Abstract
Almost all cellular functions are powered by a continuous energy supply derived from cellular metabolism. However, it is little understood how cellular energy production is coordinated with diverse energy-consuming cellular functions. Here, using the cardiac muscle system, we demonstrate that nuclear receptors estrogen-related receptor α (ERRα) and ERRγ are essential transcriptional coordinators of cardiac energy production and consumption. On the one hand, ERRα and ERRγ together are vital for intact cardiomyocyte metabolism by directly controlling expression of genes important for mitochondrial functions and dynamics. On the other hand, ERRα and ERRγ influence major cardiomyocyte energy consumption functions through direct transcriptional regulation of key contraction, calcium homeostasis, and conduction genes. Mice lacking both ERRα and cardiac ERRγ develop severe bradycardia, lethal cardiomyopathy, and heart failure featuring metabolic, contractile, and conduction dysfunctions. These results illustrate that the ERR transcriptional pathway is essential to couple cellular energy metabolism with energy consumption processes in order to maintain normal cardiac function.
INTRODUCTION
Every cell's own survival and vital functions are supported by energy-generating metabolic pathways. The cellular energy supply and demand must be coordinated, and an imbalance results in cellular dysfunctions and diseases from heart failure to obesity (1, 2). Although the regulation of cellular energy production and consumption individually are focuses of intensive research, it is little understood how these two processes are coordinated. One possible mechanism lies at the level of transcription where the expression of genes critical in both cellular energy production and utilization processes can be regulated in an orchestrated manner. However, such transcription coordinators that directly regulate multiple energy-generating cellular metabolic pathways and energy-consuming cellular functions remain to be established.
The heart offers an ideal system for studying coordination of energy production and consumption. It continuously pumps blood to all the organs, involving energy-demanding processes such as myocardial contraction and electrical conduction (3). Accordingly, cardiomyocytes maintain an exceedingly high metabolic rate and depend on vigorous fatty acid oxidation (FAO), oxidative phosphorylation (OxPhos), and dynamic mitochondrial networks to generate energy that supports these functions (4, 5). Indeed, defects in cardiomyocyte metabolism and mitochondrial function are underlying causes of, or are associated with, many cardiac diseases, including cardiomyopathy and heart failure, that affect millions of people (6–9).
Nuclear receptors (NRs) are ligand-activated transcription factors with important roles in both physiological and pathological settings (10–12). Among the 48 NRs in the human genome, several NRs and their coactivators have been identified as key regulators of cardiac metabolism (13–16). In particular, recent work has revealed important roles for the estrogen-related receptor (ERR) subfamily of NRs, especially ERRα and ERRγ (ERRα/γ), in regulating cellular metabolism (17–19). Genomic studies have found that ERRα and ERRγ target a common set of promoters of genes related to FAO, OxPhos, and muscle contraction (20). Whole-body ERRα knockout (KO) mouse hearts exhibit defects in the bioenergetic and functional adaptation to cardiac pressure overload, but their development and function under normal, unstressed conditions remain intact (21). Whole-body ERRγ KO mice display neonatal cardiac defects, demonstrating the importance of ERRγ in supporting the transition to oxidative metabolism in the perinatal heart (22). Unfortunately, the neonatal lethality (100% within 48 h) of the whole-body ERRγ KO mice excluded further study of ERRγ. In addition, it is unclear whether these cardiac phenotypes are owing to cell-autonomous functions of cardiomyocyte ERRγ. Furthermore, due to the potential overlapping target genes of ERRα and ERRγ (20), their in vivo physiological importance remains to be determined.
Here, we generated mice that specifically lack cardiac ERRγ or both ERRα and cardiac ERRγ to address these questions. While mice lacking either ERRα or cardiac ERRγ exhibited normal survival and cardiac functions, mice lacking both ERRα and cardiac ERRγ died within the first month of life with evident cardiomyopathy and heart failure. Their hearts displayed multiple metabolic defects, including mitochondrial fragmentation and significantly decreased OxPhos activity, accompanied with reduced expression of related genes which are ERR targets. Importantly, the dynamic mitochondrial networks were significantly disrupted, revealing an essential role for ERRα and ERRγ in controlling mitochondrial dynamics. We further showed that this effect was mediated at least partially through direct transcriptional regulation of critical mitochondrial fusion proteins Mfn1 and Mfn2 by ERRα and ERRγ. We also demonstrated that ERRα and ERRγ were required for integral cardiac contractile function by regulating genes important in contraction and calcium homeostasis. In addition, mouse hearts lacking ERRα and ERRγ exhibited severe bradycardia and abnormal electrocardiography (ECG), revealing their vital roles in myocardial conduction. Mechanistically, we showed that ERRα and ERRγ directly bound to and regulated the transcription of many potassium, sodium, and calcium channel genes implicated in human cardiac conduction disorders. Together, these studies reveal the fundamental roles of ERRα and ERRγ in coordinating the cellular energy production and consumption in the heart through orchestrated transcriptional regulation of both processes. These studies also highlight the therapeutic potential of targeting the ERRα and ERRγ pathway for treating cardiac diseases such as cardiomyopathy and heart failure.
MATERIALS AND METHODS
Animal studies.
All animal studies were approved by and carried out under the guidelines of the Institutional Animal Care and Use Committee of the Children's Hospital of Philadelphia and the Salk Institute for Biological studies. Mice were maintained in a temperature- and light-controlled environment with ad libitum access to water. Mice in holding cages (after weaning) received a standard chow diet (lab diet 5L0D; 58% of calories from carbohydrate, 13.5% of calories from fat, and 28.5% of calories from proteins), and breeder mice and their pups before weaning received a breeder diet (lab diet 5058; 55% of calories from carbohydrate, 22% of calories from fat, and 23% of calories from proteins). ERRα KO and ERRγflox/flox (exon 2 is floxed) mice were previously described (23, 24). All mice were backcrossed at least six generations to and maintained in the C57BL6/J background (JAX). For survival rate analysis, the breeding pairs were monitored daily for birth of pups. The first day we observed new pups born was deemed as passage 0 (P0), and the pups were toe clipped for identification and genotyping. Since toe clipping could disturb the mother, resulting in inadequate nurturing, we excluded all pups that died before P4 (all genotypes were represented in these pups) in our survival analysis (Fig. 1C). Both male and female pups were included in the study. All tissues were harvested at between 2 and 5 p.m. of the day to avoid the impact of circadian rhythm.
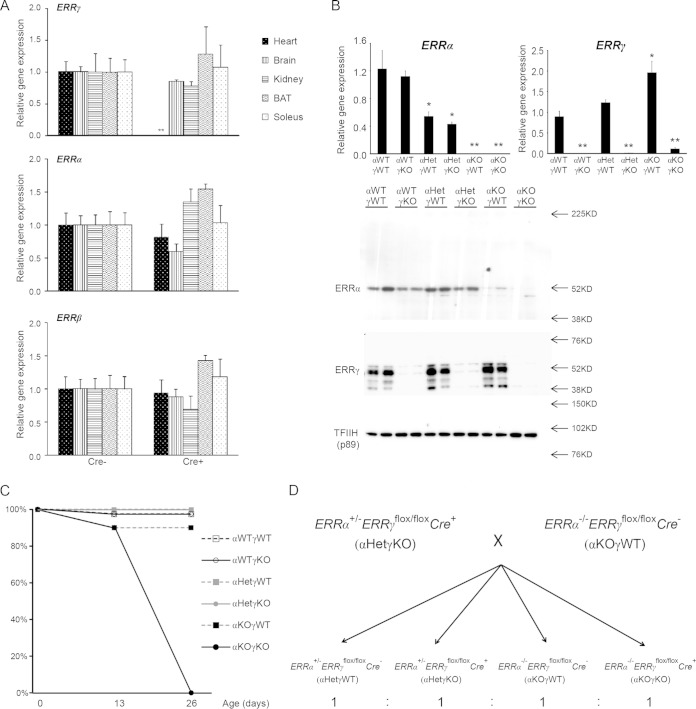
FIG 1.
Mice lacking cardiac ERRα and ERRγ die postnatally. (A) Myh6-Cre-mediated cardiac tissue-specific loss of ERRγ. ERRγ, ERRα, and ERRβ RNAs in different tissues from 2-month-old control (Cre−) and cardiac ERRγ KO (Cre+) mice (n = 4 or 5) were determined by qRT-PCR. **, P < 0.01, between Cre− and Cre+ mice. (B) ERRα and ERRγ RNA (top; n = 4 to 8) and nuclear protein (bottom; n = 2) levels in 3-day-old mouse hearts was determined by qRT-PCR and Western blotting, respectively. *, P < 0.05; **, P < 0.01, between indicated genotype and αWTγWT mice. (C) Survival rate of pups at 0, 13, and 26 days of age (n = 10 to 20). (D) Breeding strategy to generate experimental cohorts. All values are means plus standard errors of the means.
Gene expression analysis.
We isolated total RNA from mouse tissues or cells using RNAzol reagent (Molecular Research Center) according to the manufacturer's instructions. We synthesized cDNA from 1 μg of total RNA using an iScript cDNA synthesis kit (Bio-Rad) and quantified mRNA levels by real-time quantitative reverse transcription-PCR (qRT-PCR) using SYBR green (Bio-Rad) (25, 26). We calculated relative mRNA levels using a standard curve and normalized levels to 36b4 mRNA levels in the same samples. The quantitative PCR (qPCR) primer sequences are listed in Table 1.
TABLE 1.
Sequences of mouse qPCR Primers used in ChIP, mtDNA, and gene expression analysis
| Primer function and namea | Sequence |
|---|---|
| ChIP | |
| Mfn1 For | TGCATGTTTCACCACAGTTTC |
| Mfn1 Rev | GTAGCTCACAACCACCTGTAA |
| Mfn2 For | TCCAATGCAGTATCCCAGTTC |
| Mfn2 Rev | CCAGGACATTCAGGACATGATTA |
| Kcnq1 For | CCCGCAGCTAATTGCTTTAGA |
| Kcnq1 Rev | CATAAACAGACCTCTGGACAACC |
| Kcnh2 For | CTGCCAGATGACCTTGAGTG |
| Kcnh2 Rev | GCCCTGTAGTTTATCACCTTGT |
| Tnnt2 For | CAAAGGGAATTATGTTCTGGGAAA |
| Tnnt2 Rev | GGAAAGAGTAAGGTCTCGGTATG |
| Tnnc1 For | CCCACACACCTGTAACCC |
| Tnnc1 Rev | TGCTGAAAGCTGAGACCATAC |
| mtDNA/nDNA analysis | |
| Cytb For | CATTTATTATCGCGGCCCTA |
| Cytb Rev | TGTTGGGTTGTTTGATCCTG |
| Cox1 For | TGCTAGCCGCAGGCATTACT |
| Cox1 Rev | CGGGATCAAAGAAAGTTGTGT |
| Glucagon For | CAGGGCCATCTCAGAACC |
| Glucagon Rev | GCTATTGGAAAGCCTCTTGC |
| Globin For | GAAGCGATTCTAGGGAGCAG |
| Globin Rev | GGAGCAGCGATTCTGAGTAGA |
| qRT-PCR | |
| ERRα For | CTCAGCTCTCTACCCAAACGC |
| ERRα Rev | CCGCTTGGTGATCTCACACTC |
| ERRβ For | CAGATCGGGAGCTTGTGTTC |
| ERRβ Rev | TGGTCCCCAAGTGTCAGACT |
| ERRγ For | GAATCTTTTTCCCTGCACTACGA |
| ERRγ Rev | GCTGGAATCAATGTGTCGATCTT |
| ANP For | GCTTCCAGGCCATATTGGAG |
| ANP Rev | GGGGGCATGACCTCATCTT |
| BNP For | GAGGTCACTCCTATCCTCTGG |
| BNP Rev | GCCATTTCCTCCGACTTTTCTC |
| CS For | GGACAATTTTCCAACCAATCTGC |
| CS Rev | TCGGTTCATTCCCTCTGCATA |
| Ndufa4 For | TCCCAGCTTGATTCCTCTCTT |
| Ndufa4 Rev | GGGTTGTTCTTTCTGTCCCAG |
| Sdhb For | CTGAATAAGTGCGGACCTATGG |
| Sdhb Rev | AGTATTGCCTCCGTTGATGTTC |
| Cox5a For | GCCGCTGTCTGTTCCATTC |
| Cox5a Rev | GCATCAATGTCTGGCTTGTTGAA |
| Atp5b For | ACGTCCAGTTCGATGAGGGAT |
| Atp5b Rev | TTTCTGGCCTCTAACCAAGCC |
| Cpt1b For | GCACACCAGGCAGTAGCTTT |
| Cpt1b Rev | CAGGAGTTGATTCCAGACAGGTA |
| Cpt2 For | CAGCACAGCATCGTACCCA |
| Cpt2 Rev | TCCCAATGCCGTTCTCAAAAT |
| Slc25a20 For | GACGAGCCGAAACCCATCAG |
| Slc25a20 Rev | AGTCGGACCTTGACCGTGT |
| Acadm For | AGGGTTTAGTTTTGAGTTGACGG |
| Acadm Rev | CCCCGCTTTTGTCATATTCCG |
| Echs1 For | AGCCTGTAGCTCACTGTTGTC |
| Echs1 Rev | ATGTACTGAAAGTTAGCACCCG |
| Hadha For | TGCATTTGCCGCAGCTTTAC |
| Hadha Rev | GTTGGCCCAGATTTCGTTCA |
| Gabpa For | CCAAGCACATTACGACCATTTC |
| Gabpa Rev | CCGTGGACCAGCGTATAGGA |
| Tfam For | CCACAGAACAGCTACCCAAATTT |
| Tfam Rev | TCCACAGGGCTGCAATTTTC |
| Mfn1 For | TGCAATCTTCGGCCAGTTACT |
| Mfn1 Rev | CTCGGATGCTATTCGATCAAGTT |
| Mfn2 For | AGAACTGGACCCGGTTACCA |
| Mfn2 Rev | CACTTCGCTGATACCCCTGA |
| Opa1 For | TGGAAAATGGTTCGAGAGTCAG |
| Opa1 Rev | CATTCCGTCTCTAGGTTAAAGCG |
| Drp1 For | CAGGAATTGTTACGGTTCCCTAA |
| Drp1 Rev | CCTGAATTAACTTGTCCCGTGA |
| Myh6 For | GCCCAGTACCTCCGAAAGTC |
| Myh6 Rev | GCCTTAACATACTCCTCCTTGTC |
| Actc1 For | CTGGATTCTGGCGATGGTGTA |
| Actc1 Rev | CGGACAATTTCACGTTCAGCA |
| Tnni3 For | TCTGCCAACTACCGAGCCTAT |
| Tnni3 Rev | CTCTTCTGCCTCTCGTTCCAT |
| Tnnt2 For | CAGAGGAGGCCAACGTAGAAG |
| Tnnt2 Rev | CTCCATCGGGGATCTTGGGT |
| Tnnc1 For | GCGGTAGAACAGTTGACAGAG |
| Tnnc1 Rev | CCAGCTCCTTGGTGCTGAT |
| Atp2a2 For | GAGAACGCTCACACAAAGACC |
| Atp2a2 Rev | CAATTCGTTGGAGCCCCAT |
| Pln For | AAAGTGCAATACCTCACTCGC |
| Pln Rev | GGCATTTCAATAGTGGAGGCTC |
| Ckmt2 For | ACACCCAGTGGCTATACCCTG |
| Ckmt2 Rev | CCGTAGGATGCTTCATCACCC |
| Mb For | CTGTTTAAGACTCACCCTGAGAC |
| Mb Rev | GGTGCAACCATGCTTCTTCA |
| Kcnq1 For | ACCTCATCGTGGTTGTAGCCT |
| Kcnq1 Rev | GGATACCCCTGATAGCTGATGT |
| Kcnh2 For | GTGCTGCCTGAGTATAAGCTG |
| Kcnh2 Rev | CCGAGTACGGTGTGAAGACT |
| Kcnj2 For | ATGGGCAGTGTGAGAACCAAC |
| Kcnj2 Rev | TGGACTTTACTCTTGCCATTCC |
| Scn5a For | ATGGCAAACTTCCTGTTACCTC |
| Scn5a Rev | CCACGGGCTTGTTTTTCAGC |
| Scn4b For | GGAACCGAGGCAATACTCAGG |
| Scn4b Rev | CCGTTAATAGCGTAGATGGTGGT |
| Cacna1c For | CCTGCTGGTGGTTAGCGTG |
| Cacna1c Rev | TCTGCCTCCGTCTGTTTAGAA |
For, forward; Rev, reverse.
Protein analysis.
Nuclear extracts from mouse hearts were isolated, and Western blotting was performed as previously described (27). The primary antibodies used were ERRα (sc-32971; Santa Cruz), ERRγ (20), and TFIIH p89 (sc-293; Santa Cruz).
Histology.
Sixteen-day-old mice were euthanized and perfused with phosphate-buffered saline (PBS) and then 4% paraformaldehyde (1 ml/min for 5 min). The tissues were then dissected and fixed in 4% paraformaldehyde overnight. Tissues were embedded in paraffin, and 5-μm sections were used for hematoxylin and eosin (H&E) staining according to standard procedures.
EM and mitochondrial size analysis.
We performed electron microscopy (EM) as previously described with minor modifications (26). Sample preparation was performed at the Electron Microscopy Resource Laboratory of the University of Pennsylvania, and the sectioned samples were imaged using a Jeol-1010 transmission electron microscope. For mitochondrial two-dimensional size and perimeter analysis, five imaging fields (magnification of ×10,000) of longitudinal sections per genotype were used, and each field contained at least 100 mitochondria. We used the ImageJ freehand line tool to draw the outline of each mitochondrion and added each as a region of interest (ROI) with the ROI Manager function in ImageJ. The size and perimeter were then calculated with the measure function.
mtDNA/nDNA analysis.
To quantify the relative mitochondrial DNA/native (mtDNA/nDNA) ratio, we isolated total DNA from cells or hearts using a DNA isolation kit (Qiagen) and used qPCR to quantify two mitochondrial genes (Cytb and Cox1) and two nuclear genes (Glucagon and βGlobin). The relative quantities of Cytb and Cox1 and of Glucagon and βGlobin were highly comparable. The mtDNA/nDNA was calculated as the ratio of the average amount of Cytb/Cox1 to the average amount of Glucagon/βGlobin. The primer sequences are listed in Table 1.
Mitochondrial enzyme activity.
Hearts from 16-day-old pups were collected, weighed, and ground in 20 volumes (vol/wt) of homogenization buffer (1 mM EDTA and 50 mM triethanolamine in water) on ice. Citrate synthase (CS) enzymatic activity was determined by the change in absorbance of 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB; Ellman's reagent) measured at 412 nm. Complex I (NADH dehydrogenase) activity was determined by the change in absorbance of NADH measured at 340 nm. Complex II (succinate dehydrogenase) activity was determined by the change in absorbance of 2,6-dichlorophenolindophenol (DCIP) measured at 600 nm. Complex IV (cytochrome c oxidase) enzymatic activity was determined by the change in absorbance of cytochrome c measured at 550 nm. All assays were performed in 96-well plates with the kinetic function of a SpectraMax Paradigm multimode microplate detection platform (Molecular Devices). The linear slopes (change in optical density [ΔOD]/min) were calculated. The molar extinction coefficients used to calculate enzyme activity were 13.6 OD units/mmol/cm (DTNB for CS), 6.22 OD units/mmol/cm (NADH for complex I), 16.3 OD units/mmol/cm (DCIP for complex II), and 29.5 OD units/mmol/cm (cytochrome c for complex IV).
ChIP.
We performed chromatin immunoprecipitation (ChIP) in HL-1 cells as previously described (26). The antibodies used were IgG (sc-2027; Santa Cruz), ERRα (ab16363; Abcam), ERRγ (20), and acetylated histone H3 (positive control) (06-599; Millipore). The ChIP qPCR primer sequences are listed in Table 1.
Transfections.
We PCR amplified and cloned the cross-species conserved mouse Mfn2 promoter region (bp −722 to −503) into the BglII-XhoI sites and the conserved mouse Mfn1 (bp +1035 to +1280), Kcnq1 (bp +1192 to +1438), and Kcnh2 (bp +1290 to +2485) intron regions into the BamHI-SalI sites of the pGL4.10 basic luciferase reporter vector (Promega). 293 cells were transfected using Fugene HD (Promega) in 48-well plates with 100 ng of luciferase reporter, 150 ng of ERR expression vector or pcDNA3.1, and 10 ng of Renilla control. Two days later cells were lysed. The luciferase activity was measured and normalized to that of the Renilla control.
MEFs.
We derived primary mouse embryonic fibroblasts (MEFs) from embryos of ERRα+/− ERRγ+/− (nonfloxed strain) mouse breeding as previously described (26). We used MEFs within five passages for all the experiments.
Retroviral infection.
To produce retrovirus, control mitochondrially targeted DsRed (mtDsRed) or Mfn1 retroviral expression vectors were transfected with the packaging vector pCLEco (all gifts from David Chan, Caltech) into 293 cells. Virus-containing medium was harvested at 48 h to 96 h after transfection and concentrated with an Amicon Ultra-15 centrifugal filter (Millipore). The virus was diluted with fresh medium and used to infect MEF cells with 5 μg/ml Polybrene. Mitochondrial morphology and mtDNA/nDNA analysis were performed 3 and 12 days after the infection.
Mitochondrial morphology analysis.
MEFs were fixed with methanol for 10 min at −20°C. After incubation with an ATP5b antibody (A21351; Life Technologies) and fluorescence-labeled secondary antibody (Jackson ImmunoResearch), the mitochondrial morphology was imaged using a Zeiss LSM710 confocal microscope with a 63× oil immersion lens (28). For mitochondrial size and perimeter analysis, 20 to 25 images per group were used. We subtracted the background with the threshold function of ImageJ to delete pixel intensities less than 70 (background) and then changed all the pixel intensities to 255 with the binary function. We then set up the size and perimeter in the “set measurements” step and calculated the individual mitochondrial morphological parameters with the “analyze particles” function.
Echocardiography.
Mice 15 to 16 days old were anesthetized with isoflurane (3% induction for 3 min and then maintained under 2% isoflurane). After their body weights were measured, the anesthetized mice were subjected to echocardiography using a Vevo2100 Imaging system (VisualSonics). The ambient temperature was maintained with a heating pad and lamp to avoid a decrease in body temperature and bradycardia. The hearts were detected through the parasternal long axis and A2 short axis midventricle view. Two-dimensional (2D) measurements were performed to measure the left ventricle (LV) epicardial area at the end of diastole (LVAepid), the LV endocardial area at the end of diastole (LVAendd), the LV endocardial area at the end of systole (LVAends), the LV length from the plane of the mitral valve to the apical endocardial surface during diastole (LVLd), and the LV length from the plane of the mitral valve to the apical endocardial surface during systole (LVLs). M-mode measurements were performed to measure the thickness of the interventricular septum in diastole (IVSd), LV posterior wall thickness in diastole (LVPWd), LV internal dimension in diastole (LVIDd), and LV internal dimension in systole (LVIDs). The other parameters were calculated as follows:
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
| (6) |
| (7) |
where EDV is end-diastolic volume, ESV is end-systolic volume, SV is stroke volume, CO is cardiac output, HR is heart rate, and EF is ejection fraction.
ECG.
ECG in conscious, ambulatory mice was recorded using ECGenie (Mouse Specifics) according to the manufacturer's instructions. We collected at least 10 s of stable ECG recordings (more than 50 heartbeats), and the software generated the ensemble averaged signal that depicted the ECG morphology, from which P, Q, R, S, and T waves were clearly identifiable. The heart rate, PR interval, QRS complex, and QT interval were then calculated.
Ventricular cardiomyocyte isolation and potassium current recordings.
Twelve- to 16-day-old mice were heparinized (50 units intraperitoneally [i.p.]) and anesthetized with pentobarbital (50 mg/kg), and their hearts were excised through a sternotomy (n = 5 to 7 per group). The hearts were then mounted on a Langendorff apparatus and perfused with Ca2+-free Tyrode's solution for 6 min at 3.0 to 3.5 ml/min and at a temperature of 36 to 37°C, followed by 12 to 15 min of perfusion with Ca2+-free Tyrode's solution containing collagenase plus protease. The atria were dissected away, and the ventricles were kept in Ca2+-free Tyrode's solution with 1 mg/ml bovine serum albumin. Sections of ventricular tissue were then triturated gently with a Pasteur pipette to dissociate individual myocytes. Whole-cell patch clamp recordings were obtained from single ventricular myocytes at room temperature (22 to 24°C) within 12 h of being isolated, as previously described (29). Experiments were performed using an Axopatch 200B amplifier interfaced to a PC computer via a 12-bit analog-to-digital (A/D) interface running the pClamp, version 9.2, software. Potassium currents were elicited in response to 5-s voltage steps, from −60 to +70 mV in 10-mV increments, from a holding potential of −80 mV. Each trial was preceded by a 20-ms depolarization to −20 mV to help eliminate contamination from voltage-gated inward Na+ currents that were not completely inhibited by tetrodotoxin. The bath solution contained 136 mM NaCl, 4 mM KCl, 2 mM MgCl2, 1 mM CaCl2, 10 mM glucose, and 10 mM HEPES, pH 7.4, with NaOH; tetrodotoxin (20 μM) and CdCl2 (200 μM) were also added to suppress voltage-dependent Na+ and Ca2+ currents, respectively. The pipette solution contained 135 mM KCl, 4 mM NaCl, 10 mM EGTA, 10 mM HEPES, 5 mM glucose, 3 mM MgATP, and 0.5 mM Na3GTP, pH 7.2, with KOH. Traces were digitized at 20 kHz and filtered at 5 kHz prior to storage for off-line analysis. Series resistances were compensated electronically (75 to 90%), resulting in uncompensated voltage errors of less than 5 mV. Patch pipettes were fashioned from borosilicate glass and fire polished to a final resistance of 2.0 to 2.5 MΩ.
Statistical analysis.
Statistical significance was calculated by one-way analysis of variance (ANOVA), followed by Tukey's multiple-comparison test (see Fig. 7), two-tailed ANOVA (see Fig. 9F), or a two-tailed, unpaired, unequal variance t test (the remaining figures) and was indicated when the P value was less than 0.05.
FIG 7.
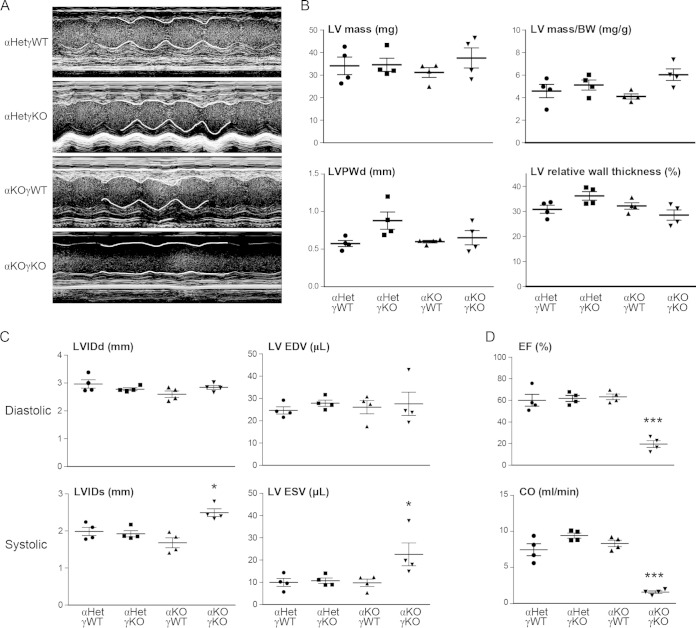
ERRα and ERRγ are vital for cardiac contractile function. (A) Representative echocardiography images (M-mode) of 15- to 16-day-old mice (n = 4). Part of the contraction track was marked by white lines for easy visualization. Please note that since αKOγKO mice have lower heart rates (Fig. 9A and B), the x axis time scale of the αKOγKO echocardiography images was different from scales of other genotypes. (B) LV mass and wall thickness in 15- to 16-day-old mice measured by echocardiography. LVPWd, diastolic LV posterior wall thickness. (C) Cardiac dimensions and volumes in 15- to 16-day-old mice (n = 4) measured by echocardiography. LVIDd, left ventricle internal dimension, diastolic; LV EDV, left ventricle volume, end of diastolic; LVIDs, left ventricle internal dimension, systolic; LV ESV, left ventricle volume, end of systolic. (D) Ejection fraction (EF) and cardiac output (CO) in 15- to 16-day-old mice (n = 4) measured by echocardiography. *, P < 0.05; ***, P < 0.001, between αKOγKO and the other three genotypes. Values are means plus standard errors of the means.
FIG 9.
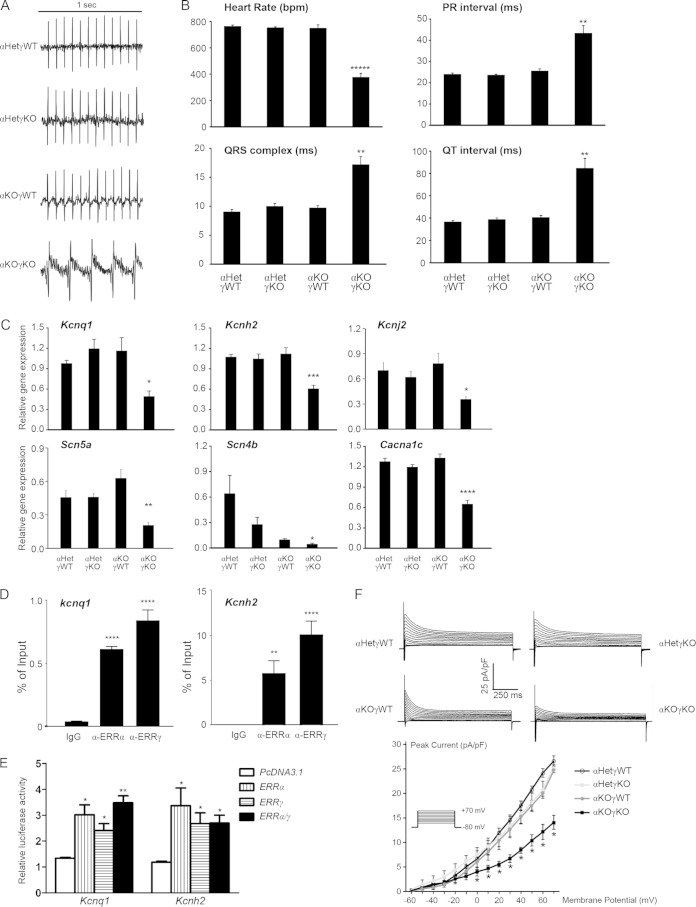
ERRα and ERRγ are essential for normal myocardial conduction through transcriptional regulation of key potassium, sodium, and calcium channels. (A) Representative ECG of 16-day-old mice. (B) Heart rate, PR interval, QRS complex, and QT interval in 16-day-old mice (n = 7 to 9) measured by ECG. **, P < 0.01; *****, P < 0.00001, between αKOγKO and the other three genotypes. (C) Expression of ion channel genes implicated in human conduction disorders in 16-day-old mouse hearts (n = 6 to 8). *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001, between αKOγKO and the other three genotypes. (D) ERRα and ERRγ bound to ERRE within the first intron of the mouse Kcnq1 and Kcnh2 genes. ChIP was performed in HL-1 cardiomyocytes. **, P < 0.01; ****, P < 0.0001, compared to the IgG control. (E) ERRα and ERRγ directly activate the ERRE of the mouse Kcnq1 and Kcnh2 genes. Transient transfection was performed in 293 cells. The value was normalized to the pcDNA3.1/PGL4.10 group. *, P < 0.05; **, P < 0.01, compared to pcDNA3.1 empty plasmid control. In panels B to E, all values are means + standard errors of the means. (F) Global potassium currents recorded from a single ventricular myocyte isolated from 12- to 16-day-old hearts (n = 5). Shown on the top are representative single-cell potassium currents recorded from each of the four different genotypes. The plot on the bottom shows the normalized peak current density versus membrane potential, and the inset depicts the pulse protocol applied to elicit the family of currents. Values are means ± standard errors of the means. *, P < 0.05, between αKOγKO and the other three genotypes.
RESULTS
Mice lacking both ERRα and ERRγ in the heart die postnatally with cardiomyopathy.
The whole-body ERRα KO mice exhibit no cardiac defects under normal, unstressed conditions (21). The whole-body ERRγ KO mice die within 48 h after birth (22), thus preventing the investigation of ERRγ function in the postnatal heart. To circumvent this early lethality, we generated cardiac tissue-specific ERRγ KO (ERRγflox/flox Cre+) mice by crossing mice possessing floxed ERRγ alleles with Myh6-Cre mice (24, 30). All mice were backcrossed at least 5 to 6 generations and maintained in the C57BL6/J background. qRT-PCR and Western blot analysis confirmed the almost complete loss of ERRγ RNA and protein in the hearts but not in other abundantly expressed tissues (brain, kidney, brown adipose tissue, and soleus) (Fig. 1A and B), consistent with the reported cardiac tissue-specific recombination mediated by Myh6-Cre (30). Expression of ERRα and ERRβ was not significantly changed in any of these tissues including the heart (Fig. 1A). The cardiac tissue-specific ERRγ KO mice showed normal survival (Fig. 1C), appeared normal, and displayed no cardiac or other abnormalities at least during the first month of life (see below). Therefore, the lethality phenotype of the whole-body ERRγ KO mice was not due to the loss of cardiac ERRγ itself.
These cardiac tissue-specific ERRγ KO (hereafter referred to as αWTγKO for simplicity, where WT is wild type) mice were further bred with whole-body ERRα KO mice to generate mice lacking both ERRα and ERRγ in the heart (ERRα−/− ERRγflox/flox Cre+, or αKOγKO). Both ERRα and ERRγ RNA and protein were barely detectable in the hearts of the αKOγKO mice by 3 days of age (Fig. 1B). The αKOγKO mice were born at close to the predicted Mendelian ratio. However, the αKOγKO pups exhibited early postnatal lethality, and none of them survived past the first month of life, with most of them dying between 13 and 26 days of age (Fig. 1C). In contrast, all littermates lacking 0 to 3 alleles of ERRα or ERRγ (αWTγWT, ERRα+/+ ERRγflox/flox Cre−; αWTγKO, ERRα+/+ ERRγflox/flox Cre+; αHetγWT, ERRα+/− ERRγflox/flox Cre−; αHetγKO, ERRα+/− ERRγflox/flox Cre+; and αKOγWT, ERRα−/− ERRγflox/flox Cre−; Het indicates heterozygous) had normal survival rates. Due to this early lethality of αKOγKO pups and the fact that no physiological or phenotypical difference between ERRα WT and heterozygous mice was observed by us nor previously reported (21, 23), we bred αHetγKO with αKOγWT mice to generate αHetγWT, αHetγKO, αKOγWT, and αKOγKO littermates (1:1:1:1 expected ratio) (Fig. 1D) for all of the following studies.
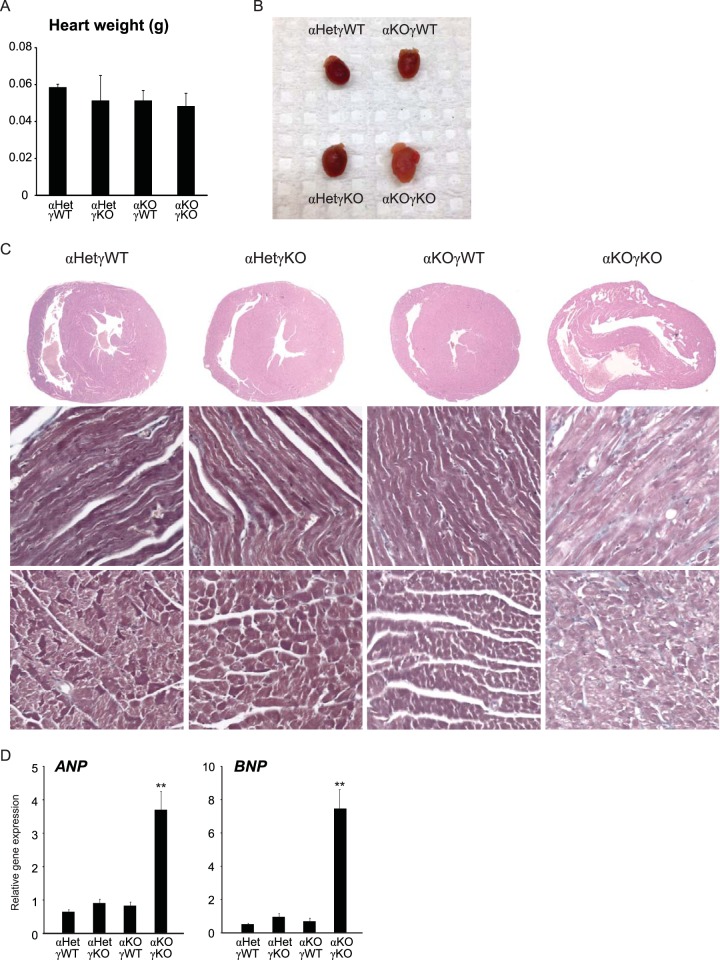
Since loss of ERRγ in the whole-body ERRα KO background was cardiac tissue specific and since only αKOγKO pups exhibited this postnatal lethality, we focused our study on the hearts of these mice. Their heart weights and left ventricle (LV) masses were comparable to those of their littermates at 16 days of age (Fig. 2A; see also Fig. 7B). Compared to control αHetγWT hearts, we observed normal histology in αHetγKO and αWTγKO mouse hearts, consistent with a previous report (21). In contrast, αKOγKO mouse hearts exhibited features of developing dilated cardiomyopathy, including enlargement of both ventricles but no significant changes in the ventricle wall and septum thickness (Fig. 2B and C; see also Fig. 7). In support of these histological observations, expression of atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP), markers associated with cardiomyopathy and heart failure (31, 32), were significantly elevated in αKOγKO hearts (Fig. 2D). These results reveal the essential role of ERRα and ERRγ in postnatal cardiac health.
FIG 2.
Mice lacking cardiac ERRα and ERRγ die postnatally with cardiomyopathy. (A) Heart weight of 16-day-old mice (n = 7 to 11). (B) Representative picture of hearts of 16-day-old mice. (C) Representative pictures of H&E-stained heart sections of 16-day-old mice, as follows: cross-section of the heart (top row), longitudinal view of the muscle fibers (middle row), and transverse view of the muscle fibers (bottom row). (D) Expression of ANP and BNP in 16-day-old mouse hearts (n = 6 to 8). **, P < 0.01, between αKOγKO mice and the other three genotypes. All values are means plus standard errors of the means.
ERRα and ERRγ regulate cardiomyocyte metabolism, especially mitochondrial functions.
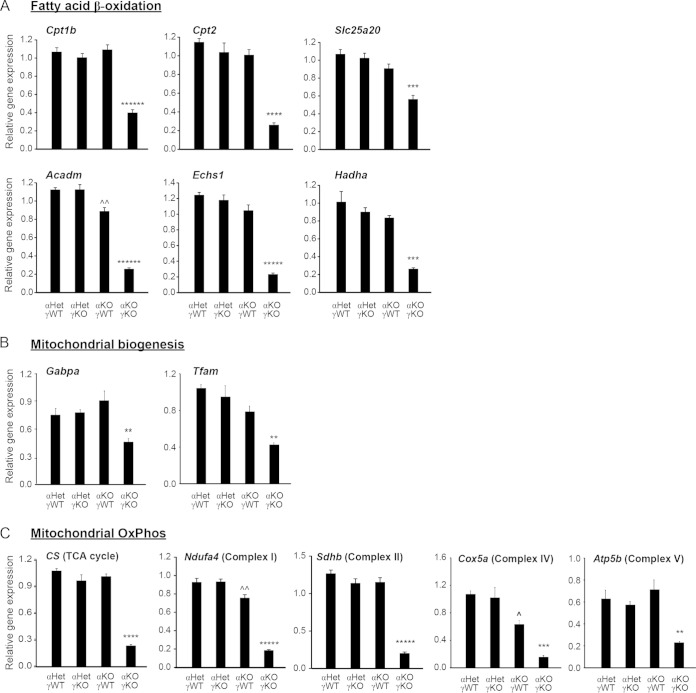
FAO and the ensuing OxPhos in the mitochondria provide a major source of energy needed for adult myocardium (2). Previous studies combining ChIP with microarray technology (ChIP-on-chip) revealed that promoters of many cardiac FAO and OxPhos genes were bound by both ERRα and ERRγ, establishing them as direct transcriptional targets of ERRα and ERRγ (20). However, expression of only some of these genes was changed in the hearts of adult whole-body ERRα KO or neonatal whole-body ERRγ KO mice (20–22). More importantly, it was not determined whether OxPhos or other mitochondrial functions were changed in hearts of mice with KO of either ERRα or ERRγ (single KO), considering the significant overlap of target genes between ERRα and ERRγ (20). We therefore first used qRT-PCR to examine the expression of genes important in cardiac metabolism in the αKOγKO hearts and found that expression of a large number of genes was significantly decreased compared to levels in the littermate controls. These covered both known and previously unknown ERR target genes and included almost all genes in the mitochondrial FAO pathway (Cpt1b, Cpt2, Slc25a20, Acadm, Echs1, and Hadha) (Fig. 3A) and genes important in the transcriptional control of mitochondrial biogenesis (Gabpa and Tfam) (Fig. 3B), as well as over 40 genes encoding proteins of the mitochondrial tricarboxylic acid (TCA) cycle and electron transport chain (ETC) complexes, a subset of which are shown in Fig. 3C (citrate synthase [CS], Ndufa4, Sdhb, Cox5v, and Atp5b).
FIG 3.
ERRα and ERRγ are essential for expression of genes important in cardiomyocyte metabolism, especially mitochondrial functions. Expression levels of genes important in mitochondrial fatty acid oxidation (A), biogenesis (B), and OxPhos (C) were determined in 16-day-old mouse hearts (n = 6 to 8). **, P < 0.01; ***, P < 0.001; ****, P < 0.0001; *****, P < 0.00001; ******, P < 0.000001, between αKOγKO mice and the other three genotypes; ^, P < 0.05; ^, P < 0.01, between αKOγWT and αHetγWT/αHetγKO. All values are means plus standard errors of the means.
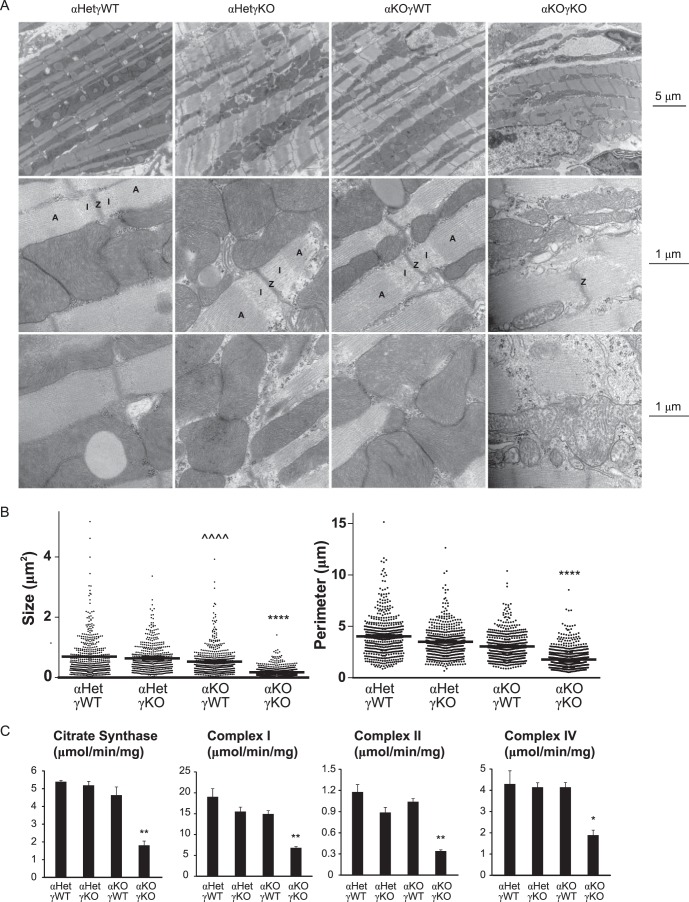
We then evaluated the mitochondrial morphology using EM. Previous studies found no structural changes of mitochondria and sarcomeres in whole-body ERRα or ERRγ single-KO mouse hearts (21, 22). Consistent with these reports and our gene expression studies (Fig. 3), we found no defects in cardiac mitochondrial and sarcomeric ultrastructures in any of the genotypes except the αKOγKO hearts (Fig. 4A). The αKOγKO hearts exhibited several notable ultrastructural abnormalities, including distorted myofibrils (Fig. 4A, top row), consistent with histological observations (Fig. 2C). In particular, compared to the cardiac sarcomeres of control littermates displaying clear A and I bands, many αKOγKO heart sarcomeres seemed to lack clear boundaries between the A band and I band (Fig. 4A, middle row). In contrast to well-organized, densely packed mitochondria along the myofibrils in hearts of the littermate controls, αKOγKO heart mitochondria appeared severely fragmented and showed significant loss of matrix density and clear crista membranes (Fig. 4A, middle and bottom rows). In support of this observation of mitochondrion fragmentation, αKOγKO heart mitochondria were significantly smaller, as quantified by their size and perimeter (Fig. 4B). In line with these gene expression and structural changes, only the αKOγKO hearts exhibited significant loss of the mitochondrial functions essential for energy generation, including decreased enzymatic activities of the TCA cycle (citrate synthase) and ETC complexes (Fig. 4C). These results indicate that ERRα and ERRγ together are essential transcriptional regulators of cardiomyocyte metabolism.
FIG 4.
ERRα and ERRγ are essential for normal mitochondrial functions. (A) Representative EM pictures of hearts of 16-day-old mice, as follows: magnification of ×10,000 to show the overall cardiac myofibril structure including the sarcomeres and the mitochondria (top row); magnification of ×60,000 to show the mitochondrial ultrastructure and the sarcomere Z line (Z), I band (I), and A band (A) (middle row); magnification of ×60,000 to focus on the mitochondrial ultrastructure (bottom row). (B) Mitochondrial size (two-dimensional area) and perimeter were quantified from five EM fields (magnification of ×10,000 with at least 100 mitochondria per field) using ImageJ. (C) Activity of TCA cycle enzyme citrate synthase and different mitochondrial ETC complexes in 16-day-old mouse hearts was measured by enzymatic assays (n = 3). *, P < 0.05; **, P < 0.01; ****, P < 0.0001, between αKOγKO and the other three genotypes; ^^^^, P < 0.0001, between αKOγWT and αHetγWT/αHetγKO mice. All values are means plus standard errors of the means.
ERRα and ERRγ are important for integral mitochondrial dynamics.
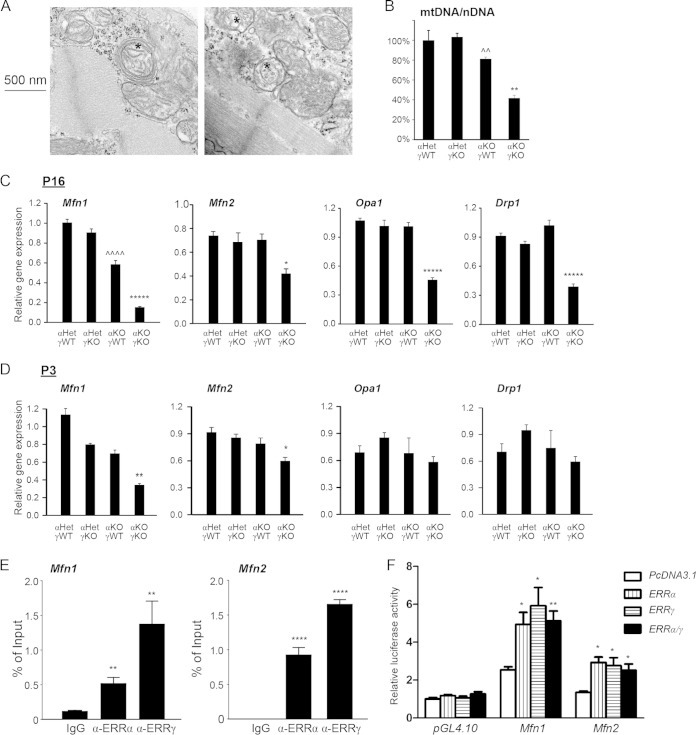
Mitochondria inside a healthy cell form a dynamic network, which is essential for mitochondrial quality control, by constantly fusing and dividing to exchange contents. In addition, mitochondrial fusion and fission are important and conserved mechanisms from yeast (Saccharomyces cerevisiae) to mammals that ensure integral mitochondrial function, balancing cellular energy demand and supply, apoptosis, and other cellular functions (33–39). Mutation of genes involved in mitochondrial fusion and fission causes a variety of diseases, including cardiomyopathy, optic atrophy, and axonal neuropathy in humans and other animals (40–42). We noticed that the mitochondrial fragmentation and other ultrastructural defects in αKOγKO hearts were similar to those observed in mitochondrial dynamics-defective animal models (43–47). We also observed that some mitochondria in the αKOγKO hearts were wrapped by multiple double membranes (Fig. 5A), indicating mitochondrial dynamics defects (46, 47). Consistent with the notion that mitochondrial dynamics is essential to maintain mitochondrial DNA (mtDNA) stability and quantity (43, 44), the mtDNA amount decreased by about 60% in the αKOγKO hearts (Fig. 5B). Accordingly, we found that expression of almost all genes essential for mitochondrial fusion and fission was significantly reduced in 16-day-old αKOγKO hearts (Fig. 5C). These included critical mitochondrial fusion genes Mfn1, Mfn2, and Opa1 as well as the key mitochondrial fission gene Drp1. Expression of Mfn1 and Mfn2 (but not Opa1 and Drp1) was also significantly decreased in αKOγKO hearts at a much younger age (3 days old) (Fig. 5D), suggesting that they are likely direct transcriptional targets of ERRα and ERRγ. We found conserved ERR response elements (ERREs) located within the first intron of the mouse Mfn1 gene and in the promoter of the mouse Mfn2 gene. A ChIP assay confirmed that ERRα and ERRγ directly bound to these ERREs (Fig. 5E). Furthermore, both ERRα and ERRγ activated these ERRE-driven luciferase reporters (Fig. 5F), establishing Mfn1 and Mfn2 as direct ERRα and ERRγ target genes in the heart.
FIG 5.
ERRα and ERRγ are important for integral mitochondrial dynamics. (A) Representative EM pictures of 16-day-old αKOγKO hearts. Magnification of ×75,000 to show some mitochondria surrounded by multiple double membranes (*). (B) mtDNA/nDNA content in 16-day-old mouse hearts (n = 4). **, P < 0.01, between αKOγKO and the other three genotypes; ^^, P < 0.01, between αKOγWT and αHetγKO only. (C and D) Expression of Mfn1, Mfn2, Opa1, and Drp1 in 16-day-old (C) and 3-day-old (D) mouse hearts (n = 6 to 8). *, P < 0.05; **, P < 0.01; *****, P < 0.00001, between αKOγKO and the other three genotypes; ^^^^, P < 0.0001 between αKOγWT and αHetγWT/αHetγKO. (E) ERRα and ERRγ bind to ERRE within the first intron of the mouse Mfn1 gene and in the promoter region of the mouse Mfn2 gene. ChIP was performed in mouse HL-1 cardiomyocytes. **, P < 0.01; ****, P < 0.0001, compared to IgG control. (F) ERRα and ERRγ directly activate the ERRE of the mouse Mfn1 and Mfn2 genes. Transient transfection was performed in 293 cells. The value was normalized to the pcDNA3.1/PGL4.10 group. *, P < 0.05; **, P < 0.01, compared to pcDNA3.1 empty plasmid control. All values are means plus standard errors of the means.
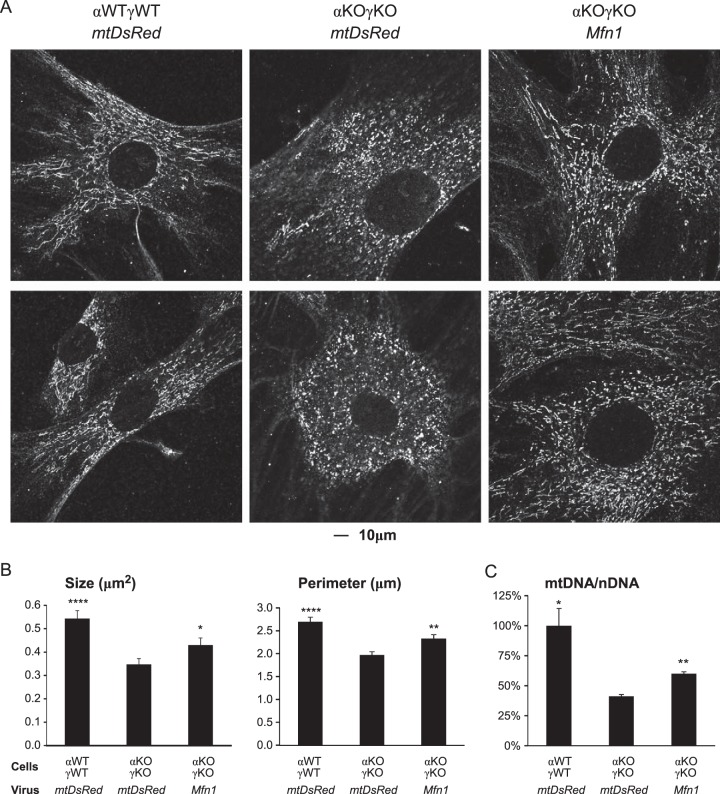
Next, we investigated whether the reduced levels of these genes were responsible for the mitochondrial dynamics defects and mtDNA loss in the αKOγKO hearts. We first confirmed that the αKOγKO cells in vitro recapitulated the mitochondrial defects in vivo, including fragmented mitochondria (compare Fig. 6A and B to 4A and B) and loss of mtDNA (compare Fig. 6C to 5B). We then tested whether restored expression of these mitochondrial dynamics genes would rescue the mitochondrial phenotype. Overexpression of Mfn1 alone significantly alleviated mitochondrial fragmentation (Fig. 6A), as quantified by the size and perimeters of mitochondria (Fig. 6B) and the mtDNA quantity (Fig. 6C) in the αKOγKO cells. The rescue was not complete, probably because the expression levels of other genes important in mitochondrial dynamics (Fig. 5) and mtDNA quantity (such as Tfam) (Fig. 3B) were not restored or because retroviral infection was incomplete (about 70 to 80% of cells were infected, as judged by mtDsRed expression). These results demonstrate the important role of ERRα and ERRγ in controlling mitochondrial dynamics.
FIG 6.
Overexpression of Mfn1 partially rescues the mitochondrial morphology and mtDNA defects in αKOγKO MEFs. (A) Representative confocal microscopy images show the mitochondrial morphology (revealed via ATP5b protein staining) in WT (αWTγWT) and ERRα−/− ERRγ−/− (αKOγKO) MEFs infected with a control mtDsRed or Mfn1 retrovirus expression vector. (B) Mitochondrion (ATP5b-positive staining) size (two-dimensional area) and perimeter in αWTγWT and αKOγKO MEFs were quantified (20 to 25 images per group). (C) mtDNA/nDNA content in αWTγWT and αKOγKO MEFs (n = 3). *, P < 0.05; **, P < 0.01; ****, P < 0.0001, between indicated genotype/treatment and the αKOγKO MEFs with mtDsRed. All values are means plus standard errors of the means.
ERRα and ERRγ are vital for cardiac contractile function.
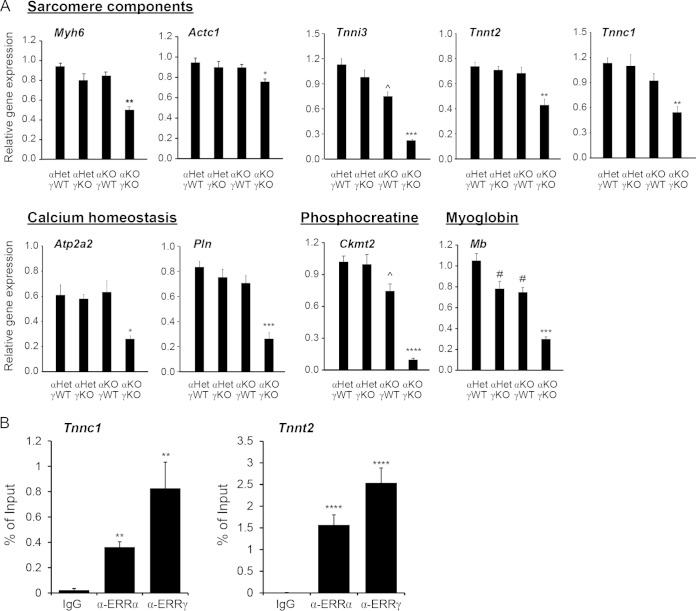
Previous ChIP-on-chip and gain-of-function studies have found that ERRα and ERRγ directly regulate the expression of cardiac contractile genes, including Myh6, Acta1, Tnni3, phospholamban (Pln), and Atp2a2 (also known as Serca2) (20). However, it is unclear whether ERRα and ERRγ are absolutely required for their expression in a loss-of-function context as expression of these genes was either unchanged or only slightly changed in ERRα KO or ERRγ KO hearts at basal states (20–22). More importantly, no cardiac contractile defects were previously observed in these ERRα KO or ERRγ KO hearts at basal states. We therefore used echocardiography to determine the cardiac contractile function in αKOγKO mice and their littermates (Fig. 7A). Although the absolute and relative (normalized to body weight) LV mass, absolute and relative (normalized to LV total dimension) LV wall thickness (Fig. 7B), and diastolic LV dimension (LVIDd) and volume (LV EDV) remained similar among all genotypes (Fig. 7C), the systolic LV dimension (LVIDs) and volume (LV ESV) were significantly increased in αKOγKO hearts but not in hearts lacking either ERRα or ERRγ alone (Fig. 7C). These findings were consistent with the histological observations of developing dilated cardiomyopathy (Fig. 2C). More importantly, the αKOγKO hearts exhibited significantly decreased cardiac contractile function as measured by the ejection fraction (EF) and cardiac output (CO) (Fig. 7D). For example, compared to the 60% EF, which is within the normal range in the control animal hearts, αKOγKO hearts have an EF of only 20%, a value indicating severe cardiomyopathy or heart failure clinically. In line with these functional changes, αKOγKO hearts had significantly reduced expression of Myh6, Actc1, Tnni3, Mb, Atp2a2, Pln, and Ckmt2, genes that encode proteins of sarcomere components and myoglobin or that are important in calcium homeostasis and the phosphocreatine system (Fig. 8A). We also identified additional, previously unknown ERRα and ERRγ target genes important in cardiac contraction. These include Tnnt2 and Tnnc1, whose expression levels were decreased in αKOγKO hearts (Fig. 8A). We found conserved ERREs located within the first intron of both the Tnnt2 and Tnnc1 genes and confirmed by ChIP that ERRα and ERRγ directly bound to these ERREs (Fig. 8B). Together, these studies establish that ERRα and ERRγ are crucial transcriptional regulators of cardiac contractile function.
FIG 8.
ERRα and ERRγ regulate cardiac contraction through transcriptional modulation of muscle contractile genes. (A) Expression of genes important in cardiac contraction in 16-day-old mouse hearts (n = 6 to 8). *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001, between αKOγKO and the other three genotypes; ^, P < 0.05, between αKOγWT and αHetγWT/αHetγKO mice; #, P < 0.05, between αKOγWT/αHetγKO and αHetγWT mice. (B) ERRα and ERRγ bound to ERREs within the first introns of the mouse Tnnc1 and Tnnt2 genes. ChIP was performed in HL-1 cardiomyocytes. **, P < 0.01; ****, P < 0.0001, compared to the IgG control. All the values are means plus standard errors of the means.
ERRα and ERRγ are essential for normal myocardial conduction through transcriptional regulation of key potassium, sodium, and calcium channel genes.
In addition to contraction, electric conduction is another essential and major energy-consuming cardiac function (3). Defects in heart rate, myocardial conduction, and repolarization that reduce metabolic demand by the weakened myocardium are often associated with the onset of cardiomyopathy (48). We previously reported that whole-body ERRγ KO mice exhibited neonatal lethality and slightly prolonged QRS complex and QT intervals (22, 49). However, we now found that mice lacking cardiac ERRγ alone (in both ERRα WT and heterozygous backgrounds) were viable, with normal cardiac structure, metabolism, mitochondrial dynamics, and contractile function (Fig. 1 to 8). This raised the question of whether the long QT intervals and associated gene expression changes in the neonatal whole-body ERRγ KO pups was due to a cardiac tissue-autonomous defect or was impacted by loss of ERRγ in other tissues such as the brain. We used ECG to investigate this in conscious, ambulatory cardiac tissue-specific ERRγ KO and αKOγKO pups. Compared to control αHetγWT littermates, we found no ECG defects in mice lacking either only ERRα (αKOγWT) or cardiac ERRγ (αHetγKO) (Fig. 9A and B). Therefore, cardiac ERRγ itself was not required for maintaining normal myocardial conduction.
In sharp contrast, mice lacking both ERRα and cardiac ERRγ (αKOγKO) exhibited abnormal ECGs and severe bradycardia, with a heart rate of only about half that of their littermate controls (Fig. 9A and B). This notable bradycardia phenotype was not seen in either αKOγWT or αHetγKO littermates, nor was it previously reported in whole-body adult ERRα or neonatal ERRγ KO mice (20–22). In addition, the QRS complex and the PR and QT intervals were significantly prolonged in the αKOγKO hearts compared to those of all other genotypes (Fig. 9B).
In the setting of cardiomyopathy, transcriptional alterations that directly affect cardiac ion channel expression and function contribute to the electrophysiological remodeling (50). Therefore, we measured the expression of important cardiac ion channel genes, especially those implicated in human conduction disorders (51–53). We found that expression levels of multiple cardiac potassium (Kcnq1, Kcnh2, and Kcnj2), sodium (Scn5a and Scn4b), and calcium (Cacna1c) channel genes were significantly decreased in αKOγKO hearts compared to control levels (Fig. 9C). This is clinically relevant as mutations of all of these genes are known to cause conduction disorders with similar symptoms, including prolonged QT intervals, in humans (51–53). Next, we examined whether transcription of these ion channel genes was directly controlled by ERRα and ERRγ. Testing this for the genes encoding Kcnq1 and Kcnh2, two of the most abundantly expressed voltage-dependent potassium channel proteins essential for myocardial repolarization (54), we found that ERRα and ERRγ directly bound to a conserved region located in the first intron of both genes (Fig. 9D). In addition, we demonstrated that ERRα and ERRγ could activate these enhancers in a luciferase reporter assay (Fig. 9E). Finally, we recorded potassium currents from acutely isolated αKOγKO and control ventricular cardiomyocytes to determine whether the potassium currents were altered. These studies revealed that peak global potassium currents were reduced by about 50% in αKOγKO cardiomyocytes compared to those isolated from hearts of control littermates (Fig. 9F), consistent with cardiomyocyte-autonomous defects of potassium channels. Taken together, these data indicate that ERRα and ERRγ are essential transcriptional regulators for normal myocardial conduction.
DISCUSSION
We previously reported that whole-body ERRγ KO pups died shortly after birth (22). This neonatal lethality (100% penetrance within 48 h) happened in both the originally reported ICR outbred and C57BL6/J inbred backgrounds. The neonatal ERRγ KO pups showed altered expression of some cardiac metabolic and contractile genes but few functional consequences, and they maintained normal cardiac structure. It was not completely clear at that time whether the neonatal lethality was caused by these cardiac problems or by other unidentified defects in other tissues. Our current finding of the normal survival and cardiac function of cardiac tissue-specific ERRγ KO Foundation for Medical Research, the Ellison Medical of ERRγ are critical in supporting neonatal survival in mice. Notably, in addition to the heart, ERRγ is also abundantly expressed in other tissues, including the brain and kidney. Whole-body ERRγ KO mice also displayed defects in embryonic kidney development (55). Future studies of other tissue-specific ERRγ KO mice are needed to determine which organ's ERRγ function is essential for the neonatal survival of mice.
In vitro studies have revealed ERRα and ERRγ as critical regulators of cardiac metabolism (17–19). However, neither ERRα KO nor cardiac tissue-specific ERRγ KO mice exhibited any major cardiac structural or functional defects at basal states. In line with the genomic studies (20), our current genetic studies using αKOγKO mice firmly establish the essential roles of ERRα and ERRγ together in maintaining intact cardiac metabolism and function. In addition to providing definitive evidence supporting the importance of ERRα and ERRγ in regulating cellular oxidative metabolism and cardiac contractile function hinted at in these earlier studies, our current study has also revealed that ERRα and ERRγ are essential for other aspects of cardiac physiology. First, they regulate mitochondrial dynamics through direct transcriptional regulation of key mitochondrial fusion genes. αKOγKO hearts exhibit loss of mtDNA and defective mitochondrial dynamics with concomitant decreased expression of key mitochondrial dynamics genes such as Mfn1. Importantly, these defects can be partially rescued by restored expression of Mfn1, at least in vitro. Second, ERRα and ERRγ directly control the expression of many ion channel genes essential for intact myocardial conduction. Although the potential role of ERRα in regulating the expression of some of these genes was implicated from gain-of-function studies in different cell types (47, 49, 56–59), our study provides the first definitive evidence that ERRα and ERRγ together are required for expression of these genes in a loss-of-function context and are absolutely essential for integral mitochondrial dynamics and myocardial conduction in vivo. Since cardiac bioenergetic deficiency, contractile dysfunction, or a conduction defect alone can result in cardiomyopathy and associated cardiac dysfunctions (6, 60–62), it is likely that both such indirect effects and ERR-dependent direct transcriptional regulation contribute to the overall cardiomyopathy phenotype of αKOγKO mice.
The phenotypes of our αKOγKO mice are strikingly similar to those reported in whole-body Pgc1α and Pgc1β KO (or cardiac Pgc1β KO in the whole-body Pgc1α KO background) or cardiac Mfn1 and Mfn2 KO mice (44, 47, 63). These include early onset, 100% penetrant postnatal lethality, cardiomyopathy, bradycardia, and cardiac dysfunction, together with defects in cellular metabolism and mitochondrial structure and function. Intriguingly, the Mfn1 locus was recently found to impact heart rate in humans through genome-wide association studies (GWAS), and its knockdown reduced heart rate in both fruit fly and zebrafish (64). These findings raise the possibility that these cardiac phenotypes are controlled by a common cellular pathway involving ERR, Pgc1, and Mfn proteins. Pgc1α and Pgc1β are coactivators of many transcription factors, including ERRα and ERRγ, playing important roles in many physiological and pathological conditions (65). The phenotypic similarity between Pgc1α and Pgc1β KO mice and our cardiac ERRα and ERRγ KO mice suggest that ERRα and ERRγ are the principal transcription factor partners of the Pgc1 proteins in the developing mouse heart. In addition, Mfn1 and Mfn2 are downstream targets of both ERRα/ERRγ (our study) and Pgc1α/Pgc1β signaling (47, 57–59). These observations support a hypothesis that ERRα/ERRγ together with coactivators Pgc1α/Pgc1β control cardiac metabolism and function at least partially through Mfn1/Mfn2. Future studies will determine the exact contribution of Mfn1/Mfn2 toward the heart phenotypes of cardiac ERRα and ERRγ KO and Pgc1α and Pgc1β KO mice.
Cellular energy production and consumption must be coordinated to support various cellular functions. Through regulating multiple processes involved in cellular energy production (FAO, OxPhos, and mitochondrial dynamics) and consumption (cardiac contraction, calcium homeostasis, and electrical conduction) at the same time, ERRα and ERRγ offer the critical assistance to this supply-and-demand relationship. Importantly, mechanistic studies from us and others (20–22) demonstrate that a large number of genes critical in all these pathways are direct transcriptional targets of ERRα and ERRγ. In addition, ERRα transcriptional activity can be modulated by multiple signaling pathways that reflect either cellular physiological/energy status or metabolic demand from distinct cellular functions (17, 18). Less is known about ERRγ, and future studies will need to determine whether and how ERRγ protein level or activity is regulated under these similar conditions.
Our studies also advocate the therapeutic potential of ERRα and ERRγ in treating heart diseases such as cardiomyopathy and heart failure. Altered expression of ERRα and ERRγ as well as mutations of their target genes have been associated with cardiomyopathy, heart failure, and conduction disorders in humans (66–68). Like many other nuclear receptors, the activities of ERRα and ERRγ can be modulated by available small-molecule ligands (C29, XCT790, GSK5182, GSK4716, etc.) (68–71). Future efforts to test ERRα and ERRγ ligands in animal models or even clinical settings of cardiac disease will help uncover their therapeutic values.
ACKNOWLEDGMENTS
We are grateful to Johan Auwerx for the ERRγ floxed mouse, Michael Schneider for the Myh6-Cre mouse (JAX 011038), David Chan for retroviral plasmids (mtDsRed control, Mfn1 and Mfn2), Bill Claycomb for the HL-1 cells, Jamie Whyte for discussion and assistance with the ECG study, Tiffany Tseng for technical assistance, Ning Zhou at the Penn CVI Mouse Cardiovascular Physiology and Microsurgery Core for help with the echocardiography study, and Ray Meade and Biao Zuo at the Electron Microscopy Resource Laboratory of the University of Pennsylvania for help with the EM study. We thank Douglas Wallace, Jonathan Epstein, Elizabeth Goldmuntz, Matthew Weitzman, Marc Vermulst, Michael Downes, Jeremy Leipzig, Will Alaynick, Martin Picard, Ryan Morrow, Catherine Dufour, and Benjamin Wilkins for critical discussion of the project and experiments.
This work was supported by National Institutes of Health grants HL105734 (V. V. Patel), HL105278 and DK057978 (R. M. Evans), HD026979 (Core Facilities Utilization Grant, Intellectual and Developmental Disabilities Center and the Metabolomics Core of CHOP and CHOP/PENN Mitochondria Research Affinity Group), and OD016393 (VisualSonics Vevo 2100 imaging system), American Heart Association grant 11IRG4930008 (V. V. Patel), the Glenn Foundation for Medical Research, the Ellison Medical Foundation, and the Leona M. and Harry B. Helmsley Charitable Trust 2012-PG-MED002 (all R. M. Evans), and pilot funds from the Research Institute of the Children's Hospital of Philadelphia (L. Pei). R. M. Evans is an investigator of the Howard Hughes Medical Institute at the Salk Institute for Biological Studies and March of Dimes Chair in Molecular and Developmental Biology.
We declare that we have no conflicts of interest.
L. Pei directed the project. Ting Wang and L. Pei performed most of the experiments and analyzed the results. C. McDonald provided technical assistance. N. B. Petrenko performed the K+ current recordings; N. B. Petrenko and V. V. Patel analyzed the result. M. Leblanc is a pathologist who performed the histology studies. Tao Wang performed echocardiography and analyzed the results. Ting Wang and L. Pei wrote and M. Leblanc, V. Giguere, V. V. Patel, and R. M. Evans edited the manuscript.
REFERENCES
- 1.Spiegelman BM, Flier JS. 2001. Obesity and the regulation of energy balance. Cell 104:531–543. doi: 10.1016/S0092-8674(01)00240-9. [DOI] [PubMed] [Google Scholar]
- 2.Huss JM, Kelly DP. 2005. Mitochondrial energy metabolism in heart failure: a question of balance. J Clin Invest 115:547–555. doi: 10.1172/JCI24405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cortassa S, Aon MA, O'Rourke B, Jacques R, Tseng HJ, Marban E, Winslow RL. 2006. A computational model integrating electrophysiology, contraction, and mitochondrial bioenergetics in the ventricular myocyte. Biophys J 91:1564–1589. doi: 10.1529/biophysj.105.076174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kane LA, Youle RJ. 2010. Mitochondrial fission and fusion and their roles in the heart. J Mol Med (Berl) 88:971–979. doi: 10.1007/s00109-010-0674-6. [DOI] [PubMed] [Google Scholar]
- 5.Jafri MS, Dudycha SJ, O'Rourke B. 2001. Cardiac energy metabolism: models of cellular respiration. Annu Rev Biomed Eng 3:57–81. doi: 10.1146/annurev.bioeng.3.1.57. [DOI] [PubMed] [Google Scholar]
- 6.Wallace DC. 2000. Mitochondrial defects in cardiomyopathy and neuromuscular disease. Am Heart J 139:S70–85. doi: 10.1067/mhj.2000.103934. [DOI] [PubMed] [Google Scholar]
- 7.Griffiths EJ. 2012. Mitochondria and heart disease. Adv Exp Med Biol 942:249–267. doi: 10.1007/978-94-007-2869-1_11. [DOI] [PubMed] [Google Scholar]
- 8.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. 2010. Heart disease and stroke statistics–2010 update: a report from the American Heart Association. Circulation 121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 9.Harvey PA, Leinwand LA. 2011. The cell biology of disease: cellular mechanisms of cardiomyopathy. J Cell Biol 194:355–365. doi: 10.1083/jcb.201101100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evans R. 2004. A transcriptional basis for physiology. Nat Med 10:1022–1026. doi: 10.1038/nm1004-1022. [DOI] [PubMed] [Google Scholar]
- 11.Sonoda J, Pei L, Evans RM. 2008. Nuclear receptors: decoding metabolic disease. FEBS Lett 582:2–9. doi: 10.1016/j.febslet.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evans RM, Mangelsdorf DJ. 2014. Nuclear receptors, RXR, and the BIG Bang. Cell 157:255–266. doi: 10.1016/j.cell.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huss JM, Kelly DP. 2004. Nuclear receptor signaling and cardiac energetics. Circ Res 95:568–578. doi: 10.1161/01.RES.0000141774.29937.e3. [DOI] [PubMed] [Google Scholar]
- 14.Leone TC, Lehman JJ, Finck BN, Schaeffer PJ, Wende AR, Boudina S, Courtois M, Wozniak DF, Sambandam N, Bernal-Mizrachi C, Chen Z, Holloszy JO, Medeiros DM, Schmidt RE, Saffitz JE, Abel ED, Semenkovich CF, Kelly DP. 2005. PGC-1α deficiency causes multi-system energy metabolic derangements: muscle dysfunction, abnormal weight control and hepatic steatosis. PLoS Biol 3:e101. doi: 10.1371/journal.pbio.0030101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burkart EM, Sambandam N, Han X, Gross RW, Courtois M, Gierasch CM, Shoghi K, Welch MJ, Kelly DP. 2007. Nuclear receptors PPARβ/δ and PPARα direct distinct metabolic regulatory programs in the mouse heart. J Clin Invest 117:3930–3939. doi: 10.1172/JCI32578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arany Z, He H, Lin J, Hoyer K, Handschin C, Toka O, Ahmad F, Matsui T, Chin S, Wu PH, Rybkin II, Shelton JM, Manieri M, Cinti S, Schoen FJ, Bassel-Duby R, Rosenzweig A, Ingwall JS, Spiegelman BM. 2005. Transcriptional coactivator PGC-1α controls the energy state and contractile function of cardiac muscle. Cell Metab 1:259–271. doi: 10.1016/j.cmet.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Giguere V. 2008. Transcriptional control of energy homeostasis by the estrogen-related receptors. Endocr Rev 29:677–696. doi: 10.1210/er.2008-0017. [DOI] [PubMed] [Google Scholar]
- 18.Villena JA, Kralli A. 2008. ERRα: a metabolic function for the oldest orphan. Trends Endocrinol Metab 19:269–276. doi: 10.1016/j.tem.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eichner LJ, Giguere V. 2011. Estrogen related receptors (ERRs): a new dawn in transcriptional control of mitochondrial gene networks. Mitochondrion 11:544–552. doi: 10.1016/j.mito.2011.03.121. [DOI] [PubMed] [Google Scholar]
- 20.Dufour CR, Wilson BJ, Huss JM, Kelly DP, Alaynick WA, Downes M, Evans RM, Blanchette M, Giguere V. 2007. Genome-wide orchestration of cardiac functions by the orphan nuclear receptors ERRα and γ. Cell Metab 5:345–356. doi: 10.1016/j.cmet.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 21.Huss JM, Imahashi K, Dufour CR, Weinheimer CJ, Courtois M, Kovacs A, Giguere V, Murphy E, Kelly DP. 2007. The nuclear receptor ERRα is required for the bioenergetic and functional adaptation to cardiac pressure overload. Cell Metab 6:25–37. doi: 10.1016/j.cmet.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 22.Alaynick WA, Kondo RP, Xie W, He W, Dufour CR, Downes M, Jonker JW, Giles W, Naviaux RK, Giguere V, Evans RM. 2007. ERRγ directs and maintains the transition to oxidative metabolism in the postnatal heart. Cell Metab 6:13–24. doi: 10.1016/j.cmet.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 23.Luo J, Sladek R, Carrier J, Bader JA, Richard D, Giguere V. 2003. Reduced fat mass in mice lacking orphan nuclear receptor estrogen-related receptor alpha. Mol Cell Biol 23:7947–7956. doi: 10.1128/MCB.23.22.7947-7956.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gan Z, Rumsey J, Hazen BC, Lai L, Leone TC, Vega RB, Xie H, Conley KE, Auwerx J, Smith SR, Olson EN, Kralli A, Kelly DP. 2013. Nuclear receptor/microRNA circuitry links muscle fiber type to energy metabolism. J Clin Invest 123:2564–2575. doi: 10.1172/JCI67652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang T, Yu Q, Chen J, Deng B, Qian L, Le Y. 2010. PP2A mediated AMPK inhibition promotes HSP70 expression in heat shock response. PLoS One 5:e13096. doi: 10.1371/journal.pone.0013096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pei L, Leblanc M, Barish G, Atkins A, Nofsinger R, Whyte J, Gold D, He M, Kawamura K, Li HR, Downes M, Yu RT, Powell HC, Lingrel JB, Evans RM. 2011. Thyroid hormone receptor repression is linked to type I pneumocyte-associated respiratory distress syndrome. Nat Med 17:1466–1472. doi: 10.1038/nm.2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pei L, Waki H, Vaitheesvaran B, Wilpitz DC, Kurland IJ, Tontonoz P. 2006. NR4A orphan nuclear receptors are transcriptional regulators of hepatic glucose metabolism. Nat Med 12:1048–1055. doi: 10.1038/nm1471. [DOI] [PubMed] [Google Scholar]
- 28.Wang T, Yanger K, Stanger BZ, Cassio D, Bi E. 2014. Cytokinesis defines a spatial landmark for hepatocyte polarization and apical lumen formation. J Cell Sci 127:2483–2492. doi: 10.1242/jcs.139923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang D, Patel VV, Ricciotti E, Zhou R, Levin MD, Gao E, Yu Z, Ferrari VA, Lu MM, Xu J, Zhang H, Hui Y, Cheng Y, Petrenko N, Yu Y, FitzGerald GA. 2009. Cardiomyocyte cyclooxygenase-2 influences cardiac rhythm and function. Proc Natl Acad Sci U S A 106:7548–7552. doi: 10.1073/pnas.0805806106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Agah R, Frenkel PA, French BA, Michael LH, Overbeek PA, Schneider MD. 1997. Gene recombination in postmitotic cells. Targeted expression of Cre recombinase provokes cardiac-restricted, site-specific rearrangement in adult ventricular muscle in vivo. J Clin Invest 100:169–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boomsma F, van den Meiracker AH. 2001. Plasma A- and B-type natriuretic peptides: physiology, methodology and clinical use. Cardiovasc Res 51:442–449. doi: 10.1016/S0008-6363(01)00195-X. [DOI] [PubMed] [Google Scholar]
- 32.Maisel A. 2001. B-type natriuretic peptide in the diagnosis and management of congestive heart failure. Cardiol Clin 19:557–571. doi: 10.1016/S0733-8651(05)70243-5. [DOI] [PubMed] [Google Scholar]
- 33.Chen H, McCaffery JM, Chan DC. 2007. Mitochondrial fusion protects against neurodegeneration in the cerebellum. Cell 130:548–562. doi: 10.1016/j.cell.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 34.Okamoto K, Shaw JM. 2005. Mitochondrial morphology and dynamics in yeast and multicellular eukaryotes. Annu Rev Genet 39:503–536. doi: 10.1146/annurev.genet.38.072902.093019. [DOI] [PubMed] [Google Scholar]
- 35.Suen DF, Norris KL, Youle RJ. 2008. Mitochondrial dynamics and apoptosis. Genes Dev 22:1577–1590. doi: 10.1101/gad.1658508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Westermann B. 2010. Mitochondrial fusion and fission in cell life and death. Nat Rev Mol Cell Biol 11:872–884. doi: 10.1038/nrm3013. [DOI] [PubMed] [Google Scholar]
- 37.Schneeberger M, Dietrich MO, Sebastian D, Imbernon M, Castano C, Garcia A, Esteban Y, Gonzalez-Franquesa A, Rodriguez IC, Bortolozzi A, Garcia-Roves PM, Gomis R, Nogueiras R, Horvath TL, Zorzano A, Claret M. 2013. Mitofusin 2 in POMC neurons connects ER stress with leptin resistance and energy imbalance. Cell 155:172–187. doi: 10.1016/j.cell.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liesa M, Shirihai OS. 2013. Mitochondrial dynamics in the regulation of nutrient utilization and energy expenditure. Cell Metab 17:491–506. doi: 10.1016/j.cmet.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dietrich MO, Liu ZW, Horvath TL. 2013. Mitochondrial dynamics controlled by mitofusins regulate Agrp neuronal activity and diet-induced obesity. Cell 155:188–199. doi: 10.1016/j.cell.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liesa M, Palacin M, Zorzano A. 2009. Mitochondrial dynamics in mammalian health and disease. Physiol Rev 89:799–845. doi: 10.1152/physrev.00030.2008. [DOI] [PubMed] [Google Scholar]
- 41.Chan DC. 2012. Fusion and fission: interlinked processes critical for mitochondrial health. Annu Rev Genet 46:265–287. doi: 10.1146/annurev-genet-110410-132529. [DOI] [PubMed] [Google Scholar]
- 42.Dorn GW., II 2013. Mitochondrial dynamics in heart disease. Biochim Biophys Acta 1833:233–241. doi: 10.1016/j.bbamcr.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen H, Vermulst M, Wang YE, Chomyn A, Prolla TA, McCaffery JM, Chan DC. 2010. Mitochondrial fusion is required for mtDNA stability in skeletal muscle and tolerance of mtDNA mutations. Cell 141:280–289. doi: 10.1016/j.cell.2010.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Papanicolaou KN, Kikuchi R, Ngoh GA, Coughlan KA, Dominguez I, Stanley WC, Walsh K. 2012. Mitofusins 1 and 2 are essential for postnatal metabolic remodeling in heart. Circ Res 111:1012–1026. doi: 10.1161/CIRCRESAHA.112.274142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen Y, Liu Y, Dorn GW II. 2011. Mitochondrial fusion is essential for organelle function and cardiac homeostasis. Circ Res 109:1327–1331. doi: 10.1161/CIRCRESAHA.111.258723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Otera H, Wang C, Cleland MM, Setoguchi K, Yokota S, Youle RJ, Mihara K. 2010. Mff is an essential factor for mitochondrial recruitment of Drp1 during mitochondrial fission in mammalian cells. J Cell Biol 191:1141–1158. doi: 10.1083/jcb.201007152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martin OJ, Lai L, Soundarapandian MM, Leone TC, Zorzano A, Keller MP, Attie AD, Muoio DM, Kelly DP. 2014. A role for PGC-1 coactivators in the control of mitochondrial dynamics during postnatal cardiac growth. Circ Res 114:626–636. doi: 10.1161/CIRCRESAHA.114.302562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nass RD, Aiba T, Tomaselli GF, Akar FG. 2008. Mechanisms of disease: ion channel remodeling in the failing ventricle. Nat Clin Pract Cardiovasc Med 5:196–207. doi: 10.1038/ncpcardio1130. [DOI] [PubMed] [Google Scholar]
- 49.Alaynick WA, Way JM, Wilson SA, Benson WG, Pei L, Downes M, Yu R, Jonker JW, Holt JA, Rajpal DK, Li H, Stuart J, McPherson R, Remlinger KS, Chang CY, McDonnell DP, Evans RM, Billin AN. 2010. ERRγ regulates cardiac, gastric, and renal potassium homeostasis. Mol Endocrinol 24:299–309. doi: 10.1210/me.2009-0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nattel S, Maguy A, Le Bouter S, Yeh YH. 2007. Arrhythmogenic ion-channel remodeling in the heart: heart failure, myocardial infarction, and atrial fibrillation. Physiol Rev 87:425–456. doi: 10.1152/physrev.00014.2006. [DOI] [PubMed] [Google Scholar]
- 51.Campuzano O, Beltran-Alvarez P, Iglesias A, Scornik F, Perez G, Brugada R. 2010. Genetics and cardiac channelopathies. Genet Med 12:260–267. doi: 10.1097/GIM.0b013e3181d81636. [DOI] [PubMed] [Google Scholar]
- 52.Cerrone M, Priori SG. 2011. Genetics of sudden death: focus on inherited channelopathies. Eur Heart J 32:2109–2118. doi: 10.1093/eurheartj/ehr082. [DOI] [PubMed] [Google Scholar]
- 53.Bokil NJ, Baisden JM, Radford DJ, Summers KM. 2010. Molecular genetics of long QT syndrome. Mol Genet Metab 101:1–8. doi: 10.1016/j.ymgme.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 54.Schmitt N, Grunnet M, Olesen SP. 2014. Cardiac potassium channel subtypes: new roles in repolarization and arrhythmia. Physiol Rev 94:609–653. doi: 10.1152/physrev.00022.2013. [DOI] [PubMed] [Google Scholar]
- 55.Berry R, Harewood L, Pei L, Fisher M, Brownstein D, Ross A, Alaynick WA, Moss J, Hastie ND, Hohenstein P, Davies JA, Evans RM, FitzPatrick DR. 2011. Esrrg functions in early branch generation of the ureteric bud and is essential for normal development of the renal papilla. Hum Mol Genet 20:917–926. doi: 10.1093/hmg/ddq530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tremblay AM, Dufour CR, Ghahremani M, Reudelhuber TL, Giguere V. 2010. Physiological genomics identifies estrogen-related receptor alpha as a regulator of renal sodium and potassium homeostasis and the renin-angiotensin pathway. Mol Endocrinol 24:22–32. doi: 10.1210/me.2009-0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liesa M, Borda-d'Agua B, Medina-Gomez G, Lelliott CJ, Paz JC, Rojo M, Palacin M, Vidal-Puig A, Zorzano A. 2008. Mitochondrial fusion is increased by the nuclear coactivator PGC-1β. PLoS One 3:e3613. doi: 10.1371/journal.pone.0003613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cartoni R, Leger B, Hock MB, Praz M, Crettenand A, Pich S, Ziltener JL, Luthi F, Deriaz O, Zorzano A, Gobelet C, Kralli A, Russell AP. 2005. Mitofusins 1/2 and ERRα expression are increased in human skeletal muscle after physical exercise. J Physiol 567:349–358. doi: 10.1113/jphysiol.2005.092031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Soriano FX, Liesa M, Bach D, Chan DC, Palacin M, Zorzano A. 2006. Evidence for a mitochondrial regulatory pathway defined by peroxisome proliferator-activated receptor-γ coactivator-1α, estrogen-related receptor-α, and mitofusin 2. Diabetes 55:1783–1791. doi: 10.2337/db05-0509. [DOI] [PubMed] [Google Scholar]
- 60.Wang J, Wilhelmsson H, Graff C, Li H, Oldfors A, Rustin P, Bruning JC, Kahn CR, Clayton DA, Barsh GS, Thoren P, Larsson NG. 1999. Dilated cardiomyopathy and atrioventricular conduction blocks induced by heart-specific inactivation of mitochondrial DNA gene expression. Nat Genet 21:133–137. doi: 10.1038/5089. [DOI] [PubMed] [Google Scholar]
- 61.Moore JR, Leinwand L, Warshaw DM. 2012. Understanding cardiomyopathy phenotypes based on the functional impact of mutations in the myosin motor. Circ Res 111:375–385. doi: 10.1161/CIRCRESAHA.110.223842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Akar FG, Tomaselli GF. 2005. Conduction abnormalities in nonischemic dilated cardiomyopathy: basic mechanisms and arrhythmic consequences. Trends Cardiovasc Med 15:259–264. doi: 10.1016/j.tcm.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 63.Lai L, Leone TC, Zechner C, Schaeffer PJ, Kelly SM, Flanagan DP, Medeiros DM, Kovacs A, Kelly DP. 2008. Transcriptional coactivators PGC-1α and PGC-lβ control overlapping programs required for perinatal maturation of the heart. Genes Dev 22:1948–1961. doi: 10.1101/gad.1661708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.den Hoed M, Eijgelsheim M, Esko T, Brundel BJ, Peal DS, Evans DM, Nolte IM, Segre AV, Holm H, Handsaker RE, Westra HJ, Johnson T, Isaacs A, Yang J, Lundby A, Zhao JH, Kim YJ, Go MJ, Almgren P, Bochud M, Boucher G, Cornelis MC, Gudbjartsson D, Hadley D, van der Harst P, Hayward C, den Heijer M, Igl W, Jackson AU, Kutalik Z, Luan J, Kemp JP, Kristiansson K, Ladenvall C, Lorentzon M, Montasser ME, Njajou OT, O'Reilly PF, Padmanabhan S, St Pourcain B, Rankinen T, Salo P, Tanaka T, Timpson NJ, Vitart V, Waite L, Wheeler W, Zhang W, Draisma HH, et al. 2013. Identification of heart rate-associated loci and their effects on cardiac conduction and rhythm disorders. Nat Genet 45:621–631. doi: 10.1038/ng.2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lin J, Handschin C, Spiegelman BM. 2005. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab 1:361–370. doi: 10.1016/j.cmet.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 66.Gupte AA, Hamilton DJ, Cordero-Reyes AM, Youker KA, Yin Z, Estep JD, Stevens RD, Wenner B, Ilkayeva O, Loebe M, Peterson LE, Lyon CJ, Wong ST, Newgard CB, Torre-Amione G, Taegtmeyer H, Hsueh WA. 2014. Mechanical unloading promotes myocardial energy recovery in human heart failure. Circ Cardiovasc Genet 7:266–276. doi: 10.1161/CIRCGENETICS.113.000404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hu X, Xu X, Lu Z, Zhang P, Fassett J, Zhang Y, Xin Y, Hall JL, Viollet B, Bache RJ, Huang Y, Chen Y. 2011. AMP activated protein kinase-α2 regulates expression of estrogen-related receptor-α, a metabolic transcription factor related to heart failure development. Hypertension 58:696–703. doi: 10.1161/HYPERTENSIONAHA.111.174128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kwon DH, Eom GH, Kee HJ, Nam YS, Cho YK, Kim DK, Koo JY, Kim HS, Nam KI, Kim KK, Lee IK, Park SB, Choi HS, Kook H. 2013. Estrogen-related receptor gamma induces cardiac hypertrophy by activating GATA4. J Mol Cell Cardiol 65:88–97. doi: 10.1016/j.yjmcc.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 69.Kim DK, Jeong JH, Lee JM, Kim KS, Park SH, Kim YD, Koh M, Shin M, Jung YS, Kim HS, Lee TH, Oh BC, Kim JI, Park HT, Jeong WI, Lee CH, Park SB, Min JJ, Jung SI, Choi SY, Choy HE, Choi HS. 2014. Inverse agonist of estrogen-related receptor gamma controls Salmonella typhimurium infection by modulating host iron homeostasis. Nat Med 20:419–424. doi: 10.1038/nm.3483. [DOI] [PubMed] [Google Scholar]
- 70.Patch RJ, Searle LL, Kim AJ, De D, Zhu X, Askari HB, O'Neill JC, Abad MC, Rentzeperis D, Liu J, Kemmerer M, Lin L, Kasturi J, Geisler JG, Lenhard JM, Player MR, Gaul MD. 2011. Identification of diaryl ether-based ligands for estrogen-related receptor alpha as potential antidiabetic agents. J Med Chem 54:788–808. doi: 10.1021/jm101063h. [DOI] [PubMed] [Google Scholar]
- 71.Chaveroux C, Eichner LJ, Dufour CR, Shatnawi A, Khoutorsky A, Bourque G, Sonenberg N, Giguere V. 2013. Molecular and genetic crosstalks between mTOR and ERRα are key determinants of rapamycin-induced nonalcoholic fatty liver. Cell Metab 17:586–598. doi: 10.1016/j.cmet.2013.03.003. [DOI] [PubMed] [Google Scholar]