Abstract
Objective
To assess the relative effectiveness of an interdisciplinary, family-centered, tertiary-care pediatric weight management program for the treatment of patients with and without cognitive disabilities (CD).
Methods
Retrospective analysis of the clinical database of a tertiary-care pediatric weight management clinic (N=453), extracting data from electronic health records including longitudinal change in weight status (BMI z-score) and frequency of attrition from treatment. Upon review of medical records, children enrolled in the treatment program were classified as having no CD (N=342) or CD (N=111).
Results
At baseline, there were no between-group differences in body mass index (BMI) or BMI z-score. After 4 months of treatment, 66% (299) of patients remained enrolled, and complete case-data was available for 219 children in final analyses. There were no statistically significant differences in attrition between the two groups (no CD vs. CD). Mean change in BMI z-score across all groups was −0.03 ± 0.13, p < 0.001. Change in BMI z-score was significantly greater among patients with cognitive disabilities (−0.07 ± 0.15) compared to those without disabilities (−0.03 ± 0.12) (difference: 0.04, 95% CI: 0.005 to 0.08, p = 0.029). These change estimates were observed after adjusting for processes potentially associated with attrition.
Conclusions
Children with CD treated in an interdisciplinary, family-centered obesity clinic had similar or better outcomes compared to peers without CD. This success may be attributable to the patient-centered nature of this behavioral weight management program, which focused on leveraging the unique strengths and capabilities of each individual patient and family.
Keywords: childhood, obesity, treatment, disabilities
INTRODUCTION
Approximately one-third of children in the United States are either overweight or obese.1 Among children with cognitive disabilities (CD), these rates are significantly higher:2, 3 in one study 17.5% of adolescents with CD were considered obese compared to 13% of adolescents without disabilities.4 More than 2.5 million children in the U.S. have disabilities, and many have disability-associated conditions that can be exacerbated by excess body weight.5 Although obesity treatment within a family-based, multidisciplinary team approach is considered the gold standard,6 there are no official recommendations regarding treatment of obesity in children with disabilities.7
Across the various types of cognitive disabilities there is no consensus on nomenclature, including: developmental disability, neurodevelopmental disability, learning disability, mental retardation, and intellectual disability. Learning disabilities are defined as a discrepancy between academic ability and academic achievement regarding reading, writing, or math;8 intellectual disability is defined as an impaired intellectual functioning (typically measured by an IQ <70) with limited adaptive behaviors.9 For the purposes of this paper, we will use the term ‘cognitive disability’ broadly to designate limitations in children’s ability to process information, which includes specific diagnoses such as autism spectrum disorder, attention deficit hyperactivity disorder (ADHD), specific learning disabilities, and intellectual disabilities.
Obesity can be difficult to manage in patients with disabilities due to inherent differences in nutritional challenges, social factors, and cognitive skills.10 Sensory sensitivities are common among children with disabilities, particularly autism, and families report frequent struggles with behavioral patterns associated with eating (e.g., food refusal and using food as a reward for certain behaviors)11. Social limitations may also influence the ability to manage weight, as families may experience difficulties with transportation, decreased access to medical facilities and health resources, frequent medical appointments, increased time away from work or school, and financial strain.12 Involvement of parents and caregivers in the treatment process may become even more critical in the event that a child is resistant or has a diminished capacity to effectively engage in behavior change strategies.
In 2005, the Surgeon General released a call to action to improve the health of persons with disabilities, including a specific reference to the need for further research and programs relating to obesity.5 However, very little is known regarding optimal management of children with disabilities who also have obesity. Understanding the effectiveness of current weight loss programs for children with disabilities is an important first step in the development of recommendations for management of obesity in children with disabilities.
The purpose of this study was to assess whether an interdisciplinary, family-based, weight management clinic was effective in treating patients with disabilities, with particular attention to change in body mass index (BMI) z-score and attrition. We hypothesized that children with disabilities do as well as children without disabilities, as measured by a similar decrease in BMI z-score, due to the individualized and family-centered nature of the program. BMI13 z-score is commonly chosen as the primary outcome measure for obesity research to account for expected changes over time related to age and sex14. However, due to the additional barriers patients with disabilities face (e.g., appropriateness of treatment facilities, frequent medical appointments, and family stress), we also hypothesized greater attrition for patients with disabilities compared to those without disabilities.
METHODS
Selection Criteria
A retrospective chart review was performed for patients who enrolled in the Brenner FIT (Families in Training) Program between November 7, 2007 and February 11, 2012. Records were examined from the Brenner FIT clinical database and electronic medical records. All patients completing an initial intake visit were included regardless of total number of additional visits completed or total time spent in the program. During the chart review, a single reviewer (CLB) classified all 455 patients according to their disability status. This classification was based on records from physicians and family counselors (licensed clinical social worker or family therapist) during an intensive intake appointment, parent questionnaire responses, and any other health records (i.e., general pediatrician or subspecialty records). During the intake appointment physicians discussed medical history and family counselors discussed how the child was doing in school, whether they received special assistance in schools, and whether the child received any additional services outside of the home. The questionnaire asked parents about past medical history, current medical problems, whether the child sees subspecialist physicians, and whether the child receives any therapies such as physical therapy, occupational therapy, or speech therapy. The Wake Forest University Health Sciences Institutional Review Board (IRB 00007733) approved all procedures.
Brenner FIT
Brenner FIT is an interdisciplinary, family-centered, pediatric weight management clinic in Northwest North Carolina. Children between 2 and 18 years old are referred by their primary care physicians and are seen if they are obese (have a BMI ≥ 95th percentile for age and gender) and have one or more obesity-related medical comorbidities. For 12 months, children and their families meet regularly with a pediatrician, family counselor, dietician, physical therapist, and exercise specialist. After an intake appointment, families participate in biweekly appointments for the first four months of treatment (six treatment visits), and a four-month review visit with the physician to assess weight, treatment progress, and laboratory studies.
The treatment team assists families in identifying unhealthy habits, and provides guidance to help them set realistic goals for changing behaviors and managing their unique weight-related challenges. Based on developmental appropriateness and ability to participate, patients are invited to engage in and direct treatment discussions regarding goal setting and behavioral modification. Families receive individualized instruction to facilitate behavior change, including guidance for healthy eating, grocery shopping, meal tracking, beverage choices, physical activity, and more, based on each family’s needs. Motivational interviewing is used to encourage families to choose and set goals, and to maintain a positive and empathetic discussion. Further details of the Brenner FIT program have been described previously.15,16
Data Collection
Brenner FIT maintains an updated, secure database containing patients’ socio-demographic information, anthropometrics, laboratory work, pertinent medical history, comorbidities and family characteristics. Detailed records are kept in the EMR pertaining to every patient contact, including information regarding progress at the visit, plans for future visits, laboratory results, physical exam findings, and any parent report of interval change in history.
Definitions
Based on information obtained in the chart review, patients were classified for the purposes of this study as having no disability, a cognitive disability, and/or a physical disability. The broad category of cognitive disabilities was chosen because there is no consensus of nomenclature in the literature and it was felt that enough information was not present in the medical records to accurately classify the severity of the CD. Patients with CD were further categorized according to their disability type (listed in Table 2). Patients with a documented IQ < 70 or evidence of severe developmental delay were categorized as intellectually disabled9. Children with a reading disorder (including dyslexia), disorder of written expression, or math disability were classified as learning disabled8. Children with ADHD were included in the cognitive disability category because it was surmised that their inattention would make it more difficult for them to participate effectively in their treatment, similar to other CD. Patients were also classified according to any other diagnoses noted in their record, such as autism, cerebral palsy, Down syndrome, Williams syndrome, or auditory processing disorder, as appropriate. When applicable, patients received more than one classification.
Table 2.
Outcomes by cognitive disability subtypesb at 4 months as compared to patients without that specific disability subtype (N = 219)
| N (%) | Change in Body Mass Index z- score ± SD |
P-Value | |
|---|---|---|---|
| Brenner FIT Study Population | 453 (100) | −0.03 ± 0.13 | |
| Cognitive Disabilities | 111 (24.5) | −0.09 ± 0.17 | 0.03 |
| Attention Deficit Hyperactivity Disorder | 72 (15.9) | −0.06 ± 0.15 | 0.26 |
| Learning disabilities | 54 (11.9) | −0.08 ± 0.16 | 0.07 |
| Intellectual disabilities | 23 (5.1) | −0.13 ± 0.18 | 0.11 |
| Autism spectrum disorder | 12 (2.6) | −0.10 ± 0.2 | 0.64 |
| Cerebral palsy | 4 (0.9) | −0.23 ± 0.29 | 0.36 |
| Auditory processing disorder | 3 (0.7) | NA | NA |
| Down syndrome | 2 (0.4) | −0.03 | NA |
| Williams syndrome | 1 (0.2) | −0.10 | NA |
Some patients were given more than one diagnosis when appropriate
Data Extraction
Data was obtained from the Brenner FIT database including age, gender, language spoken in the home, race, BMI (calculated from height and weight that were measured in clinic), BMI z-score, health insurance status, number of caregivers in the home, number of specialist physicians, number of stressors (although these were not stratified by type of severity), glucose, insulin, total cholesterol, low density lipoprotein cholesterol (LDL-C), high density lipoprotein cholesterol (HDL-C), triglycerides (TG), aspartate aminotransferase (AST), alanine aminotransferase (ALT), systolic blood pressure (SBP), and diastolic blood pressure (DBP).
Outcome Measures
Change in BMI z-score and attrition at four months were the primary outcome measures. Given the wide range of patient ages (2–18 years), BMI z-score was chosen as the primary weight-related outcome, as it provides a more accurate depiction of significant changes in weight status for patients with a very high BMI. Additional outcome variables included change in BMI, and change in laboratory values and vital signs.
Children diagnosed with ADHD accounted for approximately two-thirds of patients in the cognitive disability group. While it was important to include them in this category due to the challenges they present during treatment, as discussed above, the above analyses were also separately performed to a cognitive disability group that did not include children diagnosed with ADHD.
Statistical Analysis
No a priori statistical power calculation was conducted for this analysis. Sample size considerations are based on the available data at the time of analysis. For the purposes of analysis, data were stratified by no disability, physical disability, and/or cognitive disability. Chi-square, Fisher’s exact test, or two-sample t-tests were used as appropriate to compare groups.
Differential dropout could substantially impact change scores, so change estimates are reported using a complete case analysis (i.e., treatment completers) as well as using inverse probability weighting17. Due to small sample sizes, change estimates and inverse probability weighting were not performed on patients with physical disabilities. Inverse probability weighting reduces the potential bias of complete case analyses by estimating the probability that each patient will complete treatment, and then using the inverse of this probability weight to increase the representation of patients who were not likely to have completed treatment. In this way, the processes that influence dropout can be incorporated into a model that adjusts change estimates for missing data. The weights for this dropout model (i.e., yes or no) were estimated using a logistic regression model with the following a priori selected predictors: baseline BMI, age, gender, ethnicity (white or non-white), English language, Medicaid insurance, number of cognitive disabilities, and parental drug, alcohol or cigarette use.
SAS Enterprise Guide© version 4 (Cary, NC) was used to perform all statistical analyses. Results were considered statistically significant if a two-tailed p-value ≤0.05.
RESULTS
Patient Characteristics
During the study period 453 patients were seen for an initial evaluation at Brenner FIT, 111 of which had cognitive disabilities (CD; 24% of the study population) and seven had physical disabilities (1.6%). Due to the lower numbers of patients with physical disabilities, further analysis is not reported for that subgroup. Table 2 describes demographics of the patient population and of patients with CD. There were no statistical differences between groups for gender, insurance status, families with single caregivers, presence of stress, or number of stressors. Patients with CD were predominantly white and English-speaking, and on average were older and had more specialist physicians than patients without CD.
There were no significant differences between patients with CD and patients without CD on initial BMI or BMI z-score (Table 2). Patients with CD had higher initial DBP, total cholesterol, LDL-C, and TG than patients without CD. There were no significant differences between patients with or without CD in initial glucose, insulin, HDL-C, AST, ALT, or SBP.
Patients with ADHD were examined separately from those with CD without ADHD and were found to be older, with an average age of 12.8 years compared to 11.2 years for those with CD and not ADHD (p=0.024). Otherwise, there were no significant differences between patients with ADHD and patients with CD without ADHD in regards to gender, primary language spoken, race, health insurance status, or initial BMI.
Attrition
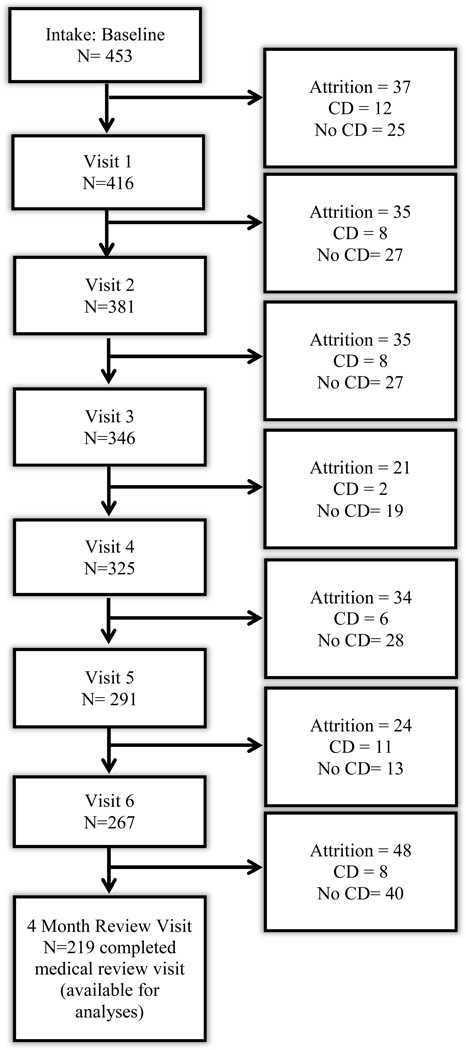
Of the 453 patients who completed a baseline intake visit, 299 (66%) remained active in the treatment program for at least four months, of which 219 completed a medical review visit (Figure 1). Patients were considered active if they attended the intake appointment and their four month visit, regardless of number of additional interim visits.
Figure 1.
Attrition of Study Population at Each Visit
The probability of patient attrition could be predicted with modest accuracy (AUC: 0.652 95% CI: 0.601 to 0.703). Although there were no statistically significant differences in attrition between patients with CD (39% attrition) and other patient groups (33% attrition), several predictors were meaningfully related to patient dropout. Non-white groups were more likely to drop out of treatment (OR: 1.56, 95%CI: 1.0 to 2.4, p = 0.048), as were non-English speakers (OR: 3.5, 95%CI: 1.8 to 6.5, p < 0.0001), and those with Medicaid insurance (OR: 1.7, 95%CI: 1.1 to 2.6, p = 0.013).
Outcomes
Outcomes analyses were available for patients who completed a medical review visit after four months of treatment (n = 219). Table 1 displays the changes in BMI z-score, mean change in BMI z-score across all groups was −0.03 ± 0.13, p < 0.001. This change was within 0.0001 z-score points after adjusting for the inverse probability of attrition (i.e., after considering what the population change might be if those with missing data had completed treatment). Changes in BMI z-score were greater among patients with CD (−0.07±0.15) than those without disabilities (−0.03±0.12) and this difference was statistically significant (difference: 0.04, 95% CI: 0.005 to 0.08, p = 0.029). This difference between disability groups was somewhat diminished after adjusting for attrition, but remained statistically significant (difference: 0.03, 95% CI: 0.008 to 0.06, p = 0.011).
Table 1.
Characteristics of Study Population who Attended an Intake Visit (N = 453)
|
Brenner FIT Totala (N=453) |
Patients with Cognitive Disabilities (N=111) |
Patients without Cognitive Disabilities (N=342) |
P-Value | |
|---|---|---|---|---|
| Mean age, years ± SD* | 11.8 ± 3.4 | 12.4 ± 3.3 | 11.6 ± 3.3 | 0.02 |
| Male Gender, % (N) | 42% (190) | 44% (49) | 41% (141) | 0.58 |
| Primary Language Spoken*, % (N) | 0.02 | |||
| English | 87% (391) | 95% (105) | 84% (286) | |
| Spanish | 13% (60) | 5% (6) | 16% (54) | |
| Other | 0.2% (1) | 0% (0) | 0.2% (1) | |
| Health Insurance, % (N) | 0.91 | |||
| Private | 36% (160) | 36% (40) | 36% (120) | |
| Medicaid | 60% (267) | 60% (66) | 60% (201) | |
| Other | 4% (17) | 4% (5) | 4% (12) | |
| Race *, % (N) | 0.02 | |||
| White | 45% (202) | 57% (63) | 41% (139) | |
| Black | 32% (147) | 29% (32) | 34% (115) | |
| Hispanic/Latino | 18% (81) | 11% (12) | 20% (69) | |
| Other | 5% (23) | 4% (4) | 6% (19) | |
| Single Caregiver, % (N) | 26% (117) | 23% (25) | 27% (92) | 0.36 |
| Number of Specialist Physicians involved in care* ± SD | 0.9 ± 1.2 | 1.3 ± 1.3 | 0.8 ± 1.0 | 0.0003 |
| Presence of Stress, % (N) | 71% (323) | 74% (82) | 70% (241) | 0.49 |
| Number of Stressors ± SD | 1.1 ± 0.9 | 1.2 ± 0.9 | 1.1 ± 0.9 | 0.64 |
| Mean Body Mass Index (BMI), kg/m2 ± SD | 35.9 ± 8.6 | 36.9 ± 9.2 | 35.6 ± 8.4 | 0.17 |
| BMI Z-score ± SD | 2.6 ± 0.5 | 2.5 ± 0.5 | 2.6 ± 0.5 | 0.64 |
Brenner FIT Total includes all patients with and without cognitive disabilities.
Significant difference of cognitive disabilities as compared to patients without cognitive disabilities by chi-square or Fisher’s exact test at p<0.05
There were no statistically significant differences among patients with or without CD regarding changes in most laboratory values (Table 2). However, patients with CD demonstrated significantly greater decreases in DBP. Patients with CD also demonstrated an average 22-point decrease in insulin levels, although this difference was not statistically significant due to the large 95% confidence interval.
Change in BMI z-score was calculated for the various subtypes of CD (Table 3). While each subtype demonstrated the same or greater change in BMI z-score than the overall mean, none of these differences were statistically significant. Patients with ADHD were examined separately from those with CD without ADHD and were found to be have a BMI z-score change that was 0.000015 different from those with CD without ADHD (p=0.9997).
Table 3.
Outcomes of Study Population who Completed Review Visits at 4 months
|
Brenner FIT Total N=219 |
Patients with Cognitive Disabilities N=56 |
Patients without Cognitive Disabilities N=163 |
P-Value | |
|---|---|---|---|---|
| Change in BMI Z-score ± SD | −0.03 ± 0.13 | −0.07 ± 0.15 | −0.03 ± 0.12 | 0.03 |
| Change in BMI ± SD* | 0.2 ± 1.5 | −0.37 ± 1.6 | 0.34 ± 1.3 | 0.001 |
| BMI Z-score improved | 66% (144) | 64% (36) | 66% (108) | 0.79 |
| BMI Improved | 42% (92) | 55% (31) | 37% (61) | 0.01 |
| Enrolled at 4 months | 66% (163) | 61% (34) | 67% (109) | 0.24 |
| Change in Diastolic Blood Pressure* | 0.01 ± 8.4 | −3.8 ± 8.4 | 1.4 ± 7.9 | 0.005 |
| Change in Pulse | −1.6 ± 11.9 | −3.7 ± 10.1 | −0.6 ± 12.7 | 0.30 |
| Change in Glucose | 0.04 ± 11.0 | −2.1 ± 13.8 | 0.8 ± 9.7 | 0.19 |
| Change in Insulin | −8.0 ± 58.4 | −22.6 ± 113.1 | −3.4 ± 20.6 | 0.34 |
| Change in Total Cholesterol | −7.7 ± 24.5 | −11.6 ± 28.3 | −6.1± 22.4 | 0.20 |
| Change in HDL Cholesterol | −2.2 ± 7.2 | −1.9 ± 6.1 | −2.3 ± 7.5 | 0.75 |
| Change in Triglycerides | −16.6 ± 74.4 | −5.5 ± 67.9 | −20.5 ± 76.1 | 0.20 |
| Change in Aspartate Aminotransferase | −2.4 ± 15.7 | 0.5 ± 9.5 | −3.2 ± 17.3 | 0.12 |
Body Mass Index (BMI)
High Density Lipoprotein (HDL)
Significant difference of cognitive disabilities as compared to patients without cognitive disabilities by chi-square or Fisher’s exact test at p<0.05
DISCUSSION
Among children enrolled in weight management, those with cognitive disabilities (CD) exhibited similar or improved outcomes compared to children without disabilities, particularly regarding attrition and change in weight outcomes. Children with CD demonstrated a significantly greater decrease in BMI z-score and mean reduction in BMI overall compared to patients without disabilities. A significantly larger percentage of patients with CD reduced their BMI during four months of treatment compared to children without CD. Contrary to our secondary hypothesis, however, there was no significant difference between-group differences in attrition among those with and without cognitive or physical disabilities.
The change in BMI z-score, both for patients with and without disabilities, is similar to other studies with mean BMI z-score changes between −0.04 and −0.2.18,19,20,21,22 Kirk et al demonstrated that a decrease in BMI z-score (mean −.15±0.15) was significantly related to an improvement in insulin and lipid values21. There is currently no established threshold for clinical significance in change of BMI or BMI z-score in pediatrics. Previous studies have suggested that a decrease in BMI z-score of 0.1020 or simply maintenance of BMI23 may have clinical relevance. In our study, patients with CD had a mean decrease in BMI z-score of 0.07. For 8 and 15 year old boys with a height at the 95th percentile and a BMI at the 99th percentile, a change in BMI z-score of 0.07 would translate to a 3 and 7 pound weight loss, respectively.
We hypothesized that patients with CD would have more dropouts due to differences in social and medical factors; however, attrition was similar for patients whether or not they had CD. The attrition in our study was similar to those of other pediatric weight management clinics.14,24, 25 Although patients with CD did have significantly more specialist physicians (and presumably more frequent doctor appointments and more missed work and school time), we suspect that these families may be more accustomed to scheduling and attending medical appointments due to the nature of their specific health conditions. Our finding of higher attrition among Spanish speakers, non-whites, and Medicaid recipients is contrary to other studies20. This is likely because much of this data was obtained before the addition of a bilingual case manager, and different analytic approaches were used.
Intuitively, patients with disabilities have many barriers to overcome to achieve weight loss compared to patients without disabilities, such as differences in nutritional adaptations and cognitive skills. While these differences can be substantial, our results indicate that they do not seem to diminish patients’ and families’ ability to successfully participate in weight management. This success may be due to the individualized and family-centered approach to care in the Brenner FIT clinic. Given that there is likely a high degree of variability in the needs and behaviors between families, treatment programs that are tailored to each patient and family may help them successfully identify health concerns and ways to modify behaviors unique to their own situations and goals. Implementing strategies to engage other family members is imperative and should be based on their specific needs, such as establishing healthy routines and schedules, setting a realistic pace of treatment progression, and minimizing disruptions in the behavior change process. By providing a diverse range of education topics to address specific topic areas (i.e., parenting, cooking skills, grocery shopping, etc.), all families are capable of achieving healthier lifestyles specific to their interests and needs. Additionally, patient-centered techniques such as motivational interviewing may encourage families to identify realistic goals to pursue.
To our knowledge, this is the first study to examine weight loss outcomes of patients with disabilities in existing pediatric weight management programs. Other authors have recommended implementation of new programs targeted solely towards children with disabilities;10 however, our results indicate that children with disabilities can achieve the same or better outcomes compared to children without disabilities through an existing clinic. Given that children with disabilities and obesity have more secondary complications than their healthy weight cohorts10 it is imperative that specialists and primary care physicians collaborate with their patients to adopt healthy weight behaviors early on in order to prevent the onset of weight related comorbidities. Based on the findings presented here, patients with CD who are overweight or obese can achieve successful weight outcomes relative to their peers without disabilities within a family-centered, interdisciplinary, pediatric weight management clinic.
Limitations
This study has limitations, including a short treatment period. The sustainability of patient weight loss is especially pertinent for children with disabilities, given the importance of establishing healthy habits during childhood to ease the transition to adulthood.26 While developing individualized goals and treatment plans is a strength of Brenner FIT, it may also lead to a treatment-by-indication bias.
Only one reviewer, based on extensive medical records and patient reports, made disability classifications; however, there was a lack of sufficient information to classify all patients by the myriad of all possible diagnoses. An ascertainment bias exists because certain billable conditions, such as ADHD, may be more likely to be listed in patients’ medical record than other problems not requiring medical management, such as developmental delay. The term cognitive disability included a wide range of disability, from ADHD to severe intellectual disability, and it is unclear if the severity of disability is related to the change in BMI z-score or attrition. Additionally, it is not known which patients were on medications that could impact their weight status. For example, children with ADHD may be on stimulants that have been known to cause weight loss, and children with behavioral problems may be on antipsychotic medications that are known to cause weight gain.
The study was retrospective, which is not ideal for studying treatment outcomes, though such designs can provide useful information for treatment programs. The small number of patients with physical disabilities precluded analysis of that population in this study. Lastly, this study only addressed outcome measures obtained from one program, whose approach may not accurately reflect that of other clinics
Future Directions for Research
Although attrition was not different between children with or without CD, it is not known if families had similar reasons for dropping out of the program. Future studies should contain qualitative work to assess what factors influence adherence, with a particular focus on transportation and other well-known barriers in disabled populations. Further studies are needed regarding efficacy of other approaches to pediatric weight management in children with disabilities to truly understand the generalizability of the data and the success of the outcomes reported.
Conclusion
Obesity disproportionately affects children with disabilities and is associated with considerable medical, psychological, and social comorbidities. This study finds that children with CD can be successfully treated in existing family-based, tertiary-care weight management clinics, with comparable outcomes to children without CD. This success is likely to be maximized by focusing treatment on the entire family, concentrating on the strengths and capabilities of each patient, and using behavioral intervention techniques such as motivational interviewing. Children with disabilities should have regular counseling regarding the importance of a healthy diet and physical activity, and children with cognitive disabilities requiring weight management can receive effective treatment within family-centered, interdisciplinary obesity treatment programs like the one presented here.
WHAT’S NEW.
Children with obesity and with cognitive disabilities can be treated effectively in an existing interdisciplinary, family-centered obesity clinic with similar frequency of attrition and greater reductions in BMI z-score than children without CD.
Acknowledgements
The authors would like to thank Karen Klein (Translational Science Institute, Wake Forest School of Medicine) and the NRSA fellows at the University of North Carolina at Chapel Hill for providing helpful edits of this manuscript.
Funding Sources: Supported in part by a grant from NICHD/NIH Mentored Patient-Oriented Research Career Development Award K23 HD061597 (JAS) and from the Health Recourses and Service Administration National Research Service Award (NRSA) grant T32 HP14001 (CLB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ogden CL, Carroll MD, Curtin LR, et al. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. JAMA. 2012 Feb 1;307(5):483–490. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bandini LG, Curtin C, Hamad C, et al. Prevalence of overweight in children with developmental disorders in the continuous national health and nutrition examination survey (NHANES) 1999–2002. J Pediatr. 2005;146:738–743. doi: 10.1016/j.jpeds.2005.01.049. [DOI] [PubMed] [Google Scholar]
- 3.Curtin C, Anderson SE, Must A, et al. The prevalence of obesity in children with autism: a secondary data analysis using nationally representative data from the National Survey of Children’s Health. BMC Pediatr. 2010;10:11. doi: 10.1186/1471-2431-10-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rimmer JH, Yamaki K, Davis BM, et al. Obesity and overweight prevalence among adolescents with disabilities. Prev Chronic Dis. 2011;8(2):A41. [PMC free article] [PubMed] [Google Scholar]
- 5.US Department of Health and Human Services, Office of the Surgeon General. The 2005 Surgeon General’s call to action to improve the health and wellness of persons with disabilities. Rockville (MD): 2005. [PubMed] [Google Scholar]
- 6.Barlow SE. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120(Suppl 4):S164–S192. doi: 10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]
- 7.U.S. Department of Education, Office of Special Education and Rehabilitative Services. Twenty-eighth Annual Report to Congress on the Implementation of the Individuals with Disabilities Education Act. Washington, DC: 2006. [Google Scholar]
- 8.First ME, editor. Diagnostic and Statistical Manual - Text Revision (DSM-IV-TR, 2000) 4th ed. Washington, DC: American Psychiatric Association; 2000. Learning Disorders. [Google Scholar]
- 9.Schalock RL, Borthwick-Duffy SA, Buntinx WHE, et al. Intellectual Disability: Definition, Classification, and Systems of Supports. 11th ed. Washington, DC: American Association on Intellectual and Developmental Disabilities; 2010. [Google Scholar]
- 10.Rimmer JH, Yamaki K, Davis Lowry BM, et al. Obesity and obesity-related secondary conditions in adolescents with intellectual/developmental disabilities. J Intellect Disabil Res. 2010;54:787–794. doi: 10.1111/j.1365-2788.2010.01305.x. [DOI] [PubMed] [Google Scholar]
- 11.Bandini LG, Anderson SE, Curtin C. Food Selectivity in Children with Autism Spectrum Disorders and Typically Developing Children. J Pediatr. 2010;157(2):259–264. doi: 10.1016/j.jpeds.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wells N, Kraus MV, Anderson B, et al. What Do Families Say About Health Care for Children With Special Health Care Needs? Your Voice Counts!! Boston, MA: Family Voices at the Federation for Children with Special Health Care Needs; 2000. [Google Scholar]
- 13.Centers for Disease Control and Prevention, National Center for Health Statistics. CDC growth charts: United States. 2000 May 30; http://www.cdc.gov/growthcharts/.
- 14.Zeller M, Kirk S, Claytor R, Khoury P, et al. Predictors of attrition from a pediatric weight management program. J Pediatr. 2004;144:466–470. doi: 10.1016/j.jpeds.2003.12.031. [DOI] [PubMed] [Google Scholar]
- 15.Irby M, Kaplan S, Garner-Edwards D, et al. Motivational interviewing in a family-based pediatric obesity program: a case study. Fam Syst Health. 2010 Sep;28(3):236–246. doi: 10.1037/a0020101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halvorsen EE, Skelton JA. Appointment Attendance in a Pediatric Weight Management Clinic. Clin Pediatr. 2012 Sep;51(9):888–891. doi: 10.1177/0009922811410876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seaman SR, White IR. Review of inverse probability weighting for dealing with missing data. Statistical Methods in Medical Research. 2013;22(3):278–295. doi: 10.1177/0962280210395740. [DOI] [PubMed] [Google Scholar]
- 18.Oude Luttikhuis H, Baur L, Jansen H, et al. Interventions for treating obesity in children. Cochrane Database Syst Rev. 2009 Jan 21;(1):CD001872. doi: 10.1002/14651858.CD001872.pub2. [DOI] [PubMed] [Google Scholar]
- 19.Cheng JK, Wen X, Coletti KD, et al. 2-Year BMI Changes of Children Referred for Multidisciplinary Weight Management. Int J Pediatr. 2014 doi: 10.1155/2014/152586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haemer MA, Ranade D, Barón AE, Krebs NF. A clinical model of obesity treatment is more effective in preschoolers and Spanish speaking families. Obesity (Silver Spring) 2013 May;21(5):1004–1012. doi: 10.1002/oby.20192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kirk S, Zeller M, Claytor R, Santangelo M, Khoury PR, Daniels SR. The relationship of health outcomes to improvement in BMI in children and adolescents. Obes Res. 2005 May;13(5):876–882. doi: 10.1038/oby.2005.101. [DOI] [PubMed] [Google Scholar]
- 22.Siwik V1, Kutob R, Ritenbaugh C, et al. Intervention in overweight children improves body mass index (BMI) and physical activity. J Am Board Fam Med. 2013 Mar-Apr;26(2):126–137. doi: 10.3122/jabfm.2013.02.120118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Whitlock EP, O’Connor EA, Williams SB, et al. Effectiveness of weight management interventions in children: a targeted systematic review for the USPSTF. Pediatrics. 2010;125(2):396–418. doi: 10.1542/peds.2009-1955. [DOI] [PubMed] [Google Scholar]
- 24.Skelton JA, Beech BM. Attrition in pediatric weight management: a review of the literature and new directions. Obes Rev. 2011 May;12(5):e273–e281. doi: 10.1111/j.1467-789X.2010.00803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williams NA, Coday M, Somes G, et al. Risk Factors for Poor Attendance in a Family-based Pediatric Obesity Intervention Program for Young Children. J Dev Behav Pediatr. 2010 Nov-Dec;31(9):705–712. doi: 10.1097/DBP.0b013e3181f17b1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fleming RK, Stokes EA, Curtin C, et al. Behavioral Health in Developmental Disabilities: A Comprehensive Program of Nutrition, Exercise, and Weight Reduction. Int J Behav Consult Ther. 2008 Jan 1;4(3):287–296. doi: 10.1037/h0100858. [DOI] [PMC free article] [PubMed] [Google Scholar]