Abstract
This study investigated the mechanism underlying Echinacea-mediated induction of CYP1A2, CYP3A4 and MDR1 in terms of human pregnane X receptor (PXR) activation.
Crude extracts and fractions of Echinacea purpurea were tested for PXR activation in HepG2 cells by a reporter gene assay. Quantitative real-time PCR was carried out to determine their effects on CYP1A2 and CYP3A4 mRNA expressions. Capsules and fractions were risk ranked as high, intermediate and remote risk of drug-metabolizing enzymes induction based on EC50 values determined for respective CYPs.
Fractions F1, F2 and capsule (2660) strongly activated PXR with 5-, 4- and 3.5-fold increase in activity, respectively. Echinacea preparations potentiated up-regulation of CYP1A2, CYP3A4 and MDR1 via PXR activation.
Thus E. purpurea preparations cause herb–drug interaction by up-regulating CYP1A2, CYP3A4 and P-gp via PXR activation.
Keywords: Activation, cytochrome P450 enzymes (CYPs), E. purpurea, human liver carcinoma (HepG2), human pregnane xenobiotic receptor (hPXR)
Introduction
Despite the roll out of free combination antiretroviral (ARV) therapy in recent years, the use of herbal products has increased among HIV-infected patients. It is estimated that 50% of HIV-infected patients admit consuming herbal products once in their life time (Behm et al., 2004; Hsiao et al., 2003). Patients claim using these products to ameliorate the purported side effects secondary to ARV therapy and/or improve general well-being. Unfortunately, patients are at risk of experiencing clinically significant herb–drug interactions (HDIs) due to possible concomitant use of ARV and herbal products. For example, disposition of ARV therapies belonging to class of non-nucleotide reverse transcriptase inhibitors, such as nevirapine and efavirenz, and protease inhibitors (PIs), such as ritonavir, are influenced by CYP3A4 and CYP2B6 and P-glycoprotein modulators. Induction of drug-metabolizing enzymes (DME) by herbal products has the potential to reduce plasma concentration of co-administered ARV therapies rendering it inefficacious and/or increase metabolite production to cause systemic toxicity. An investigation by Piscitelli et al. showed that St. John’s wort (SJW) reduced the plasma exposure of indinavir by 57% when co-administered (Piscitelli et al., 2000a). Immunosuppressive agent, cyclosporine, is a substrate for P-gp and CYP3A4, widely used after transplantation with cases of allograft rejection reported when the plasma concentration decline below the effective level in patients taking SJW (Akhlaghi & Trull, 2002).
In spite of their potential for clinically significant interaction with ARV therapies, few herbal products have been tested using in vitro and in vivo models to evaluate their likelihood to cause HDIs via induction pathway. Echinacea species are example of herbal products with an overwhelming popularity in both developed and developing countries. Echinacea preparations are ranked as one of the most popular herbal medications used globally for the management of upper respiratory infections, influenza and common cold. In the United States, the sale of echinacea is ranked number 2 with its sale reaching above $36 million in the year 2006 (SPINS Inc., 2007). Though there are nine different species of Echinacea, most preparations are made from three species, namely Echinacea purpurea, Echinacea angustifolia and Echinacea pallida. Liquid extracts, capsules, tablets, dried roots, creams, gels and teas are commercially available in both pharmacy shops and mushroom stores as over-the-counter preparations. It is, however, estimated that about 80% of Echinacea products currently in the markets are prepared from E. purpurea (Li, 1998). In HIV-infected patients, E. purpurea preparations are predominantly consumed for its immunomodulatory and antiviral properties (Guiotto et al., 2008). The practice of E. purpurea consumption for its purported benefit is gaining popularity in HIV-patients of some sub-Saharan African countries.
Literature search shows that extensive studies have been done on the potential of E. purpurea to inhibit the Cytochrome P450 enzymes (Raner et al., 2007; Yale & Glurich, 2005). However, the mechanism responsible for the potential of E. purpurea to cause HDI in vitro via induction of drug metabolizing enzymes and transporters is unknown. Studies conducted in healthy subjects by Penzak et al. (2010) revealed that E. purpurea significantly induced CYP3A4. Another study performed in healthy volunteers indicated significant systemic clearance of midazolam (CYP3A4 substrates) and oral clearance of caffeine (CYP1A2 substrate) in the presence of E. purpurea (Gorski et al., 2004). However, Echinacea had non-significant effect on the clearance of tolbutamide (CYP2C19 substrate) and dextromethorphan (CYP2D6 substrate). Initial studies by Moradai et al. (2011) indicated that E. purpurea and its constituents have nonsignificant influence on steady state expression of CYP3A4 mRNA in a non-transfected HepG2 cell. This study therefore aims to investigate the plausible underlying mechanism for Echinacea-mediated activation of CYP1A2, CYP3A4 and P-gp using HepG2 cells transfected with HepG2 cell line (Bjornsson et al., 2003).
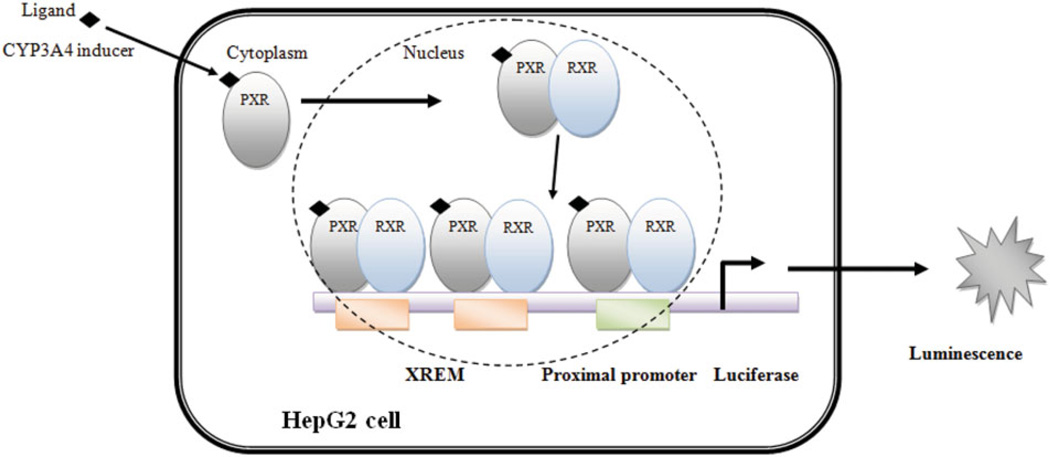
The nuclear receptor, PXR, has been identified as a master regulator of DME (Kliewer et al., 1998). Upon activation by a ligand, PXR translocates from the cytosol to the nucleus of the cell where it forms heterodimer with retinoid X receptor (RXR) (Synold et al., 2001). The PXR/RXR heterodimer complex activates response elements present in the regulatory region of the target genes (Pascussi et al., 2003). Once the transcription of target genes is activated, CYP3A4 expression level increases as illustrated in Figure 1. PXR also regulates the aryl hydrocarbon receptor and its target genes – CYP1A1 and CYP1A2 (Maglich et al., 2002). It is therefore hypothesized that since PXR is the major transcriptional regulator of CYP3A4 and CYP1A2 genes, induction of these target genes by E. purpurea in HIV-infected patients could reduce the systemic exposure of coadministered ARV therapies. CYP3A4 and CYP1A2 account for the metabolism of 54% of the current marketed drugs (Paine et al., 2006).
Figure 1.
Measurement of PXR activation via luciferase reporter gene assay.
Materials and methods
Reagents and biochemicals
Dulbecco’s Modified Eagle medium (DMEM-F12), Minimal Essential medium, Hanks Balanced Salt Solution, HEPES, Trypsin EDTA, penicillin–streptomycin and sodium pyruvate were purchased from GIBCO BRL (Invitrogen Corp., Grand Island, NY). Fetal bovine serum (FBS) was obtained from Hyclone Lab Inc. (Logan, UT). All plastic-wares for mammalian cell culture were purchased from Corning Costar Corp. (Corning, NY). Qiagen Rneasy® mini and micro kits was from Qiagen Ltd. (Austin, TX). TaqMan® Universal PCR Master Mix Reagents, SYBR® Green PCR Master Mix Reagents and TaqMan® probes were obtained from Bio-Rad Laboratories (Hercules, CA). Solid-phase extraction (SPE) cartridges (silica gel 10 g/60 mL, code 57134) were purchased from Supelco (Bellefonte, PA). Organic solvents (chloroform and methanol) were purchased from Fisher Scientific (Fair Lawn, NJ).
Plant material and commercial product
The root of E. purpurea was obtained from the National Centre for Natural Products Research (NCNPR), The University of Mississippi, Oxford. A sample specimen (voucher number 13117) is available at NCNPR. The plant material was air-dried at room temperature and ground into a fine powder. Capsule of E. purpurea root with batch number 130114 was obtained from Herbal solutions (Harare, Zimbabwe).
Capsule extraction
Capsule preparations (200.0 mg) of Echinacea was transferred into test tube and extracted with 1 mL of methanol. The tube containing methanol and the capsule was first shaken by hand, then vortexed for several minutes. The mixture was centrifuged at 16 000 rpm for five minutes at 25 °C. The supernatant was transferred into clean glass tube, and the process was repeated until exhaustive extraction occurred. The supernatant was dried using vacuum pump to obtain dry Echinacea extract. The dried extract reconstituted with methanol extract of E. purpurea capsules (2660) was stored at −20 °C for further use.
Extraction and fractionation of plant material
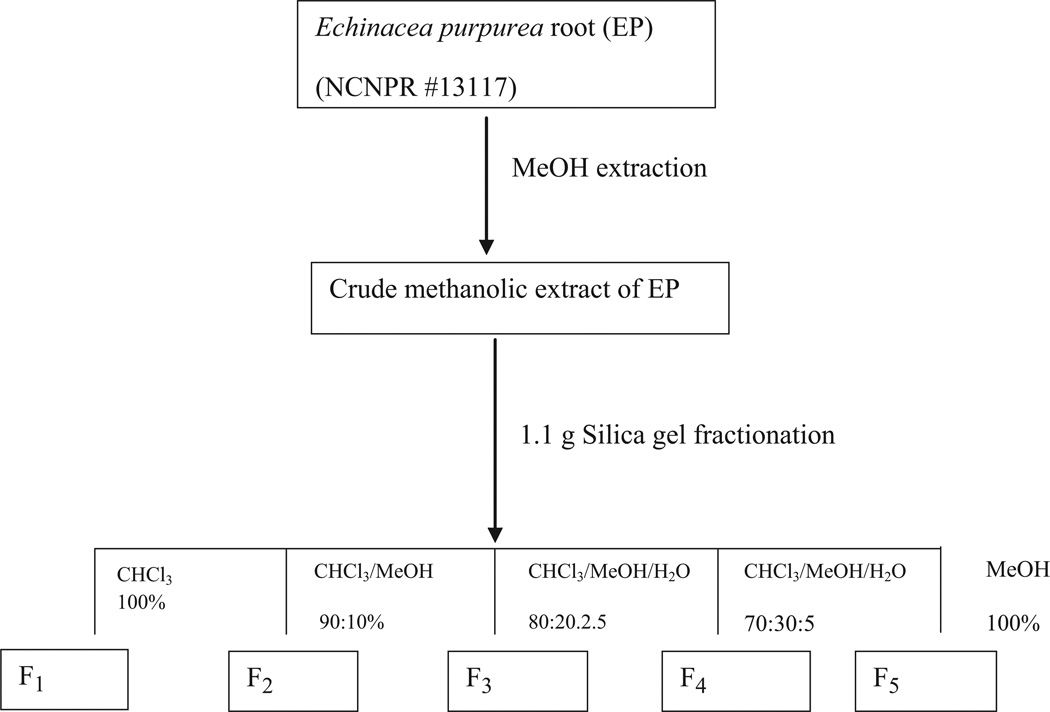
Previously dried and powdered root of E. purpurea (10.0 g) was extracted four times with 100 mL of methanol by sonication (1 h) and filtered. The solvent was evaporated under reduced pressure at 37 °C to give a dried methanolic crude extract (1.15 g). One gram of the obtained methanolic extract was suspended in methanol (10 mL) and adsorbed on celite (1 g). The solvent was evaporated, and the residual material was powdered, loaded onto a 10 g SPE silica gel cartridge and fractionated using CHCl3 (125 mL), CHCl3/MeOH (90:10, 100 mL), CHCl3/MeOH/H2O (80:20:2.5, 200 mL), CHCl3/ MeOH/H2O (70:30:5, 200 mL) and MeOH (150 mL). Fractions of 25 mL were collected and combined according to their TLC profile to give five main fractions: F1 (46.1 mg), F2 (62.1 mg), F3 (75.8 mg), F4 (120.5 mg) and F5 (642.2 mg) as shown in Figure 2. These fractions were tested for their potential to activate PXR in HepG2 cell.
Figure 2.
Schematic representation of fractionation of methanolic crude extract of E. purpurea.
Cell culture
HepG2 cells were cultured in DMEM/F12 supplemented with 10% FBS, 2.4 g/L sodium bicarbonate, 100 U/ml penicillin-G and 100 µg/ml streptomycin at 37 °C, 95% relative humidity and 5% CO2. The culture medium was refreshed every three days. After reaching 80% confluence, the cells were trypsinized and washed twice with DMEM-F12 culture medium before transfection.
Plasmids
The pSG5-PXR expression vector was obtained from Dr. Steven Kliewer (University of Texas Southwestern Medical Center, Dallas, TX; Lehmann et al., 1998), and the reporter plasmid CYP3A4-PXR response element (PXRE)-LUC (containing the proximal 0/−362 and distal 7208/7797 PXRE regions fused upstream of luciferase; Goodwin et al., 1999) was a kind gift from Dr. Christopher Liddle (University of Sydney, Westmead, NSW, Australia). Forward and reverse primers for CYP1A2, CYP3A4 and MDR1 were purchased from Bio-Rad Laboratories.
Evaluation of the cytotoxicity of E. purpurea to HepG2
Cell viability was examined using the MTT assay kit (Life Technologies, Grand Island, NY). HepG2 cells were seeded at 1.0 × 104 cell/100 µL/well and cultured in DMEM/F12 medium containing 10% fetal calf serum, 2 mM glutamine, 100 IU/mL penicillin and 100 µg/mL streptomycin. Cells were grown to 70–80% confluence and placed in serum-free solution for 24 h. The cells were treated with 100, 50 and 25 µg/mL of test samples for 24 h. After the treatment period, the media was aspirated and the cell viability was immediately assessed by first incubating the cells in MTT tetrazolium solution for two hours, followed by measurement of absorbance at 570 nm after removal of untreated dye and addition of 200 µL of DMSO. Cell viability was calculated in comparison to untreated control (Mosmann, 1983).
Transient transfection and luciferase gene reporter assays
The assay was performed as reported earlier (Manda et al., 2014). HepG2 cells (10 × 106 cells) transfected with a luciferase reporter construct (0.4 µg/well) and expression plasmid encoding pSG5-PXR (50 ng/well) by electroporation at 180 V, 1 pulse for 70 msec, were seeded into 96-well plate at a density of 5 × 104 per well. The cells were subsequently maintained in a medium supplemented with 10% FBS containing 2.4 g/L sodium bicarbonate, 100 U/ml penicillin-G and 100 µg/ml streptomycin for 24 h. Test samples and rifampicin (positive control drug) were added to transfected cells at different concentrations and further incubated for 24 h. The media was aspirated from the cells and 40 µL of luciferase reagent (Promega Corporation, Madison, WA) was added to each well, and luminescence was measured using SpectraMax M5 plate reader (Molecular Devices, Sunnyvale, CA).
For time-dependent induction, the aforementioned protocol was repeated. The luciferase activity was determined after 24 and 48 h of incubation with different concentrations of test samples. Luminescence of test samples was determined as background that was subtracted in the final calculation from luminescence of cell samples transiently transfected with reporter plasmids containing response elements of tested genes. The fold induction of each sample was calculated as a ratio between the luminescence of test samples to that of the vehicle-treated cells.
Real-time RT-PCR analysis of CYP3A4, CYP1A2 and P-gp mRNA
HepG2 cells transfected with PXR expression plasmid (400 ng/well) were seeded into 96-well plate at a density of 1.5 × 105 per well. The transfected cells were exposed to different concentrations of test samples for 48 h.
The cells were washed with PBS, and total RNA was extracted using the Quick-start protocol (Qiagen®). The following primers were used for:
CYP3A4, forward (5′-TTCAGCAAGAAGAACAAGGA CAA-3′) and reverse (5′-GGTTGAAGAAGTCCTCCTAA GC-3′) primers; CYP1A2, forward (5′-AAC-AAG-GGA-CAC-AAC-GCT-GAA-T-3′) and reverse (5′-GGA-AGA-GAA-ACA-AGG-GCT-GAG-T-3′) primers; MDR1, forward (5′-TGCTCAGACAGGATGTGAGTTG-3′) and reverse (5′-AATTACAGCAAGCCTGGAACC-3′) primers; and housekeeping genes HPRT (hypoxanthine-guanine phosphoribosyl-transferase), forward (5′-CTGGAAAGAATGTCTTGAT TGTGG-3′), reverse (5′-TTTGGATTATACTGCCTGACCA AG-3′) primers.
cDNA was prepared from 1 µg of total RNA with iScript MMLV-RT (RNaseH+) from Bio-Rad using oligo (dT) random primers and porcine RNase inhibitor. Real-time quantitative polymerase chain reaction (RT-qPCR) was performed in a 96-well plate employing an iCycler iQ™ realtime PCR detector system (Bio-Rad). cDNA (40 ng of reversetranscribed RNA) was amplified with hot-start iTaq DNA polymerase (QIAGEN, Valencia, CA) under the following conditions: 3 mM MgCl2, 0.2 mM deoxynucleoside-5-triphosphate, 0.3 µM each primer, 0.025 U/l polymerase, SYBR Green I in 1:100 000 dilution and fluorescein (10 nM). The thermal cycler was programmed on the realtime PCR instrument using the following experimental conditions: 3 min of polymerase activation and denaturation at 95 °C, DNA amplification consisting of 10–15 s denaturation at 95 °C and 30–60 s annealing/extension with plate reading at optimized temperature (55–60 °C), cycles at 35–40 min and melt curve analysis between 2 and 5 s at 55–95 °C. Test samples were run in triplicate with negative controls. The processing of real-time amplification curves was performed on iCycler software version 4.6 (Bio-Rad). Pfaffl’s method was applied for relative quantification of gene expression normalized to endogenous control (housekeeping) gene (Pfaffl, 2001). Results are presented as the means of at least three experiments. Fold induction was expressed as the ratio between the mRNA expression of treated samples and that of the untreated sample.
Measurement of CYP3A4 and CYP1A2 enzymatic activity
The transfected HepG2 cells were plated with 96-well plates, incubated treated with capsules and fractions of Echinacea (1–100 µg/mL) for 48 h. CYP3A4 and CYP1A2 enzymatic activities were measured using fluorescent probe substrates kits (BD Gentest, Woburn, MA) according to the manufacturer’s instructions. In brief, 100 µL of cofactors mixture, control protein (0.05 mg of protein/mL) and G-6-PDH were added to each well. Initial readings were taken to record any autofluorescence, and the plates were incubated at 37 °C for 30 min after addition of 3 µM 7-ethoxy-3-cyanocoumarin (CYP1A2 substrates) or 10 µM 7-benzyloxy-4-trifluoromethylcoumarin (CYP3A4 substrate) (100 mL) to respective wells (Awortwe et al., 2014; Renwick et al., 2000). The reaction was terminated by the addition of 50 µL of ice-cold acetonitrile/0.5 M Tris base (80:20). Fluorescence was measured on Spectramax M5 plate reader (Molecular Devices) at specified excitation and emission wavelengths for CYP1A2 (410/460 nm) and CYP3A4 (409/530 nm). The EC50 for each fraction was determined by plotting the fold induction against log concentrations of the test samples and the curve fitted by employing non-linear regression using GraphPad Prism.
Data analysis
The values represent the mean ± SEM from three independent experiments except for cytotoxicity testing where each experiment was repeated twice. One-way analysis of variance (ANOVA) was used to determine the significance of difference between untreated group and the treated groups. The Benferroni multi-comparison test was used for group comparisons. Statistical significance was accepted for p values <0.05. One tailed t-test was used to compare time dependent activation of hPXR by the test samples.
Results
Assessment of Echinacea-mediated cytotoxicity
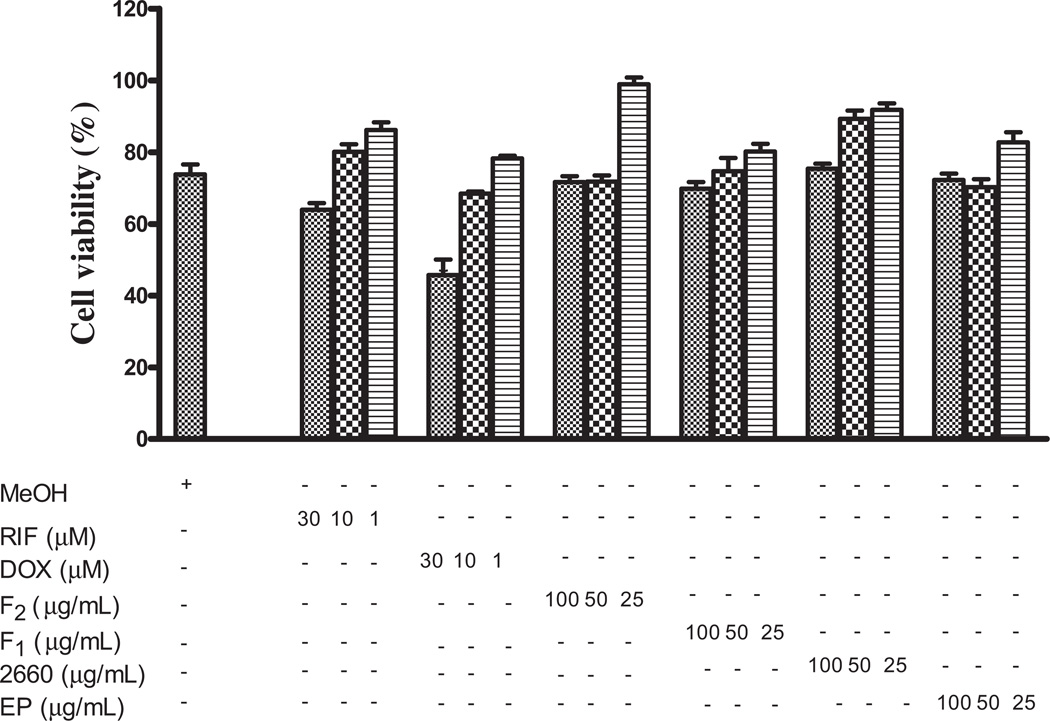
HepG2 cells were exposed to 100, 50 and 25 µg/mL of methanol extracts of E. purpurea plant material, capsules and fractions to assess the effect on HepG2 cell mitochondrial activity applying MTT assay. Echinacea demonstrated non-cytotoxic effect on HepG2 at the concentrations tested as shown in Figure 3.
Figure 3.
Effect of Echinacea on HepG2 cell viability. HepG2 cells were treated for 24 h Echinacea (100, 50 and 25 µg/mL) or rifampicin or doxorubicin (30, 10 and 1 µM). At the end of this period, cell viability was measured as described under Materials and methods. The figure shows the mean of duplicate treatments expressed as a percentage of the value in MeOH-treated cells.
PXR activation by Echinacea fractions
Concentration-dependent PXR activation
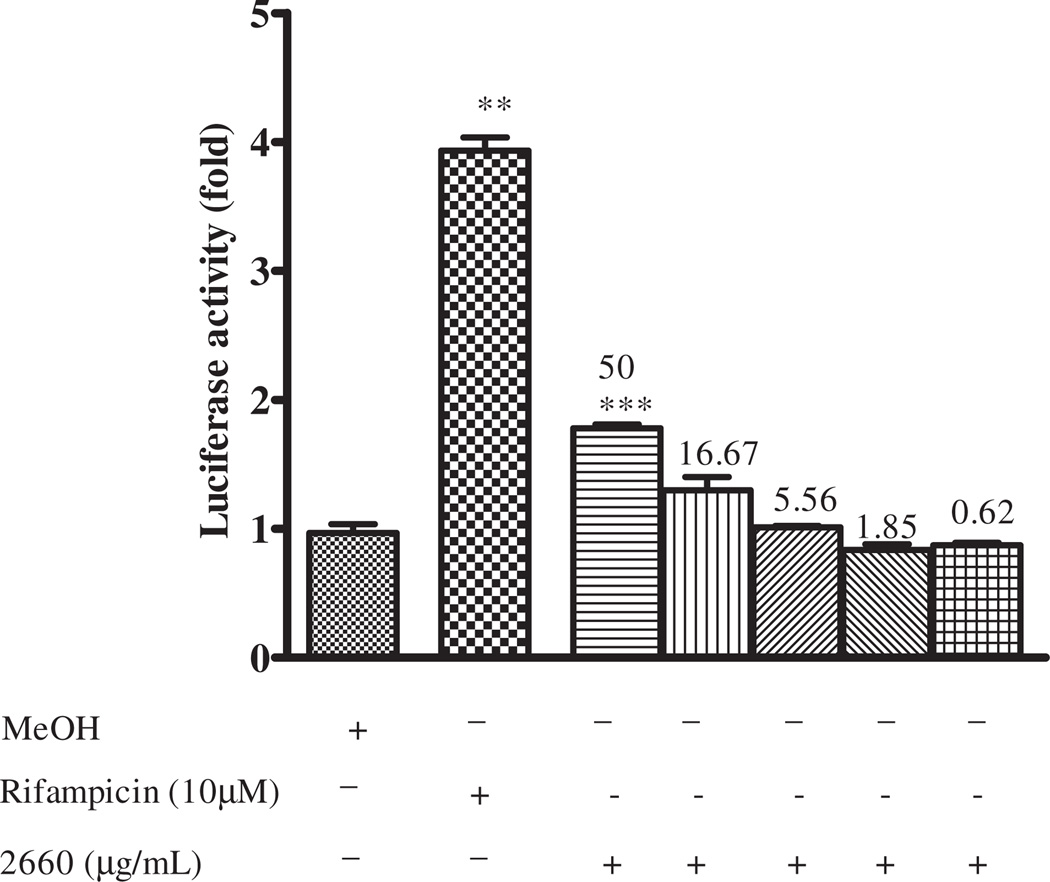
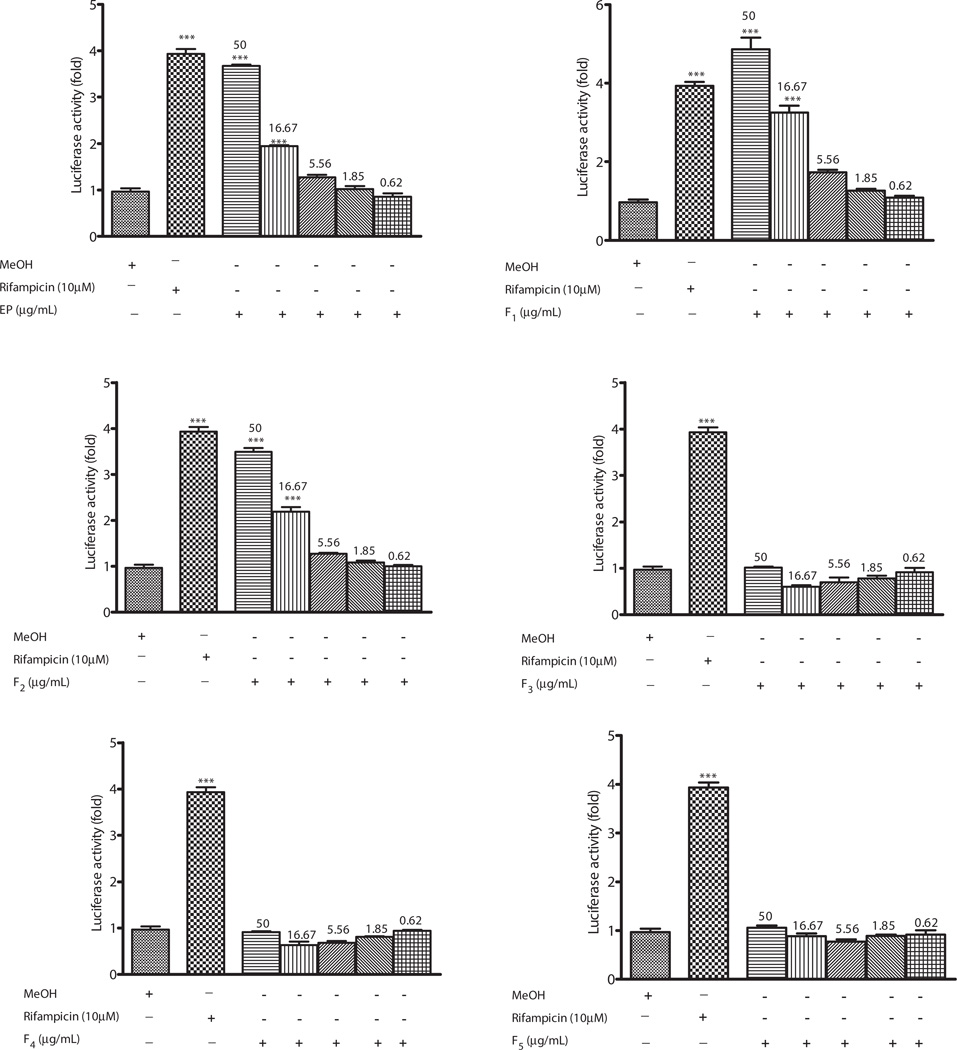
The effects of methanol extract of Echinacea capsule (2660), crude methanol extract of Echinacea (EP) and fractions of Echinacea; F1–F5 on hPXR were assessed in HepG2 cells. PXR-transfected HepG2 cells were treated with test samples for 24h, and fold increase in luciferase activity was measured as an index of PXR activation. Fractions with fold increase of >3, 2.9–2 and <2 were categorized as strong, mild and non activators of PXR. Cells treated with methanol and rifampicin served as vehicle and positive controls, respectively. The methanol extract of Echinacea purpurea capsule activated hPXR with fold induction of 1.9 compared to the vehicle control (Figure 4). To ascertain the active fractions responsible for activation of hPXR, fractions of Echinacea of different polarity were prepared from the methanol extract of whole E. purpurea. EP, F1 and F2 strongly activated hPXR with fold induction of 4-, 5.5-, 3.5-fold, respectively, compared to the vehicle control at 50-µg/mL. At 16.67 µg/mL, F1 caused strong activation of hPXR activity (3.8-fold increase), while EP and F2 mildly activated hPXR (2-fold increase) compared to the vehicle. However, F3, F4 and F5 showed no activation of hPXR. Cytotoxic analysis revealed Echinacea as non-toxic on HepG2 cell (Figure 3).
Figure 4.
Effect of Echinacea purpurea on PXR-mediated transactivation of CYP3A4 promoter PXR response elements. HepG2 cells were transiently transfected with p3A4-luc reporter construct containing the basal promoter (−362/−53) with proximal PXR response element and the distal xenobiotic responsive enhancer module (−7836/−7208) of CYP3A4 (0.4 µg/well) and pSG5-PXR expression vector (50 ng) using the manufacturer’s instructions. Transfected HepG2 cells were maintained in medium containing Echinacea purpurea at the indicated concentrations for 24 h. Luciferase activities are normalized to protein concentration and expressed as fold activation of non-treated cells transfected with p3A4-luc. Results are expressed as mean ± SEM. One-way ANOVA was used to compare the mean value between the vehicle and the treatment groups and Bonferroni multiple comparison was conducted for multiple comparison between the treatment groups when the p < 0.05. **p = 0.01 and ***p = 0.001; 2660 refers to methanol extract of Echinacea purpurea capsule.
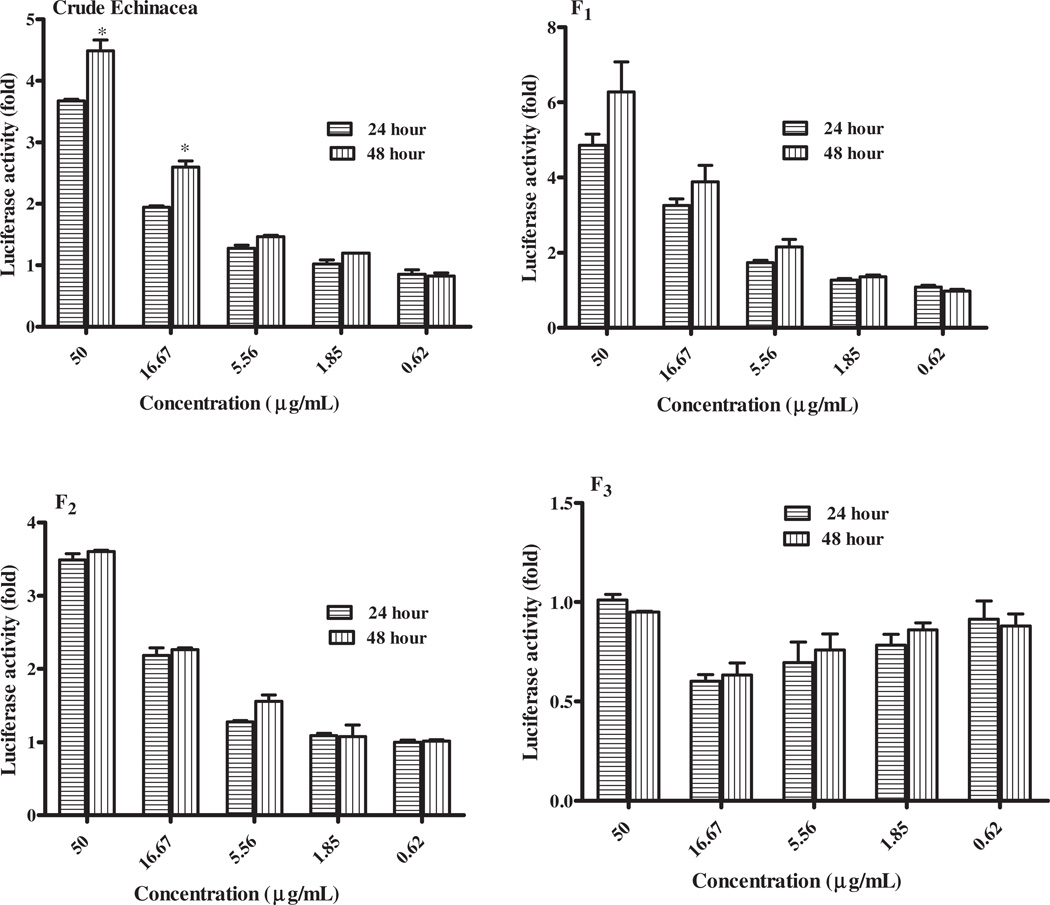
Time-dependent PXR activation
Unlike inhibition, the onset of PXR induction is generally relatively slow and it may take days or even weeks for the full effects to manifest. The effects of various fractions on hPXR activation after 24- and 48-h treatment in HepG2 cells were analyzed. EP and F1 showed 8–10% increase in PXR activation at 48 h compared to 24 h at concentrations ranging from 1.85 to 50 µg/mL. No difference was noted for F2 and F3 at 24 and 48 h (Figure 5). One-tailed t test showed nonsignificant effect of time on activation of PXR by fractions except EP tested at 50 µg/mL and 16.65 µg/mL with p value of 0.047.
Figure 5.
Effect of time on PXR- Echinacea purpurea mediated transactivation of CYP3A4. HepG2 cells were transiently transfected with p3A4-luc reporter construct containing the basal promoter (−362/−53) with proximal PXR response element and the distal xenobiotic-responsive enhancer module (−7836/−7208) of CYP3A4 (0.4 µg/well) and pSG5-PXR expression vector (50 ng) using the manufacturer’s instructions. Transfected HepG2 cells were maintained in medium containing Echinacea purpurea at the indicated concentrations for 24 and 48 h. Luciferase activities are normalized to protein concentration and expressed as fold activation of non-treated cells transfected with p3A4-luc. Two-tailed t test was performed to compare the PXR activation for 24 and 48 h, respectively. *p = 0.047 was noted for EP.
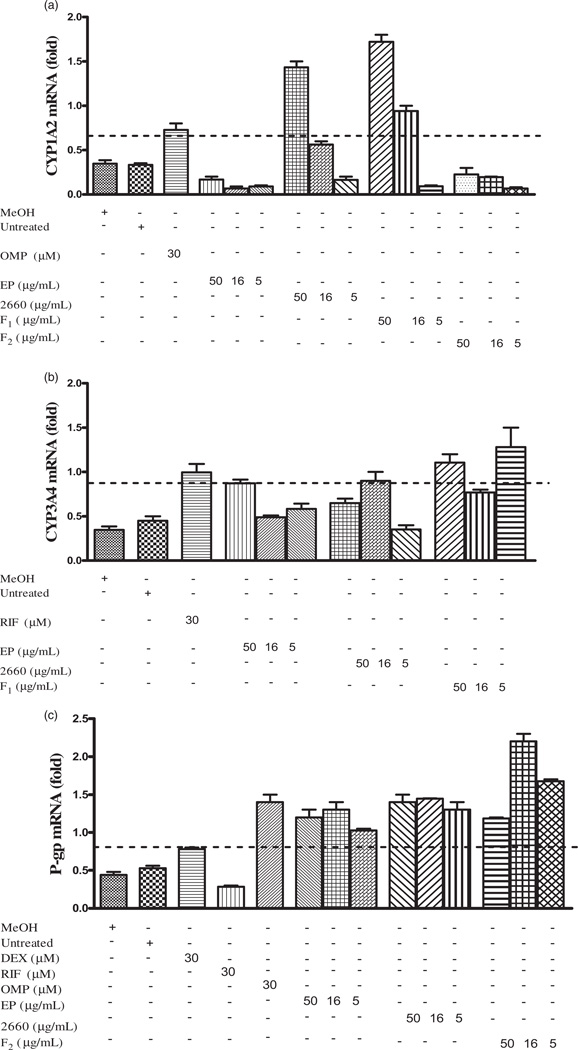
Regulation of CYP1A2, CYP3A4 and P-gp mRNA expression
Crude extracts and fractions of E. purpurea were tested for their potential to activate mRNA expression of CYP1A2, CYP3A4 and P-gp in PXR co-transfected HepG2 cells. The expression of mRNA were used to differentiate CYP or P-gp inducers and non-inducers based on calculation using the normalized ratio of the housekeeping gene after 48-h exposure of hPXR-transfected HepG2 cells to the test samples using RT-qPCR (Figure 6). Test samples with mRNA expression ≥2-fold of the non-treated group were classified as inducers whilst others with mRNA expression cut-off <2-fold of non-treated group were tagged as non-inducers. Crude extract of Echinacea and fraction F2 indicated a non-inducing effect on CYP1A2 mRNA expression in PXR. However, crude extract of Echinacea capsule and active fraction F1 exhibited an induction effect on CYP1A2 mRNA expression at 50 µg/mL. The crude extracts of Echinacea plant material and the capsule demonstrated strong induction effect on CYP3A4 and P-gp mRNA expression in PXR. In addition, a similar effect was observed for the active fraction F1 on both CYP3A4 and P-gp mRNA expressions.
Figure 6.
Analysis of Echinacea-mediated up-regulation of CYP3A4, CYP1A2 and P-gp mRNAs. HepG2 cells were transfected with pSG5-PXR expression plasmids (400 ng/well) using the manufacturer’s instructions and exposed to different concentrations of Echinacea (5.6–50 µg/mL) for 48 h. mRNA expression of tested genes was determined using real-time RT-PCR and normalized to HPRT housekeeping gene. The effect of Echinacea on CYP1A2 (A), CYP3A4 (B) and P-gp (C) mRNA expression is presented as fold increase to HPRT. Test samples with mRNA expression ≥2-fold of untreated is considered as an inducer.
Measurement of CYP3A4 and CYP1A2 enzymatic activity
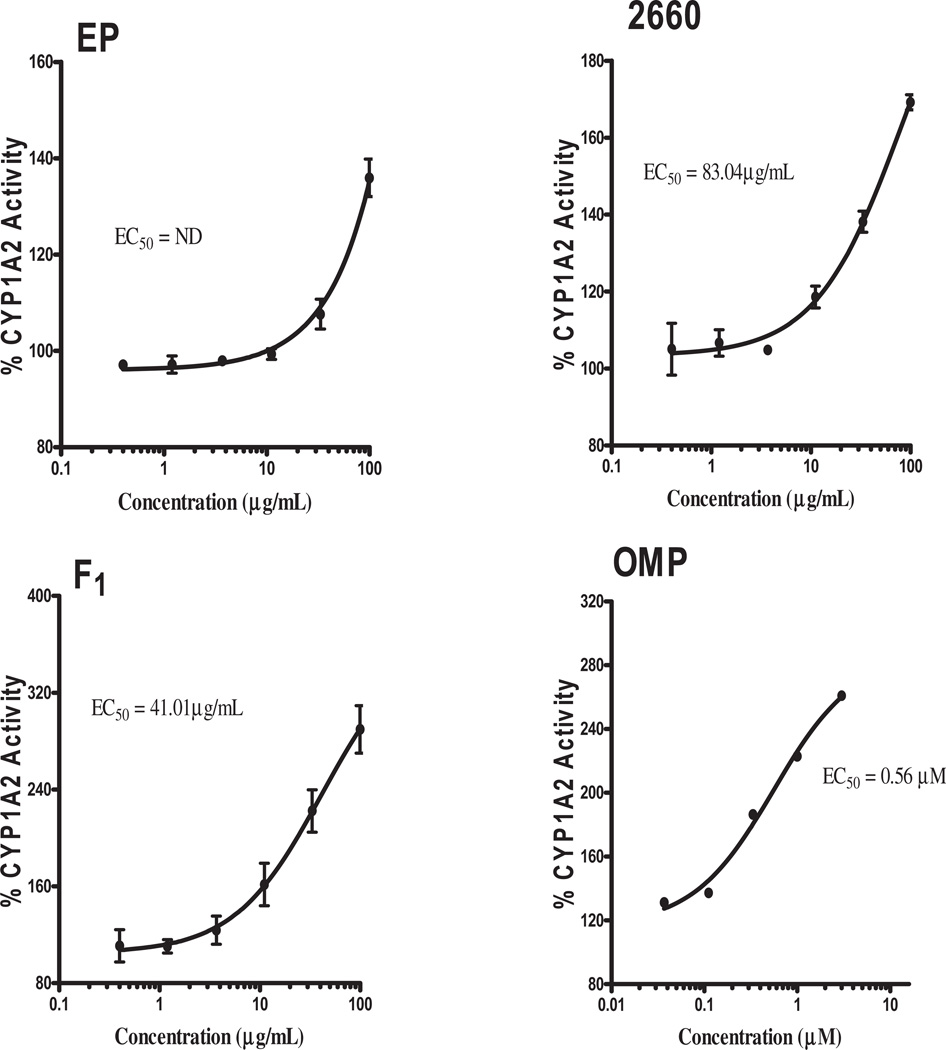
To examine the effect of the test samples on CYP1A2 and CYP3A4, the activities of the enzymes were determined in PXR-transfected HepG2 cells; omeprazole and rifampicin were used as inducers for CYP1A2 and CYP3A4, respectively, using specific fluorescent substrates. Significant induction of CYP1A2 by F1 (EC50 = 41.01 µg/mL) and 2660 (EC50 = 83.04 µg/mL) were observed (Figure 7). None of the fractions of Echinacea showed positive activity on CYP3A4 (data not shown). The Echinacea capsule showed no activity on CYP1A2 at the concentration range tested.
Figure 7.
Determination of CYP1A2 catalytic activity. PXR-transfected cell was incubated with EP fractions for 48 h and CYP1A2 fluorescence substrate –NADPH mixture prepared according to BD Gentest protocol added to the cell for 30 min after aspirating the media containing test samples. Fluorescence of 3-cyano-7-hydroxycoumarin (CHC) was measured and expressed as a percentage of the untreated group to determine fold induction. Log transformation of test sample concentrations against % activity was fitted using non-linear regression and EC50 determined. ND: not determined.
In vivo prediction of HDI from in vitro data
One of the objectives of this study is to rank the potential risk of HDIs according to the US FDA guidelines based on the [I]/ EC50 ratio where [I] is the concentration of the inducer (drug or herb) an individual is exposed to and EC50 is the half of the maximum inducer concentration. In the absence of knowledge of the chemical constituents in the herbs or how much would be absorbed to finally reach the liver and interact with the CYP, we made the worst case assumption that all herbal extract administered would be absorbed. We therefore estimated this concentration by dividing the administered dose by the estimated gastrointestinal tract (GIT) volume of 250 mL. The estimated concentration for each herb is listed in Table 1.
Table 1.
Calculation of herbal medicine concentration in the gut.
| Fraction | Yield (%W/W) |
Estimated extract per dose (mg/mL) |
Putative GIT concentration (µg/mL) |
|---|---|---|---|
| EP | 58.38 | 186.80 | 747.2 |
| 2660 | 23.17 | 74.14 | 296.58 |
| F1 | 4.19 | 13.41 | 53.63 |
Usual human dose (320 mg single dose).
To determine the yield of each sample, specific quantity of test sample was weighed and exhaustively extracted in appropriate solvent followed by drying in a vacuum. The weight of dried sample (extract) was expressed as a percentage of initial material (W/W%) to obtain the yield.
With both the estimated EC50 and [I] values, the [I]/EC50 induction ratio was used to rank the herbs with respect to risk for HDI based on FDA guidelines where, I/EC50 > 1.0 is associated with high risk for drug–drug interaction (DDI), I/EC50 = 0.1–1 is associated with intermediate risk for DDI and I/EC50 < 0.1 is unlikely to result in DDI (Hewitt et al., 2007). The methanol extract of Echinacea capsule and the fraction F1 indicated likely risk of inducing CYP1A2 with I/EC50 values of 3.57 and 1.3, respectively, as listed in Table 2.
Table 2.
In vivo prediction of HDI from in vitro data.
| Test sample | Inhibitor concentration (µg/mL) |
EC50 (µg/mL) |
Induction potency [I]/EC50 |
Risk for HDI |
|---|---|---|---|---|
| EP | 742.20 | ND | ND | |
| 2660 | 296.58 | 83.04 | 3.57 | Likely |
| F1 | 53.63 | 41.01 | 1.3 | Likely |
ND, not determined.
Discussion
Evaluation of the potential of a new drug candidate to cause DDI or HDI has become an integral part of drug development and regulatory review prior to market approval. Although drug interactions can be evaluated using specific clinical studies in healthy subjects or patients, in vitro approaches are now adopted by pharmaceutical and research companies as a critical first step in the assessment of drug interaction potential through specific pathways. Results obtained from in vitro studies can be employed to predict in vivo interaction and guide the need for further in vivo study evaluation. The application of luciferase reporter genes for investigation of mechanisms underlining the induction potential of drugs or xenobiotics have become a routine practice in pharmaceutical industries and dedicated laboratories.
Human PXR-transfected HepG2 cells have been utilized as an in vitro tool to evaluate the potential of new drug candidates to cause clinically significant induction of DME or transporter proteins (Chu et al., 2009). As the use of herbal medicines either as nutritional supplements or medication is on the increase and well established in developing countries, concerns on the risk of HDIs have increased studies on induction effects on CYPs based on the pharmaceutical industry and FDA guidelines. Long-term treatment of rifampicin, an hPXR agonist, in hypertensive patients taking verapamil caused increase hepatic and gastrointestinal levels of CYP3A4, which culminated to a decline in oral bioavailability of (S)-verapamil by 96%, abolishing the anti-hypertensive effect in patients (Fuhr, 2000). SJW and garlic are examples of herbal products that have drawn a lot of attention because of HDI. Previous studies have demonstrated that concurrent use of SJW and garlic can significantly reduce plasma concentration of unboosted PIs (Piscitelli et al., 2000a,b). PXR was identified as a key mediator in SJW interaction with conventional drugs Mannel, 2004). Other herbal products such as Ginkgo biloba, Tian Xian and Coleus forskohlii have been reported as herbs that activate PXR (Ding & Staundinger, 2005; Li et al., 2009). In recent times, E. purpurea has become a popular herbal supplement used as immunostimulant in HIV/AIDS patients. Although in vitro studies have reported E. purpurea ability to induce CYP3A4 and P-gp employing different models, the underlining mechanism is unknown. In this study, we show for the first time (to our knowledge) that E. purpurea up-regulates the CYP1A2, CYP3A4 and MDR1 (P-gp) gene expression via activation of PXR nuclear receptor.
Echinacea purpurea demonstrated a non-cytotoxic effect on HepG2 cell. In addition, it was found in this study that 0.8% methanol had no significant effect on PXR activation. This makes methanol a good solvent to dissolve E. purpurea capsule and fractions. The final concentration did not exceed 0.8% in the experiments. Hence, subsequent outcomes observed in the assays were presumably due to activation or induction effect of Echinacea on PXR. Initial screening of the methanolic extract of E. purpurea (EP) plant material and capsule indicated significant activation of PXR with 3.5- and 2.0-fold increase in luciferase activities, respectively (Figure 4). An herbal supplements showing ≥2-fold increase in luciferase activity is considered as an activator of PXR with the potential to cause HDI (Shimizu, 2010). However, unlike conventional medications, herbal supplements consist of chemical composition, which may differ depending on the place of collection, the season and extraction process. Profiling the chemicals in an herbal supplement is therefore a critical step in understanding the mechanism for HDI. In this study, silica gel fractionation was used to collect six different fractions of increasing polarity. Each fraction was evaluated for its potential to activate PXR. The non-polar fractions; F1 and F2 were the most potent activators of PXR with fold increase in luciferase activity of 5 and 3.5, respectively. The activation effects of F1 and F2 on PXR were significant at 50 µg/mL and 16.67 µg/mL, although dose-dependent activation was observed. However, the activation of PXR by F3, F4 and F5 were non-significant when compared with the control vehicle. The fractions F1–F2 of E. purpurea root showed evidence of alkamides as the principal phytochemicals in a chromatographic scanning (Bauer & Remiger, 1989). Chloroform is the best solvent for the extraction of alkamide, even though both methanol and ethanol can be used. It is therefore not surprising that chloroform fraction F1 showed the strongest activation of PXR.
The activation of CYP and drug transporter is time and concentration dependent. Therefore, the effect of time on the activation of PXR by the fractions of Echinacea was investigated. The results revealed 30–50% increase in PXR activation at 48 h compared to 24 h by EP, F1 and F2 at 50 µg/ mL and 16.67 µg/mL. Comparison of PXR activation between 24 and 48 h showed significant difference at 50 µg/mL for the crude EP. Most HIV/AIDS patients consume E. purpurea as a whole herbal extract or capsule for long duration. The observed significant time-dependent activation of PXR by the crude EP is an indication of its potential to cause HDI during chronic administration.
To analyze the effect of E. purpurea on expression of CYP1A2, CYP3A4 and P-gp genes, primers of respective DME and transporter were introduced into PXR co-transfected HepG2 cell model using the manufacturer’s protocol. In this study, it was observed that Echinacea significantly potentiated up-regulation of CYP1A2, CYP3A4 and MDR1 (P-gp) in PXR co-transfected HepG2 cell model. The outcome of this study agrees with that of Naspinski et al. (2008), which reported that PXR up-regulates the expression of CYP1A2 and CYP3A4. However, in their study, the expression of MDR1 (P-gp) was down-regulated by PXR co-transfected HepG2 (Naspinski et al., 2008). Thus this study speculates that up-regulation of CYP1A2, CYP3A4 and MDR1 genes in activated PXR to be presumably the underlining mechanism of Echinacea-mediated induction reported in humans and other in vitro studies (Gorski et al., 2004; Gurley et al., 2004; Penzak et al., 2010; Yale & Glurich, 2005). However, the observed differences in the activation of CYP1A2 genes by the Echinacea capsule compared to the EP authenticated plant material warrants further investigation to ascertain a complete phytochemical profile. Cases of capsules and tablets of herbal supplements surreptitious with conventional drugs such as benzodiazepine, fluoxetine and furosemide as weight loss products have been reported (de Carvalho et al., 2011).
The adoption of current industry and FDA guidelines for classification of herbal supplements’ potential to cause drug interactions comes with a number of challenges. First, in industry, pure compounds are evaluated whereas for herbal supplements, mixtures of unknown chemical composition are used, making it impossible to know the identity or the concentration of the inducing or activating components. The use of the total GIT concentration of the herbal extract (µg/mL) as the assumed absorbed amount and also responsible for interacting with the CYPs is therefore a gross exaggeration of the likely concentration of inducer or activator components to interact with CYPs. Accounting for plasma protein binding is also not possible with herbal extracts. Second, the EC50 used for herbal extracts is an apparent values associated with complex mixtures of chemicals hence likely to also be a gross exaggeration of these kinetic parameters. Third, industry uses the metabolism based DDI studies to both guide the molecular design of new chemical entity devoid of potential DDI risks during the early hit and lead identification and lead optimization stages. At candidate drug selection stage, the DDI data are used to select compounds devoid of such DDI risk or the design of in vivo DDI studies to determine the extent of the risk of DDI in compounds which still bear the CYP-induction effects. For herbal extracts already in use, the HDI studies are therefore mainly to assess risk, guide the design of in vivo HDI studies and possibly revise product labels to highlight the risk of co-administering some herbs with certain conventional drugs. The effect of SJW on the PI, indinavir is a good example of the likely use of results from this study through the observations done in reverse, that is, observations of PK interactions in vivo (Piscitelli et al., 2001) and working backwards to in vitro studies to understand the underlying mechanism, and eventually have product label recommendation to avoid such HDI (Clauson et al., 2008). The mechanistic approach of using in vitro systems used in this study allows for rapid evaluation of many herbal medicines and it allows for general or class label recommendations with respect to HDI, which can guide patients and clinicians in avoiding drug–herb combinations likely to result in HDI (Awortwe et al., 2014).
Several approaches based on results from the PXR reported gene assay can be used to risk rank the potential of fractions tested to cause HDI. The I/EC50 induction ratio was used to rank the herbs with respect to risk for HDI based on FDA guidelines where, I/EC50 > 1.0 is associated with high risk for DDI, I/EC50 = 0.1–1 is associated with intermediate risk for DDI and I/EC50 < 0.1 is unlikely to result in DDI on CYP1A2. The inclusion of inducer or activator plasma concentration in the risk ranking of compounds reduces the tendency of false-positive or false-negative estimation. Echinacea capsule (2660) and F1 was ranked with high risk to cause DDI in vivo with a ratio of 3.57 and 1.3, respectively, in CYP1A2. The risk ranking correlated with the earlier prediction observed when mRNA expression of CYP1A2 based on positive control. The up-regulation of CYP1A2 mRNA in the presence of Echinacea could presumably reduce systemic bioavailability of conventional drugs such as amitriptyline, acetaminophen and diazepam metabolized by CYP1A2, which are used for the management of peripheral neuropathy in HIV/AIDS patients. Such effect may result to inefficacy, which may warrant the need for increasing the dose of such drugs and therefore likely to precipitate tolerance and on the other hand generate systemic toxicity due to increase metabolite generation.
Conclusion
The high risk of E. purpurea preparations to cause HDI via CYP3A4 and CYP1A2 based on this study demonstrate the PXR-Echinacea mediated induction of DME observed in other studies. Therapeutic agents such as amitriptyline (antidepressants), haloperidol and olanzapine (antipsychotics), theophylline, zileutin (antiasthmatics), efavirenz and nevirapine are CYP1A2 and CYP3A4 substrates employed for management of HIV/AIDS and associated disorders. It is therefore recommend that clinicians should advice HIV/AIDS patients to avoid concurrent intake of E. purpurea and conventional drugs due to possible HDI although the outcome could be delayed.
Acknowledgements
We acknowledge the technical assistance of Olivia R. Dale at NCNPR, USA.
This study was funded by NIH-Fogarty International Center training grant-Brown AIDS International Training and Research Program (Grant# D43TW000237). The United States Department of Agriculture, Agricultural Research Service, Specific Cooperative Agreement No. 58-6408-1-603 is also acknowledged for partial support of this work.
Footnotes
Declaration of interest
All authors have completed the unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare no support from any organization for the submitted work; no financial relationship with any organization that might have interest in the submitted work in the previous three years; and no other relationships or activities that could appear to have influenced the submitted work.
References
- Akhlaghi F, Trull AK. Distribution of cyclosporin in organ transplant recipients. Clin Pharmacokinet. 2002;41:615–637. doi: 10.2165/00003088-200241090-00001. [DOI] [PubMed] [Google Scholar]
- Awortwe C, Bouic PJ, Masimirembwa CM, Rosenkranz B. Inhibition of major drug metabolizing CYPs by common herbal medicines used by HIV/AIDS patients in Africa: implications for herb-drug interactions. Drug Metab Lett. 2014;7:83–95. doi: 10.2174/1872312808666140129123210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer R, Remiger P. TLC and HPLC analysis of alkamides in Echinacea drugs. Planta Med. 1989;55:367–371. doi: 10.1055/s-2006-962030. [DOI] [PubMed] [Google Scholar]
- Behm Dillon DM, Penzak SR, Bailey Klepser T. The use of herbals by patients with HIV. Adv Pharm. 2004;2:41–60. [Google Scholar]
- Bjornsson TD, Callaghan JT, Einolf HJ, et al. Pharmaceutical Research and Manufacturers of America (PhRMA) Drug Metabolism/Clinical Pharmacology Technical Working Group; FDA Center for Drug Evaluation and Research (CDER) The conduct of in vitro and in vivo drug-drug interaction studies: a Pharmaceutical Research and Manufacturers of America (PhRMA) perspective. Drug Metab Dispos. 2003;31:815–832. doi: 10.1124/dmd.31.7.815. [DOI] [PubMed] [Google Scholar]
- Chu V, Einolf HJ, Evers R, et al. In vitro and in vivo induction of cytochrome p450: a survey of the current practices and recommendations: a pharmaceutical research and manufacturers of America perspective. Drug Metab Dispos. 2009;37:1339–1354. doi: 10.1124/dmd.109.027029. [DOI] [PubMed] [Google Scholar]
- Clauson KA, Santamarina ML, Rutledge JC. Clinically relevant safety issues associated with St. John’s wort product labels. BMC Complement Altern Med. 2008;8:42. doi: 10.1186/1472-6882-8-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Carvalho LM, Martini M, Moreira AP, et al. Presence of synthetic pharmaceuticals as adulterants in slimming phytotherapeutic formulations and their analytical determination. Forensic Sci Int. 2011;204:6–12. doi: 10.1016/j.forsciint.2010.04.045. [DOI] [PubMed] [Google Scholar]
- Ding X, Staudinger JL. Induction of drug metabolism by forskolin: the role of the pregnane X receptor and the protein kinase a signal transduction pathway. J Pharmacol Exp Ther. 2005;312:849–856. doi: 10.1124/jpet.104.076331. [DOI] [PubMed] [Google Scholar]
- Fuhr U. Induction of drug metabolising enzymes: pharmacokinetic and toxicological consequences in humans. Clin Pharmacokinet. 2000;38:493–504. doi: 10.2165/00003088-200038060-00003. [DOI] [PubMed] [Google Scholar]
- Goodwin B, Hodgson E, Liddle C. The orphan human pregnane X receptor mediates the transcriptional activation of CYP3A4 by rifampicin through a distal enhancer module. Mol Pharmacol. 1999;56:1329–1339. doi: 10.1124/mol.56.6.1329. [DOI] [PubMed] [Google Scholar]
- Gorski JC, Huang SM, Pinto AP, et al. The effect of echinacea (Echinacea purpurea root) on cytochrome P450 activity in vivo. Clin Pharmacol Ther. 2004;75:89–100. doi: 10.1016/j.clpt.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Guiotto P, Woelkart K, Grabnar I, et al. Pharmacokinetics and immunomodulatory effects of phytotherapeutic lozenges (bonbons) with Echinacea purpurea extract. Phytomedicine. 2008;15:547–554. doi: 10.1016/j.phymed.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Gurley BJ, Gardner SF, Hubbard MA, et al. In vivo assessment of botanical supplementation on human cytochrome P450 phenotypes: Citrus aurantium, Echinacea purpurea, milk thistle, and saw palmetto. Clin Pharmacol Ther. 2004;76:428–440. doi: 10.1016/j.clpt.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Hewitt NJ, de Kanter R, LeCluyse E. Induction of drug metabolizing enzymes: a survey of in vitro methodologies and interpretations used in the pharmaceutical industry - do they comply with FDA recommendations? Chem Biol Interact. 2007;168:51–65. doi: 10.1016/j.cbi.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Hsiao AF, Wong MD, Kanouse DE. Complementary and alternative medicine use and substitution for conventional therapy by HIV-infected patients. J Acquir Immune Defic Syndr. 2003;33:157–165. doi: 10.1097/00126334-200306010-00007. [DOI] [PubMed] [Google Scholar]
- Kliewer SA, Moore JT, Wade L. An orphan nuclear receptor activated by pregnanes defines a novel steroid signaling pathway. Cell. 1998;92:73–82. doi: 10.1016/s0092-8674(00)80900-9. [DOI] [PubMed] [Google Scholar]
- Lehmann JM, Mckee DD, Watson MA, et al. The human orphan nuclear receptor PXR is activated by compounds that regulate CYP3A4 gene expression and cause drug interactions. J Clin Invest. 1998;102:1016–1023. doi: 10.1172/JCI3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Stanton JD, Tolson AH, et al. Bioactive terpenoids and flavonoids from Ginkgo biloba extract induce the expression of hepatic drug-metabolizing enzymes through pregnane X receptor, constitutive androstane receptor, and aryl hydrocarbon receptor mediated pathways. Pharm Res. 2009;26:872–882. doi: 10.1007/s11095-008-9788-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li TSC. Echinacea: cultivation and medicinal value. Hort Technol. 1998;8:122–129. [Google Scholar]
- Maglich JM, Stoltz CM, Goodwin B, et al. Nuclear pregnane x receptor and constitutive androstane receptor regulate overlapping but distinct sets of genes involved in xenobiotic detoxification. Mol Pharmacol. 2002;62:638–646. doi: 10.1124/mol.62.3.638. [DOI] [PubMed] [Google Scholar]
- Manda VK, Dale OR, Awortwe C, et al. Evaluation of drug interaction potential of Labisia pumila (Kacip Fatimah) and its constituents. Front Pharmacol. 2014;5:178. doi: 10.3389/fphar.2014.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannel M. Drug interactions with St John’s wort: mechanisms and clinical implications. Drug Saf. 2004;27:773–797. doi: 10.2165/00002018-200427110-00003. [DOI] [PubMed] [Google Scholar]
- Moradai M, Silva E, Suter A, et al. Safety of herbal medicinal products: Echinacea and selected alkylamides do not induce CYP3A4 mRNA expression. Evid Based Complement Alternat Med. 2011;2011:213021. doi: 10.1093/ecam/nep174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Naspinski C, Gu X, Zhou GD, et al. Pregnane X receptor protects HepG2 cells from BaP-induced DNA damage. Toxicol Sci. 2008;104:67–73. doi: 10.1093/toxsci/kfn058. [DOI] [PubMed] [Google Scholar]
- Paine MF, Hart HL, Ludington SS, et al. The human intestinal cytochrome P450 “pie”. Drug Metab Dispos. 2006;34:880–886. doi: 10.1124/dmd.105.008672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascussi JM, Busson-Le Coniat M, Maurel P, Vilarem MJ. Transcriptional analysis of the orphan nuclear receptor constitutive androstane receptor (NR1I3) gene promoter: identification of a distal glucocorticoid response element. Mol Endocrinol. 2003;17:42–55. doi: 10.1210/me.2002-0244. [DOI] [PubMed] [Google Scholar]
- Penzak SR, Robertson SM, Hunt JD, et al. Echinacea purpurea significantly induces cytochrome P450 3A activity but does not alter lopinavir-ritonavir exposure in healthy subjects. Pharmacotherapy. 2010;30:797–805. doi: 10.1592/phco.30.8.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piscitelli SC, Burstein AH, Chaitt D, et al. Indinavir concentrations and St. John’s wort. Lancet. 2000a;355:547–548. doi: 10.1016/S0140-6736(99)05712-8. [DOI] [PubMed] [Google Scholar]
- Piscitelli SC, Burstein AH, Chaitt D, et al. Indinavir concentrations and St John’s wort. Lancet. 2001;14:357. doi: 10.1016/S0140-6736(99)05712-8. (9263) [DOI] [PubMed] [Google Scholar]
- Piscitelli SC, Burstein AH, Welden N, et al. The effect of garlic supplements on the pharmacokinetics of saquinavir. Clin Infect Dis. 2000b;34:234–238. doi: 10.1086/324351. [DOI] [PubMed] [Google Scholar]
- Raner G, Cornelious S, Moulick K, Wang Y. Effects of herbal products and their constituents on human cytochrome P4502E1 activity. Food Chem Toxicol. 2007;45:2359–2365. doi: 10.1016/j.fct.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renwick AB, Surry D, Price RJ, et al. Metabolism of 7-benzyloxy-4-trifluoromethyl-coumarin by human hepatic cytochrome P450 isoforms. Xenobiotica. 2000;30:955–969. doi: 10.1080/00498250050200113. [DOI] [PubMed] [Google Scholar]
- Shimizu M. Interaction between food substances and the intestinal epithelium. Biosci Biotechnol Biochem. 2010;74:232–241. doi: 10.1271/bbb.90730. [DOI] [PubMed] [Google Scholar]
- SPINS Inc. Schaumburg, Echinacea Sales Report. Philadelphia; 2007. [Google Scholar]
- Synold TW, Dussault I, Forman BM. The orphan nuclear receptor SXR coordinately regulates drug metabolism and efflux. Nat Med. 2001;7:584–590. doi: 10.1038/87912. [DOI] [PubMed] [Google Scholar]
- Yale SH, Glurich I. Analysis of the inhibitory potential of Ginkgo biloba, Echinacea purpurea, and Serenoa repens on the metabolic activity of cytochrome P450 3A4, 2D6, and 2C9. J Altern Complement Med. 2005;11:433–439. doi: 10.1089/acm.2005.11.433. [DOI] [PubMed] [Google Scholar]