas the CYP2C9*2 variant appears to have no significant impact on the pharmacokinetics of ibuprofen [88–90]. One study reported that the reduction in ibuprofen clearance in CYP2C9*3 variant allele carriers was accompanied by an increased pharmacodynamic effect, namely, prolonged inhibition of TxB2 and PGE2 synthesis, indices of COX-1 and COX-2 inhibition, respectively [90], but another study found no differences in the degree of COX-1 or COX-2 inhibition in variant allele carriers [88]. Similarly, conflicting data have been reported on the relationship between the CYP2C8*3 variant and interindividual variability in ibuprofen pharmacokinetics [88,89,91,92]. To date, most studies have suggested that ibuprofen clearance is reduced by the CYP2C8*3 allele [89,91,92], but one study reported 20% higher ibuprofen clearance in CYP2C8*3 variant allele carriers compared with CYP2C8*1/*1 individuals [88].
A few studies have suggested that a decrease in clearance, leading to sustained ibuprofen levels, may increase the risk for gastrointestinal bleeding in CYP2C8 and CYP2C9 variant allele carriers. In a small study of Italian NSAID users who experienced gastroduodenal bleeding after short-term NSAID use (< 1 month; all NSAIDs: n = 26; ibuprofen: n = 3), significantly higher frequencies of CYP2C9*1/*2 and CYP2C9*1/*3 genotypes were reported in cases versus controls [94]. A French study representing nonaspirin NSAID users of various ethnicities (all NSAIDs: n = 57; ibuprofen: n = 11) similarly found a greater risk for acute gastrointestinal bleeding in patients heterozygous [odds ratio (OR) (95% confidence interval (CI)): 4.0 (1.7–9.5)] and homozygous [OR (95% CI): 15.7 (1.8–138.0)] for the CYP2C9*3 variant allele, but did not replicate the association for CYP2C9*2 [95]. A study in Spanish NSAID users (all NSAIDs: n = 94; ibuprofen: n = 9) reported an increased risk for acute gastrointestinal bleeding in CYP2C9*2 variant allele carriers [OR (95% CI): 1.92 (1.14–3.25), P = 0.009], but found no association with CYP2C9*3 [96]. A subsequent study by the same group repeated this analysis and investigated the effect of the CYP2C8*3 variant allele in an expanded population (all NSAIDs: n = 134; ibuprofen: n = 14) [93]. The greatest risk for NSAID-related gastrointestinal bleeding was observed in individuals who carried both the CYP2C8*3 and CYP2C9*2 variant alleles [OR (95% CI): 3.73 (1.57–8.88), P = 0.003], whereas there was no elevation in risk among individuals who carried only the CYP2C8*3 [OR (95% CI): 1.36 (0.39–4.66), P = 0.646] or CYP2C9*2 [OR (95% CI): 0.73 (0.22–2.51), P = 0.637] variant allele in isolation [93]. In contrast to these findings, no significant differences in the frequency of CYP2C9*2 and CYP2C9*3 variant alleles were observed between patients with NSAID-induced gastric ulceration and controls (all NSAIDs: n = 54; ibuprofen: n = 5) in a predominantly Caucasian cohort from New Zealand [97]. Notably, all studies to date have enrolled patients taking a variety of NSAIDs; thus, it is unclear to what degree the potentially increased risk for gastrointestinal bleeding in CYP2C8 and CYP2C9 variant allele carriers is specifically related to ibuprofen use.
To date few studies have evaluated the effect of genetic variation on the therapeutic efficacy of ibuprofen [3,98]. One study has investigated the effect of polymorphisms in COX-1 (PTGS1) and COX-2 (PTGS2) on pain perception with either ibuprofen or rofecoxib after third molar (i.e. wisdom tooth) extraction [98]. The authors also quantified the mRNA expression level of COX-1, COX-2, and other related genes in mucosal biopsies before and 2–4 h after the oral surgery. No significant associations were observed with regard to variants in PTGS1. However, one variant located in the PTGS2 promoter, rs20417 (-765G > C), was associated with both lower PTGS2 mRNA expression in mucosal tissue and greater analgesic response to ibuprofen in variant allele carriers. At 48 h after surgery, patients who carried the minor allele for rs20417 (CC +CG) reported significantly lower pain scores on a visual analog scale (100 mm) following treatment with ibuprofen compared with rofecoxib (ibuprofen: 7.0 ± 1.9 mm vs. rofecoxib: 37.0 ± 6.8 mm, P <0.01), whereas patients homozygous for the major allele (GG) had a better response to rofecoxib than to ibuprofen (ibuprofen: 31.3 ± 6.7 mm vs. rofecoxib: 7.2 ± 2.5 mm, P <0.01; note: this gene is on the minus chromosomal strand, complemented on PharmGKB to the plus strand; in the paper this is reported on the minus strand) [98]. These results suggest that the PTGS2 rs20417 variant may have utility in guiding the selection of COX-2-selective versus traditional NSAID therapy following third molar extraction, but additional studies are necessary to validate these findings. Another study evaluated the relationship between CYP2C8 and CYP2C9 variants and the response to ibuprofen for PDA closure in preterm neonates because higher ibuprofen serum concentrations had been previously associated with higher response rates. No significant associations between the CYP2C8 or CYP2C9 genotype and ibuprofen response were observed after multivariate adjustment, which may reflect the substantial clinical heterogeneity in this patient population, as well as the potential influence of development on the expression and catalytic activity of CYP2C enzymes [3].
Numerous studies have investigated the effect of genetic variants on the efficacy of NSAIDs for cancer chemoprevention, but have yielded conflicting information [99 –107]. One study observed no significant role of interactions between NSAID use and polymorphisms in CYP2C8, CYP2C9, PPARD, PPARG, and UGT1A6 in modifying the risk for colorectal cancer, but it did report a nonsignificant trend (P for interaction = 0.24) toward a greater protective effect of nonaspirin NSAIDs, including ibuprofen, in carriers of the PPARG Ala12 variant allele [103]. A subsequent study in a larger population validated this potential interaction between ibuprofen use and the PPARG Pro12Ala variant in modifying rectal cancer risk (P for interaction =0.03) [107]. Other studies in colorectal cancer patients have reported significant interactions between ibuprofen use and genetic variants of CYP2C9 (CYP2C9*2 and *3) [105], SMAD7 (rs4939827 and rs4464148) [104], and UGT2B4 (rs1131878, rs1966151, and rs13119049) [106], but not PTGS2 (rs68946, rs20432, and rs5275) [99]. Studies in men with advanced prostate cancer have suggested that the protective effect of ibuprofen may be modified by the LTA C +80A (P for interaction =0.008) [102] and PTGS2 rs2745557 (P for interaction =0.12) [101] variants, but the numbers of ibuprofen users in these studies were relatively small. In contrast, no significant interactions between ibuprofen use and genetic variation in PTGS2 were observed in women with breast cancer [100].
An interesting area for future research with respect to ibuprofen is how the drug may interact with AMACR variants and modulate cancer risk [8]. Elevated protein levels of AMACR, which converts R-ibuprofen to S-ibuprofen, have been detected in prostate cancer cells and a number of other cancers, and variants and alternative splice forms of AMACR have been associated with cancer risk. However, the potential for ibuprofen to modify these relationships has not been explored to date [8].
Conclusion
To date, the most robust finding with regard to the pharmacogenomics of ibuprofen has been the relationship between the CYP2C9*3 variant and decreased ibuprofen clearance. Given ibuprofen’s wide therapeutic window, the clinical significance of this relationship is unclear, but CYP2C9*3 variant allele carriers may be at a greater risk for adverse events or drug–drug interactions, particularly with concomitant use of other CYP2C9 substrates (i.e. warfarin). Although some associations between genetic variation and therapeutic efficacy of ibuprofen have been reported, further study is necessary to validate these findings, as well as to define the role of pharmacogenomics in guiding ibuprofen therapy.
Acknowledgments
The authors thank Feng Liu for assistance with the graphics. This study is supported by the NIH/NIGMS R24 GM61374, Personalized NSAID Therapeutics Consortium (PENTACON: HL117798), and by HL007954.
Footnotes
Conflicts of interest
R.B.A. and T.E.K. are stockholders in Personalis Inc. For the remaining authors, there are no conflicts of interest.
References
- 1.Davies NM. Clinical pharmacokinetics of ibuprofen. The first 30 years. Clin Pharmacokinet. 1998;34:101–154. doi: 10.2165/00003088-199834020-00002. [DOI] [PubMed] [Google Scholar]
- 2.Rainsford KD. Ibuprofen: pharmacology, efficacy and safety. Inflammopharmacology. 2009;17:275–342. doi: 10.1007/s10787-009-0016-x. [DOI] [PubMed] [Google Scholar]
- 3.Durrmeyer X, Hovhannisyan S, Médard Y, Jacqz-Aigrain E, Decobert F, Barre J, et al. Are cytochrome P450 CYP2C8 and CYP2C9 polymorphisms associated with ibuprofen response in very preterm infants? PLoS One. 2010;5:e12329. doi: 10.1371/journal.pone.0012329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pierce CA, Voss B. Efficacy and safety of ibuprofen and acetaminophen in children and adults: a meta-analysis and qualitative review. Ann Pharmacother. 2010;44:489–506. doi: 10.1345/aph.1M332. [DOI] [PubMed] [Google Scholar]
- 5.Evans AM, Nation RL, Sansom LN, Bochner F, Somogyi AA. The relationship between the pharmacokinetics of ibuprofen enantiomers and the dose of racemic ibuprofen in humans. Biopharm Drug Dispos. 1990;11:507–518. doi: 10.1002/bdd.2510110605. [DOI] [PubMed] [Google Scholar]
- 6.Hersh EV, Pinto A, Moore PA. Adverse drug interactions involving common prescription and over-the-counter analgesic agents. Clin Ther. 2007;29 (Suppl):2477–2497. doi: 10.1016/j.clinthera.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 7.Dasgupta A, Timmerman TG. In vitro displacement of phenytoin from protein binding by nonsteroidal antiinflammatory drugs tolmetin, ibuprofen, and naproxen in normal and uremic sera. Ther Drug Monit. 1996;18:97–99. doi: 10.1097/00007691-199602000-00016. [DOI] [PubMed] [Google Scholar]
- 8.Lloyd MD, Yevglevskis M, Lee GL, Wood PJ, Threadgill MD, Woodman TJ. α-Methylacyl-CoA racemase (AMACR): metabolic enzyme, drug metabolizer and cancer marker P504S. Prog Lipid Res. 2013;52:220–230. doi: 10.1016/j.plipres.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 9.Rudy AC, Knight PM, Brater DC, Hall SD. Stereoselective metabolism of ibuprofen in humans: administration of R-, S- and racemic ibuprofen. J Pharmacol Exp Ther. 1991;259:1133–1139. [PubMed] [Google Scholar]
- 10.Hall SD, Rudy AC, Knight PM, Brater DC. Lack of presystemic inversion of (R)- to (S)-ibuprofen in humans. Clin Pharmacol Ther. 1993;53:393–400. doi: 10.1038/clpt.1993.42. [DOI] [PubMed] [Google Scholar]
- 11.Jamali F, Mehvar R, Russell AS, Sattari S, Yakimets WW, Koo J. Human pharmacokinetics of ibuprofen enantiomers following different doses and formulations: intestinal chiral inversion. J Pharm Sci. 1992;81:221–225. doi: 10.1002/jps.2600810306. [DOI] [PubMed] [Google Scholar]
- 12.Kepp DR, Sidelmann UG, Hansen SH. Isolation and characterization of major phase I and II metabolites of ibuprofen. Pharm Res. 1997;14:676–680. doi: 10.1023/a:1012125700497. [DOI] [PubMed] [Google Scholar]
- 13.Hamman MA, Thompson GA, Hall SD. Regioselective and stereoselective metabolism of ibuprofen by human cytochrome P450 2C. Biochem Pharmacol. 1997;54:33–41. doi: 10.1016/s0006-2952(97)00143-3. [DOI] [PubMed] [Google Scholar]
- 14.Chang SY, Li W, Traeger SC, Wang B, Cui D, Zhang H, et al. Confirmation that cytochrome P450 2C8 (CYP2C8) plays a minor role in (S)-(+)- and (R)-(−)-ibuprofen hydroxylation in vitro. Drug Metab Dispos. 2008;36:2513–2522. doi: 10.1124/dmd.108.022970. [DOI] [PubMed] [Google Scholar]
- 15.Andrade C, Sandarsh S, Chethan KB, Nagesh KS. Serotonin reuptake inhibitor antidepressants and abnormal bleeding: a review for clinicians and a reconsideration of mechanisms. J Clin Psychiatry. 2010;71:1565–1575. doi: 10.4088/JCP.09r05786blu. [DOI] [PubMed] [Google Scholar]
- 16.Schmider J, Greenblatt DJ, von Moltke LL, Karsov D, Shader RI. Inhibition of CYP2C9 by selective serotonin reuptake inhibitors in vitro: studies of phenytoin p-hydroxylation. Br J Clin Pharmacol. 1997;44:495–498. doi: 10.1046/j.1365-2125.1997.00601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tornio A, Niemi M, Neuvonen PJ, Backman JT. Stereoselective interaction between the CYP2C8 inhibitor gemfibrozil and racemic ibuprofen. Eur J Clin Pharmacol. 2007;63:463–469. doi: 10.1007/s00228-007-0273-9. [DOI] [PubMed] [Google Scholar]
- 18.Kuehl GE, Lampe JW, Potter JD, Bigler J. Glucuronidation of nonsteroidal anti-inflammatory drugs: identifying the enzymes responsible in human liver microsomes. Drug Metab Dispos. 2005;33:1027–1035. doi: 10.1124/dmd.104.002527. [DOI] [PubMed] [Google Scholar]
- 19.Sakaguchi K, Green M, Stock N, Reger TS, Zunic J, King C. Glucuronidation of carboxylic acid containing compounds by UDP-glucuronosyltransferase isoforms. Arch Biochem Biophys. 2004;424:219–225. doi: 10.1016/j.abb.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 20.Turgeon D, Carrier JS, Chouinard S, Bélanger A. Glucuronidation activity of the UGT2B17 enzyme toward xenobiotics. Drug Metab Dispos. 2003;31:670–676. doi: 10.1124/dmd.31.5.670. [DOI] [PubMed] [Google Scholar]
- 21.Basu NK, Kubota S, Meselhy MR, Ciotti M, Chowdhury B, Hartori M, Owens IS. Gastrointestinally distributed UDP-glucuronosyltransferase 1A10, which metabolizes estrogens and nonsteroidal anti-inflammatory drugs, depends upon phosphorylation. J Biol Chem. 2004;279:28320–28329. doi: 10.1074/jbc.M401396200. [DOI] [PubMed] [Google Scholar]
- 22.Sallustio BC, Sabordo L, Evans AM, Nation RL. Hepatic disposition of electrophilic acyl glucuronide conjugates. Curr Drug Metab. 2000;1:163–180. doi: 10.2174/1389200003339153. [DOI] [PubMed] [Google Scholar]
- 23.Castillo M, Lam YW, Dooley MA, Stahl E, Smith PC. Disposition and covalent binding of ibuprofen and its acyl glucuronide in the elderly. Clin Pharmacol Ther. 1995;57:636–644. doi: 10.1016/0009-9236(95)90226-0. [DOI] [PubMed] [Google Scholar]
- 24.Grillo MP, Lohr MT, Khera S. Interaction of γ-glutamyltranspeptidase with ibuprofen-S-acyl-glutathione in vitro and in vivo in human. Drug Metab Dispos. 2013;41:111–121. doi: 10.1124/dmd.112.048645. [DOI] [PubMed] [Google Scholar]
- 25.Grillo MP. Drug-S-acyl-glutathione thioesters: synthesis, bioanalytical properties, chemical reactivity, biological formation and degradation. Curr Drug Metab. 2011;12:229–244. doi: 10.2174/138920011795101886. [DOI] [PubMed] [Google Scholar]
- 26.Khamdang S, Takeda M, Noshiro R, Narikawa S, Enomoto A, Anzai N, et al. Interactions of human organic anion transporters and human organic cation transporters with nonsteroidal anti-inflammatory drugs. J Pharmacol Exp Ther. 2002;303:534–539. doi: 10.1124/jpet.102.037580. [DOI] [PubMed] [Google Scholar]
- 27.Chu XY, Bleasby K, Yabut J, Cai X, Chan GH, Hafey MJ, et al. Transport of the dipeptidyl peptidase-4 inhibitor sitagliptin by human organic anion transporter 3, organic anion transporting polypeptide 4C1, and multidrug resistance P-glycoprotein. J Pharmacol Exp Ther. 2007;321:673–683. doi: 10.1124/jpet.106.116517. [DOI] [PubMed] [Google Scholar]
- 28.Itagaki S, Gopal E, Zhuang L, Fei YJ, Miyauchi S, Prasad PD, Ganapathy V. Interaction of ibuprofen and other structurally related NSAIDs with the sodium-coupled monocarboxylate transporter SMCT1 (SLC5A8) Pharm Res. 2006;23:1209–1216. doi: 10.1007/s11095-006-0023-1. [DOI] [PubMed] [Google Scholar]
- 29.Omkvist DH, Brodin B, Nielsen CU. Ibuprofen is a non-competitive inhibitor of the peptide transporter hPEPT1 (SLC15A1): possible interactions between hPEPT1 substrates and ibuprofen. Br J Pharmacol. 2010;161:1793–1805. doi: 10.1111/j.1476-5381.2010.01000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tamai I, Takanaga H, Maeda H, Sai Y, Ogihara T, Higashida H, Tsuji A. Participation of a proton-cotransporter, MCT1, in the intestinal transport of monocarboxylic acids. Biochem Biophys Res Commun. 1995;214:482–489. doi: 10.1006/bbrc.1995.2312. [DOI] [PubMed] [Google Scholar]
- 31.Wang J, Hughes TP, Kok CH, Saunders VA, Frede A, Groot-Obbink K, et al. Contrasting effects of diclofenac and ibuprofen on active imatinib uptake into leukaemic cells. Br J Cancer. 2012;106:1772–1778. doi: 10.1038/bjc.2012.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Honjo H, Uwai Y, Aoki Y, Iwamoto K. Stereoselective inhibitory effect of flurbiprofen, ibuprofen and naproxen on human organic anion transporters hOAT1 and hOAT3. Biopharm Drug Dispos. 2011;32:518–524. doi: 10.1002/bdd.779. [DOI] [PubMed] [Google Scholar]
- 33.Kindla J, Müller F, Mieth M, Fromm MF, König J. Influence of non-steroidal anti-inflammatory drugs on organic anion transporting polypeptide (OATP) 1B1- and OATP1B3-mediated drug transport. Drug Metab Dispos. 2011;39:1047–1053. doi: 10.1124/dmd.110.037622. [DOI] [PubMed] [Google Scholar]
- 34.Maeda A, Tsuruoka S, Kanai Y, Endou H, Saito K, Miyamoto E, Fujimura A. Evaluation of the interaction between nonsteroidal anti-inflammatory drugs and methotrexate using human organic anion transporter 3-transfected cells. Eur J Pharmacol. 2008;596:166–172. doi: 10.1016/j.ejphar.2008.08.023. [DOI] [PubMed] [Google Scholar]
- 35.Takeda M, Khamdang S, Narikawa S, Kimura H, Hosoyamada M, Cha SH, et al. Characterization of methotrexate transport and its drug interactions with human organic anion transporters. J Pharmacol Exp Ther. 2002;302:666–671. doi: 10.1124/jpet.102.034330. [DOI] [PubMed] [Google Scholar]
- 36.El-Sheikh AA, van den Heuvel JJ, Koenderink JB, Russel FG. Interaction of nonsteroidal anti-inflammatory drugs with multidrug resistance protein (MRP) 2/ABCC2- and MRP4/ABCC4-mediated methotrexate transport. J Pharmacol Exp Ther. 2007;320:229–235. doi: 10.1124/jpet.106.110379. [DOI] [PubMed] [Google Scholar]
- 37.Angelini A, Iezzi M, Di Febbo C, Di Ilio C, Cuccurullo F, Porreca E. Reversal of P-glycoprotein-mediated multidrug resistance in human sarcoma MES-SA/Dx-5 cells by nonsteroidal anti-inflammatory drugs. Oncol Rep. 2008;20:731–735. [PubMed] [Google Scholar]
- 38.Clemente MI, Alvarez S, Serramía MJ, Turriziani O, Genebat M, Leal M, et al. Non-steroidal anti-inflammatory drugs increase the antiretroviral activity of nucleoside reverse transcriptase inhibitors in HIV type-1-infected T-lymphocytes: role of multidrug resistance protein 4. Antivir Ther. 2009;14:1101–1111. doi: 10.3851/IMP1468. [DOI] [PubMed] [Google Scholar]
- 39.Mulato AS, Ho ES, Cihlar T. Nonsteroidal anti-inflammatory drugs efficiently reduce the transport and cytotoxicity of adefovir mediated by the human renal organic anion transporter 1. J Pharmacol Exp Ther. 2000;295:10–15. [PubMed] [Google Scholar]
- 40.Boneberg EM, Zou MH, Ullrich V. Inhibition of cyclooxygenase-1 and -2 by R(−)- and S(+)-ibuprofen. J Clin Pharmacol. 1996;36 (12 Suppl):16S–19S. [PubMed] [Google Scholar]
- 41.Gierse JK, Koboldt CM, Walker MC, Seibert K, Isakson PC. Kinetic basis for selective inhibition of cyclo-oxygenases. Biochem J. 1999;339 (Pt 3):607–614. [PMC free article] [PubMed] [Google Scholar]
- 42.Neupert W, Brugger R, Euchenhofer C, Brune K, Geisslinger G. Effects of ibuprofen enantiomers and its coenzyme A thioesters on human prostaglandin endoperoxide synthases. Br J Pharmacol. 1997;122:487–492. doi: 10.1038/sj.bjp.0701415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smyth EM, Grosser T, Wang M, Yu Y, FitzGerald GA. Prostanoids in health and disease. J Lipid Res. 2009;50 (Suppl):S423–S428. doi: 10.1194/jlr.R800094-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blain H, Boileau C, Lapicque F, Nédélec E, Loeuille D, Guillaume C, et al. Limitation of the in vitro whole blood assay for predicting the COX selectivity of NSAIDs in clinical use. Br J Clin Pharmacol. 2002;53:255–265. doi: 10.1046/j.0306-5251.2001.01533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rainsford KD. Discovery, mechanisms of action and safety of ibuprofen. Int J Clin Pract Suppl. 2003;135:3–8. [PubMed] [Google Scholar]
- 46.Dawood MY, Khan-Dawood FS. Clinical efficacy and differential inhibition of menstrual fluid prostaglandin F2alpha in a randomized, double-blind, crossover treatment with placebo, acetaminophen, and ibuprofen in primary dysmenorrhea. Am J Obstet Gynecol. 2007;196:35e1–5. doi: 10.1016/j.ajog.2006.06.091. [DOI] [PubMed] [Google Scholar]
- 47.Ricciotti E, FitzGerald GA. Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol. 2011;31:986–1000. doi: 10.1161/ATVBAHA.110.207449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.De la Cruz JP, Reyes JJ, Ruiz-Moreno MI, Lopez-Villodres JA, Jebrouni N, Gonzalez-Correa JA. Differences in the in vitro antiplatelet effect of dexibuprofen, ibuprofen, and flurbiprofen in human blood. Anesth Analg. 2010;111:1341–1346. doi: 10.1213/ANE.0b013e3181f7b679. [DOI] [PubMed] [Google Scholar]
- 49.Kaplan HB, Edelson HS, Korchak HM, Given WP, Abramson S, Weissmann G. Effects of non-steroidal anti-inflammatory agents on human neutrophil functions in vitro and in vivo. Biochem Pharmacol. 1984;33:371–378. doi: 10.1016/0006-2952(84)90228-4. [DOI] [PubMed] [Google Scholar]
- 50.Smith RJ, Iden SS. Pharmacological modulation of chemotactic factor-elicited release of granule-associated enzymes from human neutrophils. Effects of prostaglandins, nonsteroid anti-inflammatory agents and corticosteroids. Biochem Pharmacol. 1980;29:2389–2395. doi: 10.1016/0006-2952(80)90274-9. [DOI] [PubMed] [Google Scholar]
- 51.Angst MS, Clark JD, Carvalho B, Tingle M, Schmelz M, Yeomans DC. Cytokine profile in human skin in response to experimental inflammation, noxious stimulation, and administration of a COX-inhibitor: a microdialysis study. Pain. 2008;139:15–27. doi: 10.1016/j.pain.2008.02.028. [DOI] [PubMed] [Google Scholar]
- 52.Menzel EJ, Burtscher H, Kolarz G. Inhibition of cytokine production and adhesion molecule expression by ibuprofen is without effect on transendothelial migration of monocytes. Inflammation. 1999;23:275–286. doi: 10.1023/a:1020230220971. [DOI] [PubMed] [Google Scholar]
- 53.Villanueva M, Heckenberger R, Strobach H, Palmér M, Schrör K. Equipotent inhibition by R(−)-, S(+)- and racemic ibuprofen of human polymorphonuclear cell function in vitro. Br J Clin Pharmacol. 1993;35 :235–242. doi: 10.1111/j.1365-2125.1993.tb05690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 55.Costa D, Moutinho L, Lima JL, Fernandes E. Antioxidant activity and inhibition of human neutrophil oxidative burst mediated by arylpropionic acid non-steroidal anti-inflammatory drugs. Biol Pharm Bull. 2006;29:1659–1670. doi: 10.1248/bpb.29.1659. [DOI] [PubMed] [Google Scholar]
- 56.Menzel JE, Kolarz G. Modulation of nitric oxide synthase activity by ibuprofen. Inflammation. 1997;21:451–461. doi: 10.1023/a:1027374605731. [DOI] [PubMed] [Google Scholar]
- 57.Vandivier RW, Eidsath A, Banks SM, Preas HL, 2nd, Leighton SB, Godin PJ, et al. Down-regulation of nitric oxide production by ibuprofen in human volunteers. J Pharmacol Exp Ther. 1999;289:1398–1403. [PubMed] [Google Scholar]
- 58.Fowler CJ, Björklund E, Lichtman AH, Naidu PS, Congiu C, Onnis V. Inhibitory properties of ibuprofen and its amide analogues towards the hydrolysis and cyclooxygenation of the endocannabinoid anandamide. J Enzyme Inhib Med Chem. 2013;28:172–182. doi: 10.3109/14756366.2011.643304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fowler CJ, Tiger G, Stenström A. Ibuprofen inhibits rat brain deamidation of anandamide at pharmacologically relevant concentrations. Mode of inhibition and structure-activity relationship. J Pharmacol Exp Ther. 1997;283:729–734. [PubMed] [Google Scholar]
- 60.Guindon J, De Léan A, Beaulieu P. Local interactions between anandamide, an endocannabinoid, and ibuprofen, a nonsteroidal anti-inflammatory drug, in acute and inflammatory pain. Pain. 2006;121:85–93. doi: 10.1016/j.pain.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 61.Holt S, Paylor B, Boldrup L, Alajakku K, Vandevoorde S, Sundström A, et al. Inhibition of fatty acid amide hydrolase, a key endocannabinoid metabolizing enzyme, by analogues of ibuprofen and indomethacin. Eur J Pharmacol. 2007;565:26–36. doi: 10.1016/j.ejphar.2007.02.051. [DOI] [PubMed] [Google Scholar]
- 62.Ward KE, Archambault R, Mersfelder TL. Severe adverse skin reactions to nonsteroidal antiinflammatory drugs: a review of the literature. Am J Health Syst Pharm. 2010;67:206–213. doi: 10.2146/ajhp080603. [DOI] [PubMed] [Google Scholar]
- 63.Bhala N, Emberson J, Merhi A, Abramson S, Arber N, Baron JA, et al. Coxib and traditional NSAID Trialists’ (CNT) Collaboration. Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials. Lancet. 2013;382:769–779. doi: 10.1016/S0140-6736(13)60900-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schnitzer TJ, Burmester GR, Mysler E, Hochberg MC, Doherty M, Ehrsam E, et al. TARGET Study Group. Comparison of lumiracoxib with naproxen and ibuprofen in the Therapeutic Arthritis Research and Gastrointestinal Event Trial (TARGET), reduction in ulcer complications: randomised controlled trial. Lancet. 2004;364:665–674. doi: 10.1016/S0140-6736(04)16893-1. [DOI] [PubMed] [Google Scholar]
- 65.Farkouh ME, Kirshner H, Harrington RA, Ruland S, Verheugt FW, Schnitzer TJ, et al. TARGET Study Group. Comparison of lumiracoxib with naproxen and ibuprofen in the Therapeutic Arthritis Research and Gastrointestinal Event Trial (TARGET), cardiovascular outcomes: randomised controlled trial. Lancet. 2004;364:675–684. doi: 10.1016/S0140-6736(04)16894-3. [DOI] [PubMed] [Google Scholar]
- 66.Grosser T, Fries S, FitzGerald GA. Biological basis for the cardiovascular consequences of COX-2 inhibition: therapeutic challenges and opportunities. J Clin Invest. 2006;116:4–15. doi: 10.1172/JCI27291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hippisley-Cox J, Coupland C. Risk of myocardial infarction in patients taking cyclo-oxygenase-2 inhibitors or conventional non-steroidal anti-inflammatory drugs: population based nested case-control analysis. BMJ. 2005;330:1366. doi: 10.1136/bmj.330.7504.1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Graham DJ, Campen D, Hui R, Spence M, Cheetham C, Levy G, et al. Risk of acute myocardial infarction and sudden cardiac death in patients treated with cyclo-oxygenase 2 selective and non-selective non-steroidal anti-inflammatory drugs: nested case-control study. Lancet. 2005;365:475–481. doi: 10.1016/S0140-6736(05)17864-7. [DOI] [PubMed] [Google Scholar]
- 69.García Rodríguez LA, Varas-Lorenzo C, Maguire A, González-Pérez A. Nonsteroidal antiinflammatory drugs and the risk of myocardial infarction in the general population. Circulation. 2004;109:3000–3006. doi: 10.1161/01.CIR.0000132491.96623.04. [DOI] [PubMed] [Google Scholar]
- 70.García Rodríguez LA, González-Pérez A. Long-term use of non-steroidal anti-inflammatory drugs and the risk of myocardial infarction in the general population. BMC Med. 2005;3:17. doi: 10.1186/1741-7015-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Catella-Lawson F, Reilly MP, Kapoor SC, Cucchiara AJ, DeMarco S, Tournier B, et al. Cyclooxygenase inhibitors and the antiplatelet effects of aspirin. N Engl J Med. 2001;345:1809–1817. doi: 10.1056/NEJMoa003199. [DOI] [PubMed] [Google Scholar]
- 72.Ellison J, Dager W. Recent FDA warning of the concomitant use of aspirin and ibuprofen and the effects on platelet aggregation. Prev Cardiol. 2007;10:61–63. doi: 10.1111/j.1520-037x.2007.06496.x. [DOI] [PubMed] [Google Scholar]
- 73.Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324:71–86. doi: 10.1136/bmj.324.7329.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.MacDonald TM, Wei L. Effect of ibuprofen on cardioprotective effect of aspirin. Lancet. 2003;361:573–574. doi: 10.1016/s0140-6736(03)12509-3. [DOI] [PubMed] [Google Scholar]
- 75.Hudson M, Baron M, Rahme E, Pilote L. Ibuprofen may abrogate the benefits of aspirin when used for secondary prevention of myocardial infarction. J Rheumatol. 2005;32:1589–1593. [PubMed] [Google Scholar]
- 76.Curtis JP, Wang Y, Portnay EL, Masoudi FA, Havranek EP, Krumholz HM. Aspirin, ibuprofen, and mortality after myocardial infarction: retrospective cohort study. BMJ. 2003;327:1322–1323. doi: 10.1136/bmj.327.7427.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schulman S, Henriksson K. Interaction of ibuprofen and warfarin on primary haemostasis. Br J Rheumatol. 1989;28:46–49. doi: 10.1093/rheumatology/28.1.46. [DOI] [PubMed] [Google Scholar]
- 78.Juel J, Pedersen TB, Langfrits CS, Jensen SE. Administration of tramadol or ibuprofen increases the INR level in patients on warfarin. Eur J Clin Pharmacol. 2013;69:291–292. doi: 10.1007/s00228-012-1325-3. [DOI] [PubMed] [Google Scholar]
- 79.Phelan KM, Mosholder AD, Lu S. Lithium interaction with the cyclooxygenase 2 inhibitors rofecoxib and celecoxib and other nonsteroidal anti-inflammatory drugs. J Clin Psychiatry. 2003;64:1328–1334. doi: 10.4088/jcp.v64n1108. [DOI] [PubMed] [Google Scholar]
- 80.Ragheb M, Ban TA, Buchanan D, Frolich JC. Interaction of indomethacin and ibuprofen with lithium in manic patients under a steady-state lithium level. J Clin Psychiatry. 1980;41:397–398. [PubMed] [Google Scholar]
- 81.Bailey CE, Stewart JT, McElroy RA. Ibuprofen-induced lithium toxicity. South Med J. 1989;82:1197. doi: 10.1097/00007611-198909000-00042. [DOI] [PubMed] [Google Scholar]
- 82.Ragheb M. Ibuprofen can increase serum lithium level in lithium-treated patients. J Clin Psychiatry. 1987;48:161–163. [PubMed] [Google Scholar]
- 83.Khan IH. Lithium and non-steroidal anti-inflammatory drugs. BMJ. 1991;302:1537–1538. doi: 10.1136/bmj.302.6791.1537-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Radack KL, Deck CC, Bloomfield SS. Ibuprofen interferes with the efficacy of antihypertensive drugs. A randomized, double-blind, placebo-controlled trial of ibuprofen compared with acetaminophen. Ann Intern Med. 1987;107:628–635. doi: 10.7326/0003-4819-107-5-628. [DOI] [PubMed] [Google Scholar]
- 85.Gurwitz JH, Everitt DE, Monane M, Glynn RJ, Choodnovskiy I, Beaudet MP, Avorn J. The impact of ibuprofen on the efficacy of antihypertensive treatment with hydrochlorothiazide in elderly persons. J Gerontol A Biol Sci Med Sci. 1996;51:M74–M79. doi: 10.1093/gerona/51a.2.m74. [DOI] [PubMed] [Google Scholar]
- 86.Fournier JP, Sommet A, Bourrel R, Oustric S, Pathak A, Lapeyre-Mestre M, Montastruc JL. Non-steroidal anti-inflammatory drugs (NSAIDs) and hypertension treatment intensification: a population-based cohort study. Eur J Clin Pharmacol. 2012;68:1533–1540. doi: 10.1007/s00228-012-1283-9. [DOI] [PubMed] [Google Scholar]
- 87.MacDonald TM, Richard D, Lheritier K, Krammer G. The effects of lumiracoxib 100 mg once daily vs. ibuprofen 600 mg three times daily on the blood pressure profiles of hypertensive osteoarthritis patients taking different classes of antihypertensive agents. Int J Clin Pract. 2010;64:746–755. doi: 10.1111/j.1742-1241.2010.02346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.López-Rodríguez R, Novalbos J, Gallego-Sandín S, Román-Martínez M, Torrado J, Gisbert JP, Abad-Santos F. Influence of CYP2C8 and CYP2C9 polymorphisms on pharmacokinetic and pharmacodynamic parameters of racemic and enantiomeric forms of ibuprofen in healthy volunteers. Pharmacol Res. 2008;58:77–84. doi: 10.1016/j.phrs.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 89.García-Martín E, Martínez C, Tabarés B, Frías J, Agúndez JA. Interindividual variability in ibuprofen pharmacokinetics is related to interaction of cytochrome P450 2C8 and 2C9 amino acid polymorphisms. Clin Pharmacol Ther. 2004;76:119–127. doi: 10.1016/j.clpt.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 90.Kirchheiner J, Meineke I, Freytag G, Meisel C, Roots I, Brockmöller J. Enantiospecific effects of cytochrome P450 2C9 amino acid variants on ibuprofen pharmacokinetics and on the inhibition of cyclooxygenases 1 and 2. Clin Pharmacol Ther. 2002;72:62–75. doi: 10.1067/mcp.2002.125726. [DOI] [PubMed] [Google Scholar]
- 91.KaraŸniewicz-Łada M, Luczak M, Główka F. Pharmacokinetic studies of enantiomers of ibuprofen and its chiral metabolites in humans with different variants of genes coding CYP2C8 and CYP2C9 isoenzymes. Xenobiotica. 2009;39:476–485. doi: 10.1080/00498250902862705. [DOI] [PubMed] [Google Scholar]
- 92.Martínez C, García-Martín E, Blanco G, Gamito FJ, Ladero JM, Agúndez JA. The effect of the cytochrome P450 CYP2C8 polymorphism on the disposition of (R)-ibuprofen enantiomer in healthy subjects. Br J Clin Pharmacol. 2005;59:62–69. doi: 10.1111/j.1365-2125.2004.02183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Blanco G, Martínez C, Ladero JM, Garcia-Martin E, Taxonera C, Gamito FG, et al. Interaction of CYP2C8 and CYP2C9 genotypes modifies the risk for nonsteroidal anti-inflammatory drugs-related acute gastrointestinal bleeding. Pharmacogenet Genomics. 2008;18:37–43. doi: 10.1097/FPC.0b013e3282f305a9. [DOI] [PubMed] [Google Scholar]
- 94.Pilotto A, Seripa D, Franceschi M, Scarcelli C, Colaizzo D, Grandone E, et al. Genetic susceptibility to nonsteroidal anti-inflammatory drug-related gastroduodenal bleeding: role of cytochrome P450 2C9 polymorphisms. Gastroenterology. 2007;133:465–471. doi: 10.1053/j.gastro.2007.05.025. [DOI] [PubMed] [Google Scholar]
- 95.Carbonell N, Verstuyft C, Massard J, Letierce A, Cellier C, Deforges L, et al. CYP2C9*3 loss-of-function allele is associated with acute upper gastrointestinal bleeding related to the use of NSAIDs other than aspirin. Clin Pharmacol Ther. 2010;87:693–698. doi: 10.1038/clpt.2010.33. [DOI] [PubMed] [Google Scholar]
- 96.Martínez C, Blanco G, Ladero JM, García-Martín E, Taxonera C, Gamito FG, et al. Genetic predisposition to acute gastrointestinal bleeding after NSAIDs use. Br J Pharmacol. 2004;141:205–208. doi: 10.1038/sj.bjp.0705623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Martin JH, Begg EJ, Kennedy MA, Roberts R, Barclay ML. Is cytochrome P450 2C9 genotype associated with NSAID gastric ulceration? Br J Clin Pharmacol. 2001;51:627–630. doi: 10.1046/j.0306-5251.2001.01398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lee YS, Kim H, Wu TX, Wang XM, Dionne RA. Genetically mediated interindividual variation in analgesic responses to cyclooxygenase inhibitory drugs. Clin Pharmacol Ther. 2006;79:407–418. doi: 10.1016/j.clpt.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 99.Ali IU, Luke BT, Dean M, Greenwald P. Allellic variants in regulatory regions of cyclooxygenase-2: association with advanced colorectal adenoma. Br J Cancer. 2005;93:953–959. doi: 10.1038/sj.bjc.6602806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Brasky TM, Bonner MR, Moysich KB, Ochs-Balcom HM, Marian C, Ambrosone CB, et al. Genetic variants in COX-2, non-steroidal anti-inflammatory drugs, and breast cancer risk: the Western New York Exposures and Breast Cancer (WEB) Study. Breast Cancer Res Treat. 2011;126:157–165. doi: 10.1007/s10549-010-1082-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cheng I, Liu X, Plummer SJ, Krumroy LM, Casey G, Witte JS. COX2 genetic variation, NSAIDs, and advanced prostate cancer risk. Br J Cancer. 2007;97:557–561. doi: 10.1038/sj.bjc.6603874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liu X, Plummer SJ, Nock NL, Casey G, Witte JS. Nonsteroidal antiinflammatory drugs and decreased risk of advanced prostate cancer: modification by lymphotoxin alpha. Am J Epidemiol. 2006;164:984–989. doi: 10.1093/aje/kwj294. [DOI] [PubMed] [Google Scholar]
- 103.McGreavey LE, Turner F, Smith G, Boylan K, Timothy Bishop D, Forman D, et al. No evidence that polymorphisms in CYP2C8, CYP2C9, UGT1A6, PPARdelta and PPARgamma act as modifiers of the protective effect of regular NSAID use on the risk of colorectal carcinoma. Pharmacogenet Genomics. 2005;15:713–721. doi: 10.1097/01.fpc.0000174786.85238.63. [DOI] [PubMed] [Google Scholar]
- 104.Passarelli MN, Coghill AE, Hutter CM, Zheng Y, Makar KW, Potter JD, Newcomb PA. Common colorectal cancer risk variants in SMAD7 are associated with survival among prediagnostic nonsteroidal anti-inflammatory drug users: a population-based study of postmenopausal women. Genes Chromosomes Cancer. 2011;50:875–886. doi: 10.1002/gcc.20913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Samowitz WS, Wolff RK, Curtin K, Sweeney C, Ma KN, Andersen K, et al. Interactions between CYP2C9 and UGT1A6 polymorphisms and nonsteroidal anti-inflammatory drugs in colorectal cancer prevention. Clin Gastroenterol Hepatol. 2006;4:894–901. doi: 10.1016/j.cgh.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 106.Scherer D, Koepl LM, Poole EM, Balavarca Y, Xiao L, Baron JA, et al. Genetic variation in UGT genes modify the associations of NSAIDs with risk of colorectal cancer: colon cancer family registry. Genes Chromosomes Cancer. 2014;53:568–578. doi: 10.1002/gcc.22167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Slattery ML, Curtin K, Wolff R, Ma KN, Sweeney C, Murtaugh M, et al. PPARgamma and colon and rectal cancer: associations with specific tumor mutations, aspirin, ibuprofen and insulin-related genes (United States) Cancer Causes Control. 2006;17:239–249. doi: 10.1007/s10552-005-0411-6. [DOI] [PubMed] [Google Scholar]