Abstract
Background: Endomyocardial fibrosis (EMF) is the most common form of restrictive cardiomyopathy worldwide. It has been linked to poverty and various environmental factors, but—for unknown reasons—only some people who live in similar conditions develop the disease. EMF cases cluster within both families and ethnic groups, suggesting a role for a genetic factor in host susceptibility. The human leukocyte antigen (HLA) system is associated with predisposition to various diseases. This two-center study was designed to investigate variation in the HLA system between EMF patients and unaffected controls. We provide the first genetic investigation of patients with EMF, as well as a comprehensive review of the literature. Methods: HLA class I (HLA-A, -B, -C) and class II (DRB1, DQB1) types were determined in 71 patients with severe EMF and 137 controls from Uganda and Mozambique. Chi Square analysis was used to identify any significant difference in frequency of class I and class II HLA types between cases and controls. Results: Compared to ethnically matched controls, HLA-B*58 occurred more frequently in Mozambique patients with EMF and HLA-A*02:02 occurred more frequently in Ugandan patients with EMF. Conclusions: Ample subjective evidence in the historical literature suggests the importance of a genetically susceptible host in EMF development. In this first formal genetic study, we found HLA alleles associated with cases of EMF in two populations from sub-Saharan Africa, with EMF patients being more likely than controls to have the HLA-B*58 allele in Mozambique (p-0.03) and the HLA-A*02:02 in Uganda (p = 0.005). Further investigations are needed to more fully understand the role of genetics in EMF development.
Keywords: Cardiomyopathy, Endomyocardial Fibrosis, Genetic Suceptibility
Background
Endomyocardial fibrosis (EMF) is the most common cause of restrictive cardiomyopathy worldwide1,2. It is characterized by patchy fibrosis of the endocardial surface, mural thrombi in the apical portion of the ventricles, partial cavity obliteration and atrioventricular valve dysfunction3–6. Cases have been reported from around the globe, centering in tropical and subtropical regions around the equator2, especially sub-Saharan Africa, and in particular Mozambique7,8, and Uganda1,9. EMF has repeatedly been shown to cluster both within families8,10,11 and within select ethnic groups12–15, suggesting the possible importance of a genetically susceptible host.
The human leukocyte antigens (HLA) play an essential role in immune function and differences in HLA phenotypes between individuals can both protect and predispose to disease development. In recent years, susceptibility to infections and autoimmune diseases has been found to be associated with increased frequencies of various HLA types in populations of Africa16–19. This study was designed to investigate the association between EMF and HLA types in patients from Mozambique and Uganda.
Methods
Echocardiographic definitions
The diagnosis of EMF was confirmed using a previously published evaluation scale (Table 1)8 shown to have high reliability and validity for moderate to severe EMF when compared to surgical pathology and direct visualization20. Subjects who scored < 8 were classified as having mild EMF, 8–15 moderate EMF, and >15 severe disease. Only subjects with severe EMF were included in this analysis in order to strengthen the clinical phenotype. Distribution of EMF was recorded as bi-ventricular when lesions involved both ventricles without predominance of one side, right-ventricular when lesions affected only or predominately only the right ventricle, or left-ventricular when lesions affected only or predominately only the left ventricle. A representative echocardiographic image of right-sided EMF can be seen in Figure 1A/B.
Table 1.
Criteria for assessment of the severity of endomyocardial fibrosis.
| Criterion | Score |
| Major Criteria | |
| Endomyocardial plaques >2mm in thickness | 2 |
| Thin ( < 1mm) endomyocardial patches affecting more than one ventricular wall | 3 |
| Obliteration of the right ventricular or left ventricular apex | 4 |
| Thrombi or spontaneous contrast without severe ventricular dysfunction | 4 |
| Retraction of the right ventricular apex (right ventricular apical notch) | 4 |
| AVV dysfunction due to adhesion of the valvular apparatus to the ventricular wall | 1–4* |
| Minor Criteria | |
| Thin endomyocardial patches localized to one ventricular wall | 1 |
| Restrictive flow pattern across mitral or tricuspid valves | 2 |
| Pulmonary-valve diastolic opening | 2 |
| Diffuse thickening of the anterior mitral leaflet | 1 |
| Enlarged atrium with normal sized ventricle | 2 |
| M-movement of the interventricular septum and flat posterior wall | 1 |
| Enhanced density of the moderator or other intraventricular bands | 1 |
* The score is assigned according to the severity of atrioventricular regurgitation8, AVV: Atrioventricular valve.
Figure 1.
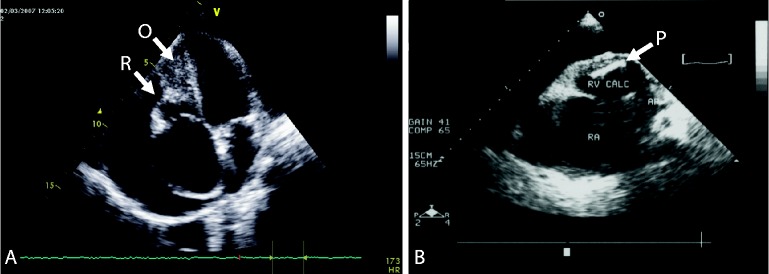
Endomyocardial fibrosis of the right ventricle. 1A: Echocardiogram in apical 4-chamber view with characteristic features of (O) obliteration and (R) retraction of the right ventricle with reduction of the right ventricular cavity size. Notice thickening of the tricuspid valve, dilatation of the right atrium and pericardial effusion 1B: Echocardiogram in paraternal short axis view shows a large fibrotic plaque with marked fibrosis and calcification. Again, noted are the enlarged right atrium and large pericardial effusion.
Study population
Our study was conducted in Mozambique and Uganda, two countries with endemic areas for EMF (Figure 2). Patients who underwent echocardiography and were diagnosed with EMF according to the previously described criteria were approached for participation (Table 1). There were no exclusion criteria for those with a positive echocardiogram. The first study was at the Mozambique Heart Institute between November 2004 and August 2006. Eighty consecutive patients with clinical evaluation and detailed echocardiographic diagnosis of EMF were recruited from outpatient clinics and wards. Of these, 40 had mild or moderate disease and were excluded from this analysis. Eighty-nine controls, matched for age and ethnic group, documented by echocardiogram to be EMF negative, were recruited from blood donors at a neighboring hospital.
Figure 2.
The study was conducted in 2 countries with high prevalence rates of EMF – Uganda and Mozambique.
The second study was carried out in Uganda from April 2009 to April 2011. Fifty consecutive patients with EMF confirmed by echocardiography were recruited from the outpatient cardiology clinics at the Ugandan Heart Institute and the cardiology ward at Mulago Hospital. Eight were found to have moderate disease and were excluded from this analysis. Fifty control subjects, matched for age and ethnic group, documented by echocardiogram to be EMF negative, were recruited from among the patients being evaluated at the Uganda Heart Institute.
Ethics statement
The national bioethical committee of Mozambique, and the institutional review boards of Children's National Medical Center (Washington, DC) and Makerere University (Kampala, Uganda) approved the study. All subjects signed written informed consent, or for participants who were minors, parents or guardians provided written informed consent.
HLA typing
For the Mozambican samples, whole blood was transferred to the Histocompatibility and Immunogenetics Department, National Health Service Blood and Transplant in Colindale, London, England for processing. DNA was extracted and HLA class I (HLA-A, -B, -C) and class II (DRB1 and DQB1) typing was performed using PCR-SSOP kits (Reli™, Invitrogen). Sequence-based typing was used to determine the HLA-B*58 allele present in relevant samples.
For the Ugandan patients, whole blood was frozen and shipped to Histogenetics, an HLA-laboratory in Ossining, NY, for processing. Sequence-based typing (SBT) was used to identify HLA class I (HLA-A, -B, -C) and class II (DRB1 and DQB1) alleles, and sequencing was performed on both strands.
Statistical analysis
Frequencies are given as absolute numbers and percentages; continuous data are reported as median (range). The differences in frequency of HLA types between patients and controls were tested for significance (p < 0.05) by means of the chi-square test (Fisher's Exact Test). The p values have not been corrected.
Results
Both populations had more females and predominance of right EMF. Demographic data for the two groups can be found in Table 2.
Table 2.
Demographic characteristics of patients with severe EMF and successful HLA typing.
| Mozambique (n = 40) | Uganda (n = 31) | |
| Age (mean, range) | 14.9 (5-45) | 23 (15–35) |
| Gender (% female) | 21 (52.5%) | 21 (68.0%) |
| Disease Distribution | ||
| Right-ventricular EMF | 23 (57.5%) | 18 (58.0%) |
| Bi-ventricular EMF | 17 (42.5%) | 13 (42.0%) |
Disease distribution represented by total number per cohort (percentage of patients for each cohort).
No differences were found between cases and controls for either population in HLA-C, -DR, -DQ frequencies and these are not shown. Table 3 shows the phenotype frequencies of HLA-A and HLA-B alleles occurring in more than 5% of either cases or controls. In the two control groups the frequencies of some HLA types (e.g. HLA-A*02:01, -A*23*01, -B*15:10, -B*42:01) are comparable, however those of others (e.g. HLA-A*29, -A*74, -B*15:03, -B*44:03) are appreciably different. Therefore the HLA data from the two studies could not be combined for analysis.
Table 3A.
Selected HLA-A (3A) and HLA-B (3B) phenotype frequencies in EMF cases and controls for two populations.
| HLA-A* | Uganda controls n = 48 | Uganda patients n = 31 | Mozambique controls n = 89 | Mozambique patients n = 40 |
| % phenotype frequency | ||||
| 01:01 | 8.3 (4) | 16.1 (5) | 5.6 (5) | 12.5 (5) |
| 02:01 | 20.8 (10) | 22.6 (7) | 18.0 (16) | 10.0 (4) |
| 02:02 | 6.2 (3) | 29.0 (9)† | 5.6 (5) | 5.0 (2) |
| 02:05/08 | 2.1 (1) | 0 | 9.0 (8) | 17.5 (7) |
| 03:01 | 12.5 (6) | 9.7 (3) | 6.7 (6) | 12.5 (5) |
| 23:01 | 25.0 (12) | 9.7 (3) | 22.5 (20) | 12.5 (5) |
| 29:01/02 | 10.4 (5) | 16.1 (5) | 23.6 (21) | 27.5 (11) |
| 30:01 | 6.2 (3) | 9.7 (3) | 15.7 (14) | 10.0 (4) |
| 30:02 | 18.7 (9) | 9.7 (3) | 25.8 (23) | 15.0 (6) |
| 32:01 | 6.2 (3) | 6.4 (2) | 2.2 (2) | 0 |
| 33:01/03 | 8.3 (4) | 6.4 (2) | 6.7 (6) | 17.5 (7) |
| 34:02 | 6.2 (3) | 0 | 1.1 (1) | 7.5 (3) |
| 36:01 | 6.3 (3) | 9.7 (3) | 3.4 (3) | 2.5 (1) |
| 66:01 | 8.3 (4) | 6.4 (2) | 3.4 (3) | 5.0 (2) |
| 68:02 | 14.6 (7) | 12.9 (4) | 18.0 (16) | 7.5 (3) |
| 74:01/02/03 | 20.8 (10) | 29.0 (9) | 9.0 (8) | 20.0 (8) |
3A: Selected HLA-A phenotype frequencies (n)) in EMF cases and controls for two populations.
† p = < 0.01.
Mozambique
Blood samples were obtained from 40 patients with severe EMF and 89 ethnically matched controls. All samples were successfully transported to London for analysis.
Table 3 shows selected HLA-A, -B frequencies in the Mozambique patients severe EMF (n = 40) and controls. The frequency of HLA-A*02:02 was comparable in patients and controls. HLA-B*58 was more frequent in patients compared with controls (37.5% vs. 14.6%, p = 0.03, Figure 3a. Patients and controls positive for HLA-B*58 were tested further to determine which allele of HLA-B*58 was present. Although both HLA-B*58:01 and HLA-B*58:02 were both more frequent in the patients than the controls the differences were not significant. The frequencies of the HLA types found in the groups of EMF patients with bi-ventricular EMF (n = 17), right-ventricular EMF (n = 23) were compared. The frequency of HLA-A*02 in patients with bi-ventricular EMF was reduced when compared to patients with right-ventricular EMF (4/17 vs. 8/23, respectively) and the frequency of HLA-B*58 in patients with bi-ventricular EMF was increased when compared to patients with right-ventricular EMF (8/17 vs. 7/23 respectively) but neither difference is statistically significant.
Figure 3.
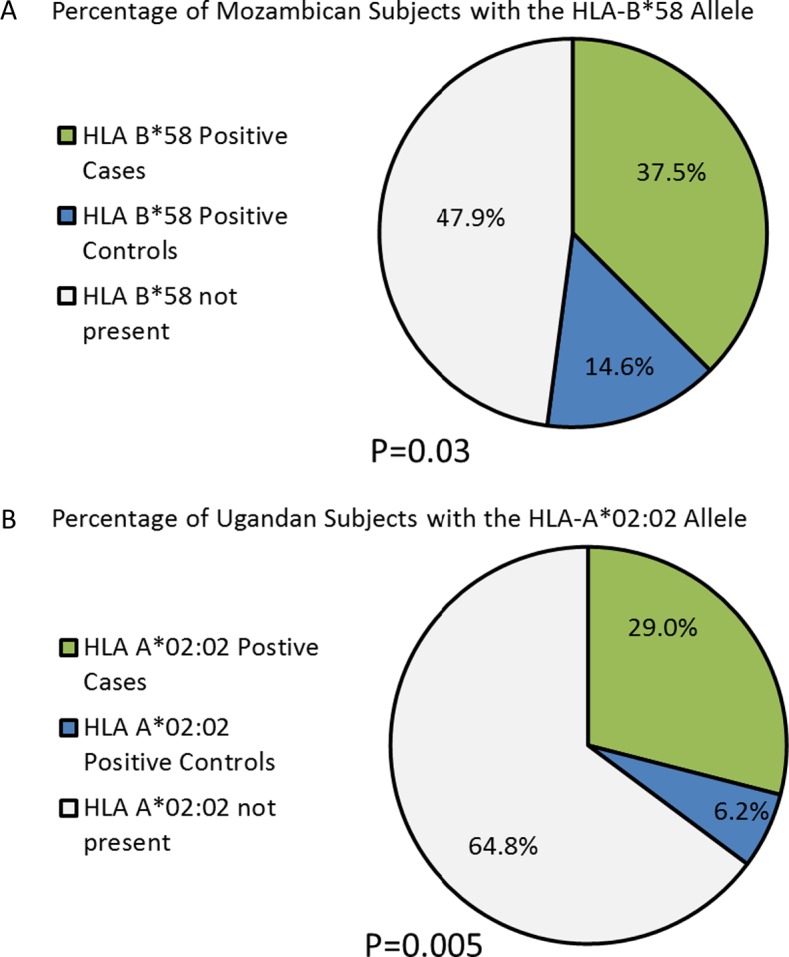
Distribution of HLA alleles determined to have significance for the 2 populations. 3A: Mozambique; 3B: Uganda.
Uganda
Blood samples were obtained from 42 Ugandan patients with severe EMF and 50 ethnically matched controls. After storage and shipment to the United States for sample processing, 31 EMF samples and 48 control samples were viable for HLA typing.
HLA-A*02:02 was more frequent in cases than in controls (29.0% in cases compared to 6.2% in controls). In light of this difference in allele frequency, a secondary analysis was performed to distinguish those with the allele from those without. The new analysis revealed a significant association (p = 0.005) between disease development and presence of the HLA-A*02:02 allele, Figure 3b. HLA-B*58 occurred more frequently in patients than in controls (35.5% vs. 20.8%), though this difference was not significant (p = 0.19). HLA-B*58 was more frequent in bilateral disease (38.9%) than in right-sided disease (30.8%), but this difference did not reach statistical significance (p = 0.72).
Discussion
Our study, the first to formally examine genetic predisposition to EMF, found differences in the frequency of certain HLA alleles between patients with severe EMF and EMF-negative controls in two sub-Saharan populations. HLA-A*02:02 which is predominantly found in African populations21 occurred more frequently in Ugandan patients with EMF and HLA-B*58 was found more frequently in Mozambique patients with EMF compared to ethnically matched controls. In the Mozambique population, a sub-analysis to look at the specific HLA-B*58 allele (either HLA-B*58:01 or B*58:02) between cases and controls was also conducted, showing no difference between cases and controls. However, once sub-analysis was performed, sample size was quite small. HLA-B*58:01 and B*58:02 differ by three amino acids, which has been shown to affect their peptide-binding motifs related to HIV. However the differences are predicted to only affect the C pocket of the peptide binding groove and may not be significant in the context of different antigens22. Three different HLA-B27 alleles, HLA-B*27:02, HLA-B*27:04 and HLA-B*27:05, are associated with susceptibility to ankylosing spondylitis23.
A review of existing literature reveals widespread, if not airtight, support for a genetic susceptibility to EMF. The possibility of host susceptibility in EMF development was first raised over 50 years ago. Physicians at Mulago Hospital (Kampala, Uganda) noted that immigrants from Rwanda-Burundi were similar to their Ugandan neighbors in admission for chronic RHD management, but were represented almost three times as often in admissions for EMF24. An 11-year review of autopsy records at the same institution (1950-1961) examined demographic data on 124 patients diagnosed with EMF. 5.4% of autopsies on patients from Rwanda-Burundi showed evidence of EMF, compared to only 0.73% of those from Uganda12. While much of this difference was attributed to the extreme poverty of these immigrants, the disparity raised the question of disease predisposition.
Around the same time, a series of case presentations reported occurrences of EMF among family members. Cases among siblings were reported from Nigeria25, India26, and northern Zambia11, the first two reporting concurrent cases, and the latter with cases separated by 8 years. In a more extensive family review, Patel and colleagues reported nine cases of EMF among Rwanda-Burundi immigrants to Uganda, including one sibling pair, two mother-child sets, and one family with three affected members10. And, more recently, a case study of EMF in a 22-month-old child, one of the youngest patients known, was reported. While the child's father was from a high-prevalence area of Mozambique, the child had never traveled to the region himself, suggesting genetic susceptibility, not environmental factors may drive EMF development27.
Mocumbi and colleagues conducted the first systematic epidemiological study of EMF. Using echocardiography, they examined 1063 people in 214 households across the Inharrime district of Mozambique. The overall prevalence of EMF was 19.8%. Risk of EMF was found to increase with each additional affected family member within a household, with 24% prevalence among those with one or more affected family members, 28.3% prevalence among those with two or more affected family members, and 38.8% among those with three or more affected family members8. While this study could not separate the impact of environmental and genetic factors, it confirmed the familial occurrence of EMF, previously only reported by case series.
A single study reporting a murine model of EMF also provides support for genetic susceptibility to disease development. Eling and colleagues found that mice infected with murine malaria (Plasmodium berghei) showed changes characteristic of EMF from the second week of infection onwards. When treatment was given before 4 weeks, lesions were likely to resolve; both chronic and repeated infection, however, led to irreversible fibrosis and thrombus formation. Notably, some strains of mice developed disease, while others did not. Offspring of crosses, between strains susceptible to EMF and those not susceptible, developed disease. The authors concluded these mice demonstrated a genetic susceptibility to disease development with a dominant inheritance28.
The case-control model used in this study has numerous strengths. As EMF is diagnosed exclusively by phenotype, only severe cases were included in the HLA analysis to ensure diagnostic accuracy, making misclassification of cases unlikely. EMF is a rare disease and is found most commonly in resource-poor settings. Given this, the sample size of both populations is quite reasonable. Additionally, through partnerships abroad, state of the art HLA typing was used for allelic identification, ensuring scientifically rigorous results. Finally, this collaboration is a good example of how South-South cooperation within Africa can address the challenges of non-communicable and rare disease.
It is also important, however, to point out several weakness of this study. First, it is well documented that Africa has one of the most genetically diverse populations29. The baseline allelic frequencies for HLA type differed between the two populations, and thus, the data could not be combined for analysis. Similarly, it is important to point out that this manuscript represents the combination of two distinct case-control studies. While similar methodology and analysis were performed, the studies were designed independently, and combined only for convenience of reporting and discussing a rare disease. Secondly, while a reasonable sample size has been achieved for a rare disease, the study population is small enough to recognize that type I error (false positive results) could occur. Additionally, while the control populations for both sites represent healthy volunteers screened by echocardiogram to confirm absence of EMF– blood donors in Mozambique and relatives of patients being evaluated for cardiac disease in Uganda – control subjects could have unforeseen similarities or differences that could introduce bias. Finally, this study examines the prevalence of HLA alleles in isolation. It is possible that due to linkage disequilibrium, the HLA findings could be spurious, and only a marker for another, unmeasured, predictor allele. This study was not designed to detect these associations.
Conclusions
The review of the literature suggests that EMF development may be the result of a complex interplay between environmental factors and a genetically susceptible host. Our data suggest that the HLA-system or other MHC encoded factors may contribute to this complex pathway. The cascade of events in a susceptible individual who develops EMF remains to be fully delineated. Future studies are needed to confirm and clarify the role of HLA associated predisposition to EMF development; this should also expand the search for candidate genes. We hope this manuscript generates interest in the genetic aspects of EMF development. A better understanding of host susceptibility could provide important insights into the development of EMF, which in turn could inform early diagnosis and improve therapeutics for this devastating tropical cardiomyopathy.
List of abbreviations
EMF: Endomyocardial Fibrosis
HLA: Human Leukocyte Antigen
Competing interests
None.
Funding sources
None.
Authors contributions
-
[1]
Andrea Beaton participated in all aspects of study design, data acquisition and interpretation, drafting of manuscript, and approval of final draft.
-
[2]
Craig Sable participated in data interpretation, drafting of manuscript, and approval of final draft.
-
[3]
Juliette Brown participated in data analysis, drafting of manuscript, and approval of final draft.
-
[4]
Joshua Hoffman participated in data analysis, drafting of manuscript, and approval of final draft.
-
[5]
Michael Mungoma participated in data collection, drafting of manuscript, and approval of final draft.
-
[6]
Charles Mondo participated in study design, data collection, drafting of manuscript, and approval of final draft.
-
[7]
Nezith Cereb participated in data analysis, drafting of manuscript, and approval of final draft.
-
[8]
Colin Brown participated in data analysis, drafting of manuscript, and approval of final draft.
-
[9]
Marshall Summar participated in data analysis, drafting of manuscript, and approval of final draft.
-
[10]
Jurgen Freers participated in study design, data analysis, drafting of manuscript, and approval of final draft.
-
[11]
Maria Beatriz Ferreira participated in data collection, drafting of manuscript, and approval of final draft.
-
[12]
Magdi Yacoub participated in study design, drafting of manuscript, and approval of final draft.
-
[13]
Ana Olga Mocumbi participated in all aspects of study design, data collection, data analysis, drafting of manuscript, and approval of final draft.
All authors read and approved the final manuscript.
Table 3B.
Selected HLA-A (3A) and HLA-B (3B) phenotype frequencies in EMF cases and controls for two populations.
| HLA-B* | Uganda controls n = 48 | Uganda patients n = 31 | Mozambique controls n = 89 | Mozambique patients n = 40 |
| % phenotype frequency | ||||
| 07:02 | 6.2 (3) | 3.2 (1) | 9.0 (8) | 5.0 (2) |
| 08:01 | 16.7 (8) | 6.4 (2) | 11.2 (10) | 15.0 (6) |
| 13:02 | 2.1 (1) | 0 | 5.6 (5) | 2.5 (1) |
| 14:02 | 4.2 (2) | 19.3 (6) | 7.9 (7) | 7.5 (3) |
| 15:03 | 10.4 (5) | 12.9 (4) | 19.1 (17) | 17.5 (7) |
| 15:10 | 10.4 (5) | 6.4 (2) | 12.4 (11) | 7.5 (3) |
| 15:17 | 0 | 6.4 (2) | 0 | 0 |
| 18:01 | 8.3 (4) | 6.4 (2) | 3.4 (3) | 2.5 (1) |
| 35:01 | 6.2 (3) | 6.4 (1) | 11.2 (10) | 2.5 (1) |
| 39:10 | 0 | 0 | 5.6 (5) | 0 |
| 42:01 | 14.6 (7) | 16.1 (5) | 14.6 (13) | 5.0 (2) |
| 44:03 | 8.3 (4) | 0 | 15.7 (14) | 17.5 (7) |
| 44:15 | 0 | 9.7 (3) | 0 | 0 |
| 45:01 | 16.7 (8) | 12.9 (4) | 7.9 (7) | 5.0 (2) |
| 49:01 | 8.3 (4) | 9.7 (3) | 7.9 (7) | 7.5 (3) |
| 51:01 | 4.2 (2) | 6.4 (2) | 2.2 (2) | 0 |
| 53:01 | 14.6 (7) | 12.9 (4) | 10.1 (9) | 17.5 (7) |
| 57:02 | 6.2 (3) | 0 | 0 | 0 |
| 57:03 | 0 | 6.4 (2) | 6.7 (6) | 5.0 (2) |
| 58 | 20.8 (10) | 35.5 (11) | 14.6 (17) | 37.5 (15)† |
| 58:01 | 8.3 (4) | 16.1 (5) | 13.5 (12) | 25.0 (10) |
| 58:02 | 12.5 (6) | 25.8 (8) | 6.7 (6) | 15.0 (6) |
| 81:01/02 | 12.5 (6) | 9.7 (3) | 9.0 (8) | 15.0 (6) |
3B: Selected HLA-B phenotype frequencies in EMF cases and controls for two populations.
† p = 0.03.
Acknowledgements
We are indebted to the blood donors at the Instituto do Coração in Mozambique and the Uganda Heart Institute. We would also like to thank the staff from the H&I Laboratory NHSBT Colindale in London, and Histogenetics in New York who extracted DNA and performed HLA typing.
References
- 1.Bukhman G, Ziegler J, Parry E. Endomyocardial fibrosis: Still a mystery after 60 years. PLoS Neglected Tropical Diseases. 2008;2:e97. doi: 10.1371/journal.pntd.0000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hutt MS. Epidemiology aspects of endomyocardial fibrosis. Postgraduate Medical Journal. 1983;59:142–146. doi: 10.1136/pgmj.59.689.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hernandez-Pieretti O. Echocardiographic diagnosis and evaluation of cardiomyopathies: Idiopathic hypertrophic subaortic stenosis, chagas' heart disease and endomyocardial fibrosis. Postgraduate Medical Journal. 1977;53:533–536. doi: 10.1136/pgmj.53.623.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davies J, Gibson DG, Foale R, Heer K, Spry CJ, Oakley CM, Goodwin JF. Echocardiographic features of eosinophilic endomyocardial disease. British Heart Journal. 1982;48:434–440. doi: 10.1136/hrt.48.5.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tello R, Cuan V, Abundes A, Navarro J, Garcia Lara J, Astudillo R, Ariza H, Cuan M. Doppler echocardiography in endomyocardial fibrosis. Archivos del Instituto de Cardiologia de Mexico. 1994;64:251–255. [PubMed] [Google Scholar]
- 6.Olsen EG. Pathological aspects of endomyocardial fibrosis. Postgraduate Medical Journal. 1983;59:135–141. doi: 10.1136/pgmj.59.689.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferreira B, Matsika-Claquin MD, Hausse-Mocumbi AO, Sidi D, Paquet C. Geographic origin of endomyocardial fibrosis treated at the central hospital of Maputo (Mozambique) between 1987 and 1999. Bull Soc Pathol Exot. 2002;95:276–279. [PubMed] [Google Scholar]
- 8.Mocumbi AO, Ferreira MB, Sidi D, Yacoub MH. A population study of endomyocardial fibrosis in a rural area of Mozambique. The New England Journal of Medicine. 2008;359:43–49. doi: 10.1056/NEJMoa0708629. [DOI] [PubMed] [Google Scholar]
- 9.Freers J, Mayanja-Kizza H, Ziegler JL, Rutakingirwa M. Echocardiographic diagnosis of heart disease in Uganda. Tropical Doctor. 1996;26:125–128. doi: 10.1177/004947559602600310. [DOI] [PubMed] [Google Scholar]
- 10.Patel AK, Ziegler JL, D'Arbela PG, Somers K. Familial cases of endomyocardial fibrosis in Uganda. British Medical Journal. 1971;4:331–334. doi: 10.1136/bmj.4.5783.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lowenthal MN. Endomyocardial fibrosis: Familial and other cases from northern Zambia. Medical Journal of Zambia. 1978;12:2–7. [PubMed] [Google Scholar]
- 12.Shaper AG, Coles RM. The tribal distribution of endomyocardial fibrosis in Uganda. British Heart Journal. 1965;27:121–127. doi: 10.1136/hrt.27.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Somers K. Proceedings of the 3rd asian pacific congress on cardiology. 1964;1:162.
- 14.Hutt MS. Pathology of African cardiomyopathies. Pathologia et Microbiologia. 1970;35:37–43. doi: 10.1159/000162197. [DOI] [PubMed] [Google Scholar]
- 15.Rutakingirwa M, Ziegler JL, Newton R, Freers J. Poverty and eosinophilia are risk factors for endomyocardial fibrosis (EMF) in Uganda. Tropical Medicine & International Health: TM & IH. 1999;4:229–235. doi: 10.1046/j.1365-3156.1999.43376.x. [DOI] [PubMed] [Google Scholar]
- 16.Diaz-Pena R, Ouedraogo DD, Lopez-Vazquez A, Sawadogo SA, Lopez-Larrea C. Ankylosing spondylitis in three sub-Saharan populations: HLA-B*27 and HLA-B*14 contribution. Tissue Antigens. 2012;80:14–15. doi: 10.1111/j.1399-0039.2012.01879.x. [DOI] [PubMed] [Google Scholar]
- 17.Mkhize-Kwitshana ZL, Taylor M, Jooste P, Mabaso ML, Walzl G. The influence of different helminth infection phenotypes on immune responses against HIV in co-infected adults in South Africa. BMC Infectious Diseases. 2011;11:273. doi: 10.1186/1471-2334-11-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.El-Hagrassy N, El-Chennawi F, Zaki Mel S, Fawzy H, Zaki A, Joseph N. HLA class I and class II HLA-DRB profiles in Egyptian children with rheumatic valvular disease. Pediatric Cardiology. 2010;31:650–656. doi: 10.1007/s00246-010-9663-3. [DOI] [PubMed] [Google Scholar]
- 19.Lombard Z, Brune AE, Hoal EG, Babb C, Van Helden PD, Epplen JT, Bornman L. HLA class II disease associations in southern Africa. Tissue Antigens. 2006;67:97–110. doi: 10.1111/j.1399-0039.2006.00530.x. [DOI] [PubMed] [Google Scholar]
- 20.Mocumbi AO, Carrilho C, Sarathchandra P, Ferreira MB, Yacoub M, Burke M. Echocardiography accurately assesses the pathological abnormalities of chronic endomyocardial fibrosis. The International Journal of Cardiovascular Imaging. 2011;27:955–964. doi: 10.1007/s10554-010-9753-6. [DOI] [PubMed] [Google Scholar]
- 21.Krausa P, Brywka M, 3rd, Savage D, Hui KM, Bunce M, Ngai JL, Teo DL, Ong YW, Barouch D, Allsop CE, Hill AVS, McMichael AJ, Bodmer JG, Browning MJ. Genetic polymorphism within HLA-A*02: Significant allelic variation revealed in different populations. Tissue Antigens. 1995;45:223–231. doi: 10.1111/j.1399-0039.1995.tb02444.x. [DOI] [PubMed] [Google Scholar]
- 22.Kiepiela P, Leslie AJ, Honeyborne I, Ramduth D, Thobakgale C, Chetty S, Rathnavalu P, Moore C, Pfafferott KJ, Hilton L, Zimbwa P, Moore S, Allen T, Brander C, Addo MM, Altfeld M, James I, Mallal S, Bunce M, Barber LD, Szinger J, Day C, Klenerman P, Mullins J, Korber B, Coovadia HM, Walker BD, Goulder PJ. Dominant influence of HLA-B in mediating the potential co-evolution of HIV and HLA. Nature. 2004;432:769–775. doi: 10.1038/nature03113. [DOI] [PubMed] [Google Scholar]
- 23.Marcilla M, Lopez de Castro JA. Peptides: The cornerstone of HLA-B27 biology and pathogenetic role in spondyloarthritis. Tissue Antigens. 2008;71:495–506. doi: 10.1111/j.1399-0039.2008.01051.x. [DOI] [PubMed] [Google Scholar]
- 24.Shaper AG, Williams AW. Cardiovascular disorders at an African hospital in Uganda. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1960;54:12–32. doi: 10.1016/0035-9203(60)90207-8. [DOI] [PubMed] [Google Scholar]
- 25.Adi FC. Endomyocardial fibrosis in two brothers. British Heart Journal. 1963;25:684–688. doi: 10.1136/hrt.25.5.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mehrotra AN, Maheshwari HB, Khosla SN, Kumar S. Endomyocardial fibrosis. (A report of two cases in brothers) The Journal of the Association of Physicians of India. 1964;12:845–850. [PubMed] [Google Scholar]
- 27.Cilliers AM, Adams PE, Mocumbi AO. Early presentation of endomyocardial fibrosis in a 22-month-old child: A case report. Cardiology in the Young. 2011;21:101–103. doi: 10.1017/S1047951110001460. [DOI] [PubMed] [Google Scholar]
- 28.Eling WM, Jerusalem CR, Heinen-Borries UJ, Hermsen CC, van Run-van Breda JJ. Is malaria involved in the pathogenesis of tropical endomyocardial fibrosis? Acta Leidensia. 1988;57:47–52. [PubMed] [Google Scholar]
- 29.Cao K, Moormann AM, Lyke KE, Masaberg C, Sumba OP, Doumbo OK, Koech D, Lancaster A, Nelson M, Meyer D, Single R, Hartzman RJ, Plowe CV, Kazura J, Mann DL, Sztein MB, Thomson G, Fernandez-Vina MA. Differentiation between African populations is evidenced by the diversity of alleles and haplotypes of HLA class I loci. Tissue Antigens. 2004;63:293–325. doi: 10.1111/j.0001-2815.2004.00192.x. [DOI] [PubMed] [Google Scholar]


