Abstract
Methylglyoxal is a typical 2-oxoaldehyde derived from glycolysis. We show here that methylglyoxal activates the Pkc1-Mpk1 mitogen-activated protein (MAP) kinase cascade in a target of rapamycin complex 2 (TORC2)-dependent manner in the budding yeast Saccharomyces cerevisiae. We demonstrate that TORC2 phosphorylates Pkc1 at Thr1125 and Ser1143. Methylglyoxal enhanced the phosphorylation of Pkc1 at Ser1143, which transmitted the signal to the downstream Mpk1 MAP kinase cascade. We found that the phosphorylation status of Pkc1T1125 affected the phosphorylation of Pkc1 at Ser1143, in addition to its protein levels. Methylglyoxal activated mammalian TORC2 signaling, which, in turn, phosphorylated Akt at Ser473. Our results suggest that methylglyoxal is a conserved initiator of TORC2 signaling among eukaryotes.
INTRODUCTION
All biological activities in organisms must be accompanied by an ample supply of nutrients, which warrants energy production to meet the demand for biochemical reactions in cells. Therefore, the machinery for sensing and transducing nutritional conditions inside and outside the cell plays crucial roles. Nutrients and hormones are pivotal signal initiation molecules that regulate cellular energy metabolism in animals. Although nutrients are essential for living cells, several adverse by-products, such as reactive oxygen species (ROS), are formed during energy metabolism (1). ROS are inevitably formed by oxygen respiration in accordance with ATP production and cause oxidative stress, which damages cellular components (2).
We have been focusing on methylglyoxal (MG) and its role as a metabolic stressor. MG (CH3COCHO) is a typical 2-oxoaldehyde, the main source of which in the cell is glycolysis, a ubiquitous energy-producing pathway (3, 4). Since MG is highly reactive because of its two carbonyl groups in one molecule, it reacts with DNA/RNA and proteins to make adducts that change their functions, and this change of function sometimes leads to several diseases, such as diabetes and Alzheimer's disease (5–7). MG and other carbonyl compounds produce various carbonyl compound-modified proteins through a process that is sometimes referred to as carbonyl stress. Carbohydrate-derived carbonyl compounds produce advanced glycation end products (AGEs) via spontaneous reactions with the amino groups of proteins and carbonyl compounds. However, even though the reactivity of MG with proteins is >20,000-fold higher than that of glucose, the formation of AGEs has been shown to occur on a time scale of days, weeks, or longer (8, 9). The harmful effects of MG on cellular functions have been attributed to MG-derived AGEs (4–9). We previously reported that MG causes acute cellular responses in yeasts (10, 11). For example, the exogenous addition of MG activated yeast AP-1-like transcription factors (Yap1 in Saccharomyces cerevisiae and Pap1 in Schizosaccharomyces pombe) through the reversible modification of their critical Cys residues within 45 min (10, 11). MG was also found to enhance the influx of extracellular Ca2+ into the cell, thereby activating the Ca2+/calcineurin system in S. cerevisiae (12). In the present study, we found that MG enhances the target of rapamycin (TOR) complex 2 (TORC2)-protein kinase C (Pkc1) signaling pathway in S. cerevisiae.
The TOR signaling network is evolutionally conserved among eukaryotes and is involved in diverse cellular activities (13, 14). TOR is a Ser/Thr kinase that forms two distinct protein kinase complexes, TORC1 and TORC2 (15). The TOR complex phosphorylates protein kinases involved in the AGC (named initially for cyclic AMP- and cyclic GMP-dependent protein kinases and protein kinase C) kinase family (16). S. cerevisiae TORC1 has been shown to phosphorylate Sch9, whereas TORC2 phosphorylates Ypk1 and Ypk2 (17–19), both of which belong to the AGC kinase family. A previous study demonstrated that TOR complexes phosphorylate conserved Thr/Ser residues within the turn motif (TM) and hydrophobic motif (HM) of AGC kinase (16). PKC1 is the only gene that encodes protein kinase C in S. cerevisiae (20, 21), and Pkc1 structurally belongs to the AGC kinase family. Thr/Ser residues in the TM and HM were also found to be conserved in Pkc1 (Thr1125 in TM and Ser1143 in HM). Genetic interactions between PKC1 and some genes (TOR2, AVO1, and AVO3), the products of which are involved in the assembly of TORC2, have been reported previously (15, 22–24); however, it still remains unclear whether Pkc1 is a direct target of TORC2 (25).
TORC1 is involved in events concerning cell growth, such as protein synthesis, while TORC2 plays a role in actin organization (14). In the present study, we demonstrate that the organization of actin is perturbed by an MG treatment, thereby inhibiting the polarized cell growth of S. cerevisiae. Since TORC2 is involved in actin organization, we investigated the relationship between MG and TORC2 signaling. We show that Thr1125 and Ser1143 in Pkc1 are phosphorylated by TORC2 both in vivo and in vitro. We also found that MG enhances TORC2-Pkc1 signaling, and the downstream Mpk1 mitogen-activated protein (MAP) kinase cascade was subsequently activated following the treatment of yeast cells with MG. Furthermore, we show that MG activates TORC2 signaling in mammalian cells. A physiological trigger that has been shown to activate TORC2 signaling is insulin (and insulin-like growth factor) (26–28); however, lower eukaryotes, such as yeast, do not have a hormonal system. Therefore, it remains unclear whether the ubiquitous physiological molecules that activate TORC2 exist among eukaryotes. Our results suggest that MG is a common initiator of TORC2 signaling.
MATERIALS AND METHODS
Media.
The media used were synthetic dextrose (SD) medium (2% glucose, 0.67% yeast nitrogen base without amino acids) and synthetic complete (SC)-Gal medium (2% galactose, 3% glycerol, 0.67% yeast nitrogen base without amino acids). Appropriate amino acids and bases were added to SD or SC-Gal medium as necessary. Cells were cultured at 28°C unless otherwise stated.
Strains.
The yeast strains and PCR primers used are summarized in Tables S1 and S2 in the supplemental material. The deletion allele of MID2 with KanMX or his5+ was amplified by PCR with primer sets mid2-F and mid2-R from S. cerevisiae BY4741-based deletion mutants (29). The corresponding loci of S. cerevisiae YPH250 were disrupted using PCR products. To construct wsc1Δ and mpk1Δ mutants, the wsc1Δ::CgLEU2 allele of YOC2573 (30) and the mpk1Δ::HIS3 allele of TNP46 (31) were amplified by PCR with primers WSC1F and WSC1R and primers MPK1FSalI and MPK1REcoRI, respectively. The disruption of MKK1, MKK2, and BCK1 was performed as described previously (32).
Plasmids.
The plasmids used are summarized in Table S3 in the supplemental material. Details for the construction of plasmids are described in the supplemental material.
Actin staining.
Cells were cultured in SD medium until the A610 reached 0.3 to 0.5. MG was then added. Cells were fixed with formaldehyde (final concentration, 4%) for 1 h. After fixation, the cells were harvested, washed twice with phosphate-buffered saline (PBS; pH 7.4), and suspended in 30 μl of PBS. Rhodamine-phalloidin (Molecular Probes) was added to the cell suspension to a final concentration of 33 units/ml (1.1 μM), and the cell suspension was then incubated at 4°C in the dark overnight. Cells were collected by centrifugation and washed twice with PBS, and the distribution of actin was observed using a fluorescence microscope.
Immunoprecipitation.
Cells were cultured in SD medium until the A610 reached 0.5. Cells were then collected, washed with a 0.85% NaCl solution, and suspended in lysis buffer B (50 mM Tris-HCl [pH 7.5], 150 mM NaCl, 0.1 mM EDTA, 0.5% Tween 20) containing 2.5 mM phenylmethylsulfonyl fluoride, 50 mM NaF, and protease inhibitor cocktail (Nacalai Tesque). Cells were disrupted with glass beads using a Beads Smash 12 cell disrupter (Wakenyaku), and cell homogenates were centrifuged at 700 × g for 10 min at 4°C to remove cell debris. The protein concentrations in the cell extracts were determined using a DC protein assay (Bio-Rad Laboratories). The Avo3-13myc, Pkc1 tagged with a 3× hemagglutinin tag (Pkc1-3HA), or Pkc1 tagged with a 3× FLAG tag (Pkc1-3FLAG) protein was immunoprecipitated from the cell extracts (1.5 or 1 mg protein) by incubation with anti-c-myc antibodies coupled with agarose resin (Nacalai Tesque), anti-HA antibodies coupled with agarose resin (MBL), or anti-FLAG antibody-conjugated resin (Sigma) for 2 h at 4°C in lysis buffer B. After being incubated, the agarose resins were precipitated by centrifugation, washed four times with lysis buffer B, and suspended in SDS-PAGE sample buffer. SDS-PAGE was then performed, followed by Western blotting.
Bacterial expression and purification of Ypk2.
Escherichia coli BL21(DE3) cells carrying pET-15b-Ypk2 were grown in LB medium containing ampicillin at 37°C until the A610 reached 0.3, and isopropyl-β-d-thiogalactopyranoside was added to a final concentration of 0.2 mM. Cells were incubated at 25°C overnight and then disrupted by sonication at 4°C in 20 mM sodium phosphate buffer (pH 7.4) containing 0.5 mM NaCl and 10 mM imidazole. Cell homogenates were centrifuged to remove cell debris, cell extracts were applied to a HisTrapTM HP column (GE Healthcare) equilibrated with sonication buffer, and the column was washed with the same buffer. The proteins that were adsorbed were eluted with 20 mM sodium phosphate buffer (pH 7.4) containing 0.5 mM NaCl and 250 mM imidazole. Fractions containing Ypk2 were dialyzed against 20 mM HEPES-KOH buffer (pH 7.4) containing 150 mM NaCl and 2 mM dithiothreitol.
In vitro protein kinase assay.
An in vitro protein kinase assay for TORC2 was performed as described previously (17), with some modifications. Briefly, TORC2 was immunopurified using myc-tagged Avo3 (Avo3-myc) instead of HA-tagged Tor2 (HA-Tor2), which was used in a previous study (17). Immunopurified TORC2 was incubated with 5 μg of Pkc1 peptides (for wild-type [WT] Pkc1 [Pkc1WT], APPTLTPLPSVLTTSQQEEFRGFSFMPDDL; for Pkc1 with the T1125A and S1143A mutations [Pkc1T1125A/S1143A], APPTLAPLPSVLTTSQQEEFRGFAFMPDDL) or 4 μg of recombinant Ypk2 protein. Synthetic Pkc1 peptides were produced by the GenScript Corporation (Piscataway, NJ). The reaction was initiated by adding [γ-32P]ATP (10 μCi). After being incubated for 30 min at 30°C, the reaction was terminated by the addition of SDS-PAGE sample buffer, and the samples were then incubated for 5 min at 65°C. Samples were subjected to Tricine-SDS-PAGE (33) or SDS-PAGE, and phosphorylated peptides were detected by autoradiography.
Western blotting of Mpk1.
Cells were collected and washed with lysis buffer A (50 mM Tris-HCl [pH 7.5], 150 mM NaCl, 5 mM EDTA, 1% Nonidet P-40, 1 mM sodium pyrophosphate, 1 mM phenylmethylsulfonyl fluoride, 1 mM sodium orthovanadate, 20 mM NaF, 2 μg each of pepstatin A and leupeptin per ml), and cell pellets were frozen with liquid nitrogen. Cell pellets were suspended in lysis buffer A and agitated with glass beads using a Beads Smash 21 cell disrupter (Wakenyaku). Cell homogenates were centrifuged for 5 min at 15,000 × g and 4°C, and the protein concentration in clear supernatants was determined using the DC protein assay (Bio-Rad Laboratories). Samples were subjected to SDS-PAGE, and the separated proteins were transferred to a polyvinylidene difluoride membrane (Millipore). The blots were incubated with appropriate dilutions of the primary antibodies (anti-phospho-p44/42 MAP kinase [Thr202/Tyr204; Cell Signaling] and anti-Mpk1 [yC-20; catalog number sc-6803; Santa Cruz Biotechnology]). Immunoreactive bands were visualized with a 5-bromo-4-chloro-3-indolylphosphate–nitroblue tetrazolium solution kit for alkaline phosphatase (Nacalai Tesque) or Immobilon Western chemiluminescent horseradish peroxidase (HRP) substrate (Millipore) using a LAS-4000 mini-imaging system (Fujifilm).
Western blotting of Pkc1.
Total protein extracts were prepared using the trichloroacetic acid extraction method (12). The protein concentrations of the samples were determined using an RC DC protein assay kit (Bio-Rad Laboratories). The antibodies used for Western blotting were anti-Pkc1 antibodies.
Anti-Pkc1, anti-p-T1125, and anti-p-S1143 antibodies.
Anti-Pkc1 polyclonal antibodies were raised by immunizing rabbits with a peptide corresponding to amino acid residues 470 to 488 in Pkc1. Antisera were used to detect Pkc1 in Western blots. Anti-phospho-Pkc1 polyclonal antibodies were raised by immunizing rabbits with Pkc1 containing a phosphorylated Thr1125 peptide (p-T1125) in the turn motif [NH2-C+APPTL(pT)PLPSVLTTSQQEE-COOH] or a phosphorylated Ser1143 peptide (p-S1143) in the hydrophobic motif [NH2-C+EFRGF(pS)FMPD-COOH]. Antisera were loaded onto a column packed with the resin conjugated with each of the phosphorylated peptides. After the adsorbed fractions were recovered, they were passed through a column packed with the resin conjugated with each of the unphosphorylated peptides. The fractions were recovered and used as anti-phospho-Pkc1 at Thr1125 (anti-p-T1125) or anti-phospho-Pkc1 at Ser1143 (anti-p-S1143) antibodies.
Culture conditions for mammalian cells.
Mouse 3T3-L1 cells were maintained in maintenance medium (10% fetal bovine serum and 10 mg/ml penicillin-streptomycin in Dulbecco modified Eagle medium [DMEM]) at 37°C with 5% CO2. Preadipocytes were differentiated to adipocytes by the established protocol (34) as briefly described below. 3T3-L1 preadipocytes were grown for 2 days until postconfluence, and medium was switched to a differentiation medium consisting of the maintenance medium supplemented with 0.25 μM dexamethasone, 10 μg/ml insulin, and 0.5 mM 3-isobutyl-1-methylxanthine. After 48 h, the medium was replaced with a postdifferentiation medium consisting of the maintenance medium supplemented with 5 μg/ml insulin. Medium was exchanged every 2 days with a fresh postdifferentiation medium until the cells were ready for experimentation.
3T3-L1 adipocytes were incubated in the maintenance medium for 20 h in DMEM for Western blotting and then starved of serum for 5 h in DMEM. Cells were lysed with SDS-PAGE sample buffer, and the protein concentrations of the samples were determined using the RC DC protein assay kit (Bio-Rad Laboratories). The antibodies used for Western blotting were anti-Akt antibodies (Cell Signaling) to detect the bulk protein levels of Akt, anti-phospho-Akt (Ser473) antibodies (Cell Signaling) to detect the phosphorylation of Ser473 in the HM of Akt, and anti-phospho-PKCα/βII (Thr638/641) antibodies (Cell Signaling) to detect the phosphorylation of Thr450 in the TM of Akt (35). Immunoreactive bands were visualized with a kit (Immobilon Western chemiluminescent HRP substrate; Millipore) and an LAS-4000 mini-imaging system (Fujifilm).
RESULTS
MG perturbs actin organization.
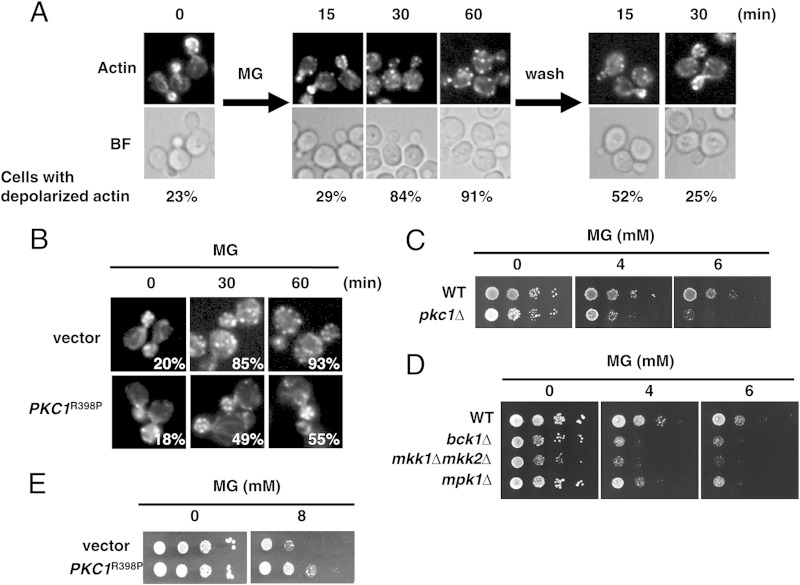
We previously demonstrated that a moderate concentration of MG reversibly inhibits the growth of S. cerevisiae cells without decreasing cell viability (12). S. cerevisiae exhibits polarized growth, and actin is known to play crucial roles in determining cellular polarity (36). To gain insight into the MG-induced inhibition of growth, we examined the organization of actin. Actin patches accumulated in the buds of logarithmically growing cells; however, actin patches were almost completely (84 to 91%) and uniformly dispersed in both the mother cell and bud 30 to 60 min after the MG treatment (Fig. 1A). The depolarization of actin patches by the MG treatment was reversible; i.e., the patches returned to the bud 30 min after the removal of MG from the medium (Fig. 1A).
FIG 1.
MG depolarizes the actin cytoskeleton. (A) Cells (YPH250) were cultured in SD medium until the A610 was 0.3 and were then treated with 10 mM MG for 60 min. Cells were stained for actin with rhodamine-phalloidin and observed using a fluorescence microscope. The proportion of cells with depolarized actin was determined by counting the cells in which actin had not accumulated in the bud. More than approximately 200 cells were counted in each experiment. BF, bright field. (B) Cells (YPH250) carrying YCp50 (vector) or YCp50-PKC1R398P were cultured in SD medium until the A610 was 0.3 and were then treated with 10 mM MG for the times indicated. Cells were stained for actin with rhodamine-phalloidin. The proportion of cells with depolarized actin is indicated for each image. (C) Wild-type (DL100) and pkc1Δ (DL376) cells were cultured in SD medium containing 0.5 M sorbitol until the log phase of growth and serially diluted (1:10) with 0.5 M sorbitol solution. An aliquot (4 μl) of each cell suspension was spotted onto SD agar plates containing 0.5 M sorbitol with or without MG. (D) Cells (YPH250) defective in the components of the Mpk1 MAP kinase cascade (bck1Δ, mkk1Δ mkk2Δ, and mpk1Δ cells) were cultured in SD medium until the log phase of growth and serially diluted (1:10) with 0.85% NaCl solution, and 4 μl of each cell suspension was then spotted onto SD agar plates containing MG. (E) Cells (YPH250) carrying YCp50 (vector) or YCp50-PKC1R398P were cultured in SD medium until the log phase of growth. The cell suspension was diluted as described in the legend to panel D, and 4 μl of each suspension was spotted onto SD agar plates containing MG.
PKC1 with the R398P mutation (PKC1R398P) is a constitutively active allele of PKC1 that has been shown to suppress the depolarization of actin patches in a rho1-2 temperature-sensitive mutant at nonpermissive temperatures when PKC1R398P is expressed using its own promoter in a single-copy plasmid (23, 37). Therefore, we investigated whether the depolarization of actin patches in the presence of MG is alleviated by the expression of PKC1R398P. As shown in Fig. 1B, Pkc1R398P partially repressed the depolarization of actin patches in cells treated with MG.
Pkc1-Mpk1 MAP kinase cascade mutants are sensitive to MG.
The organization of actin was previously shown to be regulated by the Pkc1-Mpk1 MAP kinase cascade (38, 39). To verify whether MG influences the growth of mutants defective in the Pkc1-Mpk1 MAP kinase cascade, we conducted spot assays on SD agar plates containing MG. pkc1Δ cells are not able to grow without sorbitol (38) because synthesis of the cell wall is defective in this mutant and cell lysis occurs without an osmoprotectant. Therefore, spot assays were performed using SD agar plates containing sorbitol. As shown in Fig. 1C, the pkc1Δ mutant exhibited susceptibility to MG. Similarly, bck1Δ, mkk1Δ mkk2Δ, and mpk1Δ mutants, defective in the MAP kinase cascade, also showed susceptibility to MG (Fig. 1D). Wild-type cells displayed an increased tolerance toward MG when PKC1R398P was expressed from its own promoter using a single-copy plasmid (Fig. 1E).
MG activates the Mpk1 MAP kinase cascade.
Since mutants defective in the Pkc1-Mpk1 MAP kinase cascade were sensitive to MG (Fig. 1C and D), we speculated that MG may activate this signaling pathway to combat MG-induced cellular stress. We monitored the phosphorylation levels of Mpk1. As shown in Fig. 2A, Mpk1 was phosphorylated following treatment with MG, whereas no phosphorylated band was detected in pkc1Δ cells or in any of the Mpk1 MAP kinase cascade mutants (Fig. 2B and C). These results indicate that MG activates the Pkc1-Mpk1 MAP kinase cascade.
FIG 2.
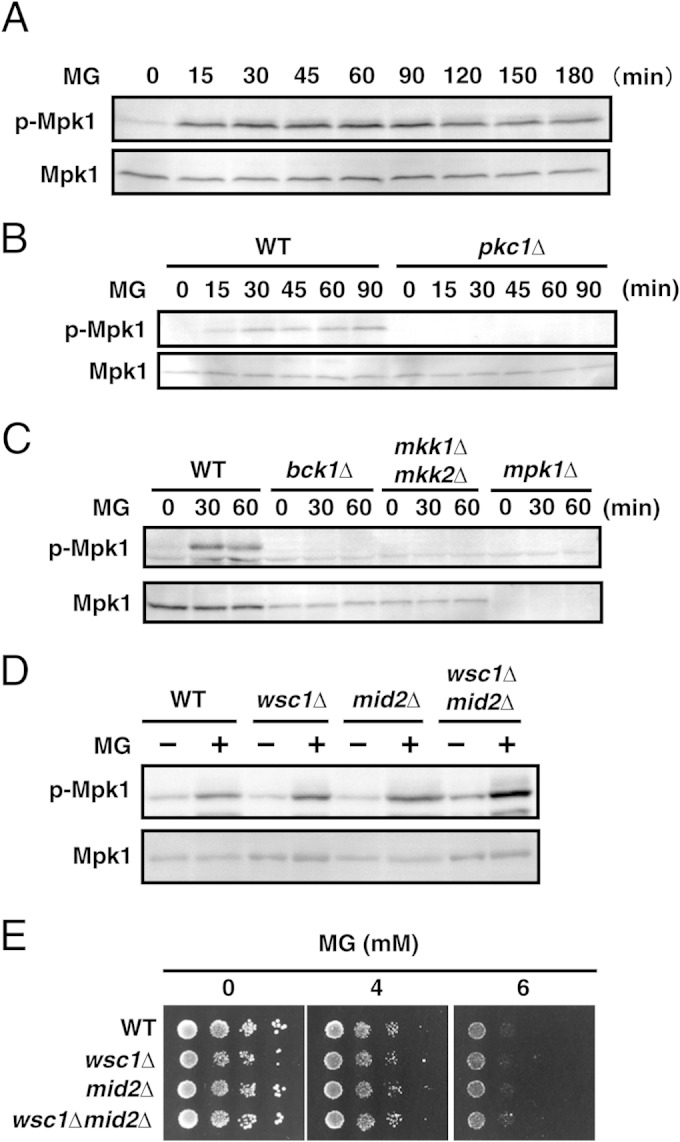
Effects of MG on activation of the Mpk1 MAP kinase cascade. (A) Cells (YPH250) were cultured in SD medium until the A610 was 0.3, and 10 mM MG was added. The levels of phosphorylation of Mpk1 (p-Mpk1) and the Mpk1 protein (Mpk1) were determined after incubation for the prescribed times. (B) Wild-type (DL100) and pkc1Δ (DL376) cells were cultured in SD medium containing 1 M sorbitol until the A610 was 0.3 to 0.5 and were treated with 10 mM MG for the prescribed times. (C) Cells (YPH250) defective in the components of the Mpk1 MAP kinase cascade (bck1Δ, mkk1Δ mkk2Δ, and mpk1Δ cells) were cultured in SD medium containing 1 M sorbitol until the A610 was 0.3 to 0.5, and 10 mM MG was added. (D) Wild-type (YPH250), wsc1Δ, mid2Δ, and wsc1Δ mid2Δ cells were cultured in SD medium until the A610 was 0.3 to 0.5 and treated with 10 mM MG for 30 min, and the phosphorylation of Mpk1 was then determined. (E) The cells of each mutant in the YPH250 background were cultured in SD medium until the log phase of growth and serially diluted (1:10) with a 0.85% NaCl solution, and 4 μl of each cell suspension was then spotted onto SD agar plates containing MG.
The membrane-integrated proteins Wsc1 and Mid2 act as sensors for the heat shock-induced activation of the Mpk1 MAP kinase cascade (see Fig. 8) (38–40). To explore whether these proteins function as sensors for MG-triggered stress, we disrupted WSC1 and MID2 and determined the phosphorylation of Mpk1 in the resultant mutants. However, as shown in Fig. 2D, Mpk1 was phosphorylated following treatment with MG in wsc1Δ, mid2Δ, and wsc1Δ mid2Δ cells. Subsequently, susceptibility to MG was not increased by the deletion of WSC1 and/or MID2 (Fig. 2E). Hence, the MG-induced activation of the Pkc1-Mpk1 MAP kinase cascade occurred irrespective of the Wsc1/Mid2 pathway. Besides Wsc1/Mid2, Wsc2 and Mtl1 have also been reported to act as upstream sensors for the Pkc1-Mpk1 pathway (41, 42). Therefore, we determined whether these proteins are involved in the MG-induced phosphorylation of Mpk1. As shown in Fig. S1A in the supplemental material, the phosphorylation of Mpk1 occurred in wsc2Δ and mtl1Δ mutants following treatment with MG, and these mutants did not subsequently exhibit increased sensitivity to MG (see Fig. S1B in the supplemental material).
FIG 8.
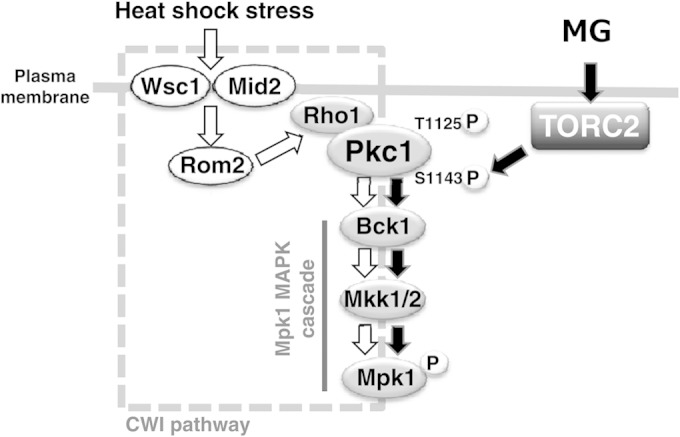
Schematic model for the integration of TORC2 into the Pkc1-Mpk1 MAP kinase (MAPK) cascade in S. cerevisiae. Details are described in the text. Briefly, heat shock stress is sensed by the stress sensor proteins Wsc1 and Mid2 on the cytoplasmic membrane, and the heat shock signal is transmitted to Rom2 (guanine-nucleotide exchange factor for Rho1), which in turn activates Rho1. GTP-bound Rho1 interacts with Pkc1, and Pkc1 activates the Mpk1 MAP kinase cascade (the so-called cell wall integrity [CWI] pathway). TORC2 phosphorylates Pkc1 at Thr1125 in the turn motif and at Ser1143 in the hydrophobic motif. The phosphorylation of these amino acid residues is necessary for transmitting the MG signal downstream to the Mpk1 MAP kinase cascade. The phosphorylation level of Ser1143 in Pkc1 increases following treatment with MG.
Yeast TORC2 phosphorylates Pkc1.
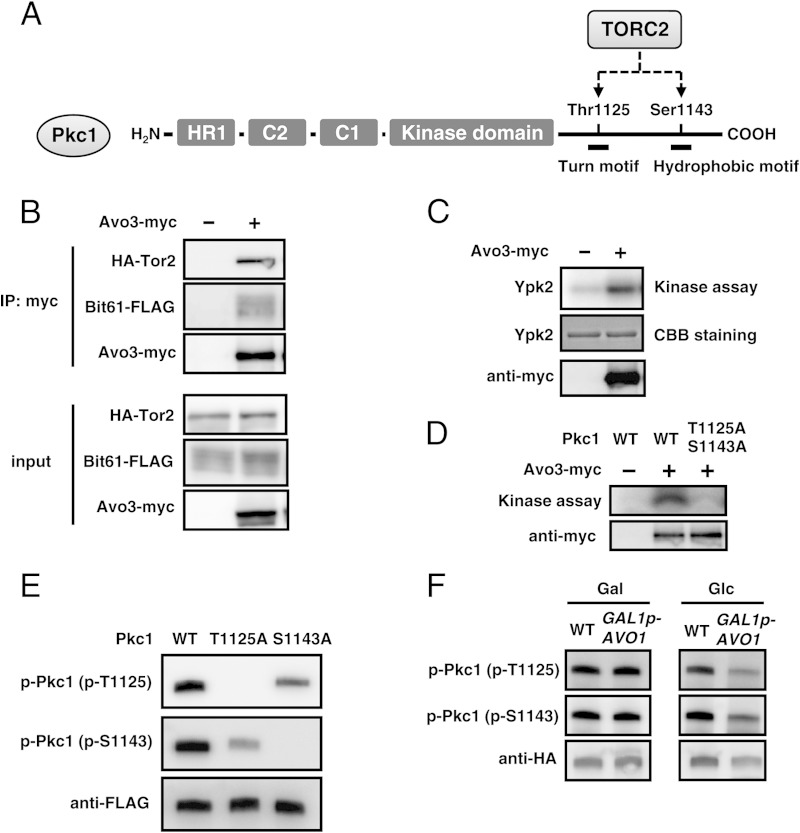
Previous studies suggested that genetic interactions may occur between TORC2 and the Pkc1-Mpk1 signaling pathway (22–24, 43). For example, the temperature sensitivity of a temperature-sensitive (ts) tor2 (tor2ts) mutant was suppressed by the overexpression of RHO1, PKC1, and MPK1 (22, 23, 43). Pkc1 belongs to the AGC kinase family, and AGC kinases are generally the targets of TOR kinases (16). Potential phosphorylation sites (Thr1125 in TM and Ser1143 in HM) are conserved in Pkc1 (Fig. 3A) (16, 35, 44); however, it currently remains unknown whether yeast TORC2 directly phosphorylates Pkc1. To gain insight into the MG-induced activation of the Pkc1-Mpk1 MAP kinase cascade, we investigated whether Pkc1 is a substrate of TORC2.
FIG 3.
TORC2 phosphorylates Pkc1. (A) Diagram of Pkc1. The HR1 domain, C1 domain, and C2 domain represent homology region 1, the cysteine-rich domain, and the lipid-binding domain, respectively. AGC kinases contain conserved Ser/Thr in the turn motif (TM) and the hydrophobic motif (HM), which are phosphorylated by TOR kinases. Pkc1 also contains Thr1125 in TM and Ser1143 in HM. Both are potential phosphorylation sites of TORC2; however, TORC2-dependent phosphorylation of these sites has not yet been biochemically confirmed. (B) TORC2 components were immunoprecipitated (IP) using anti-myc antibodies from wild-type (YPH250) cells with or without Avo3-myc carrying HA-Tor2 and Bit61-FLAG. Each TORC2 component was determined using the respective antibodies for each tag. (C) Immunoprecipitated TORC2 containing Avo3-myc from wild-type (YPH250) cells was incubated with [γ-32P]ATP and recombinant Ypk2. The phosphorylation of Ypk2 was detected by autoradiography. CBB, Coomassie brilliant blue. (D) The WT Pkc1 peptide or an Ala-substituted variant (T1125A/S1143A) was incubated with immunopurified TORC2 and [γ-32P]ATP. After Tricine-SDS-PAGE, the phosphorylation of each Pkc1 peptide was detected by autoradiography. (E) Cells (YPH250) carrying PKC1-3FLAG (WT), PKC1T1125A-3FLAG (T1125A), or PKC1S1143A-3FLAG (S1143A) were cultured in SD medium until the A610 was 0.4 to 0.6. After the immunoprecipitation of FLAG-tagged Pkc1 using anti-FLAG antibody-conjugated resin, samples were subjected to SDS-PAGE followed by Western blotting using anti-phosphorylated Pkc1T1125 antibodies [p-Pkc1 (p-T1125)], anti-phosphorylated Pkc1S1143 antibodies [p-Pkc1 (p-S1143)], or an anti-FLAG monoclonal antibody (Sigma). (F) Wild-type (TB50a) and GAL1p-AVO1 (RL25-1c) cells carrying pFL39-PKC1-3HA were cultured overnight in SC-Gal medium (Gal). After being transferred to SD medium (Glc), cells were cultured for 17 h to impair TORC2 activity. After the immunoprecipitation of HA-tagged Pkc1 using the anti-HA antibody-conjugated resin, samples were subjected to SDS-PAGE followed by Western blotting using anti-phosphorylated Pkc1T1125 antibodies [p-Pkc1 (p-T1125)], anti-phosphorylated Pkc1S1143 antibodies [p-Pkc1 (p-S1143)], or the anti-HA monoclonal antibody (Cell Signaling).
TORC2 consists of Tor2, Avo1, Avo2, Avo3, Bit61, and Lst8. Tor2 and Lst8 have also been shown to be involved in TORC1 (14, 15). We carried out the immunopurification of TORC2 from yeast cells that simultaneously expressed Avo3-myc, Tor2-HA, and FLAG-tagged Bit61 (Bit61-FLAG). The immunoprecipitation of Avo3-myc was performed using anti-myc monoclonal antibody-conjugated resin, and we verified that Tor2-HA and Bit61-FLAG were coimmunoprecipitated with Avo3-myc (Fig. 3B). Since Tor2 is involved not only in TORC2 but also in TORC1 (15), we performed immunoprecipitation with myc-tagged Avo3, which was previously shown to be specifically involved in TORC2 (18, 45), in order to reduce the probability of contamination of TORC1. We then determined whether immunopurified TORC2 actually possessed TORC2 activity. Since the substrate of yeast TORC2 has been identified to be Ypk2 (17), we carried out an in vitro kinase assay using a 6×His-tagged Ypk2 protein purified from recombinant E. coli cells as a substrate. As shown in Fig. 3C, immunopurified TORC2 was able to phosphorylate 6×His-Ypk2. Therefore, the in vitro kinase assay system that we constructed was confirmed to work properly.
Using this assay system, we conducted an in vitro kinase assay to determine whether Pkc1 is phosphorylated by TORC2. As shown in Fig. 3D, the wild-type Pkc1 peptide (the C-terminal region of Pkc1 [amino acid residues 1120 to 1149] including Thr1125, Ser1143, and some other Ser and Thr residues) was phosphorylated, while the Pkc1T1125A/S1143A peptide, an Ala-substituted variant of Thr1125 and Ser1143, was not. In addition, TORC2 containing kinase-dead Tor2 did not phosphorylate the Pkc1WT peptide (see Fig. S2 in the supplemental material). These results indicate that TORC2 phosphorylates Pkc1 in vitro.
We then raised anti-phospho-Pkc1T1125 and anti-phospho-Pkc1S1143 antibodies to examine whether Pkc1 is a target of TORC2 in yeast cells. To verify the specificity of these antibodies, Pkc1 variants with Ala substitutions at Thr1125 (Pkc1T1125A) and Ser1143 (Pkc1S1143A) were constructed. As shown in Fig. 3E, each antibody specifically crossed with the respective phosphorylation site. To determine whether these amino acid residues are phosphorylated by TORC2, we used a strain carrying the AVO1 gene, which encodes an essential component of TORC2 and which is driven by the GAL1 promoter (45), and shifted the cell from a galactose medium to a glucose medium to stop the expression of AVO1, which impaired TORC2 activity. The impairment of TORC2 activity in yeast cells in the glucose medium was verified by observing the depolarization of actin (see Fig. S3 in the supplemental material). As shown in Fig. 3F, Thr1125 and Ser1143 were phosphorylated in the basal state in both wild-type and GAL1p-AVO1 cells in the galactose medium; however, the phosphorylation levels of these amino acids decreased in GAL1p-AVO1 cells in the glucose medium. These results indicate that Pkc1 is phosphorylated in a TORC2-dependent manner in yeast cells.
Involvement of Rho1 in the phosphorylation of Pkc1 at Thr1125 and Ser1143.
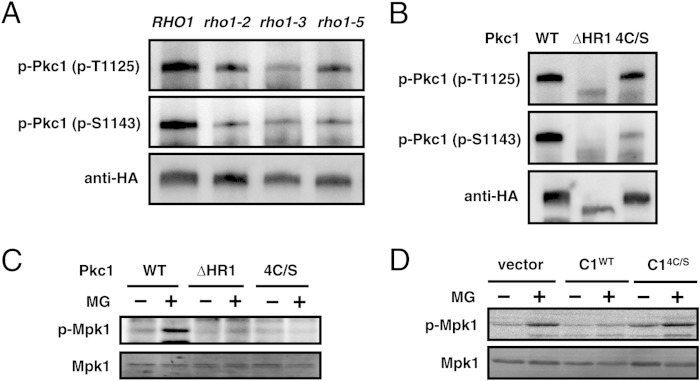
Rho1 is a small G protein that is crucially involved in the heat shock-triggered phosphorylation of Mpk1 (37, 38) (see Fig. 8). Rho1 also physically interacts with Pkc1 (37, 38). Therefore, we investigated whether Rho1 is involved in TORC2-Pkc1 signaling. Since RHO1 is an essential gene, we used temperature-sensitive rho1 mutants. Saka et al. isolated several rho1ts mutants, of which rho1-2, rho1-3, and rho1-5 were impaired in heat shock-induced phosphorylation of Mpk1 (46). These findings prompted us to examine Pkc1 phosphorylation levels in these mutants. As shown in Fig. 4A, the basal phosphorylation levels of Thr1125 and Ser1143 of Pkc1 were decreased in the temperature-sensitive RHO1 mutants at nonpermissive temperatures.
FIG 4.
Role of Rho1 in MG-triggered TORC2-Pkc1 signaling. (A) RHO1 (YOC764), rho1-2 (YOC752), rho1-3 (YOC729), and rho1-5 (YOC755) cells carrying pFL39-PKC1-3HA were cultured in SD medium at 25°C until the A610 was 0.3 to 0.5 and then shifted to 37°C for 3 h. After the immunoprecipitation of HA-tagged Pkc1 using anti-HA antibody-conjugated resin, samples were subjected to SDS-PAGE followed by Western blotting using anti-phosphorylated Pkc1T1125 antibodies [p-Pkc1 (p-T1125)], anti-phosphorylated Pkc1S1143 antibodies [p-Pkc1 (p-S1143)], or an anti-HA monoclonal antibody (Cell Signaling). (B) Cells (YPH250) carrying pFL39-PKC1-3HA (WT), pFL39-PKC1ΔHR1-3HA (ΔHR1), or pFL39-PKC14C/S-3HA (4C/S) were cultured in SD medium until the A610 was 0.4 to 0.6. Immunoprecipitation and Western blotting were performed as described in the legend to panel A. (C) pkc1Δ (DL376) cells carrying pFL39-PKC1-3HA (WT), pFL39-PKC1ΔHR1-3HA (ΔHR1), or pFL39-PKC14C/S-3HA (4C/S) were cultured in SD medium until the A610 was 0.3 to 0.5, and 10 mM MG was added. The phosphorylation of Mpk1 was determined after incubation for 30 min. (D) Cells (YPH250) carrying the vector (pRS426), ADH1 p-PKC1 C1 WT (C1WT), or ADH1 p-PKC1 C14C/S (C14C/S) were cultured in SD medium until the A610 was 0.3 to 0.5, and 10 mM MG was added. The phosphorylation of Mpk1 was determined after incubation for 30 min.
We then constructed Pkc1 mutants defective in the domains for the physical interaction with Rho1. Two domains in the N-terminal region of Pkc1, the homology region 1 (HR1) domain and the Cys-rich C1 domain, have been shown to contribute to the physical interactions with Rho1 (37, 47, 48) (Fig. 3A). Previous studies reported that the HR1 domain was deleted in the Pkc1ΔHR1 mutant and also that the Pkc14C/S mutant was a variant with Cys-to-Ser substitutions of the four Cys residues (Cys434, Cys437, Cys514, and Cys517) necessary for the zinc finger motif in the C1 domain (47–49). The basal phosphorylation levels of Thr1125 and Ser1143 were markedly decreased in the Pkc1ΔHR1 mutant (Fig. 4B). The phosphorylation level of Ser1143 was also markedly decreased in the Pkc14C/S mutant, whereas the phosphorylation level of Thr1125 was similar to that of wild-type Pkc1 (Pkc1WT) (Fig. 4B). The MG-induced phosphorylation of Mpk1 did not occur in either mutant (Fig. 4C). Additionally, overproduction of the C1 domain, which may have competed with endogenous Pkc1, inhibited the MG-induced phosphorylation of Mpk1, whereas overproduction of the C1 domain in the mutant with Cys-to-Ser substitution mutations (C14C/S) did not (Fig. 4D).
MG facilitates TORC2 signaling in yeast.
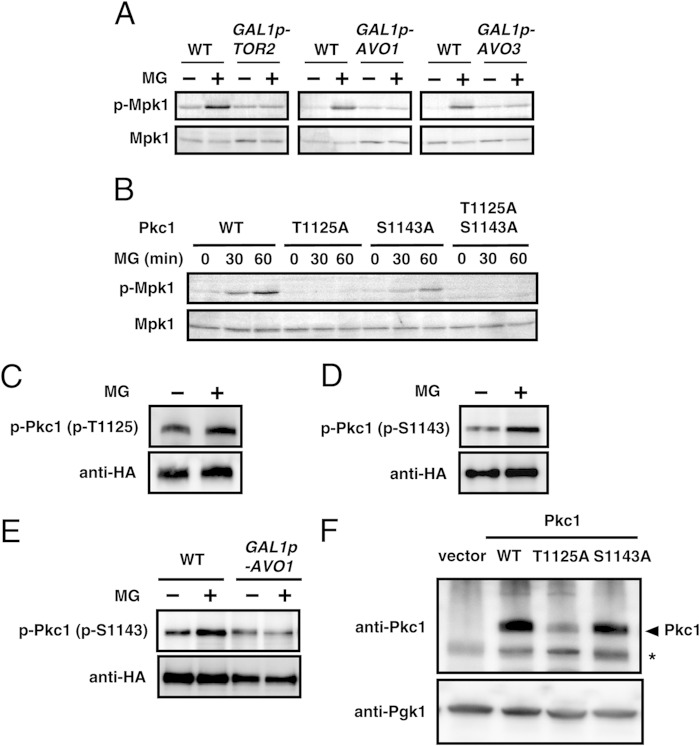
We demonstrated that MG activates the Pkc1-Mpk1 MAP kinase cascade. To assess whether TORC2 is involved in this signaling pathway triggered by MG, we first monitored the phosphorylation of Mpk1 using a tor2-21ts mutant. Although Tor2 was shown to be involved in both TORC1 and TORC2, the tor2-21ts allele was specifically defective in TORC2 function (22). The MG-induced phosphorylation of Mpk1 was observed at 28°C in tor2-21ts cells, whereas its level was greatly reduced at a nonpermissive temperature (see Fig. S4A in the supplemental material). The MG-induced phosphorylation of Mpk1 also rarely occurred in cells defective in TORC2 activity (Fig. 5A), which was achieved by transferring cells carrying the essential components of TORC2 (AVO1 or AVO3) with the GAL1 promoter from the galactose medium to the glucose medium. The same result was obtained when cells with GAL1 promoter-driven TOR2 were used (Fig. 5A). We confirmed that TORC2 function was impaired under these conditions by observing the depolarization of actin (see Fig. S4B in the supplemental material).
FIG 5.
MG functions as an initiator of the TORC2-Pkc1 signaling pathway. (A) GAL1p-TOR2 (JM340), GAL1p-AVO1 (RL25-1c), and GAL1p-AVO3 (RS61-5b) cells were cultured overnight in SC-Gal medium. After being transferred to SD medium, cells were cultured for 16 h (GAL1p-TOR2), 9 h (GAL1p-AVO1), or 10 h (GAL1p-AVO3) to impair TORC2 activity and were then treated with 10 mM MG for 30 min. (B) pkc1Δ (DL376) cells carrying YEp352-PKC1 (pFR22), YEp352-PKC1T1125A, YEp352-PKC1S1143A (pFR74), or YEp352-PKC1T1125A/S1143A were cultured in SD medium until the A610 was 0.3 to 0.5 and were then treated with 10 mM MG for the prescribed times. (C) Cells (YPH250) carrying pFL39-PKC1-3HA were cultured in SD medium until the A610 was 0.4 to 0.6, and 10 mM MG was added. After the immunoprecipitation of HA-tagged Pkc1 using the anti-HA-antibody-conjugated resin, samples were subjected to SDS-PAGE followed by Western blotting using anti-phosphorylated Pkc1T1125 antibodies [p-Pkc1 (p-T1125)] or an anti-HA monoclonal antibody (Cell Signaling). (D) Cells (YPH250) carrying YCplac111-PKC1-3HA were cultured in SD medium. Immunoprecipitation and Western blotting were performed as described in the legend to panel C, except that anti-phosphorylated Pkc1S1143 antibodies [p-Pkc1 (p-S1143)] were used. (E) Wild-type (TB50a) cells and GAL1p-AVO1 (RL25-1c) cells carrying YCplac111-PKC1-3HA were cultured overnight in SC-Gal medium. After being transferred to SD medium, cells were cultured for 15 h to impair TORC2 activity and were then treated with 10 mM MG for 60 min. Immunoprecipitation and Western blotting were performed as described in the legend to panel C. (F) pkc1Δ (DL376) cells carrying YEp352-PKC1 (pFR22), YEp352-PKC1T1125A, or YEp352-PKC1S1143A (pFR74) were cultured in SD medium until the A610 was 0.4 to 0.6. DL376 cells carrying the vector (YEp352) were cultured in SD medium containing 1 M sorbitol. Cell extracts were subjected to SDS-PAGE followed by Western blotting to determine the protein levels of Pkc1 using anti-Pkc1 antibodies. As the loading control, Pgk1 protein levels were determined using an anti-Pgk1 monoclonal antibody (Molecular Probes). The asterisk indicates a nonspecific band.
Since Thr1125 and Ser1143 in Pkc1 were the direct phosphorylation sites of TORC2 (Fig. 3), we investigated whether these amino acids are necessary for the MG-induced phosphorylation of Mpk1. As shown in Fig. 5B, Mpk1 was slightly phosphorylated in PKC1S1143A cells but not in PKC1T1125A or PKC1T1125A/S1143A cells following treatment with MG. These results indicate that Thr1125 and Ser1143 in Pkc1 are involved in the MG-induced activation of the Mpk1 MAP kinase cascade.
We next determined whether MG enhances the phosphorylation of Pkc1. As shown in Fig. 5C, the phosphorylation levels of Thr1125 in the TM of Pkc1 remained unchanged following treatment with MG. Meanwhile, the phosphorylation levels of Ser1143 in HM were increased by MG treatment (Fig. 5D). To examine whether MG directly affects the activity of TORC2 to increase the phosphorylation level of Pck1 at Ser1143, we added MG in a mixture of the in vitro kinase assay. However, the phosphorylation level of Pkc1Ser1143 was substantially unchanged in the presence of MG (see Fig. S2 in the supplemental material). Next, to verify that the increase in the phosphorylation level of Pkc1 at Ser1143 in MG-treated cells is dependent on TORC2, cells carrying the AVO1 gene driven by the GAL1 promoter were shifted from the galactose medium to the glucose medium in order to reduce the activity of TORC2 (see Fig. S5 in the supplemental material). These cells were then treated with MG. As shown in Fig. 5E, the phosphorylation levels of Ser1143 in TORC2-impaired cells were not increased by treatment with MG. These results imply that MG may have facilitated TORC2 signaling, thereby increasing the phosphorylation level of Pkc1 at Ser1143, which transmitted the signal downstream to the Mpk1 MAP kinase cascade.
The phosphorylation status of Pkc1 at Thr1125 affects its protein levels.
Although Thr1125 in the TM of Pkc1 did not respond to MG (Fig. 5C), the basal phosphorylation level of this site appeared to be necessary for transmitting the signal to the Mpk1 MAP kinase cascade (Fig. 5B). However, it currently remains unclear why the phosphorylation of Mpk1 did not occur in Pkc1T1125A cells following treatment with MG, in spite of Ser1143 being intact. Previous studies reported that the phosphorylation of Thr450 in the TM of Akt secured its stability in mammalian cells (35, 50). To verify whether the replacement of Thr1125 in TM by Ala affects the stability of Pkc1, whereby the MG-triggered signal is not adequately transmitted to the Mpk1 MAP kinase cascade, we determined Pkc1 protein levels. We raised anti-Pkc1 antibodies to monitor Pkc1 protein levels. Although some nonspecific bands appeared in the blots, the Pkc1 antibodies raised were able to detect Pkc1 proteins in cell extracts by Western blotting (see Fig. S6 in the supplemental material). As shown in Fig. 5F, the steady-state levels of the Pkc1T1125A protein were markedly lower than those of the Pkc1WT protein. This may be one of the reasons why the MG-induced phosphorylation of Mpk1 did not occur in Pkc1T1125A cells (Fig. 5B).
Role of phosphorylation of Pkc1 at Thr1125 and Ser1143 under conditions of MG stress.
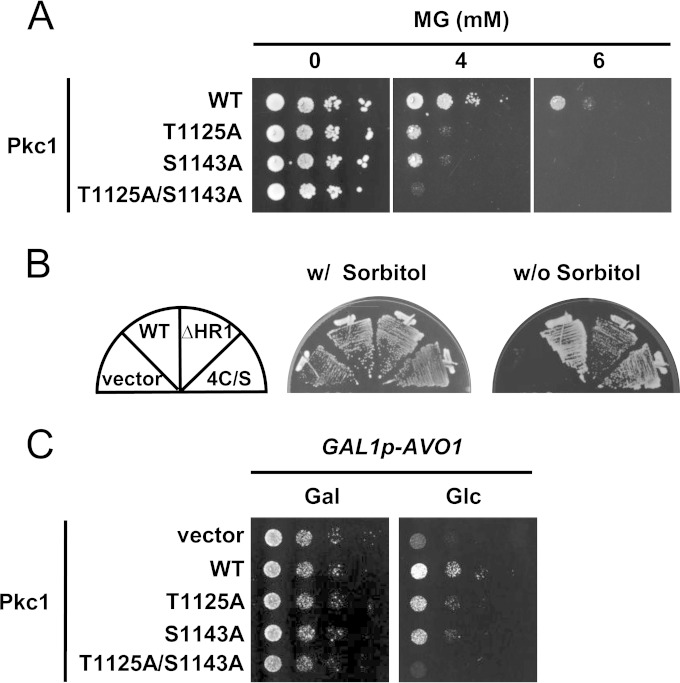
To explore its physiological relevance to the TORC2-dependent phosphorylation of Pkc1, we investigated the effects of the substitution of the phosphorylation sites of Pkc1 on growth and sensitivity to MG. The phosphorylation sites of Pkc1 were found to be irrelevant to the growth of yeast cells. The pkc1Δ mutant is unable to grow in the absence of an osmoprotectant, such as sorbitol, because cell wall biogenesis is defective (51, 52). However, we found that the Pkc1T1125A/S1143A mutant was able to grow in the medium without sorbitol (Fig. 6A). Pkc1 mutants defective in the HR1 and C1 domains, in which the phosphorylation levels of Thr1125 and Ser1143 were reduced (Fig. 4B), were also able to grow without sorbitol (Fig. 6B). These results suggest that the phosphorylation of Thr1125 and Ser1143 in Pkc1 is dispensable for the growth of yeast cells under normal osmotic conditions. However, the replacement of Thr1125 and/or Ser1143 by Ala enhanced the susceptibility of cells to MG (Fig. 6A).
FIG 6.
Physiological significance of the phosphorylation states of Pkc1 at Thr1125 and Ser1143 on the growth of yeast cells. (A) pkc1Δ (DL376) cells carrying various PKC1 alleles (WT, PKC1WT; T1125A, PKC1T1125A; S1143A, PKC1S1143A; T1125A/S1143A, PKC1T1125A/S1143A) with YCp50 (a CEN-type plasmid) were cultured in SD medium until the log phase of growth. Cells were then serially diluted (1:10) with a 0.85% NaCl solution, and 4 μl of each cell suspension was spotted onto SD agar plates containing MG. Cells were grown at 28°C for 3 days. (B) pkc1Δ cells (DL376) carrying various PKC1 alleles (WT, PKC1WT; ΔHR1, PKC1ΔHR1; 4C/S, PKC14C/S) with pFL39 (a CEN-type plasmid) were streaked onto SD agar plates with or without 1 M sorbitol and cultured at 28°C for 3 days. (C) Cells with GAL1 promoter-driven AVO1 (RL25-1C) carrying various PKC1 alleles (WT, PKC1WT; T1125A, PKC1T1125A; S1143A, PKC1S1143A; T1125A/S1143A, PKC1T1125A/S1143A) with YEp352 (a 2μ-type plasmid) were cultured in SC-Gal medium until the log phase of growth. Cells were then serially diluted (1:10) with 0.85% NaCl solution, and 4 μl of each cell suspension was spotted onto SC-Gal (Gal) or SD (Glc) agar plates. Cells were cultured at 28°C for 3 days.
The growth defect in TORC2-knockdown cells was partially suppressed by multiple copies of PKC1WT, PKC1T1125A, or PKC1S1143A without sorbitol in the medium (Fig. 6C). Since the addition of sorbitol to a medium was previously shown to suppress the growth defect in TORC2-knockdown cells (22), suppression of the growth defect in TORC2-impaired cells by multiple copies of PKC1 may be attributed to the recovery of cell wall integrity. However, the growth defect in TORC2-knockdown cells was not suppressed by multiple copies of PKC1T1125A/S1143A. To explore the physiological relationship between TORC2 and Pkc1, we examined the organization of actin. As shown in Fig. S7 in the supplemental material, the proportion of cells with depolarized actin increased when TORC2 was impaired (16.9% of the wild-type cells had depolarized actin, and 59.8% of the TORC2-impaired cells had depolarized actin); however, it reverted to nearly wild-type levels (29.3%) when PKC1 was expressed in a multicopy vector. However, multiple copies of PKC1T1125A/S1143A were not able to recover the polarization of actin. This may have been because of a failure in the growth recovery of TORC2-knockdown cells carrying multiple copies of PKC1T1125A/S1143A.
MG activates mTORC2 signaling in mammalian cells.
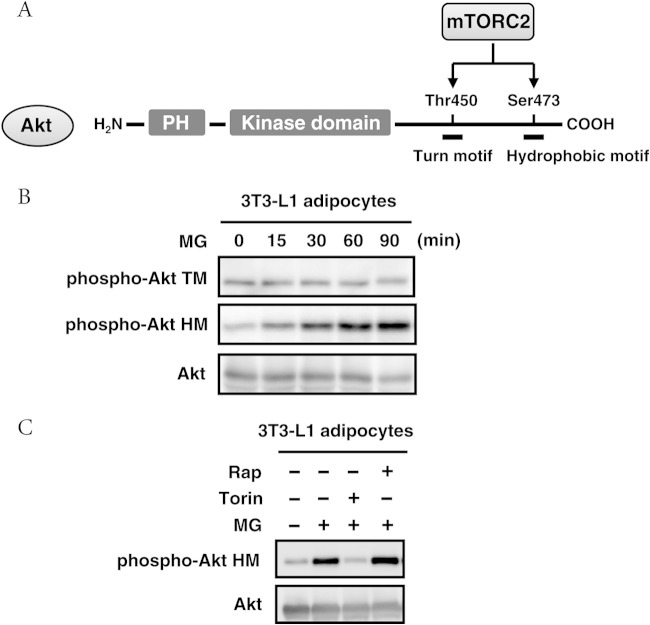
We demonstrated that MG functions as a signal initiator for TORC2 signaling using the budding yeast S. cerevisiae as a model organism. To determine whether this is also the case in higher eukaryotes, we measured the phosphorylation levels of Akt, a substrate of mammalian TORC2 (mTORC2) (Fig. 7A), using mouse 3T3-L1 adipocytes. The phosphorylation level of Ser473 in the HM of Akt increased in adipocytes following treatment with MG (Fig. 7B). Meanwhile, Thr450 in the TM of Akt was constitutively phosphorylated, and its level remained unchanged with MG treatment (Fig. 7B). To verify that the MG-induced phosphorylation of AktSer473 was dependent on mTORC2, we used torin 1 to inhibit mTOR. Rapamycin specifically inhibits TORC1, while torin 1 acts on both mTORC1 and mTORC2 (53). The MG-induced phosphorylation of Ser473 was completely inhibited in the presence of torin 1; however, rapamycin did not inhibit the MG-induced phosphorylation of Ser473 (Fig. 7C). In mouse adipocytes, the phosphorylation of Akt at Ser473 was previously shown to depend on mTORC2 (54). Therefore, these results suggest that MG may function as a signal initiator of mTORC2 signaling in mammalian cells, similar to yeast cells.
FIG 7.
Effects of MG on mTORC2 signaling. (A) Diagram of Akt. PH, the pleckstrin homology domain. (B) Mouse 3T3-L1 adipocytes were treated with 5 mM MG. After being incubated for the prescribed time, the phosphorylation of Thr450 in the turn motif (p-Akt TM) and Ser473 in the hydrophobic motif (p-Akt HM) of Akt was determined using the respective specific antibodies. (C) Mouse 3T3-L1 adipocytes were preincubated with 250 nM torin 1 or 20 nM rapamycin (Rap) for 60 min before treatment with 5 mM MG for 60 min.
DISCUSSION
In the present study, we demonstrated that MG activates the Pkc1-Mpk1 MAP kinase cascade in S. cerevisiae. The activation of this kinase cascade appeared to be necessary for yeast cells to combat MG-induced stress. An important feature of our results is that TORC2 was involved in activating the Pkc1-Mpk1 signaling pathway triggered by MG. We have demonstrated herein for the first time that Pkc1 is a substrate of TORC2; i.e., TORC2 phosphorylated Thr1125 in the TM and Ser1143 in the HM of Pkc1. Genetic studies previously reported a relationship between Tor2 and the Pkc1-Mpk1 pathway; i.e., PKC1 and MPK1 have been isolated as multicopy suppressors that rescue growth defects in the tor2-21ts mutant at nonpermissive temperatures (22, 23, 43). Since the tor2-21ts mutant was defective in TORC2 only at nonpermissive temperatures (22), TORC2 appeared to communicate with the Pkc1-Mpk1 pathway. However, the heat shock-induced activation of Mpk1 was previously shown to be controlled independently of Tor2 (23, 55). We demonstrated that TORC2-Pkc1 signaling is involved in the MG-induced activation of the Mpk1 MAP kinase cascade (a schematic model is shown in Fig. 8). However, since the MG-induced phosphorylation of Mpk1 occurred in wsc1Δ mid2Δ cells as well as in mutants defective in other sensor proteins (Wsc2 or Mtl1), MG appeared to trigger TORC2-Pkc1 signaling via routes different from the known pathway for the heat shock-induced activation of the Pkc1-Mpk1 MAP kinase cascade (Fig. 8).
We have previously demonstrated that MG treatment triggers other cellular responses, including the high-osmolarity glycerol (HOG) pathway and the Ca2+/calcineurin signaling pathway (10, 12). The cytoplasmic membrane proteins Sln1 and Sho1 acted as MG sensors in the activation of the HOG pathway, although the phosphorylation of Mpk1 following treatment with MG occurred in mutants defective in both branches (data not shown). On the other hand, it was reported that both Mpk1 and calcineurin are involved in the regulatory mechanism of G2/M delay by a high dose of Ca2+ (56). However, the MG-induced phosphorylation of Mpk1 occurred in the presence of the Ca2+ chelator EGTA (data not shown) under conditions in which we have previously shown that the uptake of Ca2+ into the cell was inhibited and activation of Ca2+/calcineurin signaling subsequently did not occur (12). Collectively, the MG-triggered stimulation of TORC2-Pkc1 signaling does not seem to cross talk with other MG-induced cellular responses.
The phosphorylation levels of Ser1143 in the HM of Pkc1 were increased following treatment with MG, whereas those of Thr1125 in the TM were not (Fig. 5C and D). Nevertheless, the MG-induced phosphorylation of Mpk1 did not occur in the Pkc1T1125A mutant (Fig. 5B). In mammalian cells, the phosphorylation of Akt at Thr450 in the TM secured the stability of the Akt protein per se (35, 50). We showed that Pkc1 protein levels were markedly decreased when Thr1125 in the TM of Pkc1 was changed to Ala (Fig. 5F). Therefore, the phosphorylation of this site appears to affect the stability of Pkc1 in S. cerevisiae cells. In addition, intriguingly, we noticed that the phosphorylation level of Ser1143 in Pkc1T1125A cells was significantly lower than that in Pkc1WT cells (Fig. 3E). This may be another reason why the phosphorylation of Mpk1 did not occur in Pkc1T1125A cells following treatment with MG. Since not only the Pkc1 protein levels but also the phosphorylation levels of Sre1143 were lower in Pkc1T1125A cells, the MG-triggered signal was not adequately transmitted to the Mpk1 MAP kinase cascade in Pkc1T1125A cells. Therefore, the maintenance of a certain level of phosphorylation at both Thr1125 and Ser1143 appeared to secure the transmittance of the signal downstream to the Mpk1 MAP kinase cascade. To the best of our knowledge, this is the first study to demonstrate that the phosphorylation status of TM affected the phosphorylation levels of HM in AGC kinases.
The heat shock-induced activation of Mpk1 occurs through the activation of Rho1 (38, 39) (Fig. 8). We showed that the basal phosphorylation levels of Pkc1 were decreased by a deficiency in the Pkc1 domains (HR1 and C1 domains) needed for the physical interaction with Rho1 (37, 47–49) (Fig. 4B). Previous studies reported that TORC2 was predominantly located in punctate structures adjacent to the cytoplasmic membrane in S. cerevisiae cells (57, 58). Since TORC2 exists beneath the cytoplasmic membrane, its substrate (Pkc1) also must be present near the cytoplasmic membrane, in which the enzyme (TORC2) exists. Rho1 is associated with the plasma membrane (38, 59); therefore, the physical interaction between Rho1 and Pkc1 may guarantee contact between TORC2 and Pkc1. In addition to the physical interaction with Rho1, the C1 domain of Pkc1 has been suggested to bind to lipids (49, 55). A previous study using multiple copies of green fluorescent protein-labeled Pkc1 showed that Pkc1 localized to the bud cortex or bud neck (60). In addition, the C1 domain of Pkc1 was shown to be sufficient for its peripheral localization in the cell (60); therefore, the C1 domain of Pkc1 may contribute to TORC2-Pkc1 signaling not only by interacting with Rho1 but also by binding to lipids in the plasma membrane, thereby allowing Pkc1 to interact with TORC2 beneath the cytoplasmic membrane. Overexpression of the C1 domain may compete with endogenous Pkc1 to interfere with the interaction with Rho1, thereby inhibiting the MG-induced phosphorylation of Mpk1 (Fig. 4D). A possible explanation for these results is that both the HR1 and C1 domains are necessary for Pkc1 to be present near the cytoplasmic membrane through a physical interaction with Rho1, whereby TORC2 phosphorylates Pkc1.
The physiological significance of the MG-induced activation of TORC2-Pkc1 signaling in yeast cells is the prevention of physiological disorders caused by MG. We demonstrated here that MG also facilitates mTORC2 signaling in mammalian cells. Although the mechanism by which MG activates TORC2 currently remains unknown, a study is now being conducted to elucidate the mechanism underlying the sensing of MG and facilitation of TORC2 signaling using yeast as a model organism and also to determine the common physiological significance of MG-induced TORC2 signaling in eukaryotes.
Supplementary Material
ACKNOWLEDGMENTS
We thank T. Kawada, N. Takahashi, T. Goto, Y. Kim, S. Izawa, and K. Maeta for their technical support and helpful discussions. We are grateful to M. N. Hall, D. E. Levin, T. Miyakawa, J. Thorner, Y. Ohya, J. J. Heinisch, M. Y. Chen, M. S. Cyert, J. R. Broach, K. Irie, and the National Bio-Resource Project (NBRP) of MEXT, Japan, for providing the plasmids and yeast strains.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/MCB.01118-14.
REFERENCES
- 1.Rochette L, Zeller M, Cottin Y, Vergely C. 2014. Diabetes, oxidative stress and therapeutic strategies. Biochim Biophys Acta 1840:2709–2729. doi: 10.1016/j.bbagen.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 2.Schieber M, Chandel NS. 2014. ROS function in redox signaling and oxidative stress. Curr Biol 24:R453–R462. doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Inoue Y, Maeta K, Nomura W. 2011. Glyoxalase system in yeasts: structure, function, and physiology. Semin Cell Dev Biol 22:278–284. doi: 10.1016/j.semcdb.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 4.Thornalley PJ. 2008. Protein and nucleotide damage by glyoxal and methylglyoxal in physiological systems—role in ageing and disease. Drug Metabol Drug Interact 23:125–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rabbani N, Thornalley PJ. 2011. Glyoxalase in diabetes, obesity and related disorders. Semin Cell Dev Biol 22:309–317. doi: 10.1016/j.semcdb.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 6.Matafome P, Sena C, Seiça R. 2013. Methylglyoxal, obesity, and diabetes. Endocrine 43:472–484. doi: 10.1007/s12020-012-9795-8. [DOI] [PubMed] [Google Scholar]
- 7.Angeloni C, Zambonin L, Hrelia S. 2014. Role of methylglyoxal in Alzheimer's disease. Biomed Res Int 2014:238485. doi: 10.1155/2014/238485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zoccali C, Mallamaci F, Tripepi G. 2000. AGEs and carbonyl stress: potential pathogenetic factors of long-term uraemic complications. Nephrol Dial Transplant 15:7–11. doi: 10.1093/ndt/15.suppl_1.7. [DOI] [PubMed] [Google Scholar]
- 9.Thornalley PJ. 2005. Dicarbonyl intermediates in the Maillard reaction. Ann N Y Acad Sci 1043:111–117. doi: 10.1196/annals.1333.014. [DOI] [PubMed] [Google Scholar]
- 10.Maeta K, Izawa S, Okazaki S, Kuge S, Inoue Y. 2004. Activity of the Yap1 transcription factor in Saccharomyces cerevisiae is modulated by methylglyoxal, a metabolite derived from glycolysis. Mol Cell Biol 24:8753–8764. doi: 10.1128/MCB.24.19.8753-8764.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takatsume Y, Izawa S, Inoue Y. 2006. Methylglyoxal as a signal initiator for activation of the stress-activated protein kinase cascade in the fission yeast Schizosaccharomyces pombe. J Biol Chem 281:9086–9092. doi: 10.1074/jbc.M511037200. [DOI] [PubMed] [Google Scholar]
- 12.Maeta K, Izawa S, Inoue Y. 2005. Methylglyoxal, a metabolite derived from glycolysis, functions as a signal initiator of the high osmolarity glycerol-mitogen-activated protein kinase cascade and calcineurin/Crz1-mediated pathway in Saccharomyces cerevisiae. J Biol Chem 280:253–260. doi: 10.1074/jbc.M408061200. [DOI] [PubMed] [Google Scholar]
- 13.Laplante M, Sabatini DM. 2012. mTOR signaling in growth control and disease. Cell 149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loewith R, Hall MN. 2011. Target of rapamycin (TOR) in nutrient signaling and growth control. Genetics 189:1177–1201. doi: 10.1534/genetics.111.133363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loewith R, Jacinto E, Wullschleger S, Lorberg A, Crespo JL, Bonenfant D, Oppliger W, Jenoe P, Hall MN. 2002. Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol Cell 10:457–468. doi: 10.1016/S1097-2765(02)00636-6. [DOI] [PubMed] [Google Scholar]
- 16.Jacinto E, Lorberg A. 2008. TOR regulation of AGC kinases in yeast and mammals. Biochem J 410:19–37. doi: 10.1042/BJ20071518. [DOI] [PubMed] [Google Scholar]
- 17.Kamada Y, Fujioka Y, Suzuki NN, Inagaki F, Wullschleger S, Loewith R, Hall MN, Ohsumi Y. 2005. Tor2 directly phosphorylates the AGC kinase Ypk2 to regulate actin polarization. Mol Cell Biol 25:7239–7248. doi: 10.1128/MCB.25.16.7239-7248.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Niles BJ, Mogri H, Hill A, Vlahakis A, Powers T. 2012. Plasma membrane recruitment and activation of the AGC kinase Ypk1 is mediated by target of rapamycin complex 2 (TORC2) and its effector proteins Slm1 and Slm2. Proc Natl Acad Sci U S A 109:1536–1541. doi: 10.1073/pnas.1117563109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Urban J, Soulard A, Huber A, Lippman S, Mukhopadhyay D, Deloche O, Wanke V, Anrather D, Ammerer G, Riezman H, Broach JR, De Virgilio C, Hall MN, Loewith R. 2007. Sch9 is a major target of TORC1 in Saccharomyces cerevisiae. Mol Cell 26:663–674. doi: 10.1016/j.molcel.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 20.Antonsson B, Montessuit S, Friedli L, Payton MA, Paravicini G. 1994. Protein kinase C in yeast. Characteristics of the Saccharomyces cerevisiae PKC1 gene product. J Biol Chem 269:16821–16828. [PubMed] [Google Scholar]
- 21.Watanabe M, Chen CY, Levin DE. 1994. Saccharomyces cerevisiae PKC1 encodes a protein kinase C (PKC) homolog with a substrate specificity similar to that of mammalian PKC. J Biol Chem 269:16829–16836. [PubMed] [Google Scholar]
- 22.Helliwell SB, Howald I, Barbet N, Hall MN. 1998. TOR2 is part of two related signaling pathways coordinating cell growth in Saccharomyces cerevisiae. Genetics 148:99–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Helliwell SB, Schmidt A, Ohya Y, Hall MN. 1998. The Rho1 effector Pkc1, but not Bni1, mediates signalling from Tor2 to the actin cytoskeleton. Curr Biol 8:1211–1214. doi: 10.1016/S0960-9822(07)00511-8. [DOI] [PubMed] [Google Scholar]
- 24.Ho HL, Shiau YS, Chen MY. 2005. Saccharomyces cerevisiae TSC11/AVO3 participates in regulating cell integrity and functionally interacts with components of the Tor2 complex. Curr Genet 47:273–288. doi: 10.1007/s00294-005-0570-8. [DOI] [PubMed] [Google Scholar]
- 25.Cybulski N, Hall MN. 2009. TOR complex 2: a signaling pathway of its own. Trends Biochem Sci 34:620–627. doi: 10.1016/j.tibs.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 26.Dazert E, Hall MN. 2011. mTOR signaling in disease. Curr Opin Cell Biol 23:744–755. doi: 10.1016/j.ceb.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 27.Zoncu R, Efeyan A, Sabatini DM. 2011. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol 12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. 2005. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 29.Gueldener U, Heinisch J, Koehler GJ, Voss D, Hegemann JH. 2002. A second set of loxP marker cassettes for Cre-mediated multiple gene knockouts in budding yeast. Nucleic Acids Res 30:e23. doi: 10.1093/nar/30.6.e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sekiya-Kawasaki M, Abe M, Saka A, Watanabe D, Kono K, Minemura-Asakawa M, Ishihara S, Watanabe T, Ohya Y. 2002. Dissection of upstream regulatory components of the Rho1p effector, 1,3-β-glucan synthase, in Saccharomyces cerevisiae. Genetics 162:663–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakamura T, Ohmoto T, Hirata D, Tsuchiya E, Miyakawa T. 1996. Genetic evidence for the functional redundancy of the calcineurin- and Mpk1-mediated pathways in the regulation of cellular events important for growth in Saccharomyces cerevisiae. Mol Gen Genet 251:211–219. doi: 10.1007/BF02172920. [DOI] [PubMed] [Google Scholar]
- 32.Irie K, Takase M, Lee KS, Levin DE, Araki H, Matsumoto K, Oshima Y. 1993. MKK1 and MKK2, which encode Saccharomyces cerevisiae mitogen-activated protein kinase-kinase homologs, function in the pathway mediated by protein kinase C. Mol Cell Biol 13:3076–3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schägger H. 2006. Tricine-SDS-PAGE. Nat Protoc 1:16–22. doi: 10.1038/nprot.2006.4. [DOI] [PubMed] [Google Scholar]
- 34.Goto T, Lee JY, Teraminami A, Kim YI, Hirai S, Uemura T, Inoue H, Takahashi N, Kawada T. 2011. Activation of peroxisome proliferator-activated receptor-alpha stimulates both differentiation and fatty acid oxidation in adipocytes. J Lipid Res 52:873–884. doi: 10.1194/jlr.M011320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Facchinetti V, Ouyang W, Wei H, Soto N, Lazorchak A, Gould C, Lowry C, Newton AC, Mao Y, Miao RQ, Sessa WC, Qin J, Zhang P, Su B, Jacinto E. 2008. The mammalian target of rapamycin complex 2 controls folding and stability of Akt and protein kinase C. EMBO J 27:1932–1943. doi: 10.1038/emboj.2008.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pruyne D, Bretscher A. 2000. Polarization of cell growth in yeast. J Cell Sci 113(Pt 4):571–585. [DOI] [PubMed] [Google Scholar]
- 37.Nonaka H, Tanaka K, Hirano H, Fujiwara T, Kohno H, Umikawa M, Mino A, Takai Y. 1995. A downstream target of RHO1 small GTP-binding protein is PKC1, a homolog of protein kinase C, which leads to activation of the MAP kinase cascade in Saccharomyces cerevisiae. EMBO J 14:5931–5938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levin DE. 2011. Regulation of cell wall biogenesis in Saccharomyces cerevisiae: the cell wall integrity signaling pathway. Genetics 189:1145–1175. doi: 10.1534/genetics.111.128264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen RE, Thorner J. 2007. Function and regulation in MAPK signaling pathways: lessons learned from the yeast Saccharomyces cerevisiae. Biochim Biophys Acta 1773:1311–1340. doi: 10.1016/j.bbamcr.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Philip B, Levin DE. 2001. Wsc1 and Mid2 are cell surface sensors for cell wall integrity signaling that act through Rom2, a guanine nucleotide exchange factor for Rho1. Mol Cell Biol 21:271–280. doi: 10.1128/MCB.21.1.271-280.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilk S, Wittland J, Thywissen A, Schmitz HP, Heinisch JJ. 2010. A block of endocytosis of the yeast cell wall integrity sensors Wsc1 and Wsc2 results in reduced fitness in vivo. Mol Genet Genomics 284:217–229. doi: 10.1007/s00438-010-0563-2. [DOI] [PubMed] [Google Scholar]
- 42.Jin C, Parshin AV, Daly I, Strich R, Cooper KF. 2013. The cell wall sensors Mtl1, Wsc1, and Mid2 are required for stress-induced nuclear to cytoplasmic translocation of cyclin C and programmed cell death in yeast. Oxid Med Cell Longev 2013:320823. doi: 10.1155/2013/320823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schmidt A, Bickle M, Beck T, Hall MN. 1997. The yeast phosphatidylinositol kinase homolog TOR2 activates RHO1 and RHO2 via the exchange factor ROM2. Cell 88:531–542. doi: 10.1016/S0092-8674(00)81893-0. [DOI] [PubMed] [Google Scholar]
- 44.Roelants FM, Torrance PD, Thorner J. 2004. Differential roles of PDK1- and PDK2-phosphorylation sites in the yeast AGC kinases Ypk1, Pkc1 and Sch9. Microbiology 150:3289–3304. doi: 10.1099/mic.0.27286-0. [DOI] [PubMed] [Google Scholar]
- 45.Wullschleger S, Loewith R, Oppliger W, Hall MN. 2005. Molecular organization of target of rapamycin complex 2. J Biol Chem 280:30697–30704. doi: 10.1074/jbc.M505553200. [DOI] [PubMed] [Google Scholar]
- 46.Saka A, Abe M, Okano H, Minemura M, Qadota H, Utsugi T, Mino A, Tanaka K, Takai Y, Ohya Y. 2001. Complementing yeast rho1 mutation groups with distinct functional defects. J Biol Chem 276:46165–46171. doi: 10.1074/jbc.M103805200. [DOI] [PubMed] [Google Scholar]
- 47.Schmitz HP, Lorberg A, Heinisch JJ. 2002. Regulation of yeast protein kinase C activity by interaction with the small GTPase Rho1p through its amino-terminal HR1 domain. Mol Microbiol 44:829–840. doi: 10.1046/j.1365-2958.2002.02925.x. [DOI] [PubMed] [Google Scholar]
- 48.Schmitz HP, Jöckel J, Block C, Heinisch JJ. 2001. Domain shuffling as a tool for investigation of protein function: substitution of the cysteine-rich region of Raf kinase and PKC eta for that of yeast Pkc1p. J Mol Biol 311:1–7. doi: 10.1006/jmbi.2001.4848. [DOI] [PubMed] [Google Scholar]
- 49.Jacoby JJ, Schmitz HP, Heinisch JJ. 1997. Mutants affected in the putative diacylglycerol binding site of yeast protein kinase C. FEBS Lett 417:219–222. doi: 10.1016/S0014-5793(97)01287-8. [DOI] [PubMed] [Google Scholar]
- 50.Ikenoue T, Inoki K, Yang Q, Zhou X, Guan KL. 2008. Essential function of TORC2 in PKC and Akt turn motif phosphorylation, maturation and signalling. EMBO J 27:1919–1931. doi: 10.1038/emboj.2008.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Levin DE, Bartlett-Heubusch E. 1992. Mutants in the S. cerevisiae PKC1 gene display a cell cycle-specific osmotic stability defect. J Cell Biol 116:1221–1229. doi: 10.1083/jcb.116.5.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Paravicini G, Cooper M, Friedli L, Smith DJ, Carpentier JL, Klig LS, Payton MA. 1992. The osmotic integrity of the yeast cell requires a functional PKC1 gene product. Mol Cell Biol 12:4896–4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thoreen CC, Kang SA, Chang JW, Liu Q, Zhang J, Gao Y, Reichling LJ, Sim T, Sabatini DM, Gray NS. 2009. An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. J Biol Chem 284:8023–8032. doi: 10.1074/jbc.M900301200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hresko RC, Mueckler M. 2005. mTOR · RICTOR is the Ser473 kinase for Akt/protein kinase B in 3T3-L1 adipocytes. J Biol Chem 280:40406–40416. doi: 10.1074/jbc.M508361200. [DOI] [PubMed] [Google Scholar]
- 55.Levin DE. 2005. Cell wall integrity signaling in Saccharomyces cerevisiae. Microbiol Mol Biol Rev 69:262–291. doi: 10.1128/MMBR.69.2.262-291.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mizunuma M, Hirata D, Miyahara K, Tsuchiya E, Miyakawa T. 1998. Role of calcineurin and Mpk1 in regulating the onset of mitosis in budding yeast. Nature 392:303–306. doi: 10.1038/32695. [DOI] [PubMed] [Google Scholar]
- 57.Berchtold D, Walther TC. 2009. TORC2 plasma membrane localization is essential for cell viability and restricted to a distinct domain. Mol Biol Cell 20:1565–1575. doi: 10.1091/mbc.E08-10-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sturgill TW, Cohen A, Diefenbacher M, Trautwein M, Martin DE, Hall MN. 2008. TOR1 and TOR2 have distinct locations in live cells. Eukaryot Cell 7:1819–1830. doi: 10.1128/EC.00088-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Inoue SB, Qadota H, Arisawa M, Watanabe T, Ohya Y. 1999. Prenylation of Rho1p is required for activation of yeast 1,3-β-glucan synthase. J Biol Chem 274:38119–38124. doi: 10.1074/jbc.274.53.38119. [DOI] [PubMed] [Google Scholar]
- 60.Denis V, Cyert MS. 2005. Molecular analysis reveals localization of Saccharomyces cerevisiae protein kinase C to sites of polarized growth and Pkc1p targeting to the nucleus and mitotic spindle. Eukaryot Cell 4:36–45. doi: 10.1128/EC.4.1.36-45.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.