Introduction
Absorption of both dietary cholesterol and cholesterol cleared from the liver through biliary secretion contributes substantially to tight control of cholesterol homeostasis. This process is mediated by a specific transporter – Niemann-Pick C1-Like 1 (NPC1L1) protein – localized to the brush border membrane of jejunal enterocytes (Figure 1, Table 1).1 NPC1L1 was first described by Davies and colleagues in 2000 while searching for proteins homologues of human Niemann-Pick type C1 protein (NPC1) – the primary causative protein for Niemann-Pick disease type C1 – that may be involved in subcellular cholesterol trafficking.2,3 Human liver express also NPC1L1, however its physiological significance in hepatocytes remains to be elucidated.
Figure 1.
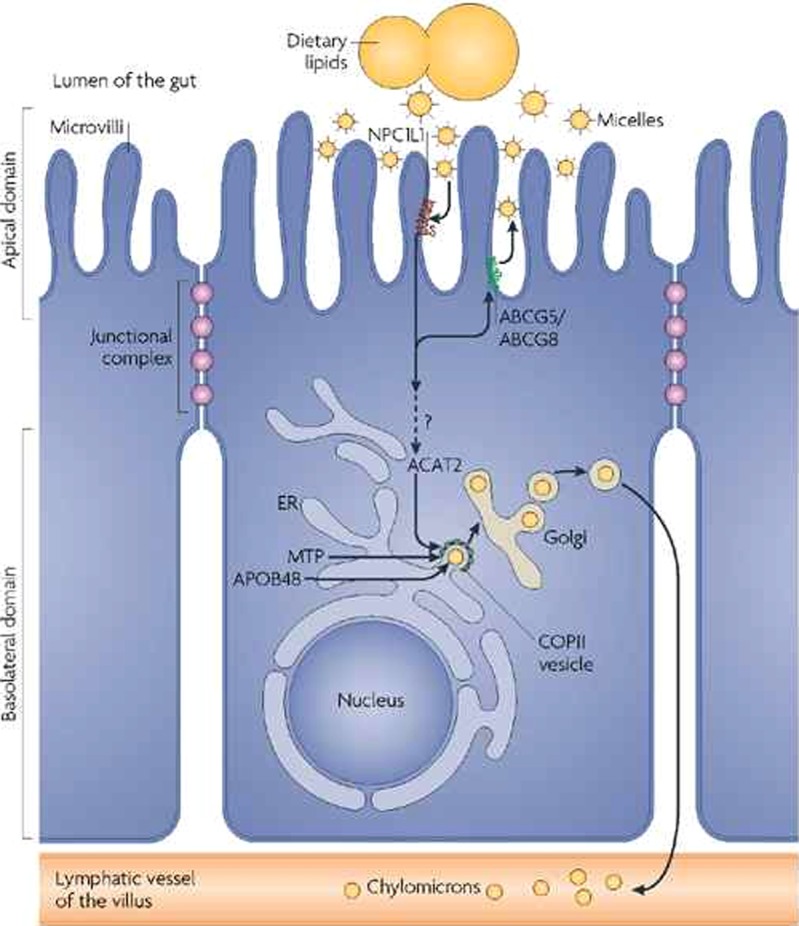
The Niemann–Pick C1-like-1(NPC1L1) protein (dark red) is located at the apical membrane of enterocytes and facilitates the uptake of cholesterol across the brush border membrane. In contrast, the ABCG5/G8 transporter (green) promotes the active transfer of cholesterol and plant sterols back into the intestinal lumen for excretion. Acyl CoA cholesterol acyltransferase isoform-2 (ACAT2) esterifies the absorbed cholesterol, which becomes incorporated into nascent chylomicron particles. Dietary fatty acids are used for triglyceride synthesis in the smooth ER and MTP (microsomal triglyceride transfer protein) transfers triglycerides and cholesteryl esters to APOB48. The nascent chylomicrons leave the ER in COPII-coated vesicles and are secreted through the Golgi complex to the basolateral side of the enterocyte and reach the venous circulation through lymphatic vessels18.
Table 1.
Comparison Between NPC1L1 and PCSK9 proteins.
| Niemman-Pick type C1 L1 (NPC1L1) | Proprotein convertase subtilisin/kexin 9 (PCSK9) | |
| Discovery | Nabil Seidah and colleagues (2003) | Davies and colleagues (2000) |
| Molecular structure | Domain A (residues 22-242), Domain B (residues 243 – 265) | Serine protease (prodomain, catalytic domain, and V domain) |
|
|
|
| Main site of production | Small intestine and liver | Liver and small intestine |
| Level of action in cholesterol metabolism | Promotes cholesterol absorption in small intestine | Promotes LDL receptor degradation and decreases the liver ability to clear LDL-C from blood |
| Prevalence of gene mutation | Inactivation mutation was detected in 1 in every 650 persons.14 | Non-sense mutations was detected in 2% of African Americans and < 0.1% of European American.4 |
| Therapeutic potential (pharmacological inhibition) | NPC1L1 inhibitors have achieved 10-20% reduction in LDL-C levels when added to background statin therapy.6–8 | PCSK9 inhibitors have achieved 60% reduction in LDL-C levels when added to background statin therapy. 9,10 |
| NPC1L1 inhibition is associated with significant decrease in adverse CV events | The results of long term clinical outcome studies have not been yet released |
Ezetimibe – a potent selective inhibitor of NPC1L1 protein activity5 – has been shown to lower plasma levels of low density lipoprotein cholesterol (LDL-C) by 12% to 20%6–8. It was approved, in 2012, by the Food and Drug Administration (FDA) for the treatment of hypercholesterolemia on the basis of its LDL-C lowering alone, despite lack of data on its effects on clinical end-points such as death, myocardial infarction (MI), or stroke in clinical outcome studies.
Ezetimibe failed to slow the progression of carotid intima-media thickness, when added to background statin therapy in patients with familial hypercholesterolemia in the landmark Ezetimibe and Simvastatin in Hypercholesterolemia Enhance Atherosclerosis Regression (ENHANCE) trial, reported in January 200811. Unfortunately these negative results cast a shadow over ezetimibe, although the ENHANCE study was heavily criticized by a significant and unexplained 18-month delay between completion of the study and publication of results. In the latest European Society of Cardiology (ESC) and American Heart Association (AHA) prevention guidelines, the use of ezetimibe alone or in combination is considered a class IIb recommendation.12,13
These results move us to more uncertainty about the benefit of this drug, and stimulated the need for conducting large genetic and clinical studies in order to test the impact of NPC1L1 inhibition on clinical outcome. Data from two large studies have been recently published and reviewed here to determine the clinical efficacy and safety of ezetimibe therapy.
NPC1L1 gene-inactivating mutation and coronary heart disease risk
This study was conducted using DNA samples from 16 case-control studies and cohort studies, and has been recently published in The New England Journal of Medicine in November 201414. During the first phase of the study, the 20 protein coding regions (exons) of NPC1L1 were initially sequenced in 7,364 patients with coronary heart disease (CHD) and in 14,728 controls who were of European, African, or South Asian ancestry. The most frequently observed NPC1L1 inactivating mutation during this phase was p.Arg406X. During the second phase of the study, p.Arg406X was genotyped in additional 91,002 participants.
Fifteen rare mutations (nonsense, splice-site, or frameshift mutations) that were expected to inactivate NPC1L1 protein were identified. Approximately 1 in every 650 persons was a heterozygous carrier for 1 of these mutations. No homozygotes or compound heterozygotes were identified. Carriers of any NPC1L1 inactivating mutations had a significantly lower plasma LDL-C level (mean adjusted difference, − 12 mg/dL; p = 0.04) and a lower risk of CHD (0.04% vs. 0.09% respectively, p = 0.008; odds ratio for carriers, 0.47, 95% confidence interval (CI), 0.25 to 0.8) than non-carriers. No significant differences in plasma triglycerides (mean difference − 12%; p = 0.11) or high-density lipoprotein cholesterol levels (mean difference 2 mg/dL, p = 0.29) were demonstrated between carriers and non-carriers.
Improve-it Study
The Improved Reduction of Outcomes: Vytorin Efficacy International Trial (IMPROVE-IT) was a phase III, multicenter, randomized, double-blind, placebo-controlled trial that was conducted at 1185 sites in 39 countries15 and was presented at the annual meeting of the AHA in November 201416. The study aimed to investigate the potential benefit from the addition of ezetimibe versus placebo to background simvastatin therapy in reducing cardiovascular (CV) events in patients at high risk. A total of 18,144 patients, aged 50 years or older, with recent acute coronary syndrome [ST-segment MI (STEMI) in 29%, Non ST-segment MI (NSTEMI) in 45%, and unstable angina (UA) in 24%] and plasma LDL-C ≤ 125 mg/dL (or ≤ 100 mg/dl if they were already taken statin) were randomized in a 1:1 fashion to either ezetimibe 10 mg/simvastatin 40 mg or simvastatin 40 mg.15 Uptitration to 80 mg simvastatin occurred in 27% of the simvastatin group and 6% of the ezetimibe/simvastatin group. The target plasma LDL-C level in simvastatin arm was < 70 mg/dL. Ezetimibe was assumed to further lower LDL-C by 15 mg/dL. The primary composite endpoint was cardiovascular (CV) death, no fatal MI, no fatal stroke, readmission for UA, and coronary revascularization ( ≥ 30 days after randomization).
Over a median follow up period of 7 years, the primary end-point was significantly lower in the ezetimibe/simvastatin arm compared with the simvastatin arm (32.7% vs. 34.7% respectively; hazard ratio [HR] 0.94; 95% CI 0.89-0.99; p = 0.016; number needed to treat [NNT] = 50). Ezetimibe/simvastatin therapy reduced LDL-C level to an average of 54 mg/dL, compared with 69 mg/dL for those treated with simvastatin alone. In terms of individual components of primary end-points, patients randomized to ezetimibe/simvastatin had significantly less incidence of MI (13.1% vs. 14.8% respectively, p = 0.002), stroke (3.4% vs. 4.1% respectively, p = 0.008), and CV death/MI/stroke (20.4% vs. 22.2% respectively, p = 0.003) compared to those randomized to simvastatin alone. No differences were detected in all-cause mortality (15.4% vs. 15.3%, p = 0.78), CV mortality (6.9% vs. 6.8%, p = 0.99) and need for coronary revascularization (21.8% vs. 23.4%, p = 0.11). Patients with diabetes had a greater benefit with ezetimibe/simvastatin (HR = 0.86, p = 0.023). No differences were observed in cancer incidence (10.2% vs. 10.2%, p = 0.57), myopathy (0.2% vs. 0.1%, p = 0.32), or transaminitis (2.5% vs. 2.3%, p = 0.43).
Discussion
Data came from these studies have emphasized the clinical benefit of genetic or pharmacological inhibition of NPC1L1 protein in reducing the risk of CHD. Naturally occurring DNA sequence variants that affect the activity of a particular protein target can be used to estimate the potential efficacy and safety of a drug targeting such proteins.17 Interestingly, rare protein-inactivating mutations in NPC1L1 have been shown to reduce both the plasma LDL-C concentration and the risk of CHD.
These findings were supported by the new results of the long-delayed and eagerly awaited IMPROVE-IT study that showed a modest benefit in reducing CV events when ezetimibe was added to background simvastatin therapy in high-risk patients. IMPROVE-IT study is the first outcome study to show an incremental clinical benefit for a non-statin agent when added to a statin in reducing CV events, especially MI and stroke. There were no significant differences in cancer, muscle and gall bladder related events, which confirm the safety profile of this drug. The mean levels of LDL-C in the ezetimibe/simvastatin arm of IMPROVE-IT study were less than 60 mg/dl which reaffirms “the lower is better” LDL hypothesis. However certain points need to be raised when these studies were thoroughly analyzed:
-
(1)
The 53% relative risk reduction (RRR) in the risk CHD that was detected in carriers of NPC1L1 inactivation mutation – as compared to only 13% RRR in the incidence of MI in the IMPROVE-IT trial – is a strong stimulus for future attempts to reproduce it clinically. However, lifelong genetic inhibition is totally different from pharmacologic inhibition that is initiated in adulthood and lasts for several years. In addition, pharmacological inhibition is counterbalanced by toxic effects that would not be tested in a genetic model.
-
(2)
The RRR in the IMPROVE-IT study is small over a long period of time. There were only 6% RRR in the primary composite end-point, primarily driven by reductions in non-fatal end points, and no mortality benefit. However NNT is only 50 to prevent one CV event. Moreover, the addition of ezetimibe was associated with 21% RRR in the incidence of stroke and 13% RRR in the incidence of MI which positively affect the quality of life of those patients.
-
(3)
The prolonged duration of the IMRPOVE-IT trial as well as the controversy surrounding the trial leads to concern that dropout and crossover rates may significantly reduce the possible benefits of ezetimibe.
-
(4)
The clinical benefit of further LDL-C lowering in patients with a baseline LDL-C < 70 mg/dl is unknown and not established, as reported in AHA guidelines.
-
(5)
The statin used in IMPROVE-IT study was simvastatin. Other available statins – such as atorvastatin or rosuvastatin – are more potent.
-
(6)
The effect of ezetimibe monotherapy in patients with higher LDL-C, particularly those who are statin-intolerant, was not assessed. No clinical outcome trials have been performed to investigate the clinical efficacy of ezetimibe in these patients.
What have we learned?
These data are another proof for how genetic studies are very helpful in clinical practice. In addition, it reaffirms the LDL hypothesis; low is good but lower is better which need to be re-addressed in future prevention guidelines. The positive results of IMPROVE-IT study support the use of ezetimibe as a reasonable adjunct to statin therapy in treating high risk patients. Moreover, it may reassure the FDA and help early approval of the PCSK9 inhibitors by accepting LDL-C lowering as a surrogate endpoint, without waiting evidence from clinical outcomes trials. Current evidence supports the future exciting role of NPC1L1 and PCSK9 inhibition in the treatment of hypercholesterolemia.
References
- 1.Davis HR, Zhu L-J, Hoos LM, Tetzloff G, Maguire M, Liu J, Yao X, Iyer SP, Lam MH, Lund EG, Detmers PA, Graziano MP, Altmann SW. Niemann-Pick C1 Like 1 (NPC1L1) is the intestinal phytosterol and cholesterol transporter and a key modulator of whole-body cholesterol homeostasis. J. Biol. Chem. 2004;279(32):33586–33592. doi: 10.1074/jbc.M405817200. [DOI] [PubMed] [Google Scholar]
- 2.Davies JP, Levy B, Ioannou YA. Evidence for a Niemann-pick C (NPC) gene family: identification and characterization of NPC1L1. Genomics. 2000;65(2):137–145. doi: 10.1006/geno.2000.6151. [DOI] [PubMed] [Google Scholar]
- 3.Carstea ED, Morris JA, Coleman KG, Loftus SK, Zhang D, Cummings C, Gu J, Rosenfeld MA, Pavan WJ, Krizman DB, Nagle J, Polymeropoulos MH, Sturley SL, Ioannou YA, Higgins ME, Comly M, Cooney A, Brown A, Kaneski CR, Blanchette-Mackie EJ, Dwyer NK, Neufeld EB, Chang TY, Liscum L, Strauss JF, 3rd, Ohno K, Zeigler M, Carmi R, Sokol J, Markie D, O'Neill RR, van Diggelen OP, Elleder M, Patterson MC, Brady RO, Vanier MT, Pentchev PG, Tagle DA. Niemann-Pick C1 disease gene: homology to mediators of cholesterol homeostasis. Science. 1997;277(5323):228–231. doi: 10.1126/science.277.5323.228. [DOI] [PubMed] [Google Scholar]
- 4.Cohen J, Pertsemlidis A, Kotowski IK, Graham R, Garcia CK, Hobbs HH. Low LDL cholesterol in individuals of African descent resulting from frequent nonsense mutations in PCSK9. Nat. Genet. 2005;37(2):161–165. doi: 10.1038/ng1509. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Calvo M, Lisnock J, Bull HG, Hawes BE, Burnett DA, Braun MP, Crona JH, Davis HR, Jr, Dean DC, Detmers PA, Graziano MP, Hughes M, Macintyre DE, Ogawa A, O'neill KA, Iyer SP, Shevell DE, Smith MM, Tang YS, Makarewicz AM, Ujjainwalla F, Altmann SW, Chapman KT, Thornberry NA. The target of ezetimibe is Niemann-Pick C1-Like 1 (NPC1L1) Proc. Natl. Acad. Sci. U.S.A. 2005;102(23):8132–8137. doi: 10.1073/pnas.0500269102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ballantyne CM, Houri J, Notarbartolo A, Melani L, Lipka LJ, Suresh R, Sun S, LeBeaut AP, Sager PT, Veltri EP. Effect of ezetimibe coadministered with atorvastatin in 628 patients with primary hypercholesterolemia: a prospective, randomized, double-blind trial. Circulation. 2003;107(19):2409–2415. doi: 10.1161/01.CIR.0000068312.21969.C8. [DOI] [PubMed] [Google Scholar]
- 7.Ezzet F, Wexler D, Statkevich P, Kosoglou T, Patrick J, Lipka L, Mellars L, Veltri E, Batra V. The plasma concentration and LDL-C relationship in patients receiving ezetimibe. J. Clin. Pharmacol. 2001;41(9):943–949. doi: 10.1177/00912700122010915. [DOI] [PubMed] [Google Scholar]
- 8.Davidson MH, McGarry T, Bettis R, Melani L, Lipka LJ, LeBeaut AP, Suresh R, Sun S, Veltri EP. Ezetimibe coadministered with simvastatin in patients with primary hypercholesterolemia. J. Am. Coll. Cardiol. 2002;40(12):2125–2134. doi: 10.1016/s0735-1097(02)02610-4. [DOI] [PubMed] [Google Scholar]
- 9.Raal FJ, Stein EA, Dufour R, Turner T, Civeira F, Burgess L, Langslet G, Scott R, Olsson AG, Sullivan D, Hovingh GK, Cariou B, Gouni-Berthold I, Somaratne R, Bridges I, Scott R, Wasserman SM, Gaudet D. PCSK9 inhibition with evolocumab (AMG 145) in heterozygous familial hypercholesterolaemia (RUTHERFORD-2): a randomised, double-blind, placebo-controlled trial. Lancet. 2014;(October) doi: 10.1016/S0140-6736(14)61399-4. doi:10.1016/S0140-6736(14)61399-4. [DOI] [PubMed] [Google Scholar]
- 10.Robinson JG, Nedergaard BS, Rogers WJ, Fialkow J, Neutel JM, Ramstad D, Somaratne R, Legg JC, Nelson P, Scott R, Wasserman SM, Weiss R. Effect of Evolocumab or Ezetimibe Added to Moderate- or High-Intensity Statin Therapy on LDL-C Lowering in Patients With Hypercholesterolemia The LAPLACE-2 Randomized Clinical Trial. JAMA. 2014;311(18):1870–1882. doi: 10.1001/jama.2014.4030. [DOI] [PubMed] [Google Scholar]
- 11.Kaye T. Simvastatin with or without ezetimibe in familial hypercholesterolemia. N. Engl. J. Med. 2008;359(5):531–532. [PubMed] [Google Scholar]
- 12.Reiner Z, Catapano AL, De Backer G, Graham I, Taskinen MR, Wiklund O, Agewall S, Alegria E, Chapman MJ, Durrington P, Erdine S, Halcox J, Hobbs R, Kjekshus J, Filardi PP, Riccardi G, Storey RF, Wood D, European Association for Cardiovascular Prevention&Rehabilitation ESC/EAS Guidelines for the management of dyslipidaemias: The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS) Eur. Heart J. 2011;32(14):1769–1818. doi: 10.1093/eurheartj/ehr158. [DOI] [PubMed] [Google Scholar]
- 13.Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC, Jr, Watson K, Wilson PW. ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults. J. Am. Coll. Cardiol. 2014;63(25):2889–2934. doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 14.The Myocardial Infarction Genetics Consortium Investigators. Inactivating Mutations in NPC1L1 and Protection from Coronary Heart Disease. N. Engl. J. Med. 2014 doi: 10.1056/NEJMoa1405386. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blazing MA, Giugliano RP, Cannon CP, Musliner TA, Tershakovec AM, White JA, Reist C, McCagg A, Braunwald E, Califf RM. Evaluating cardiovascular event reduction with ezetimibe as an adjunct to simvastatin in 18,144 patients after acute coronary syndromes: final baseline characteristics of the IMPROVE-IT study population. Am. Heart J. 2014;168(2):205–12e1. doi: 10.1016/j.ahj.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 16.http://www.cardiosource.org/science-and-quality/clinical-trials/i/improve-it.aspx?w_nav = RI [Google Scholar]
- 17.Plenge RM, Scolnick EM, Altshuler D. Validating therapeutic targets through human genetics. Nat. Rev. Drug Discov. 2013;12(8):581–594. doi: 10.1038/nrd4051. [DOI] [PubMed] [Google Scholar]
- 18.Ikonen E. Cellular cholesterol trafficking and compartmentalization. Nat. Rev. Mol. Cell Bio. 2008;9:125–138. doi: 10.1038/nrm2336. [DOI] [PubMed] [Google Scholar]


