Abstract
Purpose/Objective(s)
Imaging biomarkers of resistance to radiation therapy can inform and guide treatment management. Most studies so far have focused on assessing a single imaging biomarker. The goal of this study was to explore a number of different molecular imaging biomarkers as surrogates of resistance to radiation therapy.
Methods and Materials
Twenty-two canine patients with spontaneous sinonasal tumors were treated with accelerated hypofractionated radiation therapy, receiving either 10 fractions of 4.2 Gy or 10 fractions of 5.0 Gy to the GTV. Patients underwent FDG, FLT, and Cu-ATSM PET/CT imaging before therapy, and FLT and Cu-ATSM PET/CT imaging during therapy. In addition to traditional SUV measures (eg, maximum SUV), imaging metrics providing response and spatiotemporal information were extracted for each patient. Progression-free survival was assessed according to RECIST criteria. The prognostic value of each imaging biomarker was evaluated using univariable Cox proportional hazards regression. Multivariable analysis was also performed, but was restricted to two predictor variables due to the limited patient number. The best bivariable model was selected according to Pseudo R2.
Results
The following variables were significantly associated with poor clinical outcome following radiation therapy according to univariable analysis: tumor volume (P=0.011), midtreatment FLT SUVmean (P=0.018), and midtreatment FLT SUVmax (P=0.006). Large decreases in FLT SUVmean from pretreatment to midtreatment were associated with worse clinical outcome (P=0.013). In the bivariable model, the best two-variable combination for predicting poor outcome was high midtreatment FLT SUVmax (P=0.022) in combination with large FLT response from pretreatment to midtreatment (P=0.041).
Conclusions
In addition to tumor volume, pronounced tumor proliferative response quantified using FLT PET, especially when associated with high residual FLT PET at midtreatment, is a negative prognostic biomarker of outcome in canine tumors following radiation therapy. Neither FDG PET nor Cu-ATSM PET were predictive of outcome.
Keywords: PET, radiotherapy, biomarkers
Introduction
Outcome following radiation therapy can be highly variable across different patients, even in tumors with similar clinical presentation [1]. Due to the biological complexity of tumor resistance and its multiple underlying sources, treatment outcome remains difficult to predict. Better methods of predicting outcome following radiation therapy could greatly improve patient management by helping physicians better tailor treatments to patient-specific biology.
There is great interest in identifying biomarkers of tumor resistance to radiation therapy. Numerous studies have investigated genetic, molecular, and anatomical biomarkers of radiation resistance in different tumor types [2–3]. Many of these biomarkers are biopsy-based and therefore limited by sampling, invasiveness, and intratumor heterogeneity. Positron emission tomography (PET) imaging, however, non-invasively provides spatially-resolved quantitative measurements of tumor biological processes. Therefore, there is great promise for molecular imaging biomarkers to improve treatment outcomes in radiation oncology [4].
Several different biological properties are of particular interest as imaging biomarkers. The best established biomarker of tumor resistance to radiation therapy is tumor oxygenation. Highly hypoxic tumors have been shown to respond poorly to radiation therapy [5–7]. PET imaging of tumor hypoxia may also identify patients who will respond poorly to radiation therapy; this has been demonstrated in head-and-neck tumors using the radiotracers 18F-fluoromisonidazole (FMISO) [8] and 18F-fluoroazomycin arabinoside (FAZA) [9], and in cervical [10–11], lung [12], rectal [13], and head-and-neck [14] tumors using the radiotracer copper(II)-diacetyl-bis(N4-methylthiosemicarbazone) (Cu-ATSM).
Measurements of tumor proliferation may also be useful in predicting tumor outcome following radiation therapy. Tumor types with faster proliferation rates and higher growth fractions are generally regarded as more radiosensitive than slow-growing tumors [15]. On the other hand, radiation damage can dramatically increase tumor proliferation rates during the course of therapy, which has been shown to adversely affect patient outcome [16]. Studies have investigated using 3′-deoxy-3′-18F-fluorothymidine (FLT) PET imaging, a marker of proliferative index, at multiple time points over the course of treatment for early treatment response assessment [17–22]. These studies demonstrated that changes in FLT uptake generally precede anatomical response for both radiation therapy and chemotherapy, although the studies found variable relationships between FLT response and clinical outcome.
Baseline levels of glucose metabolism, as measured by 2-deoxy-2-[18F]fluoro-D-glucose (FDG) PET, have been shown to predict outcome following radiation therapy in some tumor sites [23–26]. However, the relationship between FDG uptake and tumor resistance is complex and not well understood, as FDG uptake is influenced by a myriad of biological processes, including hypoxia, inflammation, tumor cell density, and proliferation [27]. On the other hand, the wide availability of FDG PET makes FDG uptake an appealing imaging biomarker for radiation oncology.
Given the multiple factors affecting resistance to radiation therapy, a direct comparison of multiple imaging biomarkers may shed further light on the characteristics of tumor resistance. This study aimed to concurrently evaluate the predictive value of numerous quantitative imaging biomarkers derived from multi-tracer PET imaging in tumors before and during radiation therapy. Canines with spontaneous sinonasal tumors were used as research subjects, wherein patient motion and positioning could be carefully controlled during imaging. In addition to traditional quantitative PET imaging metrics (ie, standardized uptake values), advanced imaging metrics containing spatiotemporal and response information were also tested for their predictive value.
Methods and Materials
Patients
This study included 22 canine patients referred to the XXX. The research protocol was approved by the Animal Care and Use Committee of the XXX, and all canine owners signed a written informed consent. Patients were diagnosed using CT and biopsied for histopathologic verification. All dogs had treatment-naive nasal or paranasal sinus tumors with no evidence of distant metastases or intracranial invasion. Histopathology results were 13 adenocarcinoma, 7 chondrosarcoma, 1 squamous cell carcinoma, and 1 osteosarcoma tumor.
Treatment and imaging
Patients received intensity-modulated radiation therapy (IMRT) delivered by helical tomotherapy with curative intent. Patients were split into two treatment groups as part of a study investigating dose escalation. The first group received the veterinary standard-of-care prescription at our institution: 10 fractions of 4.2 Gy to the planning target volume (PTV) for a total of 42 Gy. The second group was prescribed 10 fractions of 4.2 Gy to the PTV with an integrated boost of 0.8 Gy per fraction to the gross tumor volume (GTV), for a total of 50 Gy to the GTV.
The imaging and treatment schedules for patients are illustrated in Figure 1. Dogs fasted for at least 12 hours before each imaging and treatment session. Patients had pretreatment [18F]FDG, [18F]FLT, and [61Cu]Cu-ATSM PET/CT scans on consecutive days beginning 3 days before the onset of therapy. Patients received a second FLT PET/CT scan after two fractions of IMRT (8.4 or 10 Gy) and a weekend break. Patients received a second Cu-ATSM PET/CT scan following the third fraction of IMRT (12.6 or 15 Gy). One patient missed the midtreatment FLT scan due to equipment failure. Patients were injected with 150–370 MBq of tracer. After injection, patients were kept in a kennel to limit physical activity. PET/CT scans were acquired on a Discovery VCT (GE Healthcare, Waukesha, WI) scanner. FDG and Cu-ATSM PET scans were acquired 60 minutes and 180 minutes post-injection, respectively; both were 20-minute 3D static acquisitions over a single 15 cm bed position. A 180 minute uptake period was used for Cu-ATSM based on preliminary analysis showing greater spatial stability of Cu-ATSM uptake at 180 minutes than at 60 minutes, as described in [28]. FLT scans were 90-minute 3D dynamic acquisitions over a single 15 cm bed position. Patients were anesthetized during imaging and treatment with an initial propofol bolus injection, and then maintained on isoflurane inhalation plus 100% oxygen. Emission data were attenuation corrected and reconstructed using ordered subset expectation maximization (2 iterations, 35 subsets, and 3 mm postfiltering). The image grid was 256 × 256 × 47 with 2.0 × 2.0 × 3.3 mm3 voxel sizes. Voxel activity measurements were converted to standardized uptake values (SUVs) for analysis. For FLT scans, SUVs were calculated by averaging frames from 60–90 minutes; we have previously shown FLT SUV to have high correlations to FLT influx (Ki) from kinetic analysis [29]. To achieve reproducible positioning across PET/CT scans and IMRT treatment sessions, patients’ maxillae were positioned into custom dental molds that were affixed to the scanner and treatment couches, and patients’ bodies were immobilized with vacuum mattresses [30].
Figure 1.
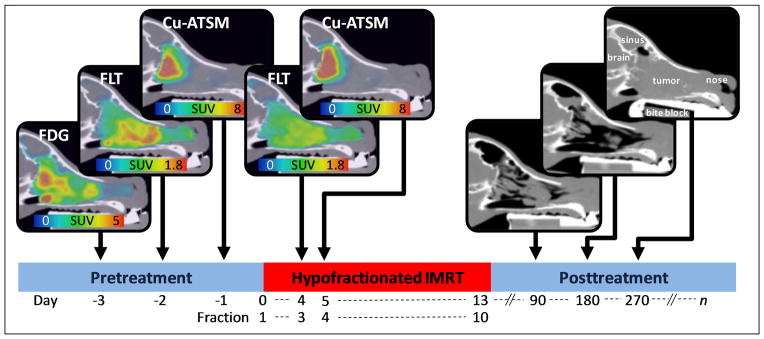
Patients underwent pretreatment FDG, FLT, and Cu-ATSM PET/CT imaging (in no particular order). Midtreatment FLT and Cu-ATSM PET/CT scans were acquired before fractions 3 and 4, respectively. Following therapy, CT scans were acquired at 3, 6, and 9 months, and at time of recurrence (up until progressive disease was detected). Sagittal slices of a canine patient’s PET/CT images are shown above.
Following treatment, patients were scheduled for follow-up CT scans every 3 months for the first 9 months or until patients had progressive disease. Patient response was classified according to the response evaluation criteria in solid tumor (RECIST) [31]. Patients without progressive disease within the first 9 months received an additional CT scan whenever they presented with clinical signs suspicious of recurrence (eg, epistaxis).
Imaging biomarkers
Numerous imaging biomarkers were extracted from each patient’s imaging set. Table 1 describes the imaging biomarkers used for analysis. These included the conventional mean SUV (SUVmean) and maximum SUV (SUVmax) measures at pretreatment and midtreatment, as well as response and spatiotemporal variables. Response variables, quantifying the relative change from pretreatment to midtreatment, were calculated according to
Table 1.
Imaging biomarker definitions.
| Variable | Description | |
|---|---|---|
| Volume | Gross tumor volume (cm3) | |
| SUVmean | Mean SUV in the GTV | |
| SUVmax | Maximum voxel SUV in the GTV | |
|
|
Fractional change in FLT SUVmean from pretreatment to midtreatment | |
|
|
Fractional change in FLT SUVmax from pretreatment to midtreatment | |
|
|
Fractional change in Cu-ATSM SUVmean from pretreatment to midtreatment | |
|
|
Fractional change in Cu-ATSM SUVmax from pretreatment to midtreatment | |
| ρFDG,FLT | Spatial (voxel) correlation between pretreatment FDG and FLT uptake distributions | |
| ρFDG,Cu-ATSM | Spatial (voxel) correlation between pretreatment FDG and Cu-ATSM uptake distributions | |
| ρ FLT,Cu-ATSM | Spatial (voxel) correlation between pretreatment FLT and Cu-ATSM uptake distributions | |
|
|
Spatial (voxel) correlation between pretreatment and midtreatment FLT uptake distributions | |
|
|
Spatial (voxel) correlation between pretreatment and midtreatment Cu-ATSM uptake distributions |
Abbreviations: SUV = standardized uptake value; GTV = gross tumor volume; FLT = 3′-deoxy-3′-18F-fluorothymidine; Cu-ATSM = copper(II)-diacetyl-bis(N4-methylthiosemicarbazone); FDG = 2-deoxy-2-[18F]fluoro-D-glucose
where X is either the SUVmean or SUVmax of tracer T, evaluated at pretreatment and midtreatment. For spatiotemporal variables, voxel-based Spearman correlation coefficients (ρ) quantified the relative spatial agreement between two different tracer distributions, T1 and T2, at baseline (ρT1,T2). Spearman correlation coefficients also quantified the relative spatial stability of a tracer T from pretreatment to midtreatment ( ). The computation of ρ has been described previously [28,32].
Statistical analysis
Progression-free survival was assessed according to the RECIST criteria. Kaplan-Meier analysis with log-rank tests for significance were applied to the following categorical variables: dose level (42 Gy vs. 50 Gy), histologic tumor type (sarcoma vs. carcinoma), sex, and the tumor stage as defined by Adams et al. [33]. For continuous variables, such as patient age and the imaging biomarkers of Table 1, Cox proportional hazards (PH) regression was used to assess their impact on progression-free survival. Univariable Cox PH regression was performed for each continuous variable, and hazard ratios (HR) with their respective 95% confidence intervals (CI) were calculated.
A full multivariable regression model was not developed in this study due to the limited number of patients and the high number of predictor variables. However, we investigated bivariable Cox PH regression models that contained 2 predictor variables, thus following the recommended ratio of ~10 patients per explanatory variable [34–35]. This was done by creating regression models for all possible combinations of 2 explanatory variables, considering both categorical and continuous variables. Of the SUV measures, only SUVmax was used due to the strong correlations between SUVmax and SUVmean. The best bivariable model was selected according to the Pseudo R2 [36]. P values less than 0.05 were considered statistically significant.
Results
The average time to disease progression was 14.3 months, the median was 12.5 months, and the range was 3 months to 35 months. Four cases were censored: two due to death unrelated to disease, and two who were alive without progressive disease at the time of the analysis but had the longest progression-free intervals.
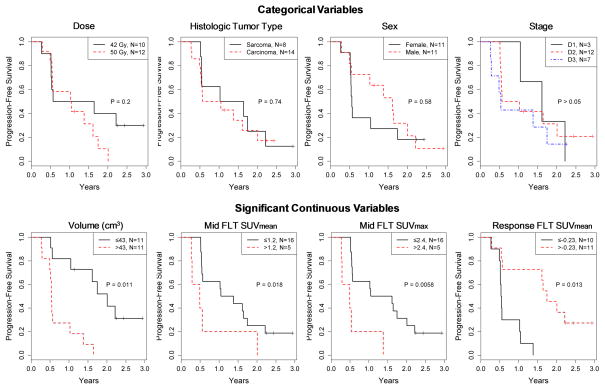
Figure 2 shows results from Kaplan-Meier analysis for categorical variables. Dose level, histologic tumor type, sex, and tumor stage were found not to be significant predictors of time to progression after radiation therapy. Table 2 summarizes the results of univariable Cox PH regression for continuous variables. Large tumor volume (HR [95% CI] = 1.01 [1.00, 1.02]; P=0.011), high midtreatment FLT SUVmean (2.76 [1.19, 6.40]; P=0.018), and high midtreatment FLT SUVmax (1.36 [1.09, 1.68]; P=0.006) were found to be significant independent predictors of worse outcome. Positive values (ie, increases in FLT SUVmean from pretreatment to midtreatment) were significantly associated with better clinical outcome (0.11 [0.02, 0.64]; P=0.013). FDG and Cu-ATSM PET imaging biomarkers were not significant predicators of patient outcome, although FDG SUVmax was borderline significant (P=0.08). None of the spatial imaging biomarkers (ie, voxel correlation coefficients) were significant predictors of outcome. Figure 2 also shows Kaplan-Meier plots (for display purposes only) for the group of continuous variables found to be significant predictors according to univariable analysis.
Figure 2.
Top: Results of Kaplan-Meier analyses with log-rank tests for categorical variables. Categorical variables were not predictive of outcome. Bottom: For display purposes only, variables that were significant predictors of outcome according to Cox proportional hazards (PH) regression were dichotomized using an optimal cut point, and plotted according to Kaplan-Meier method. P values are from Cox PH regression.
Table 2.
Results of univariable Cox proportional hazard regression for continuous variables.
| Variable | Hazard Ratio | 95% Confidence Interval | P | |
|---|---|---|---|---|
| Volume (cm3) | 1.01 | [1.00, 1.02] | 0.011 | |
| Age | 1.01 | [0.87, 1.17] | 0.911 | |
| Pre FDG* | ||||
| SUVmean | 1.12 | [0.91, 1.40] | 0.287 | |
| SUVmax | 1.09 | [0.99, 1.20] | 0.080 | |
| Pre FLT | ||||
| SUVmean | 1.07 | [0.86, 1.35] | 0.543 | |
| SUVmax | 1.02 | [0.95, 1.01] | 0.565 | |
| Pre Cu-ATSM | ||||
| SUVmean | 1.28 | [0.84, 1.94] | 0.245 | |
| SUVmax | 1.08 | [0.95, 1.23] | 0.226 | |
| Mid FLT | ||||
| SUVmean | 2.76 | [1.19, 6.40] | 0.018 | |
| SUVmax | 1.36 | [1.09, 1.68] | 0.006 | |
| Mid Cu-ATSM | ||||
| SUVmean | 1.63 | [0.88, 3.02] | 0.124 | |
| SUVmax | 1.06 | [0.96, 1.17] | 0.288 | |
|
|
0.11 | [0.02, 0.64] | 0.013 | |
|
|
0.15 | [0.02, 1.11] | 0.064 | |
|
|
0.63 | [0.10, 3.97] | 0.620 | |
|
|
0.94 | [0.19, 4.58] | 0.941 | |
| ρFDG,FLT | 5.08 | [0.41, 62.2] | 0.204 | |
| ρFDG,Cu-ATSM | 0.99 | [0.04, 25.8] | 0.997 | |
| ρFLT,Cu-ATSM | 3.82 | [0.62, 23.7] | 0.150 | |
|
|
3.28 | [0.17, 61.6] | 0.428 | |
|
|
2.36 | [0.02, 275] | 0.724 | |
see Table 1 for variable definitions
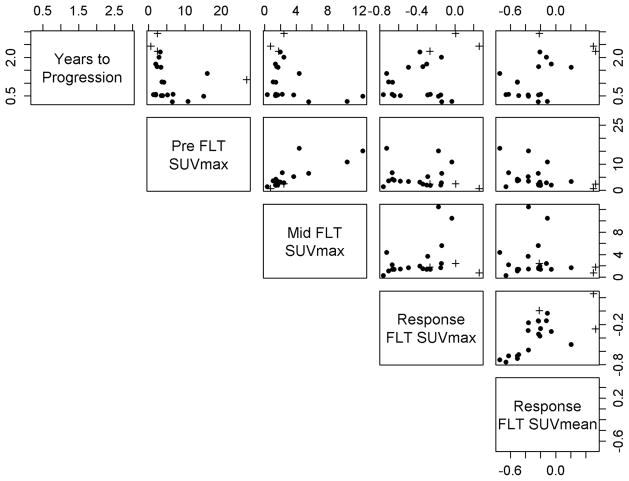
Table 3 presents the two bivariable models with the highest Pseudo R2. Both models indicate that significantly worse outcome was associated with large reductions in FLT uptake (ie, negative response values) in combination with high midtreatment FLT SUVmax. The relationships between patients’ clinical outcome and their various FLT measures are illustrated in the scatter plots of Figure 3.
Table 3.
The two bivariable models with the highest Pseudo R2.
| Variable | Hazard Ratio | P | Pseudo R2 |
|---|---|---|---|
| Model 1 | 0.44 | ||
| Mid FLT SUVmax* | 1.28 | 0.022 | |
| 0.17 | 0.041 | ||
| Model 2 | 0.43 | ||
| Mid FLT SUVmax | 1.38 | 0.002 | |
| 0.11 | 0.042 |
see Table 1 for variable definitions
Figure 3.
Scatter plot matrix illustrating the relationships between patients’ treatment outcome (top row) and various FLT measures. Each column and row corresponds to a different measure, as indicated by the labels in the diagonals. Crosses (+) indicate censored patients.
Discussion
Using multi-tracer PET imaging in canines with spontaneous tumors, we were able to directly compare numerous imaging biomarkers as predictors of time-to-progression after radiation therapy. The precise immobilization of anesthetized canine patients during imaging sessions resulted in motionless image acquisition with repeatable positioning [30]. Furthermore, the bony anatomy surrounding the nasal cavity allowed for very accurate image registration. These conditions enabled us to extract non-conventional spatiotemporal imaging measures, which we compared against conventional SUV measures for their predictive value.
The best predictors of time to progression after radiation therapy were FLT-based biomarkers. FLT response and midtreatment FLT SUV were both found to be significant predictors of outcome in univariable and multivariable analysis. Whereas high midtreatment FLT SUV was found to be associated with worse clinical outcome, the relationship between FLT-response and patient outcome was counter to our expectations: tumors with large relative reductions in FLT uptake had worse outcomes than tumors with small relative changes. FLT response during radiation therapy has been previously investigated in humans [17,19–22]; unfortunately, several of these studies were small and no consistent picture is emerging at this point in time. In a small series of 12 lung cancer patients, Trigonis et al. found that higher midtreatment FLT SUVmax, but not baseline FLT SUVmax, was associated with worse local-regional control, which is consistent with our findings. However, FLT SUV response was not a significant predictor of outcome [17]. In another small study, Wieder et al. did not find significant relationships between histopathological tumor response and FLT SUVmean at baseline or at midtreatment in 10 rectal cancer patients undergoing neoadjuvant chemoradiotherapy [19]. Hoeben et al. found that for 33 head-and-neck cancer patients, early FLT SUV response during radiation therapy was not predictive of patient outcome [21]. However, when they included 15 chemoradiotherapy patients in their analysis, large reductions in FLT SUV became associated with better 3-year disease-free survival (but not overall survival). For tumors treated with chemotherapies, studies have generally found that large FLT reductions during treatment predict better clinical outcome [37–38]. The heterogeneity between the findings of these studies could be due to their limited statistical power compounded by differences in treatment schedules (hypofractionated vs. conventional), imaging time points (our patients were imaged after the second fraction, including a weekend break), treatment type (radiotherapy alone vs. chemoradiotherapy), tumor histology, and species.
It is unclear what radiobiological principles are driving the relationships between FLT biomarkers and patient outcome. It is not surprising that baseline FLT uptake was not significantly associated with clinical outcome in this study, as high baseline proliferation rates have been previously shown to be associated with both favorable and poor clinical outcome following radiation therapy [39]. However, we did find that high FLT uptake at midtreatment was associated with worse clinical outcome. It is tempting to speculate that these tumors could have contained a greater number of remaining viable cells after receiving ~10 Gy of radiation dose, or the population of surviving cells in these tumors were rapidly proliferating. We also found that tumors with large reductions in FLT uptake at midtreatment relative to baseline had a shorter time to progression than tumors with small changes or increases in FLT uptake, and this relationship was independent of pretreatment or midtreatment FLT uptake levels (see Figure 3). This is consistent with a hypothesis where tumors with pronounced responses at the beginning of treatment could be more likely to regrow rapidly following radiation therapy, resulting in shorter progression-free intervals. Indirect support for this hypothesis comes from the recent study by Brink et al., who found that marked tumor regression during fractionated radiation therapy for non-small cell lung cancer was associated with worse clinical tumor outcome in a series of 99 patients [40].
In addition to FLT PET biomarkers, tumor volume was a predictor of adverse clinical outcome following radiation therapy. Dose level, histology, sex, age, and tumor stage were not significant predictors of outcome. Past veterinary studies have found conflicting results on the prognostic significance of tumor histology, patient age, and tumor stage in canine nasal tumors following radiation therapy [33,41–42]. It is possible that the low patient number, and thus the low statistical power, prevented us from detecting predictive relationships that would have been observed in a larger population. Interestingly, we did not find Cu-ATSM and FDG biomarkers to be significant predictors of outcome; although, FDG SUVmax was borderline significant (P=0.08). Even though Cu-ATSM uptake has been shown to have inconsistent correlations with hypoxia in rodent tumor models [43], several clinical studies have found Cu-ATSM uptake to be a prognostic biomarker of response to radiation therapy [10–14]. Therefore, it was surprising that Cu-ATSM uptake did not predict outcome in this study. It is possible that Cu-ATSM uptake in canines is not directly comparable to Cu-ATSM uptake in humans. For example, Cu-ATSM has lower binding affinity to serum albumin in canines than in humans [44]. Also, the use of anesthesia and oxygen during imaging may have reduced Cu-ATSM uptake levels, as has been demonstrated in rodents [45] (the dogs were awake and breathing air, however, for most of the 3-hour uptake period). On the other hand, Hansen et al. found that canine tumors with high levels of pimonidazole staining generally had high uptake of Cu-ATSM as well, supporting its use as a surrogate marker of hypoxia in canines [46].
None of the spatial imaging biomarkers were significantly associated with patient outcome. The baseline voxel correlation coefficients (ρFDG,FLT, ρFDG,Cu-ATSM, and ρFLT,Cu-ATSM) represent how similar the spatial distributions of the three tracers were, so that a low correlation coefficient indicates spatial heterogeneity of phenotypes within a tumor [32]. We hypothesized that higher PET heterogeneity would predict worse response to therapy; however, this was not supported by our analysis. The voxel correlations from pretreatment to midtreatment ( and ) represent the spatial stability of Cu-ATSM and FLT uptake distributions during treatment. XXX [28]. We hypothesized that larger spatial stability of a tumor’s Cu-ATSM or FLT maps during treatment might be associated with tumor resistance to radiation. However, we did not find PET spatial stability to be significantly associated with clinical outcome.
The cost and logistics of conducting multiple scans restricted the total sample size in this exploratory study. This in turn limited our ability to investigate biomarkers in subpopulations of tumors (eg, sarcomas), and to create a complex multivariable model. Multivariable models can become unstable when there are less than 10 observations per explanatory variable [34–35]. Therefore, we tested bivariable models, selected according to a goodness of fit statistic. Another caveat of the study is the large number of hypotheses tested in this study, which increases the likelihood of type I error. Consequently, these results should be considered as hypothesis-generating, and need to be tested in a larger cohort. It is also uncertain how these results will translate to humans, as canine and human tumors can differ substantially in biology. However, canine tumors are more similar in growth rate, vascularity, and treatment response to human tumors than are murine tumors, and represent a valuable model for cancer research [47].
Conclusions
Using extensive imaging of spontaneous tumors in canines, we explored a number of molecular imaging biomarkers as potential predictors of resistance to radiation therapy. In addition to tumor volume, pronounced tumor proliferative response measured with FLT PET, especially when associated with high residual FLT PET at midtreatment, was a predictive biomarker of poor outcome following radiation therapy. Neither FDG PET nor Cu-ATSM PET were significant predictors of outcome.
Summary.
Imaging biomarkers of resistance to radiation therapy can help guide treatment decisions and improve patient outcome. This study tested multiple quantitative imaging biomarkers derived from FDG, FLT, and Cu-ATSM PET imaging as predictors of resistance to radiation therapy in canines with spontaneous sinonasal tumors. We found FLT PET biomarkers, especially those acquired during treatment, to be the most predictive of patient outcome following radiation therapy.
Acknowledgments
Financial Support: The study and most authors were supported by R01 CA136927; Søren Bentzen is supported in part by grant no. P30 CA 134274-04 from the NCI
Footnotes
Conflicts of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hedman M, Bjork-Eriksson T, Mercke C, et al. Comparison of predicted and clinical response to radiotherapy: a radiobiology modelling study. Acta Oncol. 2009;48:584–590. doi: 10.1080/02841860802637757. [DOI] [PubMed] [Google Scholar]
- 2.Chen M, Jiang G-L, Fu X-L, et al. Prognostic factors for local control in non-small-cell lung cancer treated with definitive radiation therapy. Am J Clin Oncol. 2002;25:76–80. doi: 10.1097/00000421-200202000-00016. [DOI] [PubMed] [Google Scholar]
- 3.Bibault J-E, Fumagalli I, Ferte C, et al. Personalized radiation therapy and biomarker-driven treatment strategies: a systematic review. Cancer metastasis reviews. 2013;32:479–492. doi: 10.1007/s10555-013-9419-7. [DOI] [PubMed] [Google Scholar]
- 4.Van de Wiele C, Lahorte C, Oyen W, et al. Nuclear medicine imaging to predict response to radiotherapy: a review. Int J Radiat Oncol Biol Phys. 2003;55:5–15. doi: 10.1016/s0360-3016(02)04122-6. [DOI] [PubMed] [Google Scholar]
- 5.Brizel DM, Sibley GS, Prosnitz LR, et al. Tumor hypoxia adversely affects the prognosis of carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 1997;38:285–289. doi: 10.1016/s0360-3016(97)00101-6. [DOI] [PubMed] [Google Scholar]
- 6.Nordsmark M, Bentzen SM, Rudat V, et al. Prognostic value of tumor oxygenation in 397 head and neck tumors after primary radiation therapy. An international multi-center study. Radiother Oncol. 2005;77:18–24. doi: 10.1016/j.radonc.2005.06.038. [DOI] [PubMed] [Google Scholar]
- 7.Nordsmark M, Overgaard M, Overgaard J. Pretreatment oxygenation predicts radiation response in advanced squamous cell carcinoma of the head and neck. Radiother Oncol. 1996;41:31–39. doi: 10.1016/s0167-8140(96)91811-3. [DOI] [PubMed] [Google Scholar]
- 8.Rajendran JG, Schwartz DL, O’Sullivan J, et al. Tumor hypoxia imaging with [F-18] fluoromisonidazole positron emission tomography in head and neck cancer. Clin Cancer Res. 2006;12:5435–5441. doi: 10.1158/1078-0432.CCR-05-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mortensen LS, Johansen J, Kallehauge J, et al. FAZA PET/CT hypoxia imaging in patients with squamous cell carcinoma of the head and neck treated with radiotherapy: results from the DAHANCA 24 trial. Radiother Oncol. 2012;105:14–20. doi: 10.1016/j.radonc.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 10.Dehdashti F, Grigsby PW, Lewis JS, et al. Assessing tumor hypoxia in cervical cancer by PET with 60Cu-labeled diacetyl-bis(N4-methylthiosemicarbazone) J Nucl Med. 2008;49:201–205. doi: 10.2967/jnumed.107.048520. [DOI] [PubMed] [Google Scholar]
- 11.Dehdashti F, Grigsby PW, Mintun MA, et al. Assessing tumor hypoxia in cervical cancer by positron emission tomography with 60Cu-ATSM: relationship to therapeutic response-a preliminary report. Int J Radiat Oncol Biol Phys. 2003;55:1233–1238. doi: 10.1016/s0360-3016(02)04477-2. [DOI] [PubMed] [Google Scholar]
- 12.Dehdashti F, Mintun MA, Lewis JS, et al. In vivo assessment of tumor hypoxia in lung cancer with 60Cu-ATSM. Eur J Nucl Med Mol Imaging. 2003;30:844–850. doi: 10.1007/s00259-003-1130-4. [DOI] [PubMed] [Google Scholar]
- 13.Dietz DW, Dehdashti F, Grigsby PW, et al. Tumor hypoxia detected by positron emission tomography with 60Cu-ATSM as a predictor of response and survival in patients undergoing Neoadjuvant chemoradiotherapy for rectal carcinoma: a pilot study. Dis Colon Rectum. 2008;51:1641–1648. doi: 10.1007/s10350-008-9420-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Minagawa Y, Shizukuishi K, Koike I, et al. Assessment of tumor hypoxia by 62Cu-ATSM PET/CT as a predictor of response in head and neck cancer: a pilot study. Ann Nucl Med. 2011;25:339–345. doi: 10.1007/s12149-011-0471-5. [DOI] [PubMed] [Google Scholar]
- 15.Hall EJ, Giaccia AJ. Radioboiology for the Radiologist. Philadeplphia, PA: Lippincott Williams & Wilkins; 2006. [Google Scholar]
- 16.Kim JJ, Tannock IF. Repopulation of cancer cells during therapy: an important cause of treatment failure. Nat Rev Cancer. 2005;5:516–525. doi: 10.1038/nrc1650. [DOI] [PubMed] [Google Scholar]
- 17.Trigonis I, Koh PK, Taylor B, et al. Early reduction in tumour [(18)F]fluorothymidine (FLT) uptake in patients with non-small cell lung cancer (NSCLC) treated with radiotherapy alone. Eur J Nucl Med Mol Imaging. 2014;41:682–693. doi: 10.1007/s00259-013-2632-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Troost EG, Bussink J, Hoffmann AL, et al. 18F-FLT PET/CT for early response monitoring and dose escalation in oropharyngeal tumors. J Nucl Med. 2010;51:866–874. doi: 10.2967/jnumed.109.069310. [DOI] [PubMed] [Google Scholar]
- 19.Wieder HA, Geinitz H, Rosenberg R, et al. PET imaging with [18F]3′-deoxy-3′-fluorothymidine for prediction of response to neoadjuvant treatment in patients with rectal cancer. Eur J Nucl Med Mol Imaging. 2007;34:878–883. doi: 10.1007/s00259-006-0292-2. [DOI] [PubMed] [Google Scholar]
- 20.Menda Y, Boles Ponto LL, Dornfeld KJ, et al. Kinetic analysis of 3′-deoxy-3′-(18)F-fluorothymidine ((18)F-FLT) in head and neck cancer patients before and early after initiation of chemoradiation therapy. J Nucl Med. 2009;50:1028–1035. doi: 10.2967/jnumed.108.058495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoeben BAW, Troost EGC, Span PN, et al. 18F-FLT PET During Radiotherapy or Chemoradiotherapy in Head and Neck Squamous Cell Carcinoma Is an Early Predictor of Outcome. J Nucl Med. 2013;54:532–540. doi: 10.2967/jnumed.112.105999. [DOI] [PubMed] [Google Scholar]
- 22.Vera P, Bohn P, Edet-Sanson A, et al. Simultaneous positron emission tomography (PET) assessment of metabolism with 18F-fluoro-2-deoxy-d-glucose (FDG), proliferation with 18F-fluoro-thymidine (FLT), and hypoxia with 18fluoro-misonidazole (F-miso) before and during radiotherapy in patients with non-small-cell lung cancer (NSCLC): a pilot study. Radiother Oncol. 2011;98:109–116. doi: 10.1016/j.radonc.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 23.Ulger S, Demirci NY, Eroglu FN, et al. High FDG uptake predicts poorer survival in locally advanced nonsmall cell lung cancer patients undergoing curative radiotherapy, independently of tumor size. J Cancer Res Clin Oncol. 2014;140:495–502. doi: 10.1007/s00432-014-1591-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horne ZD, Clump DA, Vargo JA, et al. Pretreatment SUVmax predicts progression-free survival in early-stage non-small cell lung cancer treated with stereotactic body radiation therapy. Radiat Oncol. 2014;9:41. doi: 10.1186/1748-717X-9-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu WS, Wu MF, Tseng HC, et al. The role of pretreatment FDG-PET in nasopharyngeal carcinoma treated with intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2012;82:561–566. doi: 10.1016/j.ijrobp.2010.11.055. [DOI] [PubMed] [Google Scholar]
- 26.Allal AS, Slosman DO, Kebdani T, et al. Prediction of outcome in head-and-neck cancer patients using the standardized uptake value of 2-[18F]fluoro-2-deoxy-D-glucose. Int J Radiat Oncol Biol Phys. 2004;59:1295–1300. doi: 10.1016/j.ijrobp.2003.12.039. [DOI] [PubMed] [Google Scholar]
- 27.Bos R, van Der Hoeven JJM, van Der Wall E, et al. Biologic correlates of (18)fluorodeoxyglucose uptake in human breast cancer measured by positron emission tomography. J Clin Oncol. 2002;20:379–387. doi: 10.1200/JCO.2002.20.2.379. [DOI] [PubMed] [Google Scholar]
- 28.XXX
- 29.XXX
- 30.XXX
- 31.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 32.XXX
- 33.Adams WM, Kleiter MM, Thrall DE, et al. Prognostic significance of tumor histology and computed tomographic staging for radiation treatment response of canine nasal tumors. Vet Radiol Ultrasound. 2009;50:330–335. doi: 10.1111/j.1740-8261.2009.01545.x. [DOI] [PubMed] [Google Scholar]
- 34.Concato J, Peduzzi P, Holford TR, et al. Importance of events per independent variable in proportional hazards analysis. I. Background, goals, and general strategy. J Clin Epidemiol. 1995;48:1495–1501. doi: 10.1016/0895-4356(95)00510-2. [DOI] [PubMed] [Google Scholar]
- 35.Peduzzi P, Concato J, Feinstein AR, et al. Importance of events per independent variable in proportional hazards regression analysis. II. Accuracy and precision of regression estimates. J Clin Epidemiol. 1995;48:1503–1510. doi: 10.1016/0895-4356(95)00048-8. [DOI] [PubMed] [Google Scholar]
- 36.Cox DH, Snell EJ. Analysis of binary data. London: Chapman and Hall; 1989. [Google Scholar]
- 37.Sohn H-J, Yang Y-J, Ryu J-S, et al. [18F]Fluorothymidine positron emission tomography before and 7 days after gefitinib treatment predicts response in patients with advanced adenocarcinoma of the lung. Clin Cancer Res. 2008;14:7423–7429. doi: 10.1158/1078-0432.CCR-08-0312. [DOI] [PubMed] [Google Scholar]
- 38.Zander T, Scheffler M, Nogova L, et al. Early prediction of nonprogression in advanced non-small-cell lung cancer treated with erlotinib by using [(18)F]fluorodeoxyglucose and [(18)F]fluorothymidine positron emission tomography. J Clin Oncol. 2011;29:1701–1708. doi: 10.1200/JCO.2010.32.4939. [DOI] [PubMed] [Google Scholar]
- 39.Begg AC, Haustermans K, Hart AA, et al. The value of pretreatment cell kinetic parameters as predictors for radiotherapy outcome in head and neck cancer: a multicenter analysis. Radiother Oncol. 1999;50:13–23. doi: 10.1016/s0167-8140(98)00147-9. [DOI] [PubMed] [Google Scholar]
- 40.Brink C, Bernchou U, Bertelsen A, et al. Locoregional control of non-small cell lung cancer in relation to automated early assessment of tumor regression on cone beam computed tomography. Int J Radiat Oncol Biol Phys. 2014;89:916–923. doi: 10.1016/j.ijrobp.2014.03.038. [DOI] [PubMed] [Google Scholar]
- 41.LaDue TA, Dodge R, Page RL, et al. Factors influencing survival after radiotherapy of nasal tumors in 130 dogs. Vet Radiol Ultrasound. 1999;40:312–317. doi: 10.1111/j.1740-8261.1999.tb00367.x. [DOI] [PubMed] [Google Scholar]
- 42.Yoon JH, Feeney DA, Jessen CR, et al. External-beam Co-60 radiotherapy for canine nasal tumors: a comparison of survival by treatment protocol. Res Vet Sci. 2008;84:140–149. doi: 10.1016/j.rvsc.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 43.Hueting R, Kersemans V, Cornelissen B, et al. A comparison of the behavior of (64)Cu-acetate and (64)Cu-ATSM in vitro and in vivo. J Nucl Med. 2014;55:128–134. doi: 10.2967/jnumed.113.119917. [DOI] [PubMed] [Google Scholar]
- 44.Basken NE, Green MA. Cu(II) bis(thiosemicarbazone) radiopharmaceutical binding to serum albumin: further definition of species dependence and associated substituent effects. Nucl Med Biol. 2009;36:495–504. doi: 10.1016/j.nucmedbio.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kersemans V, Cornelissen B, Hueting R, et al. Hypoxia imaging using PET and SPECT: the effects of anesthetic and carrier gas on [Cu]-ATSM, [Tc]-HL91 and [F]-FMISO tumor hypoxia accumulation. PLoS One. 2011;6:e25911. doi: 10.1371/journal.pone.0025911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hansen AE, Kristensen AT, Jorgensen JT, et al. 64Cu-ATSM and 18FDG PET uptake and 64Cu-ATSM autoradiography in spontaneous canine tumors: comparison with pimonidazole hypoxia immunohistochemistry. Radiat Oncol. 2012;7:89. doi: 10.1186/1748-717X-7-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hansen K, Khanna C. Spontaneous and genetically engineered animal models; use in preclinical cancer drug development. Eur J Cancer. 2004;40:858–880. doi: 10.1016/j.ejca.2003.11.031. [DOI] [PubMed] [Google Scholar]