Abstract
Background: The reason why some individuals but not others are susceptible to rheumatic fever and chronic rheumatic heart disease is not understood. Because of the substantial evidence that poverty is an important determinant of the disease and must operate in early life, we have investigated the role of the early environment in an ecological study using 20th century mortality as an index of disease prevalence. Methods: We analysed 37,321 deaths from rheumatic heart disease in England and Wales during 1968–78. We compared the geographical distribution of deaths with previous infant mortality records from 1911 onwards. These records included details of mortality at different ages and from different causes. They also included data on housing and population density. Results: Mortality from rheumatic heart disease showed a strong correlation with past infant mortality that was consistently stronger with postneonatal mortality (deaths from one month to one year) than with neonatal mortality (deaths during the first month of life). Areas with high infant mortality from diarrhoea or bronchitis had the highest subsequent mortality from rheumatic heart disease. Although rheumatic heart disease was linked with early overcrowding, regression analyses suggested that overcrowding could not per se explain the infant mortality associations. Conclusions: Chronic rheumatic heart disease may have its origins in early infancy. Our findings raise the possibility that susceptibility to rheumatic fever and rheumatic heart disease may be linked with infection in the postneonatal period. Alternatively, they may be explained by the operation of environmental factors that both predispose to infection in infancy and the subsequent liability to heart disease.
Keywords: Chronic Rheumatic Heart disease, infant mortality, postneonatal mortality
Background
While it is well-recognised that chronic rheumatic heart disease results from an autoimmune response following infection with Lancefield group A, β-haemolytic streptococci in childhood or early adolescence, the influences through which some people develop this adverse reaction to a common organism remain elusive. In the Western world acute rheumatic fever and chronic rheumatic heart disease have virtually disappeared, a decline which began long before antibiotics were available.1 However, the disease remains a major public health problem in both resource poor countries and among the poor indigenous populations of some wealthy countries.2,3 Historically, rheumatic fever and its cardiac sequelae have been consistently and strongly linked with poor living conditions. Surveys carried out in the UK in the earlier years of the twentieth century demonstrated as much as a 30-fold gradient in incidence between deprived and affluent urban areas,4 and similar gradients are still found in resource-poor countries.3,5 However, the processes by which poverty increases susceptibility to this disease are not understood and are not easily explained by the biology of streptococcal infection.
Because rheumatic fever following streptococcal infection tends to occur only after the age of five, it is likely that poverty exerts its effects prior to this during early childhood. At this stage adverse influences are known to result in lifelong changes in morphology and physiology, a process is known as programming or developmental plasticity.6 These changes are thought to be important in predisposing to later life disease and underlie the “Developmental Origins Hypothesis,” which proposes that many common diseases of adult life have their origins during early development. This hypothesis had its origins in epidemiological studies demonstrating that markers of a poor early environment such as low birthweight or poor growth in early infancy correlate with the occurrence of a variety of later life conditions ranging from cardiovascular disease or stroke to chronic bronchitis. The hypothesis has been strongly supported by prospective clinical studies and experimental interventions in animal models. These studies show that the epidemiological associations are not merely due to the persistence of poor living conditions throughout the lifecourse.7 Although there are suggestions that rheumatic heart disease may be primed by conditioning factors operating in early life, for example viruses,8 nutritional factors,3 or recurrent exposure to haemolytic streptococci,9 it is not known whether the disease has a developmental origin although two recent studies have suggested that umbilical cord length, a possible marker of prenatal vascular function, is linked with the disease.10,11
The objective of the study was to investigate the possible role of developmental influences in rheumatic heart disease. We used rheumatic heart disease mortality data as an index of disease prevalence and evaluated the geographical correlations with previous infant mortality, an indicator of health in infancy. The study was based on a historical dataset of mortality in England and Wales collected between 1968 and 1978 in the 212 local authority areas into which the country was formerly divided. This dataset was compared with infant mortality records, which were maintained from 1911 onwards and included details of mortality at different ages and from different causes. Analyses of mortality from coronary heart disease and pulmonary disease based on this dataset have been published12,13 and although the study had details of a large number of deaths attributed to chronic rheumatic heart disease, no separate analysis of this condition has been carried out.
Methods
The Office of National Statistics made available extracts from all death certificates in England and Wales during 1968–78, the period covered by the eighth revision of the international classification of diseases (ICD). Death rates were based on the 1971 census and are expressed as standardised mortality ratios. In England and Wales infant mortality rates were recorded from 1911 onwards. The data were divided into neonatal mortality (deaths during the first month of life) and postneonatal mortality (deaths from one month to one year) Numbers of infant deaths by specific causes were published from 1921 and the current analysis is based on the years 1921–25. Causes of infant deaths were divided into five groups using Woolf's classification – congenital, bronchitis and pneumonia, infectious diseases, diarrhoea, and others.14
Adult mortality from chronic rheumatic heart disease (ICD 393–398) was compared with infant mortality in the four main geographical groupings used by the Registrar General – that is county boroughs (larger towns), London boroughs, urban areas (metropolitan boroughs and urban districts outside London), and rural areas. These groups divide England and Wales into 212 local authority areas comprising 80 county boroughs, 15 London boroughs, 59 urban areas and 58 rural areas.
Although data on the social class distribution were not available in these early censuses, they did include extensive data on housing. These comprised the number of rooms in each dwelling, the number of families per dwelling, the family size, and the number of rooms per person given separately for all families and families living in houses with up to 9 rooms, in order to exclude people living in boarding houses or institutions. The census reports also provided data on the population density expressed as the number of people per acre.
Standardised mortality ratios were calculated to allow for geographical differences in the age and sex distribution of the population such that the overall mean for England and Wales was 100. We studied deaths in the 55–74 age group as this was the approximate age range of the generation born on or after 1911 and excludes deaths in older age groups where the cause is less likely to have been accurately ascertained.
We used Poisson regression to model the standardised mortality ratios (SMRs) for chronic rheumatic heart disease during 1968–78. Poisson regression weights the analysis appropriately to allow for variation in population size across the 212 areas. The numerator of the SMR is an observed number of cases which is fitted using a Poisson distribution and a log link with the log of the expected number of cases as an offset. The regression coefficients of the model when exponentiated represent the multiplicative increase in SMR per unit change in the predictor variable.
Results
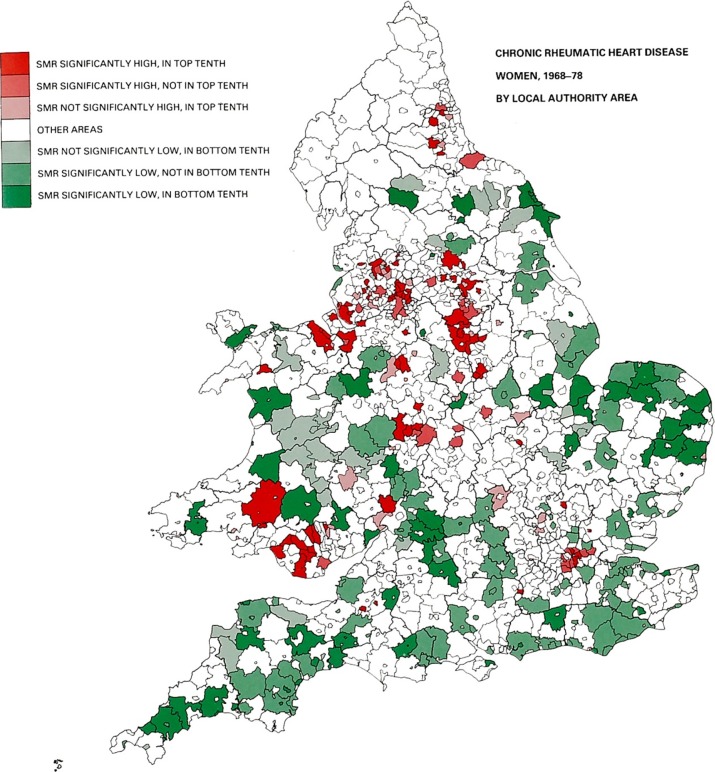
During 1968–78 there were a total of 37,321 deaths from rheumatic heart disease, a rate of 338.6 per million (301.6 in men and 338.6 in women). Of these 16,813 were due to mitral valve disease, 9734 were aortic and 3687 combined aortic and mitral disease. The remaining deaths were due to rheumatic carditis affecting other structures or the site of the disease was unspecified. Over the 11 year period the death rate fell from 372.8 to 288.3 per million. Age and sex standardised death rates were highest in the London and county boroughs (Standardised Mortality Rates (SMRs) 112.8 and 110.2 respectively) and were lowest in the urban and rural districts (SMRs 93.0 and 84.7 respectively). Figures 1 and 2 show the geographical distribution of the deaths in the men and women respectively and illustrate the similarity of trends in both genders. A pattern of high mortality rates (coloured red) is observed in the major urban concentrations, notably in South Wales, London, and the traditional industrial areas of the Midlands, the north west and north east. The lowest rates (coloured green) are mainly the rural areas. The infant mortality rate in England and Wales during 1911-15 was 102.5/1000 births and fell to 72.1/1000 in 1921–25 (Table 1). It was accompanied by similar declines in both neonatal and postneonatal mortality. Table 1 also shows the specific causes of infant mortality in 1921–25 and the data on population density and crowding from the 1921 census.
Figure 1.
Mortality from chronic rheumatic heart disease in England & Wales, 1968–78 in men by local authority areas.27
Figure 2.
Mortality from chronic rheumatic heart disease in England & Wales, 1968–78 in women by local authority areas.27
Table 1.
Mean infant, neonatal and postneonatal mortality rate per 1000 for the 212 administrative areas calculated for the years 1911–15, 1916–20 and 1921–25. The causes of postneonatal mortality in 1921–25 are also listed as are measures of population density and crowding from the 1921 census.
| Mortality rate per 1000 | Mean | Minimum, maximum |
| 1911–1915 | ||
| Infant | 102.5 | 61.8, 170.9 |
| Neonatal | 38.5 | 21.6, 61.2 |
| Postneonatal | 64.1 | 28.3, 124.1 |
| 1916–1920 | ||
| Infant | 84.7 | 50.5, 138.4 |
| Neonatal | 36.7 | 20.6, 51.4 |
| Postneonatal | 48.1 | 21.9, 90.1 |
| 1921–1925 | ||
| Infant | 72.1 | 44.0, 114.2 |
| Neonatal | 33.2 | 21.7, 48.6 |
| Postneonatal | 38.9 | 14.5, 74.5 |
| Diarrhoea | 6.8 | 2.1, 18.8 |
| Infection | 4.9 | 1.7, 13.0 |
| Bronchitis | 14.3 | 2.1, 33.0 |
| Congenital | 30.9 | 20.2, 48.2 |
| Other | 15.2 | 6.0, 30.4 |
| 1921 census | ||
| People/acre | 13.6 | 0.1, 115.0 |
| Rooms/dwelling | 5.2 | 3.3, 7.0 |
| Families/dwelling | 1.1 | 1.0, 2.1 |
| People/family | 4.1 | 3.3, 5.1 |
| Rooms per person | 1.2 | 0.7, 1.6 |
| Rooms per person* | 1.1 | 0.7, 1.5 |
* Houses with 1–9 rooms
Table 2 shows the correlation coefficients between infant mortality in the three time periods and the standardised mortality rate for chronic rheumatic heart disease at age 55–74. In the combined group of all 212 local authority areas, the correlation coefficients were high in all three time periods (r = 0.72 to 0.73), and the correlations with infant mortality were consistently higher in the postneonatal than the neonatal periods. When the correlations were analysed according to the geographical groups – i.e., county boroughs (CBs), London boroughs (LBs), urban areas and rural areas, the correlations were stronger in the CBs and LBs, although they were still evident in the urban and rural areas. In a multiple regression analysis with mortality from chronic rheumatic heart disease as the independent variable and both neonatal and postneonatal mortality as the independent variables, only the effect of postneonatal mortality remained statistically significant. Table 2 also shows that infant deaths from bronchitis and diarrhoea correlated more highly with adult chronic rheumatic heart disease than did infant deaths in any of the other four cause groups. Again these trends were paralleled in the analysis divided by geographical grouping (Table 2 and 3).
Table 2.
Correlation between death rate (standardised mortality ratio) from rheumatic heart disease in 1968–78 and infant, neonatal and postneonatal mortality rates per 1000 calculated for the years 1911–15, 1916–20 and 1921–25 according to the three geographical groupings of the 212 administrative areas (All correlation coefficients are statistically significant at p < 0.001).
| Mortality rate per 1000 | All areas | County and London boroughs | Urban areas | Rural areas |
| 1911–1915 | ||||
| Infant | 0.72 | 0.66 | 0.51 | 0.55 |
| Neonatal | 0.52 | 0.43 | 0.52 | 0.37 |
| Postneonatal | 0.72 | 0.66 | 0.49 | 0.59 |
| 1916–1920 | ||||
| Infant | 0.72 | 0.62 | 0.52 | 0.57 |
| Neonatal | 0.49 | 0.47 | 0.44 | 0.38 |
| Postneonatal | 0.73 | 0.61 | 0.52 | 0.62 |
| 1921–1925 | ||||
| Infant | 0.73 | 0.64 | 0.62 | 0.53 |
| Neonatal | 0.53 | 0.51 | 0.61 | 0.47 |
| Postneonatal | 0.73 | 0.62 | 0.58 | 0.50 |
| Diarrhoea | 0.67 | 0.52 | 0.42 | 0.54 |
| Infection | 0.58 | 0.55 | 0.21 | 0.41 |
| Bronchitis | 0.76 | 0.63 | 0.66 | 0.59 |
| Congenital | 0.43 | 0.42 | 0.46 | 0.27 |
| Other | 0.55 | 0.46 | 0.52 | 0.44 |
Figure 3.

Scatterplots showing the relationship between the infant mortality rate from diarrhoea or bronchitis in 1921–25 and the mortality rate from chronic rheumatic heart disease among men and women aged 55–74 in 1968–78.
Because of the strong correlations between infant mortality due to infectious causes and adult mortality from rheumatic heart disease, we examined the effect of early overcrowding and population density (Table 3). The strongest associations were between the number of rooms per person in 1921, either in all houses combined (r = − 0.69) or restricted to houses with up to nine rooms (r = − 0.66). The effects were evident in the separate groupings of CBs, LBs and urban and rural districts. Multiple regression models were used to explore the effects of early infection and crowding (Table 4). In models with chronic rheumatic heart disease as the dependent variable and infant mortality due to bronchitis (model 1) or diarrhoea (model 2) together with the number of persons per room and persons per family (the only measures of overcrowding which remained statistically significant in the analysis) as independent variables, both the influence of infection and overcrowding remained strongly statistically significant although the relative risk of both factors were reduced. However, in both models the effects of diarrhoea and bronchitis had by far the strongest influence on disease mortality.
Table 3.
Correlation between mortality rates for chronic rheumatic heart disease in 1968–78 and measures of population size and density from the 1921 census according to the geographical grouping of the 212 administrative areas.
| Census measure | All areas | County and London boroughs | Urban areas | Rural areas |
| Population size | 0.17* | 0.17 | 0.11 | 0.16 |
| Persons per acre | 0.51*** | 0.13 | 0.15 | 0.29* |
| Rooms per house | − 0.43*** | − 0.52*** | − 0.35** | − 0.30* |
| Families per house | 0.24** | − 0.25* | 0.01 | − 0.44** |
| Persons per family | 0.40*** | 0.31** | 0.64*** | 0.48*** |
| Rooms per person | − 0.69*** | − 0.52*** | − 0.53*** | − 0.56*** |
| Rooms per person† | − 0.66*** | − 0.50*** | − 0.55*** | − 0.54*** |
†Houses with 1–9 rooms *p < 0.05 **p < 0.01 ***p < 0.001
Table 4.
Prediction of chronic rheumatic heart disease standardised mortality ratios in men and women aged 55–74 during 1968–78 in 212 areas of England and Wales.
| Predictor (SD score) | Relative risk | 95% Confidence interval | p-value |
| Univariate analyses | |||
| Infant Diarrhoea mortality, 1921–25 | 1.185 | 1.173 to 1.198 | < 0.001 |
| Infant Bronchitis mortality, 1921–25 | 1.188 | 1.176 to 1.201 | < 0.001 |
| Persons per room, 1921 | 1.198 | 1.183 to 1.213 | < 0.001 |
| Persons per family, 1921 | 1.122 | 1.109 to 1.135 | < 0.001 |
| Multivariate analyses | |||
| Model 1 | |||
| Infant Diarrhoea mortality, 1921–25 | 1.122 | 1.105 to 1.139 | < 0.001 |
| Persons per room, 1921 | 1.072 | 1.052 to 1.092 | < 0.001 |
| Persons per family, 1921 | 1.032 | 1.018 to 1.045 | < 0.001 |
| Model 2 | |||
| Infant Bronchitis mortality, 1921–25 | 1.125 | 1.106 to 1.144 | < 0.001 |
| Persons per room, 1921 | 1.071 | 1.050 to 1.092 | < 0.001 |
| Persons per family, 1921 | 1.019 | 1.005 to 1.032 | 0.006 |
Discussion
We have shown that adult mortality from chronic rheumatic heart disease shows a strong correlation with past infant mortality rates, a trend which were observed in all three time periods, in all age groups and in the different geographical groupings. The correlations were consistently much stronger with postneonatal mortality than with neonatal mortality; detailed information on the five major causes of infant mortality showed that areas with high mortality from diarrhoea or bronchitis were those who had the highest subsequent mortality from rheumatic heart disease.
The adults dying from rheumatic heart disease were aged 55 to 74 and were, therefore, born between 1894 and 1923 and although the mean date of birth, 1908, is somewhat prior to the years in which the infant mortality data were collected, the long-term stability of the correlations in Table 2 and the high degree of correlation between the infant mortality rates between 1911 and 1921 (r>0.9) support the validity of the findings. Although mortality from rheumatic heart disease is relatively uncommon, it has been widely used as an indicator of the incidence of the disease as both geographical differences and the marked decline in disease incidence are paralleled by mortality statistics. The mortality rate has been estimated at 1.5% per year, a figure based on prospective studies from North America and the United Kingdom.15 Although historically most deaths were registered on the basis of clinical criteria, these are surprisingly accurate. A study of death certification in the United States suggest that 61% were correctly classified.16 It is unlikely that misclassification of the cause of death could result in the strong correlations seen in Table 2. It could also be argued that correlations between infant mortality rates and death rates from heart disease many decades later could be misleading because of population movements. However during the time period in question, the overwhelming majority of migration occurred over a short distance with only a small minority migrating significant distances.17 Furthermore, an analysis of over 2 million death certificates between 1969 and 1972 showed that the risk of major mortality was more strongly related to the place of birth than the place of death.18
The observation that the same areas of the UK which had high infant mortality rates early in the 20th century were those with high mortality from rheumatic heart disease some 50 to 60 years later raises the possibility that adverse influences in early childhood may be involved in determining susceptibility to developing rheumatic fever in childhood and subsequent valvular heart disease. The correlations were consistently much stronger with postneonatal mortality (deaths after the first month of life) than with neonatal mortality suggesting the influence of the postnatal environment rather than biological factors such as maternal health and the quality of the intrauterine environment, which are important determinants of early postnatal mortality. These findings contrast with other major diseases of adult life such as stroke, which was related to neonatal mortality, chronic bronchitis, which was related to postneonatal mortality and ischaemic heart disease, which was related to both.12 Longitudinal studies in Finland10 and Sweden,11 which show either no association or a weak association between birthweight or other measures of size at birth and a diagnosis of rheumatic heart disease in adult life, also suggest that prenatal factors, with the exception of cord length,11 are not major factors influencing disease susceptibility.
A major finding of this study is that past infant mortality due to infection, particularly respiratory mortality, correlated more closely with adult mortality from rheumatic heart disease than did other causes of past infant death (Table 2 and Figure 3). It is possible that these correlations are due to a direct influence of early infection on the risk of developing rheumatic fever and rheumatic heart disease. This is biologically plausible as there is evidence that infections can directly cause organ damage. In children acute infections lead to vascular endothelial damage,19 which may persist for at least a year. This finding may be relevant to rheumatic heart disease as there is increasing suspicion that the endothelial response to autoimmune-mediated injury is an important determinant of whether there is progression to clinically evident heart disease.20
It is also possible that the close geographical relationship between infant mortality from respiratory infection and adult rheumatic heart disease depends on the persistence of environmental factors that predispose to both infectious disease in infancy and facilitate the spread of streptococcal throat infection during childhood or adolescence. The risk of infant respiratory infection is reduced by breastfeeding and increased by a number of familial factors such as parental smoking, air pollution, overcrowding, or an attack of bronchitis or pneumonia in a sibling.21,22 While there is less epidemiological data on factors which facilitate the spread of group A, β-haemolytic streptococcal infection, close person to person contact with infected nasopharyngeal or oropharyngeal mucosal secretions are important23 and there is known to be an effect of overcrowding. This was demonstrated in the classical studies of military barracks during the 1950s, which showed higher rates of acquisition of streptococcal infections when beds were moved closer together.3,24 In our study we found higher disease rates in the larger urban areas (Figures 1 and 2), which is consistent with an effect of overcrowding. In addition, we found strong, statistically significant correlations between adult mortality from rheumatic heart disease and measures of overcrowding such as the number of rooms per person (Table 3). However, a multiple regression analysis (Table 4) showed that the association between infant mortality due to diarrhoea or bronchitis and adult rheumatic heart disease although reduced in strength persisted after adjustment for the level of overcrowding, suggesting that while overcrowding had an independent effect it could not itself explain the correlations in Table 2.
Our findings therefore provide support for the hypothesis that the susceptibility to chronic rheumatic heart disease has its origins in infancy. While they do not indicate a specific aetiology, the strong associations with early infectious disease and particularly with infant bronchitis raise the possibility that these early infections may act as a priming process increasing susceptibility to subsequent rheumatic fever and rheumatic heart disease. Alternatively, they suggest future research should address the environmental factors that are linked both with infection in infancy and the subsequent liability to heart disease. These are obscure, but one potentially important possibility is air pollution. This is now known to predispose to mortality from infant bronchitis25 and also has well-described immunomodulatory effects,26 which could predispose to the autoimmune processes underlying rheumatic heart disease.
Disclosures
None.
References
- 1.Lichtwitz L. Pathology and Therapy of Rheumatic Fever. New York: Grune & Stratton; 1943. [Google Scholar]
- 2.Seckeler MD, Hoke TR. The worldwide epidemiology of acute rheumatic fever and rheumatic heart disease. Clin Epidemiol. 2011;3:67–84. doi: 10.2147/CLEP.S12977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steer AC, Carapetis JR, Nolan TM, Shann F. Systematic review of rheumatic heart disease prevalence in children in developing countries: The role of environmental factors. J Paediatr Child Health. 2002;38(3):229–234. doi: 10.1046/j.1440-1754.2002.00772.x. [DOI] [PubMed] [Google Scholar]
- 4.Glover JA. Incidence of rheumatic diseases. Lancet. 1930;i:499–505. [Google Scholar]
- 5.Longo-Mbenza B, Bayekula M, Ngiyulu R, Kintoki VE, Bikangi NF, Seghers KV, Lukoki LE, Mandundu MF, Manzanza M, Nlandu Y. Survey of rheumatic heart disease in school children of Kinshasa town. Int J Cardiol. 1998;63(3):287–294. doi: 10.1016/s0167-5273(97)00311-2. [DOI] [PubMed] [Google Scholar]
- 6.Bateson P, Barker D, Clutton-Brock T, Deb D, D'Udine B, Foley RA, Gluckman P, Godfrey K, Kirkwood T, Lahr MM, McNamara J, Metcalfe NB, Monaghan P, Spencer HG, Sultan SE. Developmental plasticity and human health. Nature. 2004;430:419–421. doi: 10.1038/nature02725. [DOI] [PubMed] [Google Scholar]
- 7.Barker DJP. The Wellcome Foundation Lecture, 1994. The fetal origins of adult disease. Proc Roy Soc Lond. 1995;262:37–43. doi: 10.1098/rspb.1995.0173. [DOI] [PubMed] [Google Scholar]
- 8.Olgunturk R, Okur I, Cirak MY, Oguz AD, Akalin N, Turet S, Tunaoglu S. The role of viral agents in aetiopathogenesis of acute rheumatic fever. Clin Rheumatol. 2011;30(1):15–20. doi: 10.1007/s10067-010-1447-x. [DOI] [PubMed] [Google Scholar]
- 9.Kumar RK, Tandon R. Rheumatic fever & rheumatic heart disease: The last 50 years. Indian J Med Res. 2013;137(4):643–658. [PMC free article] [PubMed] [Google Scholar]
- 10.Eriksson JG, Kajantie E, Phillips DIW, Osmond C, Thornburg K, Barker DJ. The developmental origins of chronic rheumatic heart disease. Am J Human Biol. 2013;25:655–658. doi: 10.1002/ajhb.22425. [DOI] [PubMed] [Google Scholar]
- 11.Goodman A, Kajantie E, Osmond C, Eriksson J, Koupil I, Thornburg K, Phillips DI. The relationship between umbilical cord length and chronic rheumatic heart disease: A prospective cohort study. Eur J Prev Cardiol. 2014 doi: 10.1177/2047487314544082. [DOI] [PubMed] [Google Scholar]
- 12.Barker DJ, Osmond C, Law CM. The intrauterine and early postnatal origins of cardiovascular disease and chronic bronchitis. J Epidemiol Community Health. 1989;43(3):237–240. doi: 10.1136/jech.43.3.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barker DJ, Osmond C. Childhood respiratory infection and adult chronic bronchitis in England and Wales. Br Med J (Clin Res Ed) 1986;293(6557):1271–1275. doi: 10.1136/bmj.293.6557.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woolf B. Studies on infant mortality: Social aetiology of stillbirths and infant deaths in county boroughs of England and Wales. Br J Soc Med. 1947;1(2):73–125. doi: 10.1136/jech.1.2.73-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carapetis JR. Rheumatic heart disease in Asia. Circulation. 2008;118(25):2748–2753. doi: 10.1161/CIRCULATIONAHA.108.774307. [DOI] [PubMed] [Google Scholar]
- 16.Quinn RW, Sprague HA, Quinn JP. Mortality rates for rheumatic fever and rheumatic heart disease, 1940-65. Public Health Rep. 1970;85(12):1091–1101. [PMC free article] [PubMed] [Google Scholar]
- 17.Pooley CG, Turnbull J. Migration trends in British rural areas from the 18th to the 20th centuries. Int J Popul Geogr. 1996;2(3):215–237. doi: 10.1002/(SICI)1099-1220(199609)2:3<215::AID-IJPG35>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 18.Osmond C, Barker DJ, Slattery JM. Risk of death from cardiovascular disease and chronic bronchitis determined by place of birth in England and Wales. J Epidemiol Community Health. 1990;44(2):139–141. doi: 10.1136/jech.44.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Charakida M, Donald AE, Terese M, Leary S, Halcox JP, Ness A, Davey Smith G, Golding J, Friberg P, Klein NJ, Deanfield JE, ALSPAC (Avon Longitudinal Study of Parents and Children) Study Team Endothelial dysfunction in childhood infection. Circulation. 2005;111(13):1660–1665. doi: 10.1161/01.CIR.0000160365.18879.1C. [DOI] [PubMed] [Google Scholar]
- 20.Tandon R, Sharma M, Chandrashekhar Y, Kotb M, Yacoub MH, Narula J. Revisiting the pathogenesis of rheumatic fever and carditis. Nat Rev Cardiol. 2013;10(3):171–177. doi: 10.1038/nrcardio.2012.197. [DOI] [PubMed] [Google Scholar]
- 21.Leeder SR, Corkhill R, Irwig LM, Holland WW, Colley JR. Influence of family factors on the incidence of lower respiratory illness during the first year of life. Br J Prev Soc Med. 1976;30(4):203–212. doi: 10.1136/jech.30.4.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Backes CH, Nelin T, Gorr MW, Wold LE. Early life exposure to air pollution: How bad is it? Toxicol Lett. 2013;216(1):47–53. doi: 10.1016/j.toxlet.2012.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pichichero ME. Group A beta-hemolytic streptococcal infections. Pediatr Rev. 1998;19(9):291–302. doi: 10.1542/pir.19-9-291. [DOI] [PubMed] [Google Scholar]
- 24. Wannamaker LW. The epidemiology of streptococcal infections McCarty M, ed. Streptococcal Infections New York: Columbia University Press; 1954. [Google Scholar]
- 25.Glinianaia SV, Rankin J, Bell R, Pless-Mulloli T, Howel D. Does particulate air pollution contribute to infant death? A systematic review. Environ Health Perspect. 2004;112(14):1365–1371. doi: 10.1289/ehp.6857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ritz SA. Air pollution as a potential contributor to the ‘epidemic’ of autoimmune disease. Med Hypotheses. 2010;74(1):110–117. doi: 10.1016/j.mehy.2009.07.033. [DOI] [PubMed] [Google Scholar]
- 27.Gardner MJ, Winter PD, Barker DJP. Atlas of Mortality from Selected Diseases. Chichester: John Wiley & Sons; 1984. [Google Scholar]