Introduction
Lowering of low density lipoprotein (LDL) cholesterol (LDL-C) has been shown to be associated with significant reduction of adverse cardiovascular (CV) events.1 The 2013 ACC/AHA guidelines recommend high-intensity statin therapy - to lower LDL-C levels by ≥ 50% with no specific target goal - for adults at high risk for atherosclerotic CV diseases, and moderate-intensity statin therapy - to lower LDL-C levels by 30% - < 50% - if a high-intensity statin is not tolerated.2 Furthermore, European and Canadian guidelines recommend LDL-C goal of less than 70 mg/dL (1.8 mmol/L) in patients at very high CV risk.3,4
Despite intensive statin therapy, many patients are unable to achieve the recommended target levels of LDL-C. In addition, statin-related adverse events have been reported in up to 10% to 20% of patients,5 and stains may be not tolerated by certain subgroups of patients. This highlights the need for additional LDL-C lowering drugs. Unfortunately and up till recently, currently available non-statin LDL-C lowering therapies are either weak (ezetimibe) or poorly tolerated (niacin, and bile acid sequestrants).
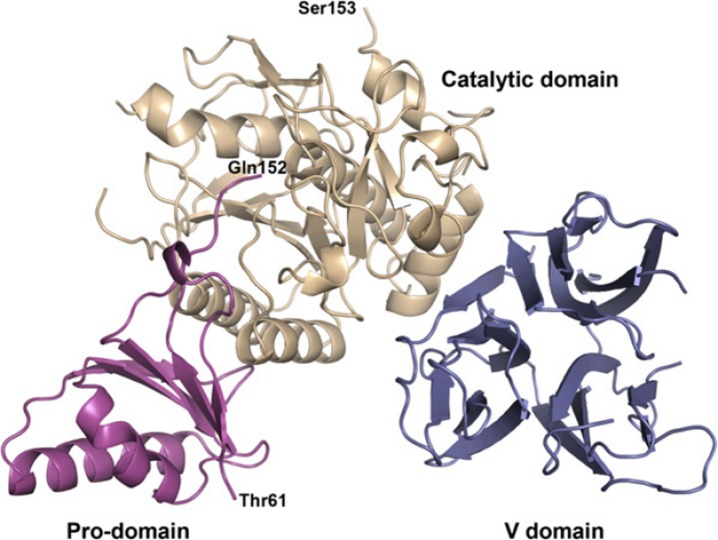
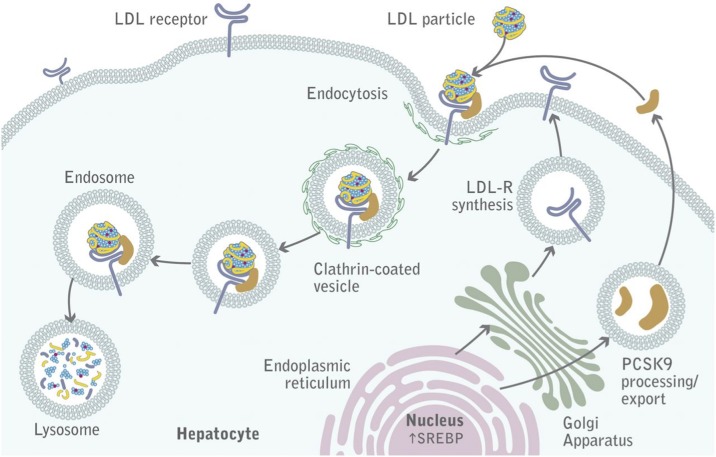
Proprotein convertase subtilisin/kexin type 9 (PCSK9) is a novel serine protease protein (Fig. 1) produced predominantly in the liver, and plays a central role in regulating LDL-C concentrations. PCSK9 binds to hepatic LDL receptors, promotes their degradation, and reduces the ability of the liver to clear LDL-C from the blood (Fig. 2).6,7 Statin use also upregulates PCSK9 levels, therefore PCSK9 inhibiton may additionally or synergistically lower LDL-C with statins.
Figure 1.
Structure of the PCSK9 protein: Ribbons diagram of PCSK9 structure with the prodomain in magenta, the catalytic domain in wheat, and the V domain in blue. Thr61 marks the first observed residue, and Gln152 marks the C terminus of the prodomain. Ser153 marks the N terminus of the catalytic domain (From Piper et al. The Crystal Structure of PCSK9: A Regulator of Plasma LDL-Cholesterol. Structure 2007; 15: 545–552).8
Figure 2.
PCSK9 mediated degradation of LDL receptors (From Lambert G et al. The PCSK9 decade. J. Lipid Res. 2012;53:2515-2524)6
Evolocumab (AMG145) – manufactured by Amgen - is a fully human monoclonal antibody that binds to PCSK9 and inhibits its interaction with LDL receptors (Fig. 2). Evolocumab has taken the lead in the race with other PCSK9 inhibitors to be the first in a new class of LDL-C lowering drugs close to the market (Table 1). Many phase II trials9–11 have evaluated the efficacy of evolocumab -including the longer term (52 weeks) OSLER study-12 and yielded robust reduction of circulating LDL-C concentration.
Table 1.
Therapeutic Approaches Targeting PCSK9. Adapted from Urban et al. Targeting the Proprotein Convertase Subtilisin/Kexin Type 9 for the Treatment of Dyslipidemia and Atherosclerosis. J Am Coll Cardiol 2013;62:1401–8.13
| Mechanism of Action | Agent | Company/Sponsor | Phase |
| Monoclonal antibodies | SAR236553/REGN727 | Sanofi/Regeneron | 3 |
| AMG 145 | Amgen | 3 | |
| RN316 | Pfizer | 2 | |
| RG7652 | Roche/Genentech | 2 | |
| LGT-209 | Novartis | 2 | |
| 1D05-lgG2 | Merck | Pre-clinical | |
| 1B20 | Merck | Pre-clinical | |
| J10, J16 | Pfizer | Pre-clinical | |
| J17 | Pfizer | Pre-clinical | |
| Adnectins | BMS-962476 | Bristol-Myers Squibb/Adnexus | 1 |
| Mimetic peptides | EGF-AB peptide fragment | Schering-Plough | Pre-clinical |
| LDLR (H306Y) subfragment | US NIH | Pre-clinical | |
| LDLR DNA construct | US NIH | Pre-clinical | |
| Small molecule inhibitors | SX-PCK9 | Serometrix | Pre-clinical |
| TBD | Shifa Biomedical | Pre-clinical | |
| Antisense oligonucleotides | ISIS 394814 | Isis | Pre-clinical |
| SPC4061 | Santaris-Pharma | Pre-clinical | |
| SPC5011 | Santaris-Pharma | 1 (terminated) | |
| RNA interference | ALN-PCS02 | Alnylam | 1 |
Data from several phase III studies have been recently released - as a part of the PROFICIO (Program to Reduce LDL-C and Cardiovascular Outcomes Following Inhibition of PCSK9 In Different POpulation) comprehensive program (Table 2). These trials serve to clarify the efficacy of evolocumab in different groups of patients, and are critically reviewed here with particular reference to their clinical utility, and their place in future clinical practice.
Table 2.
PROFICIO program
| Study | Aim |
| GAUSS-2 14 , GAUSS-3 | Statin intolerant patients |
| RUTHERFORD-2 15 | Heterozygous Familial hypercholesterolemia |
| MENDEL-2 | Evolocumab stand-alone monotherapy |
| TESLA Part B 16 | Homozygous familial hypercholesterolemia |
| LAPLACE-2 17 , YUKAWA-2 | Combination therapy with statins |
| DESCARTES 18 , OSLER-2 | Long term (52 weeks) therapy |
| TAUSSIG | Long term therapy in familial hypercholesterolemia |
| FOURIER | Secondary prevention of CV events |
| GLAGOV | Plaque regression measured by intravascular ultrasound |
PROFRICO (Latin word means: to advance or to make progress)
GAUSS-2 study
The Goal Achievement after Utilizing an Anti-PCSK9 Antibody in Statin Intolerant Subjects (GAUSS-2) study was a randomized, double-blind, phase III clinical trial that has been recently published in the Journal of the American College of Cardiology in June 2014.14 This study was designed to evaluate the efficacy and safety of subcutaneous (SC) evolocumab injection, compared to ezetemibe, in statin-intolerant (to at least 2 statins) hypercholesterolemic patients. A total of 307 patients, 18-80 years old, were randomized 2:2:1:1 to SC evolocumab 140 mg every two weeks (Q2W) or evolocumab 420 mg once monthly (QM) “both with daily oral placebo” or SC placebo Q2W or QM “both with daily oral ezetimibe 10 mg”. Co-primary endpoints were percent change from baseline in LDL-C concentration at the mean of weeks 10 and 12 and at week 12. Co-secondary efficacy endpoints at the same time points included absolute change in LDL-C from baseline, percent of patients achieved LDL-C < 70 mg/dl, and percent change of other lipoproteins. Safety endpoints included treatment emergent and serious adverse events, creatine kinase (CK) and hepatic enzyme elevations, and anti-evolocumab antibodies.
At a mean of weeks 10 and 12, evolocumab achieved mean percent reductions of LDL-C of 56.1% (Q2W dose) and 55.3% (QM dose), compared to 36.9-38.7% in ezetemibe-treated patients (p < 0.001). Furthermore, 87.5% of evolocumab treated patients achieved LCL-C < 70 mg/dl compared to only 2% in ezetemibe-treated patients. Evolocumab reduced lipoprotein(a) levels by 27% (Q2W dose) and 22% (QM dose) at week 12 compared to 1.7 - 5.8% in ezetemibe-treated patients. Evolocumab was discontinued due to adverse events in 8% of patients compared to 13% in ezetemibe treated patients. Myalgia occurred in 8% of evolocumab-treated patients and 18% of ezetimibe-treated patients. No binding or neutralizing antibodies to evolocumab were detected.
Rutherford-2 Study
The Reduction of LDL-C with PCSK9 Inhibition in Heterozygous Familial Hypercholesterolemia Disorder-2 (RUTHERFORD-2) study15 was a multicentre, randomized, double-blind, placebo-controlled trial that recruited patients at 39 sites in Australia, Asia, Europe, New Zealand, North America, and South Africa, and has been recently published in the Lancet journal in October 2014. The study aimed to assess the safety and efficacy of evolocumab in patients with heterozygous familial hypercholesterolemia and LDL-C concentrations ≥ 100 mg/dL (2.6 mmol/L) despite intense lipid-lowering therapy. A total of 331 eligible patients, 18 - 80 years old, were randomly assigned in a 2:2:1:1 ratio to receive SC evolocumab 140 mg Q2W, evolocumab 420 mg QM, placebo Q2W, or placebo QM for 12 weeks. The Co-primary and secondary endpoints were similar to that adopted in the GAUSS-2 study.
At a mean of weeks 10 and 12, evolocumab achieved mean percent reductions of LDL-C of 60.2% [95% confidence interval (CI) = 54.5–65.8] (Q2W dose) and 65.6% [95% CI = 59.8–71.3] (QM dose), compared to placebo (p < 0.0001). These effects were not related to age, gender, body-mass index, baseline LDL-C level, intensity of statin therapy, or concomitant use of ezetimibe. At week 12, LDL-C < 70 mg/dl was achieved by 68% of patients in the evolocumab Q2W group and by 63% in the evolocumab QM group, compared to only 2% in each of the placebo groups. At 12 week, evolocumab Q2W achieved significant reduction in serum triglycerides (treatment difference − 19.6% [95% CI = − 27.9 to − 11.3], p < 0.0001), and lipoprotein (a) (treatment difference − 31.6% [95% CI = –39.3 to − 23.9], p < 0.0001), as well as significant increase (9.2% [95% CI: 4.7 to 13.7], p < 0.0001) in HDL-C level compared to placebo. Similar results were detected in the evolocumab QM group. Patients with receptor-negative mutations had a similar response to treatment as those with receptor-defective mutations or those with mutations in apolipoprotein B. No serious adverse events led to study drug discontinuation. The most common adverse events in the evolocumab group were nasopharyngitis (9.0%) and muscle-related adverse events (5.0%).
Laplace-2 Study
The LDL-C Assessment with PCSK9 Monoclonal Antibody Inhibition Combined With Statin Therapy (LAPLACE-2) study was a Phase III, randomized, double-blind, placebo- and ezetimibe-controlled study that was conducted at 198 sites in 17 countries and was published in the Journal of the American Medical Association in May 2014.17 The study was designed to evaluate the efficacy and safety of evolocumab vs. placebo or ezetimibe in patients randomized to different background moderate- and high-intensity statin therapies. A total of 1899 patients, 18 to 80 years old, with hyperlipidemia [screening LDL-C level ≥ 150 mg/dL (no statin at screening), ≥ 100 mg/dL (non-intensive statin at screening), or ≥ 80 mg/dl (intensive statin at screening) provided that fasting triglyceride ≤ 400 mg/dL] were enrolled. Intensive statin therapy was defined as daily atorvastatin ( ≥ 40 mg), rosuvastatin (20 mg), simvastatin (80 mg), or any statin plus ezetimibe. Patients were randomized to 1 of 24 treatment arms. Patients were initially randomized to daily moderate-intensity statin (atorvastatin 10 mg, simvastatin 40 mg, or rosuvastatin 5 mg) or high-intensity statin (atorvastatin 80 mg, rosuvastatin 40 mg). After a 4-week lipid-stabilization period, they were randomized to receive evolocumab (140 mg Q2W or 420 mg QM), placebo (Q2W or QM; in all patients) or ezetimibe (10 mg or placebo daily; in atorvastatin patients only). The co-primary and secondary endpoints were similar to that adopted in the GAUSS-2 and RUTHERFORD-2 studies.
Compared to placebo, LDL-C concentration at the mean of weeks 10 and 12 was significantly reduced by 66% to 75% in the evolocumab Q2W group and by 63% to 75% in the evolocumab QM group in the moderate- and high-intensity statin-treated group respectively (p < 0.001). Ezetemibe reduced LDL-C concentration by 17-20% in moderate-intensity statin groups and 51-62% in high-intensity groups. In patients treated with atorvastatin (10 mg or 80 mg), the addition of evolocumab reduced LDL-C concentration by 61% to 62% (Q2W dose) and by 62% to 65% (QM dose), while ezetemibe reduced LDL-C concentration by 17% to 24% (p < 0.001).
Adverse events were reported in 36%, 40%, and 39% of evolocumab-, ezetimibe-, and placebo-treated patients, respectively. The most common adverse events in evolocumab-treated patients were back pain, arthralgia, headache, muscle spasms, and pain in extremity. Serious adverse events were reported in 2.1% of evolocumab-treated patients, 0.9% of ezetimibe-treated patients, and 2.3% of placebo-treated patients. Neurocognitive adverse events were reported in 0.1%, 1.4%, 0% of evolocumab-, ezetimibe-, and placebo-treated patients.
Descartes Study
The Durable Effect of PCSK9 Antibody Compared with Placebo Study (DESCARTES) study was a randomized, double-blind, placebo-controlled, phase III trial that was conducted at 88 centers in 9 countries, and was published in the New England Journal of Medicine in May 2014. The study compared evolocumab with placebo in patients with hyperlipidemia (LDL-C level ≥ 75 mg/dl provided that fasting triglycerides < 400 mg/dl).18 After a 4-12 weeks run-in period of open-label background lipid lowering therapy (diet alone, diet plus atorvastatin 10 mg daily, atorvastatin 80 mg daily, or atorvastatin 80 mg daily plus ezetimibe 10 mg daily), eligible patients were randomized (2:1) to SC evolocumab (420 mg) or placebo QM. No changes to the assigned background lipid-lowering therapy were permitted. The primary efficacy end-point was the percent change from baseline in the LDL cholesterol level at week 52. Secondary end-points included the absolute change in LDL-C from baseline at week 52, the percent change from baseline in the LDL-C at week 12, and the percentage of patients achieved LDL-C level of < 70 mg /dL at week 52, the percent change from baseline at week 52 in other lipids and lipoproteins. The long-term consistency of evolocumab effect was tested by comparing the percent change in the LDL-C level at week 12 with that at week 52. Among 901patients were included in the study, 800 (88.4%) completed 52 weeks of treatment.
At 52 weeks, evolocumab treatment resulted in 57% mean reduction in LDL-C versus placebo. LDL-C reduction was 55.7 ± 4.2% in the diet-alone group, 61.6 ± 2.6% in the atorvastatin 10 mg group, 56.8 ± 5.3% in the atorvastatin 80 mg group, and 48.5 ± 5.2% in the atorvastatin 80 mg plus ezetimibe 10 mg group. LDL-C concentration < 70 mg/dL was achieved in 82.3%. There were also significant reductions from baseline in apolipoprotein B, non-HDL cholesterol, lipoprotein (a), and triglycerides, as well as 5.4% and 3.0% increases in HDL-C and apoA1.
Adverse events occurred in 74.8% patients treated with evolocumab and in 74.2% treated with placebo. The most common adverse events on evolocumab were nasopharyngitis, upper respiratory tract infection, influenza, and back pain. Serious adverse events were slightly higher with evolocumab than placebo (5.5% versus 4.3%). Myalgia was reported by 4% of patients on evolocumab vs. 3% on placebo. No anti-evolocumab neutralizing antibodies were detected in any patient.
TESLA Part B study:
The Trial Evaluating PCSK9 Antibody in Subjects with LDL Receptor Abnormalities (TESLA) study was a prospective, double- blind, placebo-controlled phase III study that has been recently published in the Lancet journal in October 2014.16 The study enrolled 50 patients with homozygous familial hypercholesterolemia on lipid lowering therapy for at least 4 weeks and not receiving lipoprotein apharesis. Patients were randomized in a 2:1 ratio to receive SC evolocumab 420 mg QM or placebo for 12 weeks. The primary endpoint was percent change from baseline in LDL-C at week 12.
Compared to placebo, evolocumab significantly reduced LDL-C at 12 weeks by 30.9% (95%CI = − 43.9 to − 18%, p = 0.001). Patients with defective receptor mutation had the best response to treatment, and had significantly higher reduction in LDL-cholesterol compared to those with single LDL receptor negative mutation. Evolocumab had no effect in patients with negative/negative mutation and LDL-C increased 10% from baseline. No serious clinical or laboratory adverse events occurred, and no anti-evolocumab antibody development.
Discussion
Although statins are the mainstay of treatment for lowering LDL-C concentration, additional LDL-lowering therapies are needed to reduce residual CV risk especially in patients at very high risk, hereditary lipid disorders, or statin intolerance. PCSK9 inhibitors have taken another big step after the promising data came from the recently published phase III studies. The addition of evolocumab to existing lipid-lowering therapy has achieved significant (approximately 60%), consistent, and sustained reduction in LDL-C levels in patients with varying levels of CV risk. PCSK9 inhibitors produced also significant reduction of lipoprotein(a) concentration in all studies. The exact mechanism is unknown, and further studies exploring the benefits of evolocumab in patients with elevated lipoprotein (a) are warranted.
These results support the effectiveness of evolocumab as a treatment option in patients with hypercholesterolemia, and help to dramatically facilitate achievement of LDL-C goals. However, the effect of evolocumab on clinical outcome has not been yet confirmed and still waiting the results of the ongoing large scale FOURIER (Further Cardiovascular Outcomes Research with PCSK9 inhibition in Subjects with Elevated Risk) study which test the effect of evolocumab on primary endpoint of death, myocardial infarction and hospitalization in 22,500 patients.
In the RUTHERFORD-2 study, the response to evolocumab was unrelated to the underlying genetic mutation. However, these results should be viewed as a hypothesis generating, as it was not pre-specified in the study protocol. Therefore, the genetic analysis might not be helpful in assessment of response to evolocumab in patients with heterozygous familial hypercholesterolemia, unlike patients with homozygous familial hypercholesterolemia in the TESLA Part B study, in whom genetic information seems to be helpful in predicting response to evolocumab treatment
No synergism between statins and PCSK9 inhibition was observed in many trials. The addition of evolocumab to baseline statin therapy in the LAPLACE-2 study resulted in similar percent reductions in LDL-C levels regardless of baseline statin type, dose, or intensity. This may reflect greater up-regulation of PCSK9 levels with high-intensity statin therapy, therefore patients may require higher doses of the antibody.
Evolocumab was well tolerated in all studies and was associated with low rates of adverse event. No serious adverse events led to its discontinuation. Moreover, the low incidence of muscle related side effects gives hope that evolocumab can be used as an alternative therapy in statin-intolerant patients. However, long term studies are needed to determine whether there is potential harm from such drastic reduction in LDL-C levels. Cholesterol is an important component of cell membrane and neurons, moreover PCSK9 is involved in cortical neuron regeneration.19 Very low LDL-C level may be associated with increased incidence of hemorrhagic stroke, neurocognitive impairment, hormonal insufficiency, and hemolytic anemia.20 On the other hand, the Friedewald equation may underestimate LDL-C when the level is very low.21
In results of these 5 phase III studies, Amgen announced the submission of license application to the US Food and Drug Administration (FDA) seeking approval of evolocumab for treatment of high cholesterol. The FDA reported that evolocumab will be reviewd on the basis of LDL-C lowering alone, may be before the clinical outcome studies are completed. However, FDA has requested assessment of the potential neurocognitive adverse events across the whole program despite that no signal for such events in the all phase III trials.
What have we learned?
The PCSK9 story highlights the great importance of molecular medicine, and how it serves clinical practice and development of novel therapeutic approaches to manage a large scale of diseases. These trials showed robust efficacy and favorable safety profile that makes evolocumab a promising and attractive therapy for high risk patients, patients with statin intolerance, and those with familial hypercholesterolemia in addition to the currently available LDL-C lowering therapies. To date, no major adverse effects have been reported. Ongoing phase III trials will provide more information on the long-term safety and efficacy of PCSK9 inhibiton in reducing CV events.The long term results of these trials are eagerly awaited, with the expectations that PCSK9 inhibitors will have a major impact on the treatment of dyslipidemia and CV diseases.
References
- 1.Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, Peto R, Barnes EH, Keech A, Simes J, Collins R. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670–1681. doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stone NJ, Robinson JG, Lichtenstein AH, Merz CNB, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC, Watson K, Wilson PWF. 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults. J. Am. Coll. Cardiol. 2014;63(25_PA):2889–2934. doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Reiner Z, Catapano AL, De Backer G, Graham I, Taskinen MR, Wiklund O, Agewall S, Alegria E, Chapman MJ, Durrington P, Erdine S, Halcox J, Hobbs R, Kjekshus J, Filardi PP, Riccardi G, Storey RF, Wood D. ESC/EAS Guidelines for the management of dyslipidaemias The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS) Eur. Heart J. 2011;32:1769–1818. doi: 10.1093/eurheartj/ehr158. [DOI] [PubMed] [Google Scholar]
- 4.Anderson TJ, Grégoire J, Hegele RA, Couture P, Mancini GB, McPherson R, Francis GA, Poirier P, Lau DC, Grover S, Genest J, Jr, Carpentier AC, Dufour R, Gupta M, Ward R, Leiter LA, Lonn E, Ng DS, Pearson GJ, Yates GM, Stone JA, Ur E. 2012 Update of the Canadian Cardiovascular Society Guidelines for the Diagnosis and Treatment of Dyslipidemia for the Prevention of Cardiovascular Disease in the Adult. Can. J. Cardiol. 2013;29:151–167. doi: 10.1016/j.cjca.2012.11.032. [DOI] [PubMed] [Google Scholar]
- 5.Zhang H, Plutzky J, Skentzos S, Morrison F, Mar P, Shubina M, Turchin A. Discontinuation of statins in routine care settings: a cohort study. Ann. Intern. Med. 2013;158(7):526–534. doi: 10.7326/0003-4819-158-7-201304020-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lambert G, Sjouke B, Choque B, Kastelein JJP, Hovingh GK. The PCSK9 decade. J. Lipid Res. 2012;53(12):2515–2524. doi: 10.1194/jlr.R026658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elguindy A, Yacoub MH. The discovery of PCSK9 inhibitors: A tale of creativity and multifaceted translational research. Glob. Cardiol. Sci. Pract. 2013;4:1–5. doi: 10.5339/gcsp.2013.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piper DE, Jackson S, Liu Q, Romanow WG, Shetterly S, Thibault ST, Shan B, Walker NP. Article The Crystal Structure of PCSK9: A Regulator of Plasma LDL-Cholesterol. Structure. 2007;15:545–552. doi: 10.1016/j.str.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 9.Giugliano RP, Desai NR, Kohli P, Rogers WJ, Somaratne R, Huang F, Liu T, Mohanavelu S, Hoffman EB, McDonald ST, Abrahamsen TE, Wasserman SM, Scott R, Sabatine MS. Efficacy, safety, and tolerability of a monoclonal antibody to proprotein convertase subtilisin/kexin type9 incombination with a statin in patients with hypercholesterolaemia (LAPLACE-TIMI57): a randomised, placebo-controlled, dose- ranging, phase2 study. Lancet. 2012;380:2007–2017. doi: 10.1016/S0140-6736(12)61770-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raal F, Scott R, Somaratne R, Bridges I, Li G, Wasserman SM, Stein EA. Low-density lipoprotein cholesterol- lowering effects of AMG145, a monoclonal antibody to proprotein convertase subtilisin/ kexin type9 serine protease in patients with heterozygous familial hypercholesterolemia: theReduction of LDL-C with PCSK9 Inhibi. Circulation. 2012;126:2408–2417. doi: 10.1161/CIRCULATIONAHA.112.144055. [DOI] [PubMed] [Google Scholar]
- 11.Sullivan D, Olsson AG, Scott R, Kim JB, Xue A, Gebski V, Wasserman SM, Stein EA. Effect of a monoclonal antibody to PCSK9 on low-density lipoprotein cholesterol levels in statin-intolerant patients: the GAUSS randomized trial. JAMA. 2012;308:2497–2506. doi: 10.1001/jama.2012.25790. [DOI] [PubMed] [Google Scholar]
- 12.Koren MJ, Giugliano RP, Raal FJ, Sullivan D, Bolognese M, Langslet G, Civeira F, Somaratne R, Nelson P, Liu T, Scott R, Wasserman SM, Sabatine MS. Efficacy and Safety of Longer-Term Administration of Evolocumab (AMG 145) in Patients With Hypercholesterolemia: 52-Week Results From the Open-Label Study of Long-Term Evaluation Against LDL-C (OSLER) Randomized Trial. Circulation. 2013;129(2):234–243. doi: 10.1161/CIRCULATIONAHA.113.007012. [DOI] [PubMed] [Google Scholar]
- 13.Urban D, Pöss J, Böhm M, Laufs U. Targeting the proprotein convertase subtilisin/kexin type 9 for the treatment of dyslipidemia and atherosclerosis. J. Am. Coll. Cardiol. 2013;62(16):1401–1408. doi: 10.1016/j.jacc.2013.07.056. [DOI] [PubMed] [Google Scholar]
- 14.Stroes E, Colquhoun D, Sullivan D, Civeira F, Rosenson RS, Watts GF, Bruckert E, Cho L, Dent R, Knusel B, Xue A, Scott R, Wasserman SM, Rocco M. Anti-PCSK9 Antibody Effectively Lowers Cholesterol in Patients With Statin Intolerance. J Am Coll Cardiol. 2014;63(23):2541–2548. doi: 10.1016/j.jacc.2014.03.019. [DOI] [PubMed] [Google Scholar]
- 15.Raal FJ, Stein EA, Dufour R, Turner T, Civeira F, Burgess L, Langslet G, Scott R, Olsson AG, Sullivan D, Hovingh GK, Cariou B, Gouni-Berthold I, Somaratne R, Bridges I, Scott R, Wasserman SM, Gaudet D. PCSK9 inhibition with evolocumab (AMG 145) in heterozygous familial hypercholesterolaemia (RUTHERFORD-2): a randomised, double-blind, placebo-controlled trial. Lancet. 2014 doi: 10.1016/S0140-6736(14)61399-4. October. doi:10.1016/S0140-6736(14)61399-4. [DOI] [PubMed] [Google Scholar]
- 16.Raal FJ, Honarpour N, Blom DJ, Hovingh GK, Xu F, Scott R, Wasserman SM, Stein EA. Inhibition of PCSK9 with evolocumab in homozygous familial hypercholesterolaemia (TESLA Part B): a randomised, double-blind, placebo-controlled trial. Lancet. 2014 doi: 10.1016/S0140-6736(14)61374-X. October. doi:10.1016/S0140-6736(14)61374-X. [DOI] [PubMed] [Google Scholar]
- 17.Robinson JG, Nedergaard BS, Rogers WJ, Fialkow J, Neutel JM, Ramstad D, Somaratne R, Legg JC, Nelson P, Scott R, Wasserman SM, Weiss R. Effect of Evolocumab or Ezetimibe Added to Moderate- or High-Intensity Statin Therapy on LDL-C Lowering in Patients With Hypercholesterolemia The LAPLACE-2 Randomized Clinical Trial. JAMA. 2014;311(18):1870–1882. doi: 10.1001/jama.2014.4030. [DOI] [PubMed] [Google Scholar]
- 18.Blom DJ, Hala T, Bolognese M, Lillestol MJ, Toth PD, Burgess L, Ceska R, Roth E, Koren MJ, Ballantyne CM, Monsalvo ML, Tsirtsonis K, Kim JB, Scott R, Wasserman SM, Stein EA. A 52-Week Placebo-Controlled Trial of Evolocumab in Hyperlipidemia. NEJM. 2014;370(19):1809–1819. doi: 10.1056/NEJMoa1316222. [DOI] [PubMed] [Google Scholar]
- 19.Dadu RT, Ballantyne CM. Lipid lowering with PCSK9 inhibitors. Nat. Rev. Cardiol. 2014;11(10):563–575. doi: 10.1038/nrcardio.2014.84. [DOI] [PubMed] [Google Scholar]
- 20.Larosa JC, Pedersen TR, Somaratne R, Wasserman SM. Safety and effect of very low levels of low-density lipoprotein cholesterol on cardiovascular events. Am. J. Cardiol. 2013;111:1221–1229. doi: 10.1016/j.amjcard.2012.12.052. [DOI] [PubMed] [Google Scholar]
- 21.Martin SS, Blaha MJ, Elshazly MB, Brinton EA, Toth PP, McEvoy JW, Joshi PH, Kulkarni KR, Mize PD, Kwiterovich PO, DeFilippis AP, Blumenthal RS, Jones SR. Friedewald-estimated versus directly measured low-density lipoprotein cholesterol and treatment implications. J. Am. Coll. Cardiol. 2013;62:732–739. doi: 10.1016/j.jacc.2013.01.079. [DOI] [PubMed] [Google Scholar]