Summary
Mind bomb (Mib) proteins are large, multi-domain E3 ligases that promote ubiquitination of the cytoplasmic tails of Notch ligands. This ubiquitination step marks the ligand proteins for epsin-dependent endocytosis, which is critical for in vivo Notch receptor activation. We present here crystal structures of the substrate recognition domains of Mib1, both in isolation and in complex with peptides derived from Notch ligands. The structures, in combination with biochemical, cellular and in vivo assays, show that Mib1 contains two independent substrate recognition domains that engage two distinct epitopes from the cytoplasmic tail of the ligand Jagged1, one in the intracellular membrane proximal region and the other near the C-terminus. Together, these studies provide new insights into the mechanism of ubiquitin transfer by Mind bomb E3 ligases, illuminate a key event in ligand-induced activation of Notch receptors, and identify a potential new target for therapeutic modulation of Notch signal transduction in disease.
Introduction
Notch signaling guides cell differentiation and lineage choice in many progenitor cell populations. A distinct feature of the Notch pathway is its reliance on direct cell-cell contact, because both Notch receptors and their activating ligands on signal-sending cells are transmembrane proteins. The central importance of this pathway in mammalian development is highlighted by the embryonic lethality or developmental defects associated with ligand or receptor loss-of-function in knockout mice, and by the wide spectrum of diseases linked to Notch mutation in humans (Aster et al., 2008; Gridley, 2003; Maillard et al., 2005; Ranganathan et al., 2011).
Notch receptors are large, single-pass transmembrane proteins normally maintained in an autoinhibited “off” state by a juxtamembrane negative regulatory region that is resistant to activating proteolysis (Gordon et al., 2007). Ligand-induced release of autoinhibition permits receptor cleavage at a juxtamembrane site by ADAM-family metalloproteases, generating a truncated receptor that becomes a substrate for the intramembrane protease gamma-secretase. After gamma-secretase cleavage, the intracellular part of the receptor (NICD) is released from the membrane, migrates to the nucleus, and assembles a transcriptional activation complex with the RBPJ transcription factor and Mastermind-family co-activators to induce the transcription of Notch target genes.
Relief of receptor autoinhibition is dependent on trans-cellular interaction of Notch proteins with canonical ligands that are members of the Delta, Serrate, and Lag2 (DSL) family. In mammals, there are three Delta-like proteins (DLL1, DLL3, and DLL4) and two Jagged proteins (Jag1 and Jag2, homologues of Drosophila Serrate). Purified extracellular fragments of various ligands are sufficient to bind purified or cell-surface Notch receptors. Remarkably, however, ligand molecules that lack their endogenous cytoplasmic tails are unable to signal and can have dominant negative activity (Sun and Artavanis-Tsakonas, 1996), indicating that formation of Notch ligand:receptor complexes at sites of cell-cell contact is insufficient for release of autoinhibition and subsequent receptor activation.
The critical event that links formation of ligand:receptor complexes to productive signaling is an in vivo requirement for ubiquitination-dependent endocytosis of the ligand (reviewed by (Musse et al., 2012)). Genetic studies in Drosophila and zebrafish identified two structurally unrelated E3 ubiquitin ligases, Mind bomb (Mib) and Neuralized (Neur), that catalyze ubiquitin transfer to the cytoplasmic tails of Notch ligands (Haddon et al., 1998; Itoh et al., 2003; Lai et al., 2001). Although Mib and Neur are structurally unrelated and appear to recognize different regions of the cytoplasmic tails of Notch ligands (Daskalaki et al., 2011), the two E3 ligases can functionally substitute for each other in some cellular contexts (Le Borgne et al., 2005).
In vertebrates, Mind bomb 1 (Mib1) is the primary E3 ligase responsible for driving Notch signal transduction. Whereas both mammalian Neur genes, as well as Mib2, are dispensable for murine development (Koo et al., 2007), Mib1 knockout mice die in utero around embryonic day 10.5 and exhibit numerous developmental defects associated with loss of Notch function (Barsi et al., 2005; Koo et al., 2005). Similarly, conditional inactivation of Mib1 phenocopies loss of Notch function in a number of different tissues, including skin and the hematopoetic system (Jeong et al., 2012; Song et al., 2008; Yoon et al., 2008), and Mib1 loss-of-function mutations in humans gives rise to a left-ventricular non-compaction phenotype also attributed to Notch loss of function (Luxan et al., 2013). In addition to their role as Notch ligand effectors, Mib proteins have also been implicated as ubiquitin ligases for proteins involved in innate immunity, neuronal function, Wnt/β-catenin signaling, genomic stability, and cell death (Berndt et al., 2011; Choe et al., 2007; Jin et al., 2002; Li et al., 2011; Villumsen et al., 2013; Zhang and Gallagher, 2009), further highlighting the general importance of Mib proteins in mammalian biology.
We present here structural, biochemical, cell-based, and in vivo assays that reveal the basis for recognition of the cytoplasmic tails of Notch ligands by Mib1. These studies show that Mib1 contains two independent substrate recognition domains, which engage two separate epitopes from the Jag1 cytoplasmic tail, and that both interactions are needed for normal stimulation of Notch signal transduction.
Results
Structure of Mind Bomb 1 MZM and REP domains
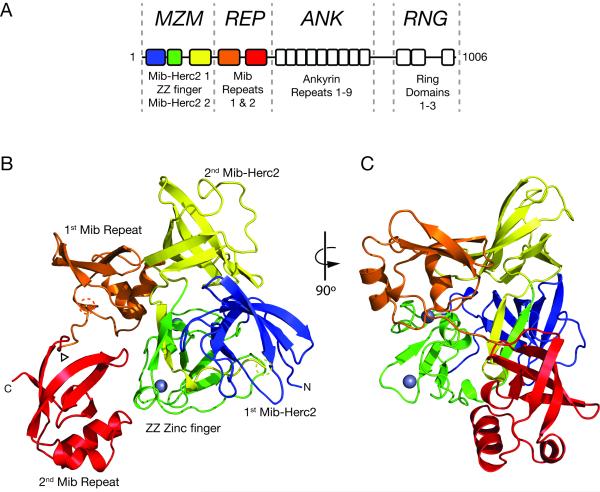
Mind Bomb 1 is a large E3 ligase with four contiguous structural elements: i) an MZM region, which contains two Mib-Herc2 domains flanking a ZZ zinc finger (ZZ), ii) a REP region, which includes two tandem repeat elements known as Mib repeats; iii) an ANK region, which spans nine ankyrin repeats, and iv) a RNG region, which contains three RING domains (Figure 1A). Prior work has established the N-terminal region encompassing the MZM and REP domains as necessary and sufficient for cellular interaction with Notch ligand molecules (Chen and Corliss, 2004; Itoh et al., 2003). Furthermore, deletion of either the MZM or REP region eliminates co-immunoprecipitation (co-IP) with Jagged 1 (Figure S1A), indicating that the presence of both regions is required for stable association of Mib1 with Jag1 in cells. In contrast, the ankyrin domain does not appear to have a role in Jag1 recognition. It may participate in binding to other substrates, subcellular localization, or as a spacer domain to facilitate ubiquitin transfer, analogous to cullin subunits of cullin-family ligases.
Figure 1. Structure of the MZM-REP region of Mib1.
A, Domain organization of Mib1, highlighting the MZM, REP, ANK, and RNG regions of the protein. B-C, Cartoon representation of the X-ray structure of the MZM-REP protein, colored by domain. Residues 315-330 of the linker connecting Mib repeats 1 and 2 were deleted to assist crystallization (arrowhead). Dashed circles indicate the residues immediately preceding (Gly235, yellow) and following (Leu251, orange) the disordered linker connecting the MZM and REP regions. The two Zinc2+ ions in the ZZ domain are represented by grey spheres. See also Figure S1.
To elucidate the overall architecture of the N-terminal region of Mib1 and to identify potential binding sites for the Jag1 cytoplasmic tail and other ligands, we determined the X-ray structure of MZM-REP (Table 1). The crystallized protein spans residues 8-402 and contains a deletion of residues 315-330 that link the two Mib repeats (Δ315-330). This deletion mirrors the shorter linker of Mind Bomb 2 (Figure S1B) and does not deleteriously affect binding to Jag1 (Table S1).
Table 1.
Crystallization Data Collection and Refinement Statistics
| Data collection | MZM-REP | Jag1 N-box | Delta N-box | REP | ||
|---|---|---|---|---|---|---|
| Space group | P 41 2 2 | P 41 2 2 | P 41 2 2 | P 21 21 2 | ||
| Cell dimensions | ||||||
| a, b, c (Å) | 73.08, 73.08, 170.96 | 73.73, 73.83, 170.28 | 74.13, 74.13, 170.17 | 51.32, 86.42, 86.60 | ||
| α, β, υ(°) | 90, 90, 90 | 90, 90, 90 | 90, 90, 90 | 90, 90, 90 | ||
| Wavelength | 1.2822 (Peak) | 1.2830 (Inflection) | 1.2562 (Remote) | 0.9792 | 0.9792 | 0.9792 |
| Resolution (Å) | 50-2.04 (2.11-2.04) | 50-2.04 (2.08-2.04) | 50-2.00 (2.03-2.00) | 50-2.05 (2.09-2.05) | 50-2.15 (2.19-2.15) | 50-2.05 (2.09-2.05) |
| R merge | 0.069 (0.377) | 0.067 (0.404) | 0.071 (0.466) | 0.092 (0.627) | 0.114 (0.993) | 0.113 (0.644) |
| I/σI | 29.0 (2.9) | 28.7 (2.6) | 26.3 (2.2) | 26.0 (4.0) | 17.6 (2.1) | 14.6 (2.6) |
| Completeness (%) | 96.5 (67.2) | 95.2 (53.6) | 93.2 (47.4) | 96.3 (94.5) | 100 (100) | 99.7 (99.8) |
| Redundancy | 8.1 (4.7) | 8.1 (4.6) | 7.9 (4.4) | 10.0 (9.8) | 7.0 (7.2) | 6.1 (6.0) |
|
| ||||||
| Refinement | ||||||
|
| ||||||
| Resolution (Å) | 44.95-2.00 | 49.85-2.05 | 45.05-2.15 | 44.15-2.06 | ||
| No. reflections | 28954 | 27746 | 24766 | 20656 | ||
| Rwork/Rfree | 0.20/0.25 | 0.20/0.22 | 0.21/0.24 | 0.19/0.23 | ||
| No. atoms | ||||||
| Protein | 2836 | 2880 | 2888 | 4918 | ||
| Ligand/ion | 12 (2 Zn2+, 2 SO42−) | 12 (2 Zn2+, 2 SO42−) | 12 (2 Zn2+, 2 SO42−) | |||
| Water | 186 | 161 | 108 | 240 | ||
| B-factors | ||||||
| Protein | 37.6 | 41.0 | 53.9 | 17.70 | ||
| Ligand/ion | 22.7 | 32.9 | 41.6 | |||
| Water | 32.4 | 35.7 | 38.0 | 28.90 | ||
| Rms deviations | ||||||
| Bond lengths (Å) | 0.018 | 0.0016 | 0.0039 | 0.012 | ||
| Bond angles (°) | 1.296 | 0.1917 | 0.7856 | 1.31 | ||
| Ramachandran (%) | ||||||
| Favored | 94.7 | 94.44 | 95.04 | 96.7 | ||
| Allowed | 5.3 | 5.00 | 4.41 | 3.3 | ||
| Outlier | 0 | 0.56 | 0.55 | 0 | ||
| Rotamer Outliers (%) | 0.66 | 0.65 | 0.98 | 0.4 | ||
| Clashscore | 4.85 | 1.59 | 1.23 | 3.21 | ||
| MolProbity Score | 1.62 | 1.28 | 1.20 | 1.43 | ||
Values in parentheses are for the highest resolution shell
The overall architecture of MZM-REP is compact, extending 70 Å in the largest dimension (Figure 1B,C). The MZM region adopts a tricorn shape derived from the integrated assembly of two Mib-Herc2 domains and a ZZ-type zinc finger module. Extensive interdomain contacts between the first Mib-Herc2 module and both the ZZ zinc finger and the second Mib-Herc2 module stabilize the tertiary arrangement of the MZM region. The relative positions of the three modules within the MZM region are locked in place by a small anti-parallel β-bridge (Figure 1C, yellow and green anti-parallel strands) formed from the linkers immediately preceding and following the second Mib-Herc2 module. The two Zn++ ions seen in the ZZ module are coordinated by cys4 and cys2his2 clusters, and play a structural role as in other ZZ-type zinc fingers. A 15-residue linker that connects the MZM and REP domains is not visible in the structure and is likely disordered (Figure 1B, dashed circles). The first Mib repeat packs against a surface derived from the second Mib-Herc2 domain and the ZZ module, while the second Mib repeat contacts only the ZZ module (Figure 1B,C). Each individual Mib repeat has a small interface area with the MZM region (336 Å2 for the first Mib repeat; 348 Å2 for the second Mib repeat). Unlike the MZM region, which constitutes an integrated structural unit of the protein, the two Mib repeats are structurally independent and make no direct contact with each other.
Whether the MZM-REP interfaces observed in the crystal have a role in Mib1 function or are simply a consequence of crystal packing is unclear. Small-angle X-ray scattering (SAXS) measurements of MZM-REP in a stringent, high salt buffer (both with the native linker intact and with the shortened linker between Mib repeats 1 and 2 used for crystallization) show that the bulk solution conformation under these conditions is less compact than predicted from the crystal structure (28.9 Å radius of gyration observed, versus 22.7 Å predicted from the crystal structure, Figure S1C,D). The SAXS data suggest that the arrangement of the two Mib repeats with respect to the MZM region is dynamic (Figure S1E), and more closely approximates a “beads on a string” model, but do not exclude the possibility that a closed conformation is populated or even dominant under physiologic conditions in the context of the full-length protein or within the cellular milieu.
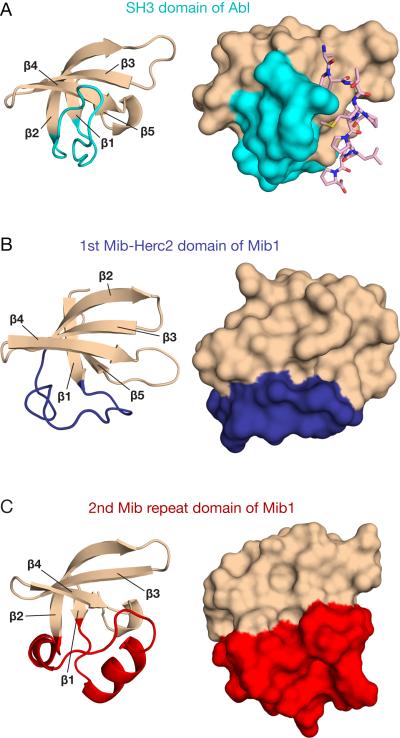
The individual Mib-Herc2 and Mib repeat folds have a five-stranded anti-parallel twisted β-sheet topology similar to that of SH3 domains (Figure 2), even though the three types of modules have no detectable sequence homology. The three folds are structurally distinguished by the segment connecting β-strands 1 and 2 (Figure 2). In SH3 domains, this connector forms a loop perpendicular to β-strand 4 and participates in recognition of proline-rich sequence motifs (Figure 2A) (Musacchio et al., 1994). In Mib-Herc2 domains, the connector is instead parallel to β-strand 4 (Figure 2B), and in the Mib repeats, the connector is a helix-turn-helix motif (Figure 2C).
Figure 2. Similarity of Mib-Herc2 and Mib repeat elements to SH3 domains.
Cartoon and surface representations of the SH3 domain from Abl tyrosine kinase PDB 1ABO (A), the first Mib-Herc2 domain of Mib1 (B), and the second Mib repeat of Mib1 (C). For each structure, the connector between β strands 1 and 2 is highlighted in color. For the SH3 domain, a bound proline-rich peptide is shown as sticks (A, pink).
Binding of Mib1 to Notch ligands
To elucidate how the MZM-REP region recognizes Notch ligands, we prepared a series of polypeptides from the Jag1 tail (Figure 3A, schematic), and analyzed their ability to bind to purified MZM-REP (residues 8-410 prepared as an N-terminal SUMO fusion protein in order to maintain solubility) using isothermal titration calorimetry (ITC). Surprisingly, it was not possible to narrow down the motif to a short linear peptide sequence. Instead, we found an N-terminal segment proximal to the membrane (Figure 3A, in blue) and a segment near the C-terminus (in orange) that each accounted for a portion of the binding enthalpy associated with the full-length tail (in black; thermogram in Figure S2A). These findings suggested that Jag1 utilizes two discontinuous peptide motifs in forming complexes with Mib1. To test this idea, we fused a short N-terminal segment of the Jag1 tail to a short C-terminal segment, and measured the affinity of this fusion protein (in green) for the MZM-REP region of Mib1 (Figure 3A). The fusion protein bound to Mib1 with an affinity comparable to that of the full Jag1 tail (Figure 3A, Table S1) indicating that a combination of N- and C-terminal motifs reconstitutes full-strength binding affinity.
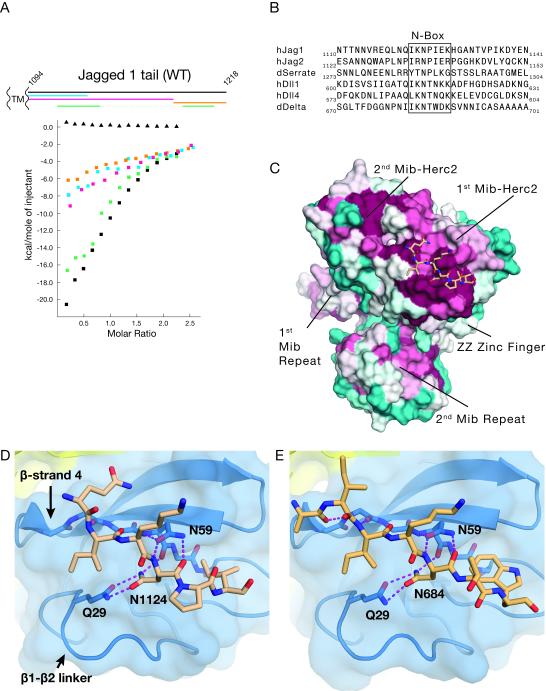
Figure 3. Structural basis for N-box binding to Mib1.
A, Upper panel: Schematic of the Jag1 intracellular tail from transmembrane (left) to C-terminus (right). Lower panel: Detection of the binding interaction between Jag1 tail polypeptides (protein indicated by color) and SUMO-MZM-REP (squares) by ITC. Full length Jag1 tail was titrated into a cell containing SUMO protein as a control (triangles). B, multiple sequence alignment of Notch ligands from human and Drosophila proteins. C, Structure of the complex between the Jag1 N-box peptide and MZM-REP. The MZM-REP protein is rendered as a molecular surface colored by conservation (cyan, least conserved to maroon, most conserved) and the N-box peptide is in CPK colors with carbon atoms in beige. D, Close-up view of the interface between Mib1 (blue) and the Jag1 N-box peptide (beige). E, Close-up view of the interface between Mib1 (blue) and the Drosophila Delta N-box peptide (gold). See also Figure S2.
The MZM region interacts with the Jag1-N epitope
To identify a potential binding motif within the Jag1 N-terminal segment, we aligned the amino acid sequences from the cytoplasmic tails of Delta and Jagged/Serrate ligands, identifying a conserved seven-residue sequence in the membrane proximal region (Figure 3B) that we will refer to as the N-box. This N-box corresponds to a Drosophila sequence identified previously (Daskalaki et al., 2011) as essential for interaction of Delta with fly Mib1 and for Mib1-dependent ubiquitination of Delta.
MZM-REP crystals soaked with a Jag1 N-box peptide and with an N-box peptide from the aligned region of Drosophila Delta (Table 1) revealed the mode of peptide binding to the MZM-REP region (Figures 3C and S2C,D). Both the Jag1 and Delta N-box peptides interact with the first Mib-Herc2 element, and are nestled in an elongated channel formed by β-strand 4 and the lengthy β1-β2 linker (Figure 3D,E). Notably, this site of binding is analogous to the site used by the topologically similar SH3 fold (Figure 2).
Anchoring the peptide:Mib1 interaction is an invariant Asn residue (peptide position “0”) present in Jag1 and conserved in the aligned sequences of all homologs (N1124 and N684 in human Jag1 and Drosophila Delta, respectively; Figure 3B). Mib1 residues Q29 and N59 form hydrogen bonds to the side-chain and main-chain, respectively, of the peptide Asn (Figure 3D,E). A shallow hydrophobic pocket created from the Q23 and T50 side chains of Mib1 is occupied by the Ile at peptide position −2 in both structures, and a second pocket formed by the aliphatic parts of the side chains from Y54 and R55 of Mib1 accommodates the hydrophobic residue (Ile in Jag1-N, and Trp in Delta-N) at the +2 position. There are also main-chain hydrogen bonds between the peptide and the fourth β-strand of the Mib-Herc2 domain. In comparison to the first Mib-Herc2 domain, a tryptophan side chain in the second Mib-Herc2 domain (as well as in the structure of the Mib-Herc2 module from HECTD1, PDB ID 3DKM) fills the pocket for the +2 peptide side-chain, potentially explaining why the peptide binds selectively to the first Mib-Herc2 (Figure S2E). Introduction of Q29A and N59A mutations into Mib1 (referred to as MZM*REP), which are predicted to eliminate hydrogen bonding interactions to the invariant Asn of the Jag1 N-box peptide, eliminates detectable interaction of the Jag1 N-terminal segment with Mib1 by ITC (Figure S2F) confirming that key interactions seen in the X-ray structure contribute to the binding affinity of Mib1 for the Jag1 N-terminal segment in solution.
The REP region interacts with the Jag1-C epitope
We next evaluated the role of the REP region in Jag1 binding and whether it could mediate recognition of the C-terminal segment of Jag1. We first expressed and purified the REP region of Mib1 (241-410), and divided the Jag1 tail into two parts: an extended N-terminal segment and a C-terminal segment. Then, we measured the affinity of the REP region of Mib1 for the full-length tail and for the two segments by ITC in a buffer approximating physiological conditions. Both the full-length tail and the Jag1 C-terminal segment bound to the REP domain (Figure 4A and S2B, Table S1), but the N-terminal segment did not (Figure 4A). These findings support the conclusion that the REP region interacts specifically with an epitope present in the C-terminal segment.
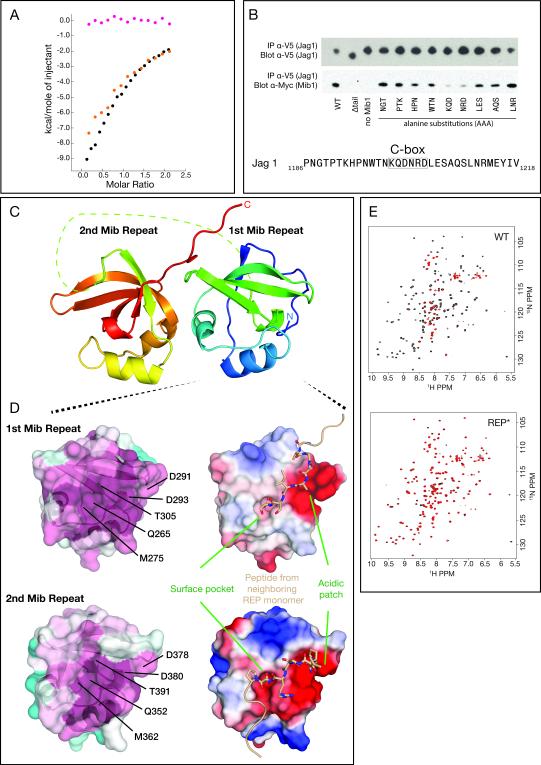
Figure 4. Basis for C-box binding to Mib1.
A, Detection of Jag1 binding to the REP domain of Mib1 by ITC (coloring as in Figure 3). B, Top: Co-immunoprecipitation of Mib1:Jag1 complexes from cells co-transfected with myc-tagged Mib1 (ΔRNG) and V5-tagged Jag1. The triplet sequences indicate the residues of Jag1 that were mutated to AAA for that lane of the blot. Bottom: Sequence of Jag1 in the region encompassing the Jag1 C-terminal peptide. C, Cartoon representation of the Mib repeats, colored on a sliding scale from blue (N-terminus) to red (C-terminus). The dotted line represents the disordered linker (residues 315-331) connecting the first and second repeats. D, Top panel: Surface representations of the first Mib repeat colored by residue conservation (left: cyan, least conserved to maroon, most conserved) or electrostatic potential (right: red, acidic to blue, basic). Residues from a neighboring symmetry-related subunit that make contacts with the α-β interface are rendered as sticks (beige). Bottom panel: Surface representations of the second Mib repeat in the same orientation as that shown for the first Mib repeat. Residues from a neighboring symmetry-related subunit that make contacts with the α-β interface are rendered as sticks (beige). E, HSQC spectra of 15N-labeled REP (left) and REP* (right) protein in the absence (black) or presence (red) of 1.5 molar equivalents of unlabeled Jag1 C-terminal peptide. See also Figure S3.
To identify the epitope recognized by the REP region, we performed alanine-scanning mutagenesis across the Jag1 C-terminal segment, in the context of full-length Jag1, and tested the ability of the mutated Jag1 proteins to co-immunoprecipitate with Mib1 (ΔRNG) in cells. The scanning experiment showed that alanine substitutions in the KQDNRD sequence (hereafter the Jag1 “C-box” epitope) greatly attenuated the association of Mib1 with Jag1 (Figure 4B). This sequence is also conserved in human Jag2 (KVDNRA).
Attempts to co-crystallize C-box peptides with MZM-REP or REP proteins were unsuccessful. A potential mode for peptide recognition, however, was revealed in the crystal structure of the unliganded REP domain (241-410, Figure 4C and Table 1). The crystal structure contains two REP domain molecules (two copies each of both the first and second Mib repeat) in the asymmetric unit. In the crystal, there are intermolecular contacts with peptide segments at the α-β interface of each Mib repeat (Figure 4D), analogous to the site of peptide recognition in SH3 domains. At the α-β interface, there is an acidic patch of surface accessible residues conserved between the repeats as well as across diverse metazoan species (Figure 4D).
To test the possibility that this conserved α-β interface is involved in peptide recognition, we first assessed whether charge reversal mutations of the C-box epitope affected peptide binding to the REP protein. When the KQDNRD epitope of the Jag1 C-box was mutated to EQDNED (K1199E/R1203E of human Jag1), binding of the Jag1 C-terminal segment to the REP protein was no longer detectable by ITC (Figure S3A). Similarly, when asparagine for aspartate substitutions were introduced at the acidic patches of both Mib repeats (D291N/D293N of Mib repeat 1 and D378N/D380N in Mib repeat 2, hereafter REP*), binding to the Jag1 C-terminal segment was also abolished (Figure S3B). To further evaluate the effect of these mutations, we carried out NMR chemical shift perturbation studies with 15N-isotopically labeled wild-type and mutated REP. Both the wild-type REP and mutant REP* proteins yielded well-dispersed, nearly superimposable 1H-15N heteronuclear single quantum coherence (HSQC) spectra (Figure 4E) indicating that the asparagine substitutions in REP* did not affect the overall structure of the protein. Titration of the Jag1 C-terminal segment into wild-type REP resulted in substantial line broadening of many peaks in the spectrum, consistent with a binding interaction between these two proteins (Figure 4E). In contrast, titration of the Jag1 C-terminal segment into REP* did not substantially perturb the HSQC spectrum, indicating a lack of interaction with the mutated REP* protein. Together with the ITC results, these data show that conserved aspartate residues at the α-β cleft are required for C-box binding.
Mutation of either Mib repeat individually resulted in partial loss of binding enthalpy as analyzed by ITC (Figure S3B) suggesting a cooperative binding event. The individual contribution of each Mib repeat was further evaluated by producing GST fusions of the full REP domain and each individual Mib repeat. The purified tandem REP assembly was sufficient for biochemical pull-down of purified Jag1 ligand (but not a ligand with alanine substitutions at the C-box epitope), whereas neither individual Mib repeat was capable of appreciable pull-down in the same assay (Figure S3C). Mib1 constructs containing Asp to Asn mutations in one or both Mib repeat(s) were also tested for cellular co-immunoprecipitation with Jag1 (Figure S3D). These results suggest that the first Mib repeat exerts a dominant role in C-box binding, at least in the context of a simultaneous and intact MZM:N-box interaction in cells. As the in vitro results indicate the presence of some degree of cooperativity in binding, all other functional experiments were conducted using proteins mutated at both Mib repeats.
We used an independent set of mutations to further probe the importance of the α-β interface in substrate binding. In the crystal, a conserved surface pocket present at the α-β interface (formed by Q265, M275, T305 in the first Mib repeat and Q352, M362, T391 in the second Mib repeat, as shown in Figure 4D) serves as a docking site for crystal contact-derived asparagine side-chains. To test the role of this pocket in substrate recognition, it was occluded by mutation of the helix linking residues G269 (first Mib repeat) and G356 (second repeat) to histidine (Figure S3E). This substitution had no apparent effect on REP secondary structure (Figure S3E-H). The mutated REP protein was then used to test interaction with the C-terminal segment of Jag1 by ITC. These substitutions abolished REP interaction with Jag1 (Figure S3I) further supporting the inference that the α-β interface participates in substrate recognition.
Functional evaluation of the MZM:Jag1-N and REP:Jag1-C interactions
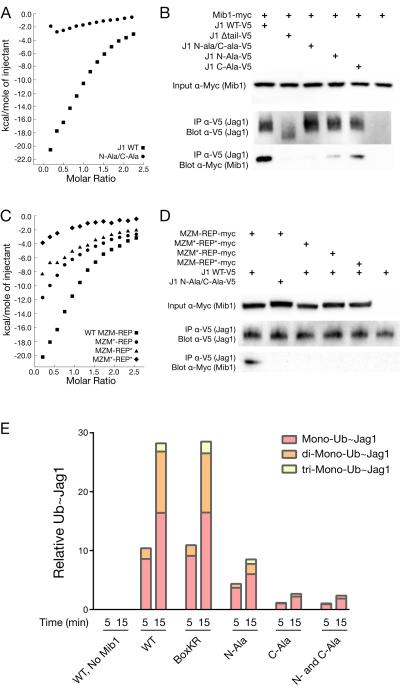
Since association of Mib1 with Notch ligands appears to rely on two independent contact sites, we next tested the effect of disrupting these interfaces using in vitro and cell-based assays. Whereas isolated Jag1 N-terminal and C-terminal segments alone show some capacity to bind the MZM-REP protein as assessed by ITC (Figure 3A), a Jag1 tail peptide with alanine residues in place of both the Jag1 N- and C-boxes (Jag1 N-ala/C-ala) exhibits greatly attenuated MIB1 binding by ITC in comparison to the wild-type Jag1 tail peptide (Figure 5A). Similarly, cellular co-IP assays show reduced binding of Jag1 to Mib1 when either epitope alone is mutated and a loss of detectable binding when both epitopes are mutated (Figure 5B). Mutating the Mib1 side of either interface also attenuates binding to the wild-type Jag1 tail and mutating both interfaces effectively eliminates detectable binding by ITC (Figure 5C). Mutations of Mib1 targeting either interface alone, or both interfaces in combination, also prevent co-immunoprecipitation with Jag1 in cells (Figure 5D).
Figure 5. Mutational analysis of the MZM:N-box and REP:C-box interfaces.
A, ITC analysis of the interaction between the MZM-REP region of Mib1 and either the wild-type (squares) or mutant (circles) Jag1 tail. B, Co-IP analysis monitoring the interaction of mutated Jag1 tails with myc-tagged Mib1 (ΔRNG). C, ITC analysis of the interaction between wild-type and mutated MZM-REP proteins and the wild-type Jag1 tail. Wild-type MZM-REP: squares, MZM mutant: circles, REP mutant: triangles, or double MZM/REP mutant: diamonds. D, Co-IP analysis monitoring the interaction of wild-type and mutated Myc-tagged Mib1 proteins (ΔRNG) with either wild-type or mutated Jag1 tails, as indicated. E, In vitro reconstitution of Mib1-dependent ubiquitin transfer to wild-type or mutated HA-tagged Jag1 tails, analyzed at 5 and 15 minute time points. The bar graph is quantified from HA-probed Western blots (Figure S4) using densitometry.
We next evaluated the contribution of each interaction site towards Mib1-dependent ubiquitin transfer. Purified, full-length Mib1 was incubated with wild-type and mutant HA-tagged Jag1 tails in the presence of lysine-less Ubiquitin and E1 (Ube1) and E2 (UbcH5B) enzymes. A time course of ubiquitin transfer was assayed by Jag1 size shift on an anti-HA probed Western blot (as lysine-less ubiquitin prohibits poly-ubiquitin chain formation, each size shift represents mono-ubiquitination at a single Jag1 lysine). Robust, Mib-dependent ubiquitin conjugation was observed for the wild-type Jag1 tail (Figures 5E and S4). When both N- and C-box epitopes were substituted with alanine residues, Jag1 ubiquitination was markedly curtailed. Individual mutation of either the N- or C-box epitope also attenuated the extent of ubiquitination, suggesting that the two binding sites cooperate to achieve efficient ubiquitin transfer. To address the possibility that the effect of these alanine mutations resulted from elimination of a critical Ub acceptor site, we substituted lysine with arginine in both the Jag1 N- and C-box epitopes. Ubiquitination of this mutated ligand (Box KR) was indistinguishable from that of the normal Jag1 tail, indicating that the alanine substitutions did not eliminate an important Ub acceptor site (Figure 5E).
To assess the effects of mutating the two interfaces in cell signaling assays, we created chimeric ligand molecules containing the extracellular and transmembrane regions of Delta-like 4 and the intracellular tail of Jag1 (the DLL4 extracellular region leads to stronger activation of Notch1 in transient transfection assays). Cells transfected with wild-type and tail mutant versions of this chimeric ligand were used as signal-sending cells in co-culture assays to stimulate Notch activity in responder cells (Malecki et al., 2006). The wild-type Jag1 tail produced a 7-fold increase in signal over the basal activity seen with vector alone, and deletion of the entire ligand tail attenuated this signal by roughly two-fold (Figure 6A). When both the Jag1 N- and C-box epitopes are replaced with alanine residues, the degree of activation was indistinguishable from that seen when the entire tail is deleted (Figure 6A). Alanine substitution of either epitope alone also attenuated the signal in comparison to the wild-type Jag1 tail (Figure 6A), with the effect of N-box substitution reaching statistical significance (p<0.001). Mutation of lysines in the N- and C-box sequences to arginine (Box KR) did not reduce ligand-induced Notch signaling (Figure 6A), as anticipated by the in vitro ubiquitin transfer results (Figure 5E). In contrast, lysine to arginine mutations in the linker region between the Jag1 N- and C-box epitopes (Linker KR) reduced signaling activity by an amount equivalent to ligand-tail deletion (Figure 6A), suggesting that one or more of the lysine residues in the linker between the epitopes acts as a functionally important Ub acceptor.
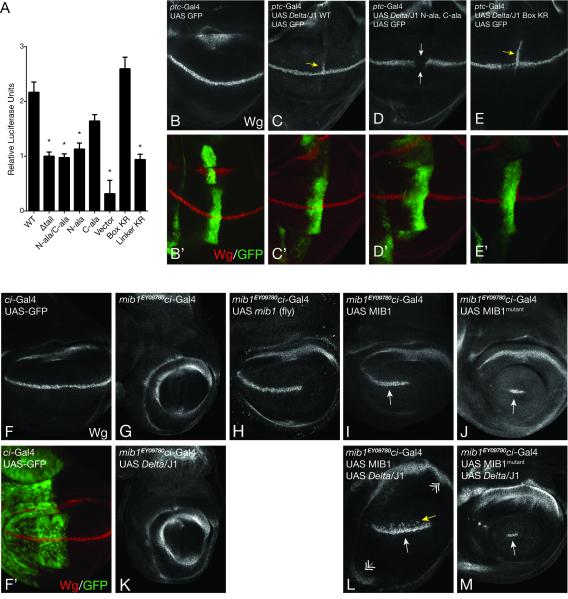
Figure 6. Mutation of MZM:N-box and REP:C-box interfaces reduce signaling in cells and flies.
A, Induction of a luciferase reporter under control of a Notch1-Gal4 chimeric protein following co-culture with cells expressing the indicated form of Jag1 (mean ± SEM, N = 12, asterisk represents p < 0.001 compared to WT). B-E, Immunostaining of the Notch target gene Wingless (Wg) in the fly instar wing disc (250x magnification). ptc-Gal4 was used to drive expression of GFP and a Delta-Jag1 chimera with the wild-type Jag1 tail (C), a Delta-Jag1 chimera with N-box and C-box alanine mutations (D), and a Delta-Jag1 chimera with arginine residues in place of lysine in the Jag1 N-box and C-box epitopes (Jag1 Box KR) (E). Ectopic Wg expression is noted by yellow arrows (C,E), and repression of Wg is noted by white arrows (D). B’-E’, Panels as in B-E, with Wg expression (red) overlaid on GFP (green). F and F’, expression of Wg and UAS-GFP using a ci-Gal4 driver (250x magnification). G-M, Wingless expression in the indicated strains. Rescue of Wg expression is noted by white arrows. Ectopic dorsal expression of Wg is indicated with a yellow arrow (L). Expansion of the wing pouch is indicated by double arrows (L).
To determine whether the Jag1 N- and C-box epitopes are required for Notch signal transduction in vivo, we developed a system to analyze wild-type and mutated forms of the Jag1 tail in a Drosophila model (Figure 6B-E). In this system, the signaling activity of Notch ligands can be interrogated by ectopically expressing the ligand and measuring its ability to induce expression of the Notch target gene Wingless in neighboring cells (Doherty et al., 1996; Speicher et al., 1994). In order to test the human Jag1 cytoplasmic tail in this assay, we created chimeric ligands that encode the extracellular and transmembrane region of fly Delta fused to either wild-type or mutated forms of the cytoplasmic tail of Jag1 and placed them under the control of a Gal4-response element. Ligands bearing wild-type and mutated forms of the Jag1 tail were ectopically expressed using a ptc-Gal4 driver (Speicher et al., 1994). Importantly, the ligand constructs (and the Mib1 constructs tested below) are inserted in the same landing site, allowing for direct comparisons between the various molecules being tested. Ectopic expression of ligand with WT Jag1 tail, or a N-box/C-box lysine to arginine control mutant, induced expression of Wingless (Wg) in the adjacent stripe of cells along the anterior/posterior boundary (Figure 6C,E). In contrast, when the Jag1 N- and C-box epitopes were replaced with alanine residues, the chimeric ligand no longer induced Wg expression and instead exhibited dominant negative and/or cis-inhibitory activity with respect to endogenous Delta (Figure 6D) consistent with the idea that the mutant ligand sequesters Notch in a non-productive ligand:receptor complex. Together, these results provide strong evidence that the Jag1 N- and C-box epitopes are critical for cellular interaction with Mib1 and for the consequent activation of Notch signaling in vivo.
To determine the effect of interface mutations in Mib1, we tested the activity of human Mib1 proteins in Mind bomb 1 deficient flies (mib1EY09780 genotype; (Bellen et al., 2004)). Wild type human Mib1 or the full-length MZM*REP* mutant were expressed using a ci-Gal4 driver (Croker et al., 2006) and Wg expression was assessed as a measure of productive Notch signaling. In the context of endogenous Drosophila ligands, wild-type human Mib1 was sufficient for robust rescue of Wg expression along the dorsal/ventral boundary in a manner similar to fly Mib1 (Compare figures 6H,I), whereas the MZM*REP* mutant resulted in a minimal Wg response (Figure 6J). When wild type Mib1 was co-expressed with the chimeric Delta/Jag1 ligand, there was substantial ectopic activation of Notch signaling, which led to dorsal Wg expression and excessive proliferation of the wing pouch (Figure 6L). In contrast, the MZM*REP* form of Mib1 failed to induce either ectopic Wg expression or excessive wing pouch proliferation when co-expressed with chimeric Delta/Jag1 suggesting that it can no longer activate the hybrid ligand (Figure 6M). These in vivo results are consistent with the structural and biochemical data and the inference that the mutated residues are indeed critical for interaction of Mib1 with Jag1 and downstream activation of Notch signaling.
Discussion
The ubiquitination of Notch ligands, thought to mark them for epsin-dependent endocytosis, is a critical event in Notch signal transduction. The dominant E3 ligase responsible for ligand ubiquitination in mammals is Mib1, which has also been implicated in the ubiquitination of many non-Notch substrates including tyrosine kinases associated with Wnt signaling (Berndt et al., 2011), innate immunity (Li et al., 2011), and the regulation of apoptosis (Jin et al., 2002).
In the work reported here, we combined structural and biochemical studies with cell-based and in vivo assays to elucidate the mode of Notch ligand tail recognition by Mib1. Mib1 uses two distinct substrate binding domains from its N-terminal region, and relies on the independent recognition of two discrete substrate epitopes by these N-terminal domains (Figure 7). These advances open new avenues in Notch pathway research and present a roadmap for understanding how Mind bomb proteins interact with a host of additional substrates and cellular pathways.
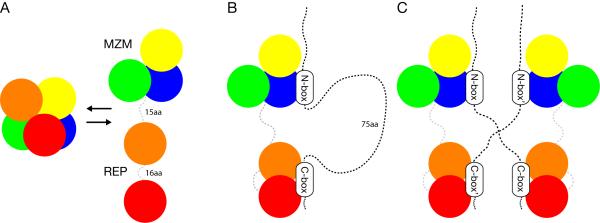
Figure 7. Model of Notch ligand recognition by Mib1.
The experimental data lead to a model in which the Mib1 MZM and REP regions act as independent substrate recognition elements. The MZM region binds the membrane proximal N-box epitope, and the REP region binds the membrane distal C-box sequence. The combination of MZM:N-box and REP:C-box interactions result in specific binding of the Jag1 tail. A, apo MZM-REP may potentially exist in either a closed conformation (observed in the crystal structure) or an open “beads on a string” model (suggested by SAXS). B, a model for Mib1:Jag1 binding in cis. C, a model for Mib1:Jag1 binding in trans.
Two structurally independent regions of Mib1 are responsible for Jag1 tail recognition. The recognition site for the Jag1 N-box epitope lies entirely within the first Mib-Herc2 module. Intriguingly, the three known examples of Mib-Herc2-containing protein families (Mind bomb, HERC2, and HECTD1) all function as E3 ubiquitin ligases, and it may be that the HERC2 and HECTD1 protein families also use their Mib-Herc2 modules in substrate recognition. Unlike these other E3 ligases, Mib1 contains a second, and also highly conserved, Mib-Herc2 module that could serve as a docking site for other, non-Notch substrates.
The REP domain of Mind bomb serves as the substrate recognition domain for a second, membrane-distal epitope of the Notch ligand (C-box). Although the first Mib repeat of REP may play a dominant role in ligand binding, the two repeats appear to cooperate in the engagement of the Jag1 tail. The two modules might “sandwich” the C-box epitope (Figure 7), or might exhibit avidity effects that also rely on backbone interactions of the second module with additional residues within a longer peptide sequence that encompasses the C-box.
On the ligand side of the binding interface, the N-box is more well-conserved than the C-box. Crystal structures of Mib1 in complex with N-box peptides from human Jag1 and fly Delta show very similar modes of interaction, and mutations within fly Serrate and fly Delta that eliminate the putative N-box epitope also disrupt ligand localization, ubiquitination, and function (Daskalaki et al., 2011; Glittenberg et al., 2006). However, sequence alignment of Delta-like and Jagged proteins does not reveal a sequence within the Delta-like proteins obviously analogous to the C-box, and future studies will be needed to elucidate the details of recognition for Delta-family proteins.
The bipartite mode of recognition of two discontinuous epitopes suggests that the second site may contribute more than just an increase in affinity. In the KEAP1-Cul3 E3 ligase, which forms a dimer, each subunit engages a different epitope on its substrate Nrf2 (Tong et al., 2006). This binding arrangement is thought to constrain the lysine-rich region between these Nrf2 epitopes in a configuration that promotes efficient ubiquitin transfer. As the intervening region between the N- and C-box epitopes of Jag1 also contains lysines critical for activation, it is possible that the bipartite mode of binding by Mib1 plays a similar mechanistic role. For example, dual engagement of proximal and distal epitopes may result in extrusion of the loop between the binding sites, facilitating ubiquitin transfer from a RING-domain associated E2 to acceptor lysine residues in the loop segment (Figure 7B). The dual-epitope, 1:1 stoichiometry also raises the possibility for trans interactions between independent Mib1 binding sites and their cognate epitopes (i.e. the N-box and C-box) on the ligand tails (Figure 7C). Binding in trans could promote clustering of Mib:ligand complexes, which might play a role in modulating the strength of the activating signal. Alternatively, Mib1 could preferentially ubiquitinate pre-clustered ligand:receptor complexes to promote internalization and activation.
More generally, it will also be important to resolve how other proteins thought to be ubiquitinated by Mib are recognized as substrates. Do they follow a dual epitope-binding model similar to Jag1? Or are they recognized exclusively by either the MZM region or the tandem Mib repeats? Most intriguing is the possibility of simultaneous engagement of two different proteins each captured by one of these two substrate recognition elements. Such a scenario would suggest that Mind bomb ligases are capable of assembling and/or targeting larger protein complexes for ubiquitination.
Experimental Procedures
Co-Immunoprecipitation
Jag1 and Mib1 constructs were co-transfected into sub-confluent HEK293T cells in a 6-well plate using Lipofectamine 2000 (Invitrogen, Carlsbad CA USA). ΔRNG Mib1 was used for all experiments except Figure S1A, which includes additional Mib1 molecules as indicated. After 48 hr, cells were lysed on ice in 500 μL buffer containing 0.5% w/v Fos-Choline 12, 50 mM HEPES pH 7.4, 150 mM NaCl, 0.5 mM DTT, 5 μM ZnCl2, 1 mM CaCl2 and EDTA-free protease inhibitors (Roche, Basel SUI). Lysates were pelleted by centrifugation and the supernatant was incubated with α-V5 agarose (Sigma-Aldrich, St. Louis MO USA) at 4 C for 1 hr. Following incubation, agarose beads were washed 3x for 5 min each and then re-suspended in SDS-PAGE loading buffer. For Western blotting, α-Myc (clone 9B11, Cell Signaling, Danvers MA USA) and α-V5 (polyclonal ab9116, Abcam, Cambridge UK) antibodies were used in 5% w/v milk TBST blocking buffer.
Isothermal Titration Calorimetry
Titrations were performed using a MicroCal iTC200 calorimeter (GE Healthcare) with 1000 RPM stirring speed. The temperature of the cell was 25 C, except for the data reported in Figure S3I, which were acquired at 20 C. MZM-REP (as an N-terminal His6-SUMO fusion) and REP titrations were performed at 42 μM or 75 μM cell concentrations, respectively. Binding data were calculated using a default one-site binding model in Origin software (OriginLab, Northampton MA USA). MZM-REP titrations were conducted in a buffer containing 25 mM HEPES pH 7.4, 500 mM NaCl, 10% w/v glucose, 100 mM L-Arginine, 10 μM ZnCl2, and 2mM TCEP. REP titrations were conducted in a buffer containing 25 mM HEPES pH 7.4, 150 mM NaCl, and 2 mM TCEP.
GST pulldowns
Pulldowns were performed in a buffer containing 25 mM HEPES pH 7.4, 250 mM NaCl, 1 mM TCEP and 0.1% v/v Nonidet P-40. GST-tagged Mib repeat proteins (60 μM) were incubated with HA-tagged Jag1 (2 μM) in the presence of glutathione-coupled beads for 1.5 hours at 25 C with agitation. After removal of an “input” aliquot, the beads were centrifuged and washed 3x for 5 minutes each and then re-suspended in SDS-PAGE loading buffer. Samples were analyzed by anti-HA Western blot using 3F10 antibody (Roche).
Ubiquitin transfer
Full-length Sumo-tagged Mib1 (0.5 μM), HA-tagged Jag1 tails (0.1 μM), His-tagged Ube1 (0.1 μM, UBPBio, Aurora CO USA), His-tagged UbcH5B (1 μM, UBPBio), and lysine-to-arginine mutant ubiquitin (25 μM) were added on ice to a final buffer consisting of 25mM HEPES pH 7.4, 160 mM NaCl, 3% w/v glucose, 30 mM L-Arginine, 1 mM ATP and 2mM TCEP. At Time 0, samples were placed in a 37 C water bath. Aliquots were removed at each indicated time-point, added to 2X SDS-PAGE buffer and heated to 95 C. Western blots were probed with HRP-conjugated anti-HA 3F10 antibody (Roche).
Notch reporter co-culture assay
On day 1, U2OS cells were reverse transfected with Notch ligand in 96 well plates using Lipofectamine 2000. On day 2, Notch1-Gal4 U2OS reporter cells, as previously described (Malecki et al., 2006), were plated onto the U2OS-ligand monolayer. On day 3, dual luciferase assays were performed using whole cell lysate.
Structure Determination
Diffraction images were indexed and integrated using HKL2000 (Otwinowski and Minor, 1997). The REP structure was solved using single-wavelength anomalous diffraction from a selenomethionine-labeled sample using SHELX experimental phasing (Sheldrick, 2008), and ARP/wARP model building/phase improvement (Langer et al., 2008). This model was then used for molecular replacement of a higher resolution dataset using Phenix (Adams et al., 2010). Refinement was performed in Phenix with manual building/review in COOT (Emsley et al., 2010). The MZM-REP structure was solved using multi-wavelength anomalous diffraction of native Zn2+ and experimental phasing/model building in Phenix. Poor density was observed for the first Mib repeat subunit. This portion of the model was built by molecular replacement using the REP structure and refined using secondary structure restraints. The REP structure coordinates should be used for detailed analysis of this domain. The model was refined in Phenix with manual building and review in Coot. N-box peptide structures were built manually into density using a combination of Fobs – Fobs, Fo – Fc, and iterative composite omit maps.
Small angle X-ray scattering
Measurements were performed at the Advanced Light Source SIBYLS beam-line as part of the high-throughput mail-in SAXS program. Data processing and comparisons were performed using Primus (Konarev et al., 2003) and Crysol (Svergun et al., 1995) software.
Drosophila expression and phenotyping
The following previously published fly stocks were used: mib1EY09780 (Bellen et al., 2004), UAS GFP (Yeh et al., 2001), UAS mib1 (Le Borgne et al., 2005), ptc-Gal4 (Speicher et al., 1994), and ci-Gal4 (Croker et al., 2006). Human Mib1 and Dl-Jag1 chimera constructs (1-607 of fly Delta and 1094-1218 of human Jag1) were generated in pUAST attB vector and inserted in attP landing sites 67Fb and 51C, respectively (Bischof et al., 2007). Antibody staining was performed according to standard protocols (anti-Wg from Developmental Hybridoma Bank). Images were obtained with a Zeiss ApoTome microscope.
Coordinates
Coordinates for the apo MZM-REP structure, the Jag1 complex, the Delta complex, and the Mib repeat (REP) region have been deposited with the PDB ID codes 4XI6, 4XI7, 4XIB, and 4TSE, respectively.
Supplementary Material
Acknowledgements
This work was supported in part by NIH grant P01 CA119070 (Subproject 3 to S.C.B.) and by American Cancer Society fellowship 120246-PF-11-044-01-DMC (to B.J.M). The work of T. K., B. S. and N. O. was supported by the Deutsche Forschungsgemeinschaft (DFG) through Sachbeihilfe KL 1028/3-1. X-ray crystallography was performed at the Advanced Photon Source, an Office of Science User Facility supported by the U.S. DOE under Contract No. DE-AC02-06CH11357. Small angle X-ray Scattering was conducted at the Advanced Light Source (ALS), a national user facility supported by the DOE. We thank Kelly Arnett for helpful discussions and critical review of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams PD, Afonine PV, Bunkoczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta crystallographica. Section D, Biological crystallography. 2010;66:213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aster JC, Pear WS, Blacklow SC. Notch signaling in leukemia. Annual review of pathology. 2008;3:587–613. doi: 10.1146/annurev.pathmechdis.3.121806.154300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsi JC, Rajendra R, Wu JI, Artzt K. Mind bomb1 is a ubiquitin ligase essential for mouse embryonic development and Notch signaling. Mechanisms of development. 2005;122:1106–1117. doi: 10.1016/j.mod.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Bellen HJ, Levis RW, Liao G, He Y, Carlson JW, Tsang G, Evans-Holm M, Hiesinger PR, Schulze KL, Rubin GM, et al. The BDGP gene disruption project: single transposon insertions associated with 40% of Drosophila genes. Genetics. 2004;167:761–781. doi: 10.1534/genetics.104.026427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berndt JD, Aoyagi A, Yang P, Anastas JN, Tang L, Moon RT. Mindbomb 1, an E3 ubiquitin ligase, forms a complex with RYK to activate Wnt/beta-catenin signaling. J Cell Biol. 2011;194:737–750. doi: 10.1083/jcb.201107021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischof J, Maeda RK, Hediger M, Karch F, Basler K. An optimized transgenesis system for Drosophila using germ-line-specific phiC31 integrases. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:3312–3317. doi: 10.1073/pnas.0611511104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Corliss DC. Three modules of zebrafish Mind bomb work cooperatively to promote Delta ubiquitination and endocytosis. Developmental biology. 2004;267:361–373. doi: 10.1016/j.ydbio.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Choe EA, Liao L, Zhou JY, Cheng D, Duong DM, Jin P, Tsai LH, Peng J. Neuronal morphogenesis is regulated by the interplay between cyclin-dependent kinase 5 and the ubiquitin ligase mind bomb 1. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:9503–9512. doi: 10.1523/JNEUROSCI.1408-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croker JA, Ziegenhorn SL, Holmgren RA. Regulation of the Drosophila transcription factor, Cubitus interruptus, by two conserved domains. Dev Biol. 2006;291:368–381. doi: 10.1016/j.ydbio.2005.12.020. [DOI] [PubMed] [Google Scholar]
- Daskalaki A, Shalaby NA, Kux K, Tsoumpekos G, Tsibidis GD, Muskavitch MA, Delidakis C. Distinct intracellular motifs of Delta mediate its ubiquitylation and activation by Mindbomb1 and Neuralized. The Journal of cell biology. 2011;195:1017–1031. doi: 10.1083/jcb.201105166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty D, Feger G, Younger-Shepherd S, Jan LY, Jan YN. Delta is a ventral to dorsal signal complementary to Serrate, another Notch ligand, in Drosophila wing formation. Genes & development. 1996;10:421–434. doi: 10.1101/gad.10.4.421. [DOI] [PubMed] [Google Scholar]
- Emsley P, Lohkamp B, Scott WG, Cowtan K. Features and development of Coot. Acta crystallographica. Section D, Biological crystallography. 2010;66:486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glittenberg M, Pitsouli C, Garvey C, Delidakis C, Bray S. Role of conserved intracellular motifs in Serrate signalling, cis-inhibition and endocytosis. EMBO J. 2006;25:4697–4706. doi: 10.1038/sj.emboj.7601337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon WR, Vardar-Ulu D, Histen G, Sanchez-Irizarry C, Aster JC, Blacklow SC. Structural basis for autoinhibition of Notch. Nature structural & molecular biology. 2007;14:295–300. doi: 10.1038/nsmb1227. [DOI] [PubMed] [Google Scholar]
- Gridley T. Notch signaling and inherited disease syndromes. Hum Mol Genet. 2003;12:R9–13. doi: 10.1093/hmg/ddg052. Spec No 1. [DOI] [PubMed] [Google Scholar]
- Haddon C, Jiang YJ, Smithers L, Lewis J. Delta-Notch signalling and the patterning of sensory cell differentiation in the zebrafish ear: evidence from the mind bomb mutant. Development. 1998;125:4637–4644. doi: 10.1242/dev.125.23.4637. [DOI] [PubMed] [Google Scholar]
- Itoh M, Kim C, Palardy G, Oda T, Jiang Y, Maust D, Yeo S, Lorick K, Wright GJ, Ariza-McNaughton L, et al. Mind bomb is a ubiquitin ligase that is essential for efficient activation of Notch signaling by Delta. Developmental cell. 2003;4:67–82. doi: 10.1016/s1534-5807(02)00409-4. [DOI] [PubMed] [Google Scholar]
- Jeong HW, Kim JH, Kim JY, Ha SJ, Kong YY. Mind bomb-1 in dendritic cells is specifically required for Notch-mediated T helper type 2 differentiation. PLoS One. 2012;7:e36359. doi: 10.1371/journal.pone.0036359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y, Blue EK, Dixon S, Shao Z, Gallagher PJ. A death-associated protein kinase (DAPK)-interacting protein, DIP-1, is an E3 ubiquitin ligase that promotes tumor necrosis factor-induced apoptosis and regulates the cellular levels of DAPK. J Biol Chem. 2002;277:46980–46986. doi: 10.1074/jbc.M208585200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konarev PV, Volkov VV, Sokolova AV, Koch MH, Svergun DI. PRIMUS: a Windows PC-based system for small-angle scattering data analysis. Journal of Applied Crystallography. 2003;36:1277–1282. [Google Scholar]
- Koo B, Lim H, Song R, Yoon M, Yoon K, Moon J, Kim Y, Kwon M, Yoo K, Kong M, et al. Mind bomb 1 is essential for generating functional Notch ligands to activate Notch. Development (Cambridge, England) 2005;132:3459–3470. doi: 10.1242/dev.01922. [DOI] [PubMed] [Google Scholar]
- Koo B, Yoon M, Yoon K, Im S, Kim Y, Kim C, Suh P, Jan YN, Kong Y. An obligatory role of mind bomb-1 in notch signaling of mammalian development. PloS one. 2007;2:e1221. doi: 10.1371/journal.pone.0001221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai EC, Deblandre GA, Kintner C, Rubin GM. Drosophila neuralized is a ubiquitin ligase that promotes the internalization and degradation of delta. Developmental cell. 2001;1:783–794. doi: 10.1016/s1534-5807(01)00092-2. [DOI] [PubMed] [Google Scholar]
- Langer G, Cohen SX, Lamzin VS, Perrakis A. Automated macromolecular model building for X-ray crystallography using ARP/wARP version 7. Nature protocols. 2008;3:1171–1179. doi: 10.1038/nprot.2008.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Borgne R, Remaud S, Hamel S, Schweisguth F. Two distinct E3 ubiquitin ligases have complementary functions in the regulation of delta and serrate signaling in Drosophila. PLoS biology. 2005;3:e96. doi: 10.1371/journal.pbio.0030096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Wang L, Berman M, Kong YY, Dorf ME. Mapping a dynamic innate immunity protein interaction network regulating type I interferon production. Immunity. 2011;35:426–440. doi: 10.1016/j.immuni.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luxan G, Casanova JC, Martinez-Poveda B, Prados B, D'Amato G, MacGrogan D, Gonzalez-Rajal A, Dobarro D, Torroja C, Martinez F, et al. Mutations in the NOTCH pathway regulator MIB1 cause left ventricular noncompaction cardiomyopathy. Nature medicine. 2013;19:193–201. doi: 10.1038/nm.3046. [DOI] [PubMed] [Google Scholar]
- Maillard I, Fang T, Pear WS. Regulation of lymphoid development, differentiation, and function by the Notch pathway. Annual review of immunology. 2005;23:945–974. doi: 10.1146/annurev.immunol.23.021704.115747. [DOI] [PubMed] [Google Scholar]
- Malecki MJ, Sanchez-Irizarry C, Mitchell JL, Histen G, Xu ML, Aster JC, Blacklow SC. Leukemia-associated mutations within the NOTCH1 heterodimerization domain fall into at least two distinct mechanistic classes. Molecular and cellular biology. 2006;26:4642–4651. doi: 10.1128/MCB.01655-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musacchio A, Saraste M, Wilmanns M. High-resolution crystal structures of tyrosine kinase SH3 domains complexed with proline-rich peptides. Nature structural biology. 1994;1:546–551. doi: 10.1038/nsb0894-546. [DOI] [PubMed] [Google Scholar]
- Musse AA, Meloty-Kapella L, Weinmaster G. Notch ligand endocytosis: mechanistic basis of signaling activity. Seminars in cell & developmental biology. 2012;23:429–436. doi: 10.1016/j.semcdb.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otwinowski Z, Minor W. Processing of X-ray diffraction data. Methods enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- Ranganathan P, Weaver KL, Capobianco AJ. Notch signalling in solid tumours: a little bit of everything but not all the time. Nature reviews. Cancer. 2011;11:338–351. doi: 10.1038/nrc3035. [DOI] [PubMed] [Google Scholar]
- Sheldrick GM. A short history of SHELX. Acta crystallographica. Section A, Foundations of crystallography. 2008;64:112–122. doi: 10.1107/S0108767307043930. [DOI] [PubMed] [Google Scholar]
- Song R, Kim YW, Koo BK, Jeong HW, Yoon MJ, Yoon KJ, Jun DJ, Im SK, Shin J, Kong MP, et al. Mind bomb 1 in the lymphopoietic niches is essential for T and marginal zone B cell development. The Journal of experimental medicine. 2008;205:2525–2536. doi: 10.1084/jem.20081344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speicher SA, Thomas U, Hinz U, Knust E. The Serrate locus of Drosophila and its role in morphogenesis of the wing imaginal discs: control of cell proliferation. Development. 1994;120:535–544. doi: 10.1242/dev.120.3.535. [DOI] [PubMed] [Google Scholar]
- Sun X, Artavanis-Tsakonas S. The intracellular deletions of Delta and Serrate define dominant negative forms of the Drosophila Notch ligands. Development. 1996;122:2465–2474. doi: 10.1242/dev.122.8.2465. [DOI] [PubMed] [Google Scholar]
- Svergun D, Barberato C, Koch M. CRYSOL-a program to evaluate X-ray solution scattering of biological macromolecules from atomic coordinates. Journal of Applied Crystallography. 1995;28:768–773. [Google Scholar]
- Tong KI, Katoh Y, Kusunoki H, Itoh K, Tanaka T, Yamamoto M. Keap1 recruits Neh2 through binding to ETGE and DLG motifs: characterization of the two-site molecular recognition model. Molecular and cellular biology. 2006;26:2887–2900. doi: 10.1128/MCB.26.8.2887-2900.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villumsen BH, Danielsen JR, Povlsen L, Sylvestersen KB, Merdes A, Beli P, Yang YG, Choudhary C, Nielsen ML, Mailand N, et al. A new cellular stress response that triggers centriolar satellite reorganization and ciliogenesis. EMBO J. 2013;32:3029–3040. doi: 10.1038/emboj.2013.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh HH, Tian T, Medina V, Falk BW. Green fluorescent protein expression from recombinant lettuce infectious yellows virus-defective RNAs originating from RNA 2. Virology. 2001;289:54–62. doi: 10.1006/viro.2001.1110. [DOI] [PubMed] [Google Scholar]
- Yoon MJ, Koo BK, Song R, Jeong HW, Shin J, Kim YW, Kong YY, Suh PG. Mind bomb-1 is essential for intraembryonic hematopoiesis in the aortic endothelium and the subaortic patches. Molecular and cellular biology. 2008;28:4794–4804. doi: 10.1128/MCB.00436-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Gallagher PJ. Mind bomb 1 regulation of cFLIP interactions. American journal of physiology. Cell physiology. 2009;297:C1275–1283. doi: 10.1152/ajpcell.00214.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.