Abstract
Niemann-Pick disease type C (NPC) is caused by defects in either the NPC1 or NPC2 gene and is characterized by accumulation of cholesterol and glycolipids in the late endosome/lysosome compartment. NPC2 is an intralysosomal protein that binds cholesterol in vitro. Previous studies demonstrated rapid rates of cholesterol transfer from NPC2 to model membranes [Cheruku, S. R., et al. (2006) J. Biol. Chem. 281, 31594–31604]. To model the potential role of NPC2 as a lysosomal cholesterol export protein, in this study we used fluorescence spectroscopic approaches to examine cholesterol transfer from membranes to NPC2, assessing the rate, mechanism, and regulation of this transport step. In addition, we examined the effect of NPC2 on the rate and kinetic mechanism of intermembrane sterol transport, to model the movement of cholesterol from internal lysosomal membranes to the limiting lysosomal membrane. The results support the hypothesis that NPC2 plays an important role in endo/lysosomal cholesterol trafficking by markedly accelerating the rates of cholesterol transport. Rates of sterol transfer from and between membranes were increased by as much as 2 orders of magnitude by NPC2. The transfer studies indicate that the mechanism of NPC2 action involves direct interaction of the protein with membranes. Such interactions were observed directly using FTIR spectroscopy and protein tryptophan spectral shifts. Additionally, cholesterol transfer by NPC2 was found to be greatly enhanced by the unique lysosomal phospholipid lyso-bisphosphatidic acid (LBPA), suggesting an important role for LBPA in NPC2-mediated cholesterol trafficking.
Niemann-Pick disease type C (NPC)1 is an inherited lipid storage disorder caused by defects in either the NPC1 or NPC2 gene. In NPC, intracellular trafficking of cholesterol is disturbed, and both cholesterol and glycolipids accumulate in late endosome/lysosomes (1–4). Mutations in NPC1, a 1278-residue transmembrane glycoprotein, account for approximately 95% of the cases (5); mutations in NPC2 account for the remaining 5% (6). While defects in either protein result in the accumulation of cholesterol, the mechanisms of action of NPC1 and NPC2 in lysosomal cholesterol trafficking are not clear.
Cholesterol enters mammalian cells following endocytosis of cholesteryl ester-rich low-density lipoprotein (LDL) via the receptor-mediated endocytic pathway (7). In the endosomal/lysosomal compartments, the cholesteryl ester is hydrolyzed to unesterified cholesterol and free fatty acids by acid lipase. LDL-derived cholesterol is then, by unknown mechanisms, transported primarily to the plasma membrane or to the endoplasmic reticulum for re-esterification. The egress of cholesterol is important for the induction of homeostatic responses which regulate the cellular cholesterol pool, including LDL receptor expression and de novo cholesterol synthesis (8).
NPC1 contains a sterol-sensing domain (SSD) located between the third and seventh of its putative 13 transmembrane domains (9). While the NPC1 protein was suggested to transport fatty acids and not cholesterol (10), others have shown this is likely not occurring in cells (11), and several reports demonstrate sterol binding, if not yet transport, by NPC1 (12–14). The NPC2 gene encodes a 132-residue soluble lysosomal glycoprotein (6), which was characterized as a cholesterol binding protein with a 1/1 stoichiometry (15–17). Depending on the experimental method used, binding affinities have been reported to range from roughly 30 nM to 2 μM (15, 16, 18); such operational differences are not uncommon for lipid-binding proteins and their small hydrophobic ligands (19). In previous work, we examined the kinetics of cholesterol transfer from NPC2 to model membranes utilizing a fluorescence dequenching assay (20). The results suggested that NPC2 transports cholesterol to phospholipid vesicles rapidly via a collisional mechanism involving direct interaction with acceptor membranes. These studies provided initial evidence of a role for NPC2 in lysosomal cholesterol trafficking and identified lysobisphosphatidic acid (LBPA; also known as bis-monoacylglycerol phosphate) as a key component of the putative protein–membrane “collisional complex” (20).
The late endosomal/lysosomal compartment is characterized by an abundance of internal membranes, as well as a limiting outer membrane that borders the cytosolic compartment (21). It is likely that in the absence of efficient egress, the lipase-liberated cholesterol partitions into the abundant internal membranes. To improve our understanding of the mechanisms of endosomal/lysosomal cholesterol transport, in this study we modeled the process of cholesterol transport from membranes to the NPC2 protein, assessing the rate, mechanism, and regulation of this transport step. In addition, we examined the effect of NPC2 on the rate and kinetic mechanism of intermembrane sterol transport, to model the movement of cholesterol from internal lysosomal membranes to the limiting lysosomal membrane. The results support the hypothesis that NPC2 plays an important role in endosomal/lysosomal cholesterol trafficking by markedly accelerating the rates of transport from and between membranes. The mechanism of NPC2 action involves direct interaction of the protein with membranes. NPC2–membrane interaction is supported by results obtained using two spectroscopic approaches, FTIR and fluorescence spectral shift. Additionally, cholesterol transfer by NPC2 is greatly enhanced by LBPA and specifically blocked by an anti-LBPA antibody, further supporting an important role for this unique phospholipid in lysosomal cholesterol trafficking, perhaps working as a localizing site for NPC2 and, thereby, enhancing the efficiency of cholesterol transport by NPC2.
MATERIALS AND METHODS
Materials
Cholesterol, dehydroergosterol (DHE), GM1 and GM2 gangliosides, lactosyl ceramide, and Ricinus communis Agent (RCA) were obtained from Sigma (St. Louis, MO). Egg phosphatidylcholine (EPC), palmitoyloleoylphosphatidylserine (POPS), oleoyl and myristoyl lysobisphosphatidic acid (LBPA; also known as bis-monoacyllgycerol phosphate or BMP), dansyl phosphatidylethanolamine (dansyl-PE), and dolichols were from Avanti Polar Lipids (Alabaster, AL). [1,2-3H]Cholesterol and cholesteryl [oleate-1-14C]oleate were from NEN Life Science Products (Boston, MA). Anti-LBPA antibody 6C4 was generously provided by J. Gruenberg (University of Geneva, Geneva, Switzerland). Cholestatrienol (CTL) was generously provided by F. Maxfield (Wiell Cornell Medical College, New York, NY).
Purification of Human and Bovine NPC2 Proteins
Human NPC2 protein was prepared from Chinese hamster ovary cells as previously described (20), using a 5 kDa cutoff flow filtration membrane to concentrate media (CDUF002 LC, Millipore, Bedford, MA). Bovine NPC2 protein was prepared from raw milk as detailed by Friedland et al. (18). Unless otherwise indicated, the monoglycosylated form of NPC2 was used.
Membrane Vesicle Preparation
Small unilamellar vesicles (SUV) were prepared by sonication and ultracentrifugation as described previously (22). Vesicles were maintained at temperatures above the phase transition temperatures of all constituent lipids. The standard vesicles were prepared to contain 100 mol % egg phosphatidylcholine (EPC). For studies of cholesterol transfer from membranes to NPC2, 10% cholesterol was substituted for EPC. For intermembrane sterol transfer experiments, 25% DHE and 3% dansyl-PE were added to donor and acceptor vesicles, respectively, also substituting for EPC. For some experiments, as indicated in the text, other lipids at 25 mol % were substituted for EPC. Vesicles were prepared in 20 mM sodium citrate, 150 mM NaCl buffer (pH 5.0).
Cholesterol Transfer from NPC2 to Membranes and from Membranes to NPC2
Human NPC2 has two tryptophan residues, at positions 109 and 122. The endogenous tryptophan fluorescence of NPC2 was used to monitor cholesterol transfer from model membranes to NPC2. As described previously, the NPC2 tryptophan signal is quenched by cholesterol binding; therefore, the transfer of cholesterol from NPC2 to membranes can be monitored by the dequenching of the NPC2 tryptophan signal (20). The reverse, i.e., cholesterol transfer from membranes to apo NPC2 protein, can be monitored by the quenching of the NPC2 tryptophan signal, as follows. Donor phospholipid vesicles containing cholesterol are added to apo NPC2, and the transfer of cholesterol from the membranes to NPC2 is monitored directly by the decrease in tryptophan fluorescence over time. For all transfer assays, membranes with a defined phospholipid composition and concentration were mixed with NPC2 using a stopped-flow mixing chamber interfaced with a DX-18MV spectrofluorimeter (Applied Photophysics Ltd.), and the time-dependent change in tryptophan signal was used to obtain the transfer rates. To ensure that unidirectional transfer of cholesterol was being monitored, an equivalent or greater amount of acceptor (NPC2) relative to donor (membrane phospholipid) was used (23, 24). This was determined by calculating the relative partitioning of cholesterol, at equilibrium, between NPC2 and phospholipid using the NPC2 tryptophan signal with stoichiometric cholesterol bound, compared to the signal in the presence of vesicles, as previously described (24, 25). For EPC vesicles, the partition of cholesterol to NPC2 relative to EPC was found to be approximately 50 (NPC2/EPC, molar ratio). Thus, for example, in a mixture of 5 μM NPC2, 5 μM cholesterol, and 400 μM EPC, approximately 3 μM cholesterol partitions to the vesicles at equilibrium while roughly 2 μM is bound to NPC2. Transfer was monitored at 25 °C, and controls to ensure the absence of photobleaching were performed before each experiment. The excitation wavelength was 280 nm, and emission was monitored using a 299 nm cutoff filter.
Data were analyzed using software provided with the Applied Photophysics stopped-flow instrument, and the cholesterol transfer rates were obtained by exponential fitting of the curves, all of which were well fit by a single-exponential function. For each experimental condition, at least five replicates were conducted, and the averages ± the standard deviation for three or more experiments are reported. It is worth noting that these transfer assays use native ligand and native protein with no exogenous probes to acquire kinetic information; thus, the absolute rates obtained are likely to be physiologically relevant.
Intermembrane Transfer of [3H]Cholesterol
We used [1,2-3H]cholesterol to study cholesterol transfer between membranes, as described by Backer and Dawidowicz (26). Briefly, donor SUV were composed of EPC and cholesterol (1/0.4 molar ratio) containing 10% (w/w) lactosoyl ceramide. A trace amount of [3H]cholesterol (25 μCi) was added to donor membranes, and 20000 cpm of [14C]cholestoryl oleate was added as a nontransferring control. Acceptor SUV were 100% EPC. In the experiment, 50 μM donor phospholipid SUV were mixed with 1.5 mM acceptor SUV in a total volume of 4 mL. The mixture was incubated at 37 °C, and at specific time points, 100 μL of the mixture was removed and vortex-mixed with 200 μL of 2.5 mg/mL RCA. The donor SUV were spun down at 14000 rpm using Eppendorf centrifuge 5415C (Brinkmann Instruments, Westbury, NY), and 200 μL of the supernatant was counted to determine the amount of cholesterol transferred to acceptor membranes. Values were corrected for volume, and as modified by 14C supernatant counts of the nontransferable cholestoryl oleate, which provides an indication of donor vesicles not removed by centrifugation.
Intermembrane Transfer of DHE
3H-labeled cholesterol can be used for monitoring the slow rate of spontaneous cholesterol transfer between membranes. In the presence of NPC2, the cholesterol transfer rates were much faster and we were unable to use the physical separation method. Therefore, fluorescence resonance energy transfer (FRET) between DHE and dansyl-PE was used to study the intermembrane transfer of DHE, a fluorescent analogue of cholesterol (27). This assay uses donor SUV containing DHE and accepter vesicles containing dansyl-PE. The fluorescence emission of DHE at 370 nm overlaps with the dansyl-PE excitation spectrum; thus, when DHE transfers to acceptor membranes containing dansyl-PE, DHE emission is quenched while the so-called sensitized emission of the dansyl moiety at 510 nm is increased. The transfer of DHE from donor to acceptor membranes is therefore monitored directly by the decrease in DHE fluorescence or the increase in dansyl fluorescence over time. The excitation wavelength was 300 nm, and emission was monitored using a 520 nm cutoff filter for the dansyl emission or a 370 nm narrow band filter for the DHE emission. Essentially identical rates were obtained using either method. Transfer was monitored at 25 °C, and controls to ensure that photobleaching was eliminated were performed before each experiment.
Cholestatrienol Transfer from NPC2 to Membranes: Effect of the Anti-LBPA Antibody
Monoclonal anti-LBPA antibody 6C4 was generously provided by J. Gruenberg. Since the monoclonal antibody itself contains tryptophan residues, it was not possible to use the NPC2 tryptophan quenching method to study cholesterol transfer from NPC2. Therefore, we utilized CTL as a cholesterol analogue and studied its transfer from NPC2 to membranes, in the absence or presence of the anti-LBPA antibody. CTL is a fluorescent sterol that mimics cholesterol behavior (28, 29) and has a fluorescence emission peak at 370–390 nm at an excitation wavelength of 340 nm. As for DHE, there is FRET between CTL and dansyl-PE. In these experiments, 2 μM apo NPC2 was incubated with 4 μM CTL for 30 min at 37 °C, and this donor complex was mixed with 500 μM SUV with the indicated composition and containing 3% dansyl-PE preincubated with 250 ng/mL 6C4, using the stopped-flow spectrofluorimeter. The excitation wavelength was 340 nm, and emission was monitored using a 370 nm narrowband filter. Transfer was monitored at 25 °C.
Fourier Transfer Infrared (FTIR) Assay for Examining Membrane–NPC2 Interactions
FTIR experiments were performed as described previously (30). Briefly, multilamellar vesicles (MLV) were prepared by dissolving POPS in a chloroform/methanol mixture (1/1, v/v), and the solvents were then evaporated under a slow stream of N2 gas followed by lyophilization for 3–4 h to remove traces of solvent. MLV were prepared by rehydrating with D2O buffer [10 mM Tris, 100 μM NaCl, and 100 μM EDTA (pD 7.4)] at a temperature well above the gel/liquid-crystalline phase transition of POPS, in sealed tubes. Throughout the heating cycle, samples were periodically vortexed to ensure complete melting and mixing of all components. After being mixed and hydrated, samples were sandwiched between heated IR windows and mounted in a temperature-controlled transmission cell holder (Harrick Scientific, Ossining, NY). For experiments with NPC2, 0.5 mg of NPC2 was added to 2.0 mg of POPS (1/60 molar ratio). FTIR spectra were collected on a Mattson spectrometer equipped with a broadband mercury- cadmium-telluride (MCT) detector. Spectra were generated by co-addition of 256 interferograms collected at 2 cm−1 resolution, apodized with a triangular function, and Fourier-transformed with one level of zero filling. Spectra were routinely acquired at 1 or 2 °C intervals from 4 to 36 °C. Data were analyzed off-line with software provided by the National Research Council of Canada (31).
RESULTS
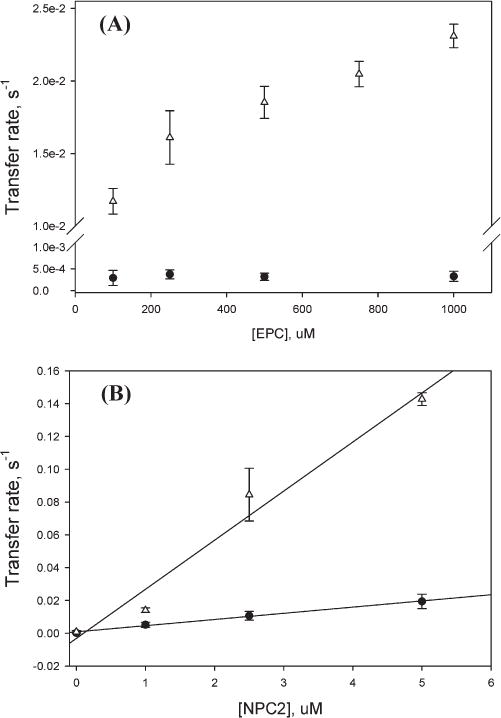
Cholesterol Transfer from Membranes to NPC2: Effects of Donor Vesicle Composition and NPC2 Concentration
Our previous studies suggested that cholesterol transfer from NPC2 to phospholipid vesicles occurs via a collisional mechanism involving direct interactions between NPC2 protein and the acceptor membranes (20). Here we examined the transfer of cholesterol from membranes to NPC2. To distinguish between collision-based and aqueous diffusion-based mechanisms, transfer was examined as a function of donor membrane composition and as a function of increasing NPC2 concentration. Figure 1A shows that the rate of cholesterol transfer from membranes containing negatively charged phospholipids to NPC2 was markedly faster than transfer from zwitterionic vesicles. The donor membranes containing 25% LBPA, a lysosome, and late endosome-specific phospholipid exhibited the highest transfer rates, ~200-fold greater than rates of transfer from EPC membranes. Figure 1B shows that when cholesterol transfers from a fixed amount of SUV to increasing amounts of NPC2, a linear increase in the cholesterol transfer rate is observed when the quantity of acceptor NPC2 is sufficient for unidirectional transfer rates to be obtained (24, 25). Thus, on the basis of the above-mentioned equilibrium partitioning of cholesterol between NPC2 and SUV, cholesterol transfer was linear from 250 μM EPC SUV at NPC2 concentrations of >5 μM. Both the composition dependence and the effect of the acceptor (NPC2) concentration strongly suggest a collisional mechanism of cholesterol transfer from membranes to NPC2. In addition, since egress of cholesterol from this compartment is likely to require movement from lysosomal internal membranes to the limiting endosomal/lysosomal membrane, the results support a physiologically specific role for LBPA in NPC2–membrane interactions and lysosomal cholesterol transport.
Figure 1.
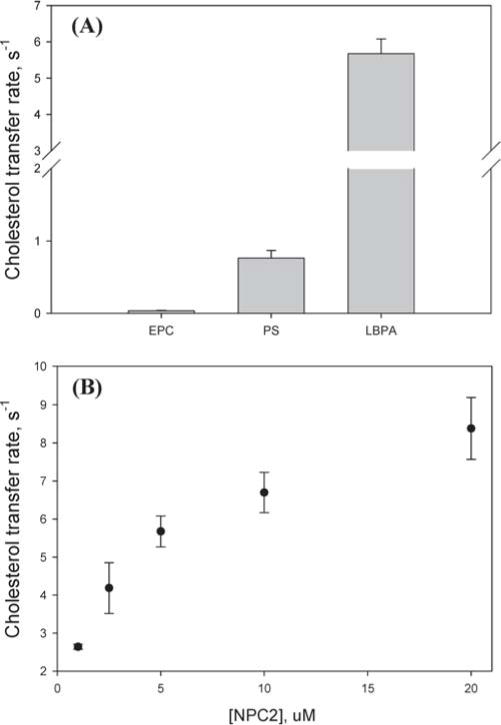
Cholesterol transfer from phospholipid membranes to NPC2. (A) Transfer of 2.5 μM cholesterol from 250 μM SUV [EPC (90% EPC/10% cholesterol), PS (65% EPC/25% PS/10% choleseterol), or LBPA (65% EPC/25% LBPA/10% cholesterol)] to 5 μM NPC2 was monitored at 25 °C and pH 5.0 using a stopped-flow fluorescence spectrometer, as described in Materials and Methods. (B) Transfer of 2.5 μM cholesterol from 250 μM LBPA SUV to NPC2 at different concentrations.
Effect of LBPA Acyl Chain Species and LBPA Stereoisomers on Cholesterol Transfer from NPC2 to Membranes
LBPA is uniquely present at high concentrations in the internal membrane network of endosomes/lysosomes (32). Unlike other mammalian phospholipids with an sn-3-glycerophosphate stereoconfiguration, LBPA in mammals favors the sn-1:sn-1′ (S,S′ isomer) headgroup configuration (33–36), yet the S,R isomer was the only commercially available LBPA isomer until very recently and, as such, was used for many of our earlier experiments. With the recent availability of additional isomers, we compared the effect of different LBPA steoroisomers on cholesterol transfer. The results in Figure 2A indicate that the S,S and R,R isomers of LBPA have essentially the same effect as the S,R isomer, with all three species equivalently enhancing the rate of cholesterol transfer from NPC2 to membranes. We also examined whether the acyl chain moieties of LBPA modified its ability to enhance cholesterol transfer from NPC2. Figure 2B shows a comparison of vesicles containing 25 mol % dioleoyl (18:1) LBPA or dimyristoyl (14:0) LBPA. The rates of cholesterol transfer from NPC2 to vesicles containing dimyristoyl LBPA are consistently ~60% slower than the rates of transfer to dioleoyl LBPA vesicles, suggesting that the acyl chain length has an effect on the membrane structure and/or the NPC2-LBPA interaction, such that the oleoyl LBPA is more effective in enhancing the rate of cholesterol transfer from NPC2.
Figure 2.
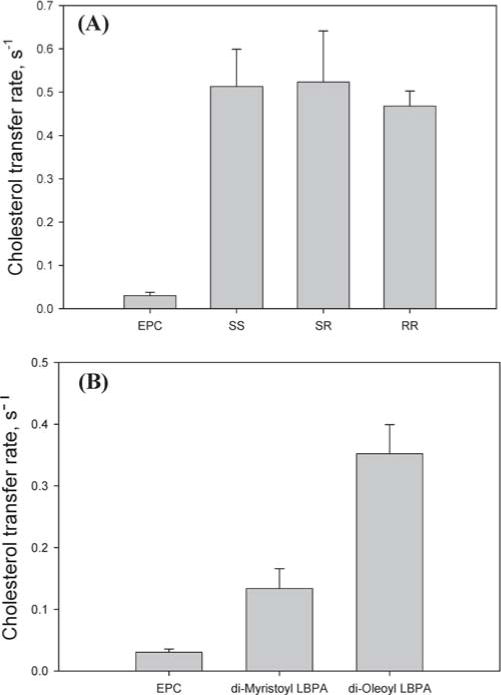
Effect of LBPA on cholesterol transfer from NPC2 to membranes. (A) Cholesterol (1 μM) transfer from 1 μM NPC2 to 50 μM SUV containing 100% EPC or with 25% EPC substituted with S,R LBPA, S,S LBPA, or R,R LBPA. (B) Cholesterol (1 μM) transfer from 1 μM NPC2 to 75% EPC/25% dioleoyl LBPA or 75% EPC/25% dimyristoyl LBPA SUV (50 μM).
Comparison of Cholesterol and Fluorescent Sterols
For technical reasons noted in Materials and Methods, several experiments could not use the endogenous NPC2 tryptophan fluorescence to track the kinetics of cholesterol transfer. A number of fluorescent sterols have been used as cholesterol analogues in biophysical, biochemical, and cellular studies by many investigators (27–29, 37–39). We therefore compared directly the rate of cholesterol transfer from NPC2 to membranes with those of DHE and CTL, two fluorescent sterols which we wished to employ in model membrane studies. DHE has been shown to be a ligand for NPC2 (17), and we found that, as for cholesterol, binding of DHE or CTL resulted in quenching of NPC2 tryptophan fluorescence and, furthermore, was accompanied by an increase in DHE or cholestatrienol fluorescence (not shown). As shown in Figure 3A, the rates of transfer from NPC2 to membranes of both fluorescent sterols, DHE and CTL, are somewhat slower but basically similar to the transfer rates of cholesterol. We also compared the rates of spontaneous transfer of cholesterol between membranes using the RCA-based physical separation method, with the rate of DHE intermembrane transfer using the FRET assay, as described in Materials and Methods. Figure 3B shows that the spontaneous intermembrane transfer rates for cholesterol and DHE are very similar to each other, 0.00023 and 0.00028 s−1, respectively. Thus, the fluorescent sterol analogues were used for several transfer experiments, as they appear to offer a reasonable approximation of the absolute rates of spontaneous and NPC2-mediated cholesterol transfer.
Figure 3.
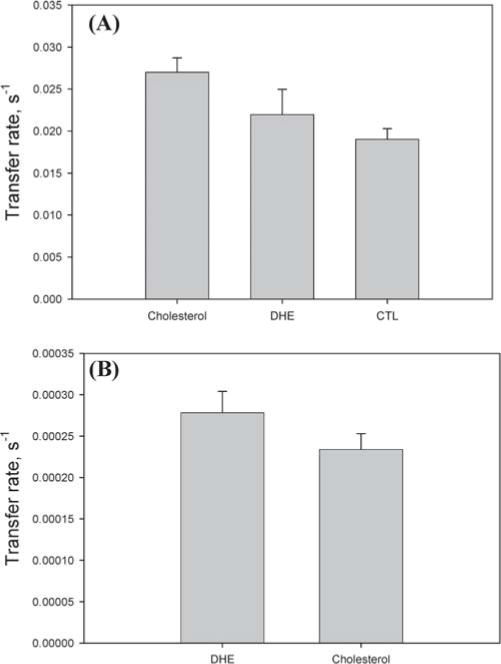
Comparison of cholesterol and fluorescent sterols. (A) Transfer of 1 μM cholesterol, DHE, or CTL from 1 μM NPC2 to 100% EPC SUV (50 μM). (B) Comparison of cholesterol and DHE intermembrane transfer rates. [3H]Cholesterol transfer from 65% EPC/25% cholesterol/10% lactosyl ceramide SUV (50 μM) to 100% EPC SUV (750 μM) and DHE transfer from 50 μM SUV (75/25 EPC/DHE) to 97% EPC/3% dansyl-PE SUV (750 μM).
Effect of the LBPA Antibody on Cholesterol Transfer
Kobayashi et al. have shown that treatment of BHK cells with anti-LBPA monoclonal antibody 6C4 results in an NPC-like phenotype, with cholesterol accumulation in late endosomes (40). To further examine the role of LBPA in NPC2-mediated cholesterol transfer, we monitored the transfer of CTL from 1 μM NPC2 to SUV containing 25% LBPA and incubated with 250 ng/mL 6C4. As shown in Figure 4, the anti-LBPA antibody decreased the CTL transfer rate by ~60% relative to that in LBPA vesicles without antibody incubation. Importantly, the antibody had no effect on the rate of CTL transfer to vesicles which contained no LBPA (Figure 4). It is likely that the antibody interferes with LBPA–NPC2 interactions, thereby diminishing the effect of LBPA in enhancing sterol transfer from NPC2 to membranes.
Figure 4.
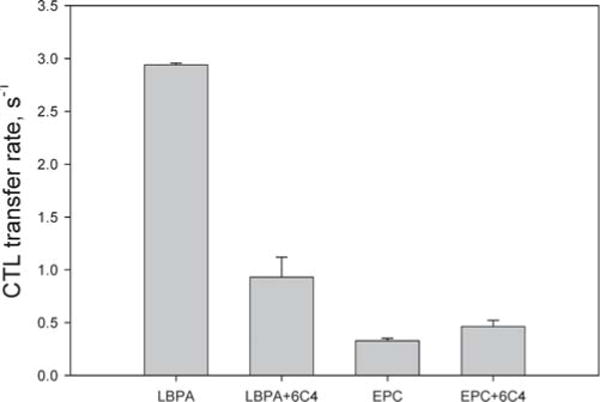
Effect of anti-LBPA antibody 6C4 on cholestatrienol transfer from NPC2 to model membranes. Transfer of 1 μM CTL from 1 μM NPC2 to 100% EPC or 25% LBPA SUV (250 μM) with or without incubation (60 min at 37 °C) with 250 ng/mL 6C4. Transfer was monitored at 25 °C.
Effect of Dolichol and Gangliosides on Cholesterol Transfer from NPC2 to Membranes
We asked whether other lysosomal lipids, in addition to LBPA, might play a role in the cholesterol transfer processes. Dolichol is a polyisoprenoid compound found in high concentrations in lysosomes, as well as the Golgi and plasma membranes (41). Further, in NPC, not only cholesterol but also other lipids such as GM2 and GM3 gangliosides accumulate in the endosomal/lysosomal compartment (42–44). To examine the effect of dolichol and gangliosides on NPC2-dependent cholesterol transfer, 25% of dolichol, lactosoyl ceramide, or gangliosides GM2 and GM3 were incorporated into EPC acceptor vesicles. Table 1 shows that the rate of cholesterol transfer from NPC2 was virtually unaffected by the presence of dolichol, lactosyl ceramide, and GM2. A 3-fold increase was observed with GM3-containing membranes, an effect almost 1 order of magnitude smaller than the effects observed for LBPA-containing membranes.
Table 1.
Rates of Cholesterol Transfer from NPC2 to SUV with Differing Compositionsa
| SUV composition | transfer rate (s−1) | SUV composition | transfer rate (s−1) |
|---|---|---|---|
| EPC | 0.028 ± 0.008 | lactosyl ceramide | 0.031±0.014 |
| GM2 | 0.035 ± 0.002 | dolichol | 0.036±0.010 |
| GM3 | 0.104 ± 0.032 | LBPA | 0.412±0.065 |
Transfer rates of 1 μM cholesterol from 1 μM NPC2 to 250 μM SUV composed of 100% EPC or 75% EPC with 25% indicated lipid (molar ratio). Average transfer rates are from five separate experiments ± the standard deviation.
Intermembrane Transfer of DHE: Effects of Acceptor Membrane Concentration, Membrane Composition, and NPC2 Concentration
The spontaneous intermembrane transfer rate of DHE was very slow, in agreement with previous studies of cholesterol transfer between membranes (26, 45), and incorporation of LBPA into the membranes had essentially no effect on the DHE transfer rate (Figure 5). In contrast, addition of NPC2 resulted in a marked increase in the rates of intermembrane DHE transfer, with 40- and 280-fold increases for EPC membranes and LBPA-containing membranes, respectively, relative to rates in the absence of NPC2 (Figures 5 and 6A). Further, in the presence of NPC2, the DHE transfer rate increased as a function of increasing acceptor membrane concentration, whereas in the absence of NPC2, the DHE transfer rate did not change as more acceptor membrane was added (Figure 6B). Addition of increasing concentrations of NPC2 led to proportional increases in DHE transfer rates for both EPC membranes and LBPA-containing membranes, with rates of transfer ~10-fold faster than those for EPC membranes (Figure 6A).
Figure 5.
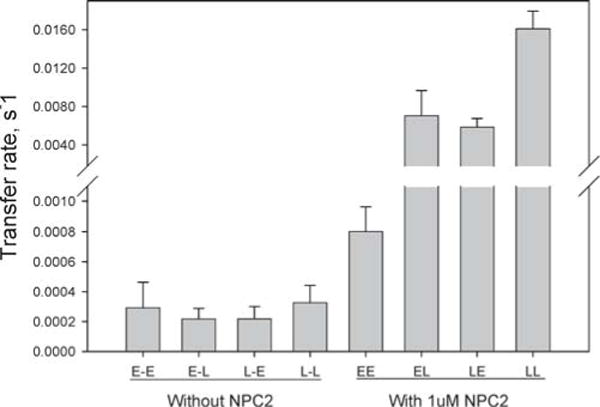
Transfer of DHE between membranes with or without NPC2. DHE transfer from 50 μM SUV with LBPA (L) or without LBPA (E) to 250 μM SUV with (L) or without LBPA (E), in the presence or absence of 1 μM NPC2.
Figure 6.
DHE transfer between membranes. (A) DHE transfer from 75% EPC/25% DHE SUV (50 μM) to 100% EPC SUV at different concentrations with (∆) or without (■) 1 μM NPC2. (B) DHE transfer from 50 μM SUV (50/25/25 EPC/LBPA/DHE) to 250 μM SUV with (∆) or without (■) LBPA in the presence of different concentrations of NPC2.
FTIR Study of the Interaction of NPC2 with Membranes
The kinetics studies strongly suggest that the cholesterol transport properties of NPC2 involve protein–membrane interactions. We therefore used FTIR spectroscopy to examine this hypothesis directly. Figure 7 shows the frequency dependence of the lipid acyl chain symmetric methylene stretching vibration (νsym, CH2) for POPS MLV as a function of temperature, in the presence and absence of NPC2 protein. The phase transition temperature of POPS, monitored by the shift of the lipid carbonyl stretching vibration, increased by ~2 °C (from ~15 to ~17 °C) in the presence of NPC2, indicating an interaction between NPC2 protein and the phospholipid membranes and suggesting that NPC2 stabilizes the bilayer gel phase. The cooperativity of the lipid phase transition was not changed by NPC2 addition.
Figure 7.
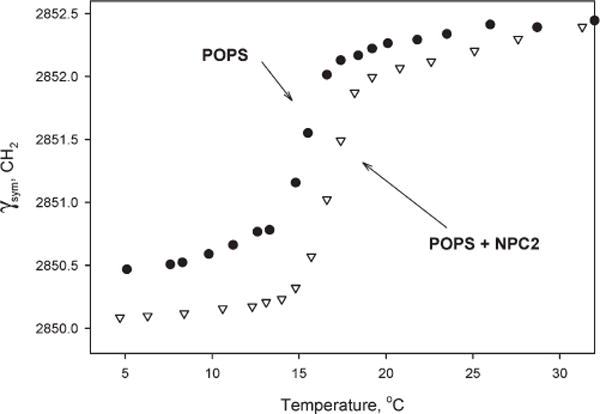
Frequencies of the υs(CH2) vibrations (±0.1 cm−1) vs temperature for 2.0 mg of pure POPS multilamellar vesicles and in the presence of NPC2 protein: pure POPS MLV (■) and POPS with 0.5 mg of NPC2 (∇) (60/1 POPS/NPC2).
Tryptophan Fluorescence Detection of NPC2–Membrane Interactions
We examined the tryptophan fluorescence spectrum of NPC2 in the absence or presence of EPC SUV. For monoglycosylated NPC2, vesicle addition quenched the tryptophan signal by approximately 10–15% but did not change the peak position of the tryptophan emission (Figure 8A). For diglycosylated NPC2, tryptophan quenching was also observed, and the emission peak in the absence of SUV (323 nm) shifted to a lower wavelength (317 nm) when EPC vesicles were added (Figure 8B), suggesting an increase in the hydrophobicity of the environment of one or more tryptophan residues and, thereby, indicating an interaction between NPC2 with the phospholipid membranes. Membranes containing 25 mol % LBPA showed the same modifications in tryptophan emission (not shown). Titration of NPC2 with increasing concentrations of phosphatidyl-choline vesicles showed that the tryptophan quenching plateaued at a PL/NPC2 molar ratio of approximately 70/1, with an apparent Kd value for phospholipid binding of 30 μM (Figure 8C).
Figure 8.
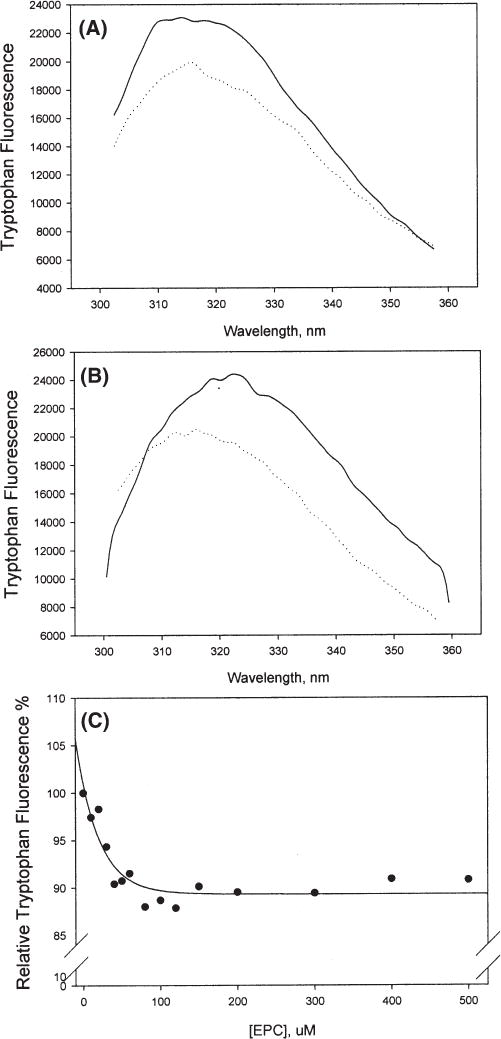
Tryptophan fluorescence of NPC2 in the absence (−) or presence (⋯) of EPC SUV: (A) 5 μM monoglycosylated NPC2 with 500 μM EPC SUV and (B) 5 μM diglycosylated NPC2 with 500 μM EPC SUV. (C) Monoglycosylated NPC2 (10 μM) was incubated with increasing concentrations of EPC SUV, and the peak tryptophan emission was obtained at ≥5 min. Results are representative of two separate experiments. The excitation wavelength was 280 nm.
DISCUSSION
Accumulation of cholesterol in the late endosome/lysosome compartment is a major cellular hallmark of Niemann-Pick disease type C. The NPC2 protein, initially identified as a cholesterol binding protein, HE1, in epidydymal fluid, was shown to be targeted to the lysosomal compartment via the mannose 6-phosphate receptor (6). The recently determined X-ray crystal structure of holo-NPC2 demonstrates that the ligand binding site closely surrounds the steroid nucleus, with the side chain buried inside the protein interior (46). In this work, we studied the absolute rates and mechanism of cholesterol transfer from model phospholipid membranes to NPC2 and, further, examined the effect of NPC2 on sterol transfer between phospholipid membranes. For many of the experiments, the intrinsic tryptophan fluorescence of NPC2 was used to monitor the transfer of unmodified cholesterol from phospholipid membranes to the protein. All studies were performed at acidic pH; thus, the rates obtained are likely to be physiologically relevant to endosomal/lysosomal cholesterol transport.
To determine the mechanism of the membrane-to-NPC2 cholesterol transfer process, we monitored the relationship between the cholesterol transfer rates and the acceptor NPC2 concentration. If transfer occurs through aqueous phase diffusion, the rates of transfer from membranes should be constant at different acceptor concentrations, as the rate of dissociation of cholesterol from membranes would be the limiting step (22, 23, 26). We observed, instead, that the transfer rates increased directly with NPC2 concentration; this increase is likely secondary to the increased frequency of NPC2–membrane interactions, supporting a collisional mechanism of cholesterol transfer from membranes to NPC2. Rates of cholesterol transfer to NPC2 were much faster from membranes containing negatively charged phospholipids than from zwitterionic vesicles. This, too, suggests that cholesterol transfer from membranes to NPC2 involves protein–membrane interactions. Further, the results indicate the likelihood of electrostatic interactions between NPC2 and membranes, which are affected by protein surface properties and membrane structure and composition.
As we initially showed for cholesterol transfer from NPC2 to membranes (20), LBPA-containing membranes exhibit the highest rates of cholesterol transfer to NPC2. Recently, Babalola et al. used an avidin and biotin-based liposome separation method to examine cholesterol transfer between membranes and found that the amount of transfer was strongly stimulated by incorporation of LBPA (47). LBPA is a unique lipid present at high concentrations in the endosome/lysosome compartment, and Kobayashi et al. showed that treatment of BHK cells with an anti-LBPA antibody, 6C4, resulted in cholesterol accumulation and an NPC-like phenotype (40). We found that the 6C4 anti-LBPA antibody substantially weakened the enhancing effect of LBPA on cholesterol transfer by NPC2, suggesting that the cholesterol accumulation in 6C4-treated BHK cells was due, at least in part, to a defect in NPC2-mediated cholesterol transport. Interestingly, LBPA was also shown to increase the extent of glycosphingolipid hydrolysis in an in vitro membrane system, acting in concert with sphingolipid activator proteins (48, 49). Taken together, the results suggest that the high level of LBPA present in endosomal/lysosomal membranes is likely to be functionally necessary for the normal trafficking and enzymatic functions of this compartment.
The three dioleoyl LBPA isomers examined [S,S (3,3′), S,R (3,1′), and R,R (1,1′)] displayed similar effects in enhancing the rate of cholesterol transport in vitro. While the S,S (3,3′) isomer is considered to be biologically stable in cells (33–36), it was also suggested that 2,2′-diacyl LBPA is dominant in cells, with a dynamic equilibrium between 2,2′ and 3,3′ (S,S′) isomers, but that the 2,2′ isomer may be thermodynamically unstable (33, 50). Membranes containing dioleoyl LBPA, which was shown to be the major LBPA species present in BHK cells (40), exhibited a faster rate of cholesterol transfer from NPC2 to membranes than membranes containing dimyristoyl LBPA. We hypothesize that LDL-derived cholesterol accumulates in internal lysosomal membranes, perhaps in LBPA-enriched lateral domains (50–52), and that NPC2 protein may remove cholesterol from these sites by a mechanism involving a direct interaction with these domains. As discussed below, our FTIR results suggest that NPC2 does not access the membrane bilayer acyl chain core but rather interacts at the membrane surface. Thus, the LBPA acyl chain composition is likely to affect the configuration of its polar headgroup, with shorter chains resulting in a configuration with weakened interaction with NPC2. In contrast, the stereochemistry of LBPA does not appear to affect its role in membranes as a putative NPC2 interaction site, suggesting that the structural effects of the isomers at the membrane surface are similar. A direct comparison between 100% 1,1′ (R,R) and 3,1′ (S,R) dimyristoyl LBPA membranes indicated that both form a compact configuration with bent headgroups and closely positioned acyl chains. However, since the capacity for hydrogen bonding was found to be greater for the 3,1′ (S,R) species and, hence, it showed tighter packing than the 1,1′ (R,R) isomer (33), we had expected to find differential effects on cholesterol transport by NPC2. The similar effects for the three LBPA stereoisomers that were observed here could have resulted from use of the dioleoyl LBPA species, which may prevent the packing differences found using disaturated LBPAs.
The membrane interaction properties of NPC2, suggested by the cholesterol kinetics results, were directly confirmed using two approaches. Titration of NPC2 with SUV resulted in saturable quenching of the tryptophan emission, suggesting protein–membrane interactions. Further, when SUV were incubated with diglycosylated NPC2, the tryptophan emission spectrum displayed a blue shift. This is generally understood as demonstrating an increase in the hydrophobicity of the environment immediately surrounding the emitting tryptophan(s). While one interpretation is that one or both of the two Trp residues on NPC2 are localized in the hydrophobic membrane bilayer, it is also possible that NPC2–membrane interaction causes a conformational change in the protein, which leads to an increased hydrophobicity of the environment surrounding the tryptophan. In either case, the blue shift likely demonstrates an interaction between NPC2 and the membrane. It is not clear why the monoglycosylated NPC2 did not demonstrate a blue-shifted tryptophan spectrum upon membrane incubation; however, given that fluorescence was quenched, that we observed collisional kinetics for both mono- and diglycosylated NPC2 (20), and that the FTIR studies presented here were conducted using the monoglycosylated protein, it is very likely that it, too, is membraneinteractive.
The melting temperature of POPS membranes increased in the presence of NPC2, but the slope of the lipid phase transition was essentially unaltered, suggesting an extrinsic interaction of NPC2 at the membrane surface without penetration into the bilayer acyl chain region. The increase in Tm indicates that binding of NPC2 leads to a stabilization of the gel phase and an increased level of ordering of the membrane phospholipids. Such behavior has been observed for a number of other surface-active proteins, including pediosin (53) and nisin (54). It is possible that the electrostatic interactions between NPC2 and acidic phospholipid headgroups may be stronger than lipid-lipid interactions (54). Promotion of a gel phase configuration rather than a fluid phase might destabilize the membrane near the site of interaction, lowering the free energy for dissociation of cholesterol from the membrane. While these studies imply a long-term interaction between NPC2 and membranes, both used apo NPC2, and we hypothesize that apo and holo NPC2, which have modest conformational differences (46), may interact differently with membranes, leading to binding or release from the membrane surface.
As both cholesterol and glycolipids build up in NPC cells, there has been discussion about whether the accumulation of cholesterol is the initial defect or whether glycosphingolipids such as gangliosides or lactosoyl ceramides accumulate first (55, 56). For example, activities of the hydrolytic enzymes sphingomyelinase and glycosylceramidase are reduced in NPC cells (57–59), which could contribute to glycosphingolipid accumulation. While the mechanism remains unclear, it has been suggested to be due to enzyme misfolding or mislocalization, or to allosteric regulation of enzyme activities by cholesterol (57, 60). Our results show that incorporation of GM2 and lactosoyl ceramide has no effect on cholesterol transfer rates, while GM3 has a relatively small effect, indirectly supporting the suggestion that cholesterol trafficking is the primary defect in NPC. Since both GM2 and GM3 contain the single N-acetylneuraminic acid (NANA) group, the differences found between the two gangliosides are not likely due to differences in net negative charge. The absence of the uncharged N-acetylgalactosamine residue that is present in GM2, however, may make the NANA carboxylate group on GM3 more accessible for membrane–NPC2 interaction.
We also examined whether dolichol, a polyisoprenoid compound abundantly present in lysosomes as well as other subcellular compartments (41), might impact the rates of cholesterol transport by NPC2. One report had indicated that NPC2 interacted with the dolichol synthetic enzyme dehydrodolichol diphosphate synthase (61); however, it is worth noting that the enzymes of dolichol synthesis are not localized in the same subcellular compartment as NPC2 (41). No effect of dolichol on cholesterol transfer kinetics was observed.
To model the transfer of LDL-derived cholesterol, much of which is likely intercalated in inner lysosomal lamellae, to the limiting organellar membrane, we developed a fluorescence resonance energy transfer (FRET) assay using the fluorescent analogue DHE as the donor fluorophore, incorporated into donor vesicles, and the dansyl moiety of dansyl-PE as the acceptor fluorophore, incorporated into acceptor membranes. Previous studies of intermembrane cholesterol transfer have been conducted by physically separating the donor and acceptor vesicles containing radio-labeled cholesterol (26, 47, 62). The main shortcoming of the separation approaches is that they limit the resolution of the time scales that can be examined to minutes or longer. For spontaneous transfer, which is very slow, we were able to use a physical separation method and obtained intermembrane transfer rates that were on the order of hours, in agreement with previous literature (26, 62). When NPC2 was added, however, only equilibrium distributions of cholesterol were able to be resolved because transfer was so rapid. Therefore, we designed the FRET method to monitor fluorescent sterol transfer between membranes and showed that the spontaneous intermembrane transfer rates for cholesterol and DHE were basically similar, with DHE transfer ~12% faster than cholesterol transfer. Using the FRET assay, we can monitor sterol transfer in real time through the decrease in DHE fluorescence or the increase in dansyl fluorescence, with a stopped-flow mixing fluorescence spectrometer. The results show that the DHE intermembrane transfer rates in the presence of NPC2 are much faster than the rates of spontaneous transfer and are markedly affected by membrane composition and concentration, supporting a collisional mechanism for sterol transfer between membranes in the presence of NPC2. In the absence of the protein, DHE transfer proceeded via aqueous diffusion, in agreement with previous studies (26, 62), and the slow transfer presumably reflects the rate of dissociation of DHE from the membrane into buffer. The dramatic, >200-fold increase in intermembrane DHE transfer rates with LBPA in the donor membranes, seen only in the presence of NPC2, further supports the importance of this lipid in sterol transfer by NPC2.
Taken together, these results suggest that NPC2 functions as an intracellular cholesterol transporter, playing an important role in the egress of cholesterol from the endosomal/lysosomal compartment. Mutations or the absence of NPC2 could thus trigger the cholesterol accumulation phenotype of NPC2 disease. The presence of LBPA in the lysosome may be important for NPC2–membrane interactions, perhaps functioning as a localization site for increasing the efficiency of cholesterol transfer by NPC2. A coordinated action of NPC1 and NPC2 is suggested by the similarity in human disease presentation and murine phenotypes caused by depletion or mutation of either gene (8, 63). Thus far, specific interactions between the two proteins have not been reported. While such protein–protein interactions may yet be demonstrated, these results suggest the possibility of a model in which NPC2 could be acting to efficiently deliver cholesterol to NPC1-containing membranes, with NPC1 then binding the sterol following its rapid lateral diffusion in the plane of the membrane.
Acknowledgments
We sincerely thank Dr. Richard Mendelsohn and his laboratory for providing the FTIR instruments. We also thank Drs. Thomas Haines, Toshihide Kobayashi, Peter Lobel, and Ann Stock for helpful discussions.
Footnotes
This work was supported by grants from the Ara Parseghian Medical Research Foundation (to J.S.) and Grant 0625994T from the American Heart Association (to Z.X.).
Abbreviations: NPC, Niemann-Pick disease type C; LDL, lowdensity lipoprotein; DHE, dehydroergosterol; RCA, Ricinus communis Agent; EPC, egg phosphatidylcholine; POPS, palmitoyloleoylphosphatidylserine; LBPA, lysobisphosphatidic acid; dansyl-PE, dansyl phosphatidylethanolamine; CTL, cholestatrienol; FRET, fluorescence resonance energy transfer; FTIR, Fourier transfer infrared.
References
- 1.Pentchev PG, Brady RO, Blanchette-Mackie EJ, Vanier MT, Carstea ED, Parker CC, Golden E, Roff CF. The Niemann-Pick C lesion and its relationship to the intracellular distribution and utilization of LDL cholesterol. Biochim Biophys Acta. 1994;1225:235–243. doi: 10.1016/0925-4439(94)90001-9. [DOI] [PubMed] [Google Scholar]
- 2.Vanier MT, Rodriguez-Lafarasse C, Rousson R, Gazzah N, Juge MC, Pentchev PG, Revol A, Louisot P. Type C Niemann-Pick disease: Spectrum of phenotypic variation in disruption of intracellular LDL-derived cholesterol processing. Biochim Biophys Acta. 1991;1096:328–337. doi: 10.1016/0925-4439(91)90069-l. [DOI] [PubMed] [Google Scholar]
- 3.Liscum L, Ruggiero RM, Faust JR. The intracellular transport of low density lipoprotein-derived cholesterol is defective in Niemann-Pick type C fibroblasts. J Cell Biol. 1989;108:1625–1636. doi: 10.1083/jcb.108.5.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cruz JC, Sugii S, Yu C, Chang TY. Role of Niemann-Pick Type C1 Protein in intracellular trafficking of low density lipoprotein-derived cholesterol. J Biol Chem. 2000;275:4013–4021. doi: 10.1074/jbc.275.6.4013. [DOI] [PubMed] [Google Scholar]
- 5.Carstea ED, Morris JA, Coleman KG, Loftus SK, Zhang D, Cummings C, Gu J, Rosenfeld MA, Pavan WJ, Krizman DB, Nagle J, Polymeropoulous MH. Niemann-Pick C1 disease gene: Homology to mediators of cholesterol homeostasis. Science. 1997;277:228–231. doi: 10.1126/science.277.5323.228. [DOI] [PubMed] [Google Scholar]
- 6.Naureckiene S, Sleat DE, Lackland H, Fensom A, Vanier MT, Wattiaux R, Jadot M, Lobel P. Identification of HE1 as the second gene of Niemann-Pick C disease. Science. 2000;290:2298–2301. doi: 10.1126/science.290.5500.2298. [DOI] [PubMed] [Google Scholar]
- 7.Goldstein JL, Brown MS. Lipoprotein receptors and the control of plasma LDL cholesterol levels. Eur Heart J. 1992;13(Suppl. B):34–36. doi: 10.1093/eurheartj/13.suppl_b.34. [DOI] [PubMed] [Google Scholar]
- 8.Patterson MC, Vanier MT, Suzuki K, Morris JA, Carstea E, Neufeld EB, Blanchette-Mackie JE, Pentchev PG. Niemann-Pick disease type C: A lipid trafficking disorder. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The metabolic and molecular bases of inherited disease. 7. II. McGraw-Hill; New York: 2001. pp. 3611–3633. [Google Scholar]
- 9.Davies JP, Ioannou YA. Topological analysis of Niemann-Pick C1 protein reveals that the membrane orientation of the putative sterol-sensing domain is identical to those of 3-hydroxy-3-methylglutaryl-CoA reductase and sterol regulatory element binding protein cleavage-activating protein. J Biol Chem. 2000;275:24367–24374. doi: 10.1074/jbc.M002184200. [DOI] [PubMed] [Google Scholar]
- 10.Davies JP, Chen FW, Ioannou YA. Transmembrane molecular pump activity of Niemann-Pick C1 protein. Science. 2000;290:2295–2298. doi: 10.1126/science.290.5500.2295. [DOI] [PubMed] [Google Scholar]
- 11.Passeggio J, Liscum L. Flux of fatty acids through NPC1 lysosomes. J Biol Chem. 2005;280:10333–10339. doi: 10.1074/jbc.M413657200. [DOI] [PubMed] [Google Scholar]
- 12.Ohgami N, Ko DC, Thomas M, Scott MP, Chang CC, Chang TY. Binding between the Niemann-Pick C1 protein and a photoactivatable cholesterol analog requires a functional sterol-sensing domain. Proc Natl Acad Sci USA. 2004;101:12473–12478. doi: 10.1073/pnas.0405255101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Infante RE, Abi-Mosleh L, Radhakrishnan A, Dale JD, Brown MS, Goldstein JL. Purified NPC2 protein: I Binding of cholesterol and oxysterols to a 1278-amino acid membrane protein. J Biol Chem. 2008;283:1052–1063. doi: 10.1074/jbc.M707943200. [DOI] [PubMed] [Google Scholar]
- 14.Infante RE, Radhakrishnan A, Abi-Mosleh L, Kinch LN, Wang ML, Grishin NV, Goldstein JL, Brown MS. Purified NPC1 protein: II. Localizarion of sterol binding to a 240-amino acid soluble luminal loop. J Biol Chem. 2008;283:1064–1075. doi: 10.1074/jbc.M707944200. [DOI] [PubMed] [Google Scholar]
- 15.Okamura N, Kiuchi S, Tamba M, Kashima T, Hiramoto S, Baba T, Dacheux F, Dacheux JL, Sugita Y, Jin YZ. A porcine homolog of the major secretory protein of human epididymis, HE1, specifically binds cholesterol. Biochim Biophys Acta. 1999;1438:377–387. doi: 10.1016/s1388-1981(99)00070-0. [DOI] [PubMed] [Google Scholar]
- 16.Ko DC, Binkley J, Sidow A, Scott MP. The integrity of a cholesterol-binding pocket in Niemann-Pick C2 protein is necessary to control lysosome cholesterol levels. Proc Natl Acad Sci USA. 2003;100:2518–2525. doi: 10.1073/pnas.0530027100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liou HL, Dixit SS, Xu S, Tint GS, Stock AM, Lobel P. NPC2, the protein deficient in Niemann-Pick C2 disease, consists of multiple glycoforms that bind a variety of sterols. J Biol Chem. 2006;281:36710–36723. doi: 10.1074/jbc.M608743200. [DOI] [PubMed] [Google Scholar]
- 18.Friedland N, Liou HL, Lobel P, Stock AM. Structure of a cholesterol-binding protein deficient in Niemann-Pick type C2 disease. Proc Natl Acad Sci USA. 2003;100:2512–2517. doi: 10.1073/pnas.0437840100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Storch J, Corsico B. The emerging functions and mechanisms of mammalian fatty acid-binding proteins. Annu Rev Nutr. 2008;28:73–95. doi: 10.1146/annurev.nutr.27.061406.093710. [DOI] [PubMed] [Google Scholar]
- 20.Cheruku SR, Xu Z, Dutia R, Lobel P, Storch J. Mechanism of cholesterol transfer from the Niemann-Pick Type C2 protein to model membranes supports a role in lysosomal cholesterol transport. J Biol Chem. 2006;281:31594–31604. doi: 10.1074/jbc.M602765200. [DOI] [PubMed] [Google Scholar]
- 21.Van Meel E, Klumperman J. Imaging and imagination: Understanding the endo-lysosomal system. Histochem Cell Biol. 2008;129:253–266. doi: 10.1007/s00418-008-0384-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Storch J, Kleinfeld AM. Transfer of long-chain fluorescent free fatty acids between unilamellar vesicles. Biochemistry. 1986;25:1717–1726. doi: 10.1021/bi00355a041. [DOI] [PubMed] [Google Scholar]
- 23.Roseman MA, Thompson TE. Mechanism of the spontaneous transfer of phospholipids between bilayers. Biochemistry. 1980;19:439–444. doi: 10.1021/bi00544a006. [DOI] [PubMed] [Google Scholar]
- 24.Storch J, Bass NM. Transfer of fluorescent fatty acids from liver and heart fatty acid-binding proteins to model membranes. J Biol Chem. 1990;265:7827–7831. [PubMed] [Google Scholar]
- 25.Herr FM, Li E, Weinberg RB, Cook VR, Storch J. Differential mechanisms of retinoid transfer from cellular retinol binding proteins types I and II to phospholipid membranes. J Biol Chem. 1999;274:9556–9563. doi: 10.1074/jbc.274.14.9556. [DOI] [PubMed] [Google Scholar]
- 26.Backer JM, Dawidowicz EA. Mechanism of cholesterol exchange between phospholipid vesicles. Biochemistry. 1981;20:3805–3810. doi: 10.1021/bi00516a021. [DOI] [PubMed] [Google Scholar]
- 27.Pipalia NH, Hao M, Mukherjee S, Maxfield FR. Sterol, protein and lipid trafficking in Chinese hamster ovary cells with Niemann-Pick Type C1 defect. Traffic. 2007;8:130–141. doi: 10.1111/j.1600-0854.2006.00513.x. [DOI] [PubMed] [Google Scholar]
- 28.Schroeder F, Dempsey ME, Fischer RT. Sterol and squalene carrier protein interactions with fluorescent Δ-5,7,9(11)-cholestatrien-3β-ol. J Biol Chem. 1985;260:2904–2911. [PubMed] [Google Scholar]
- 29.Scheidt HA, Muller P, Herrmann A, Huster D. The potential of fluorescent and spin-labeled steroid analogs to mimic natural cholesterol. J Biol Chem. 2003;278:45563–45569. doi: 10.1074/jbc.M303567200. [DOI] [PubMed] [Google Scholar]
- 30.Dieudonne D, Mendelsohn R, Farid RS, Flach CR. Secondary structure in lung surfactant SP-B peptides: IR and CD studies of bulk and monolayer phases. Biochim Biophys Acta. 2001;1551:99–112. doi: 10.1016/s0005-2736(00)00387-4. [DOI] [PubMed] [Google Scholar]
- 31.Xu Z, Brauner JW, Flach CR, Mendelsohn R. Orientation of peptides in aqueous monolayer films. Infrared reflection-absorption spectroscopy studies of a synthetic amphipathic β-sheet. Langmuir. 2004;20:3730–3733. doi: 10.1021/la0304316. [DOI] [PubMed] [Google Scholar]
- 32.Kobayashi T, Stang E, Fang KS, de Moerloose P, Parton RG, Gruenberg J. A lipid associated with the antiphospholipid syndrome regulates endosome structure and function. Nature. 1998;392:193–197. doi: 10.1038/32440. [DOI] [PubMed] [Google Scholar]
- 33.Hayakawa T, Hirano Y, Makino A, Michaud S, Lagarde M, Pageaux JF, Doutheau A, Ito K, Fujisawa T, Takahashi H, Kobayashi T. Differential membrane packing of stereoisomers of bis(monoacylglycero)phosphate. Biochemistry. 2006;45:9198–9209. doi: 10.1021/bi060722o. [DOI] [PubMed] [Google Scholar]
- 34.Brotherus J, Renkonen O, Fischer W, Herrmann J. Novel stereoconfiguration in lyso-bis-phosphatidic acid of cultured BHK-cells. Chem Phys Lipids. 1974;13:178–182. doi: 10.1016/0009-3084(74)90034-6. [DOI] [PubMed] [Google Scholar]
- 35.Joutti A, Brotherus J, Renkonen O, Laine R, Fischer W. The stereochemical configuration of lysobisphophatidic acid from rat liver, rabbit lung and pig lung. Biochim Biophys Acta. 1976;450:206–209. doi: 10.1016/0005-2760(76)90092-8. [DOI] [PubMed] [Google Scholar]
- 36.Joutti A, Renkonen O. The stereoconfiguration of bis(monoacylglycero)phosphate synthesized in vitro in lysosomes of rat liver: Comparison with the natural lipid. J Lipid Res. 1979;20:840–847. [PubMed] [Google Scholar]
- 37.John K, Kubelt J, Muller P, Wustner D, Herrmann A. Rapid transbilayer movement of the fluorescent sterol dehydroergosterol in lipid membranes. Biophys J. 2002;83:1525–1534. doi: 10.1016/S0006-3495(02)73922-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wustner D, Herrmann A, Hao M, Maxfield FR. Rapid nonvesicular transport of sterol between the plasma membrane domains of polarized hepatic cells. J Biol Chem. 2002;277:30325–30336. doi: 10.1074/jbc.M202626200. [DOI] [PubMed] [Google Scholar]
- 39.Wustner D, Mondal M, Huang A, Maxfield FR. Different transport routes for high density lipoprotein and its associated free sterol in polarized hepatic cells. J Lipid Res. 2004;45:427–437. doi: 10.1194/jlr.M300440-JLR200. [DOI] [PubMed] [Google Scholar]
- 40.Kobayashi T, Beuchat MH, Lindsay M, Frias S, Palmiter RD, Sakuraba H, Parton RG, Gruenberg J. Late endosomal membranes rich in lysobisphosphatidic acid regulate cholesterol transport. Nat Cell Biol. 1999;1:113–118. doi: 10.1038/10084. [DOI] [PubMed] [Google Scholar]
- 41.Chojnacki T, Dallner G. The biological role of dolichol. Biochem J. 1988;251:1–9. doi: 10.1042/bj2510001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vanier MT. Lipid changes in Niemann-Pick disease type C brain: Personal experience and review of the literature. Neurochem Res. 1999;24:481–489. doi: 10.1023/a:1022575511354. [DOI] [PubMed] [Google Scholar]
- 43.Wojtanik KM, Liscum L. The transport of low density lipoprotein-derived cholesterol to the plasma membrane is defective in NPC1 cells. J Biol Chem. 2003;278:14850–14856. doi: 10.1074/jbc.M300488200. [DOI] [PubMed] [Google Scholar]
- 44.Zervas M, Somers KL, Thrall MA, Walkley SU. Critical role for glycosphingolipids in Niemann-Pick disease type C. Curr Biol. 2001;11:1283–1287. doi: 10.1016/s0960-9822(01)00396-7. [DOI] [PubMed] [Google Scholar]
- 45.Neufeld EB, Cooney AM, Pitha J, Dawidowicz EA, Dwyer NK, Pentchev PG, Blanchette-Mackie EJ. Intracellular trafficking of cholesterol monitored with a cyclodextrin. J Biol Chem. 1996;271:21604–21613. doi: 10.1074/jbc.271.35.21604. [DOI] [PubMed] [Google Scholar]
- 46.Xu S, Benoff B, Liou HL, Lobel P, Stock AM. Structural basis of sterol binding by NPC2, a lysosomal protein deficient in Niemann-Pick Type C2 disease. J Biol Chem. 2007;282:23525–23531. doi: 10.1074/jbc.M703848200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Babalola JO, Wendeler M, Breiden B, Arenz C, Schwarzmann G, Locatelli-Hoops S, Sandhoff K. Development of an assay for the intermembrane transfer of cholesterol by Niemann-Pick C2 protein. Biol Chem. 2007;388:617–626. doi: 10.1515/BC.2007.063. [DOI] [PubMed] [Google Scholar]
- 48.Wilkening G, Linke T, Uhlhom-Dierks G, Sandhoff K. Degradation of membrane-bound ganglioside GM1. Stimulation by bis(monoacylglycero)phosphate and the activator proteins SAP-B and GM2-AP. J Biol Chem. 2000;275:35814–35819. doi: 10.1074/jbc.M006568200. [DOI] [PubMed] [Google Scholar]
- 49.Linke T, Wilkening G, Sadeghlar F, Mozcall H, Bernardo K, Schuchman E, Sandhoff K. Interfacial regulation of acid ceramidase activity. Stimulation of ceramide degradation by lysosomal lipids and sphingolipid activator proteins. J Biol Chem. 2001;276:5760–5768. doi: 10.1074/jbc.M006846200. [DOI] [PubMed] [Google Scholar]
- 50.Kobayashi T, Beuchat MH, Chevallier J, Makino A, Mayran N, Escola JM, Lebrand C, Cosson P, Kobayashi T, Gruenberg J. Separation and characterization of late endosomal membrane domains. J Biol Chem. 2002;277:32157–32164. doi: 10.1074/jbc.M202838200. [DOI] [PubMed] [Google Scholar]
- 51.Kobayashi T, Hirabayashi Y. Lipid membrane domains in cell surface and vacuolar systems. Glycoconjugate J. 2000;17:163–171. doi: 10.1023/a:1026528921085. [DOI] [PubMed] [Google Scholar]
- 52.Kobayashi T, Startchev K, Whitney AJ, Gruenberg J. Localization of lysobisphosphatidic acid-rich membrane domains in late endosomes. Biol Chem. 2001;382:483–485. doi: 10.1515/BC.2001.059. [DOI] [PubMed] [Google Scholar]
- 53.Gaussier H, Lefevre T, Subirade M. Binding of pediocin PA-1 with anionic lipid induces model membrane destabilization. Appl Environ Microbiol. 2003;69:6777–6784. doi: 10.1128/AEM.69.11.6777-6784.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bonev BB, Chan WC, Bycroft BW, Roberts GC, Watts A. Interaction of the lantibiotic nisin with mixed lipid bilayers: A 31P and 2H NMR study. Biochemistry. 2000;39:11425–11433. doi: 10.1021/bi0001170. [DOI] [PubMed] [Google Scholar]
- 55.Gondre-Lewis MC, McGlynn R, Walkley SU. Cholesterol accumulation in NPC1-deficient neurons is ganglioside dependent. Curr Biol. 2003;13:1324–1329. doi: 10.1016/s0960-9822(03)00531-1. [DOI] [PubMed] [Google Scholar]
- 56.te Vruchte D, Lloyd-Evans E, Veldman RJ, Neville DC, Dwek RA, Platt FM, Van Blitterswijk WJ, Sillence DJ. Accumulation of glycosphingolipids in Niemann-Pick C disease disrupts endosomal transport. J Biol Chem. 2004;279:26167–26175. doi: 10.1074/jbc.M311591200. [DOI] [PubMed] [Google Scholar]
- 57.Reagan JW, Jr, Hubbert ML, Shelness GS. Posttranslational regulation of acid sphingomyelinase in niemann-pick type C1 fibroblasts and free cholesterol-enriched chinese hamster ovary cells. J Biol Chem. 2000;275:38104–38110. doi: 10.1074/jbc.M005296200. [DOI] [PubMed] [Google Scholar]
- 58.Besley GT, Moss SE. Studies on sphingomyelinase and β-glucosidase activities in Niemann-Pick disease variants. Phosphodiesterase activities measured with natural and artificial substrates. Biochim Biophys Acta. 1983;752:54–64. doi: 10.1016/0005-2760(83)90232-1. [DOI] [PubMed] [Google Scholar]
- 59.Salvioli R, Scarpa S, Ciaffoni F, Tatti M, Ramoni C, Vanier MT, Vaccaro AM. Glucosylceramidase mass and subcellular localization are modulated by cholesterol in Niemann-Pick disease type C. J Biol Chem. 2004;279:17674–17680. doi: 10.1074/jbc.M313517200. [DOI] [PubMed] [Google Scholar]
- 60.Tamura H, Takahashi T, Ban N, Torisu H, Ninomiya H, Takada G, Inagaki N. Niemann-Pick type C disease: Novel NPC1 mutations and characterization of the concomitant acid sphingomyelinase deficiency. Mol Genet Metab. 2006;87:113–121. doi: 10.1016/j.ymgme.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 61.Kharel Y, Takahashi S, Yamashita S, Koyama T. In vivo interaction between the human dehydrodolichyl diphosphate synthase and the Niemann-Pick C2 protein revealed by a yeast two-hybrid system. Biochem Biophys Res Commun. 2004;318:198–203. doi: 10.1016/j.bbrc.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 62.Bar LK, Chong PL, Barenholz Y, Thompson TE. Spontaneous transfer between phospholipid bilayers of dehydroergosterol, a fluorescent cholesterol analog. Biochim Biophys Acta. 1989;983:109–112. doi: 10.1016/0005-2736(89)90386-6. [DOI] [PubMed] [Google Scholar]
- 63.Sleat DE, Wiseman JA, El-Banna M, Price SM, Verot L, Shen MM, Tint GS, Vanier MT, Walkley SU, Lobel P. Genetic evidence for nonredundant functional cooperativity between NPC1 and NPC2 in lipid transport. Proc Natl Acad Sci USA. 2004;101:5886–5891. doi: 10.1073/pnas.0308456101. [DOI] [PMC free article] [PubMed] [Google Scholar]


