Abstract
Penile implant usage dates to the 16th century yet penile implants to treat erectile dysfunction did not occur until nearly four centuries later. The modern era of penile implants has progressed rapidly over the past 50 years as physicians' knowledge of effective materials for penile prostheses and surgical techniques has improved. Herein, we describe the history of penile prosthetics and the constant quest to improve the technology. Elements of the design from the first inflatable penile prosthesis by Scott and colleagues and the Small-Carrion malleable penile prosthesis are still found in present iterations of these devices. While there have been significant improvements in penile prosthesis design, the promise of an ideal prosthetic device remains elusive. As other erectile dysfunction therapies emerge, penile prostheses will have to continue to demonstrate a competitive advantage. A particular strength of penile prostheses is their efficacy regardless of etiology, thus allowing treatment of even the most refractory cases.
Keywords: Erectile dysfunction, History, Penile prosthesis
INTRODUCTION
Erectile dysfunction (ED) is a multifactorial disorder that is defined as the inability to attain and maintain a penile erection sufficient for sexual intercourse [1]. A recent analysis of published work, reported by the International Consultation Committee for Sexual Medicine on Definitions/Epidemiology/Risk Factors for Sexual Dysfunction, showed that the prevalence of ED ranges from 1% to 10% in men younger than 40 years and from 2% to 9% in men between 40 and 49 years old. Prevalence increases significantly in elderly men; reported prevalences are from 20% to 40% in men aged 60 to 69 years and from 50% to 100% in men aged >70 years [2] Current first-line therapy to treat ED is the use of oral phosphodiesterase type 5 inhibitors. However, up to 35% of ED patients may fail to respond to this therapy. These patients require an alternate therapy such as intracavernosal injections, vacuum erectile devices, or a penile prosthesis. Penile prosthetics offer a surgical solution that can restore erectile function in the most refractory of cases regardless of etiology.
First, we must define a penile prosthesis and the characteristics of such a device. A penile prosthesis is a device, either external or implanted, that substitutes for or supplements the function of the erectile bodies to achieve penile rigidity, thus simulating an erection. In broad terms, prosthetics are used to restore function and make the body "whole" again. As such, the ideal penile prosthetic device for ED treatment would mimic a native physiologic erection as closely as possible, both in function and appearance. It should perform the mechanical duties necessary for successful intercourse when erect and be durable enough for many uses to match the lifespan of the patient. When not in use, the penile prosthetic would mimic the flaccid state of the penis and be discreet. The ideal prosthetic device would also not interfere with urination or other activities of daily living. As a component of the sexual experience, the prosthetic should maintain or improve the quality of the sexual experience for the patient (i.e., sensation, spontaneity). The ideal prosthesis could also be implanted in a simple surgical procedure with minimal recovery time.
While oral phosphodiesterase type 5 inhibitors have only been available since the late 1990s [3], the availability of penile implants to treat ED dates to the 1930s. Yet, it was in the late 1960s and early 1970s that the modern era of penile prostheses really began. Although present-day iterations of the penile prosthesis as characterized by models such as the American Medical Systems (AMS) 700 LGX and Coloplast Titan have made substantial progress towards the ideal penile prosthesis when compared to prostheses designed in the 1970s, significant improvements can still be made. Herein, we describe the history of penile prosthetics and the quest to constantly improve the technology.
EARLY HISTORY OF PENILE IMPLANTS
The earliest documentation of an artificial penis for medical use dates to the 16th century. Ambroise Pare is credited with making the first artificial penis from a wooden pipe for a patient to facilitate micturition [4]. The earliest documentation of a penile implant to treat ED, however, is credited to Nikolaj Bogoraz. In 1936, Bogoraz used tailored rib cartilage to provide rigidity to the penis [5]. Bergman, Howard, and Barnes also used a rib graft for penile reconstruction and in 1948 reported satisfactory intercourse results 4 months after completion of the procedure [6]. Rib cartilage was not a viable implant material, however, because if infection occurred, which it often did, the cartilage tended to curve in on itself within 18 months and become totally absorbed within several years. This unfortunately resulted in a permanently curved, nonfunctional penis [6].
Dr. Scardino, a surgeon in the United States, was likely the first person to use synthetic material for a penile implant in 1950 but the data were unpublished. Thus, the first descriptions of alloplastic implants for erectile rigidity are attributed to Goodwin and Scott who in 1952 reported on five patients with acrylic prostheses. Acrylic, a synthetic polymer, offered the advantages of being readily available, not absorbed by the body, and moldable to various shapes. The prostheses were positioned beneath Buck's fascia and there were few reported successes. Drs Loeffler and Sayegh also reported on the use of acrylic prostheses in 1960 with little discussion on placement technique except that "the prosthesis was inserted between the corpora cavernosa and fixed in position" [6,7].
In the late 1960s and early 1970s, improvements in surgical technique and the development of silicone rubber were significant advancements in the modern era of penile implant surgery [7]. Dr. Beheri, a plastic surgeon in Cairo, Egypt, is credited with the first descriptions of intracavernosal placements of penile prostheses utilizing polyethylene rods. By 1966, Beheri reported performing 700 such procedures and described his technique of placing the rod beneath the tunica albuginea and using a Hegar dilator to create a tunnel for the prosthesis. The result was an erect penis that was more rigid, less painful, and less likely to erode than previous implants. However, polyethylene was still an inadequate implant material because it was too stiff and rigid [8]. Beheri's data also did not gain much traction in the urology community because the data appeared in the plastic surgery literature and not in urology publications [7,9,10].
In the 1960s the space program developed silicone rubber for human implantation, and it was eventually determined to be a satisfactory and adequate material for penile implantation [7]. As early as 1967, Pearman described placement of a solitary silicone rod between Buck's fascia and the tunica albuginea, which caused significant pain; thus, long-term results were unsatisfactory. Pearman subsequently changed his technique and, like Beheri, placed the prosthesis beneath the tunica albuginea in a space dissected with Hegar dilators. Within the next decade, two new silicone prosthetic devices would be introduced: the inflatable penile prosthesis and the malleable prosthesis. The legacy of these two devices is indelible as the mechanics for all present-day penile implants used can be traced back to one of these prototypes.
In 1973, Dr. Scott and colleagues described a novel penile prosthetic device that used inflatable silicone cylinders, which was a significant departure from prior rod concepts. The device had three components: a reservoir, a control-pump mechanism, and two cylindrical Dacronreinforced silicone bodies all linked by tubing. The rigidity of the device came from transfer of fluid from a reservoir to intracavernosal cylinders via a pump mechanism. When not in use, the fluid was returned to the reservoir. This hydraulic mechanism better mimicked a physiologic erection in the flaccid and rigid states than did previous devices [11]. Initial devices had high mechanical failure rates, however, because the silicone was a poor material for wear and elasticity.
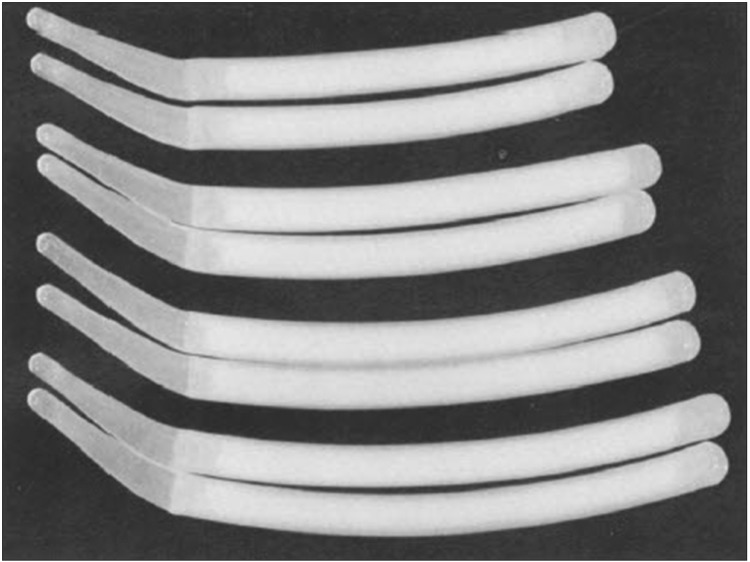
In 1974, Small and Carrion developed a penile implant made up of a pair of rods with silicone-sponge interiors and a silicone exterior. These were precursors to the modern-day malleable or semi-rigid implant. The implants were available in 16 different lengths and 3 different diameters because the appropriate size and length could not be determined before surgery (Fig. 1) [12]. Trimming the devices was eventually introduced to decrease hospital inventory. One major drawback of the Small-Carrion implant was that it would spring back and recoil, meaning that the implant would not stay in a bent position (to mimic the flaccid state) and would essentially be in a permanently erect state [13].
Fig. 1. Various sizes of Small-Carrion prosthesis [12].
DEVELOPMENT AND REFINEMENT OF MALLEABLE TECHNOLOGY SINCE THE 1970S
The permanently erect state issue of the Small-Carrion implant was addressed by both Drs. Subrini and Finney through the use of a softer silicone in the midsection of the penile device. This improvement allowed the device to be bent downward when not in use, improving concealment of the implant. Subrini described his preliminary experience in 1974, whereas Finney introduced his device, the "Flexirod," in 1977 [13]. The Flexirod device had a tapered distal tip for better glans stability, a soft hinge for improved concealment, and a tail that could be trimmed [14].
In 1978, Jonas designed a penile prosthesis that approached malleability in a different way. The Jonas implant was characterized by the silicone being wrapped around a central core of metal, such as stainless steel or twisted silver wire, which allowed the device when bent to remain in that configuration [13]. The design of the Jonas prosthesis also allowed for easier implantation into the penis.
In 1986, Dacomed introduced the Omniphase prosthesis [8]. The Omniphase (and its successor the Duraphase) was a mechanically activated penile implant that could alternate between the flaccid and the rigid state, depending on the tensions of a supporting central cable. It was specifically designed to avoid pumps, valves, and fluid and the potential danger of leakage compared with inflatable penile prostheses, which were being developed around the same time [15]. It combined the desirable properties of easy concealment, rigidity for intercourse, and a simpler surgical technique. The Omniphase and the Duraphase devices were eventually phased out of the market because of cable failure and the need for replacement [16].
In the United States, the penile prosthesis market favors inflatable penile prostheses and is dominated by two companies, AMS and Coloplast (formerly Mentor). Both companies still manufacture malleable penile implants, such as the AMS 600 and AMS 650 and Coloplast Genesis [16]. The soft silicone rods originally manufactured in France by Subrini are currently made in several countries, mainly in Europe and China, under various names [17]. These contemporary iterations of the malleable prosthesis are simple and durable and mechanical failure is quite rare. However, the tissues that support the device can be prone to erosion with direct interface of soft tissues and a more rigid mechanical prosthetic.
IMPROVEMENTS IN INFLATABLE TECHNOLOGY
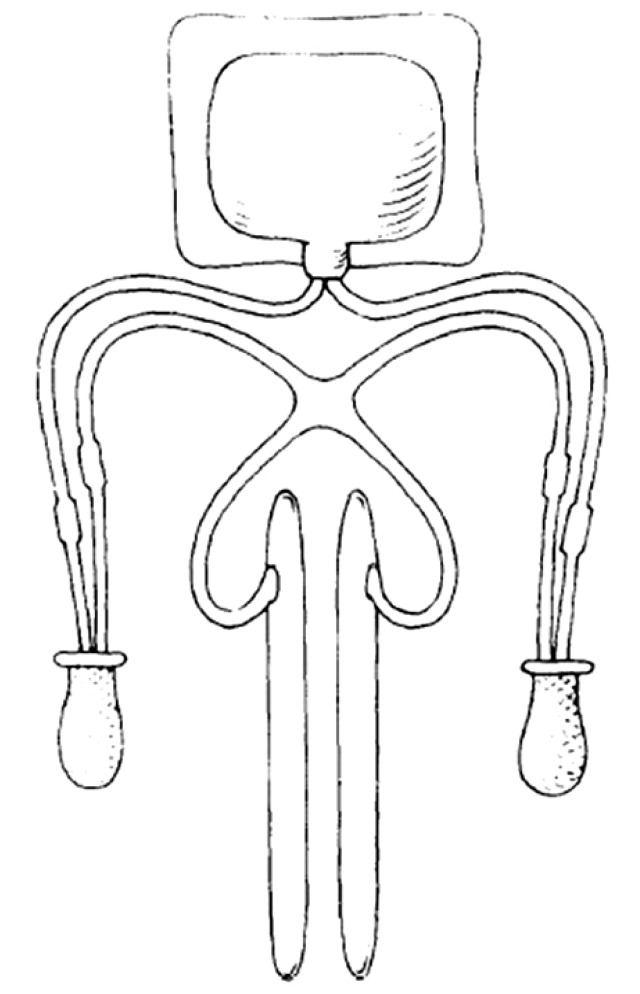
The original inflatable penile prosthesis conceived by Scott et al was manufactured and marketed by AMS, which Dr. Scott helped found. Initially referred to as inflatable penile implant, its name was changed in 1983 to the AMS 700 (Fig. 2) [10]. From 1983 to 1987, a number of design developments were made to the AMS 700. These improvements included front and rear tips, polytetrafluoroethylene (PTFE) sleeves that enhanced durability, and a suture-less connecting system that reduced the risk of device leakage. Kink-resistant tubing was added in 1986, which resulted in more forgiving measurements in tubing length and fewer complications. In 1987, the PTFE sleeves were replaced with a multilayer design in which the inner silicone tubing expanded against an inner silicone-covered woven fabric layer to better facilitate adequate expansion. A friction-reducing Parylene coating on the cylinder was added in 2000 [4,14]. These stepwise improvements increased durability and standardized implantation technique.
Fig. 2. Diagram of original inflatable penile prosthesis [34].
One noteworthy design development was the controlled expansion of the intracavernosal cylinders. Historically, the inflatable prosthesis was designed so that it expanded to a set volume and the implant would become rigid and incapable of further pumping. This was also determined by the elasticity of the material. Launched in 1987 by replacing the inner layer of woven fabric with a polypropylene resembling Dacron, controlled expansion allowed the prosthesis to be rigid enough for erection over a wider range of volumes. It has since become as well the standard for all inf latable prosthesis cylinders [10]. Further refinements with controlled expansion are characterized by the AMS LGX device, which allows for expansion in the longitudinal as well as radial dimensions. This was first described in a US patent application filed in 1985: "[the cylinders] may expand relatively freely in the longitudinal direction when the high modulus transition in radial expansion is reached (US 4651721 A)." For patients, this design allowed for a more physiologic erection that lengthened as well as radially expanded.
In 1983, Mentor, a competing manufacturer, introduced the Bioflex penile prosthesis. The AMS 700 and Bioflex prostheses were similar in structural design, but the Bioflex implant had polyurethane cylinders, which improved elasticity as well as tensile strength. The polyurethane did not allow for uncontrolled expansion, whereas cylinders made entirely f rom silicone rubber did [10]. These improvements in material and design have allowed the mechanical longevity of the device to more closely match that of the patient, with 88% of devices remaining fully functional at 10 years.
Additional implant improvements occurred in terms of user experience. Modern devices come with lock-out valves to prevent auto-inflation due to extrinsic pressure on the reservoir [14], one-touch release valves to facilitate deflation, and better designed tactile pumps that facilitate manipulation in patients with limited dexterity [18]. These incremental design changes resulted in substantial gains in how the patient interacted with and experienced the device.
Attempts to reduce the number of implanted components, yet maintain the advantages of an inflatable penile prosthesis, were undertaken in the 1990s with the introduction of the AMS Dynaflex. The Dynaflex was a unitary inflatable penile prosthesis that consisted of paired cylinders with all operating components contained within. The device was activated by manipulation of the distal tip, allowing a few milliliters of fluid from the proximal reservoir to expand the central component. It deflated by bending the device more than 55 degrees from its axis. It suffered from difficulty manipulating two separate cylinders, as well as poor resistance to buckling. An alternative approach was taken with the two-piece penile prosthesis, which did not need an abdominal reservoir, but had the pump also act as a reservoir and store fluid for transfer. Because of the scrotal location, the reservoir had a limited capacity of 15 to 20 mL. Models that characterized this approach were the AMS Ambicor and the Mentor Mark II. This approach also suffered in rigidity compared with a 3-piece model and did not become as flaccid with deflation. These devices may be of particular benefit where reservoir implantation is technically challenging or risky.
In 2000, AMS introduced the InhibiZone coating, which impregnated the external surfaces of their devices with rifampin and minocycline. This reduced infectious complications from 1.61% to 0.68% in one study [19]. Coloplast followed suit with a hydrophilic coating to adhere antibiotics to its surface, which purportedly allowed greater flexibility in antibiotic choice with reductions in infection rates to 1% to 2% in first-time implants with no risk factors [20]. Both approaches sought to address the dreaded complication of implant infection, which would necessitate removal of the whole device.
IMPROVEMENTS IN TECHNIQUE
While technological advancements were occurring, surgeons were also refining techniques for implantation. The focus turned to standardizing and simplifying the operation to allow for consistent and reproducible results. Tubing was color-coded to simplify connection. Specialized retractors such as the Scott retractor utilized hooks to allow exposure to the relevant anatomy. The Furlow insertor and Keith needle simplified the process of getting the distal tips of the cylinders into the glans without expanding the incision or risking damage to the device [10].
The Carrion-Rossello cavernotomes also added a tool to the implanter's armamentarium for fibrotic corpora. Fibrotic corpora can be particularly challenging as the planes of dissection are scarred down and inadvertent perforations are common. The cavernotome has backwards cutting teeth that allow a surgeon to tunnel a space in the fibrotic corpora more safely [21].
Increased implantation experience also resulted in refinements in technique that allowed for smaller incisions with less-invasive approaches such as single incision placement of all three components of an inflatable penile prosthesis and the use of penoscrotal, infrapubic, or even perineal approaches. With regards to reservoir placement, the most discreet position continues to be in the space of Retzius. As a result of increasing concern for injury to structures in this space related to adhesion formation, such as after robotic prostatectomy, alternate sites have been advocated. This ectopic reservoir placement is generally above the transversalis fascia. One common ectopic location is between the transversalis fascia and the rectus muscle [22,23]. This location offers the advantage of reduced risk of injury to intra-abdominal structures but may be more palpable and prone to herniation. To accommodate this ectopic location, changes in reservoir design, such as a "pancake" shaped reservoir, were developed by AMS in 2006 to make the reservoir less palpable.
EXPANDED INDICATIONS
As penile prosthesis technology has become more widely used, its indications have been expanded to treat concomitant disorders, such as Peyronie disease and priapism with concomitant ED. Peyronie disease, a scarring condition that results in penile deformity, is commonly associated with ED. Techniques to correct this deformity now include use of penile prostheses in patients with concomitant ED. An inflatable penile prosthesis is placed, the device is inflated, and the corpora are modeled around the device to correct the deformity [24]. Extended cases of ischemic priapism may also be treated with a penile prosthesis. Ischemic priapism lasting longer than 72 hours invariably results in ED, and early placement of a penile prosthesis may treat the resultant ED and priapism before severe fibrosis develops [25].
NEEDS AND FUTURE DIRECTIONS IN PENILE PROSTHETICS
Despite the numerous advances described above (Table 1), modern penile prosthetics continue to have low uptake with less than 5% of potential candidates electing to undergo placement of such devices. The reasons for this are multifactorial. On the patient side, the device is still a ways off from mimicking a natural erection. It still requires user manipulation and although fairly discrete has palpable components, which affect the users' perception of discretion. A penile prosthesis also carries risks of device malfunction, infection, and erosion. Certainly, improvements in prosthesis technology have significantly reduced these risks with device malfunction within 5 years being less than 2%. On the surgeon side, implantation continues to be a complex operation with only a small fraction of urologists undertaking these surgeries with any regular frequency. Given this, a number of surgical subtleties can affect the user satisfaction and outcomes from pump location to tubing palpability and proper device sizing. Outcomes do seem improved with high-volume implanters, but widespread adoption requires widespread support and encouragement within the medical community.
Table 1. Historical milestones in the development of penile prosthetics.
| Year | Historical milestones |
|---|---|
| 1936 | Bogaraz uses rib cartilage as penile implant to treat erectile dysfunction |
| 1952 | Goodwin & Scott report use of acrylic prostheses for erectile dysfunction |
| 1966 | Beheri uses polyethylene rods and intracavernosal placement |
| 1967 | Pearman describes placement of penile rod implant between Buck's fascia and the tunica albuginea |
| 1973 | Scott, Tim, and Bradley invent inflatable penile prosthesis |
| 1974 | Small, Carrion design silicone penile implant |
| 1978 | Furlow invents implanter tool |
| 1978 | Jonas designs penile implant characterized by silicone around metal core |
| 1983 | Mentor makes three-piece inflatable implant with Bioflex material that improves durability |
| 1985 | Surgitek and AMS introduce unitary inflatable implants |
| 1986 | Dacomed unveils Omniphase prosthesis |
| 1987 | Propylene used for inner layer of fabric, vastly improving controlled expansion design |
| 1992 | Mentor reinforces and modifies silicone tubing in inflatables prostheses, decreases mechanical failure rate |
| 1995 | Rossello invents cutting dilators and coins the term "cavernotome" |
| 2000 | Mentor designs "lock out valve" to minimize autoinflation |
| 2001 | AMS introduces InhibiZone coating on implants to prevent bacterial colonization |
| 2002 | Coloplast produces Titan implant which has hydrophilic coating |
| 2004 | AMS introduces Tactile pump design for easier inflation in 700 series |
| 2006 | AMS presents Momentary Squeeze for easier deflation |
| 2006 | AMS patents flattened reservoir design |
| 2008 | Coloplast launches One Touch Release for easier deflation |
AMS, American Medical Systems.
In the future, prosthetics will have increasing competition from emerging technologies that do not seek to replace or supplement the function of the native erectile bodies but to restore or improve dysfunctional aspects of the erectile pathway. This may come in the form of stem cell therapies, gene therapy, improved pharmaceuticals, or other forms of regenerative medicine [26,27,28,29,30,31]. The advantages of such approaches would be that the patient's physiology maintains central control over the erectile response, producing a more natural erection [31]. If such approaches prove successful, they could further narrow the market for penile prosthetics to the more severe cases of ED. Where penile prostheses will likely continue to have a role will be in refractory cases, such as situations where the physiologic pathways are so disrupted that restoration is not possible. Additionally, because penile prostheses as a treatment are not etiology specific, they will continue to have a role in conditions that may be multifactorial and not easily addressed by correction of one part of the erectile pathway, such as comorbid neurologic and venous leak etiologies of ED.
It is the opinion of the authors that the main areas of improvement for penile prostheses will be in facilitating the user interface, simplifying the operation, enhancing reliability, and better mimicking a physiologic erection. To put the market for ED in perspective, approximately 25,000 prostheses are implanted in North America versus 296,000 breast implants in the United States alone annually [32,33]. Certainly a penile prosthesis is a more complex device than a breast implant, but the argument still stands that the uptake for surgery for correction for ED is below where it could be. A large part of the reduced uptake is related to marketing, coverage issues, and physician education, but a significant part comes from the shortcomings of the device itself. An ideal device would be implanted in an outpatient or office setting in a simple fashion. The device ideally would not use a pump at all but could be under central nervous system control or be a single-touch kind of operation. This could be achieved through improvements in the area of controllable prosthetics, such as in limbs. A single-touch device would also present a significant leap forward in simplifying the user experience.
Although a hydraulic mechanism certainly is adequate, it depends on pressure and a reservoir and is prone to leakage. Valves are needed to control the flow and direction of fluid, as well as to resist forces encountered in normal use. If the end goal is to mimic a physiologic erection that expands and lengthens the penis, alternate materials may serve this purpose, from expandable foams that respond to external magnetic fields to stent technology that could expand and retract in a cage-like fashion. The future for penile prostheses will be determined by their ability to fit the need for a low-risk procedure that delivers on restoring the ability to resume sexual function.
CONCLUSIONS
Penile implant usage dates to the 16th century yet penile implants to treat ED did not occur until nearly four centuries later. The modern era of penile implants has progressed rapidly over the past 50 years as physicians' knowledge of effective materials for penile prostheses and surgical techniques has improved. Elements of the design from the first inflatable penile prosthesis by Scott and colleagues and the Small-Carrion malleable penile prosthesis are still found in present iterations of these devices. While there have been significant improvements in penile prosthesis design, the promise of an ideal prosthetic device remains elusive. As other ED therapies emerge, penile prostheses must continue to demonstrate a competitive advantage. A particular strength of prostheses is their efficacy regardless of etiology, thus allowing treatment of even the most refractory cases.
Footnotes
The authors have nothing to disclose.
References
- 1.Impotence. NIH Consens Statement. 1992;10:1–33. [PubMed] [Google Scholar]
- 2.Shamloul R, Ghanem H. Erectile dysfunction. Lancet. 2013;381:153–165. doi: 10.1016/S0140-6736(12)60520-0. [DOI] [PubMed] [Google Scholar]
- 3.Goldstein I, Lue TF, Padma-Nathan H, Rosen RC, Steers WD, Wicker PA Sildenafil Study Group. Oral sildenafil in the treatment of erectile dysfunction. N Engl J Med. 1998;338:1397–1404. doi: 10.1056/NEJM199805143382001. [DOI] [PubMed] [Google Scholar]
- 4.Hakky TS, Wang R, Henry GD. The evolution of the inflatable penile prosthetic device and surgical innovations with anatomical considerations. Curr Urol Rep. 2014;15:410. doi: 10.1007/s11934-014-0410-9. [DOI] [PubMed] [Google Scholar]
- 5.Schultheiss D, Gabouev AI, Jonas U. Nikolaj A. Bogoraz (1874-1952): pioneer of phalloplasty and penile implant surgery. J Sex Med. 2005;2:139–146. doi: 10.1111/j.1743-6109.2005.20114.x. [DOI] [PubMed] [Google Scholar]
- 6.Gee WF. A history of surgical treatment of impotence. Urology. 1975;05:401–405. doi: 10.1016/0090-4295(75)90168-5. [DOI] [PubMed] [Google Scholar]
- 7.Carson CC. Penile prostheses: state of the art. In: Kirby RS, O'Leary MP, editors. Recent advances in urology. Edinburgh: Churchill Livingstone; 1998. pp. 61–72. [Google Scholar]
- 8.Nelson RP. Pathophysiology, evalution, and treatment of erectile dysfunction. In: Rous SN, editor. Urology annual. Norwalk, CT: Appleton and Lange; 1987. pp. 139–169. [Google Scholar]
- 9.Jonas U. The history of erectile dysfunction management. Int J Impot Res. 2001;13(Suppl 3):S3–S7. doi: 10.1038/sj.ijir.3900717. [DOI] [PubMed] [Google Scholar]
- 10.Wilson SK, Delk JR., 2nd Historical advances in penile prostheses. Int J Impot Res. 2000;12(Suppl 4):S101–S107. doi: 10.1038/sj.ijir.3900586. [DOI] [PubMed] [Google Scholar]
- 11.Scott FB, Bradley WE, Timm GW. Management of erectile impotence. Use of implantable inflatable prosthesis. Urology. 1973;2:80–82. doi: 10.1016/0090-4295(73)90224-0. [DOI] [PubMed] [Google Scholar]
- 12.Small MP, Carrion HM, Gordon JA. Small-Carrion penile prosthesis. New implant for management of impotence. Urology. 1975;5:479–486. doi: 10.1016/0090-4295(75)90071-0. [DOI] [PubMed] [Google Scholar]
- 13.Jonas U. Alloplastics in the treatment of erectile dysfunction. In: Jonas U, editor. Erectile dysfunction. New York: Springer-Verlag; 1991. pp. 291–309. [Google Scholar]
- 14.Henry GD. Historical review of penile prosthesis design and surgical techniques: part 1 of a three-part review series on penile prosthetic surgery. J Sex Med. 2009;6:675–681. doi: 10.1111/j.1743-6109.2008.01145.x. [DOI] [PubMed] [Google Scholar]
- 15.Krane R. Penile prostheses in urology. In: White RW, Palmer JM, editors. New techniques in urology. Mount Kisco, NY: Futura Pub Co.; 1987. [Google Scholar]
- 16.Sadeghi-Nejad H. Penile prosthesis surgery: a review of prosthetic devices and associated complications. J Sex Med. 2007;4:296–309. doi: 10.1111/j.1743-6109.2007.00434.x. [DOI] [PubMed] [Google Scholar]
- 17.Hellstrom WJ, Montague DK, Moncada I, Carson C, Minhas S, Faria G, et al. Implants, mechanical devices, and vascular surgery for erectile dysfunction. J Sex Med. 2010;7(1 Pt 2):501–523. doi: 10.1111/j.1743-6109.2009.01626.x. [DOI] [PubMed] [Google Scholar]
- 18.Bettocchi C, Palumbo F, Spilotros M, Palazzo S, Saracino GA, Martino P, et al. Penile prostheses. Ther Adv Urol. 2010;2:35–40. doi: 10.1177/1756287209359174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carson CC., 3rd Efficacy of antibiotic impregnation of inflatable penile prostheses in decreasing infection in original implants. J Urol. 2004;171:1611–1614. doi: 10.1097/01.ju.0000118245.66976.e1. [DOI] [PubMed] [Google Scholar]
- 20.Wolter CE, Hellstrom WJ. The hydrophilic-coated inflatable penile prosthesis: 1-year experience. J Sex Med. 2004;1:221–224. doi: 10.1111/j.1743-6109.2004.04032.x. [DOI] [PubMed] [Google Scholar]
- 21.Mooreville M, Adrian S, Delk JR, 2nd, Wilson SK. Implantation of inflatable penile prosthesis in patients with severe corporeal fibrosis: introduction of a new penile cavernotome. J Urol. 1999;162:2054–2057. doi: 10.1016/S0022-5347(05)68099-8. [DOI] [PubMed] [Google Scholar]
- 22.Levine LA, Hoeh MP. Review of penile prosthetic reservoir: complications and presentation of a modified reservoir placement technique. J Sex Med. 2012;9:2759–2769. doi: 10.1111/j.1743-6109.2012.02807.x. [DOI] [PubMed] [Google Scholar]
- 23.Tausch TJ, Mauck R, Zhao LC, Morey AF. Penile prosthesis insertion for acute priapism. Urol Clin North Am. 2013;40:421–425. doi: 10.1016/j.ucl.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 24.Carson CC. Penile prosthesis implantation in the treatment of Peyronie's disease. Int J Impot Res. 1998;10:125–128. doi: 10.1038/sj.ijir.3900330. [DOI] [PubMed] [Google Scholar]
- 25.Sedigh O, Rolle L, Negro CL, Ceruti C, Timpano M, Galletto E, et al. Early insertion of inflatable prosthesis for intractable ischemic priapism: our experience and review of the literature. Int J Impot Res. 2011;23:158–164. doi: 10.1038/ijir.2011.23. [DOI] [PubMed] [Google Scholar]
- 26.Kendirci M, Gur S, Sikka SC. Gene therapy for erectile dysfunction. Front Biosci. 2005;10:2758–2769. doi: 10.2741/1733. [DOI] [PubMed] [Google Scholar]
- 27.Albersen M, Fandel TM, Lin G, Wang G, Banie L, Lin CS, et al. Injections of adipose tissue-derived stem cells and stem cell lysate improve recovery of erectile function in a rat model of cavernous nerve injury. J Sex Med. 2010;7:3331–3340. doi: 10.1111/j.1743-6109.2010.01875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bivalacqua TJ, Deng W, Champion HC, Hellstrom WJ, Kadowitz PJ. Gene therapy techniques for the delivery of endothelial nitric oxide synthase to the corpora cavernosa for erectile dysfunction. Methods Mol Biol. 2004;279:173–185. doi: 10.1385/1-59259-807-2:173. [DOI] [PubMed] [Google Scholar]
- 29.Burnett AL. Erectile dysfunction management for the future. J Androl. 2009;30:391–396. doi: 10.2164/jandrol.108.006106. [DOI] [PubMed] [Google Scholar]
- 30.Lin CS, Xin Z, Dai J, Huang YC, Lue TF. Stem-cell therapy for erectile dysfunction. Expert Opin Biol Ther. 2013;13:1585–1597. doi: 10.1517/14712598.2013.847085. [DOI] [PubMed] [Google Scholar]
- 31.Rajfer J, Magee T, Gonzalez-Cadavid N. Future strategies for treating erectile dysfunction. Rev Urol. 2002;4(Suppl 3):S48–S53. [PMC free article] [PubMed] [Google Scholar]
- 32.U.S. Food and Drug Administration, Center for Devices and Radiological Health. FDA update on the safety of silicone gelfilled breast implants. Silver Spring, MD: U.S. Food and Drug Administration; 2011. [Google Scholar]
- 33.Darouiche RO, Bella AJ, Boone TB, Brock G, Broderick GA, Burnett AL, et al. North American consensus document on infection of penile prostheses. Urology. 2013;82:937–942. doi: 10.1016/j.urology.2013.05.048. [DOI] [PubMed] [Google Scholar]
- 34.Fishman IJ, Scott FB, Light JK. Experience with inflatable penile prosthesis. Urology. 1984;23(5 Spec No):86–92. doi: 10.1016/0090-4295(84)90249-8. [DOI] [PubMed] [Google Scholar]